Polycyclic Ketone Monooxygenase (PockeMO): A Robust Biocatalyst for the Synthesis of Optically Active Sulfoxides
Abstract
1. Introduction
2. Results and Discussion
2.1. PockeMO-Catalyzed Sulfoxidations
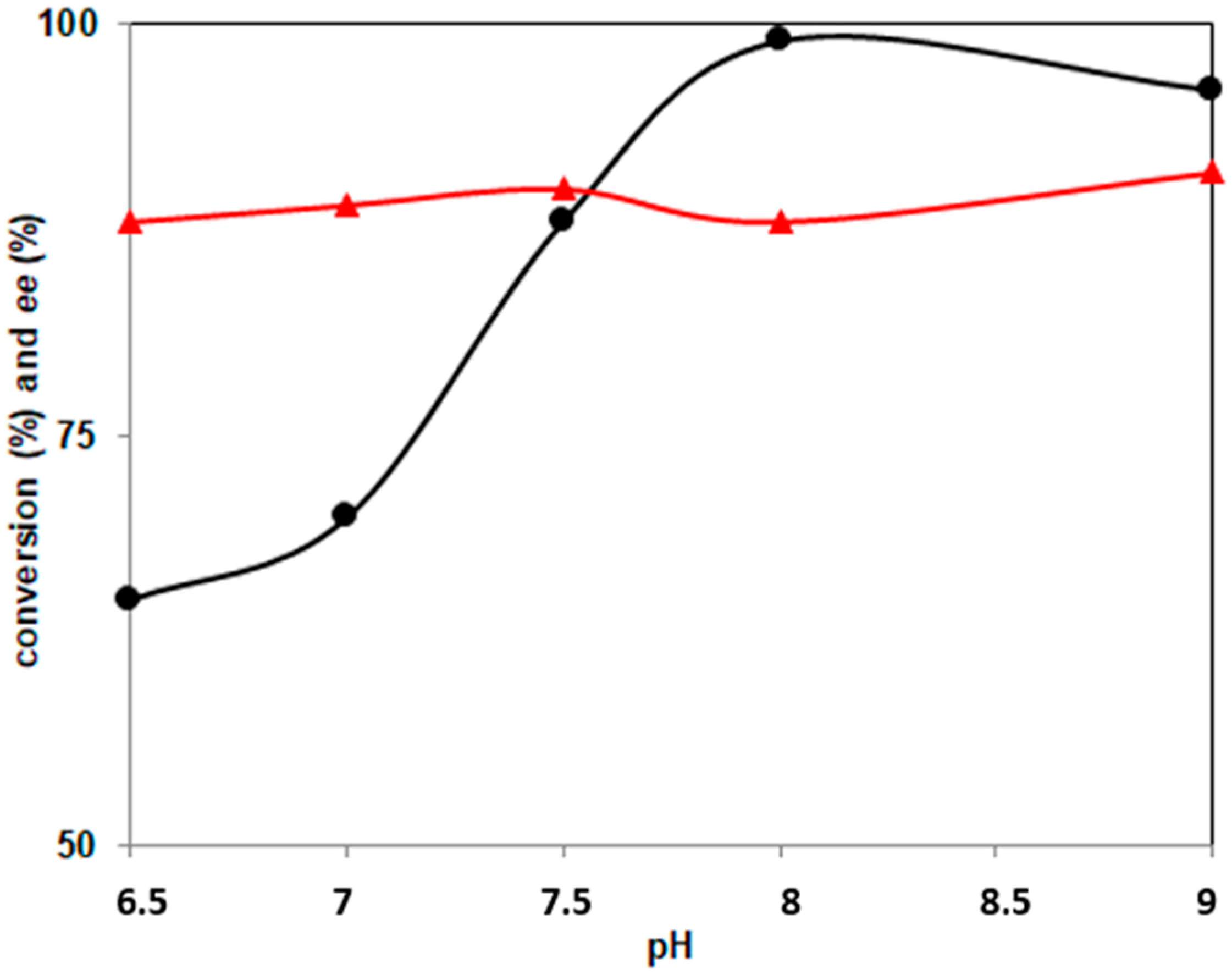
2.2. Effect of pH and Temperature on PockeMO-Catalyzed Sulfoxidations
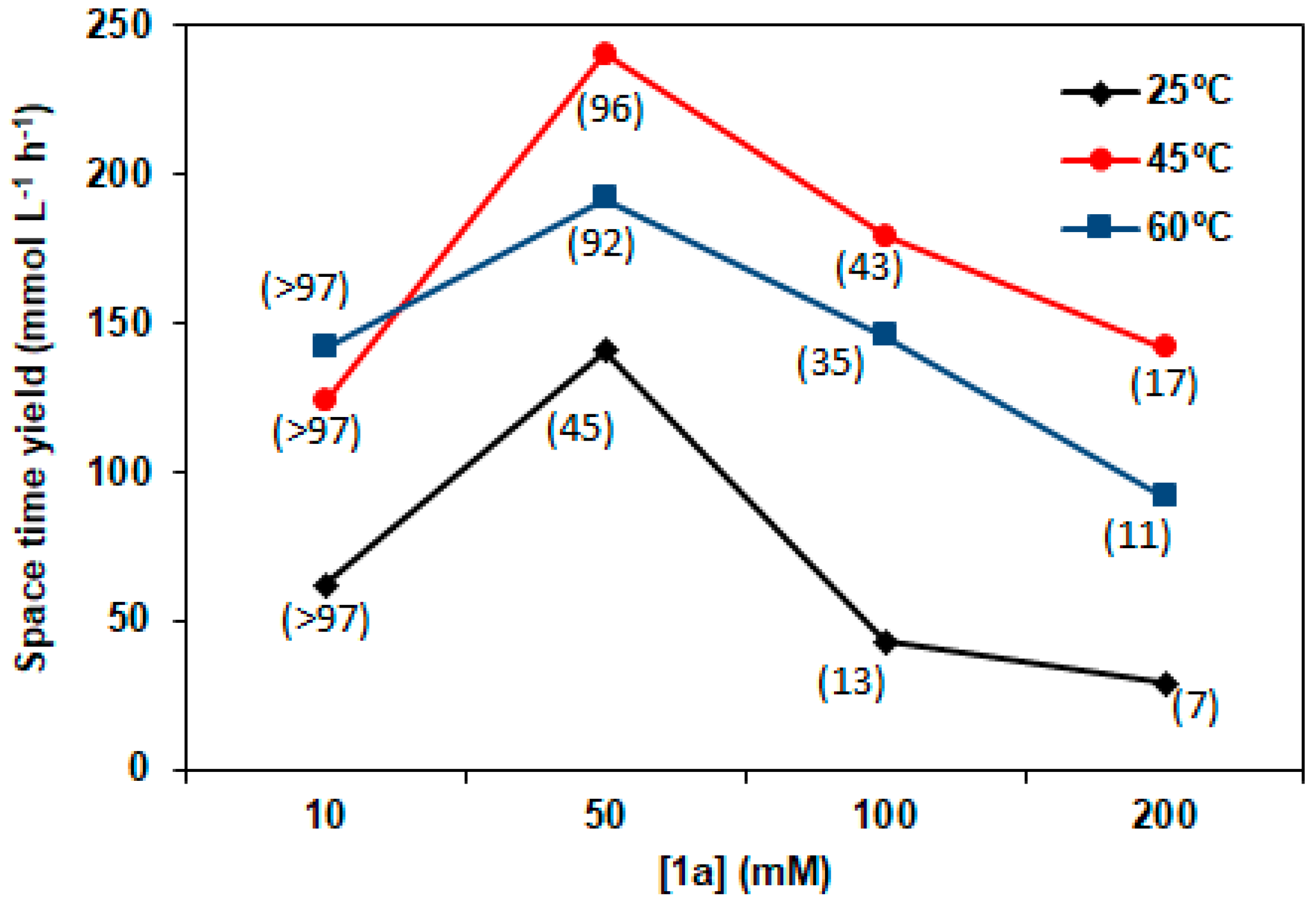
2.3. Sulfide Concentration Effect
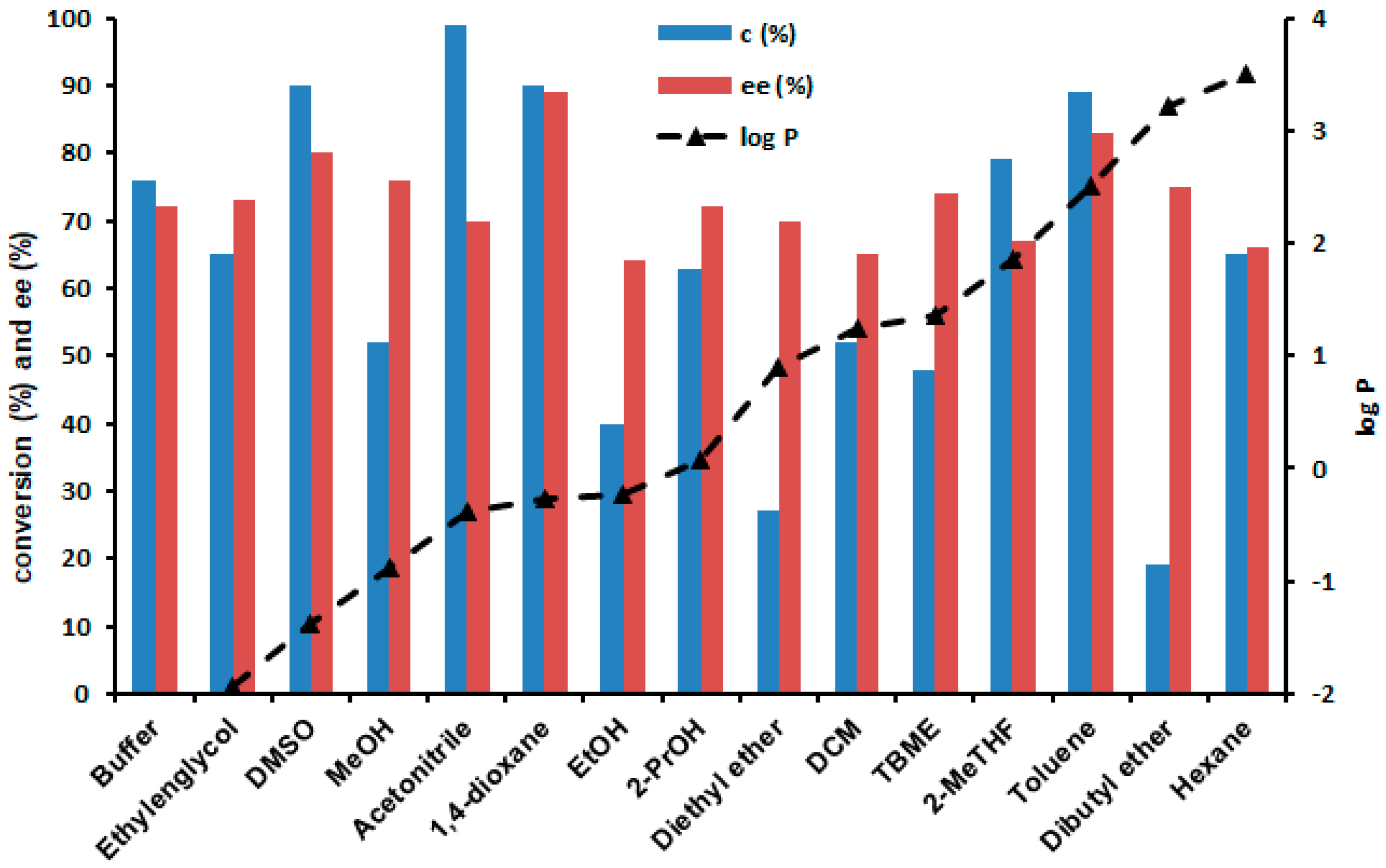
2.4. Enzymatic Sulfoxidations in the Presence of Cosolvents
3. Materials and Methods
3.1. General Materials and Methods
3.2. General Procedure for the Enzymatic Sulfoxidation of Prochiral Sulfides
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fürst, M.J.L.J.; Savino, S.; Dudek, H.M.; Gómez Castellanos, J.R.; Gutiérrez de Souza, C.; Rovida, S.; Fraaije, M.W.; Mattevi, A. Polycyclic Ketone Monooxygenase from the thermophilic fungus Thermoth elomyces thermophila: A structurally distinct biocatalyst for bulky substrates. J. Am. Chem. Soc. 2017, 139, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Pellisier, H. Use of chiral sulfoxides in asymmetric synthesis. Tetrahedron 2006, 65, 5559–5601. [Google Scholar] [CrossRef]
- Carreño, M.C.; Hernández Torres, G.; Ribagorda, M.; Urbano, A. Enantiopure sulfoxides: Recent applications in asymmetric synthesis. Chem. Commun. 2009, 41, 6129–6144. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Rao, M. Development of chiral sulfoxide ligands for asymmetric catalysis. Angew. Chem. Int. Ed. 2015, 54, 5026–5043. [Google Scholar] [CrossRef] [PubMed]
