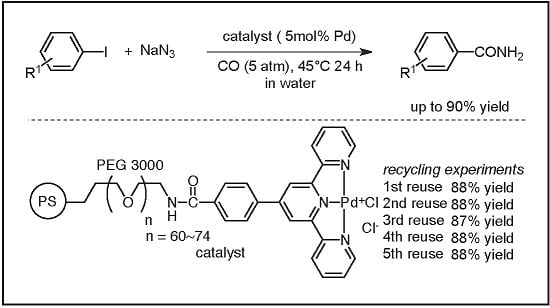
Recyclable Polymer-Supported Terpyridine–Palladium Complex for the Tandem Aminocarbonylation of Aryl Iodides to Primary Amides in Water Using NaN3 as Ammonia Equivalent
Abstract
:1. Introduction
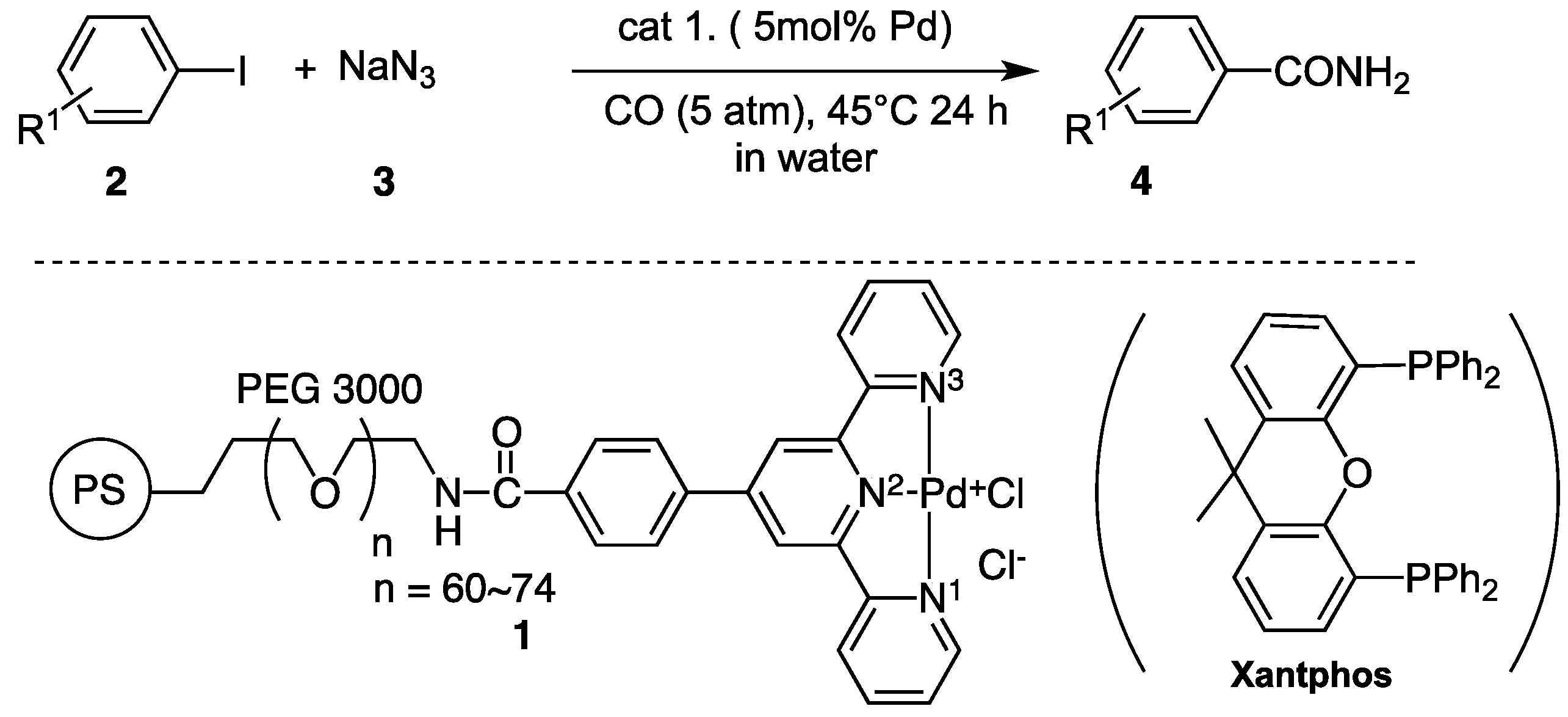
2. Results
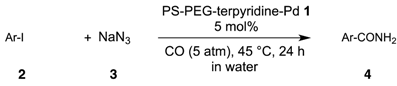
2.1. Coupling Conditions
2.2. Substrate Tolerance
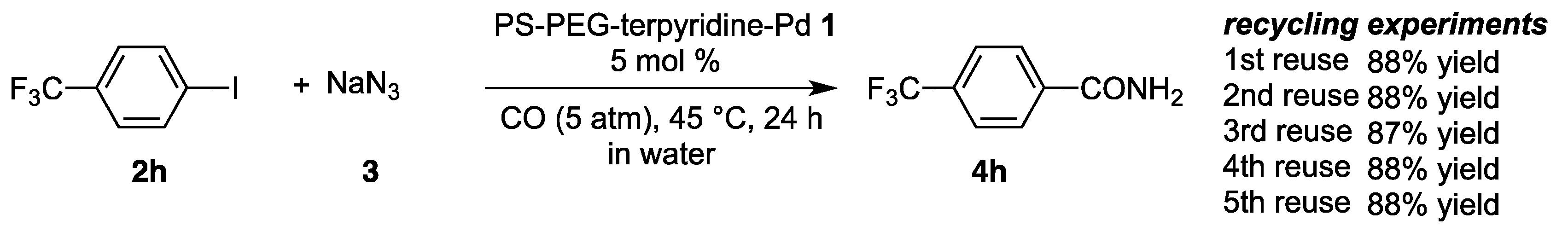
2.3. Recycling Experiments
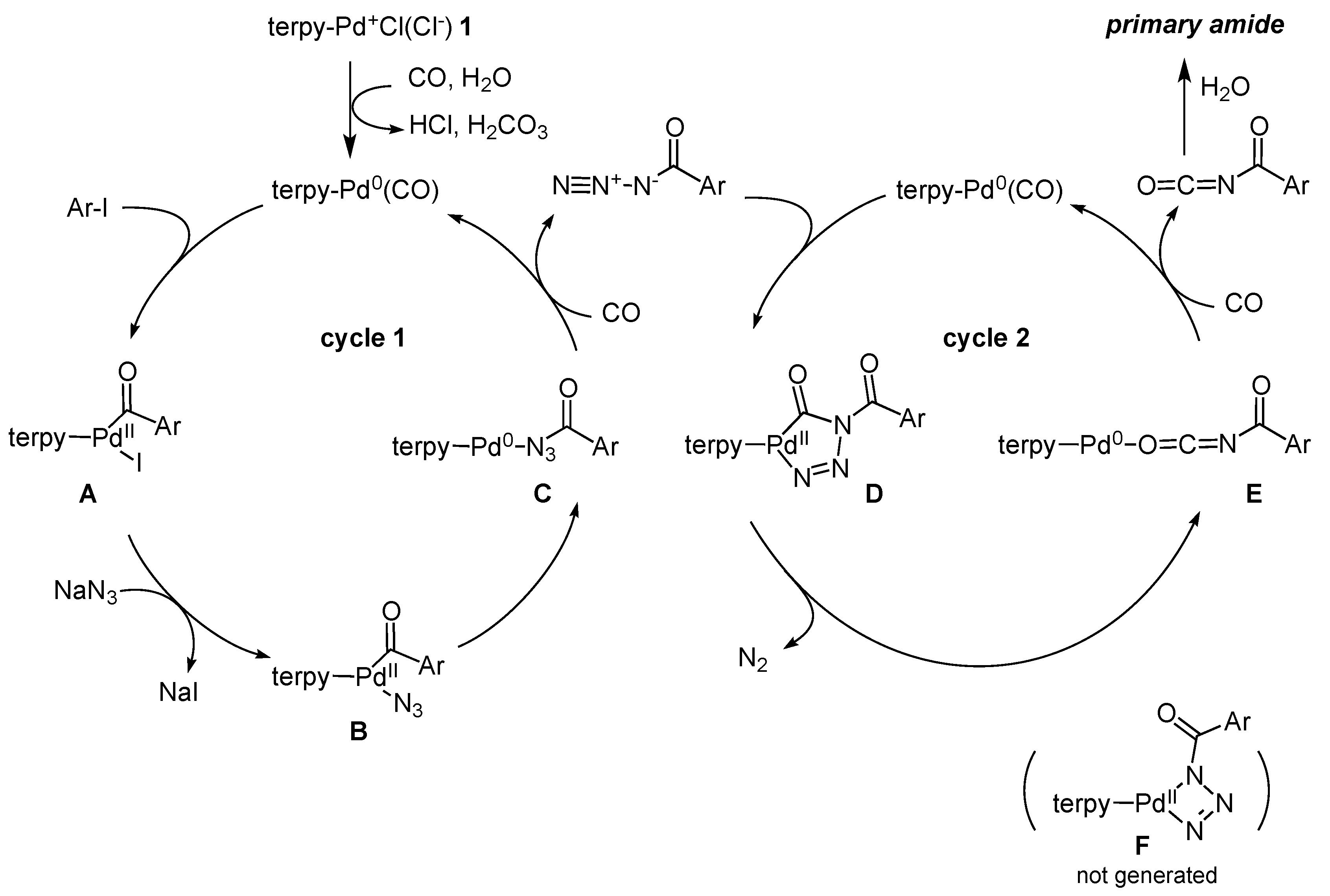
3. Discussion
Reaction Mechanism
4. Materials and Methods
4.1. General Methods
4.2. Materials
4.3. Aminocarbonylation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roy, S.; Roy, S.S.; Gribble, G.W. Metal-catalyzed amidation. Tetrahedron 2012, 68, 9867–9923. [Google Scholar] [CrossRef]
- Matsuda, F. Acrylamide production simplified. Chemtech 1977, 7, 306. [Google Scholar]
- Opsahl, R. Amide, Fatty Acid, in Encyclopedia of Chemical Technology, 1st ed.; Kroshcitz, J.I., Ed.; Wiley: New York, NY, USA, 1991; Volume 2, pp. 346–356. [Google Scholar]
- Gao, L.; Ding, J.; Gao, M.; Wang, Z.; Wu, J.; Li, A. Novel and direct transformation of methyl ketones or carbinols to primary amides by employing aqueous ammonia. Org. Lett. 2009, 11, 3810–3813. [Google Scholar]
- Soai, K.; Ookawa, A.; Hayashi, H. Novel functional group selectivity in reductions with lithium borohydride in mixed solvents containing methanol. J. Chem. Soc. Chem. Commun. 1983, 12, 668–669. [Google Scholar] [CrossRef]
- Plummer, B.F.; Menendez, M.; Songster, M. Hydration of nitriles to amides promoted by mercury(II) acetate in acetic acid. J. Org. Chem. 1989, 54, 718–719. [Google Scholar] [CrossRef]
- Blatt, A.H. The Beckmann rearrangement. Chem. Rev. 1933, 12, 215–260. [Google Scholar]
- Davenport, K.G.; Hilton, C.B. Process for Producing N-Acyl-hydroxy Aromatic Amides. U.S. Patent 4,524,217, 18 June 1985. [Google Scholar]
