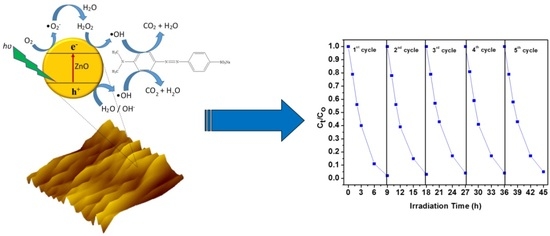
Photoactive Hybrid Film Photocatalyst of Polyethersulfone-ZnO for the Degradation of Methyl Orange Dye: Kinetic Study and Operational Parameters
Abstract
:1. Introduction
2. Results and Discussion
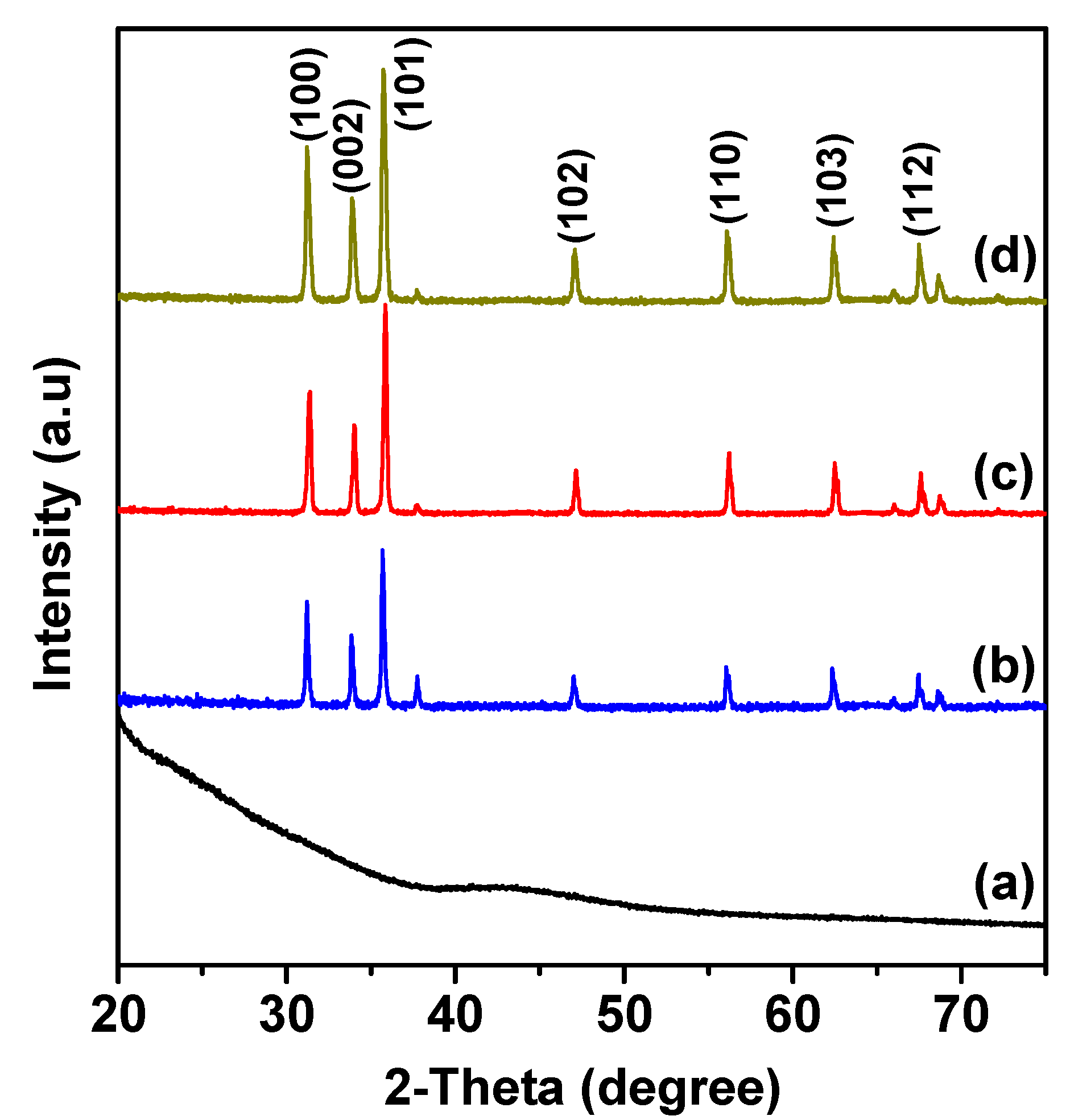
2.1. X-ray Diffraction Analysis
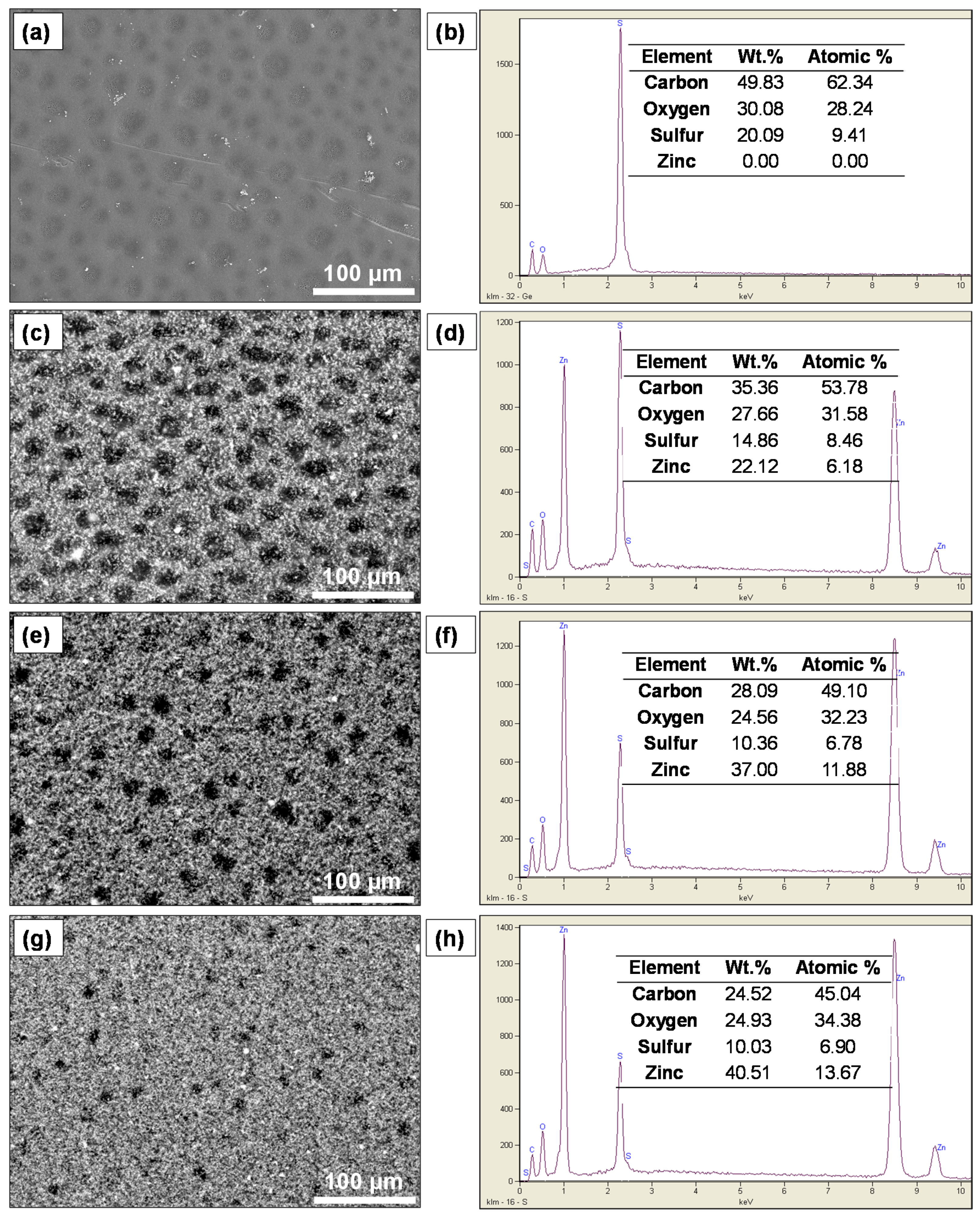
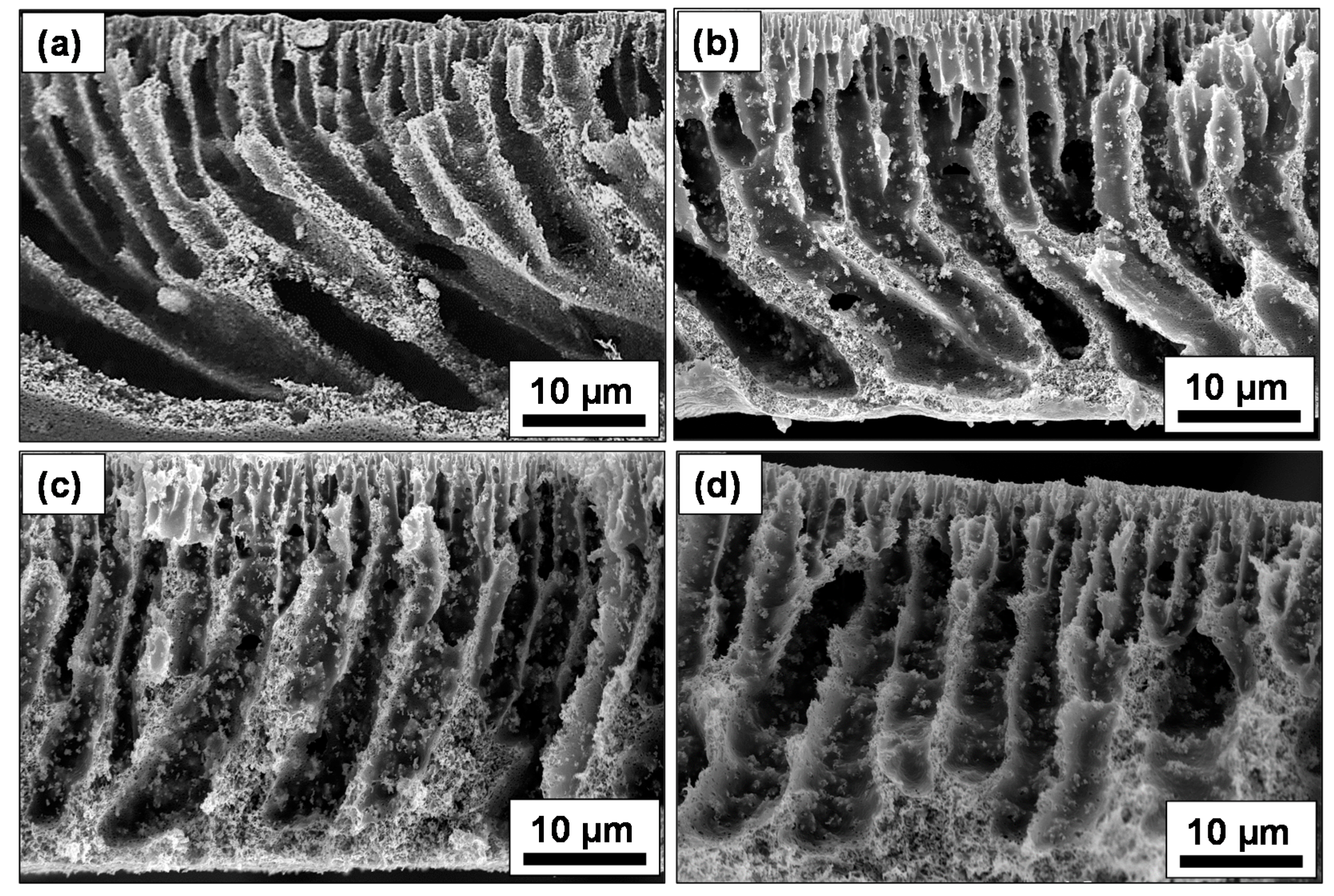
2.2. Surface Morphological and Structural Analysis
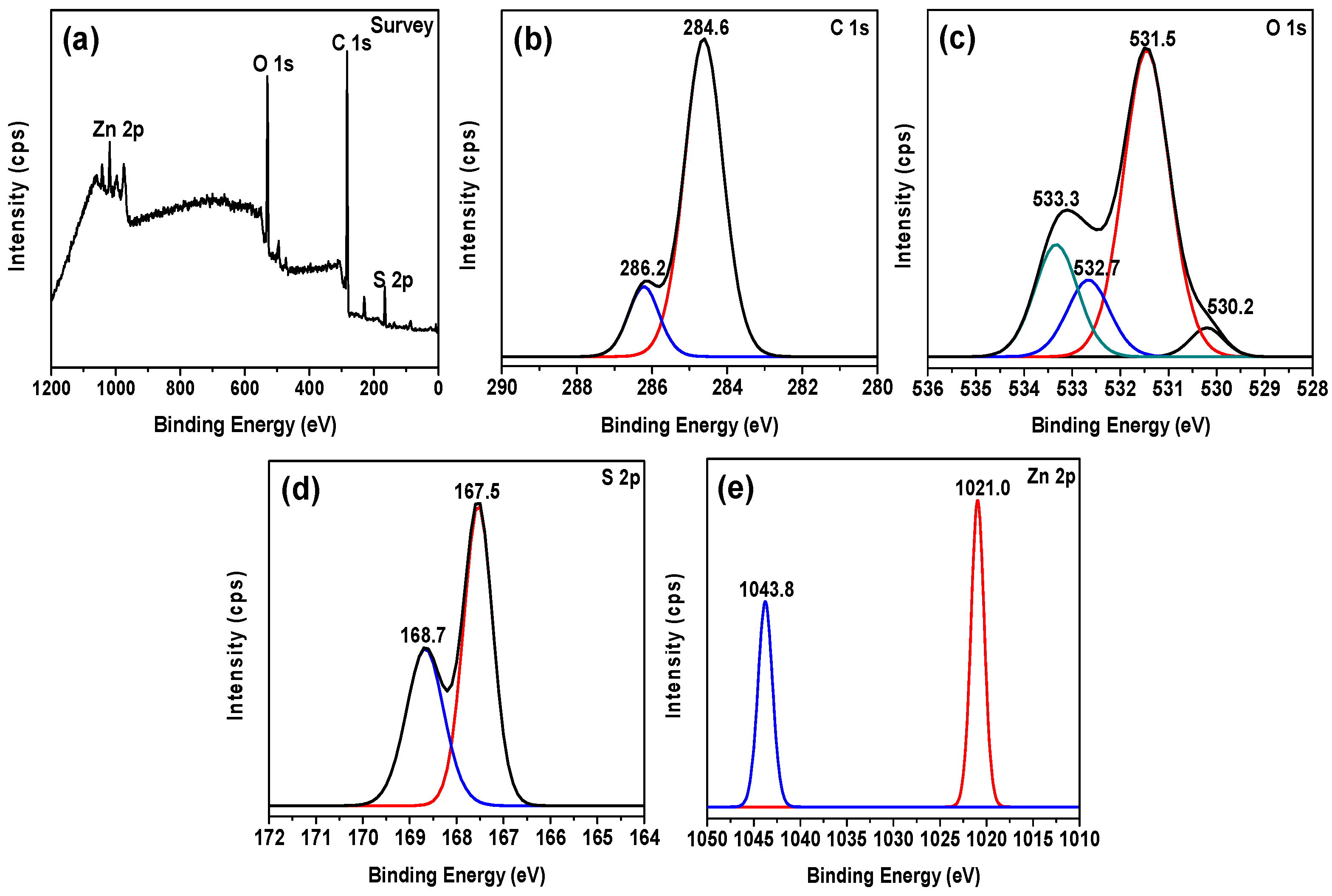
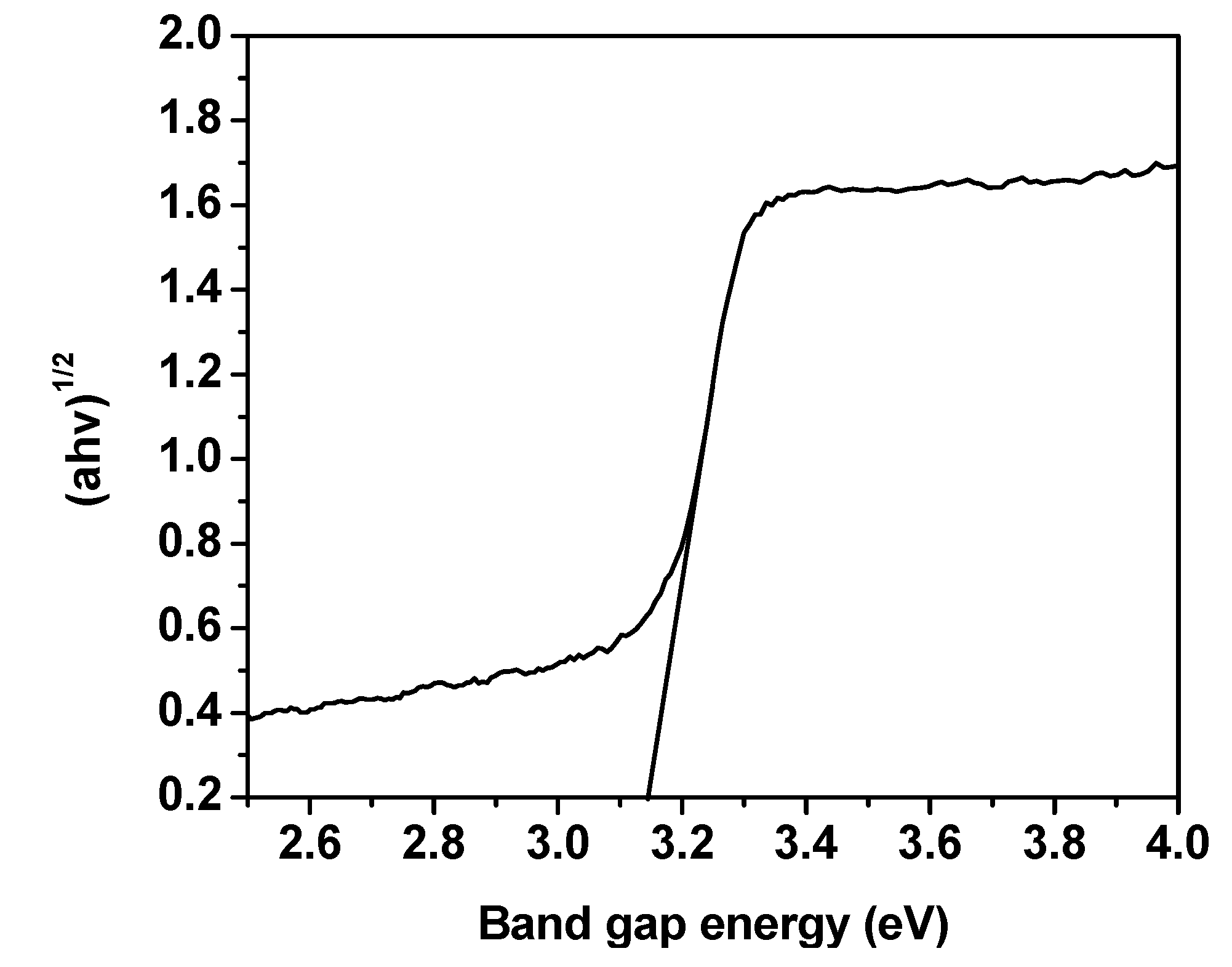
2.3. X-ray Photoelectron and Optical Property Analysis
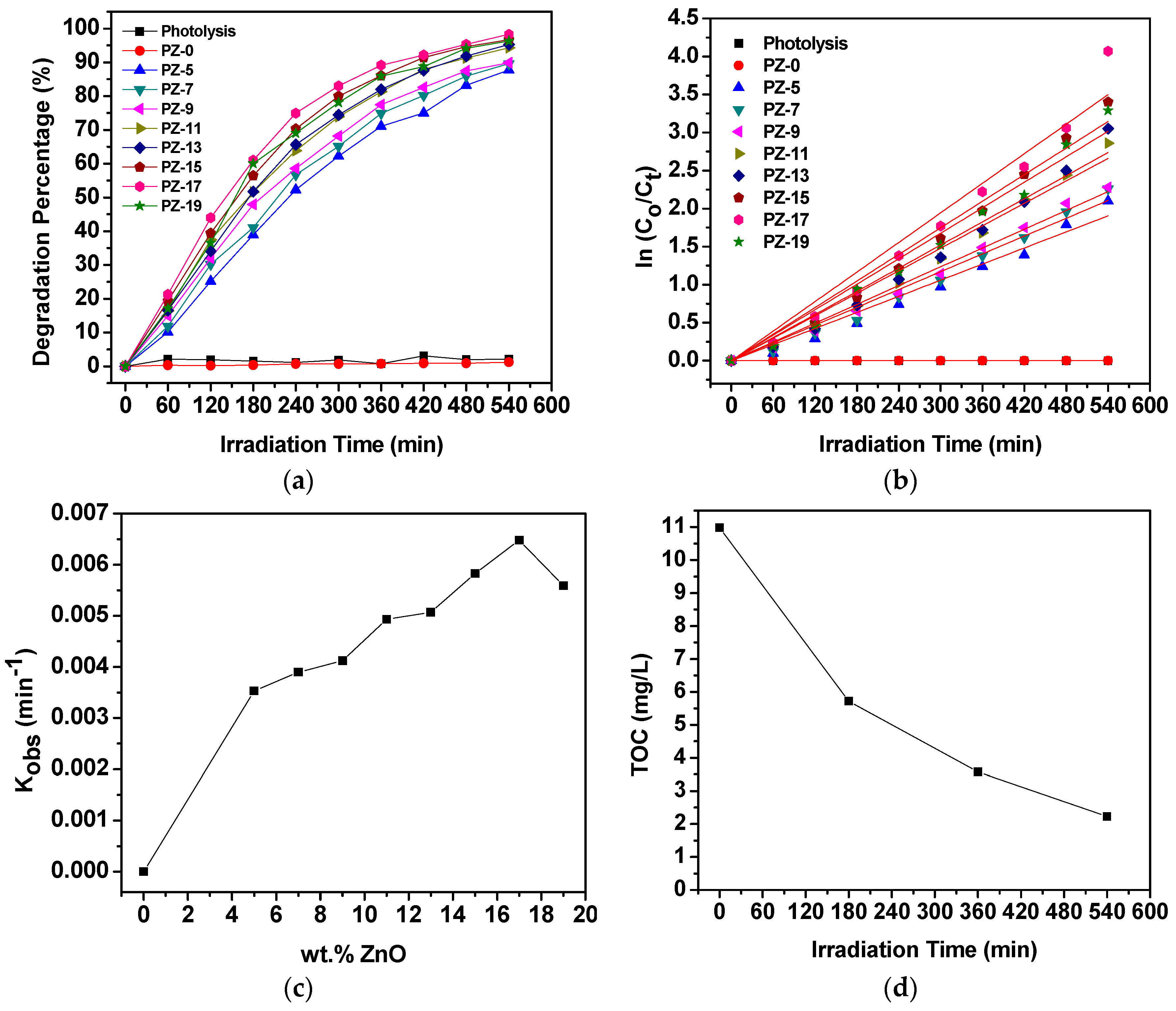
2.4. Photocatalytic Degradation Activity by the PES/ZnO Hybrid Film Photocatalysts
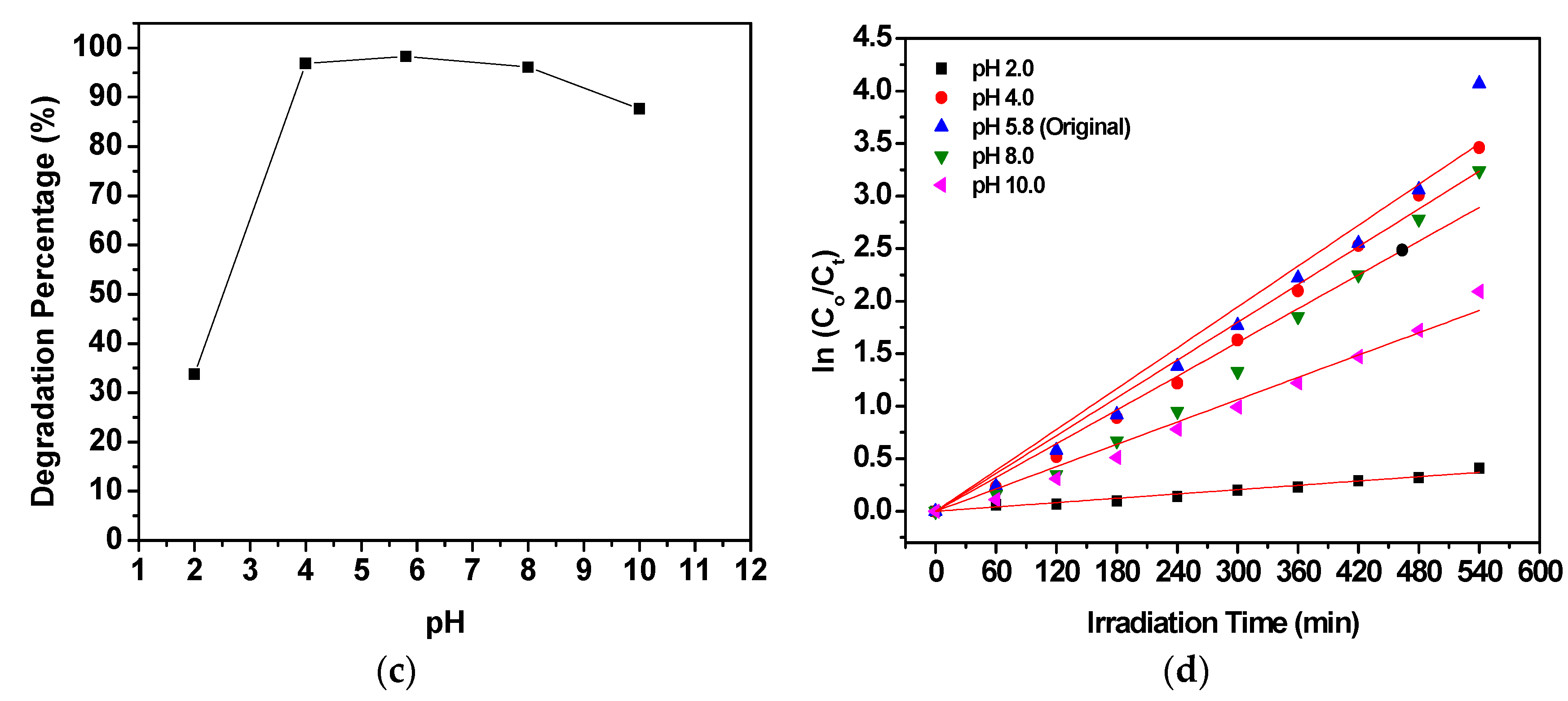
2.5. Effect of Variables in the Operating Parameters on the Degradation Percentage of MO
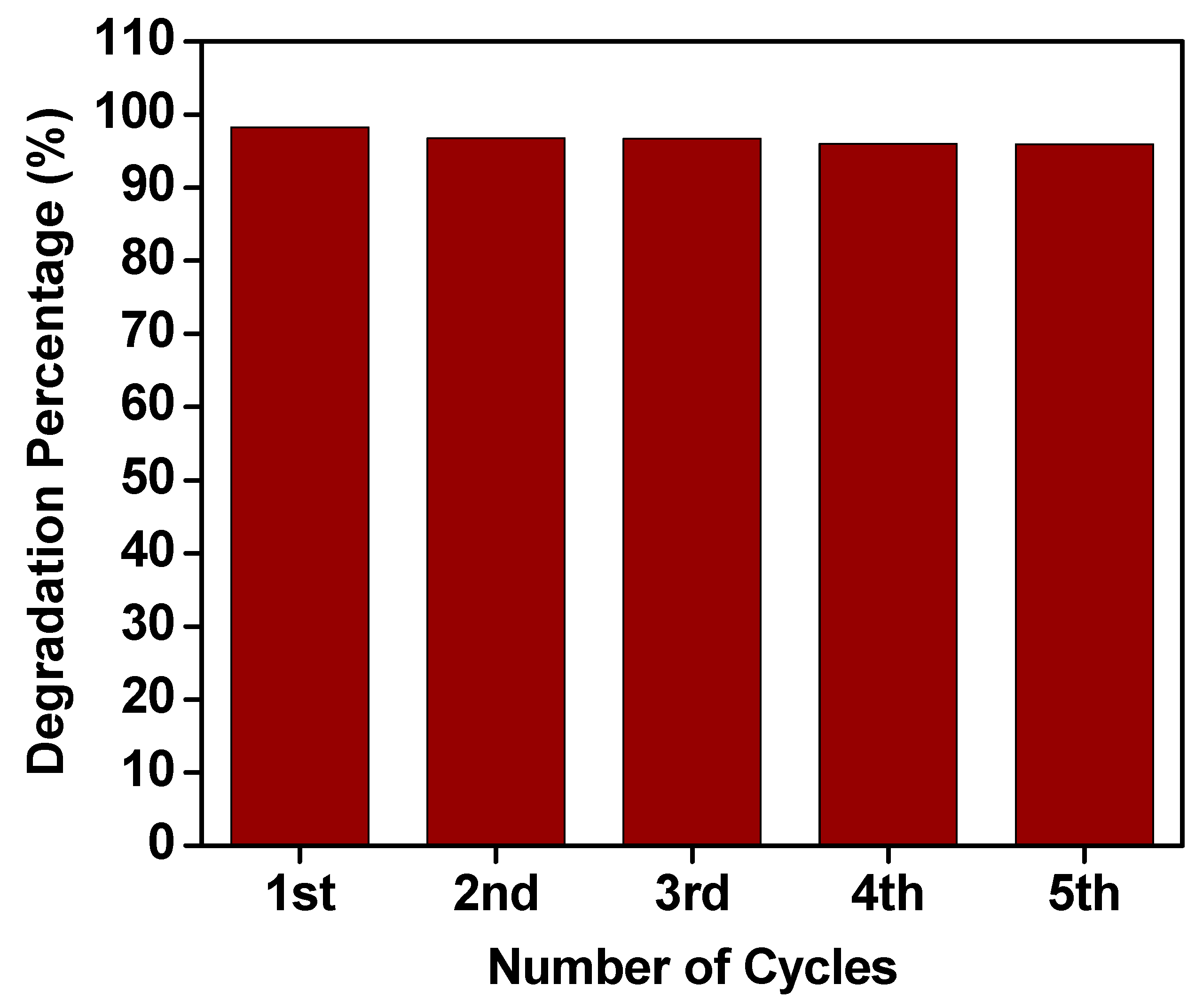
2.6. Recyclability
3. Experimental
3.1. Materials
3.2. Preparation of the PES/ZnO Hybrid Film Photocatalysts
3.3. Characterization of the PES/ZnO Hybrid Film Photocatalysts
3.4. Photocatalytic Degradation Procedure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miranda-García, N.; Suárez, S.; Sánchez, B.; Coronado, J.M.; Malato, S.; Maldonado, I.M. Photocatalytic degradation of emerging contaminants in municipal wastewater treatment plant effluents using immobilized TiO2 in a solar pilot plant. Appl. Catal. B Environ. 2011, 103, 294–301. [Google Scholar] [CrossRef]
- Vaiano, V.; Matarangolo, M.; Sacco, O.; Sannino, D. Photocatalytic treatment of aqueous solutions at high dye concentration using praseodymium-doped ZnO catalysts. Appl. Catal. B Environ. 2017, 209, 621–630. [Google Scholar] [CrossRef]