- Wojaczynska, E.; Wojaczynski, J. Enantioselective synthesis of sulfoxides: 2000–2009. Chem. Rev. 2010, 110, 4303–4356. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.-S.; Reeves, D.C.; Krishnamurthy, D.; Senanayake, C.H. Synthetically Derived Auxiliaries: Sulfur Derivatives (including Sulfilamines and Sulfoximines). In Comprehensive Chirality; Carreira, E.M., Yamamoto, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 3, pp. 560–600. [Google Scholar]
- Brondani, P.B.; de Gonzalo, G.; Fraaije, M.W. Recent developments in flavin-based catalysis: Enzymatic sulfoxidation. In Green Biocatalysis, 1st ed.; Patel, R.N., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; Volume 1, pp. 149–164. [Google Scholar]
- Matsui, T.; Dekishima, Y.; Ueda, M. Biotechnological production of chiral organic sulfoxides: Current state and perspectives. Appl. Microbiol. Biotechnol. 2014, 98, 7699–7706. [Google Scholar] [CrossRef] [PubMed]
- Ten Brink, H.B.; Holland, H.L.; Schoemaker, H.E.; van Lingen, H.; Wever, R. Probing the scope of the sulfoxidation activity of vanadium peroxidase from Ascophyllum nodosum. Tetrahedron Asymmetry 1999, 10, 4563–4572. [Google Scholar] [CrossRef]
- Pezzoti, F.; Okrasa, K.; Therisod, M. Bienzymatic synthesis of chiral heteroary-methyl-sulfoxides. Tetrahedron Asymmetry 2005, 16, 2681–2683. [Google Scholar] [CrossRef]
- Linde, D.; Canellas, M.; Coscolin, C.; Davo-Siguero, I.; Romero, A.; Lucas, F.; Ruiz-Duenas, F.J.; Guallar, V.; Martínez, A.T. Asymmetric sulfoxidation catalyzed by engineering the heme-pocket of a dye-decolorizing peroxidase. Catal. Sci. Technol. 2016, 6, 6277–6285. [Google Scholar] [CrossRef]
- Lee, K.; Brand, J.M.; Gibson, D.T. Stereospecific sulfoxidation by toluene and naphthalene dioxygenases. Biochem. Biophys. Res. Commun. 