Synthesis of Renewable Diesel Range Alkanes by Hydrodeoxygenation of Palmitic Acid over 5% Ni/CNTs under Mild Conditions
Abstract
:1. Introduction
2. Results and Discussion
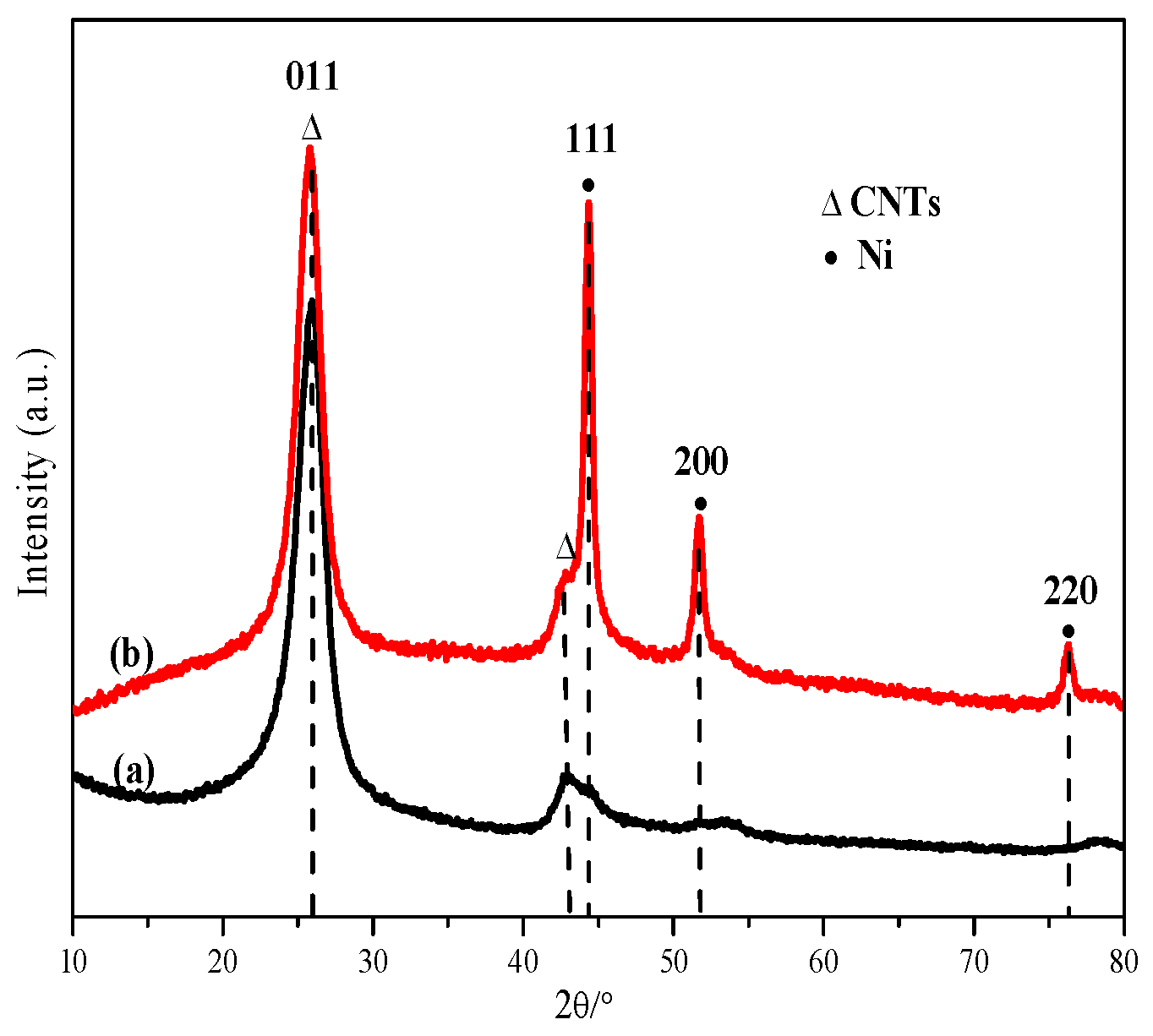
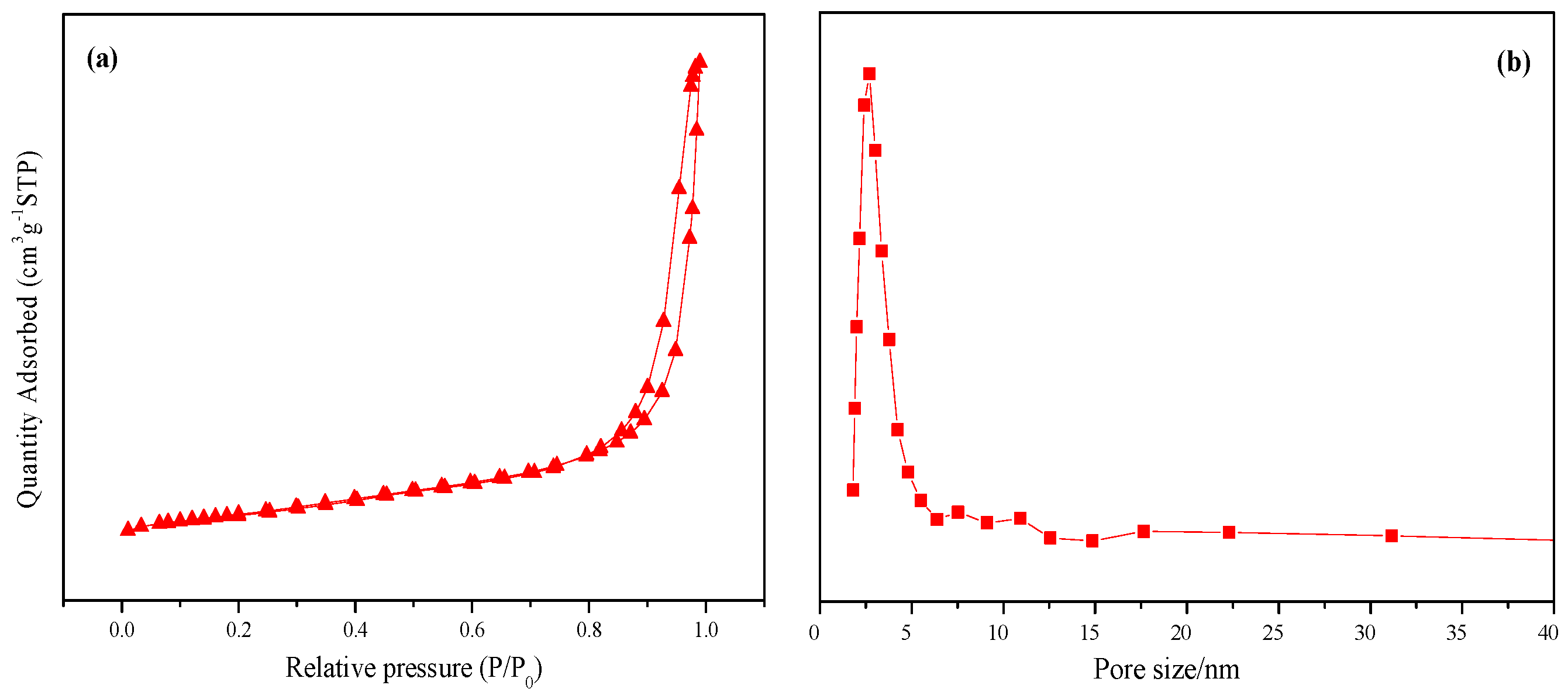
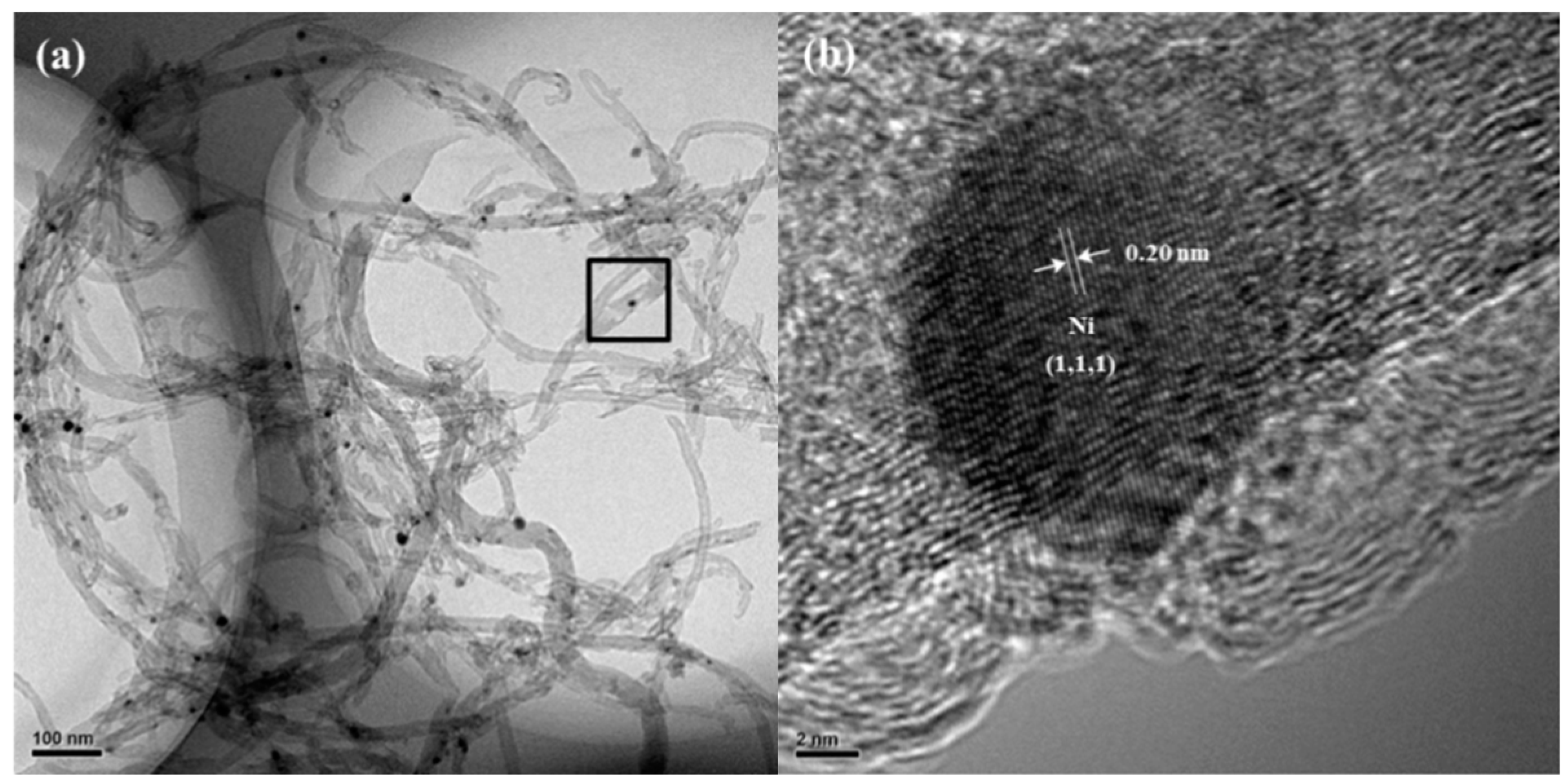
2.1. Characterization of Catalyst
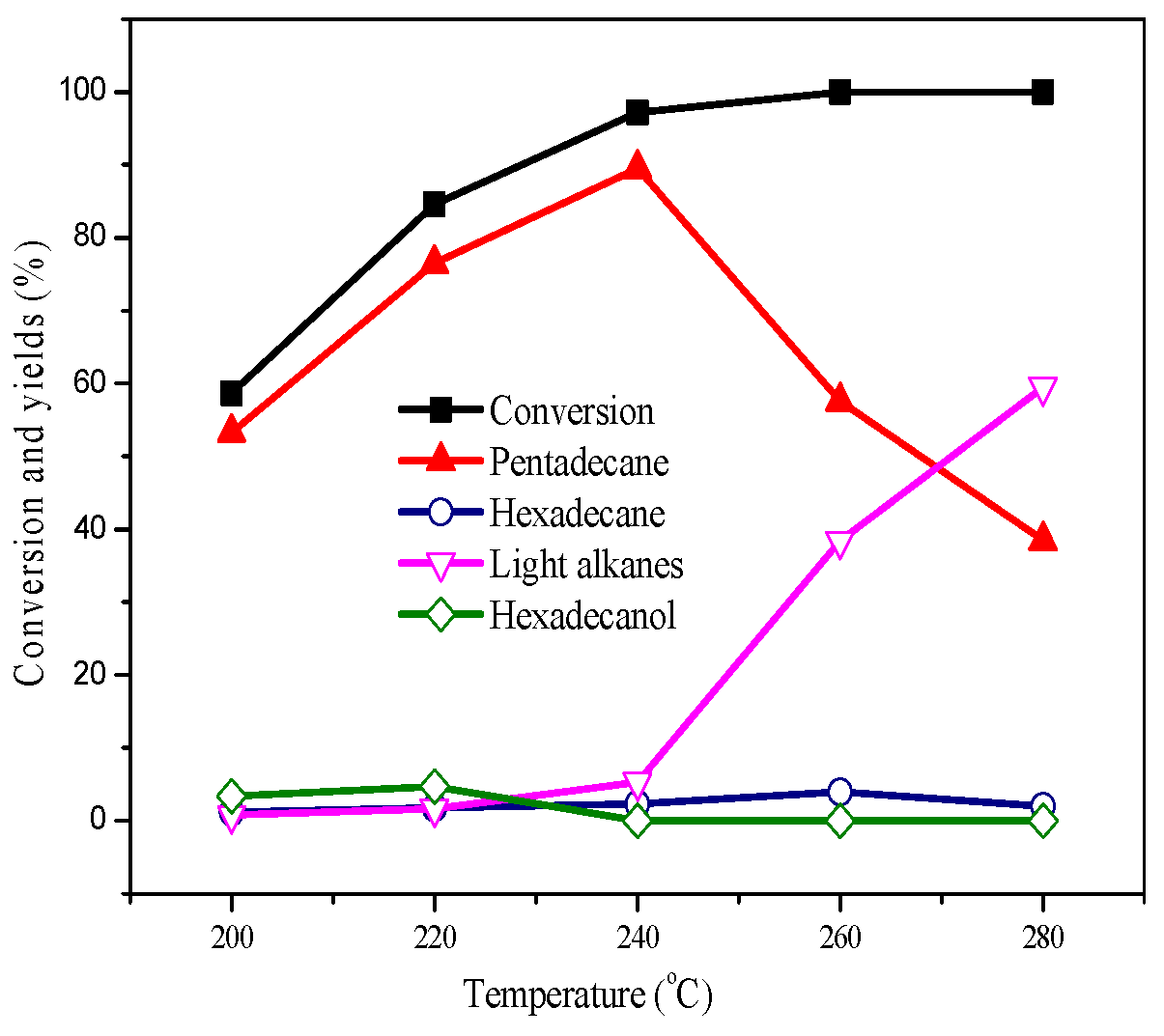
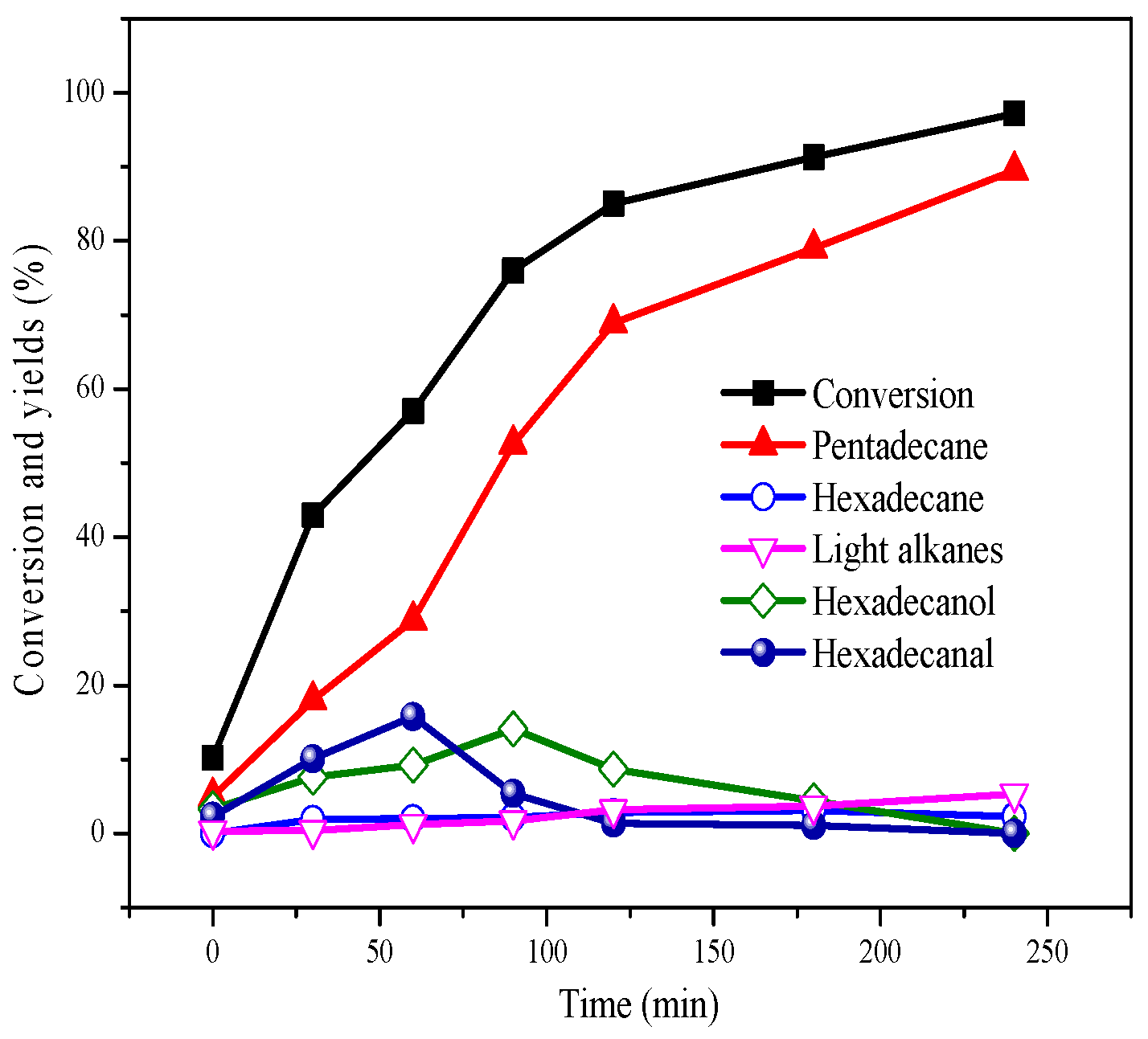
2.2. Deoxygenation at Different Temperatures
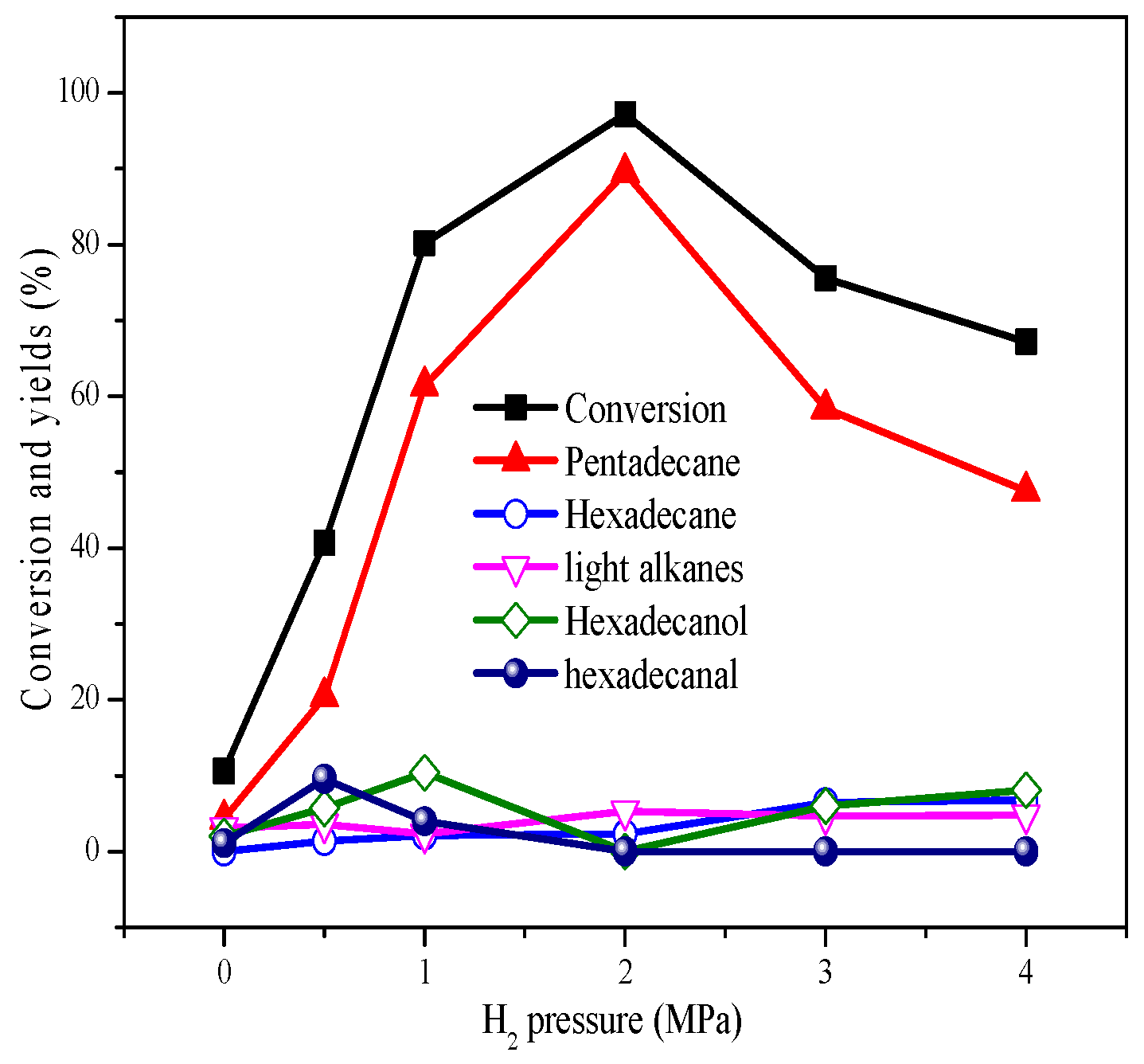
2.3. Deoxygenation at Different Hydrogen Pressures
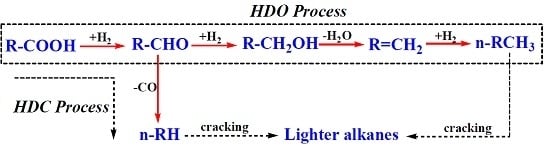
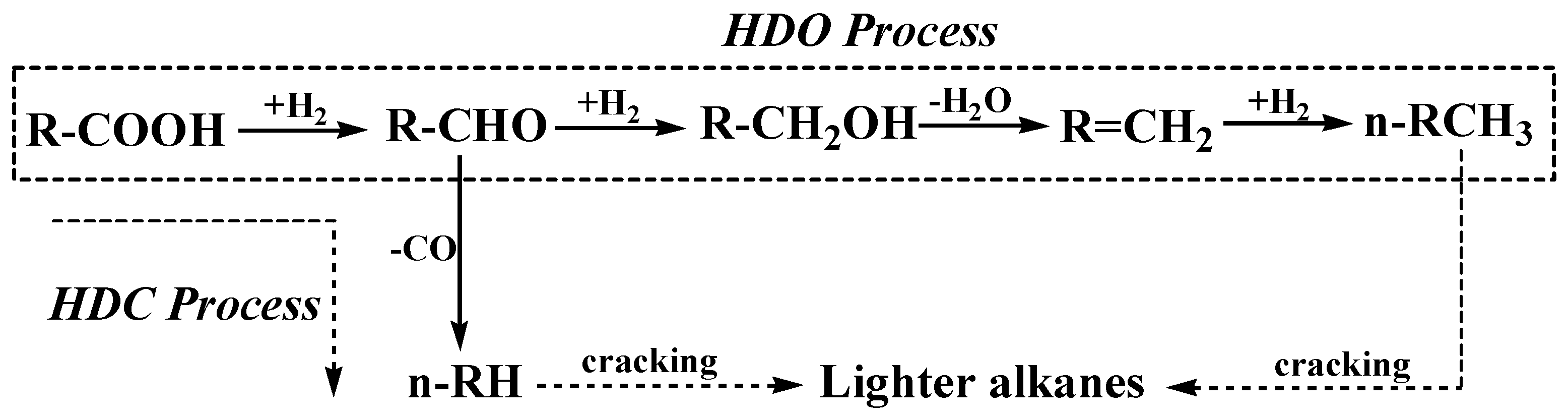
2.4. Deoxygenation Mechanism on 5% Ni/CNTs
3. Experimental
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.3. Activity Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Payormhorm, J.; Kangvansaichol, K.; Reubroycharoen, P.; Kuchonthara, P.; Hinchiranan, N. Pt/Al2O3-catalytic deoxygenation for upgrading of Leucaenaleucocephala-pyrolysis oil. Bioresour. Technol. 2013, 139, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Bruck, T.; Lercher, J.A. Catalytic deoxygenation of microalgae oil to green hydrocarbons. Green Chem. 2013, 15, 1720–1739. [Google Scholar] [CrossRef]
- Lee, E.H.; Park, R.S.; Kim, H.; Park, S.H.; Jung, S.C.; Jeon, J.K.; Kim, S.C.; Park, Y.K. Hydrodeoxygenation of guaiacol over Pt loaded zeolitic materials. J. Ind. Eng. Chem. 2016, 37, 18–21. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.M.; Lee, I.G.; Jeon, J.K.; Jung, S.C.; Chung, J.D.; Choi, W.G.; Park, Y.K. Recent advances in the catalytic hydrodeoxygenation of bio-oil. Korean J. Chem. Eng. 2016, 33, 3299–3315. [Google Scholar] [CrossRef]