- Suzuka, T.; Sueyoshi, H.; Ogiahra, K. Polymer-supported terpyridine–palladium complex for the aminocarbonylation in water of aryl iodides using methoxylamine hydrochloride as an ammonia equivalent. Trans. Mater. Res. Soc. Jpn. 2016, 41, 225–228. [Google Scholar] [CrossRef]
- Schoenberg, A.; Heck, R.F. Palladium-catalyzed formylation of aryl, heterocyclic, and vinylic halides. J. Am. Chem. Soc. 1974, 96, 7761–7764. [Google Scholar] [CrossRef]
- Brennfuhrer, A.; Neumann, H.; Beller, M. Palladium-catalyzed carbonylation reaction of aryl halides and related compounds. Angew. Chem. Int. Ed. 2009, 48, 4114–4133. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-F.; Neumann, H.; Beller, M. Development of a second generation palladium catalyst system for the aminocarbonylation of aryl halides with CO and ammonia. Chem. Asian J. 2010, 5, 2168–2172. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-F.; Neumann, H.; Beller, M. Selective palladium-catalyzed aminocarbonylation of aryl halides with CO and ammonia. Chem. Eur. J. 2010, 16, 9750–9753. [Google Scholar] [CrossRef] [PubMed]
- Morera, E.; Ortar, G. Palladium-catalyzed carbonylative route to primary amides. Tetrahedron Lett. 1998, 39, 2835–2838. [Google Scholar] [CrossRef]
- Schnyder, A.; Indolese, A.F. Synthesis of unsymmetrical aroyl acyl imides by aminocarbonylation of aryl bromides. J. Org. Chem. 2002, 67, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Sato, Y.; Mori, M. Incorporation of N2 and CO into organic molecules: Amide formation by palladium-catalyzed and nitrogenation. J. Am. Chem. Soc. 2000, 122, 10722–10723. [Google Scholar] [CrossRef]
- Gadge, S.T.; Bhanage, B.M. A phosphine-free approach to primary amides by palladium-catalyzed aminocarbonylation of aryl and heteroaryl iodides using methoxyamine hydrochloride as an ammonia equivalent. Synlett 2014, 25, 85–88. [Google Scholar]