1995, 212, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Shainsky, J.; Bernath-Levin, K.; Isaschar-Ovdat, S.; Glaser, F.; Fishman, A. Protein engineering of nitrobenzene dioxygenase for enantioselective synthesis of chiral sulfoxides. Protein Eng. Des. Sel. 2013, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, A.T.; Zhang, J.D.; Xu, J.H.; Lu, W.Y.; Lin, G.Q. Isolation of Rhodococcus sp. strain ECU0066, a new sulfide monooxygenase producing strain for asymmetric sulfoxidation. Appl. Environ. Microbiol. 2009, 75, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Nikodinovic-Runic, J.; Coulombel, L.; Francuski, D.; Sharma, N.D.; Boyd, D.R.; Ferral, R.M.O.; O’Connor, K.E. The oxidation of alkylaryl sulfides and benzo[b]thiophenes by Escherichia coli cells expressing wild-type and engineered styrene monooxygenase from Pseudomonas putida CA-3. Appl. Microbiol. Biotechnol. 2013, 97, 4849–4857. [Google Scholar] [CrossRef] [PubMed]
- Colonna, S.; Gaggero, N.; Carrea, G.; Ottolina, G.; Pasta, P.; Zambianchi, F. First asymmetric epoxidation catalysed by cyclohexanone monooxygenase. Tetrahedron Lett. 2002, 43, 1797–1799. [Google Scholar] [CrossRef]
- Leisch, H.; Morley, K.; Lau, P.C.K. Baeyer-Villiger monooxygenases: More than just Green Chemistry. Chem. Rev. 2011, 111, 4165–4222. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo, G.; van Berkel, W.J.H.; Fraaije, M.W. Baeyer-Villiger oxidations. In Science of Synthesis, Biocatalysis, 1st ed.; Faber, K., Turner, N.J., Fessner, W.D., Eds.; Georg-Thieme Verlag: Stuttgart, Germany, 2015; Volume 3, pp. 187–234. [Google Scholar]
- Bucko, M.; Gemeiner, P.; Schenkmayerova, A.; Krajcovic, T.; Rudroff, F.; Mihovilovic, M.D. Baeyer-Villiger oxidations: Biotechnological approach. Appl. Microbiol. Biotechnol. 2016, 100, 6585–6599. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.D. Cyclohexanone monooxygenase: A useful reagent for asymmetric Baeyer-Villiger reactions. Curr. Org. Chem. 1998, 2, 195–216. [Google Scholar]
