Enhanced Ricinoleic Acid Preparation Using Lipozyme TLIM as a Novel Biocatalyst: Optimized by Response Surface Methodology
Abstract
1. Introduction
2. Results and Discussion
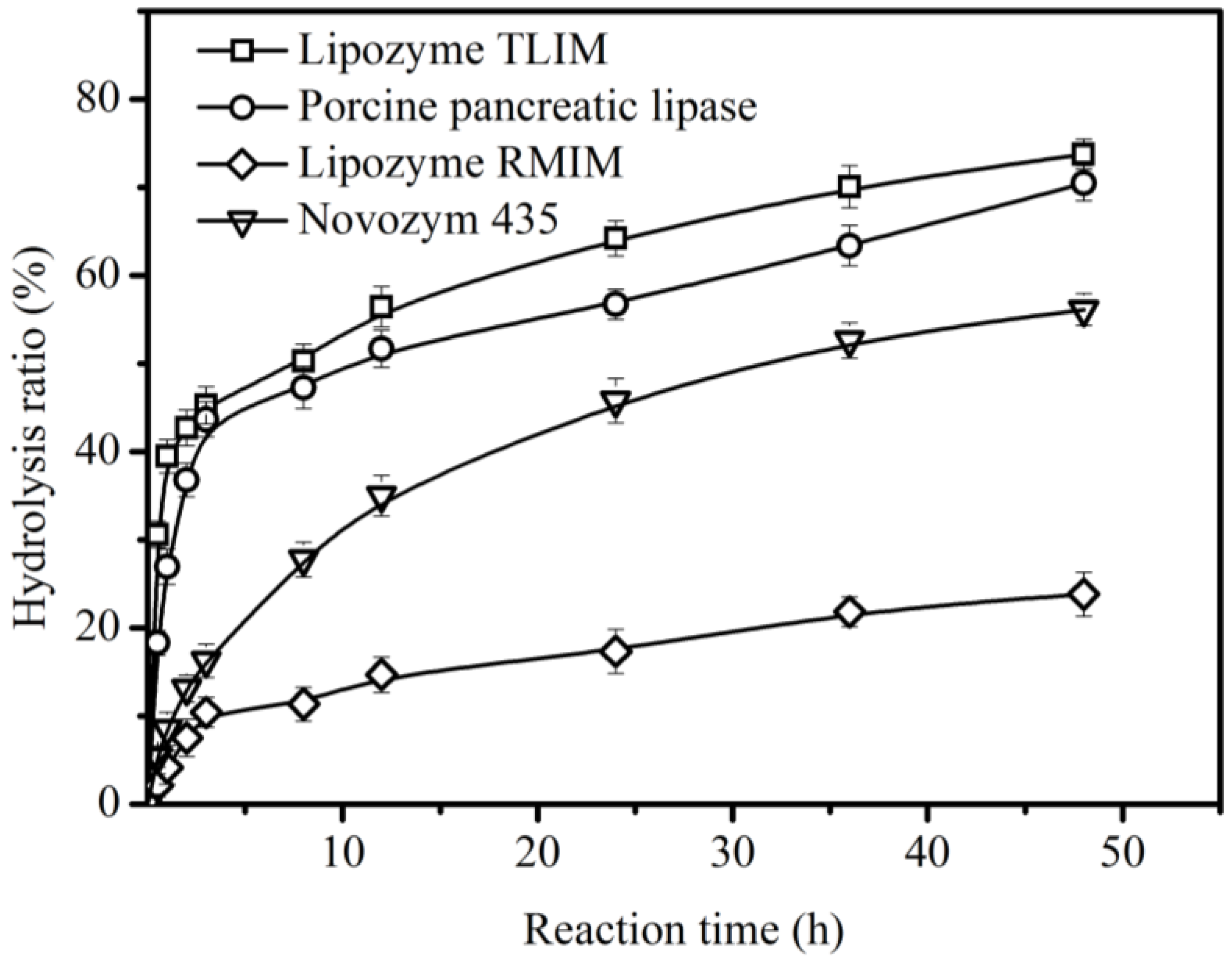
2.1. Enzyme Screening
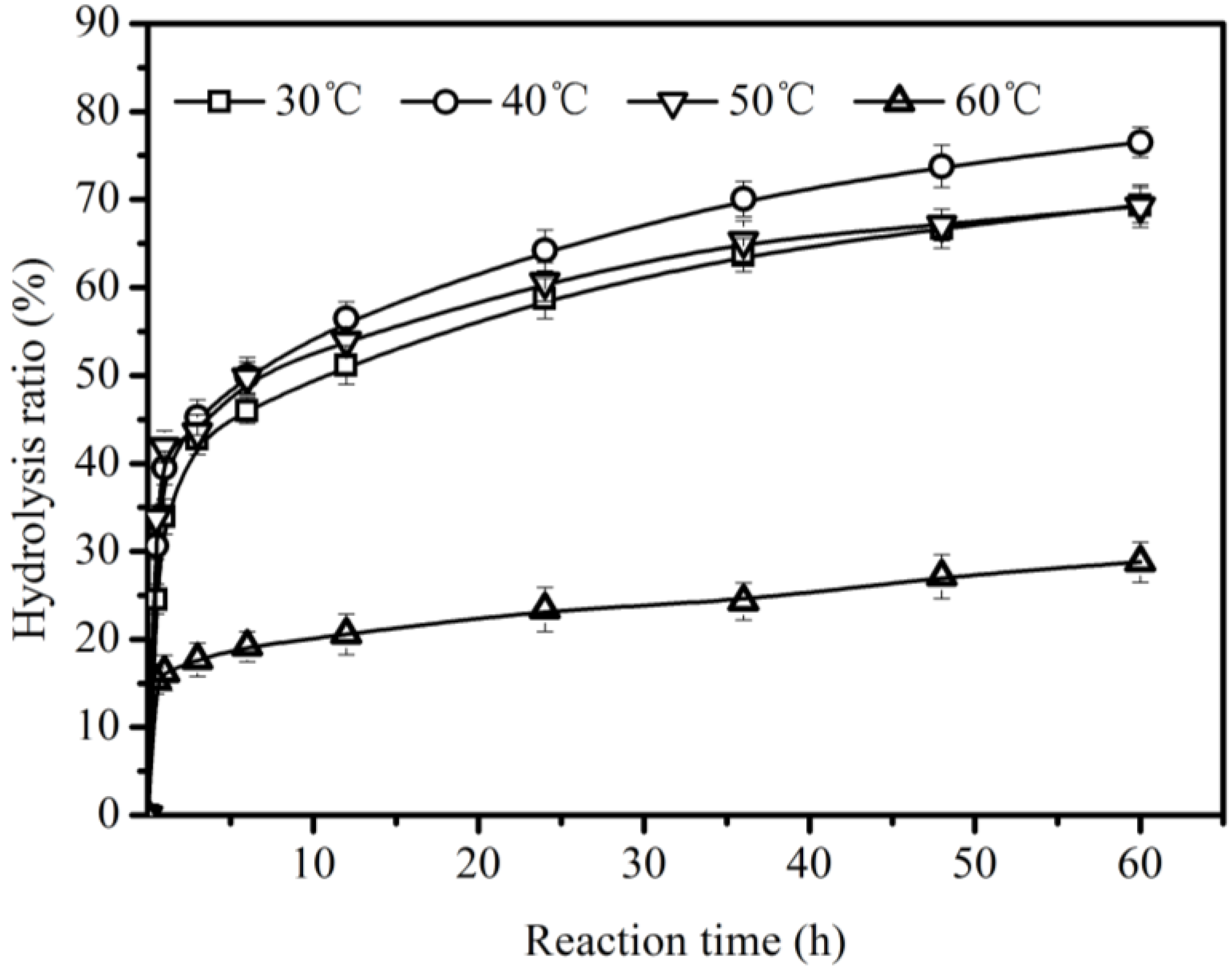
2.2. Effect of Reaction Temperature
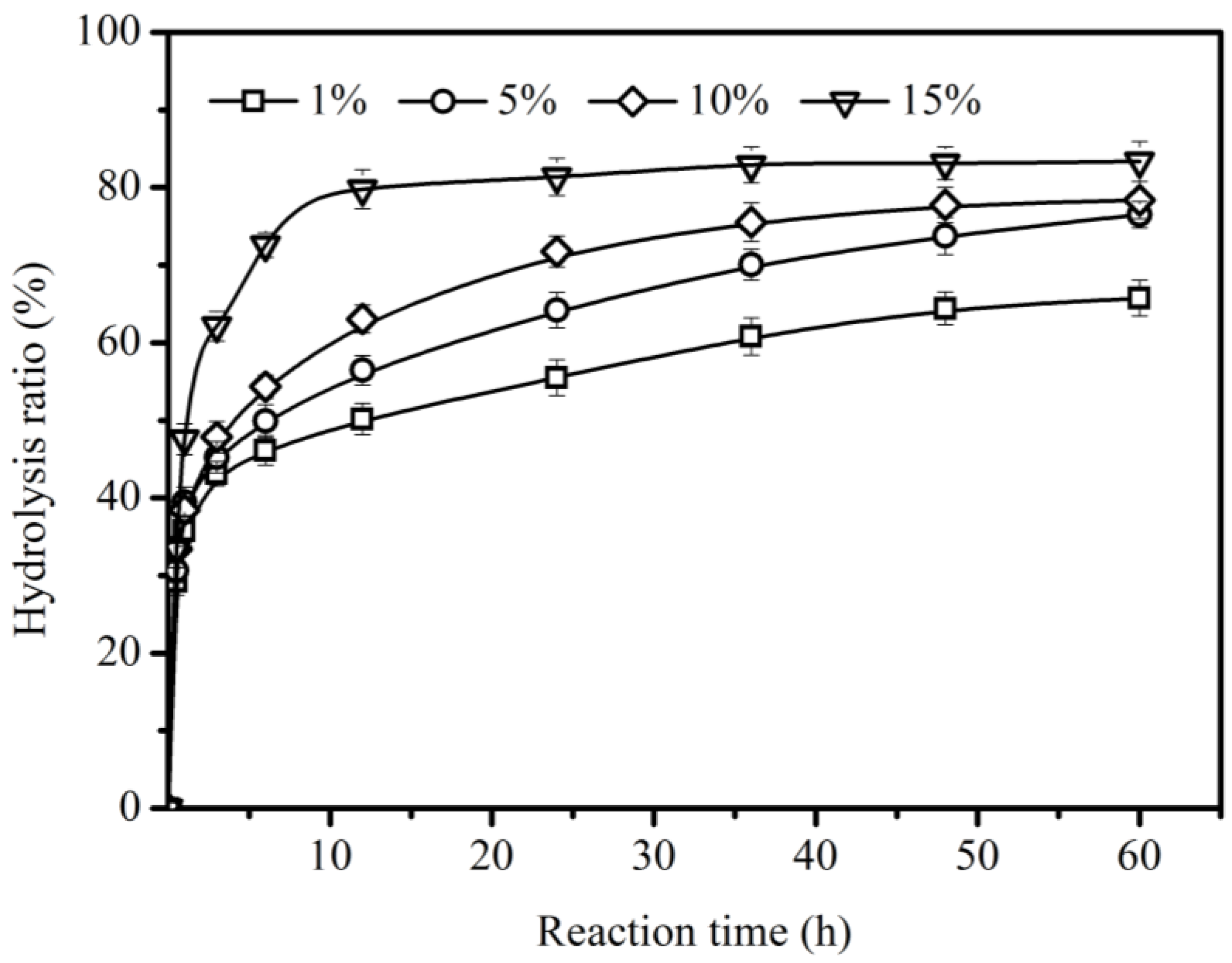
2.3. Effect of Enzyme Load
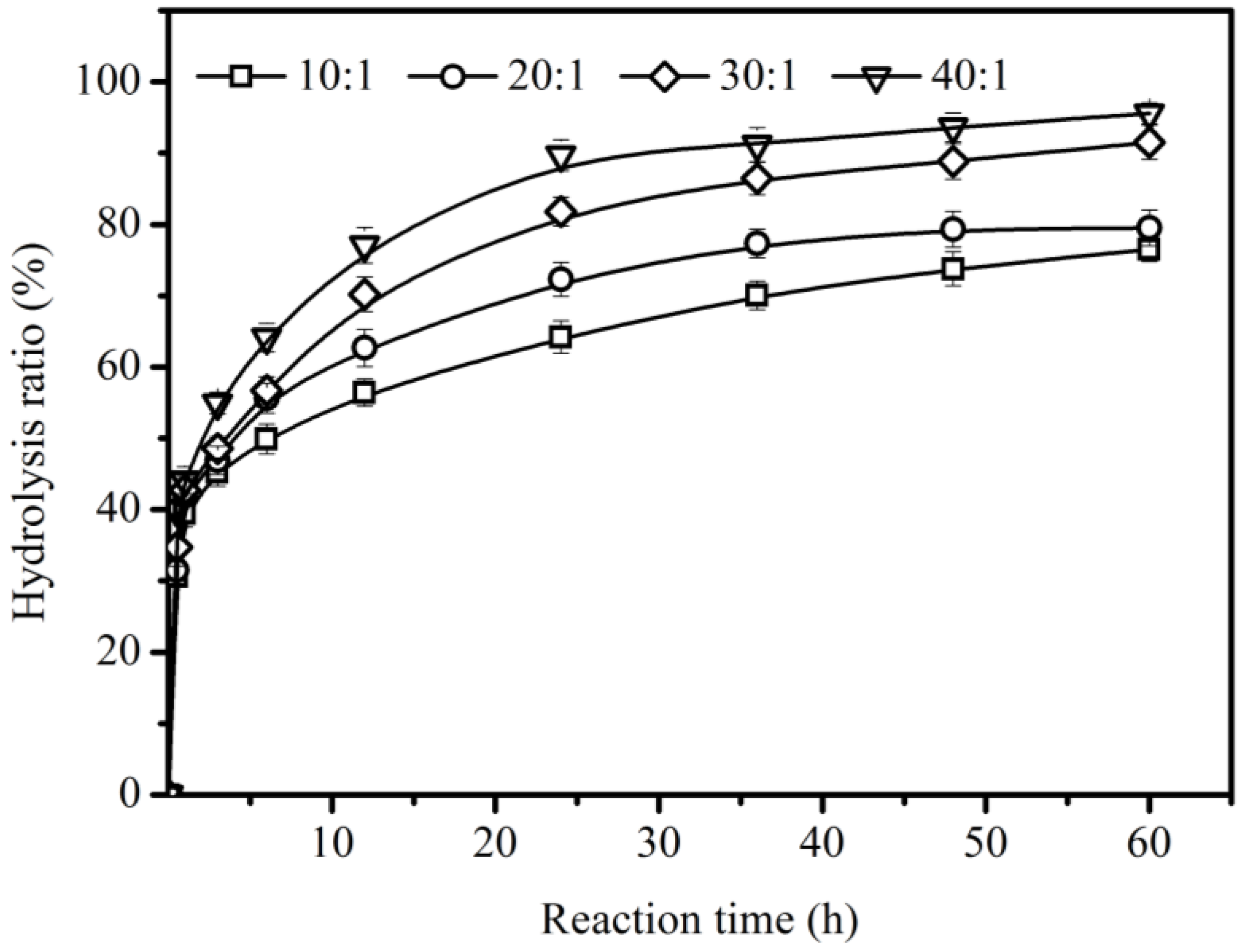
2.4. Effect of Substrate Ratio of Water to Oil
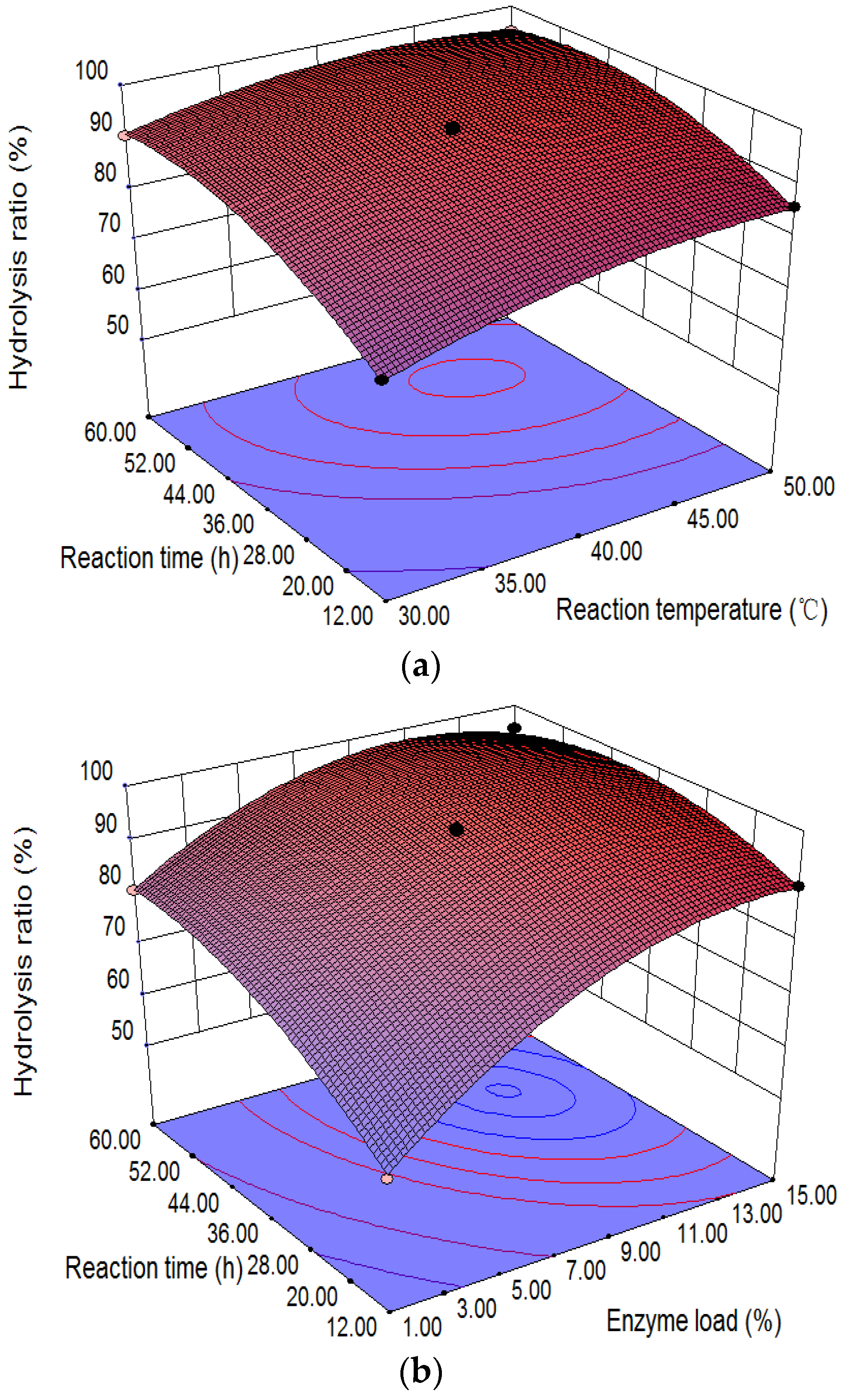
2.5. Response Surface Analysis and Model Fitting
2.6. Optimum Hydrolysis Conditions and Model Verification
3. Materials and Methods
3.1. Materials
3.2. Hydrolysis of CO
3.3. Analysis Methods
3.4. Experimental Design for RSM
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mutlu, H.; Meier, M.A.R. Castor oil as a renewable resource for the chemical industry. Eur. J. Lipid Sci. Technol. 2010, 112, 10–30. [Google Scholar] [CrossRef]
- Chen, J.; Li, K.; Wang, Y.; Huang, J.; Nie, X.; Jiang, J. Synthesis and properties of a novel environmental epoxidized glycidyl ester of ricinoleic acetic ester plasticizer for poly(vinyl chloride). Polymers 2017, 9, 640. [Google Scholar] [CrossRef]
