A Review on the Production and Purification of Biomass-Derived Hydrogen Using Emerging Membrane Technologies
Abstract
1. Introduction
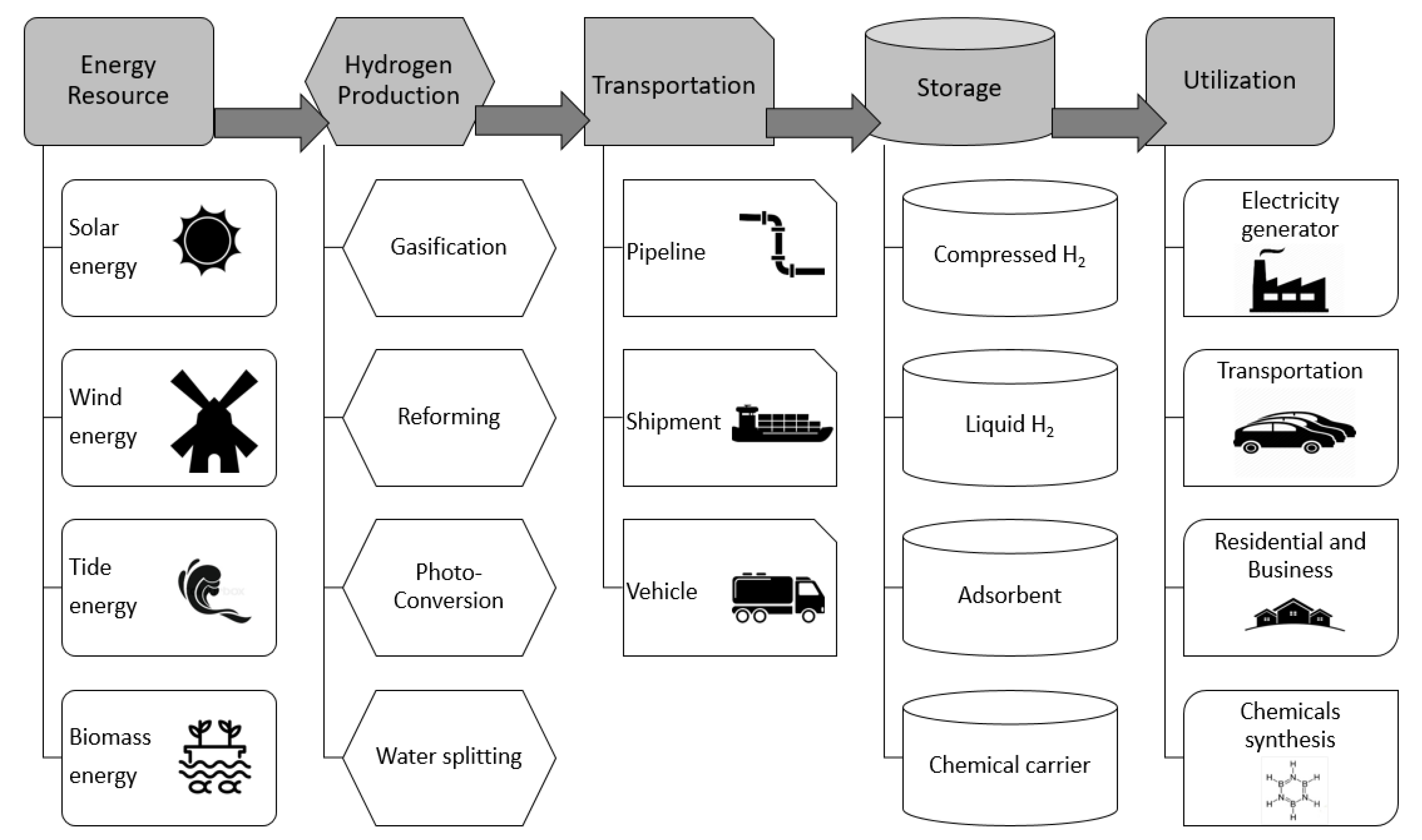
1.1. Hydrogen Energy System
1.2. Hydrogen Production from Biomass
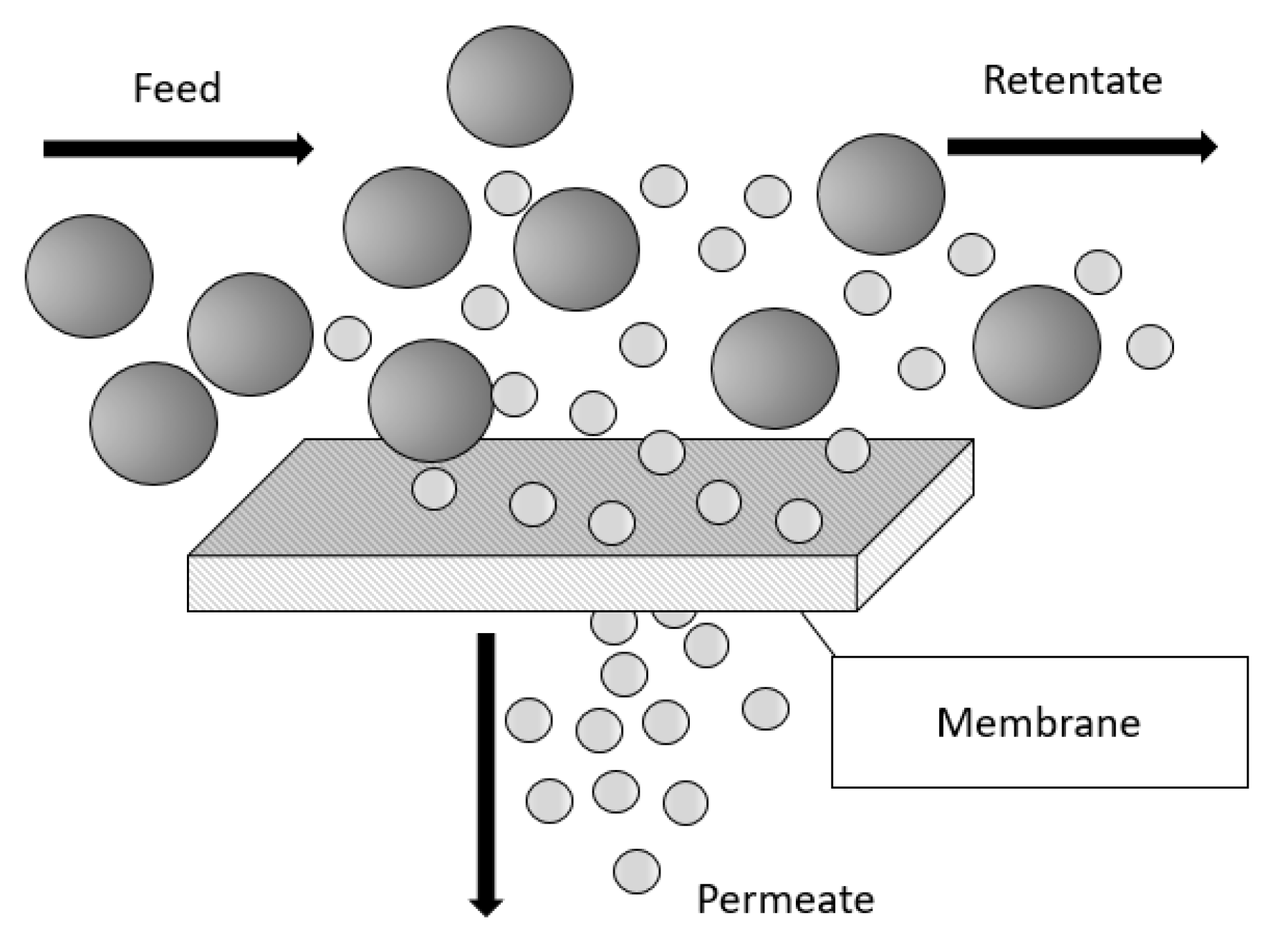
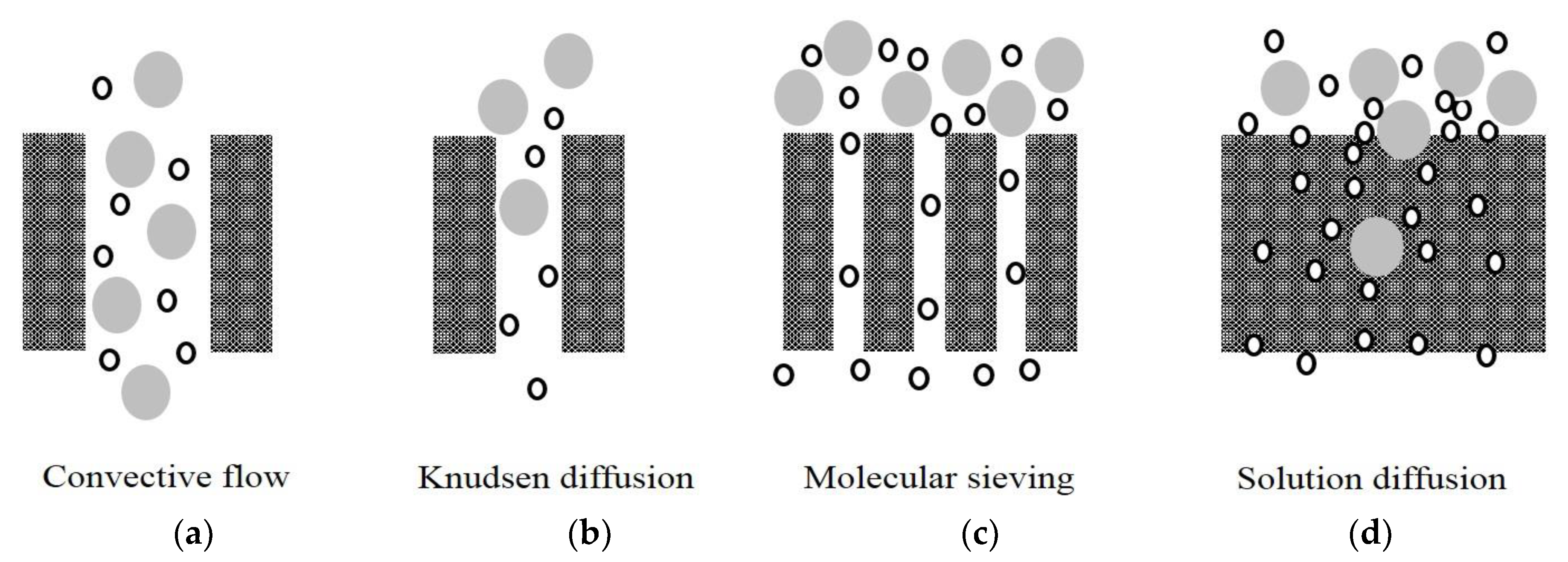
1.3. Membrane for Hydrogen Separation
2. Polymeric Membranes for Hydrogen Separation
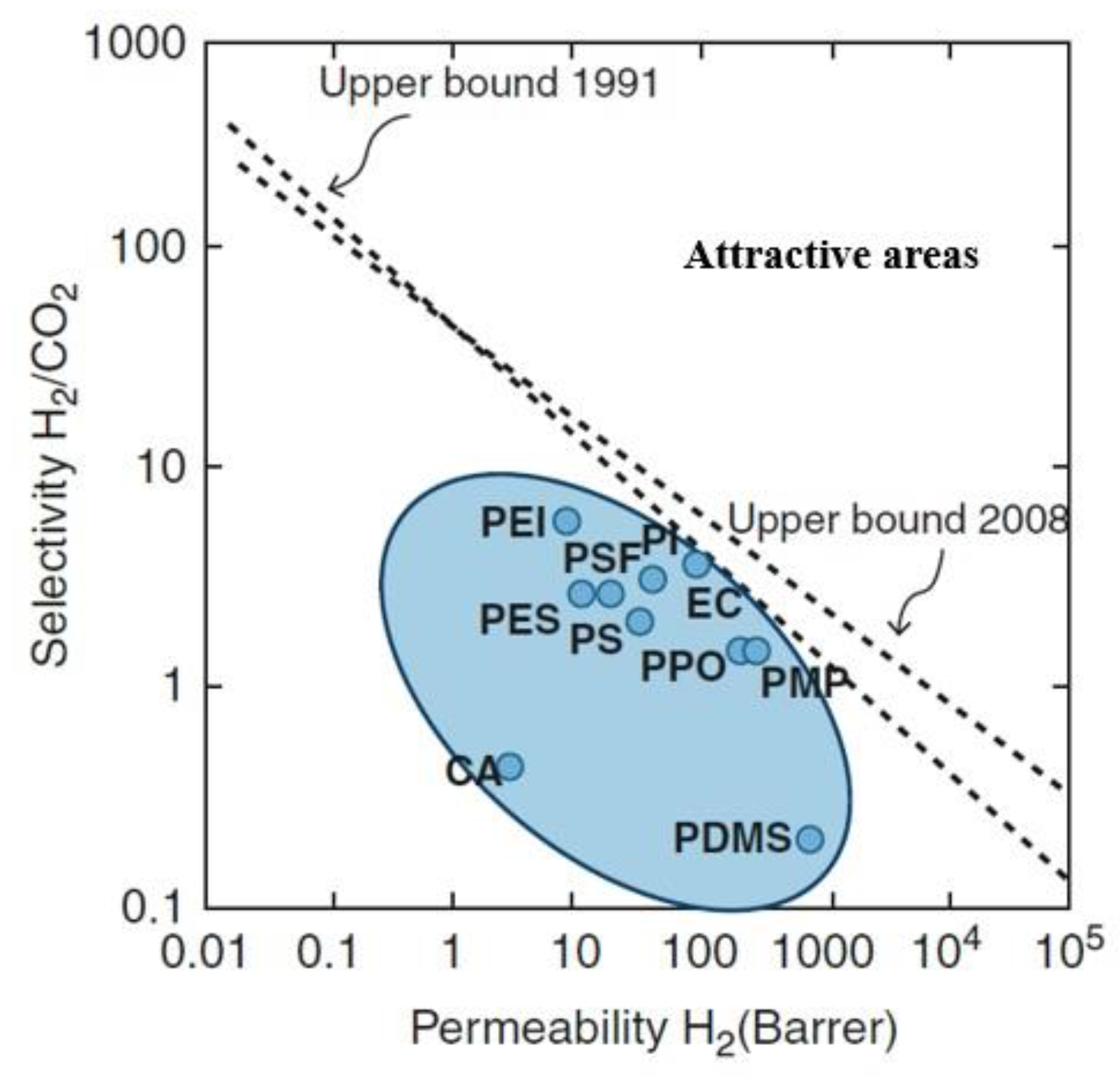
