Facile Synthesis of MnPO4·H2O Nanowire/Graphene Oxide Composite Material and Its Application as Electrode Material for High Performance Supercapacitors
Abstract
:1. Introduction
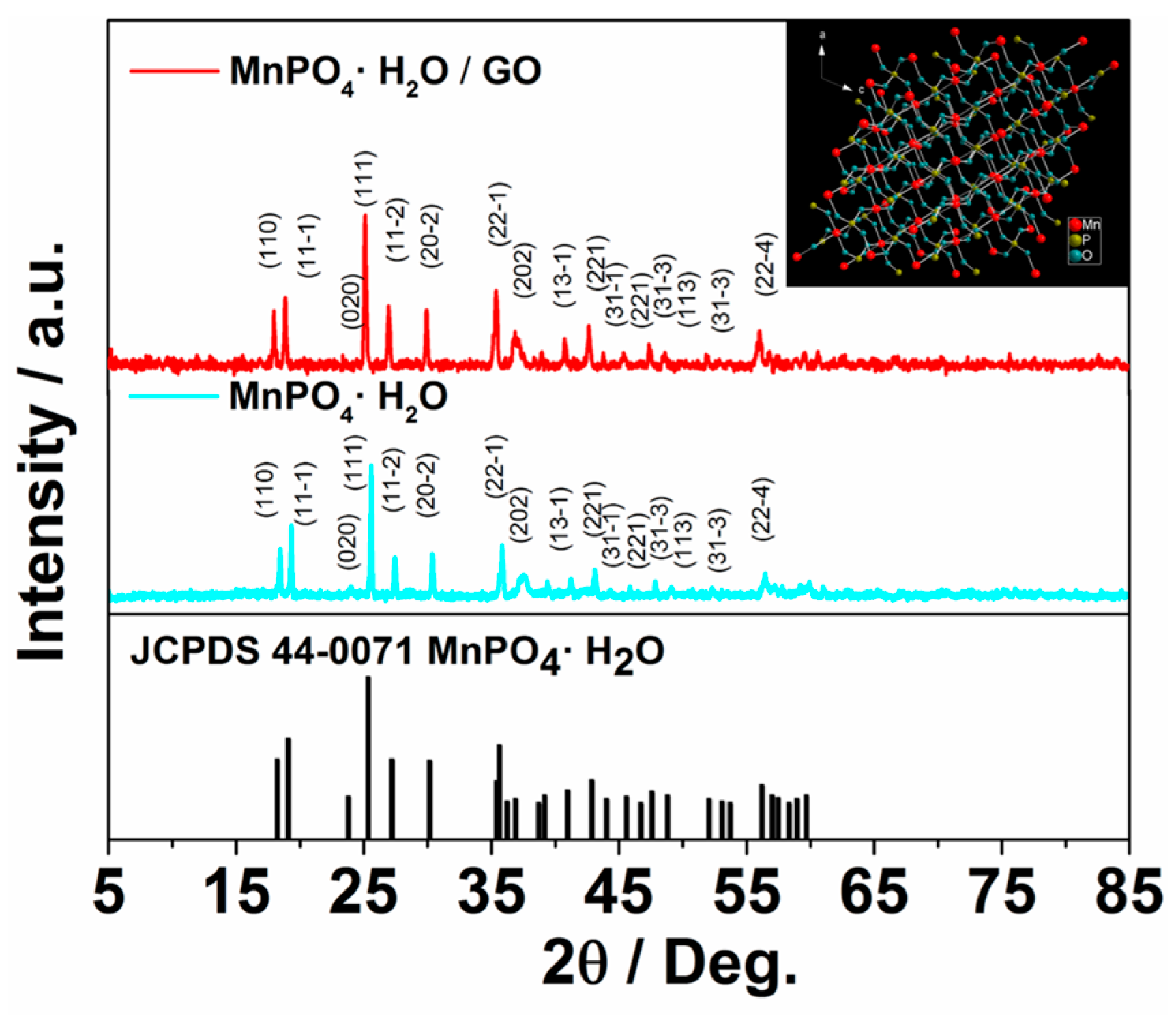
