Tuning the Activity and Selectivity of Phenylacetylene Hydrosilylation with Triethylsilane in the Liquid Phase over Size Controlled Pt Nanoparticles
Abstract
1. Introduction
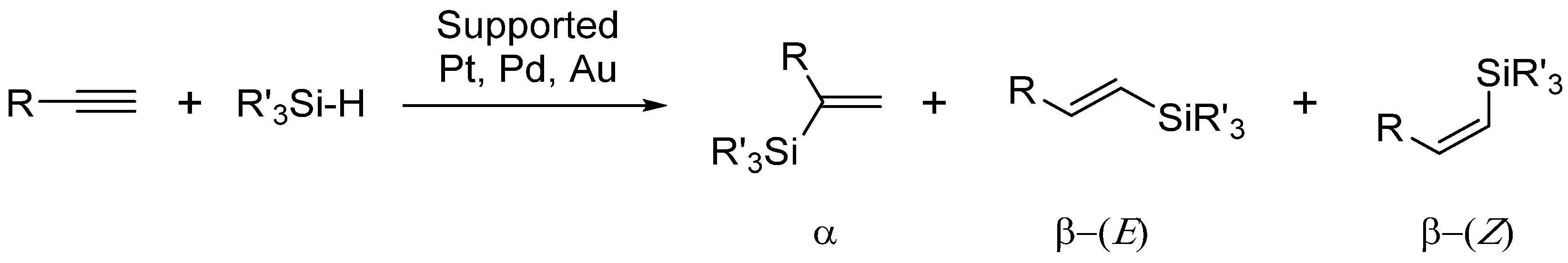
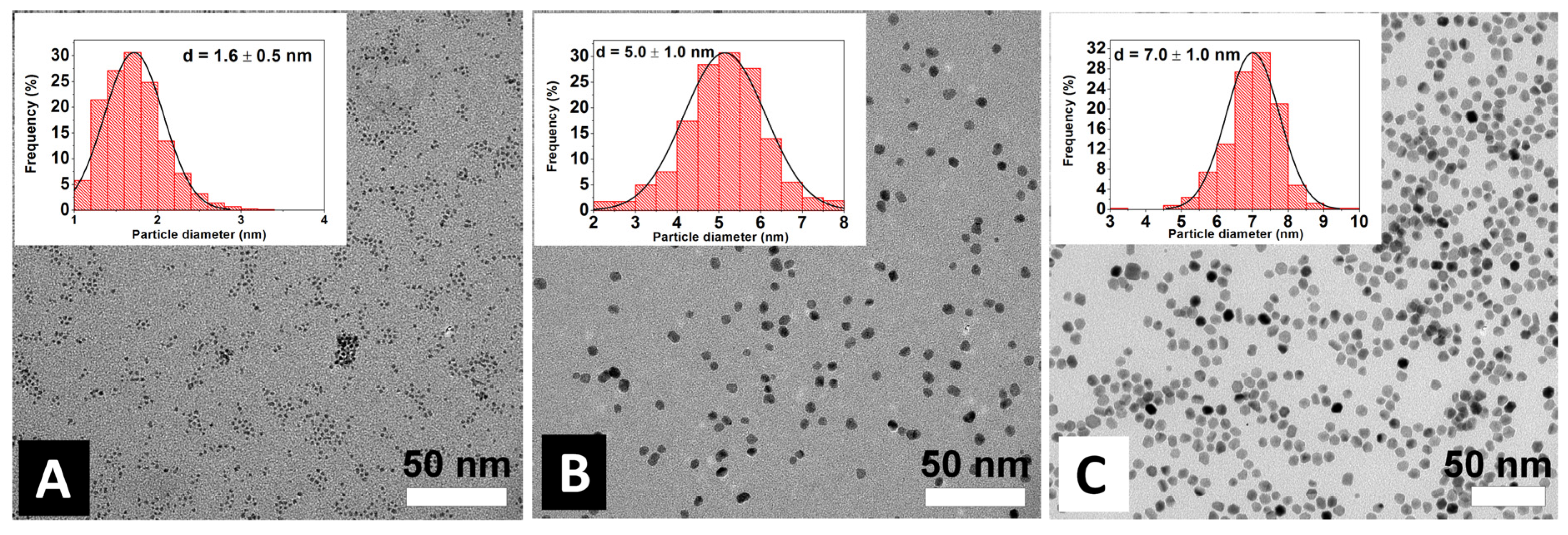
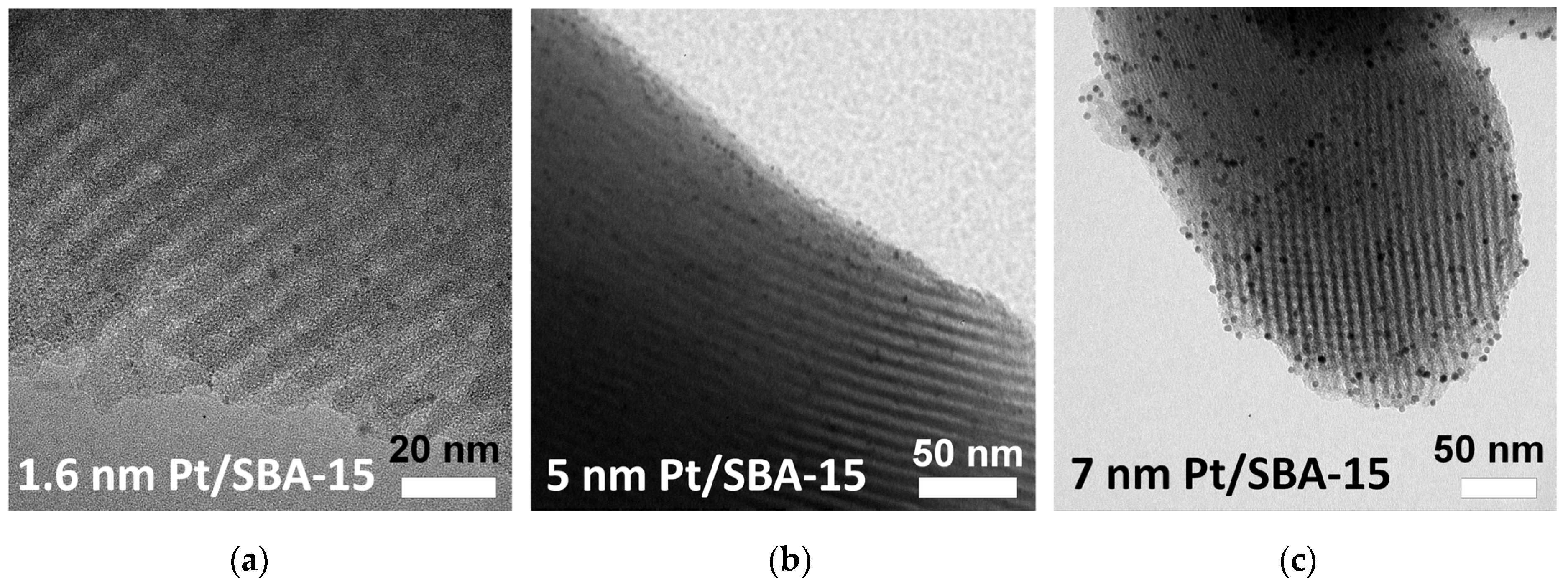
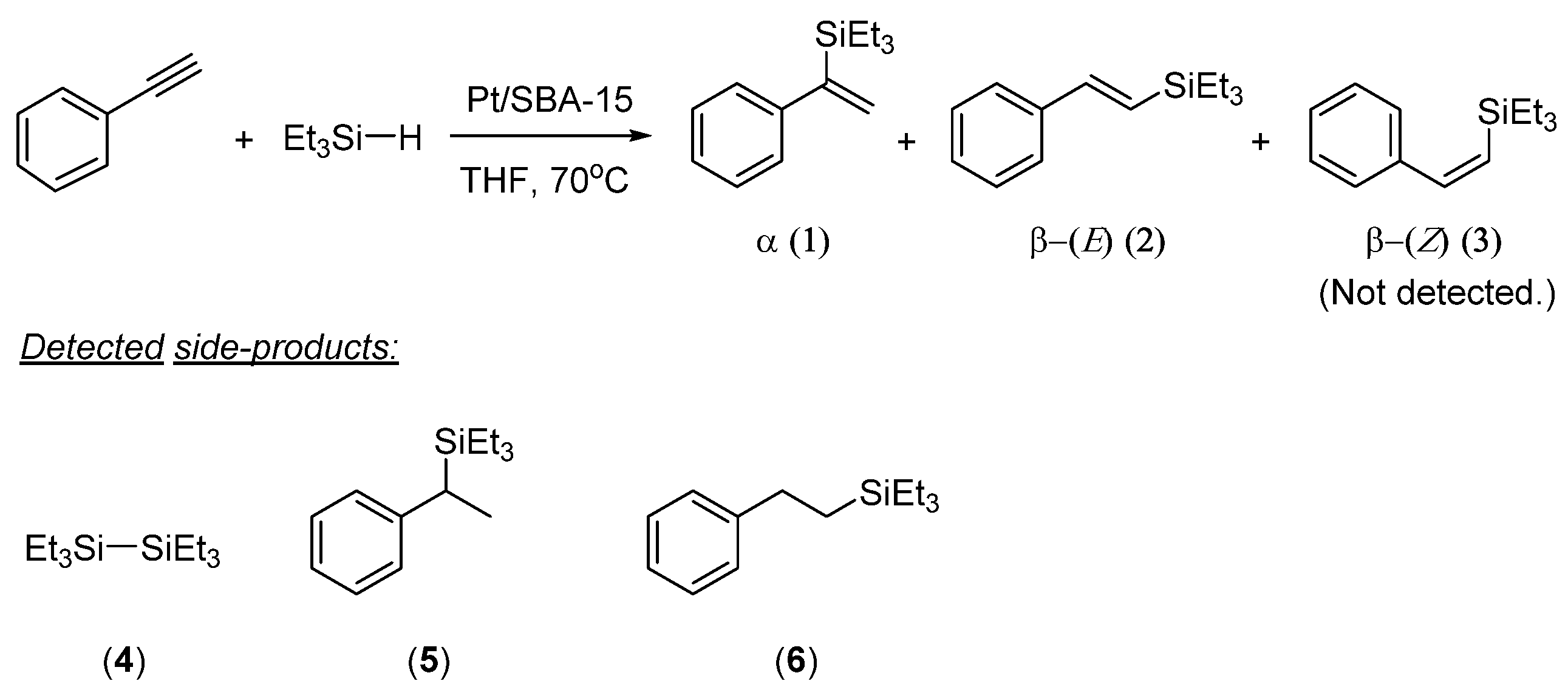
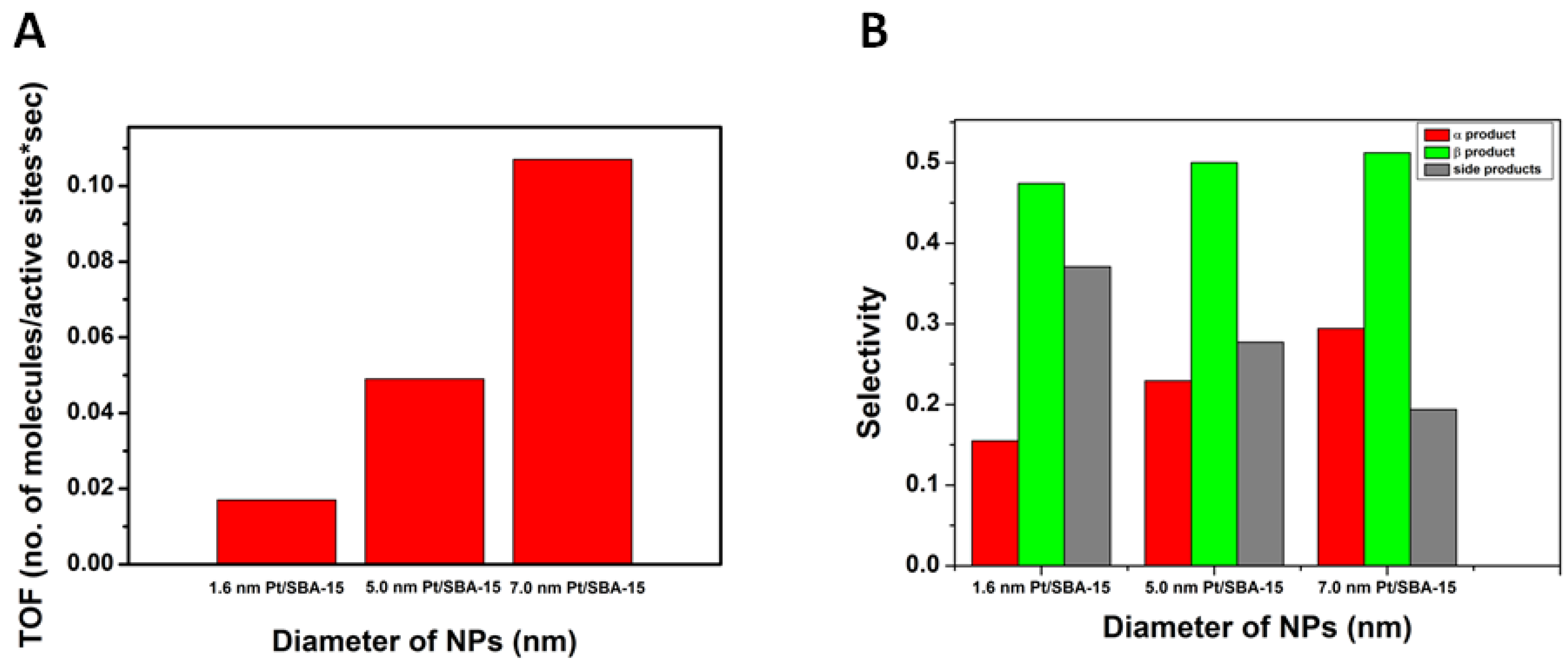
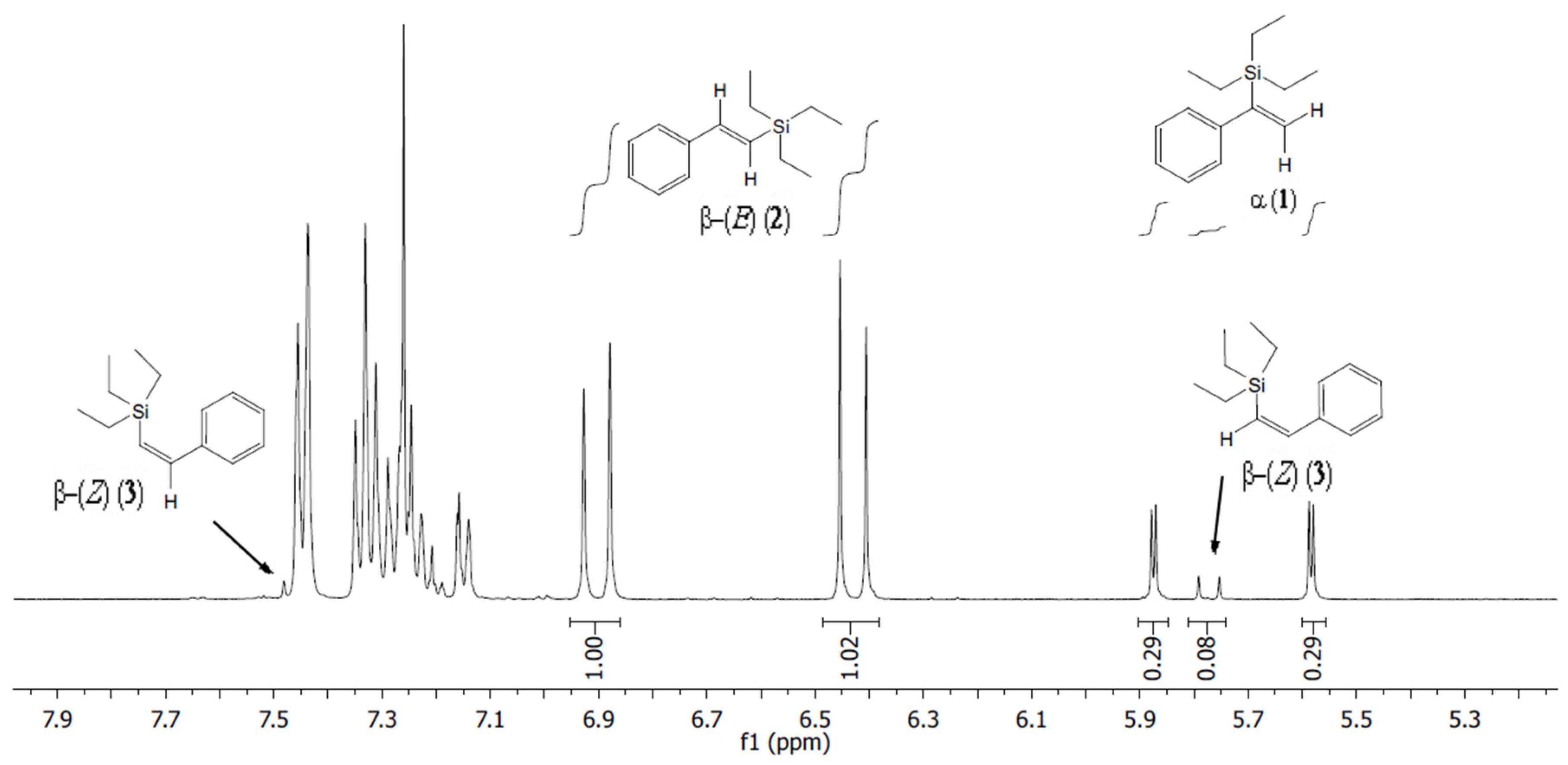
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of SBA-15 Mesoporous Silica
3.2. Synthesis of 1.6 nm Platinum Nanoparticles
3.3. Synthesis of 5.0 nm Platinum Nanoparticles
3.4. Synthesis of 7.0 nm Platinum Nanoparticles
3.5. Preparation of the Silica Supported Pt Nanoparticle Catalysts
3.6. Characterization of the Catalysts
3.7. Catalytic Hydrosilylation Reactions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ojima, I.; Li, Z.; Zhu, J. The Chemistry of Organic Silicon Compounds; ZVi, R., Apeloig, Y., Eds.; Wiley: Hoboken, NJ, USA, 1998. [Google Scholar]
- Denmark, S.; Ober, M.H. Organosilicon Reagents: Synthesis and Application to Palladium-Catalyzed Cross-Coupling Reactions. Aldrichimica Acta 2003, 3, 75–85. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Pandarus, V.; Béland, F. Platinum-Based Heterogeneously Catalyzed Hydrosilylation. Eur. J. Org. Chem. 2013, 2013, 6227–6235. [Google Scholar]