2.1. The Performance of the Polymeric Membrane for Hydrogen Separation
2.2. The Strategy of Improving the Polymeric Membrane
2.3. Current Utilization Status and Future Perspectives for Biomass Processing
3. Dense Metal Membranes for Hydrogen Separation
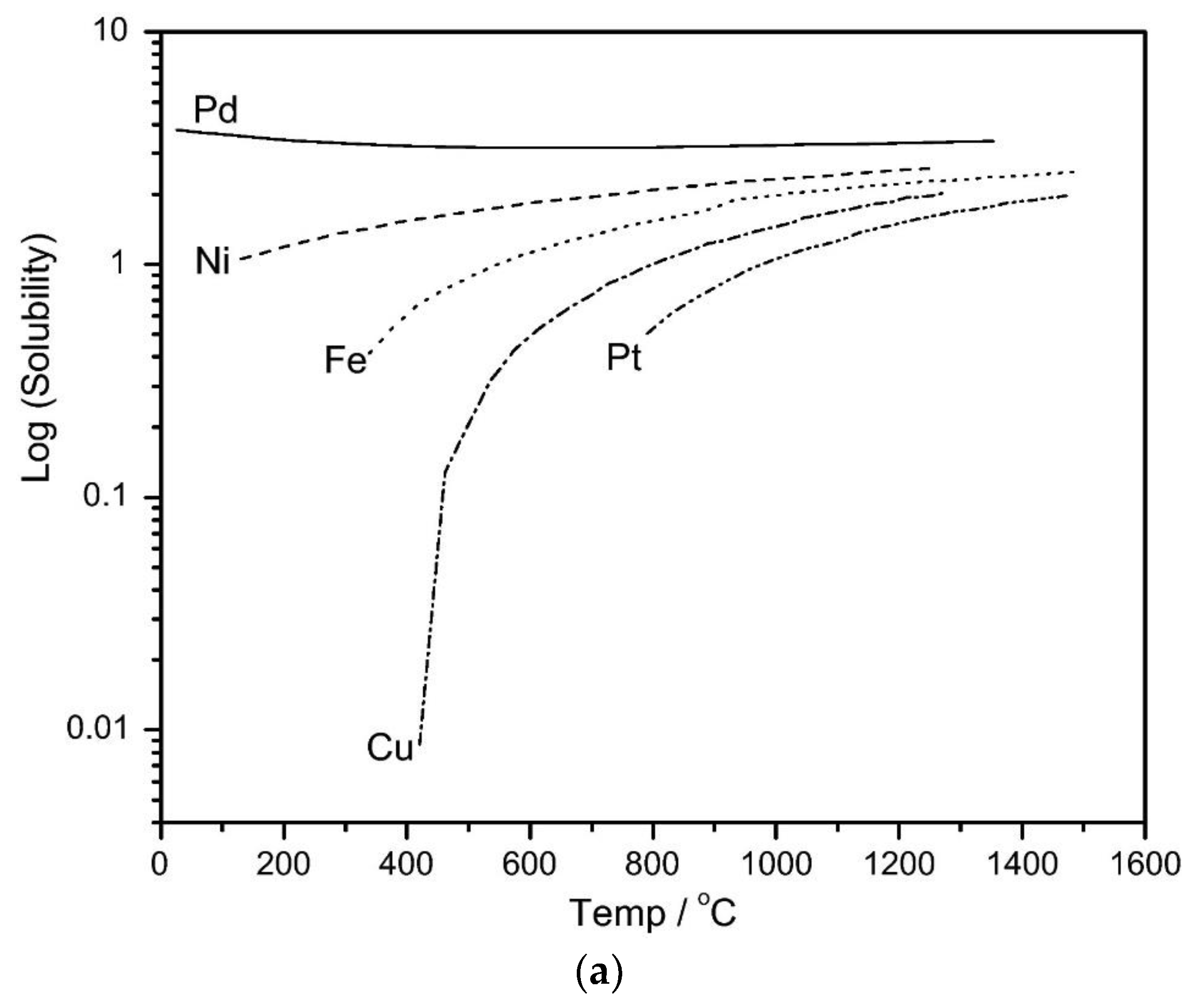
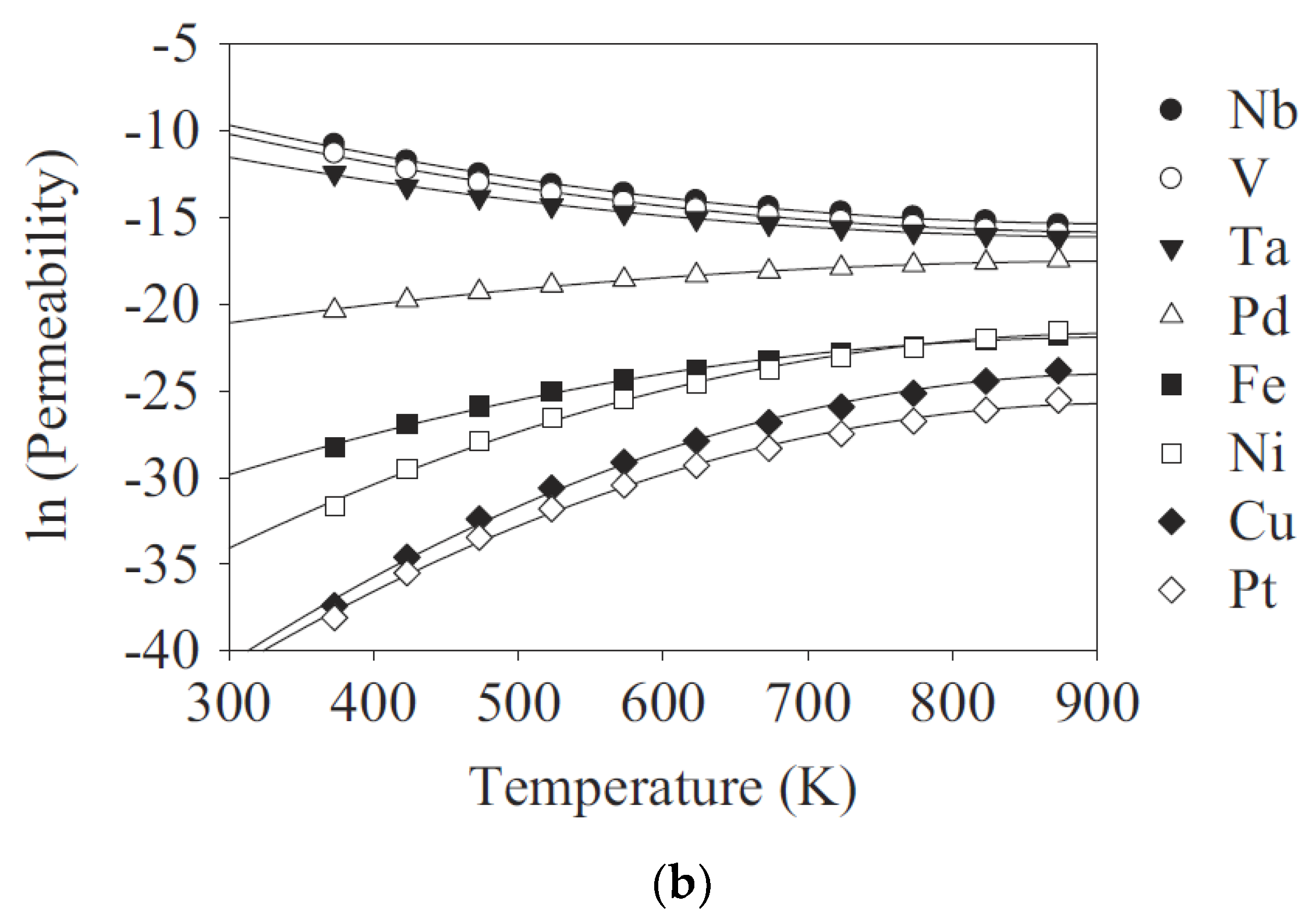
3.1. The Development of Dense Metal Membranes for Hydrogen Separation
3.2. Current Industrialization Status and Future Perspectives for Biomass Processing
