Honeycomb Structured Catalysts for H2 Production via H2S Oxidative Decomposition
Abstract
1. Introduction
2. Results and Discussion
2.1. Structured Catalyst Characterization
2.2. Catalytic Activity Test
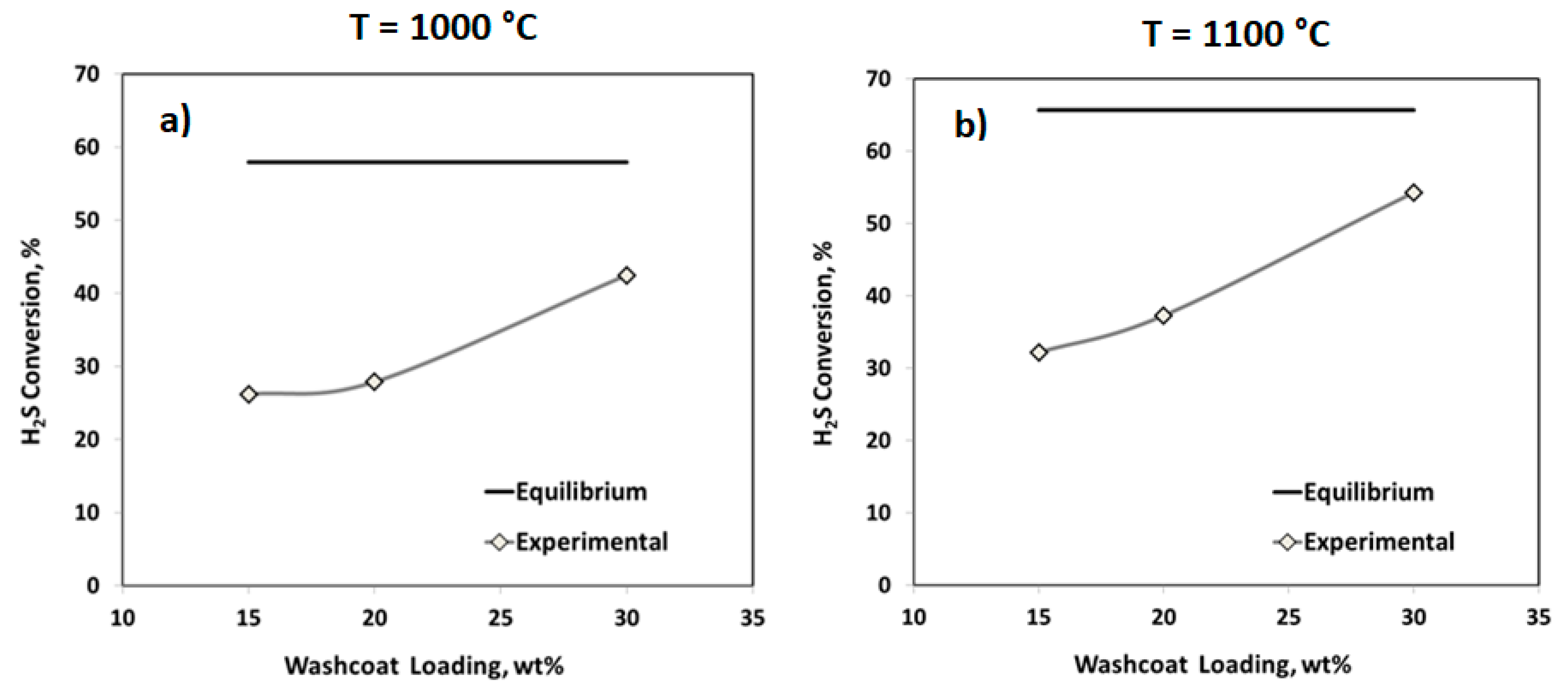
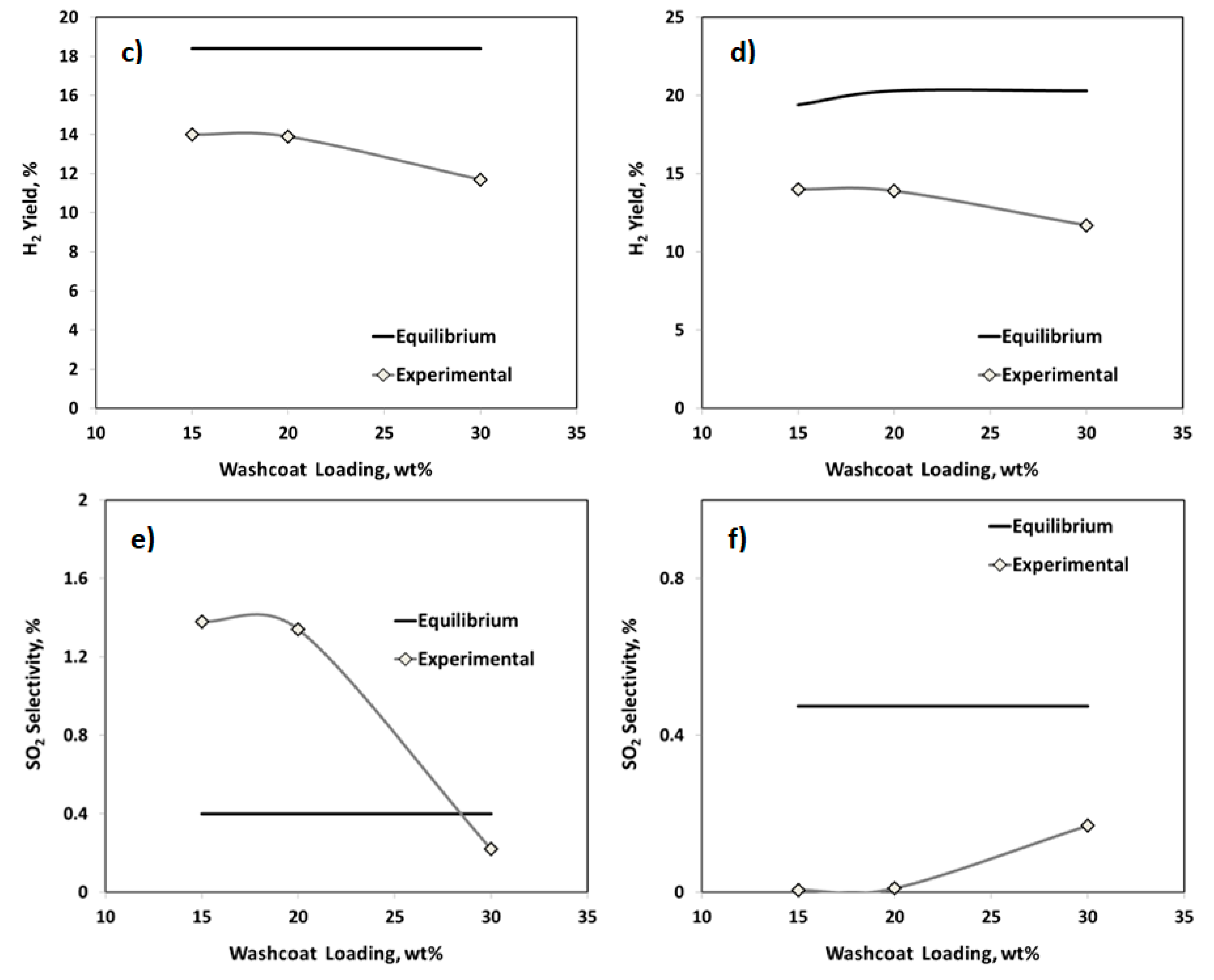
2.2.1. Effect of the Washcoat Loading
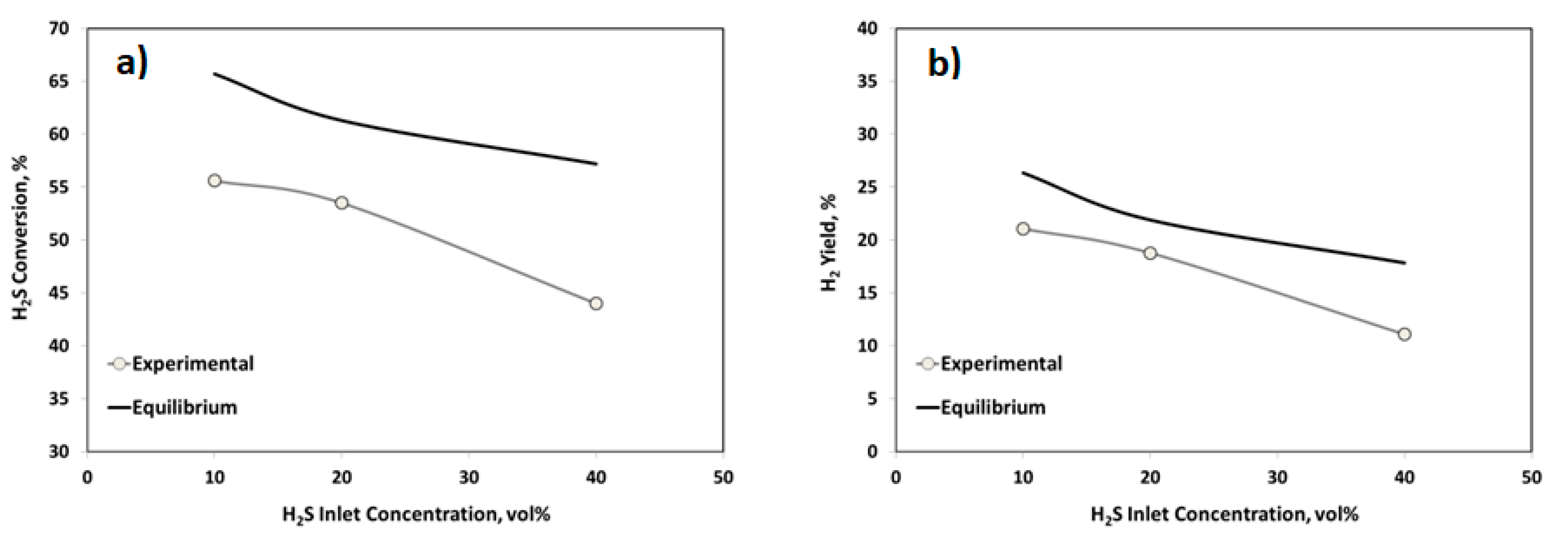
2.2.2. Influence of the Contact Time and H2S Inlet Concentration
2.2.3. Comparison between Powder and Structured Catalyst
3. Experimental and Methods
3.1. Materials
3.2. Structured Catalyst Preparation and Characterization Tests
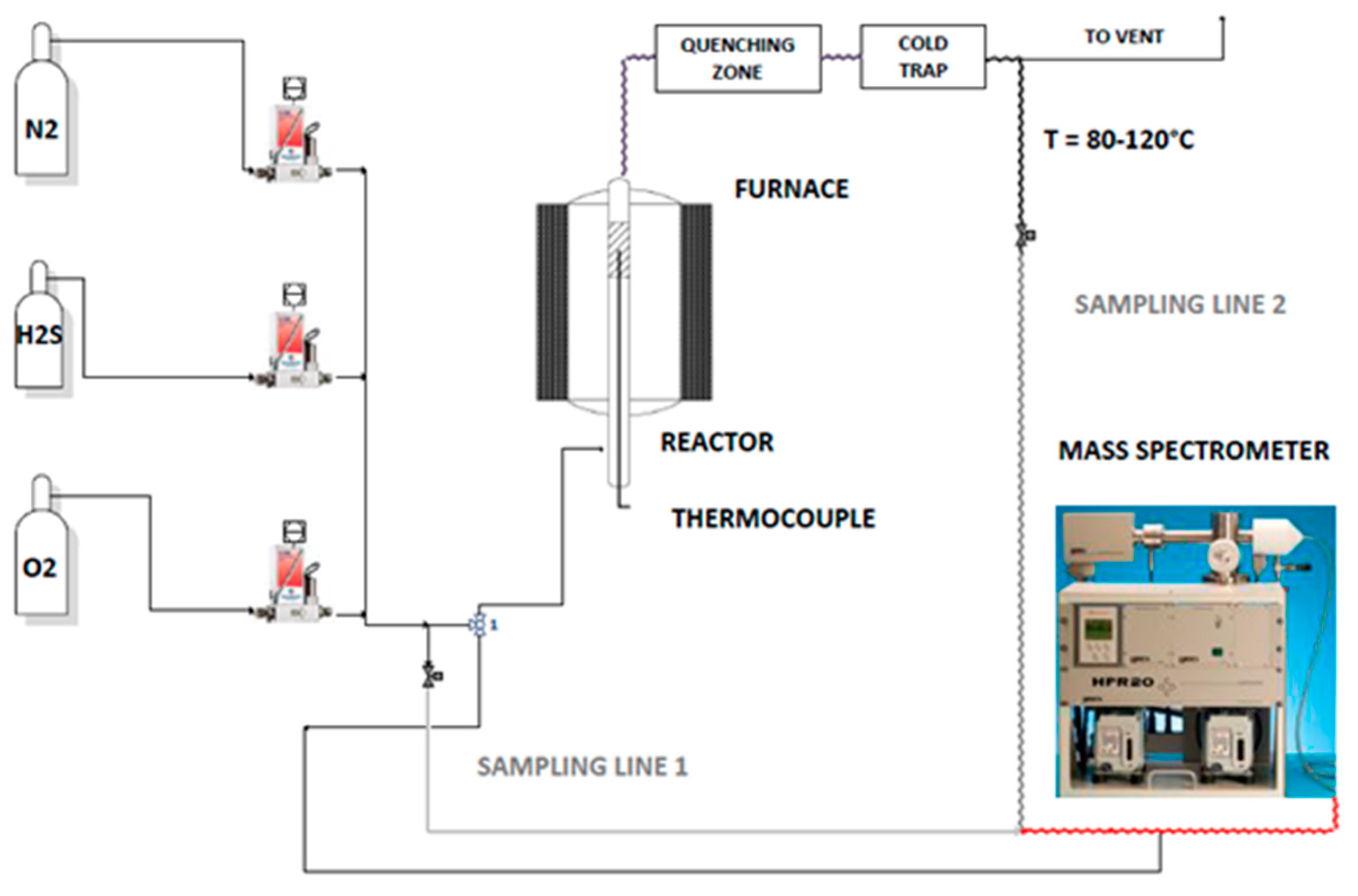
3.3. Experimental Setup
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zaman, J.; Chakma, A. Production of hydrogen and sulfur from hydrogen sulfide. Fuel Process. Technol. 1995, 41, 159–198. [Google Scholar] [CrossRef]
- Dowling, N.I.; Hyne, J.B.; Brown, D.M. Kinetics of the reaction between hydrogen and sulfur under high-temperature Claus furnace conditions. Ind. Eng. Chem. Res. 1990, 29, 2327–2332. [Google Scholar] [CrossRef]