- Tang, Y.; Yu, W.J.; Mo, L.Y.; Lou, H.; Zheng, X.M. One-Step Hydrogenation-Esterification of Aldehyde and Acid to Ester over Bifunctional Pt Catalysts: A Model Reaction as Novel Route for Catalytic Upgrading of Fast Pyrolysis Bio-Oil. Energy Fuels 2008, 22, 3484–3488. [Google Scholar] [CrossRef]
- Roh, H.S.; Eum, I.H.; Jeong, D.W.; Yi, B.E.; Na, J.G.; Ko, C.H. The effect of calcination temperature on the performance of Ni/MgO-Al2O3 catalysts for decarboxylation of oleic acid. Catal. Today 2011, 164, 457–460. [Google Scholar] [CrossRef]
- Ma, B.; Hu, J.B.; Wang, Y.M.; Zhao, C. Ni nanoparticles encapsulated into mesoporous single-crystalline HBEA: Application for drainage oil hydrodeoxygenation to diesel. Green Chem. 2015, 17, 4610–4617. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.L.; Ding, R.R.; Zhang, P.; Liu, J.; Yang, M.D. Catalytic hydrothermal liquefaction of D. tertiolecta for the production of bio-oil over different acid/base catalysts. AIChE J. 2015, 61, 1118–1128. [Google Scholar] [CrossRef]
- Duan, P.G.; Savage, P.E. Catalytic hydrotreatment of crude algal bio-oil in supercritical water. Appl. Catal. B Enviorn. 2011, 104, 136–143. [Google Scholar] [CrossRef]
- Peng, B.X.; Zhao, C.; Kasakov, S.; Foraita, S.; Lercher, J.A. Manipulating Catalytic Pathways: Deoxygenation of Palmitic Acid on Multifunctional Catalysts. Chem. Eur. J. 2013, 19, 4732–4741. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, E.S.; Crocker, M. Catalytic deoxygenation of fatty acids and their derivatives to hydrocarbon fuels via decarboxylation/decarbonylation. J. Chem. Technol. Biotechnol. 2012, 87, 1041–1050. [Google Scholar] [CrossRef]