4. Microporous Membranes for Hydrogen Separation
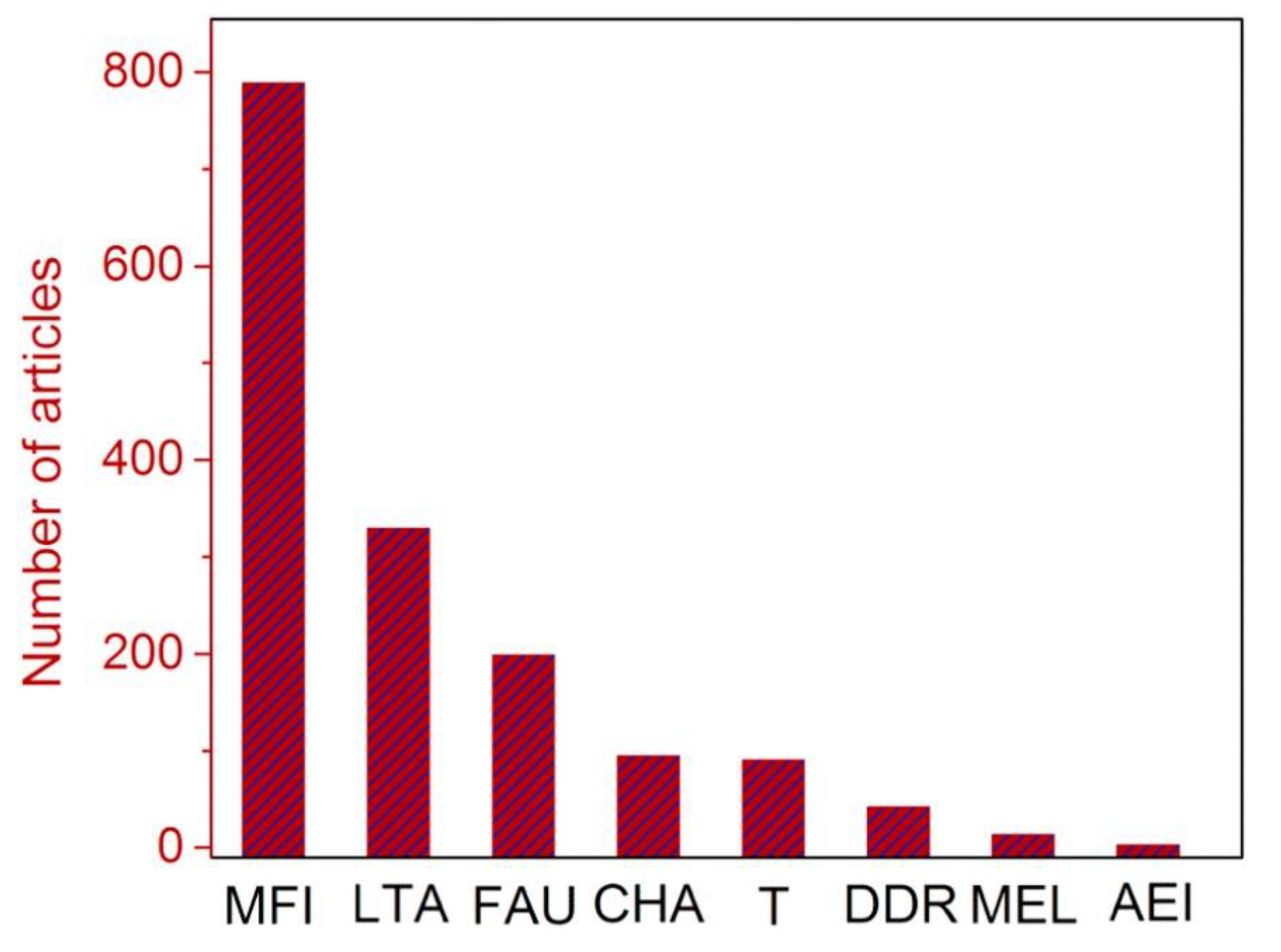
4.1. Zeolite Membranes
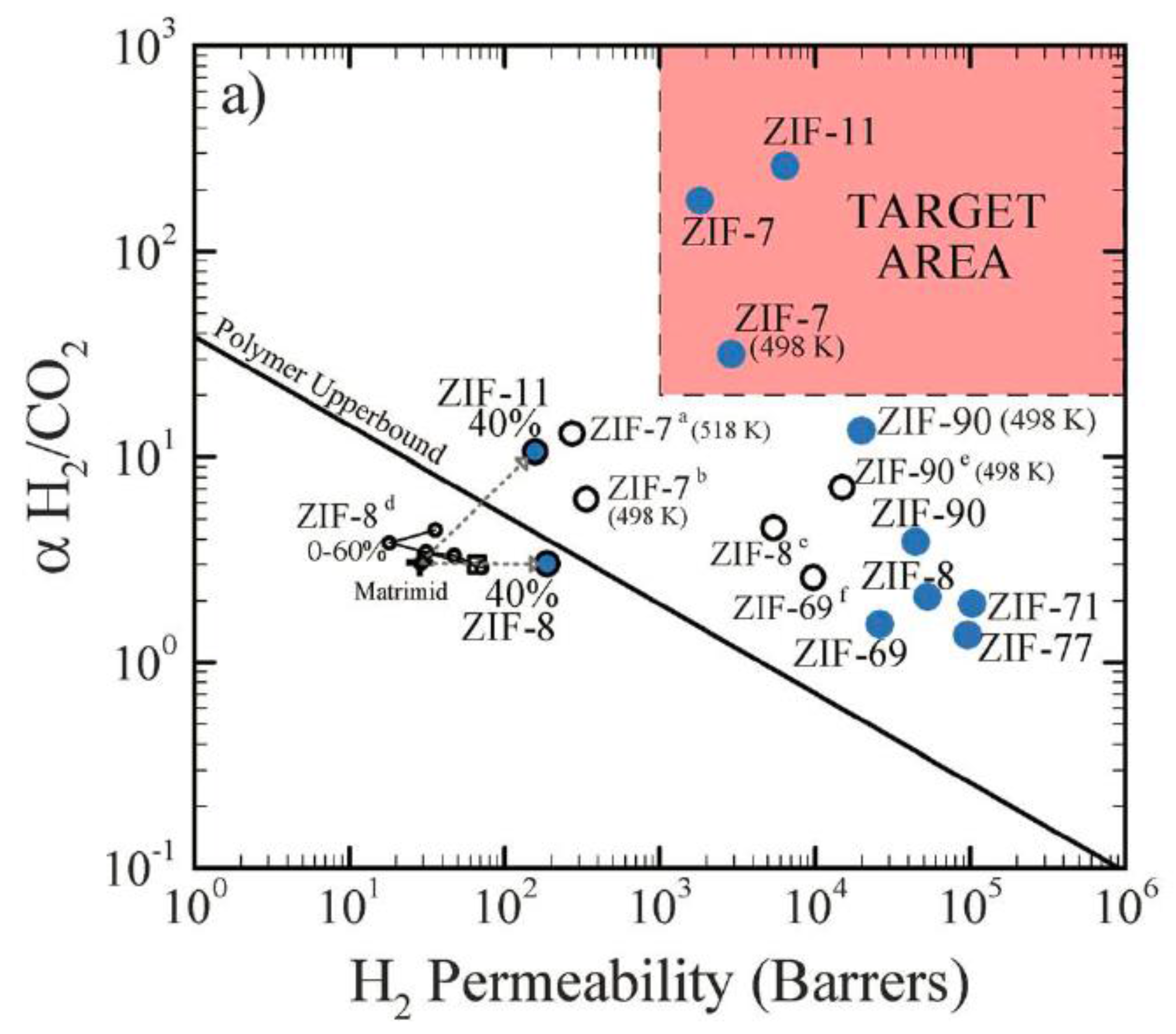
4.2. MOF Membranes
4.3. Other Microporous Membranes
5. Membrane Reactors for Hydrogen Separation
6. Conclusions and Future Perspective
Acknowledgments
Conflicts of Interest
References
- Lindstrom, P. International Energy Outlook 2016: Carbon Dioxide Emissions; U.S. Energy Information Administration: Washington, DC, USA, 2016.
