Significantly Enhanced Aqueous Cr(VI) Removal Performance of Bi/ZnO Nanocomposites via Synergistic Effect of Adsorption and SPR-Promoted Visible Light Photoreduction
Abstract
:1. Introduction
2. Results and Discussion
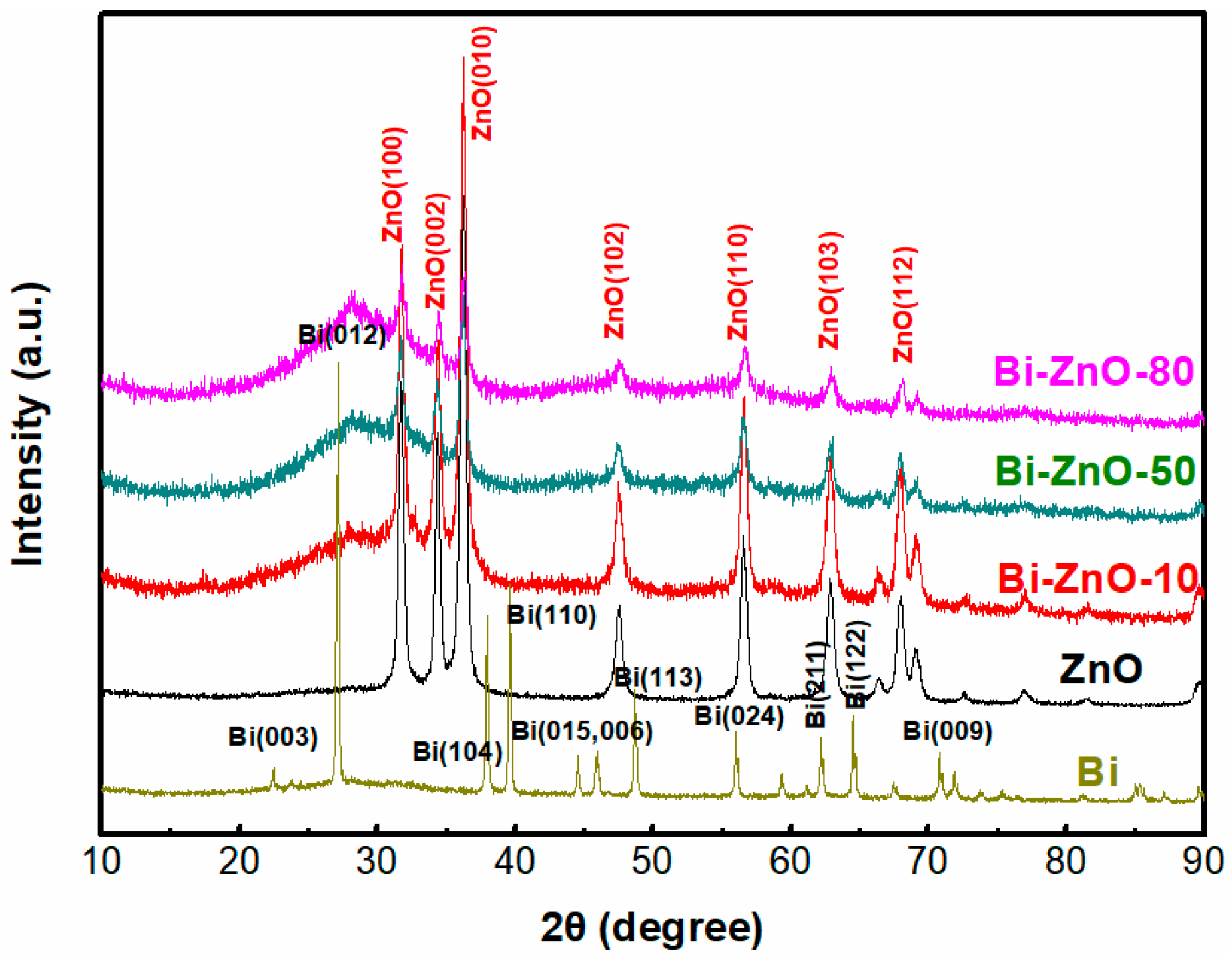
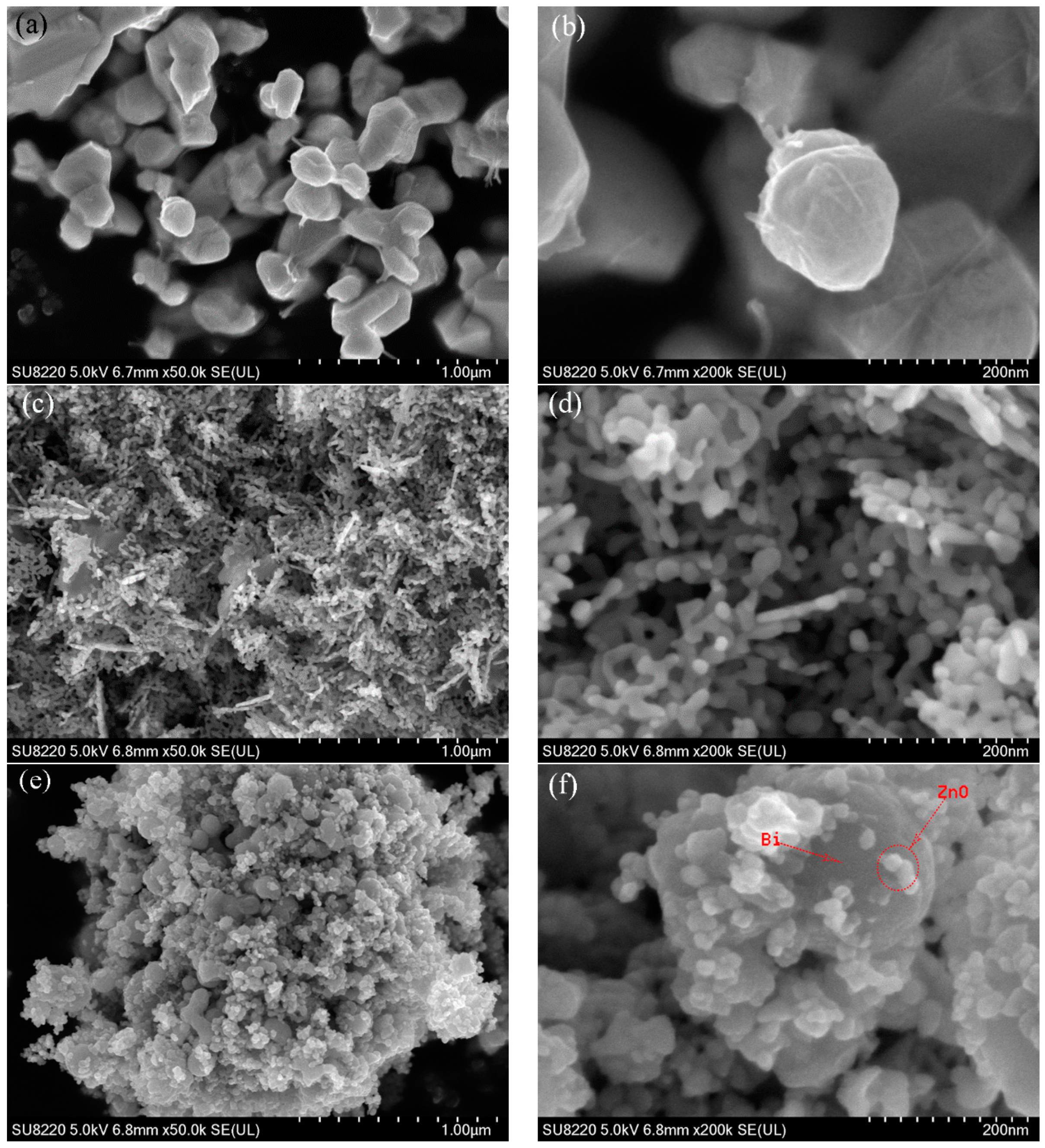
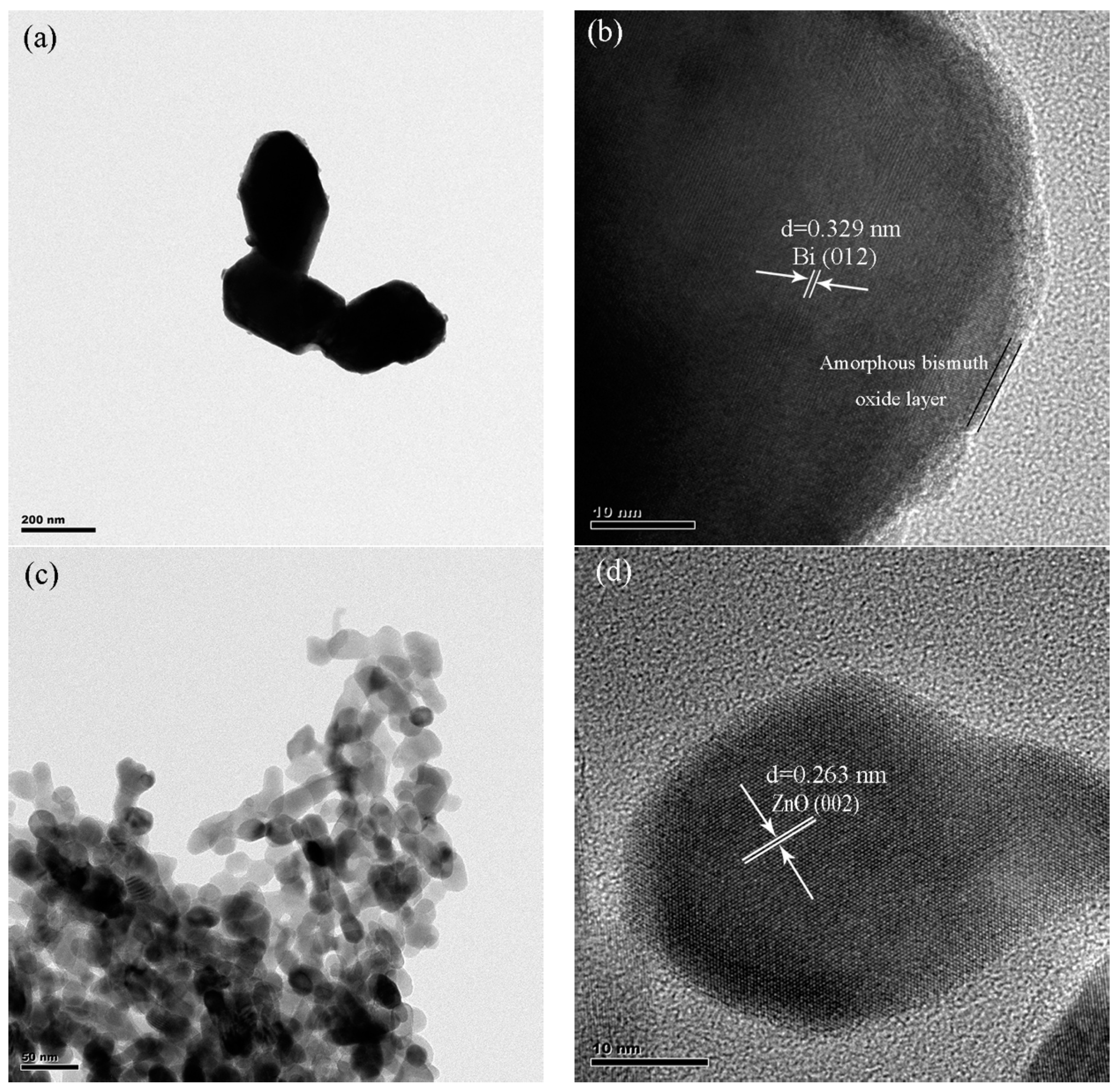
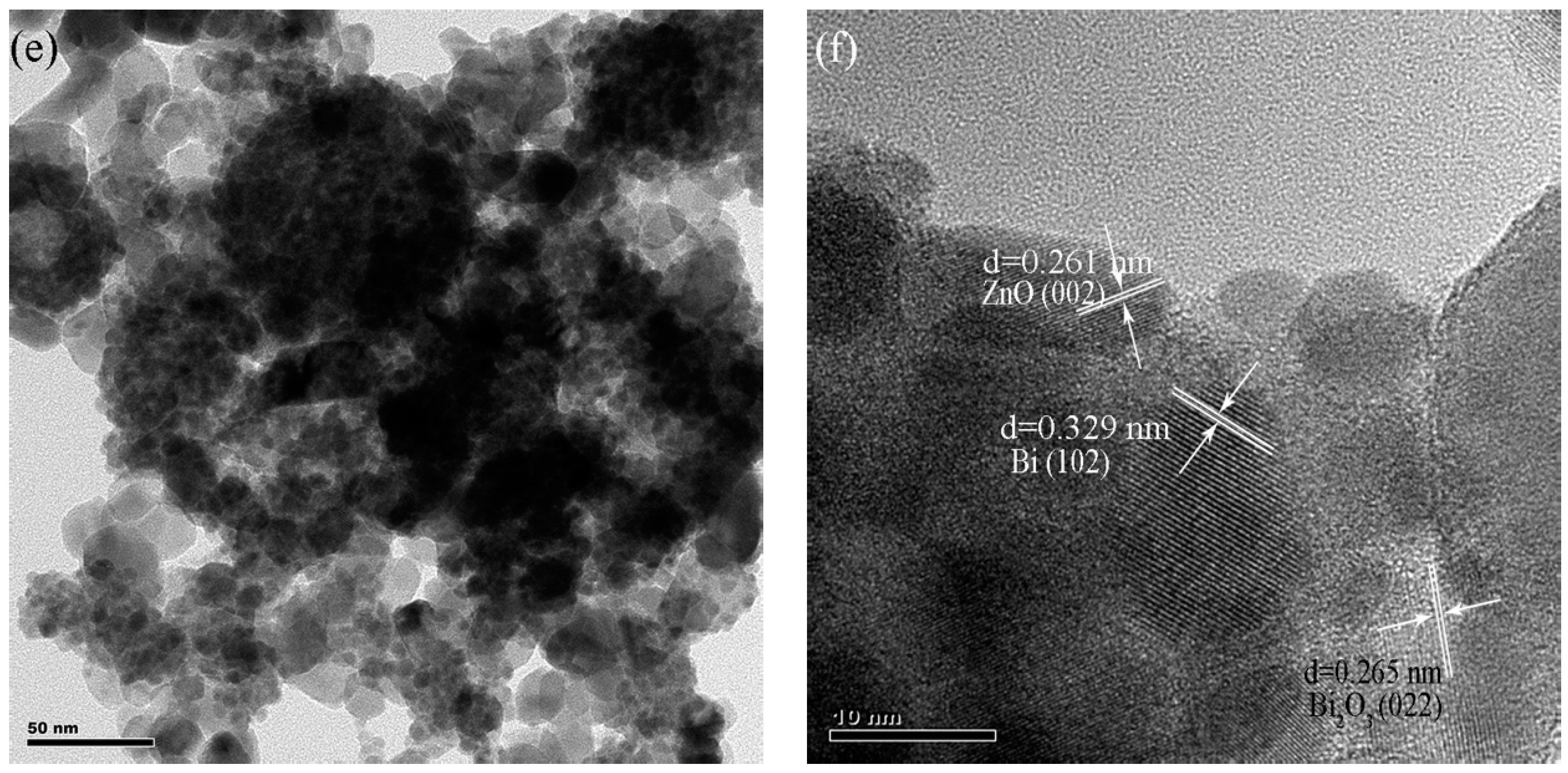
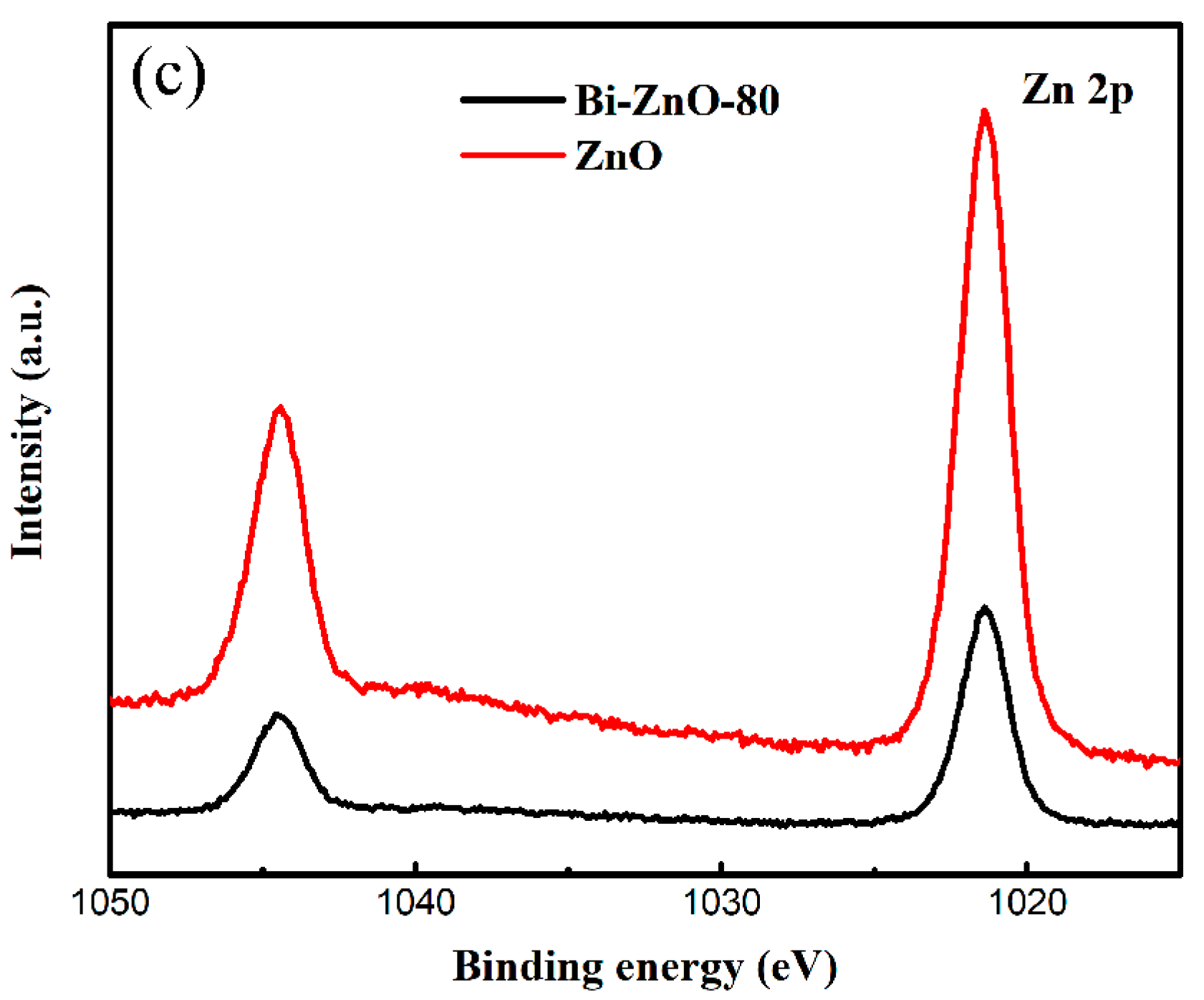
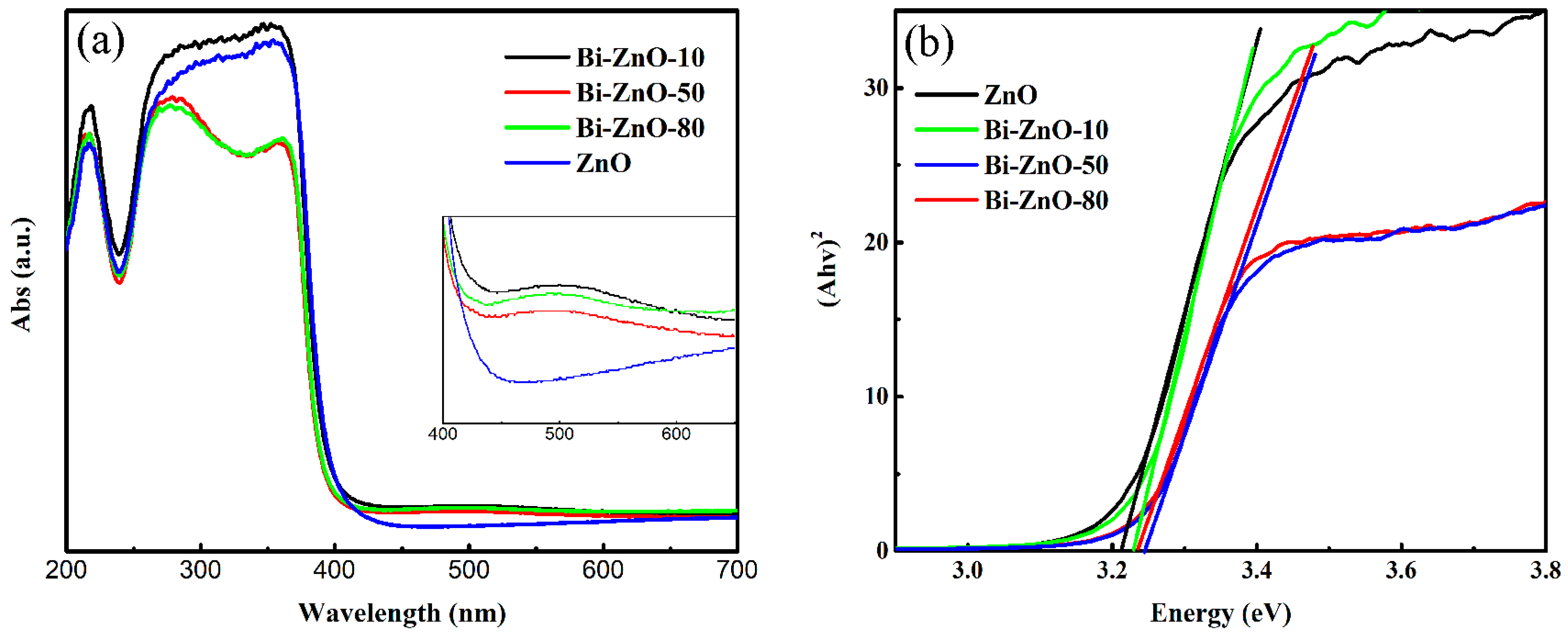
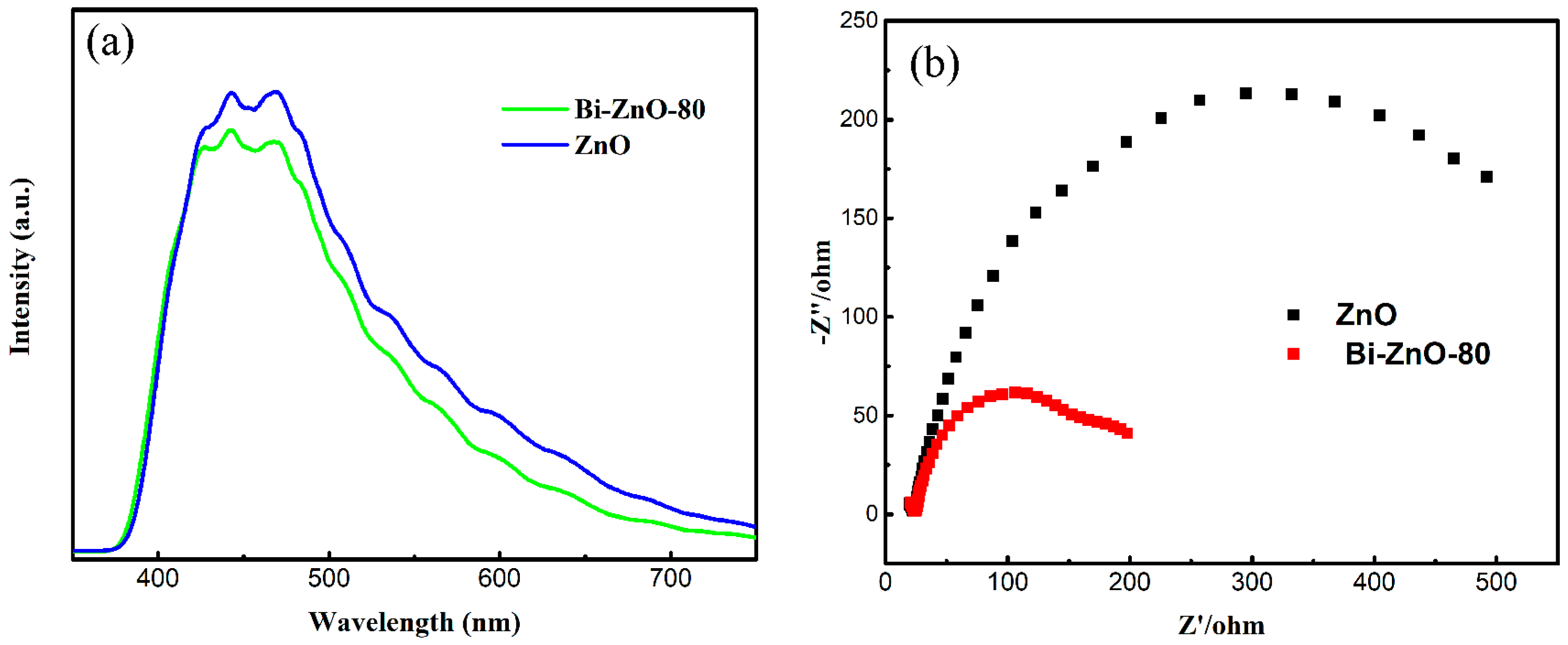
2.1. Characterization of BiNPs/ZnO Nanocomposites
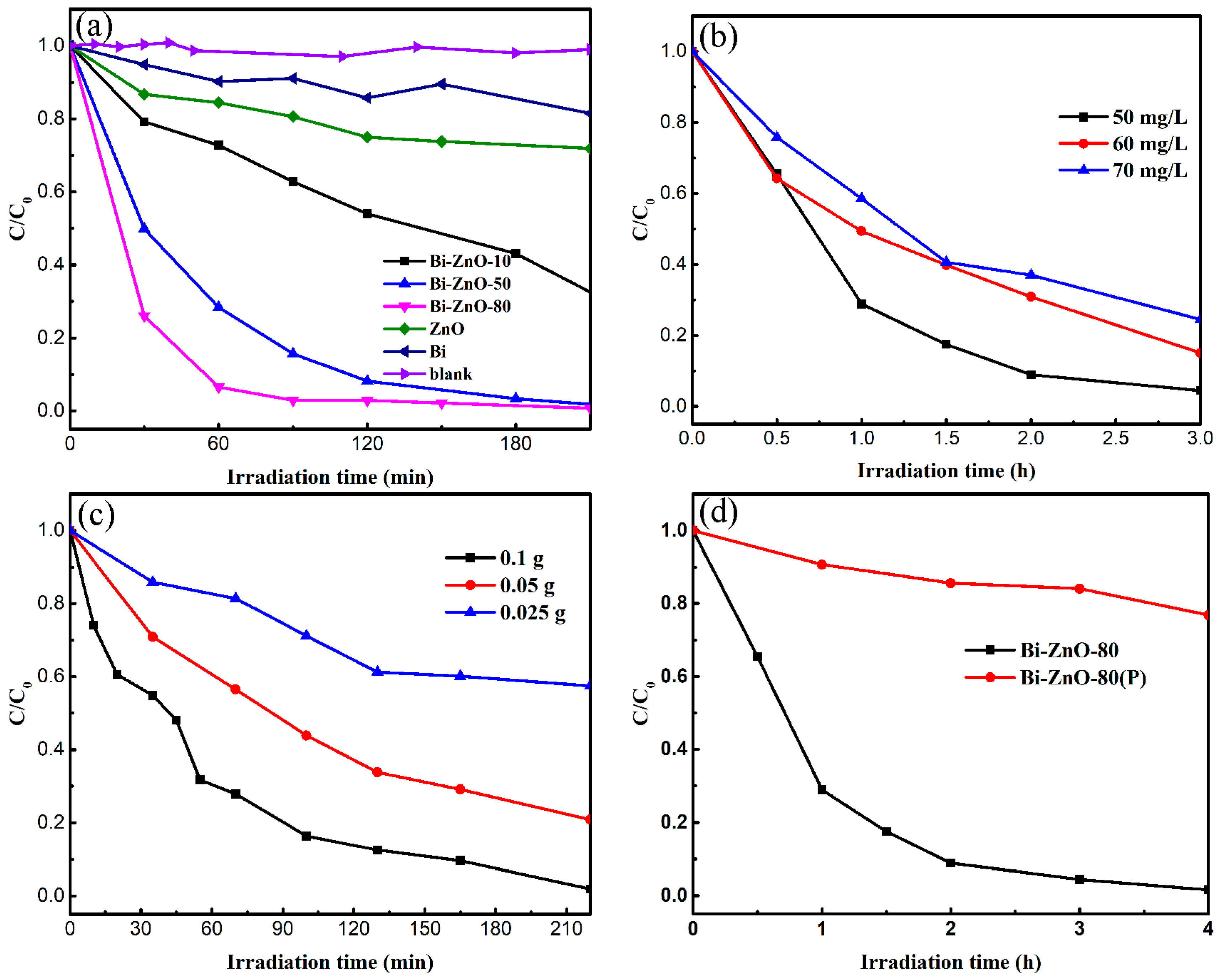
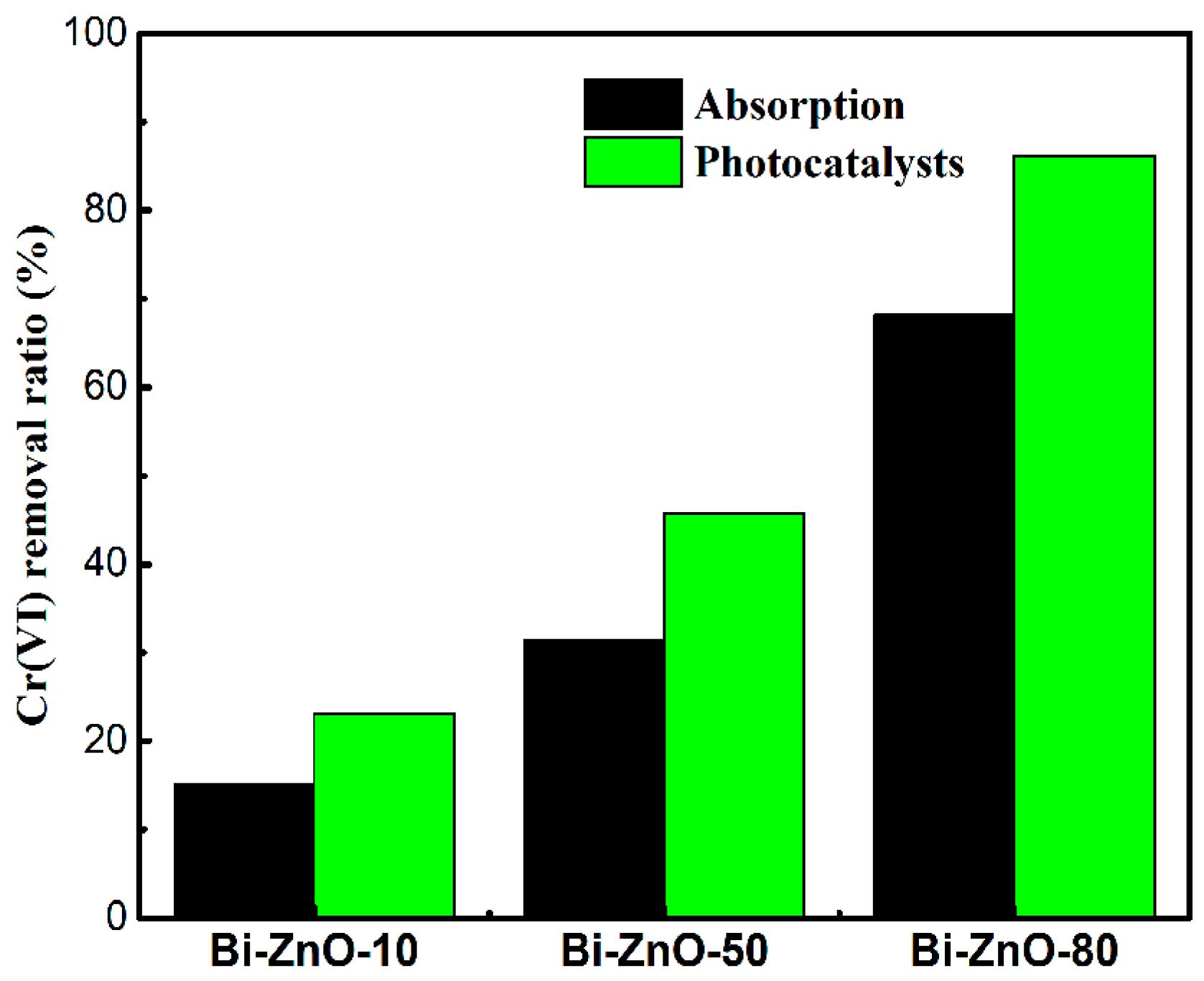
2.2. Photocatalytic Activity of Bi/ZnO Composites
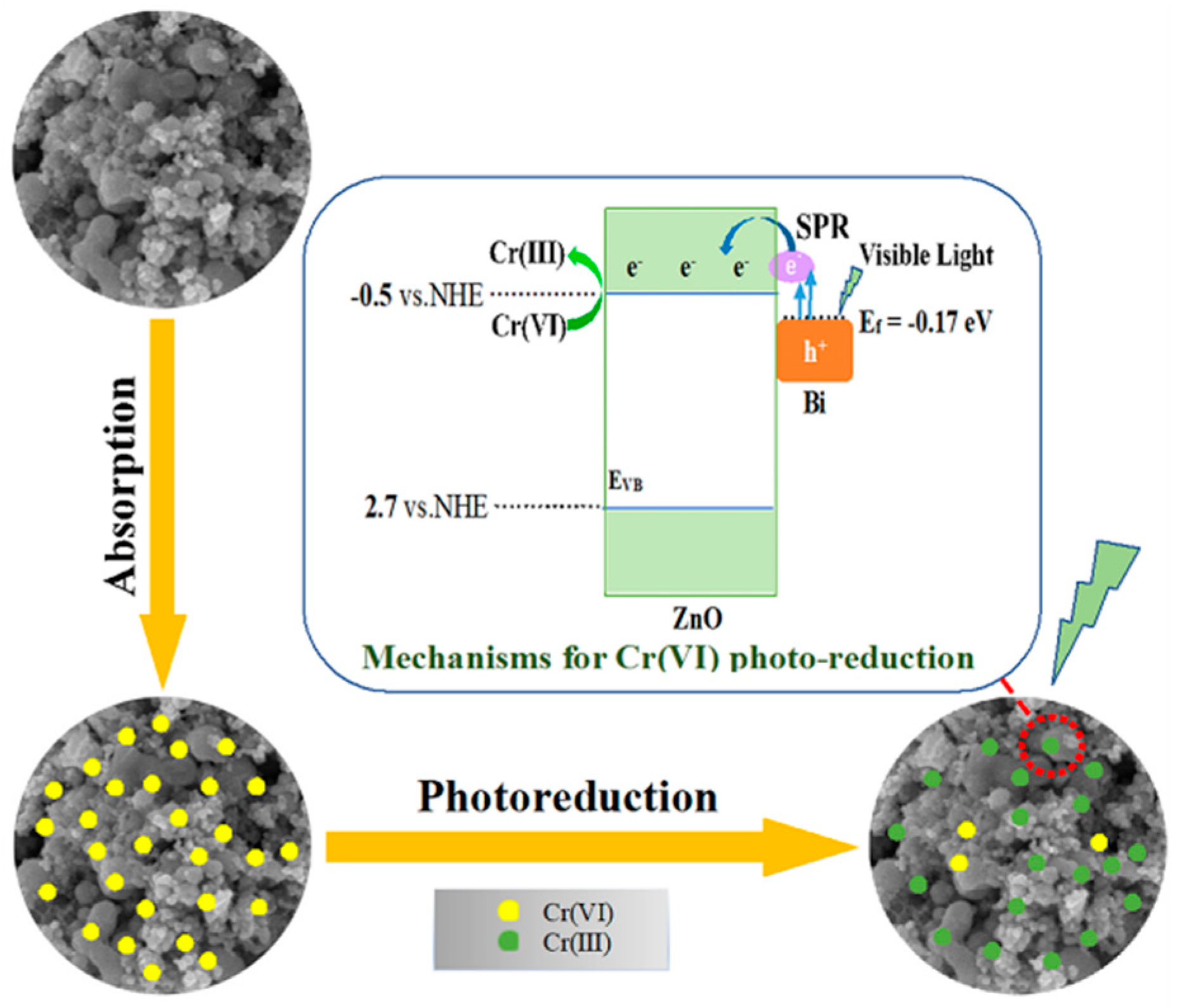
2.3. Proposed Mechanism
3. Experimental
3.1. Synthesis of BiNPs/ZnO Nanocomposite
3.2. Characterization
3.3. Photocatalytic Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Langård, S. Chromium carcinogenicity; A review of experimental animal data. Sci. Total Environ. 1988, 71, 341–350. [Google Scholar] [CrossRef]
- Katz, S.; Salem, H. The toxicology of chromium with respect to its chemical speciation: A review. J. Appl. Toxicol. 1993, 13, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Kabra, K.; Chaudhary, R.; Sawhney, R.L. Treatment of hazardous organic and inorganic compounds through aqueous-phase photocatalysis: A review. Ind. Eng. Chem. Res. 2004, 43, 7683–7696. [Google Scholar] [CrossRef]
- Barrera-Diaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012, 223, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Jiang, L.J.; Feng, C.G. Photocatalytic reduction of Cr(VI). Prog. Chem. 2013, 25, 1999–2010. [Google Scholar]