- Zhang, S.P.; Yan, Y.J.; Ren, Z.W.; Li, T.C. Study of hydrodeoxygenation of bio-oil from the fast pyrolysis of biomass. Energy Sources 2003, 25, 57–65. [Google Scholar]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Guan, J.; Peng, G.M.; Cao, Q.; Mu, X.D. Role of MoO3 on a Rhodium catalyst in the selective hydrogenolysis of biomass-derived tetrahydrofurfuryl alcohol into 1,5-pentanediol. J. Phys. Chem. C 2014, 118, 25555–25566. [Google Scholar] [CrossRef]
- Zhang, X.H.; Wang, T.J.; Ma, L.L.; Zhang, Q.; Jiang, T. Hydrotreatment of bio-oil over Ni-based catalyst. Bioresour. Technol. 2013, 127, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Donnis, B.; Egeberg, R.G.; Blom, P.; Knudsen, K.G. Hydroprocessing of bio-oils and oxygenates to hydrocarbons. Understanding the reaction routes. Top. Catal. 2009, 52, 229–240. [Google Scholar] [CrossRef]
- Liu, Y.; Sotelo, B.R.; Murata, K.; Minowa, T.; Sakanishi, K. Hydrotreatment of jatropha oil to produce green diesel over trifunctional Ni–Mo/SiO2–Al2O3 catalyst. Chem. Lett. 2009, 38, 552–553. [Google Scholar] [CrossRef]
- Immer, J.G.; Kelly, M.J.; Lamb, H.H. Catalytic reaction pathways in liquid-phase deoxygenation of C18 free fatty acids. Appl. Catal. A 2010, 375, 134–139. [Google Scholar] [CrossRef]
- Matthias, A.; Thomas, N.; Wolfgang, F.H.; Jürgen, F.; Jeremie, G. Catalytic deoxygenation of oleic acid in continuous gas flow for the production of diesel-like hydrocarbons. Appl. Catal. A Gen. 2011, 399, 198–204. [Google Scholar]