- Goswami, D.; Sen, R.; Basu, J.K.; De, S. Maximization of bioconversion of castor oil into ricinoleic acid by response surface methodology. Bioresour. Technol. 2009, 100, 4067–4073. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.G.; Mannam, V.K.; Ye, R.; Zhao, H.; Ortega, S.; Montiel, M.C. Modification of oligo-ricinoleic acid and its derivatives with 10-undecenoic acid via lipase-catalyzed esterification. Polymers 2012, 4, 1037–1055. [Google Scholar] [CrossRef]
- Naughton, F.C. Production, chemistry, and commercial applications of various chemicals from castor oil. J. Am. Oil Chem. Soc. 1974, 51, 65–71. [Google Scholar] [CrossRef]
- Hernández-Sierra, M.T.; Aguilera-Camacho, L.D.; Báez-García, J.E.; García-Miranda, J.S.; Moreno, K.J. Thermal stability and lubrication properties of biodegradable castor oil on AISI 4140 steel. Metals 2018, 8, 428. [Google Scholar] [CrossRef]
- Shombe, G.B.; Mubofu, E.B.; Mlowe, S.; Revaprasadu, N. Synthesis and characterization of castor oil and ricinoleic acid capped CdS nanoparticles using single source precursors. Mater. Sci. Semicond. Process. 2016, 43, 230–237. [Google Scholar] [CrossRef]
- Kazariya, A.; Matsumura, S. Enzymatic synthesis and crosslinking of novel high molecular weight polyepoxyricinoleate. Polymers 2012, 4, 486–500. [Google Scholar] [CrossRef]
- Sharon, C.; Furugoh, S.; Yamakido, T.; Ogawa, H.I.; Kato, Y. Purification and characterization of a lipase from Pseudomonas aeruginosa KKA-5 and its role in castor oil hydrolysis. J. Ind. Microbiol. Biotechnol. 1998, 20, 304–307. [Google Scholar] [CrossRef]
