Brønsted and Lewis Solid Acid Catalysts in the Valorization of Citronellal
Abstract
1. Introduction
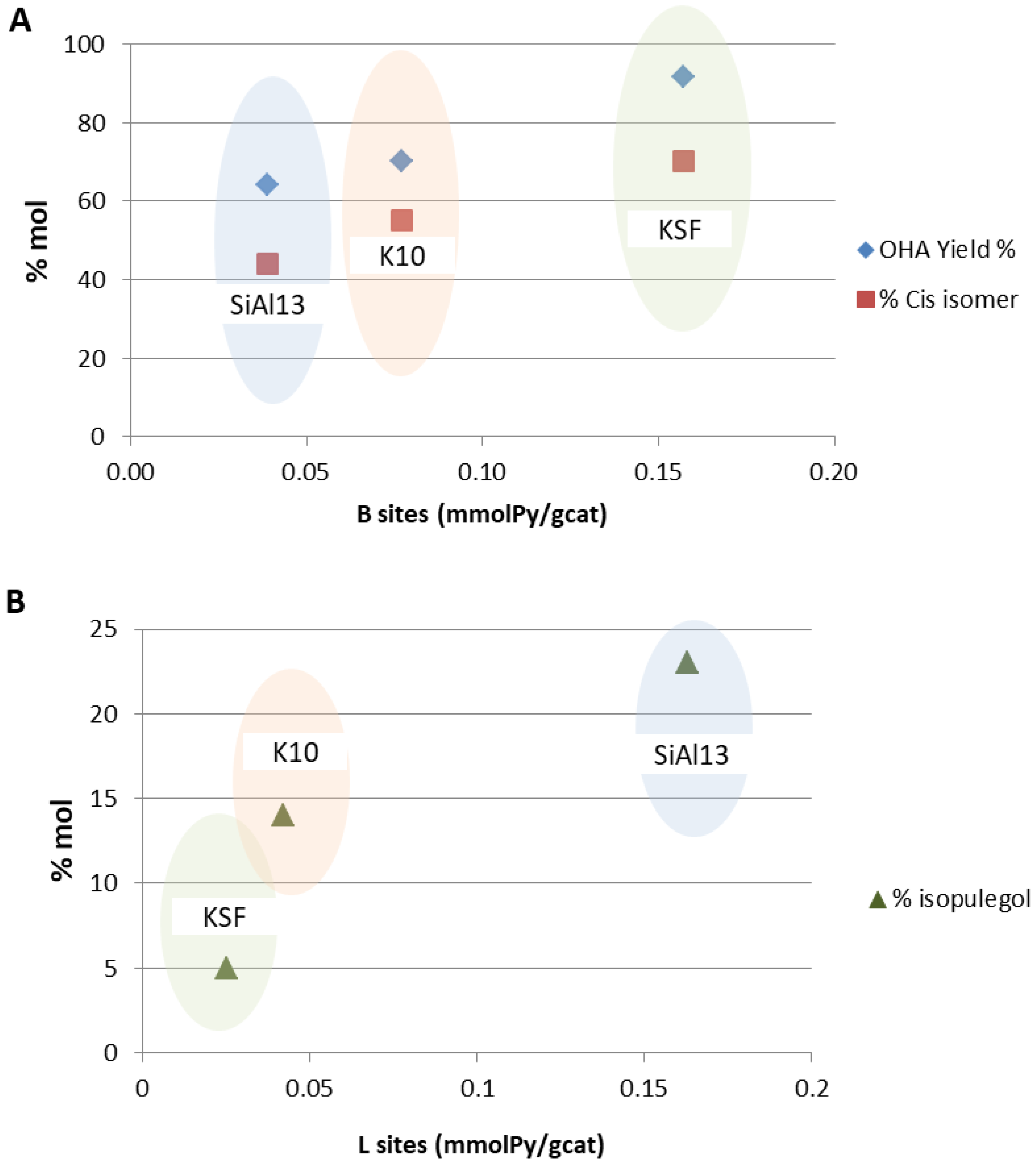
2. Results and Discussion
3. Experimental Section
3.1. Materials and Methods
3.2. Copper Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Monteiro, J.L.F.; Veloso, C.O. Catalytic conversion of terpenes into fine chemicals. Top. Catal. 2004, 27, 169–180. [Google Scholar] [CrossRef]
- Ravasio, N.; Zaccheria, F.; Guidotti, M.; Psaro, R. Mono- and bifunctional heterogeneous catalytic transformation of terpenes and terpenoids. Top. Catal. 2004, 27, 157–168. [Google Scholar] [CrossRef]
- Zhang, D.; del Rio-Chanona, E.A.; Shah, N. Screening synthesis pathways for biomass-derived sustainable polymer production. ACS Sustain. Chem. Eng. 2017, 5, 4388–4398. [Google Scholar] [CrossRef]
- Wilbon, P.A.; Chu, F.; Tang, C. Progress in renewable polymers from natural terpenes, terpenoids, and rosin. Macromol. Rapid Commun. 2013, 34, 8–37. [Google Scholar] [CrossRef] [PubMed]
- Kinanthi, R.; Puspitasari; Dwi, F.; Warsito, S.; Farid Rahman, M. Synthesis of schiff base from citronellal in kaffir lime oil (Citrus hystrix D.C) using acid catalyst. In Proceedings of the 1st International Conference of Essential Oils (ICEO2017), Malang, Indonesia, 11–12 October 2017. [Google Scholar]