- Palo, E.; Barbato, L.; Colozzi, M.; Angelini, F.; Palma, V.; Vaiano, V. Catalyst for a Sulphur Recovery Process with Concurrent Hydrogen Production, Method of Making Thereof and the Sulphur Recovery Process with Concurrent Hydrogen Production Using the Catalyst. Patent No. WO2014073966 A1, 15 May 2014. [Google Scholar]
- Reshetenko, T.V.; Khairulin, S.R.; Ismagilov, Z.R.; Kuznetsov, V.V. Study of the Reaction of High-Temperature H2S Decomposition on Metal Oxides (γ-Al2O3; α-Fe2O3;V2O5). Int. J. Hydrogen Energy 2002, 27, 387–394. [Google Scholar] [CrossRef]
- Palma, V.; Vaiano, V.; Barba, D.; Colozzi, M.; Palo, E.; Barbato, L.; Cortese, S. H2 Production by Thermal Decomposition of H2S in the Presence of Oxygen. Int. J. Hydrogen Energy 2015, 40, 106–113. [Google Scholar] [CrossRef]
- Palma, V.; Vaiano, V.; Barba, D.; Colozzi, M.; Palo, E.; Barbato, L.; Cortese, S. H2S Oxidative decomposition for the simultaneous production of sulphur and hydrogen. Chem. Eng. Trans. 2016, 52, 1201–1206. [Google Scholar]
- Palma, V.; Vaiano, V.; Barba, D.; Colozzi, M.; Palo, E.; Barbato, L.; Cortese, S. Oxidative Decomposition of H2S over Alumina-Based Catalyst. Ind. Eng. Chem. Res. 2017, 56, 9072–9078. [Google Scholar] [CrossRef]
- Heck, R.M.; Gulati, S.; Farrauto, R.J. The application of monoliths for gas phase catalytic reaction. Chem. Eng. J. 2001, 82, 149–156. [Google Scholar] [CrossRef]