- Momirlan, M.; Veziroglu, T.N. Current status of hydrogen energy. Renew. Sustain. Energy Rev. 2002, 6, 141–179. [Google Scholar] [CrossRef]
- Dunn, S. Hydrogen futures: Toward a sustainable energy system. Int. J. Hydrogen Energy 2002, 27, 235–264. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Barbir, F. Transition to renewable energy systems with hydrogen as an energy carrier. Energy 2009, 34, 308–312. [Google Scholar] [CrossRef]
- Cipriani, G.; Dio, V.D.; Genduso, F.; Cascia, D.L.; Liga, R.; Miceli, R. Perspective on hydrogen energy carrier and its automotive applications. Int. J. Hydrogen Energy 2014, 39, 8482–8494. [Google Scholar] [CrossRef]
- LeValley, T.L.; Richard, A.R.; Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies—A review. Int. J. Hydrog. Enery 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Haryanto, A.; Fernando, S.; Murali, N.; Adhikari, S. Current Status of Hydrogen Production Techniques by Steam Reforming of Ethanol: A Review. Energy Fuels 2015, 191, 2098–2106. [Google Scholar] [CrossRef]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol Steam Reforming for Hydrogen Production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Ni, M.; Leung, Y.C.; Leung, M.K.H.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Kalinci, Y.; Hepbasli, A.; Dincer, I. Biomass-based hydrogen production: A review and analysis. Int. J. Hydrogen Energy 2009, 34, 8799–8817. [Google Scholar] [CrossRef]
- Tanksale, A.; Beltramini, J.N.; Lu, G.Q.M. A review of catalytic hydrogen production processes from Biomass. Renew. Sustain. Energy Rev. 2010, 14, 166–182. [Google Scholar] [CrossRef]
- Lu, G.Q.; Costa, J.C.D.; Duke, M.; Giessler, S.; Socolow, R.; Williams, R.H.; Kreutz, T. Inorganic membranes for hydrogen production and purification: A critical review and perspective. J. Colloid Interface Sci. 2007, 314, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Strathmann, H. Membrane separation process: Current relevance and future opportunities. AIChE J. 2001, 47, 1077–1087. [Google Scholar] [CrossRef]
- Adhikari, S.; Fernando, S. Hydrogen membrane separation technology. Ind. Eng. Chem. Res. 2006, 45, 875–881. [Google Scholar] [CrossRef]
- Veziroglu, T.N.; Sahin, S. 21st Century’s energy: Hydrogen energy system. Energy Convers. Manag. 2008, 49, 1820–1831. [Google Scholar] [CrossRef]
- Jensen, M.W.; Ross, M. The ultimate challenge: Developing an infrastructure for fuel cell vehicles. Environ. Sci. Policy. Sustain. Dev. 2000, 42, 10–22. [Google Scholar] [CrossRef]
- Arnason, B.; Sigfusson, T.I. Iceland—A future hydrogen economy. Int. J. Hydrogen. Energy 2000, 25, 289–294. [Google Scholar] [CrossRef]
- Koroneos, C.; Dompros, A.; Roumbas, G. Hydrogen production via biomass gasification—A life cycle assessment approach. Chem. Eng. Process. 2008, 47, 1261–1268. [Google Scholar] [CrossRef]
- Saxena, R.C.; Seal, D.; Kumar, S.; Goyal, H.B. Thermo-chemical routes for hydrogen wich gas from biomass: A review. Renew. Sustain. Energy Rev. 2008, 12, 1909–1927. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, K.S. Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield—A review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Drioli, E.; Giorno, L. Comprehensive Membrane Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Baker, R. Future directions of membrane gas-separation technology. Membr. Technol. 2001, 138, 5–10. [Google Scholar] [CrossRef]
- Gallucci, F.; Fernandez, E.; Corengia, P.; Annaland, M.V.S. Recent advances on membranes and membrane reactors for hydrogen production. Chem. Eng. Sci. 2013, 92, 40–66. [Google Scholar] [CrossRef]
- Khatib, S.J.; Oyama, S.T. Silica membranes for hydrogen separation prepared by chemical vapor deposition CVD. Sep. Purif. Sci. 2013, 111, 20–42. [Google Scholar] [CrossRef]
- Liu, K.; Song, C.; Subramani, V. Hydrogen and Syngas Production and Purification Technologies; John Wiley & Sons Incorporation: Hoboken, NJ, USA, 2010. [Google Scholar]
- Koros, W.J.; Mahajan, R. Pushing the limits on possibilities for large scale gas separation: Which strategies? J. Membr. Sci. 2000, 175, 181–196. [Google Scholar] [CrossRef]
- Baker, R.W.; Cussler, E.L.; Eykamp, W.; Koros, W.J.; Strathmann, H. Membrane Separation System: Recent Developments and Future Directions; Noyes Data Corp: Park Ridge, NJ, USA, 1991. [Google Scholar]
- Huang, X.; Yao, H.; Cheng, Z. Hydrogen Separation Membranes of Polymeric Mateirals, Nanostructured Materials for Next-Generation Energy Storage and Conversion; Springer: Berlin, Germany, 2017. [Google Scholar]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Brinkmann, S.; Shishatskiy, S. Hydrogen Separation with Polymeric Membranes, Hydrogen Science and Engineering: Materials, Processes, Systems and Technology, 1st ed.; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2016. [Google Scholar]
- Perry, J.D.; Nagai, K.; Koros, W.J. Polymer Membranes for Hydrogen Separations. MRS Bull. 2006, 31, 745–749. [Google Scholar] [CrossRef]
- Shao, L.; Low, B.T.; Chung, T.S.; Greenberg, A.R. Polymeric membranes for the hydrogen economy: Contemporary approaches and prospects for the future. J. Membr. Sci. 2009, 327, 18–31. [Google Scholar] [CrossRef]
- Peng, N.; Widjojo, N.; Sukitpaneenit, P.; Teoh, M.M.; Lipscomb, G.G.; Chung, T.S.; Lai, J.-Y. Evolution of polymeric hollow fibers as sustainable technologies: Past, present, and future. Prog. Polym. Sci. 2012, 37, 1401–1424. [Google Scholar] [CrossRef]
- Li, D.F.; Chung, T.S.; Wang, R.; Liu, Y. Fabrication of fluoropolyimide/polyethersulfone (PES) dual-layer asymmetric hollow fiber membrane for gas separation. J. Membr. Sci. 2002, 198, 211–223. [Google Scholar] [CrossRef]
- Wan, C.F.; Yang, T.; Lipscomb, G.G.; Stookey, D.J.; Chung, T.S. Design and fabrication of hollow fiber membrane modules. J. Membr. Sci. 2017, 538, 96–107. [Google Scholar] [CrossRef]
- Kroschwitz, J.I. Encyclopedia of Polymer Science and Engineering; Jon Wiley & Sons: New York, NY, USA, 1990. [Google Scholar]
- Abetz, V.; Brinkmann, T.; Dijkstra, M.; Ebert, K.; Fritsch, D.; Ohlrogge, K.; Paul, D.; Peinemann, K.V.; Nunes, S.P.; Scharnagl, N.; et al. Developments in Membrane Research: From Material via Process Design to Industrial Application. Adv. Eng. Mater. 2006, 8, 328–358. [Google Scholar] [CrossRef]
- Chiou, J.S.; Maeda, Y.; Paul, D.R. Gas separation in polyethersulfone. J. Appl. Polym. Sci. 1987, 33, 1823–1828. [Google Scholar] [CrossRef]
- Aitken, C.L.; Koros, W.J.; Paul, D.R. Effect of Structural Symmetry on Gas Transport Properties of Polysulfones. Macromoleculars 1992, 25, 3424–3434. [Google Scholar] [CrossRef]
- Orme, C.J.; Stone, M.L.; Benson, M.T.; Peterson, E.S. Testing of polymer membranes for the selective permeability of hydrogen. Sep. Sci. Technol. 2003, 38, 3225–3238. [Google Scholar] [CrossRef]
- Roberson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Roberson, L.M. The upper bone revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Shishatskiy, S.; Nistor, C.; Popa, M.; Nunes, S.P.; Peinemann, K.V. Polyimide asymmetric membranes for hydrogen separation: Influence of formation conditions on gas transport properties. Adv. Eng. Mater. 2006, 8, 390–397. [Google Scholar] [CrossRef]
- Low, B.T.; Xiao, Y.; Chung, T.S.; Liu, Y. Simultaneous occurrence of chemical grafting, crosslinking and etching on the surface of polyimide membranes and their impact on H2/CO2 separation. Macromolecules 2008, 41, 1297–1309. [Google Scholar] [CrossRef]
- Li, X.; Singh, R.P.; Dudeck, K.W.; Berchtold, K.A.; Benicewicz, B.C. Influence of polybenzimidazole main chain structure on H2/CO2 separation at elevated temperatures. J. Membr. Sci. 2014, 461, 59–68. [Google Scholar] [CrossRef]
- Pesiri, D.R.; Jorgensen, B.R.; Dye, C. Thermal optimization of polybenzimidazole meniscus membranes for the separation of hydrogen, methane, and carbon dioxide. J. Membr. Sci. 2003, 218, 11–18. [Google Scholar] [CrossRef]
- Bondar, V.I.; Freeman, B.D.; Pinnau, I. Gas transport properties of poly (ether-b-amide) segmented block copolymers. J. Polym. Sci. B Polym. Phys. 2000, 38, 2051–2062. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K-V. Tailor-made polymeric membranes based on segmented block copolymers for CO2 separation. Adv. Funct. Mater. 2008, 18, 2815–2823. [Google Scholar] [CrossRef]