- Suzuka, T.; Adachi, M.; Yang, Z.-S.; Ogihara, K.; Higa, M. Suzuki–Miyaura cross coupling reaction in water with polymer-supported terpyridine palladium complex and application for the synthesis of 2,6-disubstitued pyrimidines. Trans. Mater. Res. Soc. Jpn. 2013, 38, 119–122. [Google Scholar] [CrossRef]
- Suzuka, T.; Adachi, M.; Nakamoto, Y.; Ogihara, K. Use of polymer-supported terpyridine–palladium complex for Mizoroki–Heck reaction in water under aerobic conditions. Trans. Mater. Res. Soc. Jpn. 2015, 40, 77–80. [Google Scholar] [CrossRef]
- Suzuka, T.; Adachi, M.; Ogihara, K. Sonogashira coupling reaction in water with polymer-supported terpyridine–palladium complex under aerobic conditions. Trans. Mater. Res. Soc. Jpn. 2015, 40, 103–106. [Google Scholar] [CrossRef]
- Suzuka, T.; Sueyoshi, H.; Maehara, S.; Ogasawara, H. Reactivity of aryl halides for transfer reduction in (sea)water using polymer-supported terpyridine palladium complex. Molecules 2015, 20, 9906–9914. [Google Scholar] [CrossRef] [PubMed]
- Miloserdov, F.M.; Grushin, V.V. Palladium-catalyzed aromatic azidocarbonylation. Angew. Chem. Int. Ed. 2012, 51, 3668–3672. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kwak, Y.S.; Lee, S.W. Synthesis and properties of arylpalladium(II) azido complexes PdAr(N3)(PR3)2. Nucleophilic reactions of the azido ligand with CO and with isocyanides to afford Pd(II) isocyanate, C-tetrazolate and carbodiimide complexes. J. Organomet. Chem. 2000, 603, 152–160. [Google Scholar] [CrossRef]
- Van Leeuwen, P.W.N.M.; Kamer, P.C.J.; Reek, J.N.H.; Dierkes, P. Lingand bite angle effects in metal-catalyzed C–C bond formation. Chem. Rev. 2000, 100, 2741–2769. [Google Scholar] [CrossRef] [PubMed]
- Yukawa, Y.; Tsuno, Y. The Crutius rearrangement. II. The decomposition of o-substituted benzazides in toluene. J. Am. Chem. Soc. 1958, 80, 6346–6350. [Google Scholar] [CrossRef]
- Chetcuti, P.A.; Walker, J.A.; Knobler, C.B.; Hawthorne, M.F. Synthesis of metallacycles by 1,3-dipolar cycloaddition reactions between low-valent metal carbonyls and aryl nitrile N-oxides. Organometallics 1988, 7, 641–680. [Google Scholar] [CrossRef]
