Improved CO Oxidation Activity of 3DOM Pr-Doped Ceria Catalysts: Something Other Than an Ordered Macroporous Structure
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Oxidation of CO
2.2. Catalyst Characterization
2.2.1. Characterization of the Catalysts’ Crystallinity by Raman Spectroscopy, X-ray Diffraction (XRD), and Helium Picnometry
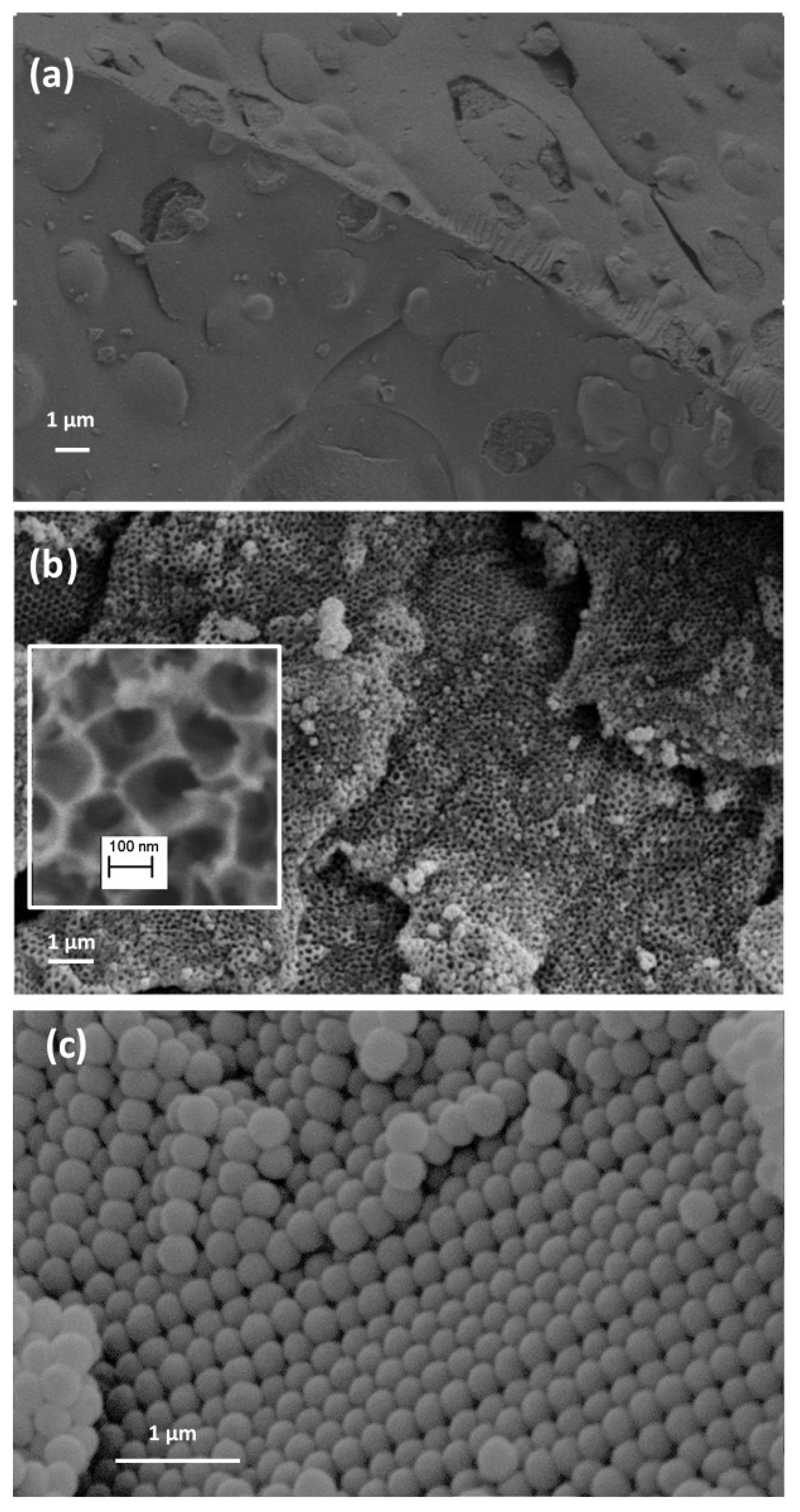
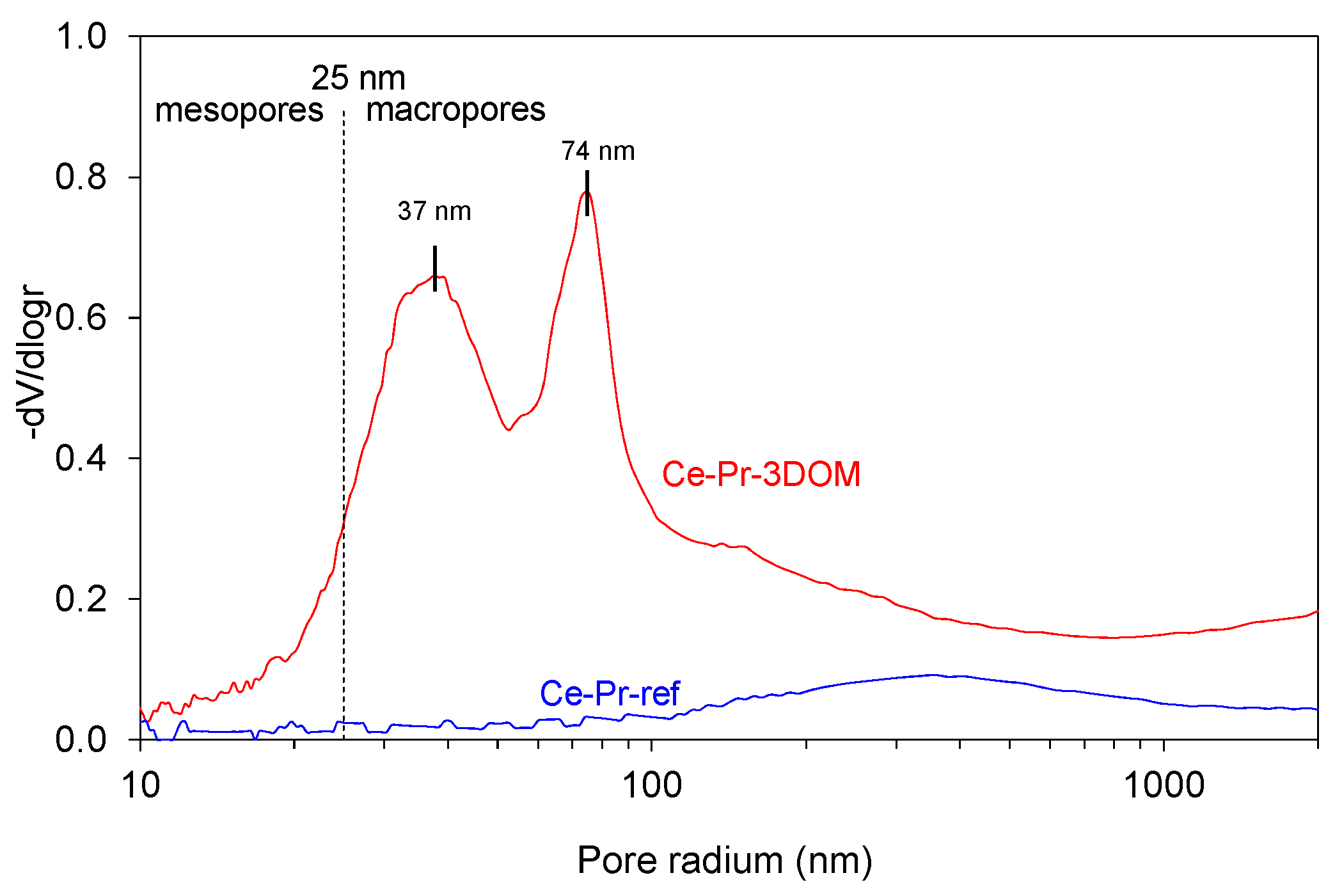
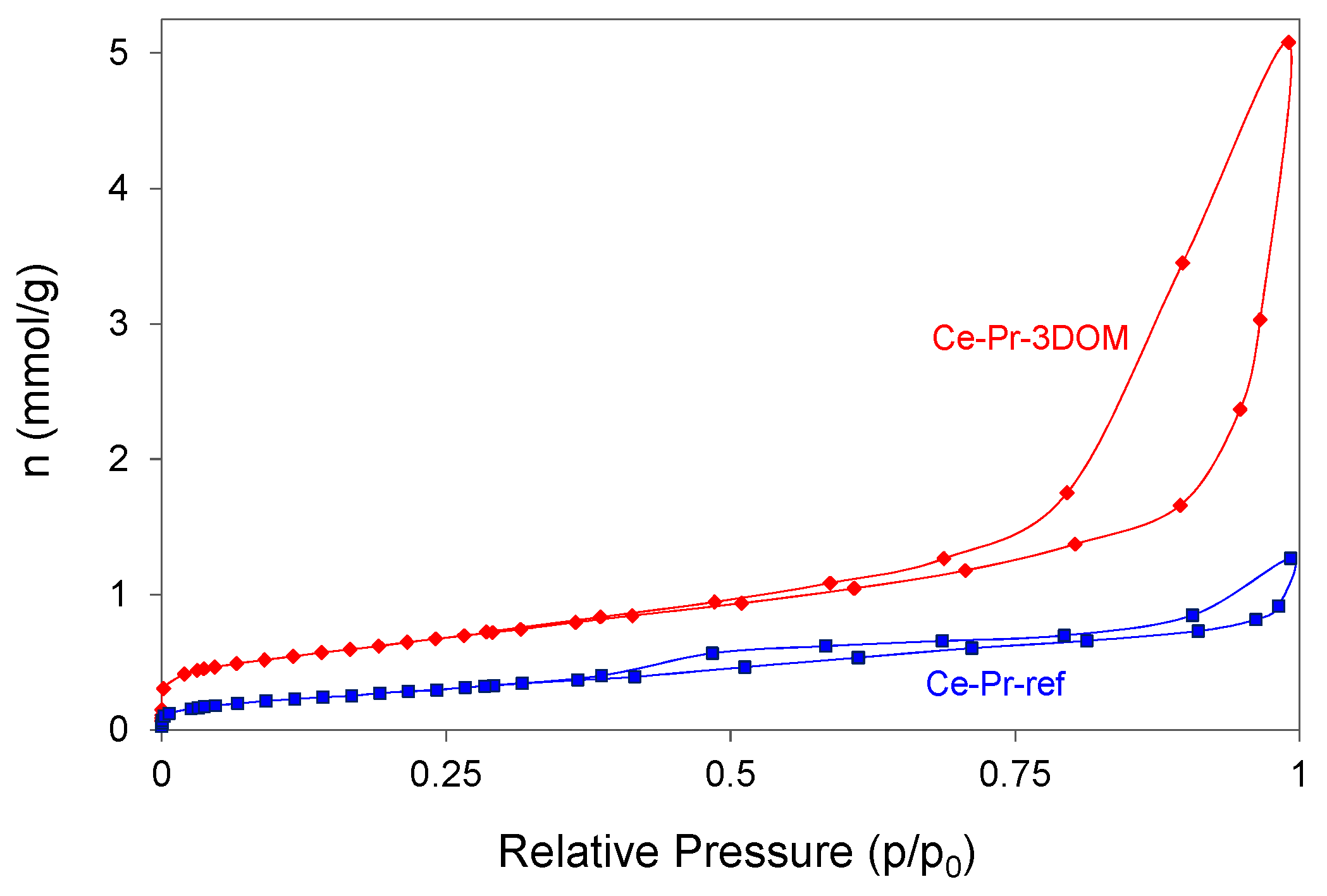
2.2.2. Characterization of the Catalysts’ Surface by Scanning electron microscopy (SEM), Mercury Intrusion Porosimetry, and N2 Adsorption
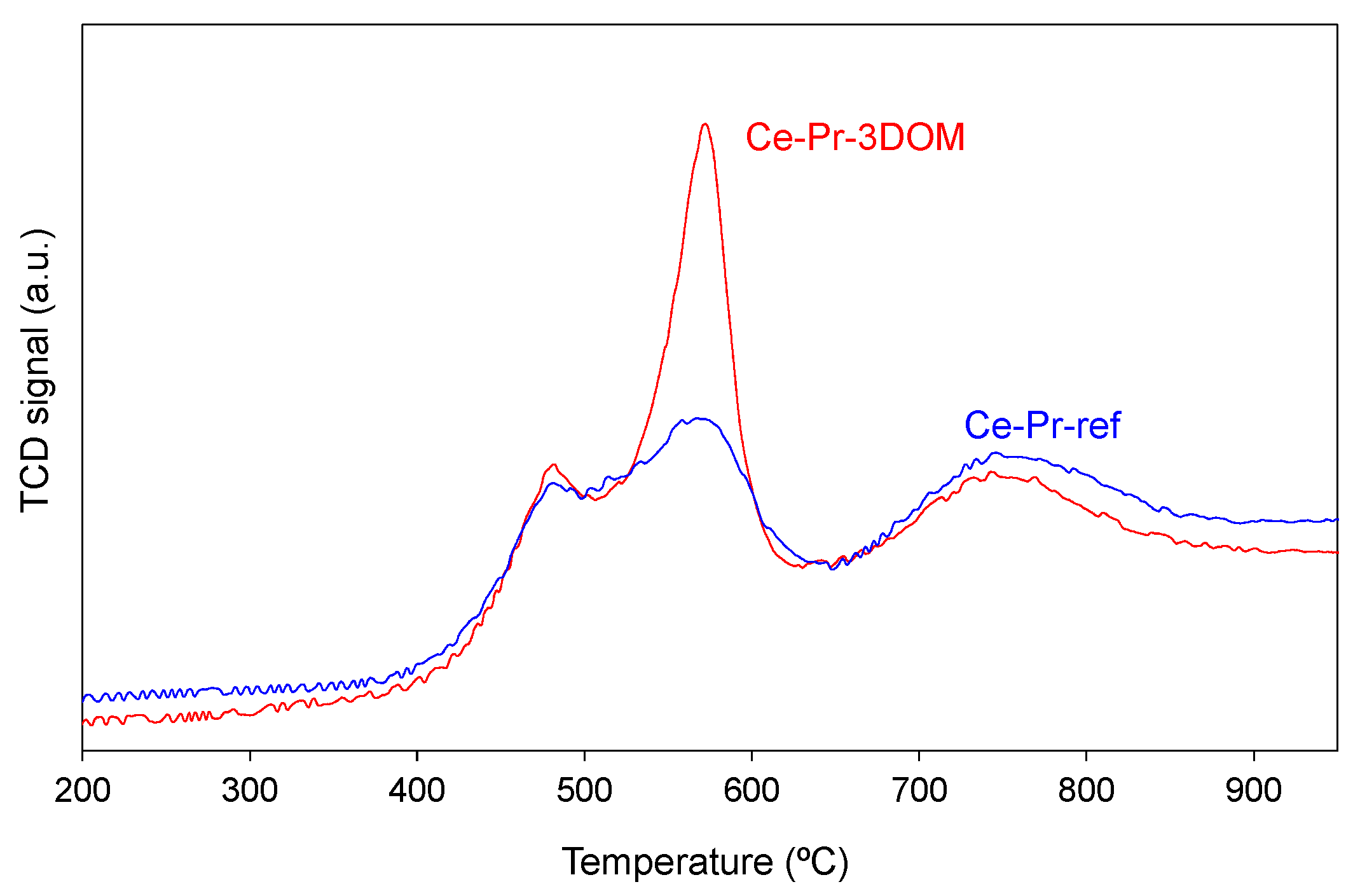
2.2.3. Characterization of the Chemical Properties of the Catalysts’ Surface by Temperature-Programmed Reduction with H2 (H2-TPR) and X-ray Photoelectron Spectroscopy (XPS).