- Budd, P.M.; Msayib, K.J.; Tattershall, C.E.; Ghanem, B.S.; Reynolds, K.J.; McKeown, N.B.; Fritsch, D. Gas separation membranes from polymers of intrinsic microporosity. J. Membr. Sci. 2005, 251, 263–269. [Google Scholar] [CrossRef]
- Carta, M.; Malpass-Evans, R.; Croad, M.; Rogan, Y.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; McKeown, N.B. An efficient polymer molecular sieve for membrane gas separation. Science 2013, 339, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Blume, I.; Pinnau, I. Composite Membrane, Method of Preparation and Use. U.S. Patent No. 4,963,165, 16 October 1990. [Google Scholar]
- Hosseini, S.S.; Teoh, M.M.; Chung, T.S. Hydrogen separation and purification in membranes of miscible polymer blends with interpretation networks. Polymer 2008, 49, 1594–1603. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K.V. Pebax®/Polyethylene glycol blend thin film composite membranes for CO2 separation: Performance with mixed gases. Sep. Purif. Technol. 2008, 62, 110–117. [Google Scholar] [CrossRef]
- Shao, L.; Liu, L.; Cheng, S.X.; Huang, Y.D.; Ma, J. Comparison of diamino crosslinking in different polyimide solutions and membranes by precipitation observation and gas transport. J. Membr. Sci. 2008, 312, 174–185. [Google Scholar] [CrossRef]
- Tin, P.S.; Chung, T.S.; Liu, Y.; Wang, R.; Liu, S.L.; Pramoda, K.P. Effects of crosslinking modification on gas separation performance of Matrimid membranes. J. Membr. Sci. 2003, 225, 77–90. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Chung, T.S.; Liu, Y. Chemical cross-linking modification of polyimide membranes for gas separation. J. Membr. Sci. 2001, 189, 231–239. [Google Scholar] [CrossRef]
- Chung, T.S.; Shao, L.; Tin, P.S. Surface modification of polyimide membranes by diamines for H2 and CO2 separation. Macromol. Rapid Commun. 2006, 27, 998–1003. [Google Scholar] [CrossRef]
- Chung, T.S.; Jiang, L.Y.; Li, Y.; Kulprathiapanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Xiao, Y.; Chung, T.S. Poly-/metal-benzimidazole nano-composite membranes for hydrogen purification. Energy Environ. Sci. 2011, 4, 4171–4180. [Google Scholar]
- Yang, T.; Shi, G.M.; Chung, T.S. Symmetric and asymmetric zeolitic imidazolate frameworks (ZIFs)/polybenzimidazole (PBI) nanocomposite membranes for hydrogen purification at high temperatures. Adv. Energy. Mater. 2012, 2, 1358–1367. [Google Scholar] [CrossRef]
- Yang, T.; Chung, T.S. Room-temperature synthesis of ZIF-90 nanocrystals and the derived nano-composite membranes for hydrogen purification. J. Mater. Chem. A 2013, 1, 6081–6090. [Google Scholar] [CrossRef]
- Dong, G.; Li, H.; Chen, V. Challenges and opportunities for mixed-matrix membranes for gas sepration. J. Mater. Chem. A 2013, 1, 4610–4630. [Google Scholar] [CrossRef]
- Henis, J.M.S.; Tripodi, M.K. Multicomponent Membranes for Gas Separations. U.S. Patent 4,230,463, 28 October 1980. [Google Scholar]
- Makino, H.; Kusuki, Y.; Yoshida, H.; Nakamura, A. Process for Preparing Aromatic Polyimide Semipermeable Membranes. U.S. Patent 4.378,324, 29 March 1983. [Google Scholar]
- Schell, W.J.; Houston, C.D. Process Gas with Selective Membranes. Chem. Eng. Prog. 1982, 78, 33–37. [Google Scholar]
- Swaidan, R.; Ghanem, B.; Litwiller, E.; Pinnau, I. Physical aging, plasticzation and their effects on gas permeation in “Rigid” polymers of intrinsic microporosity. Macromolecules 2015, 48, 6553–6561. [Google Scholar] [CrossRef]
- Conde, J.J.; Marono, M.; Sanchez-Hervas, J.M. Pd-based Membranes for Hydrogen Separation: Review of Alloying Elements and Their Influence on Membrane Properties. Sep. Pruif. Rew. 2017, 46, 152–177. [Google Scholar] [CrossRef]
- Yun, S.; Oyama, S.T. Correlations in Palladium membranes for hydrogen separation: A review. J. Membr. Sci. 2011, 375, 28–45. [Google Scholar] [CrossRef]
- LI, H.; Caravella, A.; Xu, H.Y. Recent progress in Pd-based composite membranes. J. Mater. Chem. A 2016, 4, 14069–14094. [Google Scholar] [CrossRef]
- Gryaznow, V. Membrane catalysis. Catal. Today 1999, 51, 391–395. [Google Scholar] [CrossRef]
- Lewis, F.A. The Palladium Hydrogen System; Academic Press: New York, NY, USA, 1967. [Google Scholar]
- Al-Mufachi, N.A.; Rees, N.V.; Steinberger-Wilkens, R. Hydrogen selective membranes: A review of palladium-based dense metal membranes. Renew. Sustain. Energy Rev. 2015, 47, 540–551. [Google Scholar] [CrossRef]
- Jun, C.S.; Lee, K.H. Palladium and palladium alloy composite membranes prepared by metal-organic chemical vapor deposition method (cold-wall). J. Membr. Sci. 2000, 176, 121–130. [Google Scholar] [CrossRef]
- Xomeritakis, G.; Lin, Y.S. Fabrication of thin metallic membranes by MOCVD and sputtering. J. Membr. Sci. 1997, 133, 217–230. [Google Scholar] [CrossRef]
- Paglieri, S.N.; Way, J.D. Innovations in palladium membrane research. Sep. Purif. Rew. 2002, 31, 1–169. [Google Scholar] [CrossRef]
- Melendez, J.; Fernandez, E.; Gallucci, F.; Annaland, M.V.S.; Arias, P.L.; Tanaka, D.A.P. Preparation and characterization of ceramic supported ultra-thin (~1 µm) Pd-Ag membranes. J. Membr. Sci. 2017, 528, 12–23. [Google Scholar] [CrossRef]
- Nam, S.E.; Seong, Y.K.; Lee, J.W.; Lee, K.H. Preparation of highly stable palladium alloy composite membranes for hydrogen separation. Desalination 2009, 236, 51–55. [Google Scholar] [CrossRef]
- Uemiya, S.; Sato, N.; Ando, H.; Kude, Y.; Matsuda, T.; Kikuchi, E. Separation of hydrogen through palladium thin film supported on a porous glass tube. J. Membr. Sci. 1991, 56, 303–313. [Google Scholar] [CrossRef]
- Zhao, H.; Pflanz, K.; Gu, J.; Li, A.; Stroh, N.; Brunner, H.; Xiong, G. Preparation of palladium composite membranes by modified electronless plating procedure. J. Membr. Sci. 1998, 42, 147–157. [Google Scholar] [CrossRef]
- Hunter, J.B. Silver-Palladium Film for Separation and Purification of Hydrogen. U.S. Patent 2,773,561, 11 December 1956. [Google Scholar]
- Bosko, M.L.; Lambardo, E.A.; Cornaglia, L.M. The effect of electronless plating time on the morphology, alloy formation and H2 transport properties of Pd-Ag composite membranes. Int. J. Hydrogen Energy 2011, 36, 4068–4078. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, G.; Yang, W. Hydrogen separation from the mixtures in a thin Pd-Cu alloy membrane reactor. Stud. Surf. Sci. Catal. 2007, 167, 219–224. [Google Scholar]
- Zhang, K.; Way, J.D. Palladium-copper membranes for hydrogen separation. Sep. Purif. Technol. 2017, 186, 39–44. [Google Scholar] [CrossRef]
- Roa, F.; Block, M.J.; Way, J.D. The influence of alloy composition on the H2 flux of composite PdCu membranes. Desalination 2002, 147, 411–416. [Google Scholar] [CrossRef]
- Shen, Y.; Emerson, S.C.; Magdefrau, N.J.; Opalka, S.M.; Thibaud-Erkey, C.; Vanderspurt, T.H. Hydrogen permeability of sulfur tolerant Pd-Cu alloy membranes. J. Membr. Sci. 2014, 452, 203–211. [Google Scholar]
- Flanagan, T.B.; Wang, D. Hydrogen Permeation through fcc Pd-Au Alloy Membranes. J. Phys. Chem. C 2011, 115, 11618–11623. [Google Scholar] [CrossRef]
- Braun, F.; Tarditi, A.M.; Miller, J.B.; Cornaglia, L.M. Pd-based binary and ternary alloy membranes: Morphological and perm-selectivity characterization in the present of H2S. J. Membr. Sci. 2014, 450, 299–307. [Google Scholar] [CrossRef]
- Maneerung, T.; Hidajat, K.; Kawi, S. Ultra-thin (<1 µm) internally-coated Pd-Ag alloy hollow fiber membrane with superior thermal stability and durability for high temperature H2 separation. J. Membr. Sci. 2014, 452, 127–142. [Google Scholar] [CrossRef]
- Yu, J.; Qi, C.; Zhang, J.; Bao, C.; Xu, H. Synthesis of a zeolite membrane as a protective layer on a metallic Pd composite membrane for hydrogen purification. J. Mater. Chem. A 2015, 3, 5000–5006. [Google Scholar] [CrossRef]
- Lin, Y.S. Microporous and dense inorganic membranes: Current status and prospective. Sep. Purif. Technol. 2011, 25, 39–55. [Google Scholar] [CrossRef]
- Li, H.; Haas-Santo, K.; Schygulla, U.; Dittmeyer, R. Inorganic microporous membranes for H2 and CO2 separation—Review of experimental and modeling progress. Chem. Eng. Sci. 2015, 127, 401–417. [Google Scholar] [CrossRef]
- Cronstedt, A.F. Ron och beskriting om en obekant barg ant, som kallas zeolites. Akad Handl Stackholm 1756, 17, 120–130. [Google Scholar]
- International Zeolite Association. Available online: http://www.iza-online.org/ (accessed on 23 July 2016).