- Jing, L.Q.; Zhou, W.; Tian, G.H.; Fu, H.G. Surface tuning for oxide-based nanomaterials as efficient photocatalysts. Chem. Soc. Rev. 2013, 42, 9509–9549. [Google Scholar] [CrossRef] [PubMed]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for environmental photocatalytic applications: A review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Zhang, H.J.; Chen, G.H.; Bahnemann, D.W. Photoelectrocatalytic materials for environmental applications. J. Mater. Chem. 2009, 19, 5089–5121. [Google Scholar] [CrossRef]
- Hernandez-Alonso, M.D.; Fresno, F.; Suarez, S.; Coronado, J.M. Development of alternative photocatalysts to TiO2: Challenges and opportunities. Energy Environ. Sci. 2009, 2, 1231–1257. [Google Scholar] [CrossRef]
- Peng, H.; Liu, X.; Tang, W.; Ma, R. Facile synthesis and characterization of ZnO nanoparticles grown on halloysite nanotubes for enhanced photocatalytic properties. Sci. Rep. 2017, 7, 2250. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, T.; Zhang, H.; Chen, H. Scalable and facile preparation of optical-magnetic dual function 3d Ni@graphene-ZnO for high efficiency removal of hexavalent chromium. Ceram. Int. 2017, 43, 3792–3796. [Google Scholar] [CrossRef]
- Yang, Y.; Li, H.; Hou, F.; Hu, J.; Zhang, X.; Wang, Y. Facile synthesis of ZnO/Ag nanocomposites with enhanced photocatalytic properties under visible light. Mater. Lett. 2016, 180, 97–100. [Google Scholar] [CrossRef]
- Li, S.; Cai, J.; Wu, X.; Zheng, F. Sandwich-like TiO2@ZnO-based noble metal (Ag, Au, Pt, or Pd) for better photo-oxidation performance: Synergistic effect between noble metal and metal oxide phases. Appl. Surf. Sci. 2018, 443, 603–612. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Pouran, S.R. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Gu, X.; Li, C.; Yuan, S.; Ma, M.; Qiang, Y.; Zhu, J. ZnO based heterojunctions and their application in environmental photocatalysis. Nanotechnology 2016, 27, 402001. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Xiong, T.; Sun, Y.J.; Zhao, Z.W.; Zhou, Y.; Feng, X.; Wu, Z.B. A semimetal bismuth element as a direct plasmonic photocatalyst. Chem. Commun. 