- Warsito; Ramadhan, S.D.; Al Karoma, D.; Zulfa, A. Comparison of synthesis of some benzimidazole derivatives of cytronellal and citral. In Proceedings of the 1st International Conference of Essential Oils (ICEO2017), Malang, Indonesia, 11–12 October 2017. [Google Scholar]
- Kouznetsov, V.V. Recent synthetic developments in a powerful imino Diels–Alder reaction (Povarov reaction): Application to the synthesis of N-polyheterocycles and related alkaloids. Tetrahedron 2009, 65, 2721–2750. [Google Scholar] [CrossRef]
- Canas-Rodriguez, A.; Canas, R.G.; Mateo-Bernardo, A. Tricyclic inhibitors of gastric acid secretion. Part, V. Octahydroacridines. An. Quim. Ser. C. 1987, 83, 24–27. [Google Scholar]
- Mayekar, N.V.; Nayak, S.K.; Chattopadhyay, S. Two convenient one-pot strategies for the synthesis of octahydroacridines. Synth. Commun. 2004, 34, 3111–3119. [Google Scholar] [CrossRef]
- Sakane, S.; Matsumura, Y.; Yamamura, Y.; Ishida, Y.; Maruoka, K.; Yamamoto, H. Olefinic cyclizations promoted by beckmann rearrangement of oxime sulfonate. J. Am. Chem. Soc. 1983, 105, 672–674. [Google Scholar] [CrossRef]
- Sakanishi, K.; Mochida, I.; Okazaki, H.; Soeda, M. Selective hydrogenation of 9-aminoacridine over supported noble metal catalysts. Chem. Lett. 1990, 19, 319–322. [Google Scholar]