- Alonso, F.; Buitrago, R.; Moglie, Y.; Ruiz-Martínez, J.; Sepúlveda-Escribano, A.; Yus, M. Hydrosilylation of alkynes catalysed by platinum on titania. J. Organomet. Chem. 2011, 696, 368–372. [Google Scholar] [CrossRef]
- Alonso, F.; Buitrago, R.; Moglie, Y.; Sepúlveda-Escribano, A.; Yus, M. Selective hydrosilylation of 1,3-diynes catalyzed by titania-supported platinum. Organometallics 2012, 31, 2336–2342. [Google Scholar] [CrossRef]
- Aronica, L.A.; Schiavi, E.; Evangelisti, C.; Caporusso, A.M.; Salvadori, P.; Vitulli, G.; Bertinetti, L.; Martra, G. Solvated gold atoms in the preparation of efficient supported catalysts: Correlation between morphological features and catalytic activity in the hydrosilylation of 1-hexyne. J. Catal. 2009, 266, 250–257. [Google Scholar] [CrossRef]
- Cano, R.; Yus, M.; Ramón, D.J. Impregnated Platinum on Magnetite as an Efficient, Fast, and Recyclable Catalyst for the Hydrosilylation of Alkynes. ACS Catal. 2012, 2, 1070–1078. [Google Scholar] [CrossRef]
- Hamze, A.; Provot, O.; Brion, J.D.; Alami, M. Regiochemical aspects of the platinum oxide catalyzed hydrosilylation of alkynes. Synthesis (Stuttg) 2007, 13, 2025–2036. [Google Scholar] [CrossRef]
- Hu, W.; Xie, H.; Yue, H.; Prinsen, P.; Luque, R. Super-microporous silica-supported platinum catalyst for highly regioselective hydrosilylation. Catal. Commun. 2017, 97, 51–55. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Yamamoto, Y.; Asao, N. Selective hydrosilylation of alkynes with a nanoporous gold catalyst. Catal. Sci. Technol. 2013, 3, 2902. [Google Scholar] [CrossRef]