- Auerbach, S.M.; Carrado, K.A.; Dutta, P.K. Handbook of Zeolite Science and Technology; Marcel Dekker Inc.: New York, NY, USA, 2003. [Google Scholar]
- Kulprathipanja, S. Zeolites in Industrial Separation and Catalysis, Focus on Catalysts; Viley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Regli, L.; Zecchina, A.; Vitillo, J.G.; Cocina, D.; Spoto, G.; Lamberti, C.; Lillerud, K.P.; Olsbye, U.; Bordiga, S. Hydrogen storage in Chabazite zeolite frameworks. Phys. Chem. Chem. Phys. 2005, 7, 3197–3203. [Google Scholar] [CrossRef] [PubMed]
- Mcleary, E.E.; Jansen, J.C.; Kapteijn, F. Zeolite based films, membranes and membrane reactors: Process and prospects. Microporous Mesoporous Mater. 2006, 90, 198–220. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M. Zeolite membranes—Recent developments and progress. Microporous Mesoporous Mater. 2008, 115, 215–233. [Google Scholar] [CrossRef]
- Kosinov, N.; Gascon, J.; Kapteijn, F.; Hensen, E.J.M. Recent developments in zeolite membranes for gas separation. J. Membr. Sci. 2016, 499, 65–79. [Google Scholar] [CrossRef]
- Dong, J.; Lin, Y.S. In situ synthesis of P-type zeolite membrane on porous α-alumina supports. Ind. Eng. Chem. Res. 1998, 37, 2404–2409. [Google Scholar] [CrossRef]
- Caro, J. Are MOF membranes better in gas separation than those made of zeolites? Curr. Opin. Chem. Eng. 2011, 1, 77–83. [Google Scholar] [CrossRef]
- Wang, H.; Lin, Y.S. Synthesis and modification of ZSM-5/silicate bilayer membrane with improved hydrogen separation performance. J. Membr. Sci. 2012, 396, 128–137. [Google Scholar] [CrossRef]
- Dong, X.; Wang, H.; Rui, Z.; Lin, Y.S. Tubular dual-layer MFI zeolite membrane reactor for hydrogen production via the WGS reaction: Experimental and modeling studies. Chem. Eng. J. 2015, 268, 219–229. [Google Scholar] [CrossRef]
- Kim, S-J.; Yang, S.; Reddy, G.K.; Smirniotis, P.; Dong, J. Zeolite Membrane Reactor for High Temperature Water Gas Shift Reaction: Effect of Membrane Properties and Operating Conditions. Energy Fuels 2013, 27, 4471–4480. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.; Hong, Z.; Gu, X.; Xu, N. Hydrogen-selective zeolite membrane reactor for low temperature water gas shift reation. Chem. Eng. J. 2012, 197, 314–321. [Google Scholar] [CrossRef]
- Poshusta, J.C.; Tuan, V.A.; Pape, E.A.; Noble, R.D.; Falconer, J.L. Separation of light gas mixtures using SAPO-34 membranes. AIChE J. 2000, 46, 779–789. [Google Scholar] [CrossRef]
- Hong, M.; Li, S.; Falconer, J.L.; Noble, R.D. Hydrogen purification using a SAPO-34 membrane. J. Membr. Sci. 2008, 307, 277–283. [Google Scholar] [CrossRef]
- Xu, X.; Yang, W.; Liu, J.; Lin, L. Synthsis of a high-permeance NaA zeolite membrane by microwave heating. Adv. Mater. 2000, 12, 195–198. [Google Scholar] [CrossRef]
- Michalkiewicz, B.; Koren, Z.C. Zeolite membranes for hydrogen production from nature gas: State of the art. J. Porous Mater. 2015, 22, 635–646. [Google Scholar] [CrossRef]
- Yang, S.; Cao, Z.; Arvanitis, A.; Sun, X.; Xu, Z.; Dong, J. DDR-type zeolite membrane synthesis, modification and gas permeation studies. J. Membr. Sci. 2016, 505, 194–204. [Google Scholar] [CrossRef]
- Huang, A.; Liang, F.; Steinbach, F.; Gesing, T.M.; Caro, J. Neutral and cation-free LTA-type aluminophosphate (AlPO4) molecular sieve membrane with high hydrogen permselectivity. J. Am. Chem. Soc. 2010, 132, 2040–2141. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; Li, H. Hydrothermal synthesis of a Meral-organic framework containing large rectangular channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, S.M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptional stable and highly porous metal organic framework. Nature 1999, 402, 276–279. [Google Scholar]
- Meek, S.T.; Greathouse, J.A.; Allendorf, M.D. Metal-organic frameworks: A rapidly growing class of versatile nanoporous materials. Adv. Mater. 2011, 23, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A.W.; Dubbeldam, D.; Liu, M.S.; Ladewig, B.P.; Hill, A.J.; Hill, M.R. Feasibility of zeolitic imidazolate framework membranes for clean energy applications. Energy Environ. Sci. 2012, 5, 7637. [Google Scholar] [CrossRef]
- Lee, J.Y.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.B.T.; Hupp, J.T. Metal organic framework materials as catalysts. Chem. Soc. Rew. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- James, S.L. Meral-organic frameworks. Chem. Soc. Rev. 2006, 32, 276–288. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Cote, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S. Metal organic framework membranes for separation applications. Curr. Opin. Chem. Eng. 2015, 8, 21–28. [Google Scholar] [CrossRef]
- Pan, Y.; Li, T.; Lestari, G.; Lai, Z. Effective separation of propylene/propane mixtures by ZIF-8 membranes. J. Membr. Sci. 2012, 390–391, 93–98. [Google Scholar] [CrossRef]
- Zhang, C.; Koros, W.J. Zeolitic Imidazolate Framework-Enabled Membranes: Challenges and Opportunities. J. Phys. Chem. Lett. 2015, 6, 3841–3849. [Google Scholar] [CrossRef] [PubMed]
- Melgar, V.M.A.; Kim, J.; Othman, M.R. A review of synthesis methods and gas separation performance. J. Ind. Eng. Chem. 2015, 28, 1–15. [Google Scholar] [CrossRef]