- Kouznetsov, V.; Palma, A.; Rozo, W.; Stashenko, E.; Bahsas, A.; Amaro-Luis, J. A facile Brønsted acidic-mediated cyclisation of 2-allyl-1-arylaminocyclohexanes to octahydroacridine derivatives. Tetrahedron Lett. 2000, 41, 6985–6988. [Google Scholar] [CrossRef]
- Jacob, R.G.; Perin, G.; Botteselle, G.V.; Lenardão, E.J. Clean and atom-economic synthesis of octahydroacridines: Application to essential oil of citronella. Tetrahedron Lett. 2003, 44, 6809–6812. [Google Scholar] [CrossRef]
- Sabitha, G.; Reddy, E.V.; Yadav, J.S. Bismuth(III) Chloride: An efficient catalyst for the one-pot stereoselective synthesis of octahydroacridines. Synthesis 2002, 3, 409–412. [Google Scholar] [CrossRef]
- Santoro, F.; Psaro, R.; Ravasio, N.; Zaccheria, F. Reductive amination of ketones or amination of alcohols over heterogeneous Cu catalysts: Matching the catalyst support with the N-Alkylating agent. ChemCatChem 2012, 4, 1249–1254. [Google Scholar] [CrossRef]
- Zaccheria, F.; Scotti, N.; Marelli, M.; Psaro, R.; Ravasio, N. Unravelling the properties of supported copper oxide: Can the particle size induce acidic behaviour? Dalton Trans. 2013, 42, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Zaccheria, F.; Santoro, F.; Psaro, R.; Ravasio, N. CuO/SiO2: A simple and effective solid acid catalyst for epoxides ring opening. Green Chem. 2011, 13, 545–548. [Google Scholar] [CrossRef]