- May-Lozano, M.; Mendoza-Escamilla, V.; Rojas-García, E.; López-Medina, R.; Rivadeneyra-Romero, G.; Martinez-Delgadillo, S.A. Sonophotocatalytic degradation of Orange II dye using low cost photocatalyst. J. Clean. Prod. 2017, 148, 836–844. [Google Scholar] [CrossRef]
- Bailón-García, E.; Elmouwahidi, A.; Álvarez, M.A.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J. New carbon xerogel-TiO2 composites with high performance as visible-light photocatalysts for dye mineralization. Appl. Catal. B Environ. 2017, 201, 29–40. [Google Scholar] [CrossRef]
- Intarasuwan, K.; Amornpitoksuk, P.; Suwanboon, S.; Graidist, P. Photocatalytic dye degradation by ZnO nanoparticles prepared from X2C2O4 (X = H, Na and NH4) and the cytotoxicity of the treated dye solutions. Sep. Purif. Technol. 2017, 177, 304–312. [Google Scholar] [CrossRef]
- Chowdhury, S.; Balasubramanian, R. Recent advances in the use of graphene-family nanoadsorbents for removal of toxic pollutants from wastewater. Adv. Colloid Interface Sci. 2014, 204, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Riera-Torres, M.; Gutiérrez-Bouzán, C.; Crespi, M. Combination of coagulation-flocculation and nanofiltration techniques for dye removal and water reuse in textile effluents. Desalination 2010, 252, 53–59. [Google Scholar] [CrossRef]
- Ghaedi, M.; Sadeghian, B.; Pebdani, A.A.; Sahraei, R.; Daneshfar, A.; Kinetics, C.D. thermodynamics and equilibrium evaluation of direct yellow 12 removal by adsorption onto silver nanoparticles loaded activated carbon. Chem. Eng. J. 2012, 187, 133–141. [Google Scholar] [CrossRef]
- Bagheri, S.; Hir, Z.A.M.; Yousefi, A.T.; Hamid, S.B.A. Progress on mesoporous titanium dioxide: Synthesis, modification and applications. Microporous Mesoporous Mater. 2015, 218, 206–222. [Google Scholar] [CrossRef]