2014, 50, 10386–10389. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, X.A.; Sun, Y.; Cen, W.; Dong, F. Facet-dependent interfacial charge separation and transfer in plasmonic photocatalysts. Appl. Catal. B 2018, 226, 269–277. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, Z.W.; Sun, Y.J.; Zhang, Y.X.; Yan, S.; Wu, Z.B. An advanced semimetal-organic Bi spheres-g-C3N4 nanohybrid with SPR-enhanced visible-light photocatalytic performance for no purification. Environ. Sci. Technol. 2015, 49, 12432–12440. [Google Scholar] [CrossRef] [PubMed]
- Kale, M.J.; Avanesian, T.; Christopher, P. Direct photocatalysis by plasmonic nanostructures. ACS Catal. 2014, 4, 116–128. [Google Scholar] [CrossRef]
- Li, J.T.; Cushing, S.K.; Bright, J.; Meng, F.K.; Senty, T.R.; Zheng, P.; Bristow, A.D.; Wu, N.Q. Ag@Cu2O core-shell nanoparticles as visible-light plasmonic photocatalysts. ACS Catal. 2013, 3, 47–51. [Google Scholar] [CrossRef]
- Yu, J.; Yue, L.; Liu, S.; Huang, B.; Zhang, X. Hydrothermal preparation and photocatalytic activity of mesoporous Au-TiO2 nanocomposite microspheres. J. Colloid Interface Sci. 2009, 334, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, F.; Xu, Y.; Zhang, J.W.; Zhang, D.; Li, G.; Li, H. Plasmon-induced photoelectrocatalytic activity of au nanoparticles enhanced TiO2 nanotube arrays electrodes for environmental remediation. Appl. Catal. B 2015, 164, 217–224. [Google Scholar] [CrossRef]
- Yu, J.; Sun, D.; Wang, T.; Li, F. Fabrication of Ag@AgCl/ZnO submicron wire film catalyst on glass substrate with excellent visible light photocatalytic activity and reusability. Chem. Eng. J. 2018, 334, 225–236. [Google Scholar] [CrossRef]
- Yu, J.; Dai, G.; Huang, B. Fabrication and characterization of visible-light-driven plasmonic photocatalyst Ag/AgCl/TiO2 TiO2 nanotube arrays. J. Phys. Chem. C 2009, 113, 16394–16401. [Google Scholar] [CrossRef]