- Gomez-Alvarez, P.; Hamad, S.; Haranczyk, M.; Ruiz-Salvador, A.R.; Calero, S. Comparing gas separation performance between all known zeolites and their zeolitic imidazolate framework counterparts. Dalton Trans. 2016, 45, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Bux, H.; Liang, F.; Li, Y.; Cravillon, J.; Wiebcke, M.; Caro, J. Zeolitic imidazolate framework membrane with molecular sieving properties by microwave-assisted solvothermal synthesis. J. Am. Chem. Soc. 2009, 131, 16000–16001. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-S.; Bux, H.; Feldhoff, A.; Li, G.-L.; Yang, W.-S. Controllable synthesis of Metal-Organic Frameworks: From MOF nanorods to oriented MOF memebranes. Adv. Mater. 2010, 22, 3322–3326. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.C.; Varela-Guerrero, V.; Barnett, G.V.; Jeong, H.-K. Synthesis of Zeolitic Imidazolate Framework Films and Membranes with Controlled Microstructures. Langmuir 2010, 26, 14636–14641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lively, R.P.; Zhang, K.; Johnson, J.R.; Karvan, O.; Koros, W.J. Unexpected molecular sieving properties of zeolitic imidazolate framework-8. J. Phys. Chem. Lett. 2012, 3, 2130–2134. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, Y.; Zeng, G.; Zhao, L.; Lai, Z. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Lai, Z. Sharp separation of C2/C3 hydrocarbon mixtures by zeolitic imidazolate fremawork-8 (ZIF-8) membranes synthesized in aqueous solutions. Chem. Commun. 2011, 47, 10275–10277. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, B.; Lai, Z. Synthesis of ceramic hollow fiber support zeolitic imidazolate framework-8 (ZIF-8) membranes with high hydrogen permeability. J. Membr. Sci. 2012, 421–422, 292–298. [Google Scholar] [CrossRef]
- Li, Y.; Wee, L.H.; Volodin, A.; Martens, J.A.; Vankelecom, F.J. Polymer supported ZIF-8 membranes prepared via an interfacial synthesis method. Chem. Commun. 2015, 51, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, F.; Bux, H.; Yang, W.; Caro, J. Zeolitic imidazolate frameworks ZIF-7 based molecular sieve membrane for hydrogen separation. J. Membr. Sci. 2010, 354, 48–54. [Google Scholar] [CrossRef]
- Huang, A.; Bux, H.; Steinbach, F.; Caro, J. Molecular-sieve membrane with hydrogen permselectivity: ZIF-22 in LTA topology prepared with 3-Aminopropyltriethoxysilane as covalent linker. Angew. Chem. 2010, 122, 5078–5081. [Google Scholar] [CrossRef]
- Huang, A.; Dou, W.; Caro, J. Steam-stable zeoliteic imidazolate framework ZIF-90 membrane with hydrogen selectivity through covalent functionalization. J. Am. Chem. Soc. 2010, 132, 15562–15564. [Google Scholar] [CrossRef] [PubMed]
- Nan, J.; Dong, X.; Wang, W.; Jin, W.; Xu, N. Step-by-step procedure for preparing HKUST-1 membrane on porous α-Alumina support. Langmuir 2011, 27, 4309–4312. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ng, Z.; Khan, E.A.; Jeong, H.-K.; Ching, C.-B.; Lai, Z. Synthesis of continuous MOF-5 membranes on Porous α-Alumina substrates. Microporous Mesoporous Mater. 2009, 118, 296–301. [Google Scholar] [CrossRef]
- Yin, H.; Kim, H.; Choi, J.; Yip, A.C.K. Thermal stability of ZIF-8 under oxidative and inert environments: A practical perspective on using ZIF-8 as a catalyst support. Chem. Eng. J. 2015, 278, 293–300. [Google Scholar] [CrossRef]
- Yin, H.; Lee, T.; Choi, J.; Yip, A.C.K. On the zeolitic imidazolate framework-8 (ZIF-8) membrane for hydrogen separation from simulated biomass-derived syngas. Microporous Mesoporous Mater. 2016, 233, 70–77. [Google Scholar] [CrossRef]
- Cai, W.; Lee, T.; Lee, M.; Cho, M.; Han, D-Y.; Choi, N.; Yip, A.C.K.; Choi, J. Thermal structural transitions and carbon dioxide adsorption properties of zeolitic imidazolate framework-7 (ZIF-7). J. Am. Chem. Soc. 2014, 136, 7961–7971. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, Y.; Ban, Y.; Jin, H.; Jiao, W.; Liu, X.; Yang, W. Metal-organic framework nanosheets as building blocks for molecular sieving membranes. Science 2014, 346, 1356–1359. [Google Scholar] [CrossRef] [PubMed]
- Khatib, S.J.; Oyama, S.T.; Souza, K.R.D.; Noronha, F.B. Review of silica membranes for hydrogen separation prepared by chemical vapour deposition. Inorg. Polym. Compos. Membr. 2011, 14, 25–60. [Google Scholar]
- Costa, D.C.D.; Lu, G.Q.; Rudolph, V.; Lin, Y.S. Novel molecular sieve silica (MSS) membranes: Characterisation and permeation of single step and two step sol-gel membranes. J. Membr. Sci. 2002, 198, 9–21. [Google Scholar] [CrossRef]
- Vos, R.M.D.; Verweij, H. High-selectivity, high-flux silica membranes for gas separation. Science 1998, 279, 1710–1711. [Google Scholar] [CrossRef] [PubMed]
- Tsuru, T.; Igi, R.; Kanezashi, M.; Yoshioka, T.; Fujisaki, S.; Iwamoto, Y. Permeation properties of hydrogen and water vapour through porous silica membranes at high temperatures. AIChE 2011, 57, 618–629. [Google Scholar] [CrossRef]
- Battersby, S.; Duke, M.C.; Liu, S.; Rudolph, V.; Costa, J.D.D. Metal doped silica membrane reactor: Operational effects of reaction and permeation for the water gas shift reaction. J. Membr. Sci. 2008, 316, 46–52. [Google Scholar] [CrossRef]
- Giessler, S.; Jordan, L.; Costa, J.C.D.; Lu, G.Q.M. Performance of hydrophobic and hydropholic silica membrane reactors for the water gas shift reaction. Sep. Purif. Technol. 2003, 32, 255–264. [Google Scholar] [CrossRef]
- Ismail, A.F.; David, L.I.B. A review on the latest development of carbon membranes for gas separation. J. Membr. Sci. 2001, 193, 1–18. [Google Scholar] [CrossRef]
- Gallucci, F.; Basile, A.; Iulianelli, A.; Kuipers, J.A.M. A review on patents for hydrogen production using membrane reactors. Rec. Patents Chem. Eng. 2009, 2, 207–222. [Google Scholar] [CrossRef]