- Scotti, N.; Dangate, M.; Gervasini, A.; Evangelisti, C.; Ravasio, N.; Zaccheria, F. Unraveling the role of low coordination sites in a Cu metal nanoparticle: A step forwards the selective synthesis of second generation biofuels. ACS Catal. 2014, 4, 2818–2826. [Google Scholar] [CrossRef]
- Campanati, M.; Savini, P.; Tagliani, A.; Vaccari, A.; Piccolo, O. Environmentally friendly vapour phase synthesis of alkylquinolines. Catal. Lett. 1997, 47, 247–250. [Google Scholar] [CrossRef]
- Ravasio, N.; Antenori, M.; Babudri, F.; Gargano, M. Intramolecular ene reaction promoted by mixed cogels. Stud. Surf. Sci. Catal. 1997, 108, 625–632. [Google Scholar]
- Trombetta, M.; Busca, G.; Rossini, S.; Piccoli, V.; Cornaro, U.; Guercio, A.; Catani, R.; Willey, R. FT-IR studies on light olefin skeletal isomerization catalysis: III. surface acidity and activity of amorphous and crystalline catalysts belonging to the SiO2–Al2O3 system. J. Catal. 1998, 179, 581–596. [Google Scholar] [CrossRef]
- Ravindra Reddy, C.; Bhat, Y.S.; Nagendrappa, G.; Jai Prakash, B.S. Brønsted and Lewis acidity of modified montmorillonite clay catalysts determined by FT-IR spectroscopy. Catal. Today 2009, 141, 157–160. [Google Scholar] [CrossRef]