- Jiménez, R.; Martínez-Rosales, J.M.; Cervantes, J. The activity of Pt/SiO2 catalysts obtained by the sol-gel method in the hydrosilylation of 1-alkynes. Can. J. Chem. 2003, 81, 1370–1375. [Google Scholar] [CrossRef]
- Psyllaki, A.; Lykakis, I.N.; Stratakis, M. Reaction of hydrosilanes with alkynes catalyzed by gold nanoparticles supported on TiO2. Tetrahedron 2012, 68, 8724–8731. [Google Scholar] [CrossRef]
- Reddy, C.B.; Shil, A.K.; Guha, N.R.; Sharma, D.; Das, P. Solid Supported Palladium(0) Nanoparticles: An Efficient Heterogeneous Catalyst for Regioselective Hydrosilylation of Alkynes and Suzuki Coupling of β-Arylvinyl Iodides. Catal. Lett. 2014, 144, 1530–1536. [Google Scholar] [CrossRef]
- Chauhan, B.P.S.; Sarkar, A. Functionalized vinylsilanes via highly efficient and recyclable Pt-nanoparticle catalysed hydrosilylation of alkynes. Dalton Trans. 2017, 46, 8709–8715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, G.; Cai, C.; Zhang, T.; Mou, C.-Y.; Su, D.-S.; Li, J.; Zhao, J.; Thieuleux, C.; Petit, M. Regio-and stereoselective hydrosilylation of alkynes catalyzed by SiO2 supported Pd-Cu bimetallic nanoparticles. Green Chem. 2017, 19, 2535–2540. [Google Scholar] [CrossRef]