- Ottolina, G.; Pasta, P.; Carrea, G.; Colonna, S.; Dallavalle, S.; Holland, H.L. A predictive active site model for the cyclohexanone monooxygenase oxidation of sulfides to chiral sulfoxides. Tetrahedron Asymmetry 1995, 6, 1375–1386. [Google Scholar] [CrossRef]
- Carrea, G.; Redigolo, B.; Riva, S.; Colonna, S.; Gaggero, N.; Battistel, E.; Bianchi, D. Effects of substrate structure on the enantioselectivity and stereochemical course of the sulfoxidation catalysed by cyclohexanone monooxygenase. Tetrahedron Asymmetry 1992, 8, 1063–1068. [Google Scholar] [CrossRef]
- Colonna, S.; Pironti, V.; Zambianchi, F.; Ottolina, G.; Gaggero, N.; Celentano, G. Diastereoselective synthesis of β-hydroxy sulfoxides: Enzymatic and biomimetic approaches. Eur. J. Org. Chem. 2007, 363–368. [Google Scholar] [CrossRef]
- De Gonzalo, G.; Torres Pazmiño, D.E.; Ottolina, G.; Fraaije, M.W.; Carrea, G. 4-Hydroxyacetophenone monooxygenase from Pseudomonas fluorescens ACB as an oxidative biocatalyst in the synthesis of optically active sulfoxides. Tetrahedron Asymmetry 2006, 17, 130–135. [Google Scholar] [CrossRef]
- Rioz-Martínez, A.; de Gonzalo, G.; Torres Pazmiño, D.E.; Fraaije, M.W.; Gotor, V. Enzymatic synthesis of novel chiral sulfoxides employing Baeyer-Villiger monooxygenases. Eur. J. Org. Chem. 2010, 33, 6409–6416. [Google Scholar] [CrossRef]
- Rehdorf, J.; Zimmer, C.L.; Bornscheuer, U.T. Cloning, expression, characterization and biocatalytic investigation of 4-hydroxyacetophenone monooxygenase from Pseudomonas putida JD1. Appl. Environ. Microbiol. 2009, 75, 3106–3114. [Google Scholar] [CrossRef] [PubMed]
