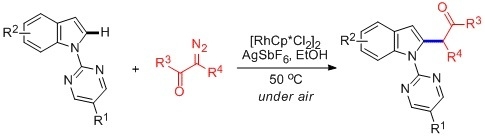
Rh(III)-Catalyzed, Highly Selectively Direct C–H Alkylation of Indoles with Diazo Compounds
Abstract
:1. Introduction
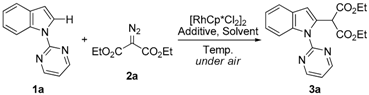
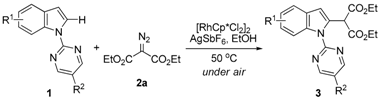
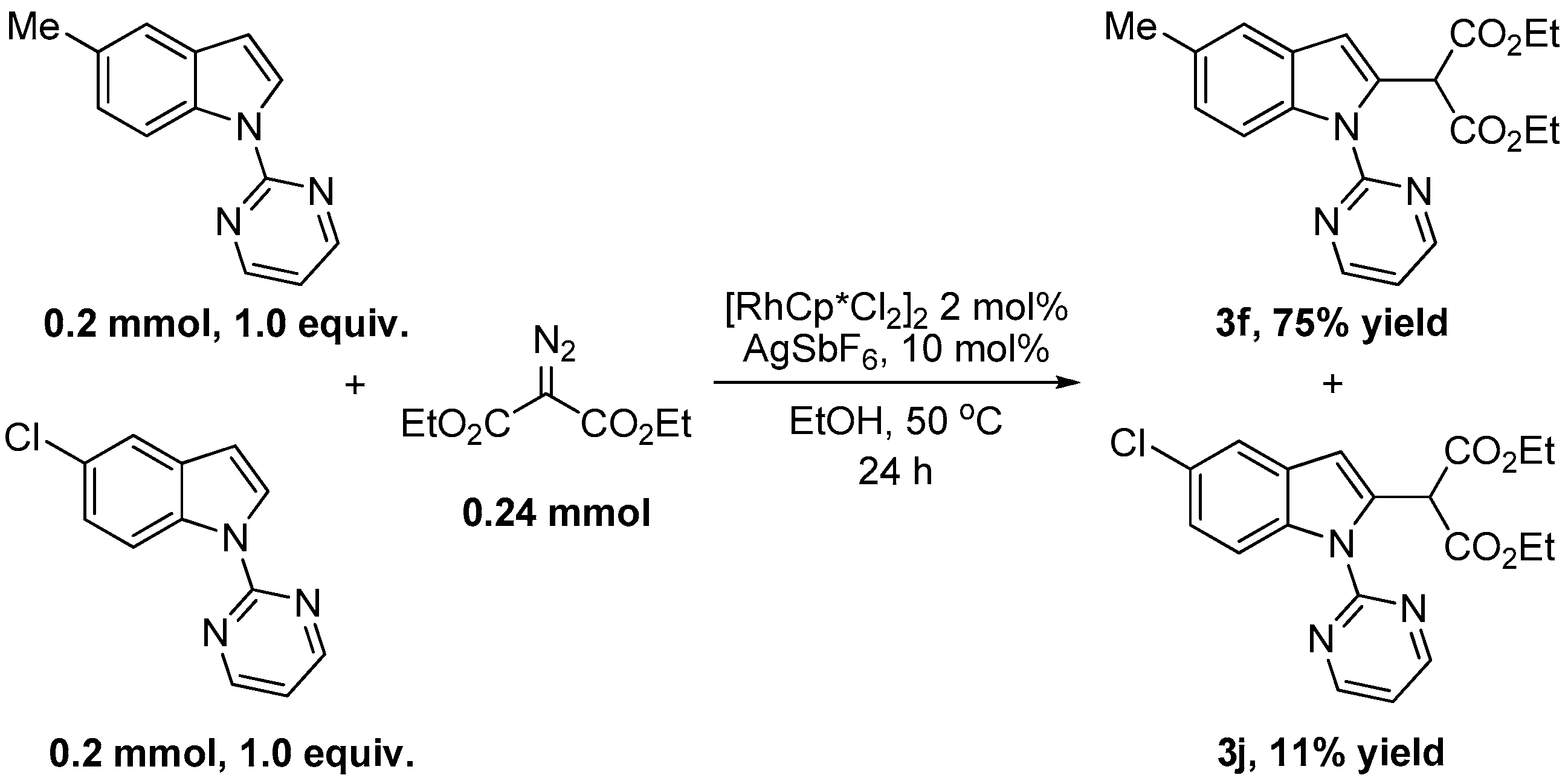
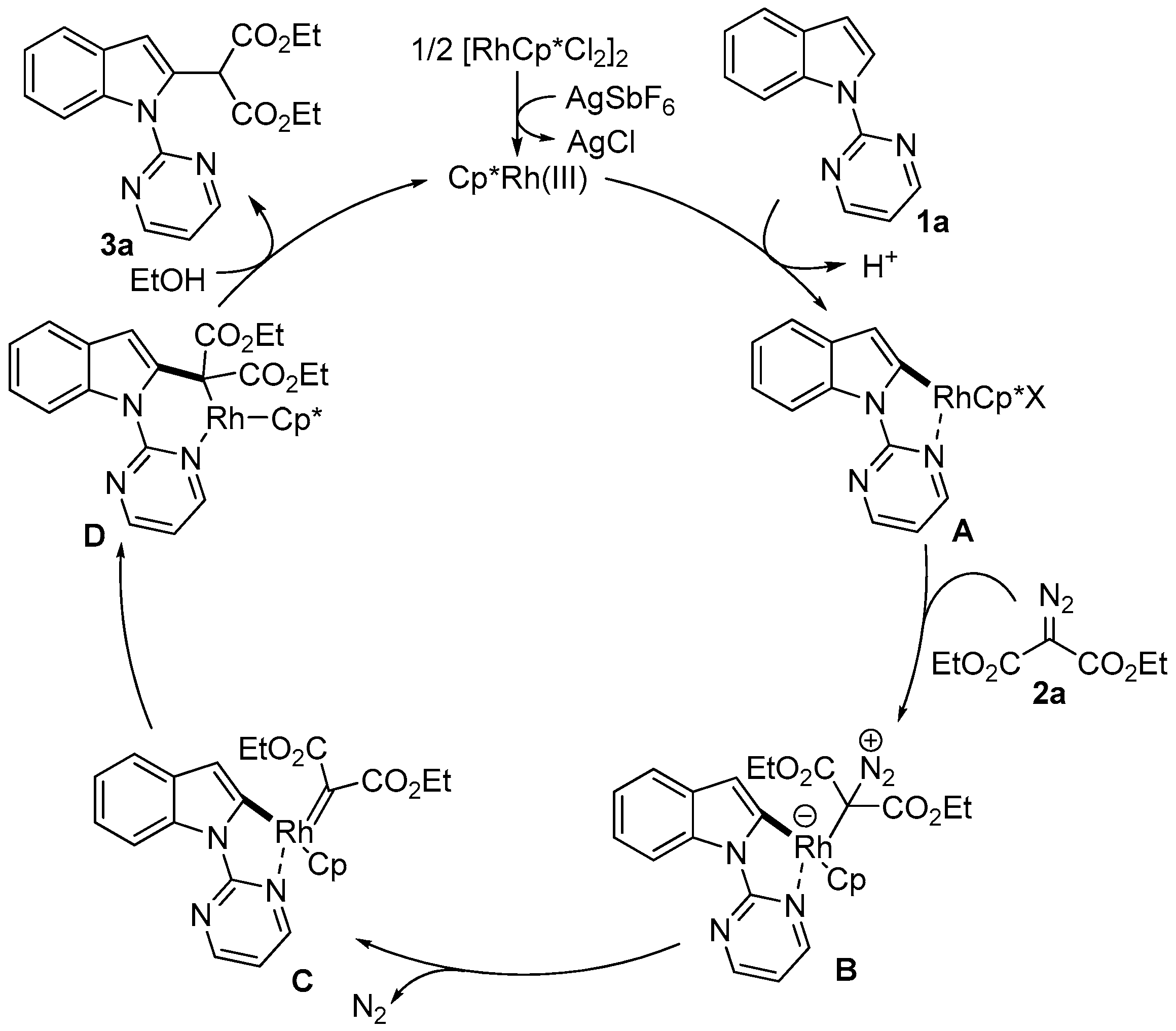
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Representative Procedure for the C–H Alkylation Reaction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Trost, B.M. On inventing reactions for atom economy. Acc. Chem. Res. 2002, 35, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Hale, C.R.; Nilewski, C.; Ioannidou, H.A. Constructing molecular complexity and diversity: Total synthesis of natural products of biological and medicinal importance. Chem. Soc. Rev. 2012, 41, 5185–5238. [Google Scholar] [CrossRef] [PubMed]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Cacchi, S.; Fabrizi, G. Synthesis and functionalization of indoles through palladium-catalyzed reactions. Chem. Rev. 2005, 105, 2873–2920. [Google Scholar] [CrossRef] [PubMed]
- Giri, R.; Shi, B.F.; Engle, K.M.; Maugel, N.; Yu, J.-Q. Transition metal-catalyzed C–H activation reactions: Diastereoselectivity and enantioselectivity. Chem. Soc. Rev. 2009, 38, 3242–3272. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.W.; Sanford, M.S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Kim, J.Y.; Kwak, J.; Chang, S. Recent advances in the transition metal-catalyzed twofold oxidative C–H bond activation strategy for C–C and C–N bond formation. Chem. Soc. Rev. 2011, 40, 5068–5083. [Google Scholar] [CrossRef] [PubMed]
- Wencel-Delord, J.; Dröge, T.; Liu, F.; Glorius, F. Towards mild metal-catalyzed C–H bond activation. Chem. Soc. Rev. 2011, 40, 4740–4761. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Wang, F.; Li, X. C–C, C–O and C–N bond formation via rhodium(III)-catalyzed oxidative C–H activation. Chem. Soc. Rev. 2012, 41, 3651–3678. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchi, F.; Murai, S. Catalytic C−H/olefin coupling. Acc. Chem. Res. 2002, 35, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jie, X.; Zhao, H.; Li, G.; Su, W. Recent advances in directed C–H functionalizations using monodentate nitrogen-based directing groups. Org. Chem. Front. 2014, 1, 843–893. [Google Scholar] [CrossRef]
- Ackermann, L. Carboxylate-assisted ruthenium-catalyzed alkyne annulations by C–H/het–H bond functionalizations. Acc. Chem. Res. 2014, 47, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Olah, G.A.; Khrishnamurti, R.; Prakash, G.K.S. Comprehensive Organic Synthesis, 1st ed.; Trost, B.M., Fleming, I., Eds.; Pergamon: Oxford, UK, 1991; Volume 3, pp. 293–335. [Google Scholar]
- Bandini, M.; Eichholzer, A. Catalytic functionalization of indoles in a new dimension. Angew. Chem. Int. Ed. 2009, 48, 9608–9644. [Google Scholar] [CrossRef] [PubMed]
- Deprez, N.R.; Kalyani, D.; Krause, A.; Sanford, M.S. Room temperature palladium-catalyzed 2-arylation of indoles. J. Am. Chem. Soc. 2006, 128, 4972–4973. [Google Scholar] [CrossRef] [PubMed]
- García-Rubia, A.; Arrayás, R.G.; Carretero, J.C. Palladium(II)-catalyzed regioselective direct C2 alkenylation of indoles and pyrroles assisted by the N-(2-pyridyl)sulfonyl protecting group. Angew. Chem. Int. Ed. 2009, 48, 6511–6515. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Qi, Z.; Yu, S.; Li, X. Rh(III)- and Ir(III)-catalyzed C–H alkynylation of arenes under chelation assistance. J. Am. Chem. Soc. 2014, 136, 4780–4787. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Zhao, M.; Qiao, X.; Zhu, Y.; Tong, X.; Shen, Z. Copper-catalyzed aromatic C–H bond cyanation by C-CN bond cleavage of inert acetonitrile. Chem. Eur. J. 2013, 19, 16880–16886. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Jin, H.; Liu, X.; Cheng, Y.; Zhu, C. Palladium-catalyzed decarboxylative C2-acylation of indoles with α-oxocarboxylic acids. Chem. Commun. 