- Miura, H.; Endo, K.; Ogawa, R.; Shishido, T. Supported Palladium–Gold Alloy Catalysts for Efficient and Selective Hydrosilylation under Mild Conditions with Isolated Single Palladium Atoms in Alloy Nanoparticles as the Main Active Site. ACS Catal. 2017, 7, 1543–1553. [Google Scholar] [CrossRef]
- Rivero-Crespo, M.A.; Leyva-Pérez, A.; Corma, A. A Ligand-Free Pt3 Cluster Catalyzes the Markovnikov Hydrosilylation of Alkynes with up to 106 Turnover Frequencies. Chem. Eur. J. 2017, 23, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Bratlie, K.M.; Kliewer, C.J.; Somorjai, G.A. Structure effects of benzene hydrogenation studied with sum frequency generation vibrational spectroscopy and kinetics on Pt(111) and Pt(100) single-crystal surfaces. J. Phys. Chem. B 2006, 110, 17925–17930. [Google Scholar] [CrossRef] [PubMed]
- Somorjai, G.A.; Davis, S.M. Surface Science Studies of Catalysed Reactions on Platinum Surfaces. Platin. Met. Rev. 1983, 27, 54–65. [Google Scholar]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. 2009, 48, 60–103. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, H.; Liu, F.; Han, H.-L.; Carl, L.M.; Sapi, A.; Somorjai, G.A. Alcohol Oxidation at Platinum–Gas and Platinum–Liquid Interfaces: The Effect of Platinum Nanoparticle Size, Water Coadsorption, and Alcohol Concentration. J. Phys. Chem. C 2017, 121, 7365–7371. [Google Scholar] [CrossRef]