- Kubiclied, I.; Snare, M.; Eranen, K.; Maki, A.P.; Murzin, D.Y. Hydrocarbons for diesel fuel via decarboxylation of vegetable oils. Catal. Today 2005, 106, 197–200. [Google Scholar]
- Kandel, K.; Anderegg, J.W.; Nelson, N.C.; Chaudhary, U.; Slowing, I.I. Supported iron nanoparticles for the hydrodeoxygenation of microalgal oil to green diesel. J. Catal. 2014, 314, 142–148. [Google Scholar] [CrossRef]
- Gusmao, J.; Brodzki, D.; Djega, M.G.; Frety, R. Utilization of vegetable oils as an alternative source for diesel-type fuel: Hydrocracking on reduced Ni/SiO2 and sulphided Ni-Mo/C-Al2O3. Catal. Today 1989, 5, 533–544. [Google Scholar] [CrossRef]
- Li, W.J.; Gao, Y.J.; Yao, S.Y.; Ma, D.; Yan, N. Effective deoxygenation of fatty acids over Ni(OAc)2 in the absence of H2 and solvent. Green Chem. 2015, 17, 4198–4205. [Google Scholar] [CrossRef]
- Han, J.X.; Duan, J.D.; Chen, P.; Lou, H.; Zheng, X.M.; Hong, H.P. Carbon-supported molybdenum carbide catalysts for the conversion of vegetable oils. ChemSusChem 2012, 5, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Flores, O.M.; Davidson, S.D.; Li, T.T.; Dong, T.; Gao, D.F.; Wang, Y.; Garcia, P.M.; Chen, S.L. Hydrothermal catalytic deoxygenation of palmitic acid over nickel catalyst. Fuel 2016, 166, 302–308. [Google Scholar] [CrossRef]
- Hachemi, I.; Jeništová, K.; Arvela, P.M.; Kumar, N.; Eränen, K.; Hemming, J.; Murzin, D.Y. Comparative study of sulfur-free nickel and palladium catalysts in hydrodeoxygenation of different fatty acid feedstocks for production of biofuels. Catal. Sci. Technol. 2016, 6, 1476–1487. [Google Scholar] [CrossRef]