- Fraaije, M.W.; Kamerbeek, N.M.; Heidekamp, A.J.; Fortin, R.; Janssen, D.B. The prodrug activator EtaA from Mycobacterium tuberculosis is a Baeyer-Villiger monooxygenase. J. Biol. Chem. 2004, 279, 3354–3360. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo, G.; Ottolina, G.; Zambianchi, F.; Fraaije, M.W.; Carrea, G. Biocatalytic properties of Baeyer-Villiger monooxygenases in aqueous-organic media. J. Mol. Catal. B Enzym. 2006, 39, 91–97. [Google Scholar] [CrossRef]
- Riebel, A.; Dudek, H.M.; de Gonzalo, G.; Stepniak, P.; Rychlewski, L.; Fraaije, M.W. Expanding the set of rhodococcal Baeyer-Villiger monooxygenases by high-throughput cloning, expression and substrate screening. Appl. Microbiol. Biotechnol. 2012, 95, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Fraaije, M.W.; Wu, J.; Heuts, D.P.; van Hellemond, E.W.; Spelberg, J.H.; Janssen, D.B. Discovery of a thermostable Baeyer-Villiger monooxygenase by genome mining. Appl. Microbiol. Biotechnol. 2005, 66, 393–400. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo, G.; Torres Pazmiño, D.E.; Ottolina, G.; Fraaije, M.W.; Carrea, G. Oxidations catalyzed by phenylacetone monooxygenase from Thermobifida fusca. Tetrahedron Asymmetry 2005, 16, 3077–3083. [Google Scholar] [CrossRef]
- Torres Pazmiño, D.E.; Snajdrova, R.; Rial, D.V.; Mihovilovic, M.D.; Fraaije, M.W. Altering the substrate specificity and enantioselectivity of phenylacetone monooxygenase by structure-inspired enzyme redesign. Adv. Synth. Catal. 2007, 349, 1361–1368. [Google Scholar] [CrossRef]
- Dudek, H.M.; de Gonzalo, G.; Torres Pazmiño, D.E.; Stepniak, P.; Wyrwicz, L.S.; Rychlewski, L.; Fraaije, M.W. Mapping the substrate binding of phenylacetone monooxygenase from Thermobifida fusca by mutational analysis. Appl. Environ. Microbiol. 2011, 77, 5730–5738. [Google Scholar] [CrossRef] [PubMed]