- Sapi, A.; Liu, F.; Cai, X.; Thompson, C.M.; Wang, H.; An, K.; Krier, J.M.; Somorjai, G.A. Comparing the Catalytic Oxidation of Ethanol at the Solid–Gas and Solid–Liquid Interfaces over Size-Controlled Pt Nanoparticles: Striking Differences in Kinetics and Mechanism. Nano Lett. 2014, 14, 6727–6730. [Google Scholar] [CrossRef] [PubMed]
- Alayoglu, S.; Krier, J.M.; Michalak, W.D.; Zhu, Z.; Gross, E.; Somorjai, G.A. In situ surface and reaction probe studies with model nanoparticle catalysts. ACS Catal. 2012, 2, 2250–2258. [Google Scholar] [CrossRef]
- Alayoglu, S.; Aliaga, C.; Sprung, C.; Somorjai, G.A. Size and shape dependence on Pt nanoparticles for the methylcyclopentane/hydrogen ring opening/ring enlargement reaction. Catal. Lett. 2011, 141, 914–924. [Google Scholar] [CrossRef]
- Grass, M.E.; Joo, S.H.; Zhang, Y.; Somorjai, G.A. Colloidally synthesized monodisperse Rh nanoparticles supported on SBA-15 for size-and pretreatment-dependent studies of CO oxidation. J. Phys. Chem. C 2009, 113, 8616–8623. [Google Scholar] [CrossRef]
- Gololobov, A.M.; Bekk, I.E.; Bragina, G.O.; Zaikovskii, V.I.; Ayupov, A.B.; Telegina, N.S.; Bukhtiyarov, V.I. Platinum nanoparticle size effect on specific catalytic activity in n-alkane deep oxidation: Dependence on the chain length of the paraffin. Kinet. Catal. 2009, 50, 864–870. [Google Scholar] [CrossRef]