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalytic Tests
3.3. Catalyst Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, G.; Zhao, Z.; Liu, J.; Jiang, G.; Duan, A.; Zheng, J.; Chen, S.; Zhou, R. Three dimensionally ordered macroporous Ce1−xZrxO2 solid solutions for diesel soot combustion. Chem. Commun. 2010, 46, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhao, Z.; Xu, J.; Zheng, J.; Liu, J.; Jiang, G.; Duan, A.; He, H. Comparative study on the preparation, characterization and catalytic performances of 3DOM Ce-based materials for the combustion of diesel soot. Appl. Catal. B 2011, 107, 302–315. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, J.; Zhao, Z.; Duan, A.; Jiang, G. The catalysts of three-dimensionally ordered macroporous Ce1−xZrxO2-supported gold nanoparticles for soot combustion: The metal–support interaction. J. Catal. 2012, 287, 13–29. [Google Scholar]
- Wei, Y.; Liu, J.; Zhao, Z.; Duan, A.; Jiang, G.; Xu, C.; Gao, J.; He, H.; Wang, X. Three-dimensionally ordered macroporous Ce0.8Zr0.2O2-supported gold nanoparticles: synthesis with controllable size and super-catalytic performance for soot oxidation. Energy Environ. Sci. 2011, 4, 2959. [Google Scholar] [CrossRef]
- Jin, B.; Wei, Y.; Zhao, Z.; Liu, J.; Yu, X.; Li, Y.; Li, J. Synthesis of three-dimensionally ordered macroporous Al–Ce mixed oxide catalysts with high catalytic activity and stability for diesel soot combustion. Catal. Today 2015, 258, 487–497. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Zhao, Z.; Xu, C.; Zheng, J.; Duan, A.; Jiang, G. Easy synthesis of three-dimensionally ordered macroporous La1−xKxCoO3 catalysts and their high activities for the catalytic combustion of soot. J. Catal. 2011, 282, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, H.; Gao, X.Y.; Liu, Y. Three-dimensionally ordered macro-porous CuO–CeO2 used for preferential oxidation of carbon monoxide in hydrogen-rich gases. Catal. Commun. 2009, 10, 1432–1436. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, Y.; Li, C.; Shen, Y.; Han, L.; Hu, Z.; Di, X.; Liu, Z. Creation of three-dimensionally ordered macroporous Au/CeO2 catalysts with controlled pore sizes and their enhanced catalytic performance for formaldehyde oxidation. Appl. Catal. B 2009, 91, 11–20. [Google Scholar] [CrossRef]
- Ji, K.; Dai, H.; Deng, J.; Li, X.; Wang, Y.; Gao, B.; Bai, G.; Au, C.T. A comparative study of bulk and 3DOM-structured Co3O4, Eu0.6Sr0.4FeO3, and Co3O4/Eu0.6Sr0.4FeO3: Preparation, characterization, and catalytic activities for toluene combustion. Appl. Catal. A 2012, 447–448, 41–48. [Google Scholar] [CrossRef]
- Si, W.; Wang, Y.; Zhao, S.; Hu, F.; Li, J. A Facile Method for in Situ Preparation of the MnO2/LaMnO3 Catalyst for the Removal of Toluene. Environ. Sci. Technol. 2016, 50, 4572–4578. [Google Scholar] [CrossRef] [PubMed]
- Trovarelli, A. Catalytic Properties of Ceria and CeO2-Containing Materials. Catal. Rev. Sci. Eng. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Sun, C.; Li, H.; Chen, L. Nanostructured ceria-based materials: Synthesis, properties, and applications. Energy Environ. Sci. 2012, 5, 8475–8505. [Google Scholar] [CrossRef]