- Johannes, T.W.; Woodyer, R.D.; Zhao, H. Efficient regeneration of NADPH using an engineered phosphite dehydrogenase. Biotechnol. Bioeng. 2007, 96, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Torres Pazmiño, D.E.; Snajdrova, R.; Baas, B.-J.; Ghobrial, M.; Mihovilovic, M.D.; Fraaije, M.W. Self-sufficient Baeyer—Villiger Monooxygenases: Effective coenzyme regeneration for biooxygenation by fusion engineering. Angew. Chem. Int. Ed. 2008, 47, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Torres Pazmiño, D.E.; Riebel, A.; de Lange, J.; Rudroff, F.; Mihovilovic, M.D.; Fraaije, M.W. Efficient biooxidations catalyzed by a new generation of self-sufficient Baeyer—Villiger monooxygenases. ChemBioChem 2009, 10, 2595–2598. [Google Scholar] [CrossRef] [PubMed]


| Sulfide | X | n | R1 | Time (h) | Conversion (%) b | % Sulfoxide c | ee (%) d | Configuration |
|---|---|---|---|---|---|---|---|---|
| 1a | H | 0 | Methyl | 16 | ≥97 | ≥97 | 91 | R |
| 2a | H | 0 | Ethyl | 24 | 76 | 94 | 72 | R |
| 3a | H | 0 | CH2CH2Cl | 24 | 27 | 81 | 76 | S e |
| 4a | p-cyano | 0 | Methyl | 24 | 43 | 88 | 91 | R |
| 5a | p-methoxy | 0 | Methyl | 24 | ≥97 | 94 | 93 | R |
| 6a | p-Cl | 0 | Methyl | 22 | 78 | ≥97 | 88 | R |
| 7a | m-Cl | 0 | Methyl | 20 | ≥97 | ≥97 | 79 | R |
| 8a | o-Cl | 0 | Methyl | 20 | 85 | 95 | 93 | R |
| 9a | H | 1 | Methyl | 16 | 70 | 82 | 68 | R |
| 10a | Phenyl | 0 | Methyl | 24 | 16 | 85 | 83 | R |
| 11a | H | 1 | Phenyl | 56 | 15 | ≥97 | 83 | R |
| Entry | Sulfide | Time (h) | Conversion (%) b | % Sulfoxide c | ee (%) d |
|---|---|---|---|---|---|
| 1 | 2a | 20 | ≥97 | 67 | 71 |
| 2 | 3a | 20 | 43 | 80 | 69 |