2013, 49, 2933–2935. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Bach, T. Palladium-catalyzed direct 2-alkylation of indoles by norbornene-mediated regioselective cascade C–H activation. J. Am. Chem. Soc. 2011, 133, 12990–12993. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Herdtweck, E.; Bach, T. Pd(II)-catalyzed regioselective 2-alkylation of indoles via a norbornene-mediated C–H activation: Mechanism and applications. J. Am. Chem. Soc. 2012, 134, 14563–14572. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Ryu, N.; Shibata, T. Ir(I)-catalyzed C–H bond alkylation of C2-position of indole with alkenes: Selective synthesis of linear or branched 2-alkylindoles. J. Am. Chem. Soc. 2012, 134, 17474–17477. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Boultadakis-Arapinis, M.; Glorius, F. Rh(III)-catalyzed dehydrogenative alkylation of (hetero)arenes with allylic alcohols, allowing aldol condensation to indenes. Chem. Commun. 2013, 49, 6489–6491. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Schröder, N.; Glorius, F. Mild rhodium(III)-catalyzed direct C–H allylation of arenes with allyl carbonates. Angew. Chem. Int. Ed. 2013, 52, 5386–5389. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, S.; Qi, Z.; Li, X. Rh(III)-catalyzed C–H alkylation of arenes using alkylboron reagents. Org. Lett. 2015, 17, 2812–2815. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhang, Y.; Wang, J. Diazo compounds and N-tosylhydrazones: Novel cross-coupling partners in transition-metal-catalyzed reactions. Acc. Chem. Res. 2013, 46, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-W.; Lo, S.-F.; Zhou, Z.; Yu, W.-Y. Rh-catalyzed intermolecular carbenoid functionalization of aromatic C–H bonds by α-diazomalonates. J. Am. Chem. Soc. 2012, 134, 13565–12568. [Google Scholar] [CrossRef] [PubMed]