- Ogunniyi, D.S. Castor oil: A vital industrial raw material. Bioresour. Technol. 2006, 97, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Madankar, C.S.; Mohanty, P.; Naik, S.N. Optimization of reactive extraction of castor seed to produce biodiesel using response surface methodology. Fuel 2012, 97, 848–855. [Google Scholar] [CrossRef]
- Prasad, L.; Das, L.M.; Naik, S.N. Effect of castor oil, methyl and ethyl esters as lubricity enhancer for low lubricity diesel fuel (LLDF). Energy Fuels 2012, 26, 5307–5315. [Google Scholar] [CrossRef]
- Rathod, V.K.; Pandit, A.B. Effect of various additives on enzymatic hydrolysis of castor oil. Biochem. Eng. J. 2009, 47, 93–99. [Google Scholar] [CrossRef]
- Puthli, M.S.; Rathod, V.K.; Pandit, A.B. Enzymatic hydrolysis of castor oil: Process intensification studies. Biochem. Eng. J. 2006, 31, 31–41. [Google Scholar] [CrossRef]
- Lakshminarayana, G.; Subbarao, R.; Sastry, Y.S.R.; Kale, V.; Rao, T.C.; Gangadhar, A. High pressure splitting of castor oil. J. Am. Oil Chem. Soc. 1983, 61, 1204–1206. [Google Scholar] [CrossRef]