- Poggio-Fraccari, E.; Irigoyen, B.; Baronetti, G.; Mariño, F. Ce-Pr mixed oxides as active supports for Water-gas Shift reaction: Experimental and density functional theory characterization. Appl. Catal. A 2014, 485, 123–132. [Google Scholar] [CrossRef]
- Weber, W.H.; Hass, K.C.; McBride, J.R. Raman study of CeO2: Second-order scattering, lattice dynamics, and particle-size effects. Phys. Rev. B 1993, 48, 178. [Google Scholar] [CrossRef]
- Nineshige, A.; Taji, T.; Muroi, Y.; Kobune, M.; Fujii, S.; Nishi, N.; Inaba, M.; Ogumi, Z. Oxygen chemical potential variation in ceria-based solid oxide fuel cells determined by Raman spectroscopy. Solid State Ion. 2000, 135, 481–485. [Google Scholar] [CrossRef]
- Twu, J.; Chuang, C.J.; Chang, K.I.; Yang, C.H.; Chen, K.H. Raman spectroscopic studies on cerium oxide. Appl. Catal. B 1997, 12, 309–324. [Google Scholar]
- Bernal, S.; Blanco, G.; Calvino, J.J.; Perez Omil, J.A.; Pintado, J.M. Some major aspects of the chemical behavior of rare earth oxides: An overview. J. Alloys Compd. 2006, 408–412, 496–502. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Liu, W.; Chen, W.; Ran, R.; Li, M.; Wen, D. Soot oxidation over CeO2 and Ag/CeO2: Factors determining the catalyst activity and stability during reaction. J. Catal. 2016, 337, 188–198. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Ce3+ and oxygen vacancy mediated tuning of structural and optical properties of CeO2 nanoparticles. Mater. Chem. Phys. 2012, 131, 666–671. [Google Scholar] [CrossRef]