- Hyster, T.K.; Ruhl, K.E.; Rovis, T. A Coupling of benzamides and donor/acceptor diazo compounds to form γ-lactams via Rh(III)-catalyzed C–H activation. J. Am. Chem. Soc. 2013, 135, 5364–5367. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Koester, D.C.; Boultadakis-Arapinis, M.; Glorius, F. Rh(III)-catalyzed synthesis of multisubstituted isoquinoline and pyridine N-oxides from oximes and diazo compounds. J. Am. Chem. Soc. 2013, 135, 12204–12207. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-W.; Yeung, S.-H.; Zhou, Z.; Chan, A.S.C.; Yu, W.-Y. Ruthenium catalyzed directing group-Free C2-selective carbenoid functionalization of indoles by α-Aryldiazoesters. Org. Lett. 2010, 12, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yan, Y.; Li, Q.; Xu, H.E.; Yi, W. Rhodium(III)-catalyzed C2-selective carbenoid functionalization and subsequent C7-alkenylation of indoles. Chem. Commun. 2014, 50, 6483–6486. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qu, X.; Li, Z.; Peng, W.-M. Rhodium-catalyzed regioselective direct C–H arylation of indoles with aryl boronic acids. Tetrahedron Lett. 2015, 56, 3754–3757. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Qu, X.; Peng, W.-M. Highly efficient synthesis of arylpyrrole derivatives via Rh(III)-catalyzed direct C–H arylation with aryl boronic acids. Chin. J. Chem. 2015, 33, 1015–1018. [Google Scholar] [CrossRef]
- Wang, L.; Qu, X.; Li, Z.; Peng, W.-M. Rhodium(III)-catalyzed C–H activation/cylization of indole-2-amides derivatives and terminal alkynes. Chin. J. Org. Chem. 2015, 35, 688–697. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Qu, X.; Peng, W.-M.; Hu, S.-Q.; Wang, H.-B. Sequential one-pot Rh(III)-catalyzed direct C2 and C7 alkylation of (hetero)aromatic C–H bonds of indoles. Tetrahedron Lett. 2015, 56, 6214–6218. [Google Scholar] [CrossRef]