- Goswami, D.; Basu, J.K.; De, S. Lipase applications in oil hydrolysis with a case study on castor oil: A review. Crit. Rev. Biotechnol. 2012, 33, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Piazza, G.J.; Farrell, H.M. Generation of ricinoleic acid from castor oil using the lipase from ground oat (Avena sativa L.) seeds as a catalyst. Biotechnol. Lett. 1991, 13, 179–184. [Google Scholar] [CrossRef]
- Khaskheli, A.A.; Talpur, F.N.; Ashraf, M.A.; Cebeci, A.; Jawaid, S.; Afridi, H.I. Monitoring the Rhizopus oryzae lipase catalyzed hydrolysis of castor oil by ATR-FTIR spectroscopy. J. Mol. Catal. B Enzym. 2015, 113, 56–61. [Google Scholar] [CrossRef]
- Ozcan, H.M.; Sagiroglu, A. Production of ricinoleic acid from castor oil by immobilised lipases. Prep. Biochem. Biotechnol. 2009, 39, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Sun, S. Enzymatic production of sterculic acid from the novel Phoenix tree seed oil: Optimization and kinetic study. Grasas Aceites 2017, 68, 197–205. [Google Scholar] [CrossRef]
- Sun, S.; Liu, J.; Li, X. A novel and rapid method for fatty acid preparation by the lipase catalyzed hydrolysis of Phoenix tree seeds. 3 Biotech 2018, 8, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Sun, S.; Liang, S.; Peng, L.; Wang, Y.; Shen, M. Lipase-catalyzed hydrolysis of linseed oil: Optimization using response surface methodology. J. Oleo Sci. 2014, 63, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.S.; Vieira, N.S.; Cunha, A.S.; Silva, A.M.; Záchia