- Flessner, U.; Jones, D.J.; Rozière, J.; Zajac, J.; Storaro, L.; Lenarda, M.; Pavanc, M.; Jiménez-López, A.; Rodrìguez-Castellón, E.; Trombetta, M.; et al. A study of the surface acidity of acid-treated montmorillonite clay catalysts. J. Mol. Catal. A Chem. 2001, 168, 247–256. [Google Scholar] [CrossRef]
- Vodnár, J.; Farkas, J.; Békássy, S. Catalytic decomposition of 1,4-diisopropylbenzene dihydroperoxide on montmorillonite-type catalysts. Appl. Catal. A 2001, 208, 329–334. [Google Scholar] [CrossRef]
- Laschat, S.; Lauterwein, J. Intramolecular hetero-Diels-Alder reaction of N-arylimines. Applications to the synthesis of octahydroacridine derivatives. J. Org. Chem. 1993, 58, 2856–2861. [Google Scholar] [CrossRef]
- Acelas, M.; Romero Bohórquez, A.R.; Kouznetsov, V.V. Highly diastereoselective synthesis of new trans-fused octahydroacridines via. intramolecular cationic imino diels–alder reaction of N-protected anilines and citronellal or citronella essential oil. Synthesis 2017, 49, 2153–2162. [Google Scholar]
- Emeis, C.A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Carey, J.S.; Laffan, D.; Thomson, C.; Williams, M.T. Analysis of the reactions used for the preparation of drug candidate molecules. Org. Biomol. Chem. 2006, 4, 2337–2347. [Google Scholar] [CrossRef] [PubMed]

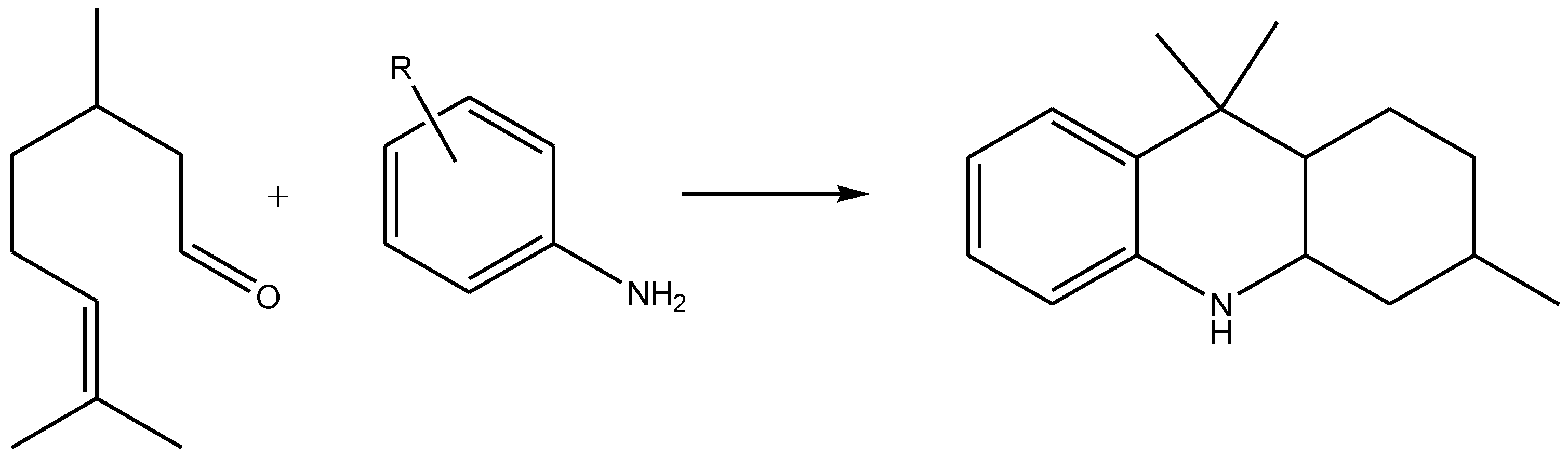

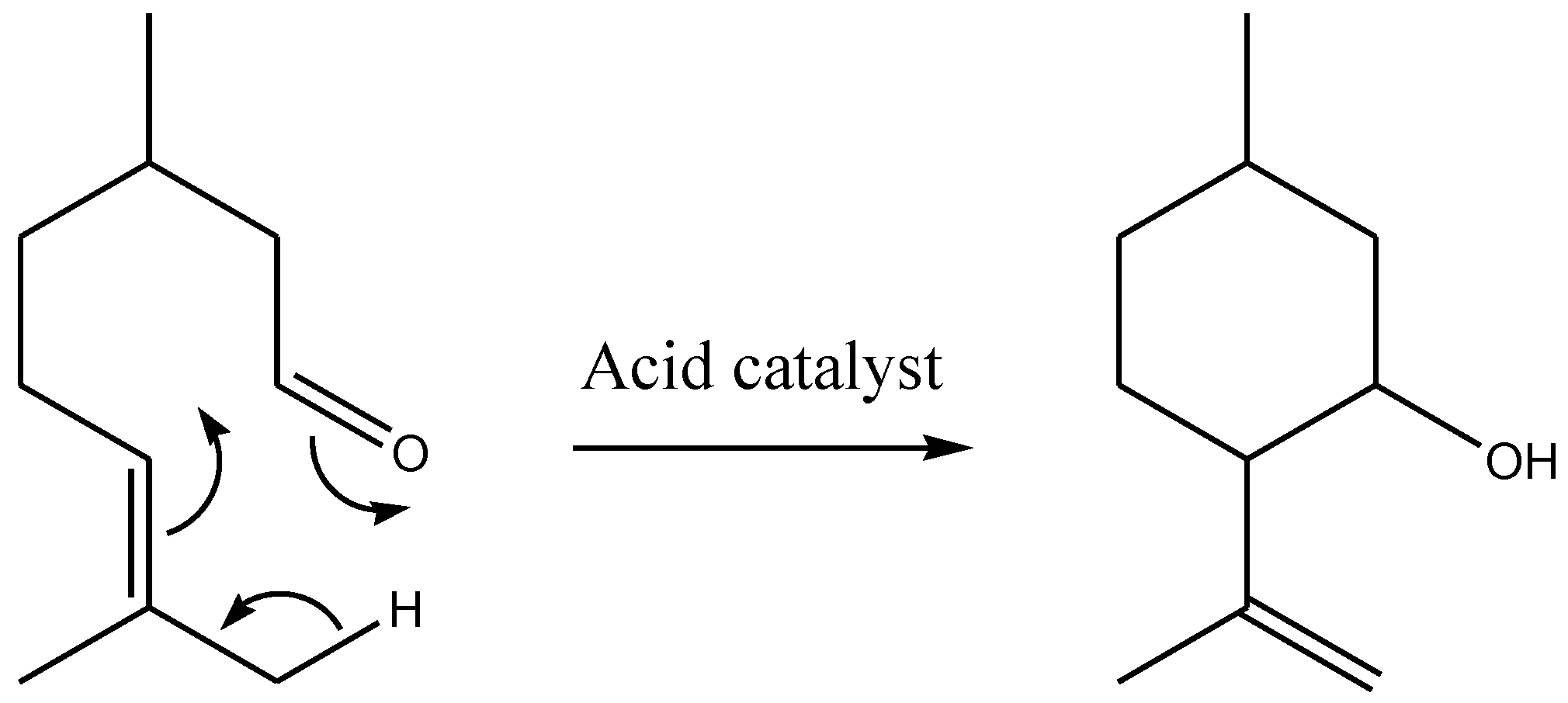