- Xin, H.; Guo, K.; Li, D.; Yang, H.Q.; Hu, C.W. Production of high-grade diesel from palmitic acid over activated carbon-supported nickel phosphide catalysts. Appl. Catal. B Enviorn. 2016, 187, 375–385. [Google Scholar] [CrossRef]
- De, S.; Jiaguang, J.G.; Luque, R.; Yan, N. Ni-based bimetallic heterogeneous catalysts for energy and environmental applications. Energy Environ. Sci. 2016, 9, 3314–3347. [Google Scholar] [CrossRef]
- Duan, Y.N.; Wu, Y.L.; Zhang, Q.H.; Ding, R.R.; Chen, Y.; Liu, J.; Yang, M.D. Towards conversion of octanoic acid to liquid hydrocarbon via hydrodeoxygenation over Mo promoter nickel-based catalyst. J. Mol. Catal. A Chem. 2015, 398, 72–78. [Google Scholar] [CrossRef]
- Ding, R.R.; Wu, Y.L.; Chen, Y.; Liang, J.M.; Liu, J.; Yang, M.D. Effective hydrodeoxygenation of palmitic acid to diesel-like hydrocarbons over MoO2/CNTs catalyst. Chem. Eng. Sci. 2015, 135, 517–525. [Google Scholar] [CrossRef]
- Ding, R.R.; Wu, Y.L.; Chen, Y.; Chen, H.; Wang, J.L.; Shi, Y.C.; Yang, M.D. Catalytic hydrodeoxygenation of palmitic acid over a bifunctional Co-doped MoO2/CNTs catalyst: An insight into the promoting effect of cobalt. Catal. Sci. Technol. 2016, 6, 2065–2076. [Google Scholar] [CrossRef]
- Liang, J.M.; Ding, R.R.; Wu, Y.L.; Chen, Y.; Wu, K.J.; Meng, Y.Q.; Yang, M.D.; Wang, Y.W. Effective conversion of heteroatomic model compounds in microalgae-based bio-oils to hydrocarbons over β-Mo2C/CNTs catalyst. J. Mol. Catal. A Chem. 2016, 411, 95–102. [Google Scholar] [CrossRef]