- Julbe, A.; Farrusseng, D.; Guizard, C. Porous ceramic membranes for catalytic reactors—Overview and new ideas. J. Membr. Sci. 2001, 18, 3–20. [Google Scholar] [CrossRef]
- Basile, A.; Criscuoli, A.; Santella, F.; Drioli, E. Membrane reactor for water gas shift reaction. Gas Sep. Purif. 1996, 10, 243–254. [Google Scholar] [CrossRef]
- Gosiewski, K.; Warmuzinski, K.; Tanczyk, M. Mathematical simulation of WGS membrane reactor for gas from coal gasification. Catal. Today 2010, 156, 229–236. [Google Scholar] [CrossRef]
- Brunetti, A.; Barbieri, G.; Drioli, E.; Lee, K.-H.; Sea, B.; Lee, D.-W. WGS Reaction in a Membrane Reactor Using a Porous Stainless Steel Supported Silica Membrane. Chem. Eng. Process. Proc. Intensif. 2007, 46, 119–126. [Google Scholar] [CrossRef]

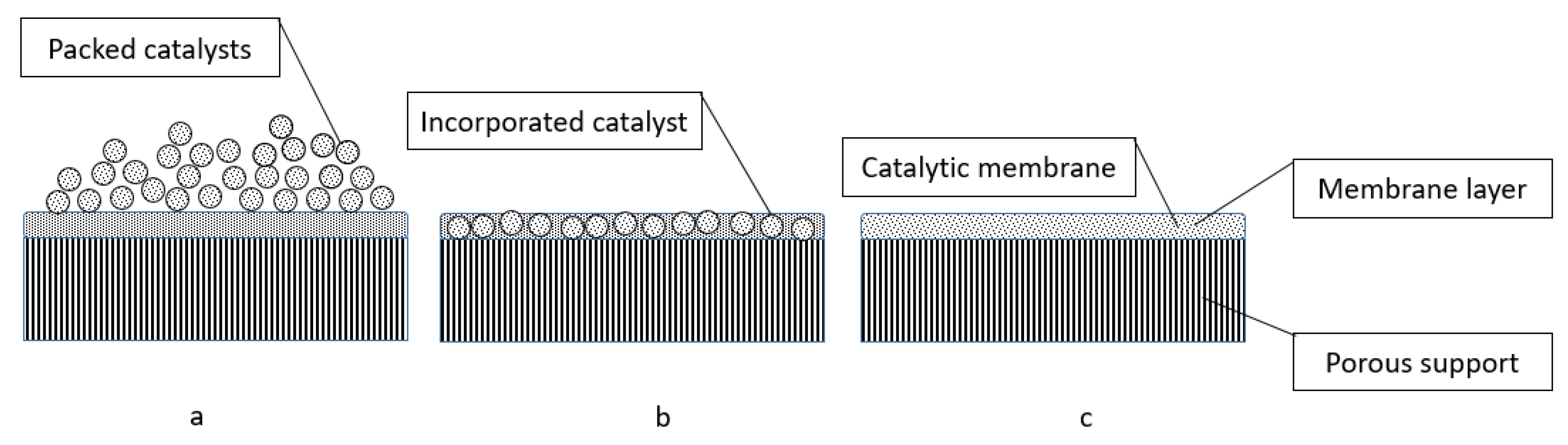
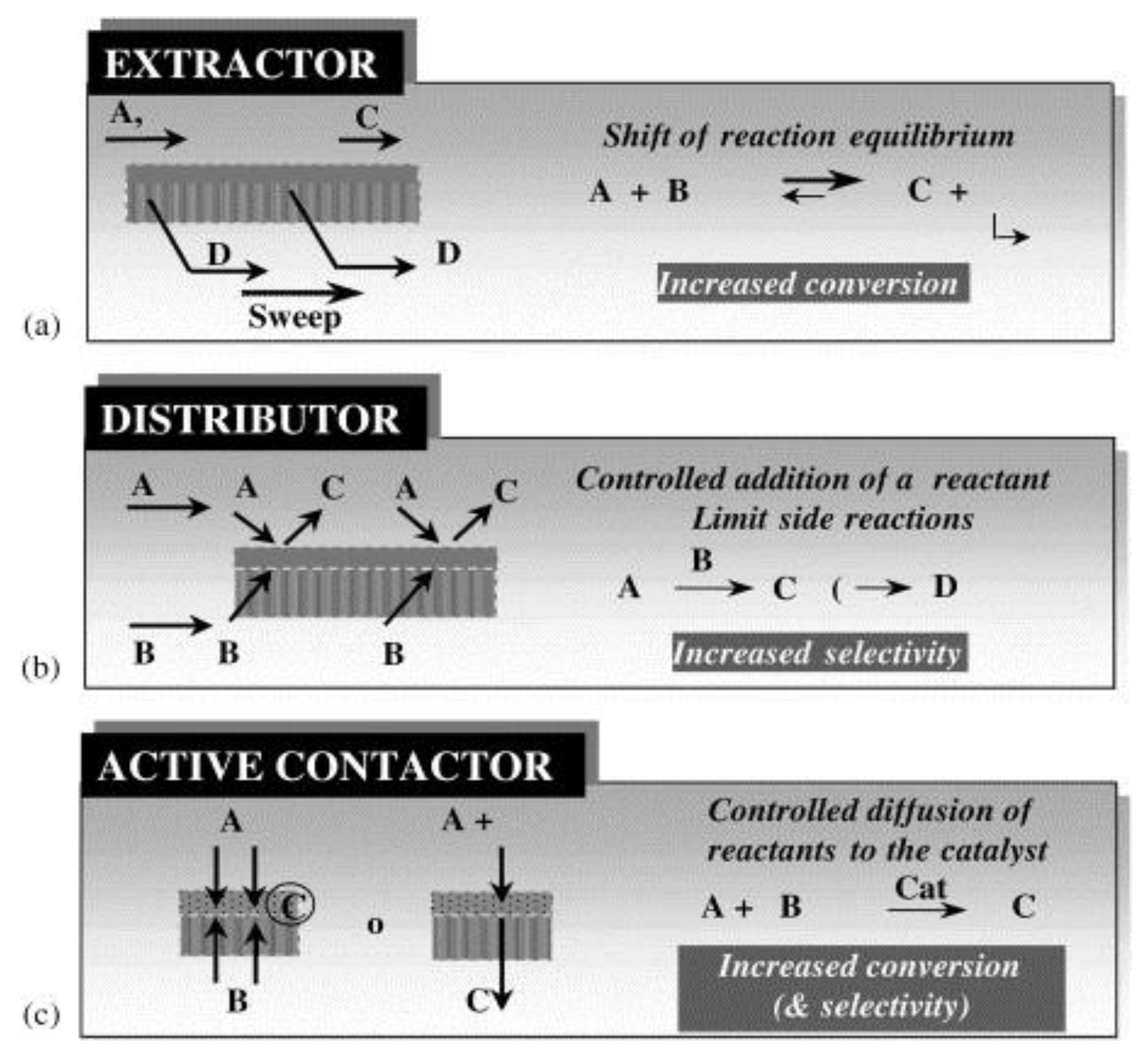
| Parameters | Membrane Type | ||
|---|---|---|---|
| Polymeric | Microporous | Dense Metal | |
| Typical composition | Polyimide; Cellulose acetate | Silica; Zeolites; Metal-organic frameworks | Palladium; Palladium alloys |
| Diffusion mechanism | Solution diffusion | Molecular sieving | Solution diffusion |
| Driving force † | Partial pressure difference | Partial pressure difference | Partial pressure difference |
| Operation temperature | ≤110 °C | ≤1000 °C | 150–700 °C |
| Relative permeability | Low—moderate | Moderate–high | Low |
| Typical selectivity | Moderate | Low—moderate | Very high |
| Relative cost | Low | Low—moderate | Moderate—High |
| (a) | |||||
| Polymers | T (°C) | P (atm) | Permeability (Barrer a) | H2/CO2 Selectivity | |
| H2 | CO2 | ||||
| Ethyl Cellulose | 30 | - | 87 | 26.5 | 3.28 |
| Polyetherimide | 30 | - | 7.8 | 1.32 | 5.91 |
| Polyphenyleneoxide | 30 | - | 113 | 75.8 | 1.49 |
| Polysulfone | 30 | - | 14 | 5.6 | 2.50 |
| Polymethylpentene | 30 | - | 125 | 84.6 | 1.48 |
| Polyimide (Matrimid®) | 30 | - | 28.1 | 10.7 | 2.63 |
| Polyethersulfone | 35 | 3.5 | 8.96 | 3.38 | 2.65 |
| Polystyrene | 30 | 1.36 | 23.8 | 10.4 | 2.29 |
| Poly(vinylidene fluoride) (Kynar) | 30 | 1.36 | 2.4 | 1.2 | 2.00 |
| Poly(methyl methacrylate) | 30 | 1.36 | 2.4 | 0.6 | 4.00 |
| (b) | |||||
| Polymers | T (°C) | P (atm) | Permeability (Barrer b) | CO2/H2 Selectivity | |
| CO2 | H2 | ||||
| Polytrimethylsilylpropyne | 25 | - | 79 | 36.1 | 2.19 |
| Poly(4-methyl-2-pentyne) | 25 | - | 10700 | 5800 | 1.84 |
| Polyphosphazene | 30 | 2.04 | 250 | 25 | 10.00 |
| Poly(tert-butylacetylene) | 25 | 1 | 560 | 300 | 1.87 |
| Poly(amide-6-b-ethylene oxide) | 25 | 4 | 132 | 20 | 6.60 |
| Poly(amide-6-b-ethylene oxide) Pebax® [51] | 35 | - | 220 | 22 | 10 |
| Poly(ethylene glycol) diacrylate | 23 | 12 | - | 83 | 11.00 |
| Crosslinked PEG copolymer b | 35 | 17 | - | - | 9.40 |
| Crosslinked PEG copolymer b | −20 | 17 | 410 | - | 31.00 |
| Polyether | - | - | 586 | 76.6 | 7.70 |
| Poly(styrene-co-butadiene) | 30 | - | 15.3 | 7.9 | 1.94 |
| poly(ethylene oxide)-poly(butylene terephthalate) [52] | 30 | - | 190 | - | 13 |
| Poly(dimethyl siloxane) (PDMS) c | 23 | 1.36 | 3200 | 950 | 3.36 |
| Supplier | Membrane Materials | Module Types | Selectivity | Reference | ||
|---|---|---|---|---|---|---|
| H2/N2 | H2/CH4 | H2/CO | ||||
| Air products | Polysulfone | Hollow fiber | 39 | 24 | 23 | [67] |
| Air liquid | Polyimide/polyaramide | Hollow fiber | - | - | - | [34] |
| Ube | Polyimide | Hollow fiber | 35.4 | - | 30 | [68] |
| UOP/Separex | Cellulose acetate | Spiral wound | 33 | 26 | 21 | [69] |
| Supplier | Material | Dimension Parameters | Applications | Performance |
|---|---|---|---|---|
| Hysep-Energy research centre of The Netherlands | Pd-Au/YSZ/SS | 0.04/0.1/0.5 m2 | Example: Coal to fuel project in New Zealand | 3.5-6 Nm3/h with 99.5–99.995% hydrogen at 21 Bar pressure difference, from 33% reforming H2 |
| Power + Energy/United Technologies Research Center | Pd-Cu trimetallic alloy | - | Coal gasification Syngas | 0.23 mol/m2 s with 99.9999% hydrogen |
| Tokyo Gas | Pd-Y(Gd)-Ag/SS | - | reforming | 40 Nm3/h with 99.99% hydrogen |
| CRI/Criterion-Shell | Pd and Pd-alloy | OD: 2 inch. L:48 inch | - | 40-70 Nm3/h m2 h1 bar0.5 with >99% hydrogen |
| REB | Pd and Pd-alloy | OD: 1/8 inch | fluidized bed membrane reactor | 0.2 mol/m2 s on Pd-Cu alloy membrane at 673K, 3.03 Bar of syngas conditions |
| Gas | Kinetic Diameter (Å) | Zeolites | Aperture Pore Size (Å) | MOFs and ZIFs | Aperture Pore Size (Å) |
|---|---|---|---|---|---|
| H2 | 2.89 | MFI | 5.4 | ZIF-7 | 3.0 |
| CO2 | 3.3 | NaA | 4.1 | ZIF-8 | 3.4 |
| N2 | 3.64 | SAPO-34 | 0.38 | ZIF-22 | 3.0 |
| CO | 3.76 | DDR | 3.6 × 4.4 | ZIF-90 | 3.5 |
| CH4 | 3.8 | NaP-GIS | 4.5 × 3.1 | MOF-5 | 15.6 |
| SF6 | 5.5 | AlPO4 | 4.0 | HKUST-1 | 9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, H.; Yip, A.C.K. A Review on the Production and Purification of Biomass-Derived Hydrogen Using Emerging Membrane Technologies. Catalysts 2017, 7, 297. https://doi.org/10.3390/catal7100297
Yin H, Yip ACK. A Review on the Production and Purification of Biomass-Derived Hydrogen Using Emerging Membrane Technologies. Catalysts. 2017; 7(10):297. https://doi.org/10.3390/catal7100297
Chicago/Turabian StyleYin, Hang, and Alex C.K. Yip. 2017. "A Review on the Production and Purification of Biomass-Derived Hydrogen Using Emerging Membrane Technologies" Catalysts 7, no. 10: 297. https://doi.org/10.3390/catal7100297
APA StyleYin, H., & Yip, A. C. K. (2017). A Review on the Production and Purification of Biomass-Derived Hydrogen Using Emerging Membrane Technologies. Catalysts, 7(10), 297. https://doi.org/10.3390/catal7100297