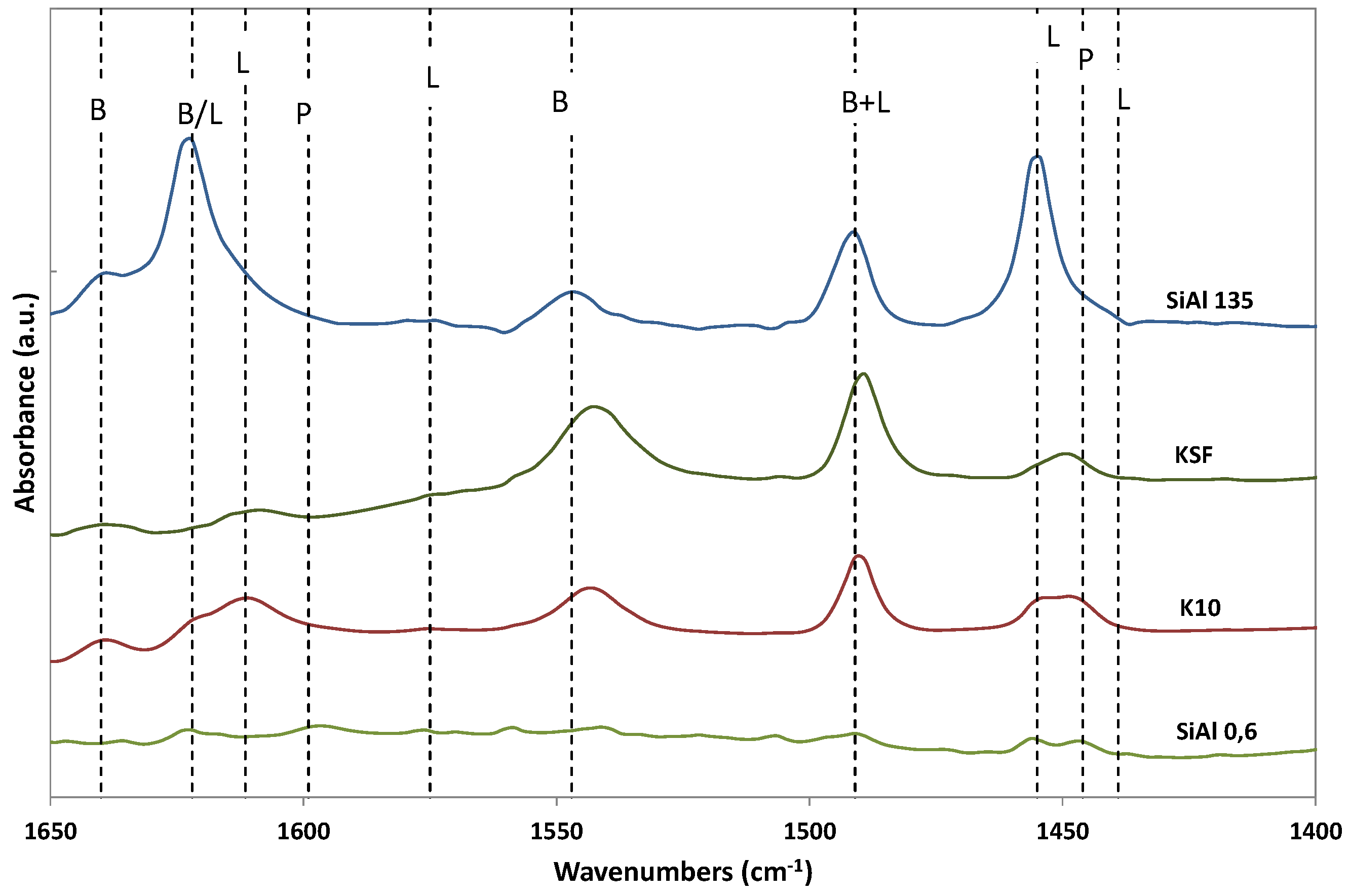
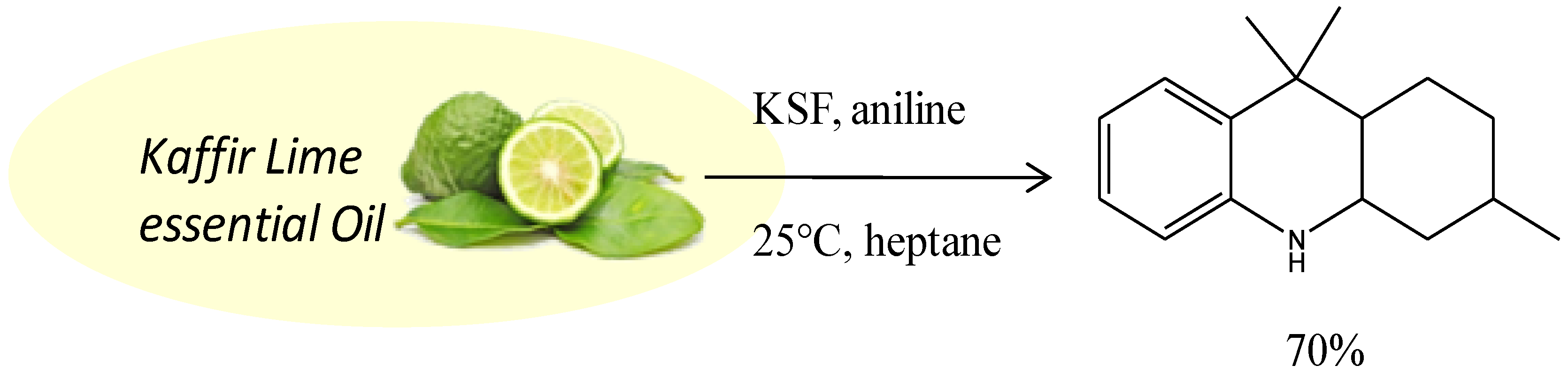
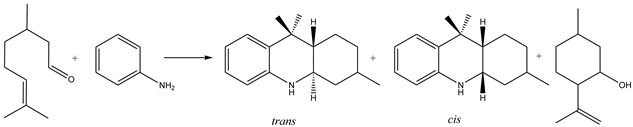
| Entry | Catalyst | Amine | Conditions | T h | Conv. % | OHAs Sel % | Cis/Trans. | Imine Sel % | Isopulegols % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | None | aniline | Toluene, 100 °C, N2 | 1 | 73 | - | - | 87 | - |
| 7.5 | 96 | 93 | |||||||
| 2 | None | aniline | Heptane, 25 °C, air | 1 | 96 | - | - | 83 | - |
| 3 | Cu/Si | aniline | Toluene, 100 °C, H2 | 1 | >99 | 97 | 37/63 | <1 | 1 |
| 4 | CuO/Si | aniline | Toluene, 25 °C, air | 1 | >99 | 96 | 45/55 | - | 2 |
| 5 | CuO/Si | p-anisidine | Toluene, 25 °C, air | 1 | >99 | 59 | 61/39 | - | 32 |
| 6 | SiAl 0.6 | aniline | Heptane, 25 °C, air | 1 | >99 | 71 | 41/59 | ˂1 | 24 |
| 7 | SiAl 0.6 | aniline | Heptane, 0 °C, air | 1 | >99 | 79 | 49/51 | - | 17 |
| 8 | SiAl 0.6 | aniline | Dioxane, 25 °C, air | 1 | >99 | 82 | 58/42 | - | 14 |
| 9 | SiAl 0.6 | p-anisidine | Heptane, 25 °C, air | 1 | >99 | 96 | 51/49 | <1 | 2 |
| 10 | SiAl 0.6 | toluidine | Heptane, 25 °C, N2 | 2 | >99 | 90 | 37/63 | - | 1 |
| 11 | SiAl 13 | aniline | Heptane, 25 °C, N2 | 1 | >99 | 64 | 41/59 | - | 23 |
| 12 | SiAl 13 | aniline | Heptane, 0 °C, air | 1 | >99 | 86 | 58/42 | - | 11 |
| 13 | Mont K10 | aniline | Heptane, 25 °C, air | 1 | >99 | 70 | 55/45 | - | 14 |