| 3 | 5a | 20 | 67 | 90 | 87 |
| 4 | 6a | 20 | ≥97 | 95 | 83 |
| 5 | 10a | 30 | 77 | 50 | 77 |
| 6 | 11a | 48 | 27 | ≥97 | 80 |
| Entry | Sulfide | Cosolvent | % Cosolvent | Time (h) | Conversion (%) b | % Sulfoxide c | ee (%) d |
|---|---|---|---|---|---|---|---|
| 1 | 2a | 1,4-dioxane | 30 | 24 | 77 | 95 | 77 |
| 2 | 2a | 1,4-dioxane | 50 | 24 | 7 | ≥97 | 39 |
| 3 | 2a | toluene | 30 | 24 | 11 | ≥97 | 74 |
| 4 | 4a | 1,4-dioxane | 10 | 24 | 88 | ≥97 | 95 |
| 5 | 6a | 1,4-dioxane | 10 | 24 | 69 | ≥97 | 97 |
| 6 | 9a | 1,4-dioxane | 10 | 24 | 95 | 72 | 31 |
| 7 | 10a | 1,4-dioxane | 10 | 30 | 29 | ≥97 | 85 |
| 8 | 11a | 1,4-dioxane | 10 | 56 | 23 | ≥97 | 87 |
| 9 | 12a | 1,4-dioxane | 10 | 24 | ≥97 | 74 | 33 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Gonzalo, G.; Fürst, M.J.L.J.; Fraaije, M.W. Polycyclic Ketone Monooxygenase (PockeMO): A Robust Biocatalyst for the Synthesis of Optically Active Sulfoxides. Catalysts 2017, 7, 288. https://doi.org/10.3390/catal7100288
De Gonzalo G, Fürst MJLJ, Fraaije MW. Polycyclic Ketone Monooxygenase (PockeMO): A Robust Biocatalyst for the Synthesis of Optically Active Sulfoxides. Catalysts. 2017; 7(10):288. https://doi.org/10.3390/catal7100288
Chicago/Turabian StyleDe Gonzalo, Gonzalo, Maximilian J. L. J. Fürst, and Marco W. Fraaije. 2017. "Polycyclic Ketone Monooxygenase (PockeMO): A Robust Biocatalyst for the Synthesis of Optically Active Sulfoxides" Catalysts 7, no. 10: 288. https://doi.org/10.3390/catal7100288
APA StyleDe Gonzalo, G., Fürst, M. J. L. J., & Fraaije, M. W. (2017). Polycyclic Ketone Monooxygenase (PockeMO): A Robust Biocatalyst for the Synthesis of Optically Active Sulfoxides. Catalysts, 7(10), 288. https://doi.org/10.3390/catal7100288