- Cai, J.; Huang, Y.; Huang, B.; Zheng, S.; Guo, Y. Enhanced activity of pt nanoparticle catalysts supported on manganese oxide-carbon nanotubes for ethanol oxidation. Int. J. Hydrogen Energy 2014, 39, 798–807. [Google Scholar] [CrossRef]
- An, Y.; Xu, B.; Liu, Y.; Wang, Z.; Wang, P.; Dai, Y.; Qin, X.; Zhang, X.; Huang, B. Photocatalytic overall water splitting over MIL-125(Ti) upon CoPi and Pt Co-catalyst deposition. Chemistryopen 2017, 6, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Chen, B.; Xie, L.; Zheng, Z.; Liu, P. Facile in situ synthesis of a Bi/BiOCl nanocomposite with high photocatalytic activity. J. Mater. Chem. A 2013, 1, 3068–3075. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Z.; Dong, F.; Zhang, W. Mechanism of visible light photocatalytic NOx oxidation with plasmonic Bi cocatalyst-enhanced (BiO)2CO3 hierarchical microspheres. Phys. Chem. Chem. Phys. 2015, 17, 10383–10390. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Feng, W.H.; Zhang, L.L.; Zhang, Y.; Huang, X.Y.; Fang, Z.B.; Liu, P. In situ construction of a novel Bi/CdS nanocomposite with enhanced visible light photocatalytic performance. Appl. Catal. B 2017, 206, 510–519. [Google Scholar] [CrossRef]
- Liu, X.; Cao, H.; Yin, J. Generation and photocatalytic activities of Bi@Bi2O3 microspheres. Nano Res. 2011, 4, 470–482. [Google Scholar] [CrossRef]
- Zou, J.-P.; Ma, J.; Luo, J.-M.; Yu, J.; He, J.; Meng, Y.; Luo, Z.; Bao, S.-K.; Liu, H.-L.; Luo, S.-L.; et al. Fabrication of novel heterostructured few layered WS2-Bi2WO6/Bi3.84W0.16O6.24 composites with enhanced photocatalytic performance. Appl. Catal. B 2015, 179, 220–228. [Google Scholar] [CrossRef]
- Zou, J.-P.; Ma, J.; Huang, Q.; Luo, S.-L.; Yu, J.; Luo, X.-B.; Dai, W.-L.; Sun, J.; Guo, G.-C.; Au, C.-T.; et al. Graphene oxide as structure-directing and morphology-controlling agent for the syntheses of heterostructured graphene-Bi2MoO6/Bi3.64Mo0.36O6.55 composites with high photocatalytic activity. Appl. Catal. B 2014, 156–157, 447–455. [Google Scholar] [CrossRef]