- Wu, Q.Z.; Shen, Y.; Liao, J.F.; Li, Y.G. Synthesis and characterization of three-dimensionally ordered macroporous rare earth oxides. Mater. Lett. 2004, 58, 2688. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution, IUPAC Technical Report. Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Romeo, M.; Bak, K.; El Fallah, J.; le Normand, F.; Hilaire, L. XPS Study of the reduction of cerium dioxide. Surf. Interf. Anal. 1993, 20, 508–512. [Google Scholar] [CrossRef]
- Borchet, H.; Frolova, Y.V.; Kaichev, V.V.; Prosvirin, I.P.; Alikina, G.M.; Lukashevich, A.I.; Zaikovskii, V.I.; Moroz, E.M.; Trukhan, S.N.; Ivanov, V.P.; et al. Electronic and Chemical Properties of Nanostructured Cerium Dioxide Doped with Praseodymium. J. Phys. Chem. B 2005, 109, 5728–5738. [Google Scholar] [CrossRef] [PubMed]
- Lowell, S.; Shields, J.; Thomas, M.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
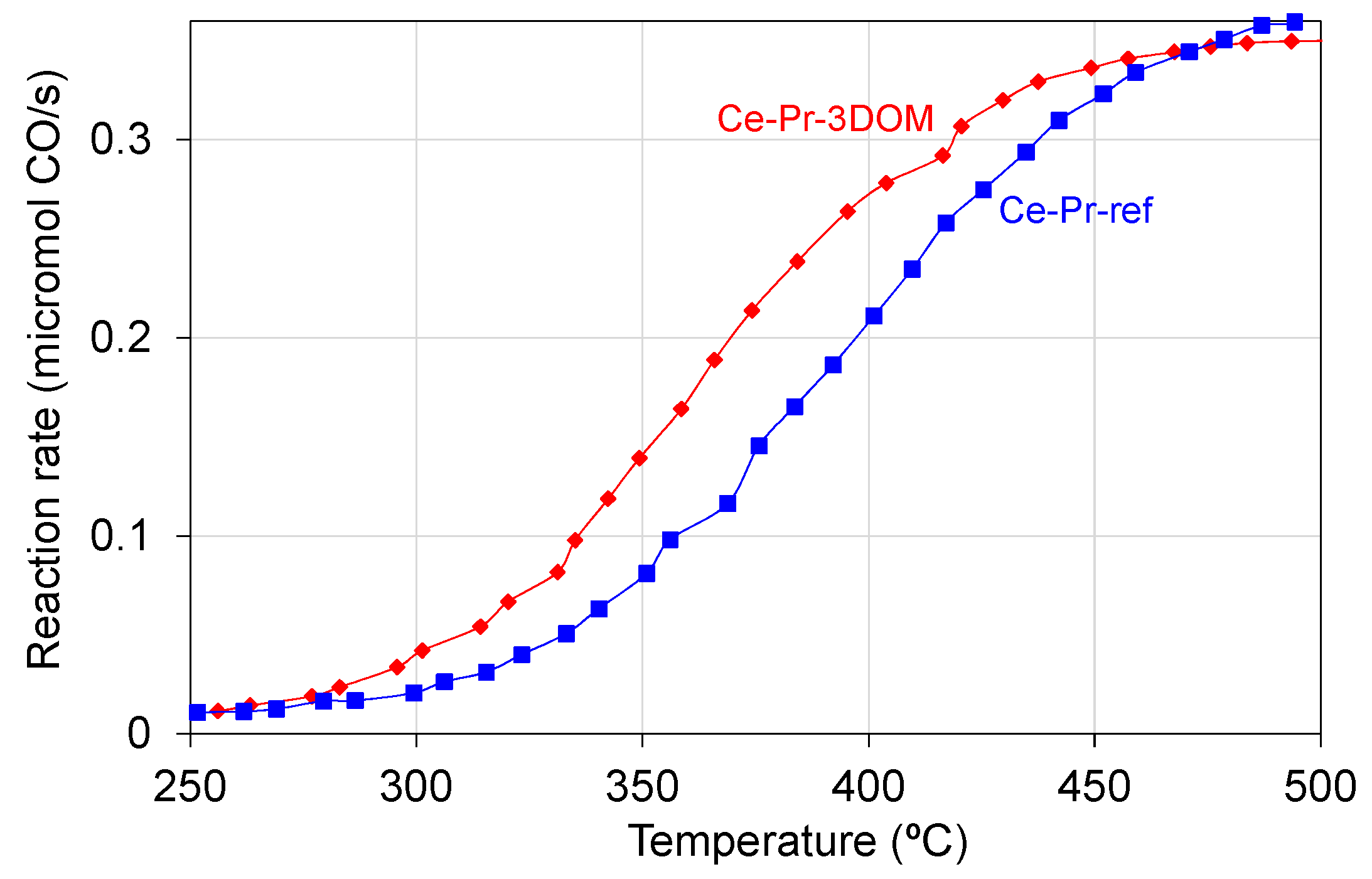
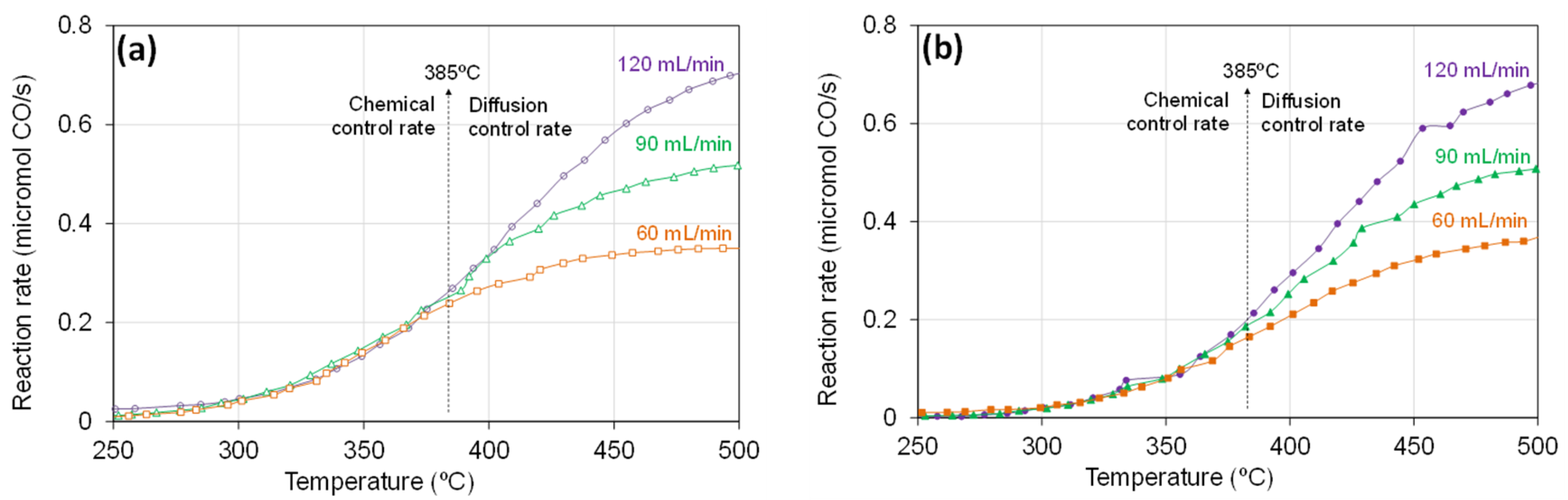
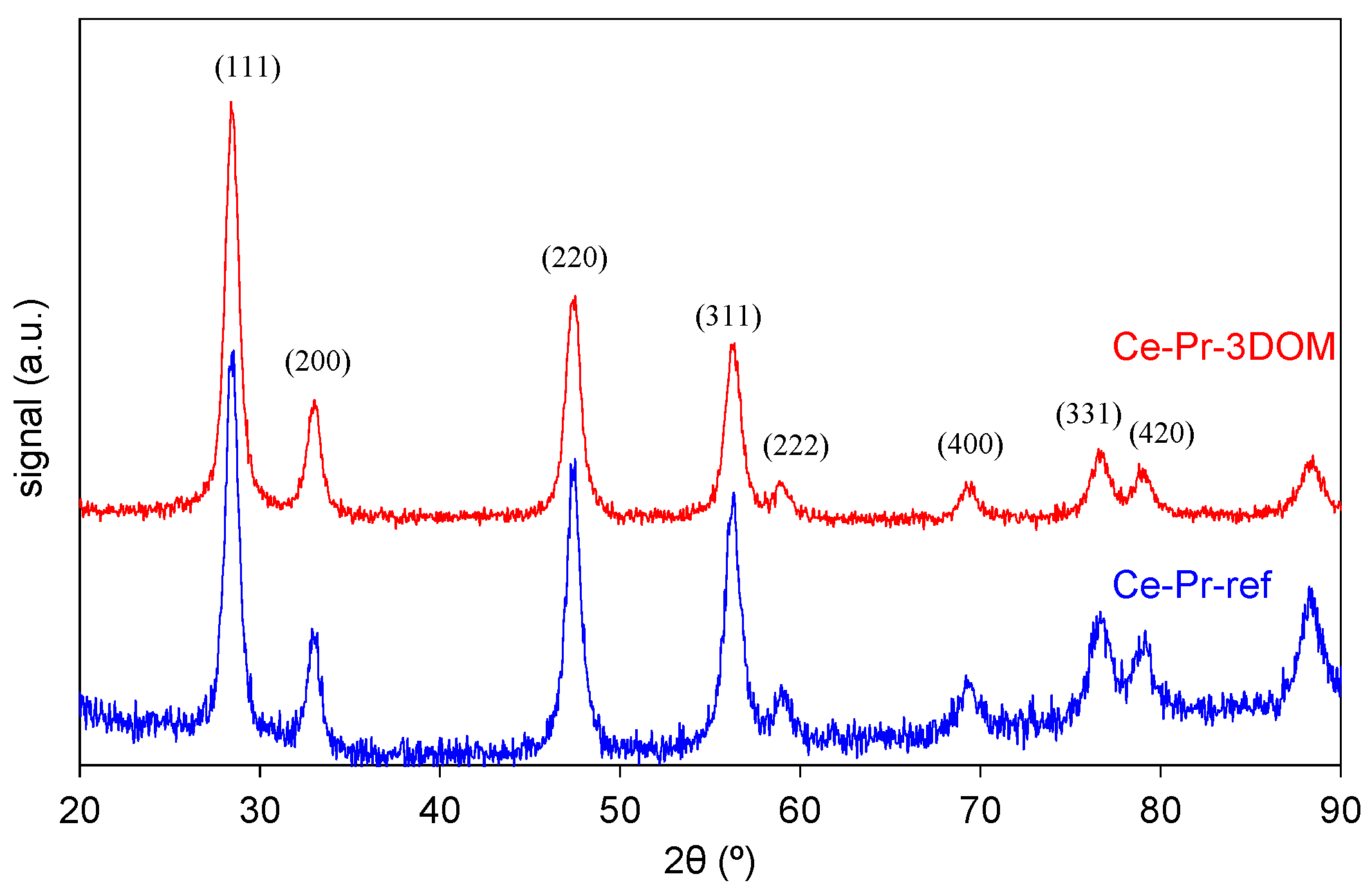
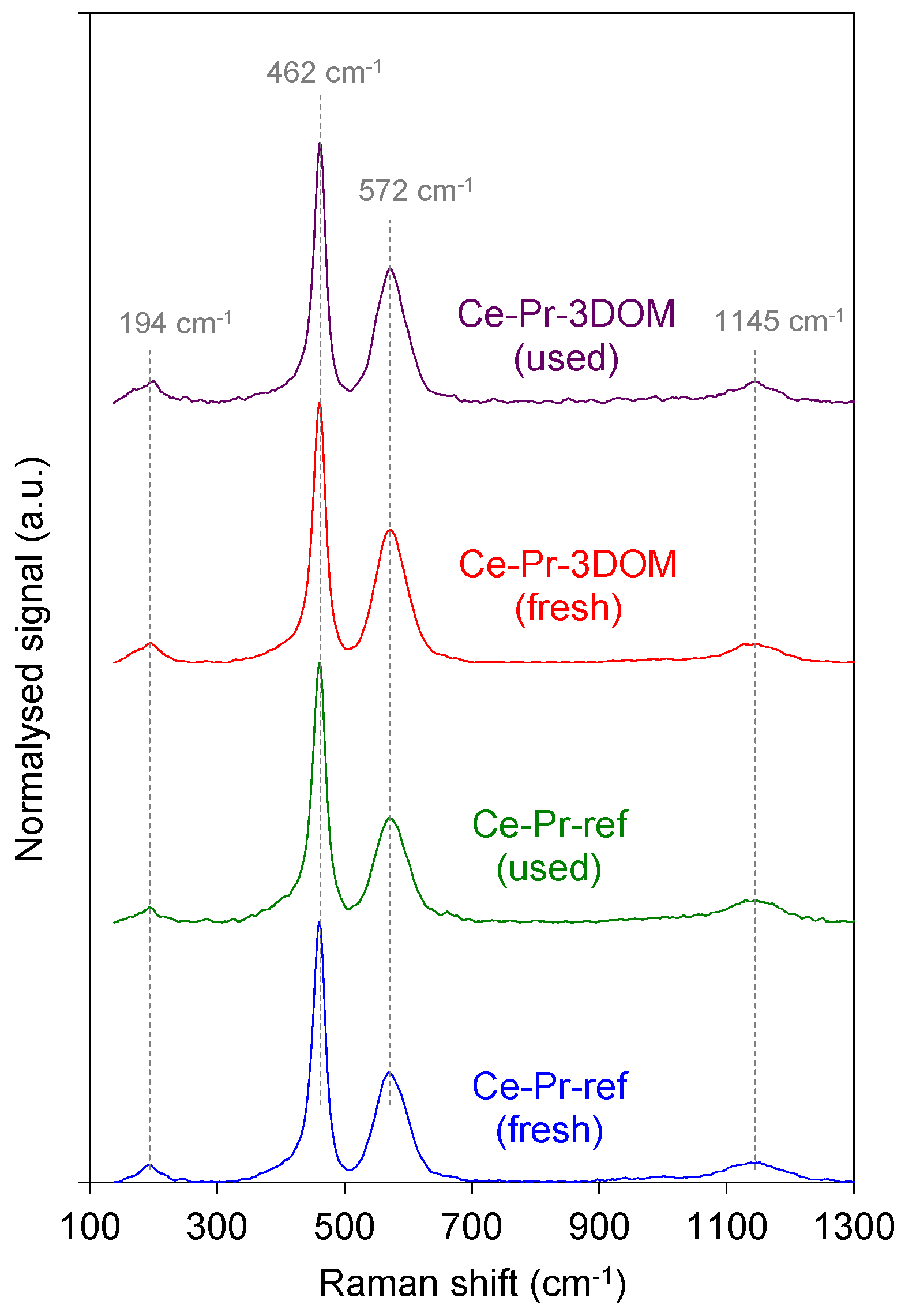
| Catalyst | Hg porosimetry Area (Meso and Macropores; m2/g) | BET * (m2/g) | Helium Density (g/cm3) | Crystallite Size Scherrer (nm) | Cell Parameter (nm) |
|---|---|---|---|---|---|
| Ce-Pr-3DOM | 41 | 51 | 6.2 | 10 | 0.5429 |
| Ce-Pr-ref | 3 | 24 | 6.3 | 10 | 0.5429 |
| Catalyst | Signal at 572 cm−1/Signal at 462 cm−1 |
|---|---|
| Ce-Pr-3DOM (fresh) | 0.51 |
| Ce-Pr-3DOM (used) | 0.52 |
| Ce-Pr-ref (fresh) | 0.42 |
| Ce-Pr-ref (used) | 0.40 |