- Li, C.; Shao, Z.F.; Pang, M.; Williams, T.C.; Zhang, X.F.; Liang, C.H. Carbon nanotubes supported mono-and bimetallic Pt and Ru catalysts for selective hydrogenation of phenylacetylene. Ind. Eng. Chem. Res. 2012, 51, 4934–4941. [Google Scholar] [CrossRef]
- Dongil, A.B.; Bachiller, B.B.; Rodriguez, I.R.; Fierro, J.L.G.; Escalona, N. The effect of Cu loading on Ni/carbon nanotubes catalysts for hydrodeoxygenation of guaiacol. RSC Adv. 2016, 6, 26658–26667. [Google Scholar] [CrossRef]
- Chen, W.; Pan, X.; Bao, X. Tuning of redox properties of iron and iron oxides via encapsulation within carbon nanotubes. JASC 2007, 129, 7421–7426. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Fan, Z.; Pan, X.; Bao, X.H. Effect of confinement in carbon nanotubes on the activity of Fischer—Tropsch iron catalyst. JASC 2008, 130, 9414–9419. [Google Scholar] [CrossRef] [PubMed]
- Charlier, J.C. Defects in carbon nanotubes Accounts. Chem. Res. 2002, 35, 1063–1069. [Google Scholar] [CrossRef]
- Prasomsr, T.; Shetty, M.; Murugappan, K.; Leshkov, Y.R. Insights into the catalytic activity and surface modification of MoO3 during the hydrodeoxygenation of lignin-derived model compounds into aromatic hydrocarbons under low hydrogen pressures. Energy Environ. Sci. 2014, 7, 2660–2669. [Google Scholar] [CrossRef]
- Yang, H.; Song, S.; Rao, R.; Wang, X.Z.; Yu, Q.; Zhang, A.M. Enhanced catalytic activity of benzene hydrogenation over nickel confined in carbon nanotubes. J. Mol. Catal. A Chem. 2010, 323, 33–39. [Google Scholar] [CrossRef]