- Labib, S. Study of photocatalytic properties of pure and doped ZnO powders. Mater. Werkst. 2016, 47, 19–28. [Google Scholar] [CrossRef]
- Yuan, X.; Zhou, C.; Jing, Q.; Tang, Q.; Mu, Y.; Du, A.-K. Facile synthesis of g-C3N4 nanosheets/ZnO nanocomposites with enhanced photocatalytic activity in reduction of aqueous chromium(VI) under visible light. Nanomaterials 2016, 6, 173. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Zhang, H.S.; Luo, J.M.; Feng, Z.J.; Huang, J.T. Hydrothermal synthesized novel nanoporous g-C3N4/MnTiO3 heterojunction with direct z-scheme mechanism. Electrochim. Acta 2017, 258, 998–1007. [Google Scholar] [CrossRef]
- Jiang, D.; Yu, H.; Yu, H. Modified g-C3N4/TiO2 nanosheets/ZnO ternary facet coupled heterojunction for photocatalytic degradation of p-toluenesulfonic acid (p-tsa) under visible light. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 85, 1–6. [Google Scholar] [CrossRef]
- Warren, S.C.; Jackson, A.C.; Cater-Cyker, Z.D.; DiSalvo, F.J.; Wiesner, U. Nanoparticle synthesis via the photochemical polythiol process. J. Am. Chem. Soc. 2007, 129, 10072–10073. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.E.; Stec, W.J.; Van Wazer, J.R. Inner-orbital binding-energy shifts of antimony and bismuth compounds. Inorg. Chem. 1973, 12, 953–955. [Google Scholar] [CrossRef]
- Dong, F.; Lee, S.C.; Wu, Z.; Huang, Y.; Fu, M.; Ho, W.-K.; Zou, S.; Wang, B. Rose-like monodisperse bismuth subcarbonate hierarchical hollow microspheres: One-pot template-free fabrication and excellent visible light photocatalytic activity and photochemical stability for no removal in indoor air. J. Hazard. Mater. 2011, 195, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, W.; Kobayashi, H.; Ma, C. Platinum-nanoparticle-loaded bismuth oxide: An efficient plasmonic photocatalyst active under visible light. Green Chem. 2010, 12, 212–215. [Google Scholar] [CrossRef]