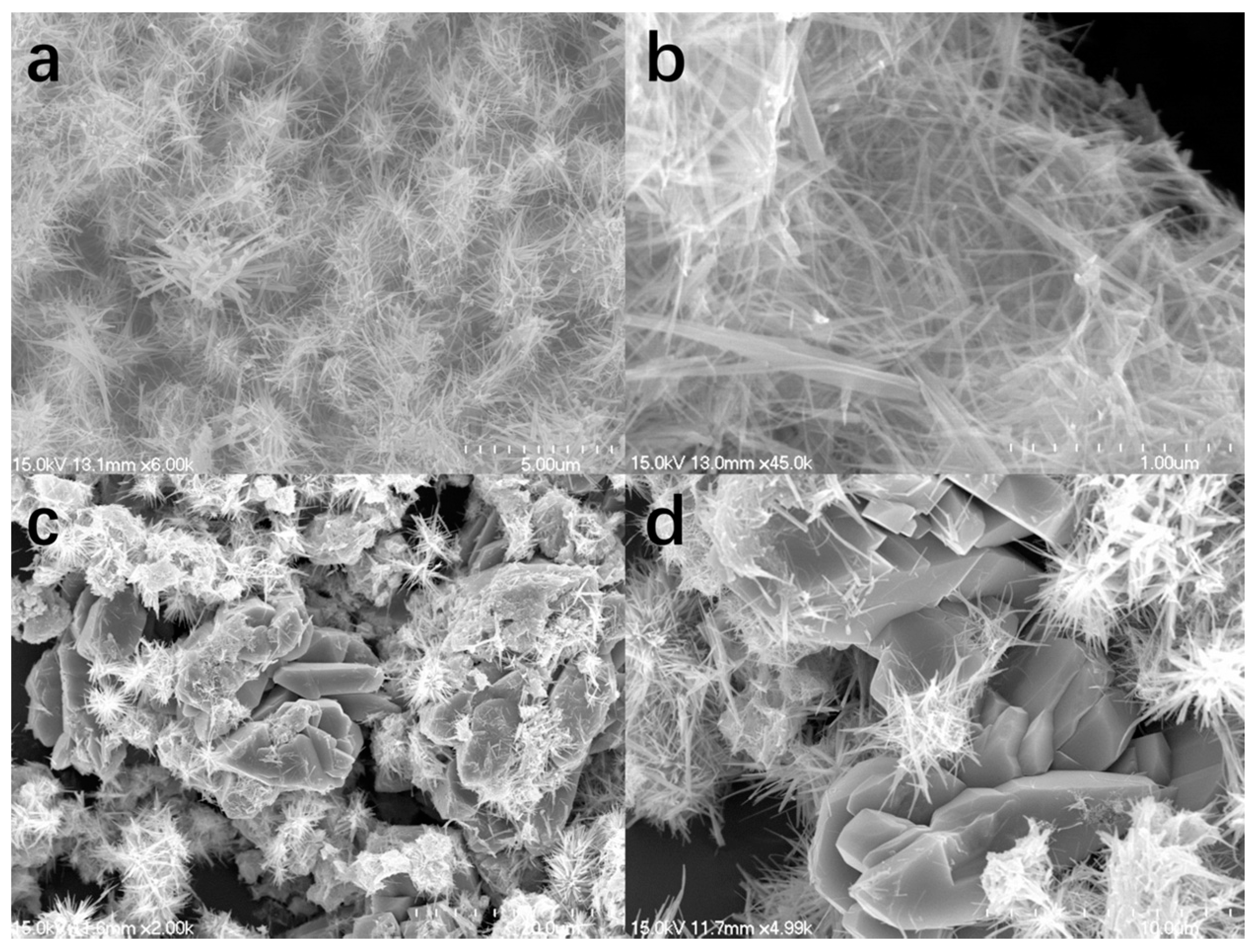
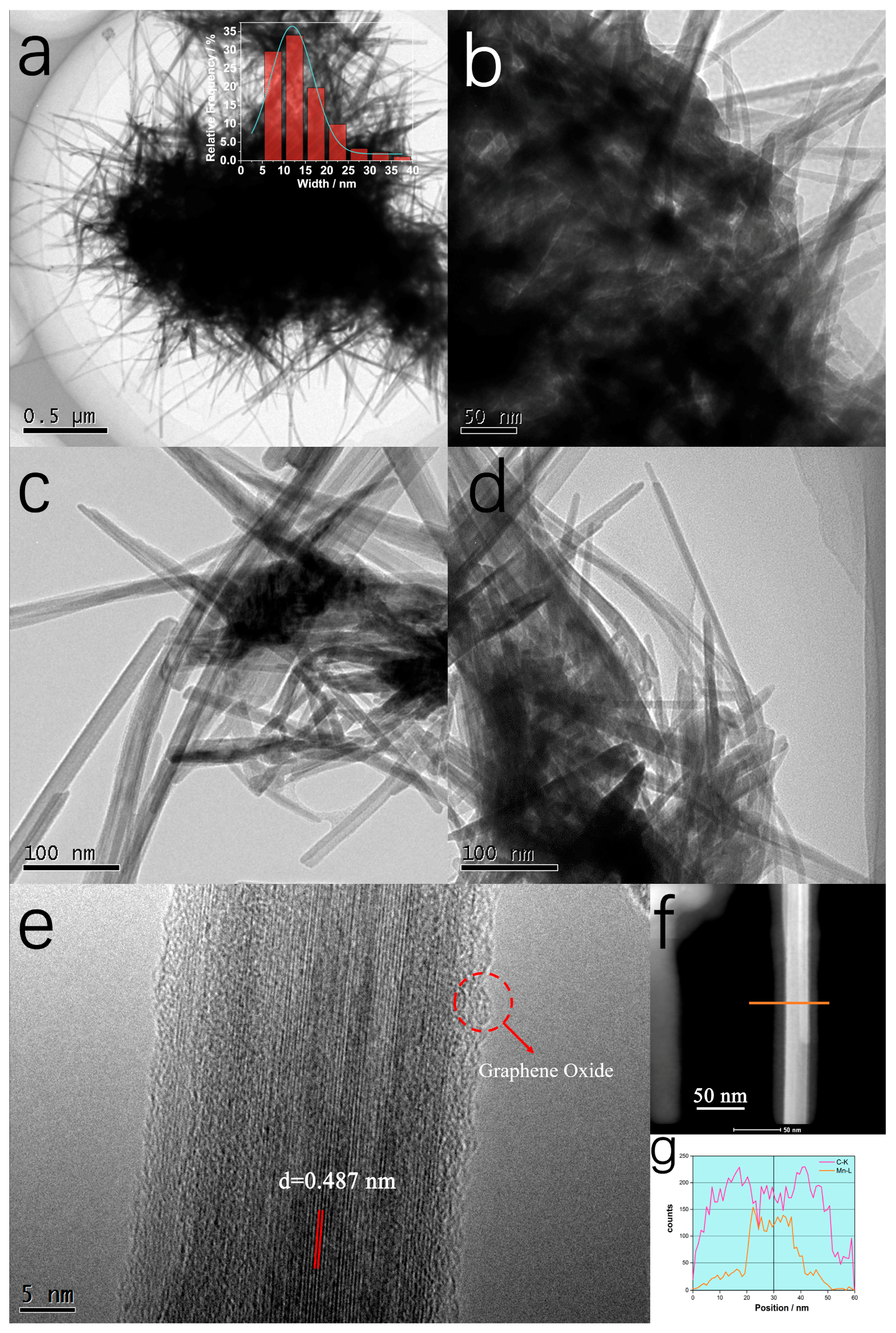