- Yamada, Y.; Tsung, C.K.; Huang, W.; Huo, Z.; Habas, S.E.; Soejima, T.; Aliaga, C.E.; Somorjai, G.A.; Yang, P. Nanocrystal bilayer for tandem catalysis. Nat. Chem. 2011, 3, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Sápi, A.; Dobó, D.G.; Sebok, D.; Halasi, G.; Juhász, K.L.; Szamosvölgyi, A.; Pusztai, P.; Varga, E.; Kálomista, I.; Galbács, G.; et al. Silica-Based Catalyst Supports Are Inert, Are They Not? Striking Differences in Ethanol Decomposition Reaction Originated from Meso-and Surface-Fine-Structure Evidenced by Small-Angle X-ray Scattering. J. Phys. Chem. C 2017, 121, 5130–5136. [Google Scholar] [CrossRef]
- Ewing, C.S.; Veser, G.; McCarthy, J.J.; Johnson, J.K.; Lambrecht, D.S. Effect of Support Preparation and Nanoparticle Size on Catalyst–Support Interactions between Pt and Amorphous Silica. J. Phys. Chem. C 2015, 119, 19934–19940. [Google Scholar] [CrossRef]
- Solomonsz, W.A.; Rance, G.A.; Harris, B.J.; Khlobystov, A.N. Competitive hydrosilylation in carbon nanoreactors: Probing the effect of nanoscale confinement on selectivity. Nanoscale 2013, 5, 12200–12205. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Sápi, A.; Varga, A.; Samu, G.F.; Dobó, D.G.; Juhász, K.L.; Takacs, B.; Varga, E.; Kukovecz, A.; Kónya, Z.; Janáky, C. Photoelectrochemistry by Design: Tailoring the Nanoscale Structure of Pt/NiO Composites Leads to Enhanced Photoelectrochemical Hydrogen Evolution Performance. J. Phys. Chem. C 2017, 121, 12148–12158. [Google Scholar] [CrossRef] [PubMed]