- Cheng, K.; Sun, W.; Jiang, H.-Y.; Liu, J.; Lin, J. Sonochemical deposition of au nanoparticles on different facets-dominated anatase TiO2 single crystals and resulting photocatalytic performance. J. Phys. Chem. C 2013, 117, 14600–14607. [Google Scholar] [CrossRef]
- Venkataswamy, P.; Jampaiah, D.; Kandjani, A.E.; Sabri, Y.M.; Reddy, B.M.; Vithal, M. Transition (Mn, Fe) and rare earth (La, Pr) metal doped ceria solid solutions for high performance photocatalysis: Effect of metal doping on catalytic activity. Res. Chem. Intermed. 2018, 44, 2523–2543. [Google Scholar] [CrossRef]
- Singh, M.; Jampaiah, D.; Kandjani, A.E.; Sabri, Y.M.; Della Gaspera, E.; Reineck, P.; Judd, M.; Langley, J.; Cox, N.; van Embden, J.; et al. Oxygen-deficient photostable Cu2O for enhanced visible light photocatalytic activity. Nanoscale 2018, 10, 6039–6050. [Google Scholar] [CrossRef] [PubMed]
- Toudert, J.; Serna, R.; Jimenez de Castro, M. Exploring the optical potential of nano-bismuth: Tunable surface plasmon resonances in the near ultraviolet-to-near infrared range. J. Phys. Chem. C 2012, 116, 20530–20539. [Google Scholar] [CrossRef]
- Dong, F.; Li, Q.Y.; Sun, Y.J.; Ho, W.K. Noble metal-like behavior of plasmonic bi particles as a cocatalyst deposited on (BiO)2CO3 microspheres for efficient visible light photocatalysis. ACS Catal. 2014, 4, 4341–4350. [Google Scholar] [CrossRef]
- Guo, L.T.; Cai, Y.Y.; Ge, J.M.; Zhang, Y.N.; Gong, L.H.; Li, X.H.; Wang, K.X.; Ren, Q.Z.; Su, J.; Chen, J.S. Multifunctional Au-Co@CN nanocatalyst for highly efficient hydrolysis of ammonia borane. ACS Catal. 2015, 5, 388–392. [Google Scholar] [CrossRef]