Ayub, M.A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Combi-lipase for heterogeneous substrates: A new approach for hydrolysis of soybean oil using mixtures of biocatalysts. RSC Adv. 2014, 4, 6863–6868. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Chen, H.-H.; Chen, J.-H.; Liu, Y.-C.; Shieh, C.-J. High yield of wax ester synthesized from cetyl alcohol and octanoic acid by lipozyme RMIM and Novozym 435. Int. J. Mol. Sci. 2012, 13, 11694–11704. [Google Scholar] [CrossRef] [PubMed]
- von der Haar, D.; Stäbler, A.; Wichmann, R.; Schweiggert-Weisz, U. Enzymatic esterification of free fatty acids in vegetable oils utilizing different immobilized lipases. Biotechnol. Lett. 2014, 37, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yang, R.; Hu, J. Lysophosphatidylcholine synthesis by lipase-catalyzed ethanolysis. J. Oleo Sci. 2015, 64, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Köse, Ö. Immobilized Candida antarctica lipase-catalyzed alcoholysis of cotton seed oil in a solvent-free medium. Bioresour. Technol. 2002, 83, 125–129. [Google Scholar] [CrossRef]
- Sun, S.; Zhu, S.; Bi, Y. Solvent-free enzymatic synthesis of feruloylated structured lipids by the transesterification of ethyl ferulate with castor oil. Food Chem. 2014, 158, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Flores, L.F.; Beltran, H.I.; Arrieta-Baez, D.; Reyes-Duarte, D. Regioselective Synthesis of Lactulose Esters by Candida antarctica and Thermomyces lanuginosus Lipases. Catalysts 2017, 7, 263. [Google Scholar] [CrossRef]
- Ruzich, N.I.; Bassi, A.S. Proposed kinetic mechanism of biodiesel production through lipase catalysed interesterification with a methyl acetate acyl acceptor and ionic liquid [BMIM][PF6] CO-solvent. Can. J. Chem. Eng. 2011, 89, 166–170. [Google Scholar] [CrossRef]
- Gamayurova, V.S.; Zinov’Eva, M.E.; Tran, H.T.T. Features of the enzymatic hydrolysis of castor oil. Catal. Ind. 2013, 5, 269–273. [Google Scholar] [CrossRef]
- Rathod, V.K.; Pandit, A.B. Enzymatic hydrolysis of oil in a spray column. J. Mol. Catal. B Enzym. 2010, 67, 1–9. [Google Scholar] [CrossRef]
- Goswami, D.; Basu, J.K.; De, S. Optimization of process variables in castor oil hydrolysis by candida rugosa lipase with buffer as dispersion medium. Biotechnol. Bioprocess Eng. 2009, 14, 220–224. [Google Scholar] [CrossRef]
- Sharon, C.; Nakazato, M.; Ogawa, H.I.; Kato, Y. Lipase-induced hydrolysis of castor oil: Effect of various metals. J. Ind. Microbiol. Biotechnol. 1998, 21, 292–295. [Google Scholar] [CrossRef]
- Goswami, D.; Sen, R.; Basu, J.K.; De, S. Surfactant enhanced ricinoleic acid production using candida rugosa lipase. Bioresour. Technol. 2010, 101, 6–13. [Google Scholar] [CrossRef] [PubMed]