- Kaur, J.; Singhal, S. Facile synthesis of ZnO and transition metal doped ZnO nanoparticles for the photocatalytic degradation of Methyl Orange. Ceram. Int. 2014, 40, 7417–7424. [Google Scholar] [CrossRef]
- Siuleiman, S.; Kaneva, N.; Bojinova, A.; Papazova, K.; Apostolov, A.; Dimitrov, D. Photodegradation of Orange II by ZnO and TiO2 powders and nanowire ZnO and ZnO/TiO2 thin films. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 408–413. [Google Scholar] [CrossRef]
- Chaker, H.; Chérif-Aouali, L.; Khaoulani, S.; Bengueddach, A.; Fourmentin, S. Photocatalytic degradation of methyl orange and real wastewater by silver doped mesoporous TiO2 catalysts. J. Photochem. Photobiol. A Chem. 2016, 318, 142–149. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, M.; Chorfi, H.; Bousselmi, L.; Bessaïs, B. Polymer supported porous TiO2: Application to photo-catalysis. Phys. Status Solidi Curr. Top. Solid State Phys. 2007, 4, 2029–2033. [Google Scholar] [CrossRef]
- Singh, S.; Mahalingam, H.; Singh, P.K. Polymer-supported titanium dioxide photocatalysts for environmental remediation: A review. Appl. Catal. A Gen. 2013, 462–463, 178–195. [Google Scholar] [CrossRef]
- Vaiano, V.; Sarno, G.; Sacco, O.; Sannino, D. Degradation of terephthalic acid in a photocatalytic system able to work also at high pressure. Chem. Eng. J. 2017, 312, 10–19. [Google Scholar] [CrossRef]
- Mozia, S.; Darowna, D.; Wróbel, R.; Morawski, A.W. A study on the stability of polyethersulfone ultrafiltration membranes in a photocatalytic membrane reactor. J. Membr. Sci. 2015, 495, 176–186. [Google Scholar] [CrossRef]
- Fischer, K.; Kühnert, M.; Gläser, R.; Schulze, A. Photocatalytic degradation and toxicity evaluation of diclofenac by nanotubular titanium dioxide–PES membrane in a static and continuous setup. RSC Adv. 2015, 5, 16340–16348. [Google Scholar] [CrossRef]