- Nijhuis, T.A.; Beers, A.E.W.; Vergunst, T.; Hoek, I.; Kapteijin, F.; Moulijn, J.A. Preparation of Monolithic Catalysts. Catal. Rev. 2001, 43, 345. [Google Scholar] [CrossRef]
- Metkar, P.S.; Harold, M.P.; Balakotaiah, V. Selective catalytic reduction of NOx on combined Fe- and Cu-zeolite monolithic catalysts: Sequential and dual layer configurations. Appl. Catal. B Environ. 2012, 111–112, 67–80. [Google Scholar] [CrossRef]
- Williams, J.L. Monolith structures, materials, properties and uses. Catal. Today 2001, 69, 3–9. [Google Scholar] [CrossRef]
- Kikuchi, R.; Maeda, S.; Sasaki, K.; Wennerstrom, S.; Ozawa, Y. Catalytic activity of oxide supported Pd catalysts on a honeycomb for low-temperature methane oxidation. Appl. Catal. A Gen. 2003, 239, 169–179. [Google Scholar] [CrossRef]
- Thi Mai, P.P.; Tien, N.T.; Le Minh, T.; Van Driessche, I. The Application of High Surface Area Cordierite Synthesized from Kaolin as a Substrate for Auto Exhaust Catalysts. J. Chin. Chem. Soc. 2015, 6, 536–546. [Google Scholar]
- Shigapov, A.N.; Graham, G.W.; McCabe, R.W.; Peck, M.P.; Plummer, H.K., Jr. The preparation of high-surface-area cordierite monolith by acid treatment. Appl. Catal. A Gen. 1999, 182, 137–146. [Google Scholar] [CrossRef]