| Trial | X1 (°C) Hydrolysis Temperature | X2 (%) Enzyme Load | X3 (h) Hydrolysis Time | Hydrolysis Ratio (%) |
|---|---|---|---|---|
| 1 | 40 (0) | 15 (1) | 12 (−1) | 90.17 ± 1.4 |
| 2 | 30 (−1) | 1 (−1) | 36 (0) | 65.33 ± 2.1 |
| 3 | 40 (0) | 8 (0) | 36 (0) | 95.73 ± 1.3 |
| 4 | 40 (0) | 1 (−1) | 12 (−1) | 58.54 ± 1.6 |
| 5 | 40 (0) | 8 (0) | 36 (0) | 94.75 ± 1.5 |
| 6 | 40 (0) | 8 (0) | 36 (0) | 93.50 ± 1.4 |
| 7 | 40 (0) | 15 (1) | 60 (1) | 95.31 ± 2.4 |
| 8 | 40 (0) | 8 (0) | 36 (0) | 96.25 ± 1.8 |
| 9 | 30 (−1) | 8 (0) | 60 (1) | 90.63 ± 2.1 |
| 10 | 30 (−1) | 8 (0) | 12 (−1) | 75.19 ± 1.6 |
| 11 | 40 (0) | 1 (−1) | 60 (1) | 80.65 ± 1.3 |
| 12 | 50 (1) | 8 (0) | 60 (1) | 95.01 ± 1.9 |
| 13 | 50 (1) | 1 (−1) | 36 (0) | 80.42 ± 1.7 |
| 14 | 30 (−1) | 15 (1) | 36 (0) | 93.88 ± 1.5 |
| 15 | 40 (0) | 8 (0) | 36 (0) | 96.09 ± 0.6 |
| 16 | 50 (1) | 8 (0) | 12 (−1) | 85.89 ± 1.4 |
| 17 | 50 (1) | 8 (0) | 60 (1) | 95.01 ± 1.3 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F Value | Prob > F Value |
|---|---|---|---|---|---|
| Model | 2087.27 | 9 | 231.92 | 182.23 | <0.0001 |
| X1 | 110.78 | 1 | 110.78 | 87.05 | <0.0001 |
| X2 | 965.80 | 1 | 965.80 | 758.86 | <0.0001 |
| X3 | 335.53 | 1 | 335.53 | 263.64 | <0.0001 |