- Hir, Z.A.M.; Moradihamedani, P.; Abdullah, A.H.; Mohamed, M.A. Immobilization of TiO2 into polyethersulfone matrix as hybrid film photocatalyst for effective degradation of methyl orange dye. Mater. Sci. Semicond. Process. 2017, 57, 157–165. [Google Scholar] [CrossRef]
- Pardeshi, S.K.; Patil, A.B. Solar photocatalytic degradation of resorcinol a model endocrine disrupter in water using zinc oxide. J. Hazard. Mater. 2009, 163, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Hou, J.; Fu, Z.; Yang, B.; Yang, P.; Liu, K.; Wen, M.; Chen, Y.; Fu, S.; Li, F. Growth and photocatalytic properties of one-dimensional ZnO nanostructures prepared by thermal evaporation. Mater. Res. Bull. 2009, 44, 1954–1958. [Google Scholar] [CrossRef]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2014, 1–20. [Google Scholar] [CrossRef]
- Rajabi, H.; Ghaemi, N.; Madaeni, S.S.; Daraei, P.; Astinchap, B.; Zinadini, S.; Hossein, S. Nano-ZnO embedded mixed matrix polyethersulfone (PES) membrane: Influence of nanofiller shape on characterization and fouling resistance. Appl. Surf. Sci. 2015, 349, 66–77. [Google Scholar] [CrossRef]
- Li, X.; Fang, X.; Pang, R.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. Self-assembly of TiO2 nanoparticles around the pores of PES ultrafiltration membrane for mitigating organic fouling. J. Membr. Sci. 2014, 467, 226–235. [Google Scholar] [CrossRef]
- De Sitter, K.; Dotremont, C.; Genne, I.; Stoops, L. The use of nanoparticles as alternative pore former for the production of more sustainable polyethersulfone ultrafiltration membranes. J. Membr. Sci. 2014, 471, 168–178. [Google Scholar] [CrossRef]