- Dongfang, W.; Zhang, H. Mechanical stability of monolithic catalysts: Scattering of washcoat adhesion and failure mechanism of active material. Ind. Eng. Chem. Res. 2013, 52, 14713–14721. [Google Scholar]
- Gonzo, E.E.; Gottifredi, J.C. Heat and Mass Transfer Limitations In Monolith Reactor Simulation With Non Uniform Washcoat Thickness. Lat. Am. Appl. Res. 2010, 40, 15–21. [Google Scholar]
- Valentini, M.; Groppi, G.; Cristiani, C.; Levi, M.; Tronconi, E.; Forzatti, P. The deposition of γ-Al2O3 layers on ceramic and metallic supports for the preparation of structured catalysts. Catal. Today 2001, 69, 307–314. [Google Scholar] [CrossRef]
- Jiang, P.; Guanzhong, L.; Guo, Y.; Guo, Y.; Zhang, S.; Wang, X. Preparation and properties of a γ-Al2O3 washcoat deposited on a ceramic honeycomb. Surf. Coat. Technol. 2005, 190, 314–320. [Google Scholar] [CrossRef]






| SAMPLE | SSA (m2/g) |
|---|---|
| Cordierite | 0.8 |
| Washcoat 15 wt% | 8 |
| Washcoat 20 wt% | 13 |
| Washcoat 30 wt% | 29.0 |
| Powder Catalyst | Structured Catalyst | |
|---|---|---|
| H2S Conversion, % | 57 ± 5 | 56 ± 5 |
| H2 Yield, % | 21 ± 3 | 21 ± 3 |
| SO2 Selectivity, % | 0.5 ± 0.05 | 0.2 ± 0.05 |