| Catalyst | Ce/Pr * | Ce3+ (%) | Pr3+ (%) | Total Ce3+ + Pr3+ (%) |
|---|---|---|---|---|
| Ce-Pr-3DOM (fresh) | 4.3 | 29 | 54 | 34 |
| Ce-Pr-3DOM (used) | 4.7 | 28 | 14 | 26 |
| Ce-Pr-ref (fresh) | 4.2 | 33 | 38 | 34 |
| Ce-Pr-ref (used) | 4.7 | 30 | 44 | 32 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davó-Quiñonero, A.; González-Mira, J.; Such-Basañez, I.; Juan-Juan, J.; Lozano-Castelló, D.; Bueno-López, A. Improved CO Oxidation Activity of 3DOM Pr-Doped Ceria Catalysts: Something Other Than an Ordered Macroporous Structure. Catalysts 2017, 7, 67. https://doi.org/10.3390/catal7020067
Davó-Quiñonero A, González-Mira J, Such-Basañez I, Juan-Juan J, Lozano-Castelló D, Bueno-López A. Improved CO Oxidation Activity of 3DOM Pr-Doped Ceria Catalysts: Something Other Than an Ordered Macroporous Structure. Catalysts. 2017; 7(2):67. https://doi.org/10.3390/catal7020067
Chicago/Turabian StyleDavó-Quiñonero, Arantxa, Jorge González-Mira, Ion Such-Basañez, Jerónimo Juan-Juan, Dolores Lozano-Castelló, and Agustín Bueno-López. 2017. "Improved CO Oxidation Activity of 3DOM Pr-Doped Ceria Catalysts: Something Other Than an Ordered Macroporous Structure" Catalysts 7, no. 2: 67. https://doi.org/10.3390/catal7020067
APA StyleDavó-Quiñonero, A., González-Mira, J., Such-Basañez, I., Juan-Juan, J., Lozano-Castelló, D., & Bueno-López, A. (2017). Improved CO Oxidation Activity of 3DOM Pr-Doped Ceria Catalysts: Something Other Than an Ordered Macroporous Structure. Catalysts, 7(2), 67. https://doi.org/10.3390/catal7020067