- Rioux, R.M.; Song, H.; Hoefelmeyer, J.D.; Yang, P.; Somorjai, G.A. High-surface-area catalyst design: Synthesis, characterization, and reaction studies of platinum nanoparticles in mesoporous SBA-15 silica. J. Phys. Chem. B 2005, 109, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
G. Dobó, D.; Sipos, D.; Sápi, A.; London, G.; Juhász, K.L.; Kukovecz, Á.; Kónya, Z. Tuning the Activity and Selectivity of Phenylacetylene Hydrosilylation with Triethylsilane in the Liquid Phase over Size Controlled Pt Nanoparticles. Catalysts 2018, 8, 22. https://doi.org/10.3390/catal8010022
G. Dobó D, Sipos D, Sápi A, London G, Juhász KL, Kukovecz Á, Kónya Z. Tuning the Activity and Selectivity of Phenylacetylene Hydrosilylation with Triethylsilane in the Liquid Phase over Size Controlled Pt Nanoparticles. Catalysts. 2018; 8(1):22. https://doi.org/10.3390/catal8010022
Chicago/Turabian StyleG. Dobó, Dorina, Dániel Sipos, András Sápi, Gábor London, Koppány L. Juhász, Ákos Kukovecz, and Zoltán Kónya. 2018. "Tuning the Activity and Selectivity of Phenylacetylene Hydrosilylation with Triethylsilane in the Liquid Phase over Size Controlled Pt Nanoparticles" Catalysts 8, no. 1: 22. https://doi.org/10.3390/catal8010022
APA StyleG. Dobó, D., Sipos, D., Sápi, A., London, G., Juhász, K. L., Kukovecz, Á., & Kónya, Z. (2018). Tuning the Activity and Selectivity of Phenylacetylene Hydrosilylation with Triethylsilane in the Liquid Phase over Size Controlled Pt Nanoparticles. Catalysts, 8(1), 22. https://doi.org/10.3390/catal8010022