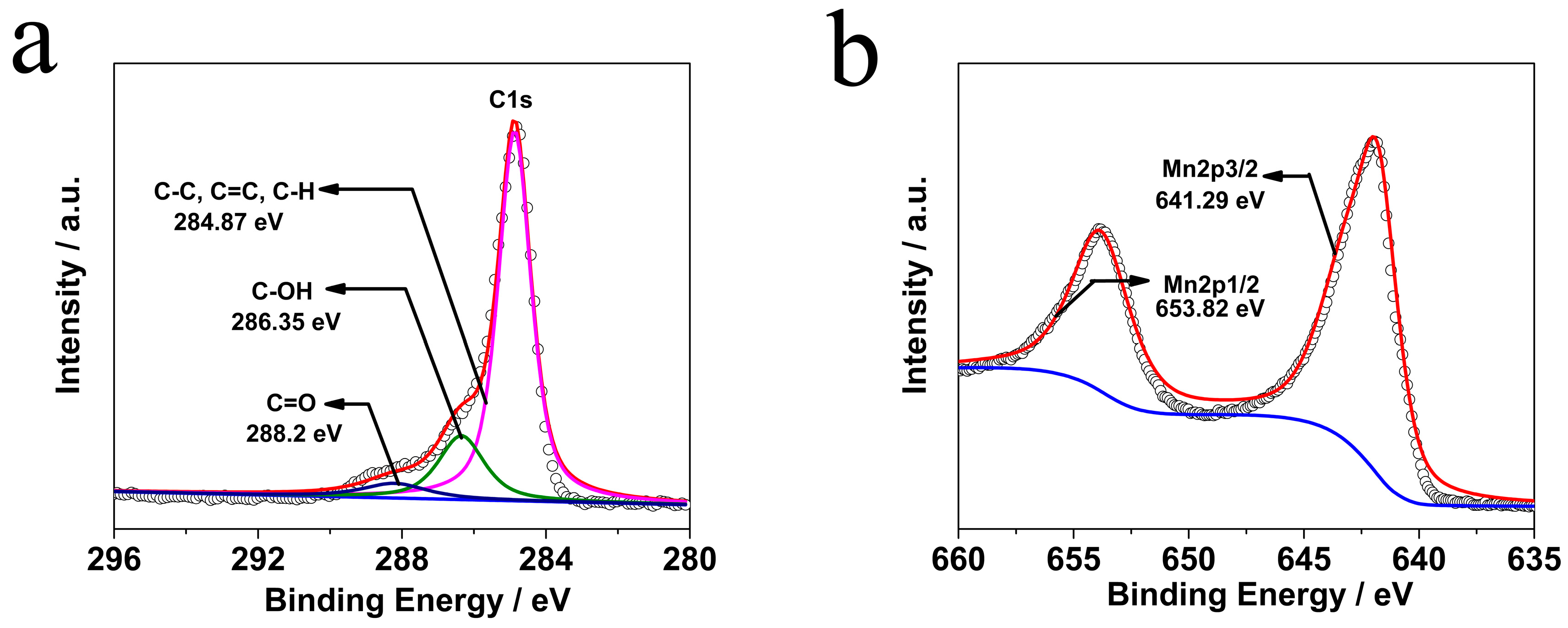