- Serp, P.; Castillejos, E. Catalysis in carbon nanotubes. ChemCatChem 2010, 2, 41–47. [Google Scholar] [CrossRef]
- Selvaraj, M.; Shanthi, K.; Maheswari, R.; Ramanathan, A. Hydrodeoxygenation of guaiacol over MoO3-NiO/mesoporous silicates: Effect of incorporated heteroatom. Energy Fuels 2014, 28, 2598–2607. [Google Scholar] [CrossRef]
- Biabani, R.A.; Rezaei, M.; Fattah, Z. Low-temperature CO oxidation over nanosized Fe-Co mixed oxide catalysts: Effect of calcination temperature and operational conditions. Chem. Eng. Sci. 2013, 94, 237–244. [Google Scholar] [CrossRef]
- Li, F.J.; Zhang, S.; Lee, J.W.; Guo, J.; White, T.J.; Li, B.; Zhao, D.L. Orientation of silicon nanowires grown from nickel-coated silicon wafers. J. Cryst. Growth 2014, 404, 26–33. [Google Scholar] [CrossRef]
- Boda, L.; Onyestyák, G.; Solt, H.; Lónyi, F.; Valyon, J.; Thernesz, A. Catalytic hydroconversion of tricaprylin and caprylic acid as model reaction for biofuel production from triglycerides. Appl. Catal. A Gen. 2010, 374, 158–169. [Google Scholar] [CrossRef]
- Peng, B.X.; Yao, Y.; Zhao, C.; Lercher, J.A. Towards quantitative conversion of microalgae oil to diesel-range alkanes with bifunctional catalysts. Angew. Chem. Int. Ed. 2012, 124, 2114–2117. [Google Scholar] [CrossRef]
| Reactant | Catalyst | Vreactor (mL) | Vsolvent (mL) | Wacid (g) | Wcatalyst (g) | T (°C) | PH2 (Mpa) | Con. (%) | Main product Distribution (%) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Octanoic acid | 5% Ni/ZrO2 | 250 | 100 | 1 | 0.5 | 320 | 3 | 92.79 | Heptane/69.68 | Octane/5.00 | [29] |