| X1 X2 | 59.99 | 1 | 59.99 | 47.13 | 0.0002 |
| X1 X3 | 9.99 | 1 | 9.99 | 7.85 | 0.0265 |
| X2 X3 | 72.00 | 1 | 72.00 | 56.57 | 0.0001 |
| X12 | 44.12 | 1 | 44.12 | 34.67 | 0.0006 |
| X22 | 322.33 | 1 | 322.33 | 253.27 | <0.0001 |
| X32 | 120.38 | 1 | 120.38 | 94.59 | <0.0001 |
| Residual | 8.91 | 7 | 1.27 | ||
| Lack of fit | 3.66 | 3 | 1.22 | 0.93 | 0.5038 |
| Total | 2096.18 | 16 | |||
| R2 = 0.9957 | RAdj2 = 0.9903 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Guo, J. Enhanced Ricinoleic Acid Preparation Using Lipozyme TLIM as a Novel Biocatalyst: Optimized by Response Surface Methodology. Catalysts 2018, 8, 486. https://doi.org/10.3390/catal8110486
Sun S, Guo J. Enhanced Ricinoleic Acid Preparation Using Lipozyme TLIM as a Novel Biocatalyst: Optimized by Response Surface Methodology. Catalysts. 2018; 8(11):486. https://doi.org/10.3390/catal8110486
Chicago/Turabian StyleSun, Shangde, and Jingjing Guo. 2018. "Enhanced Ricinoleic Acid Preparation Using Lipozyme TLIM as a Novel Biocatalyst: Optimized by Response Surface Methodology" Catalysts 8, no. 11: 486. https://doi.org/10.3390/catal8110486
APA StyleSun, S., & Guo, J. (2018). Enhanced Ricinoleic Acid Preparation Using Lipozyme TLIM as a Novel Biocatalyst: Optimized by Response Surface Methodology. Catalysts, 8(11), 486. https://doi.org/10.3390/catal8110486