- Cenini, S.; Gallo, E.; Gallo, A.; Caselli, A.; Ragaini, F.; Fantauzzi, S.; Piangiolino, C. Coordination chemistry of organic azides and amination reactions catalyzed by transition metal complexes. Coordination 2006, 250, 1234–1253. [Google Scholar] [CrossRef]
- Miloserdov, F.M.; McMullin, C.L.; Belmonte, M.M.; Benet-Buchholz, J.; Bakhmutov, V.I.; Macgregor, S.A.; Grushin, V. The challenge of palladium-catalyzed aromatic azidocarbonylation: From mechanistic and catalyst deactivation studies to a highly efficient process. Organometallics 2014, 33, 736–752. [Google Scholar] [CrossRef]
- Van Kalkeren, H.A.; Bruins, J.J.; Rutjes, F.P.J.T.; Van Delft, F.L. Organophosphorus-catalyzed Staudinger reduction. Adv. Synth. Catal. 2012, 354, 1417–1421. [Google Scholar] [CrossRef]
Sample Availability: Samples are not available from the authors. |

| Entry | NaN3 (equiv.) | Base (equiv.) | PMHS (equiv.) | Temp. (°C) | Time (h) | Yield of 4a (%) |
|---|---|---|---|---|---|---|
| 1 | 2.0 | none | 7.5 | 50 | 8 | 13 |
| 2 | 2.0 | K2CO3 | 7.5 | 50 | 8 | 9.1 |
| 3 | 2.0 | Cs2CO3 | 7.5 | 50 | 8 | 17 |
| 4 | 2.0 | Et3N | 7.5 | 50 | 8 | 3.0 |
| 5 | 2.0 | Cs2CO3 | 7.5 | 50 | 24 | 18 |
| 6 | 2.0 | none | 7.5 | 50 | 24 | 31 |
| 7 | 1.0 | none | 7.5 | 50 | 24 | 24 |
| 8 | 3.0 | none | 7.5 | 50 | 24 | 48 |
| 9 | 4.0 | none | 7.5 | 50 | 24 | 50 |
| 10 | 5.0 | none | 7.5 | 50 | 24 | 46 |
| 11 | 4.0 | none | 6.0 | 50 | 24 | 48 |
| 12 | 4.0 | none | 4.5 | 50 | 24 | 43 |
| 13 | 4.0 | none | 3.0 | 50 | 24 | 50 |
| 14 | 4.0 | none | 0.2 | 50 | 24 | 48 |
| 15 | 4.0 | none | 0.1 | 50 | 24 | 51 |
| 16 | 4.0 | none | none | 50 | 24 | 53 |
| 17 | 4.0 | none | none | 45 | 24 | 70 |
| 18 | 4.0 | none | none | 40 | 24 | 66 |
| Entry | 2 | 4 | Yield (%) a |
|---|---|---|---|
| 1 | C6H5-I: 2a | C6H5-CONH2: 4a | 70 |
| 2 | p-MeC6H4-I: 2b | p-MeC6H4-CONH2: 4b | 64 |