| Samples | Average Thickness (μm) |
|---|---|
| Washcoat 15 wt% | 46 |
| Washcoat 20 wt% | 64 |
| Washcoat 30 wt% | 90 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma, V.; Barba, D.; Vaiano, V.; Colozzi, M.; Palo, E.; Barbato, L.; Cortese, S.; Miccio, M. Honeycomb Structured Catalysts for H2 Production via H2S Oxidative Decomposition. Catalysts 2018, 8, 488. https://doi.org/10.3390/catal8110488
Palma V, Barba D, Vaiano V, Colozzi M, Palo E, Barbato L, Cortese S, Miccio M. Honeycomb Structured Catalysts for H2 Production via H2S Oxidative Decomposition. Catalysts. 2018; 8(11):488. https://doi.org/10.3390/catal8110488
Chicago/Turabian StylePalma, Vincenzo, Daniela Barba, Vincenzo Vaiano, Michele Colozzi, Emma Palo, Lucia Barbato, Simona Cortese, and Marino Miccio. 2018. "Honeycomb Structured Catalysts for H2 Production via H2S Oxidative Decomposition" Catalysts 8, no. 11: 488. https://doi.org/10.3390/catal8110488
APA StylePalma, V., Barba, D., Vaiano, V., Colozzi, M., Palo, E., Barbato, L., Cortese, S., & Miccio, M. (2018). Honeycomb Structured Catalysts for H2 Production via H2S Oxidative Decomposition. Catalysts, 8(11), 488. https://doi.org/10.3390/catal8110488