- Shen, L.; Bian, X.; Lu, X.; Shi, L.; Liu, Z.; Chen, L.; Hou, Z.; Fan, K. Preparation and characterization of ZnO/polyethersulfone (PES) hybrid membranes. Desalination 2012, 293, 21–29. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Abdulkarim, A.A.; Shafie, Z.M.H.M.; Ooi, B.S. Fouling evaluation of PES/ZnO mixed matrix hollow fiber membrane. Desalination 2017, 403, 53–63. [Google Scholar] [CrossRef]
- Wavhal, D.S.; Fisher, E.R. Membrane surface modification by plasma-induced polymerization of acrylamide for improved surface properties and reduced protein fouling. Langmuir 2003, 19, 79–85. [Google Scholar] [CrossRef]
- Liu, S.X.; Kim, J.-T. Characterization of Surface Modification of Polyethersulfone Membrane. J. Adhes. Sci. Technol. 2011, 25, 193–212. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, J.; Xue, Y.; Yu, P.; Zhang, B.; Wang, L.; Liu, R. Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Al-gaashani, R.; Radiman, S.; Daud, A.R.; Tabet, N.; Al-Douri, Y. XPS and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Rosmi, M.S.; Hir, Z.A.M.; Mutalib, M.A.; Ismail, A.F.; Tanemura, M. Carbon as amorphous shell and interstitial dopant in mesoporous rutile TiO2: Bio-template assisted sol-gel synthesis and photocatalytic activity. Appl. Surf. Sci. 2017, 393, 46–59. [Google Scholar] [CrossRef]
- Lei, P.; Wang, F.; Gao, X.; Ding, Y.; Zhang, S.; Zhao, J.; Liu, S.; Yang, M. Immobilization of TiO2 nanoparticles in polymeric substrates by chemical bonding for multi-cycle photodegradation of organic pollutants. J. Hazard. Mater. 2012, 227–228, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Zyoud, A.; Zu’bi, A.; Helal, M.H.S.; Park, D.; Campet, G.; Hilal, H.S. Optimizing photo-mineralization of aqueous methyl orange by nano-ZnO catalyst under simulated natural conditions. J. Environ. Health Sci. Eng. 2015, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Ismail, A.F.; Mutalib, M.A.; Sani, N.A.A.; Asri, S.E.A.M.; Ong, C.S. Physicochemical