- Yu, Y.; Cao, C.; Liu, H.; Li, P.; Wei, F.; Jiang, Y.; Song, W. A Bi/BiOCl heterojunction photocatalyst with enhanced electron–hole separation and excellent visible light photodegrading activity. J. Mater. Chem. A 2014, 2, 1677–1681. [Google Scholar] [CrossRef]
- Li, Y.D.; Wang, J.W.; Deng, Z.X.; Wu, Y.Y.; Sun, X.M.; Yu, D.P.; Yang, P.D. Bismuth nanotubes: A rational low-temperature synthetic route. J. Am. Chem. Soc. 2001, 123, 9904–9905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zong, R.; Zhu, Y. Photocorrosion inhibition and photoactivity enhancement for zinc oxide via hybridization with monolayer polyaniline. J. Phys. Chem. C 2009, 113, 4605–4611. [Google Scholar] [CrossRef]
- Shirzad-Siboni, M.; Farrokhi, M.; Darvishi Cheshmeh Soltani, R.; Khataee, A.; Tajassosi, S. Photocatalytic reduction of hexavalent chromium over ZnO nanorods immobilized on kaolin. Ind. Eng. Chem. Res. 2014, 53, 1079–1087. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, H.; Song, Y.; Yan, W.; Xu, Y.; Li, H.; Huang, L.; Yin, S.; Li, Y.; Zhang, Q.; et al. g-C3N4/Ag3PO4 composites with synergistic effect for increased photocatalytic activity under the visible light irradiation. Mater. Sci. Semicond. Process. 2015, 39, 726–734. [Google Scholar] [CrossRef]
- Zhang, F.-J.; Xie, F.-Z.; Zhu, S.-F.; Liu, J.; Zhang, J.; Mei, S.-F.; Zhao, W. A novel photofunctional g-C3N4/Ag3PO4 bulk heterojunction for decolorization of Rh.B. Chem. Eng. J. 2013, 228, 435–441. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Yao, L.; Zhang, G.S.; Dionysiou, D.D.; Li, J.; Du, X.H. One-step hydrothermal synthesis of high-performance visible-light-driven SnS2/SnO2 nanoheterojunction photocatalyst for the reduction of aqueous Cr(VI). Appl. Catal. B 2014, 144, 730–738. [Google Scholar] [CrossRef]
- Wang, X.J.; Wang, Q.; Li, F.T.; Yang, W.Y.; Zhao, Y.; Hao, Y.J.; Liu, S.J. Novel BiOCl-C3N4 heterojunction photocatalysts: In situ preparation via an ionic-liquid-assisted solvent-thermal route and their visible-light photocatalytic activities. Chem. Eng. J. 2013, 234, 361–371. [Google Scholar] [CrossRef]