| 14 | Mont KSF | aniline | Heptane, 25 °C, air | 1 | >99 | 92 | 71/29 | - | 5 |
| 15 | Mont KSF | aniline | Heptane, 0 °C, air | 1 | >99 | 85 | 73/27 | - | 10 |
| Catalyst | Acidic Sites (mmolpy/gcat) | % OHA | % Cis | % Isopulegols | |
|---|---|---|---|---|---|
| KSF | 0.157 | 0.025 | 92 | 71 | 5 |
| K10 | 0.077 | 0.042 | 70 | 55 | 14 |
| SiAl13 | 0.039 | 0.163 | 64 | 41 | 23 |
| SiAl 0.6 | - | 0.005 | 71 | 41 | 24 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaccheria, F.; Santoro, F.; Iftitah, E.D.; Ravasio, N. Brønsted and Lewis Solid Acid Catalysts in the Valorization of Citronellal. Catalysts 2018, 8, 410. https://doi.org/10.3390/catal8100410
Zaccheria F, Santoro F, Iftitah ED, Ravasio N. Brønsted and Lewis Solid Acid Catalysts in the Valorization of Citronellal. Catalysts. 2018; 8(10):410. https://doi.org/10.3390/catal8100410
Chicago/Turabian StyleZaccheria, Federica, Federica Santoro, Elvina Dhiaul Iftitah, and Nicoletta Ravasio. 2018. "Brønsted and Lewis Solid Acid Catalysts in the Valorization of Citronellal" Catalysts 8, no. 10: 410. https://doi.org/10.3390/catal8100410
APA StyleZaccheria, F., Santoro, F., Iftitah, E. D., & Ravasio, N. (2018). Brønsted and Lewis Solid Acid Catalysts in the Valorization of Citronellal. Catalysts, 8(10), 410. https://doi.org/10.3390/catal8100410