characteristic of regenerated cellulose/N-doped TiO2 nanocomposite membrane fabricated from recycled newspaper with photocatalytic activity under UV and visible light irradiation. Chem. Eng. J. 2016, 284, 202–215. [Google Scholar] [CrossRef]
- Moradihamedani, P.; Ibrahim, N.A.; Zin, W.; Yunus, W.; Yusof, N.A. Study of morphology and gas separation properties of polysulfone/titanium dioxide mixed matrix membranes. Polym. Eng. Sci. 2015, 367–374. [Google Scholar] [CrossRef]
- Xie, S.; Huang, P.; Kruzic, J.J.; Zeng, X.; Qian, H. A highly efficient degradation mechanism of methyl orange using Fe-based metallic glass powders. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, N.F.; Jalil, A.A.; Triwahyono, S.; Muhid, M.N.M.; Sapawe, N.; Satar, M.A.H.; Asaari, H. Photodecolorization of methyl orange over α-Fe2O3-supported HY catalysts: The effects of catalyst preparation and dealumination. Chem. Eng. J. 2012, 191, 112–122. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Jiu, H.; Qi, G.; Huang, Y. ZnO-reduced graphene oxide nanocomposites as efficient photocatalysts for photocatalytic reduction of CO2. Ceram. Int. 2015, 41, 6256–6262. [Google Scholar] [CrossRef]
- Zhang, L.; Tse, M.S.; Tan, O.K. Facile in situ synthesis of visible light-active Pt/C-TiO2 nanoparticles for environmental remediation. J. Environ. Chem. Eng. 2014, 2, 1214–1220. [Google Scholar] [CrossRef]
- Saleh, R.; Djaja, N.F. Transition-metal-doped ZnO nanoparticles: Synthesis, characterization and photocatalytic activity under UV light. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Subbaiah, M.V.; Kim, D.S. Adsorption of methyl orange from aqueous solution by aminated pumpkin seed powder: Kinetics, isotherms, and thermodynamic studies. Ecotoxicol. Environ. Saf. 2016, 128, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Hir, Z.A.M.; Yousefi, A.T.; Hamid, S.B.A. Photocatalytic performance of activated carbon-supported mesoporous titanium dioxide. Desalin. Water Treat. 