- Liu, X.-G.; Zhang, S.-S.; Wu, J.-Q.; Li, Q.; Wang, H. Cp*Co(III)-catalyzed direct functionalization of aromatic C–H bonds with a-diazomalonates. Tetrahedron Lett. 2015, 56, 4093–4095. [Google Scholar] [CrossRef]

| Entry | Additive | Solvent | Temp. | Yield b |
|---|---|---|---|---|
| 1 | AgOAc | EtOH | 60 | 86 |
| 2 | AgOAc | EtOH | 50 | 86 |
| 3 | AgOAc | EtOH | 40 | 84 |
| 4 | AgOAc | EtOH | 25 | 70 |
| 5 | AgO2CCF3 | EtOH | 50 | 85 |
| 6 | AgSbF6 | EtOH | 50 | 92 |
| 7 | AgF | EtOH | 50 | 82 |
| 8 | Ag2O | EtOH | 50 | 56 |
| 9 | AgOTf | EtOH | 50 | 75 |
| 10 | - | EtOH | 50 | 36 |
| 11 | AgSbF6 | MeOH | 50 | 90 |
| 12 | AgSbF6 | i-PrOH | 50 | 83 |
| 13 | AgSbF6 | t-AmOH | 50 | 80 |
| 14 | AgSbF6 | CH3CN | 50 | 42 |
| 15 | AgSbF6 | DCE | 50 | 54 |
| 16 | AgSbF6 | THF | 50 | 21 |
| 17 c | AgSbF6 | EtOH | 50 | 0 |
| 18 d | AgSbF6 | EtOH | 50 | 90 |
| Entry | R1 | R2 | 3 | Yield (%) b |
|---|---|---|---|---|
| 1 | 4-Me | H | 3b | 93 |
| 2 | 4-OBn | H | 3c | 92 |
| 3 | 4-CO2Me | H | 3d | 91 |
| 4 | 4-Cl | H | 3e | 87 |
| 5 | 5-Me | H | 3f | 86 |
| 6 | 5-OMe | H | 3g | 90 |
| 7 | 5-OBn | H | 3h | 94 |
| 8 | 5-Br | H | 3i | 93 |
| 9 | 5-Cl | H | 3j | 92 |
| 10 | 6-Me | H | 3k | 90 |
| 11 | 6-CO2Me | H | 3l | 90 |
| 12 | 6-F | H | 3m | 89 |
| 13 | 6-Cl | H | 3n | 89 |
| 14 | H | Me | 3o | 87 |
| 15 | H | Br | 3p | 88 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, K.; Li, Z.; Qu, X.; Wang, F.; Wang, L. Rh(III)-Catalyzed, Highly Selectively Direct C–H Alkylation of Indoles with Diazo Compounds. Catalysts 2016, 6, 89. https://doi.org/10.3390/catal6060089
Wan K, Li Z, Qu X, Wang F, Wang L. Rh(III)-Catalyzed, Highly Selectively Direct C–H Alkylation of Indoles with Diazo Compounds. Catalysts. 2016; 6(6):89. https://doi.org/10.3390/catal6060089
Chicago/Turabian StyleWan, Kang, Zhan Li, Xing Qu, Feng Wang, and Liang Wang. 2016. "Rh(III)-Catalyzed, Highly Selectively Direct C–H Alkylation of Indoles with Diazo Compounds" Catalysts 6, no. 6: 89. https://doi.org/10.3390/catal6060089