| Octanoic acid | 10% Mo-5% Ni/ZrO2 | 250 | 100 | 1 | 0.5 | 320 | 3 | 100 | Heptane/10.67 | Octane/77.07 | [29] |
| Palmitic acid | 5% MoO2/CNTs | 150 | 50 | 0.5 | 0.25 | 220 | 4 | 100 | Pentadecan/7.60 | Hexadecane/92.20 | [30] |
| Palmitic acid | 1.5% Co-5% MoO2/CNTs | 150 | 50 | 0.5 | 0.1 | 180 | 4 | 100 | Pentadecan/5.20 | Hexadecane/89.30 | [31] |
| Stearic acid | β-Mo2C/CNTs | 150 | 50 | 0. 5 | 0.25 | 180 | 4 | 100 | Heptadecan/8.76 | Octadecan/91.24 | [32] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.; Ding, R.; Shi, Y.; Fang, X.; Hu, H.; Yang, M.; Wu, Y. Synthesis of Renewable Diesel Range Alkanes by Hydrodeoxygenation of Palmitic Acid over 5% Ni/CNTs under Mild Conditions. Catalysts 2017, 7, 81. https://doi.org/10.3390/catal7030081
Duan Y, Ding R, Shi Y, Fang X, Hu H, Yang M, Wu Y. Synthesis of Renewable Diesel Range Alkanes by Hydrodeoxygenation of Palmitic Acid over 5% Ni/CNTs under Mild Conditions. Catalysts. 2017; 7(3):81. https://doi.org/10.3390/catal7030081
Chicago/Turabian StyleDuan, Yanan, Ranran Ding, Yanchun Shi, Xiao Fang, Husheng Hu, Mingde Yang, and Yulong Wu. 2017. "Synthesis of Renewable Diesel Range Alkanes by Hydrodeoxygenation of Palmitic Acid over 5% Ni/CNTs under Mild Conditions" Catalysts 7, no. 3: 81. https://doi.org/10.3390/catal7030081
APA StyleDuan, Y., Ding, R., Shi, Y., Fang, X., Hu, H., Yang, M., & Wu, Y. (2017). Synthesis of Renewable Diesel Range Alkanes by Hydrodeoxygenation of Palmitic Acid over 5% Ni/CNTs under Mild Conditions. Catalysts, 7(3), 81. https://doi.org/10.3390/catal7030081