2015, 1–7. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Mutalib, M.A.; Hir, Z.A.M.; Zain, M.F.M.; Mohamad, A.B.; Minggu, L.J.; Awang, N.A.; Salleh, W.N.W. An overview on cellulose-based material in tailoring bio-hybrid nanostructured photocatalysts for water treatment and renewable energy applicationsm. Int. J. Biol. Macromol. 2017, 103, 1232–1256. [Google Scholar] [CrossRef] [PubMed]

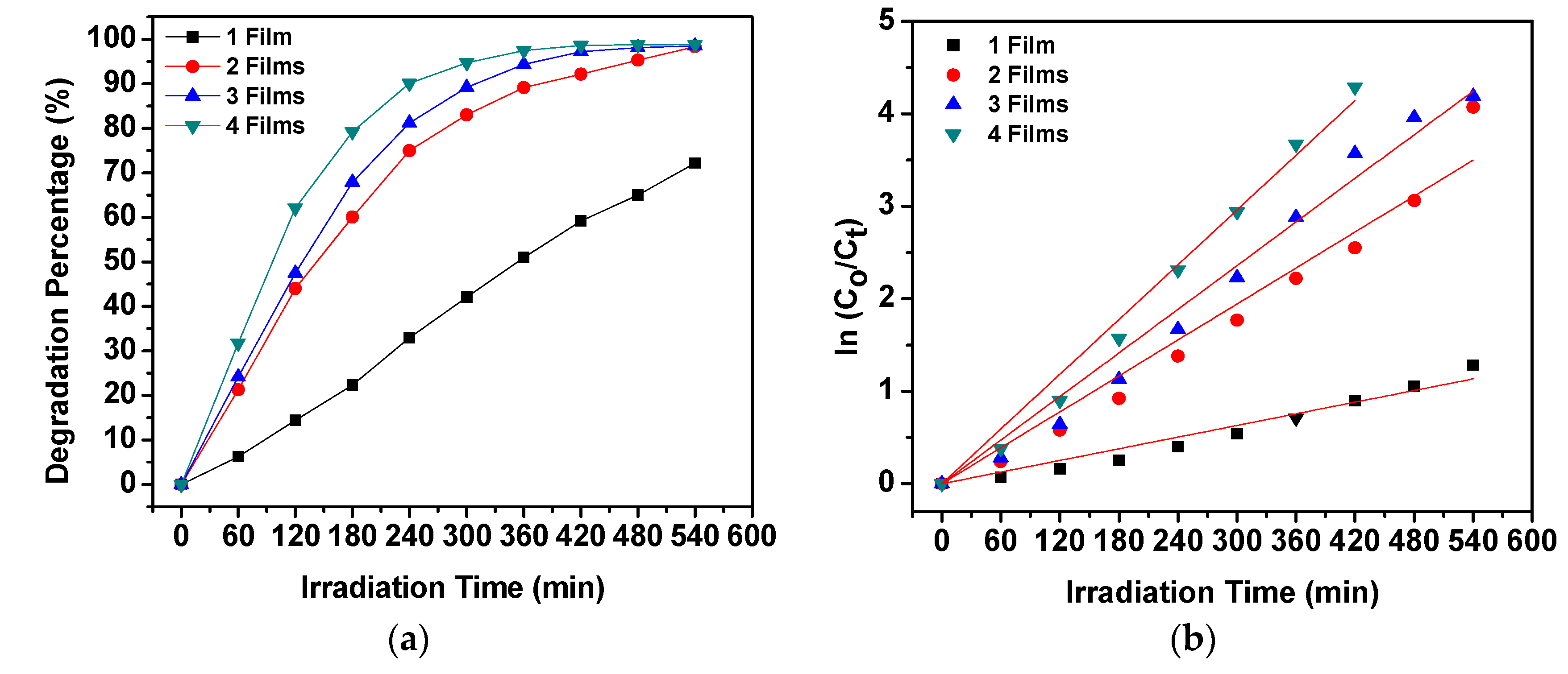
| Quantity of Film | Degradation Percentage (%) | kobs (min−1) | Rate (mg/L min) | Amount of MO Degraded (mg/g) | Correlation Factor, R2 |
|---|---|---|---|---|---|
| 1 | 72.16 | 0.00210 | 0.02090 | 17.83 | 0.98251 |
| 2 | 98.28 | 0.00648 | 0.06798 | 25.60 | 0.98591 |
| 3 | 98.49 | 0.00786 | 0.07805 | 25.67 | 0.99328 |
| 4 | 98.82 | 0.00987 | 0.09395 | 25.74 | 0.99520 |
| Initial pH of MO Solution | Degradation Percentage (%) | kobs (min−1) | Rate (mg/L min) | Amount of MO Degraded (mg/g) | Correlation Factor, R2 |
|---|---|---|---|---|---|
| 2.0 | 33.75 | 0.00069 | 0.00734 | 15.24 | 0.99167 |
| 4.0 | 96.85 | 0.00599 | 0.06079 | 24.40 | 0.99283 |
| 5.8 (original) | 98.28 | 0.00648 | 0.06798 | 25.60 | 0.98591 |
| 8.0 | 96.09 | 0.00535 | 0.05612 | 25.02 | 0.97931 |
| 10.0 | 87.69 | 0.00354 | 0.03738 | 22.99 | 0.99267 |
| Initial Concentration of MO Solution (mg/L) | Degradation Percentage (%) | kobs (min−1) | Rate (mg/L min) | Amount of MO Degraded (mg/g) | Correlation Factor, R2 |
|---|---|---|---|---|---|
| 5 | 99.41 | 0.00922 | 0.04665 | 12.49 | 0.99766 |
| 10 | 98.28 | 0.00648 | 0.06798 | 25.60 | 0.98591 |