| 3 | m-MeC6H4-I: 2c | m-MeC6H4- CONH2: 4c | 59 |
| 4 | o-MeC6H4-I: 2d | o-MeC6H4- CONH2: 4d | 26 |
| 5 | 1-iodonaphthalene: 2e | 1-naphthamide: 4e | 4.2 |
| 6 | o-NO2C6H4-I: 2f | o-NO2C6H4-CONH2: 4f | 62 b |
| 7 | p-MeOC6H4-I: 2g | p-MeOC6H4-CONH2: 4g | 41 |
| 8 | p-CF3C6H4-I: 2h | p-CF3C6H4-CONH2: 4h | 90 |
| 9 | p-NO2C6H4-I: 2i | p-NO2C6H4-CONH2: 4i | 84 b |
| 10 | p-FC6H4-I: 2j | p-FC6H4-CONH2: 4j | 82 |
| 11 | p-ClC6H4-I: 2k | p-ClC6H4-CONH2: 4k | 81 b |
| 12 | p-BrC6H4-I: 2l | p-BrC6H4- CONH2: 4l | 65 |

| Catalyst | CO (atm) | Yield (%) |
|---|---|---|
| PS–PEG–terpyridine–Pd(dba) (1′) | 5 | 95 |
| PS–PEG–terpyridine–Pd(dba) (1′) | none | 0 |
| PS–PEG–terpyridine–Pd+Cl(Cl−) (1) | 5 | 56 |
| PS–PEG–terpyridine–Pd+Cl(Cl−) (1) | none | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuka, T.; Sueyoshi, H.; Ogihara, K. Recyclable Polymer-Supported Terpyridine–Palladium Complex for the Tandem Aminocarbonylation of Aryl Iodides to Primary Amides in Water Using NaN3 as Ammonia Equivalent. Catalysts 2017, 7, 107. https://doi.org/10.3390/catal7040107
Suzuka T, Sueyoshi H, Ogihara K. Recyclable Polymer-Supported Terpyridine–Palladium Complex for the Tandem Aminocarbonylation of Aryl Iodides to Primary Amides in Water Using NaN3 as Ammonia Equivalent. Catalysts. 2017; 7(4):107. https://doi.org/10.3390/catal7040107
Chicago/Turabian StyleSuzuka, Toshimasa, Hiromu Sueyoshi, and Kazuhito Ogihara. 2017. "Recyclable Polymer-Supported Terpyridine–Palladium Complex for the Tandem Aminocarbonylation of Aryl Iodides to Primary Amides in Water Using NaN3 as Ammonia Equivalent" Catalysts 7, no. 4: 107. https://doi.org/10.3390/catal7040107
APA StyleSuzuka, T., Sueyoshi, H., & Ogihara, K. (2017). Recyclable Polymer-Supported Terpyridine–Palladium Complex for the Tandem Aminocarbonylation of Aryl Iodides to Primary Amides in Water Using NaN3 as Ammonia Equivalent. Catalysts, 7(4), 107. https://doi.org/10.3390/catal7040107