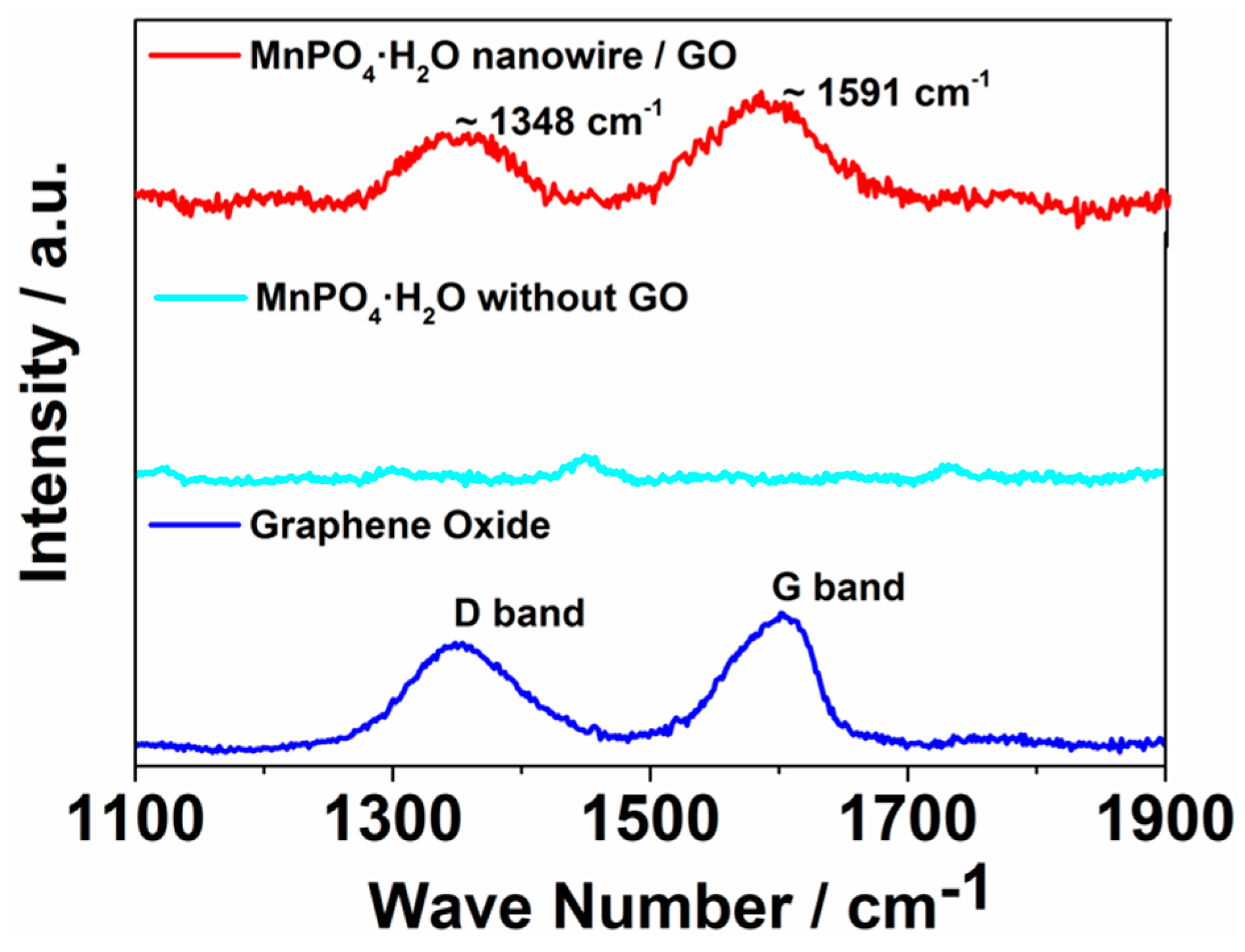
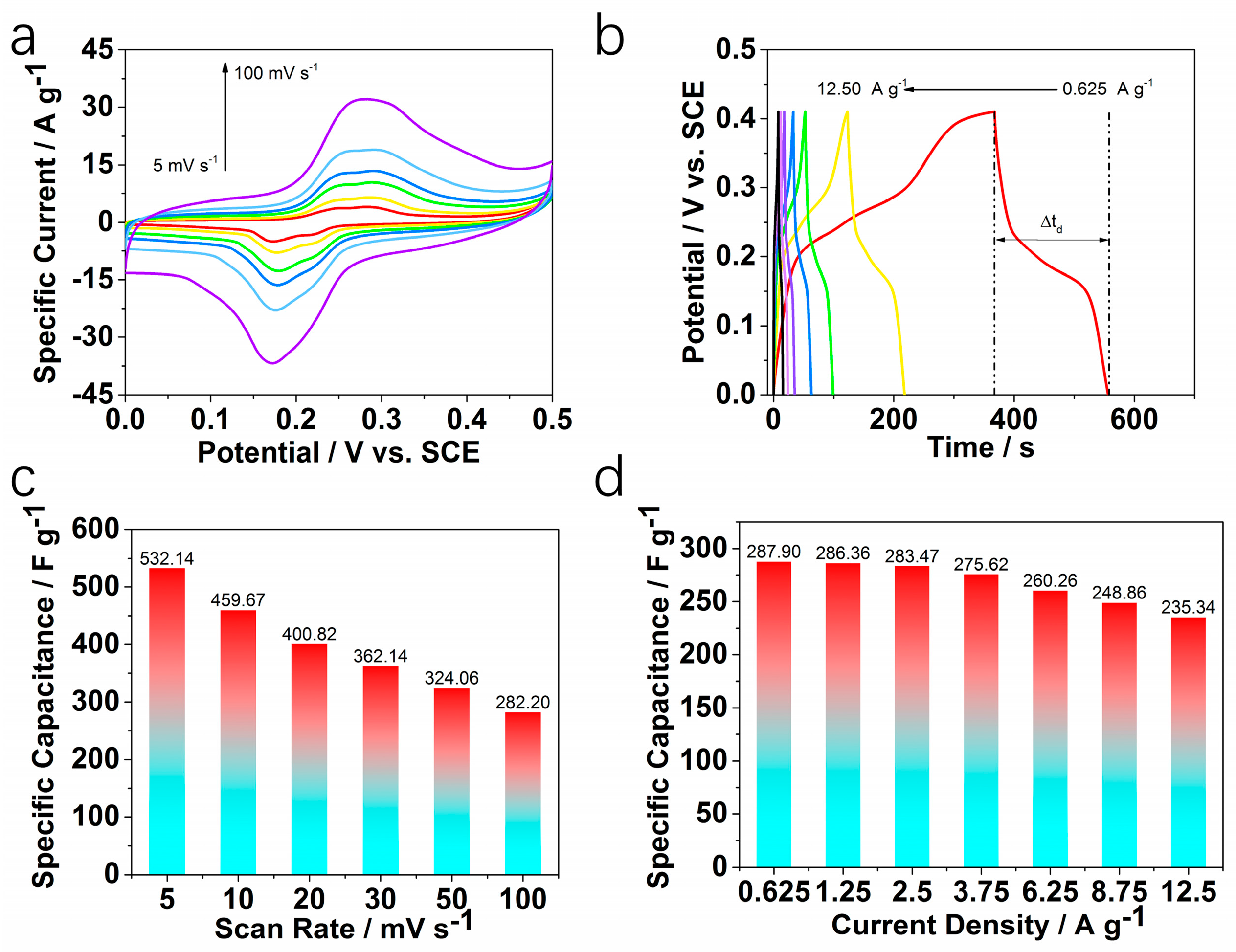