| 15 | 93.96 | 0.00474 | 0.07063 | 34.76 | 0.98936 |
| 20 | 90.60 | 0.00403 | 0.08020 | 44.76 | 0.99208 |
| Number of Cycle | Irradiation Time (h) | Degradation Percentage (%) | Amount of MO Degraded (mg/g) |
|---|---|---|---|
| 1 | 9 | 98.28 | 25.60 |
| 2 | 18 | 96.79 | 25.49 |
| 3 | 27 | 96.70 | 25.49 |
| 4 | 36 | 95.98 | 25.45 |
| 5 | 45 | 95.95 | 25.32 |
| PES wt% | ZnO wt% | NMP wt% | Labelled as |
|---|---|---|---|
| 15 | 0 | 85 | PZ-0 |
| 15 | 5 | 80 | PZ-5 |
| 15 | 7 | 78 | PZ-7 |
| 15 | 9 | 76 | PZ-9 |
| 15 | 11 | 74 | PZ-11 |
| 15 | 13 | 72 | PZ-13 |
| 15 | 15 | 70 | PZ-15 |
| 15 | 17 | 68 | PZ-17 |
| 15 | 19 | 66 | PZ-19 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Hir, Z.A.; Abdullah, A.H.; Zainal, Z.; Lim, H.N. Photoactive Hybrid Film Photocatalyst of Polyethersulfone-ZnO for the Degradation of Methyl Orange Dye: Kinetic Study and Operational Parameters. Catalysts 2017, 7, 313. https://doi.org/10.3390/catal7110313
Mohd Hir ZA, Abdullah AH, Zainal Z, Lim HN. Photoactive Hybrid Film Photocatalyst of Polyethersulfone-ZnO for the Degradation of Methyl Orange Dye: Kinetic Study and Operational Parameters. Catalysts. 2017; 7(11):313. https://doi.org/10.3390/catal7110313
Chicago/Turabian StyleMohd Hir, Zul Adlan, Abdul Halim Abdullah, Zulkarnain Zainal, and Hong Ngee Lim. 2017. "Photoactive Hybrid Film Photocatalyst of Polyethersulfone-ZnO for the Degradation of Methyl Orange Dye: Kinetic Study and Operational Parameters" Catalysts 7, no. 11: 313. https://doi.org/10.3390/catal7110313
APA StyleMohd Hir, Z. A., Abdullah, A. H., Zainal, Z., & Lim, H. N. (2017). Photoactive Hybrid Film Photocatalyst of Polyethersulfone-ZnO for the Degradation of Methyl Orange Dye: Kinetic Study and Operational Parameters. Catalysts, 7(11), 313. https://doi.org/10.3390/catal7110313