- Warren, S.C.; Thimsen, E. Plasmonic solar water splitting. Energy Environ. Sci. 2012, 5, 5133–5146. [Google Scholar] [CrossRef]
- Christopher, P.; Xin, H.; Marimuthu, A.; Linic, S. Singular characteristics and unique chemical bond activation mechanisms of photocatalytic reactions on plasmonic nanostructures. Nat. Mater. 2012, 11, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Jing, Q.; Chen, J.; Li, L. Photocatalytic Cr(VI) reduction by mixed metal oxide derived from ZnAl layered double hydroxide. Appl. Clay Sci. 2017, 143, 168–174. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Feng, Z.; Zhao, J.; Niu, J.; Liu, J.; Peng, D.; Cheng, X. Significantly Enhanced Aqueous Cr(VI) Removal Performance of Bi/ZnO Nanocomposites via Synergistic Effect of Adsorption and SPR-Promoted Visible Light Photoreduction. Catalysts 2018, 8, 426. https://doi.org/10.3390/catal8100426
Yuan X, Feng Z, Zhao J, Niu J, Liu J, Peng D, Cheng X. Significantly Enhanced Aqueous Cr(VI) Removal Performance of Bi/ZnO Nanocomposites via Synergistic Effect of Adsorption and SPR-Promoted Visible Light Photoreduction. Catalysts. 2018; 8(10):426. https://doi.org/10.3390/catal8100426
Chicago/Turabian StyleYuan, Xiaoya, Zijuan Feng, Jianjun Zhao, Jiawei Niu, Jiasen Liu, Dong Peng, and Xin Cheng. 2018. "Significantly Enhanced Aqueous Cr(VI) Removal Performance of Bi/ZnO Nanocomposites via Synergistic Effect of Adsorption and SPR-Promoted Visible Light Photoreduction" Catalysts 8, no. 10: 426. https://doi.org/10.3390/catal8100426
APA StyleYuan, X., Feng, Z., Zhao, J., Niu, J., Liu, J., Peng, D., & Cheng, X. (2018). Significantly Enhanced Aqueous Cr(VI) Removal Performance of Bi/ZnO Nanocomposites via Synergistic Effect of Adsorption and SPR-Promoted Visible Light Photoreduction. Catalysts, 8(10), 426. https://doi.org/10.3390/catal8100426