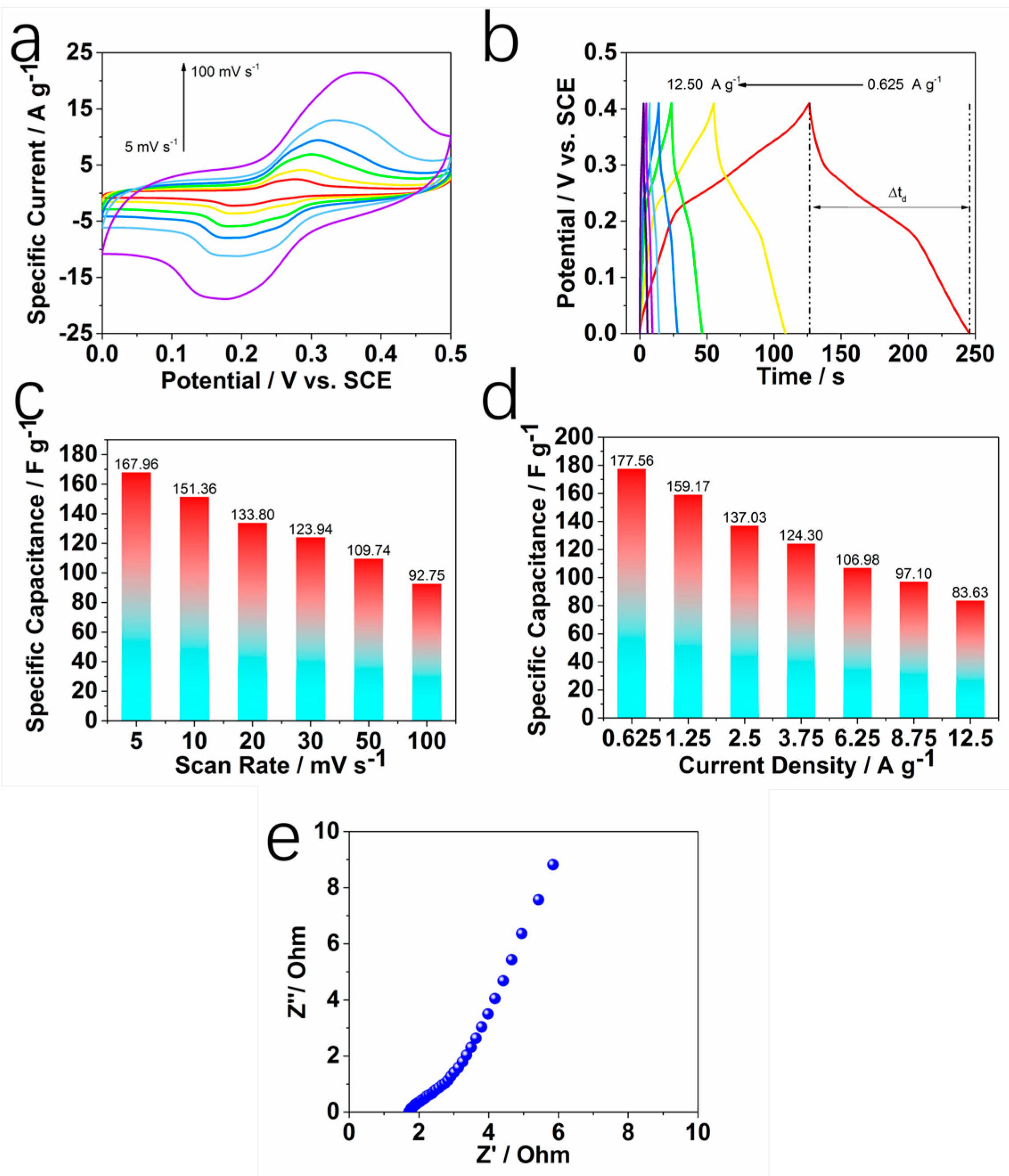
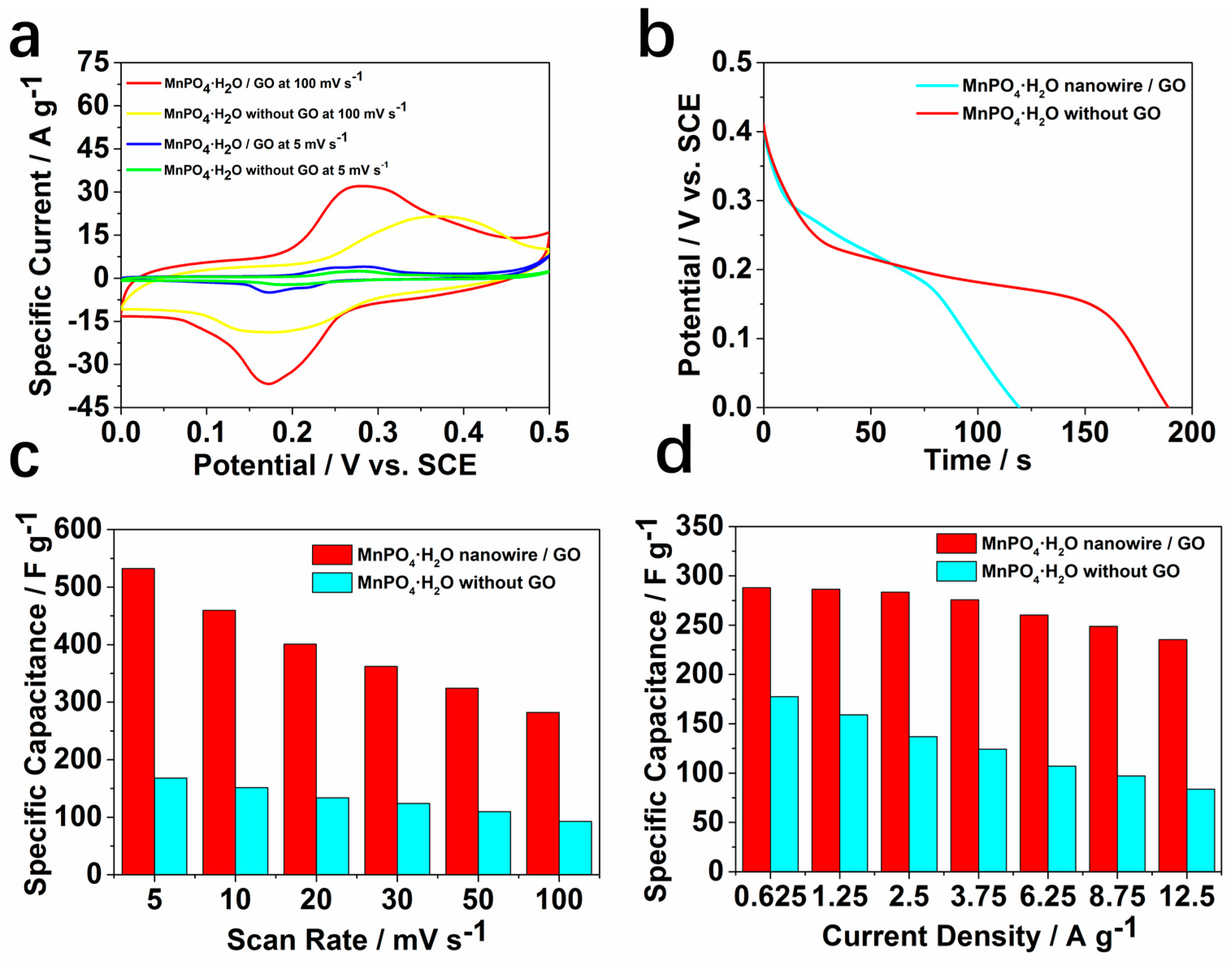
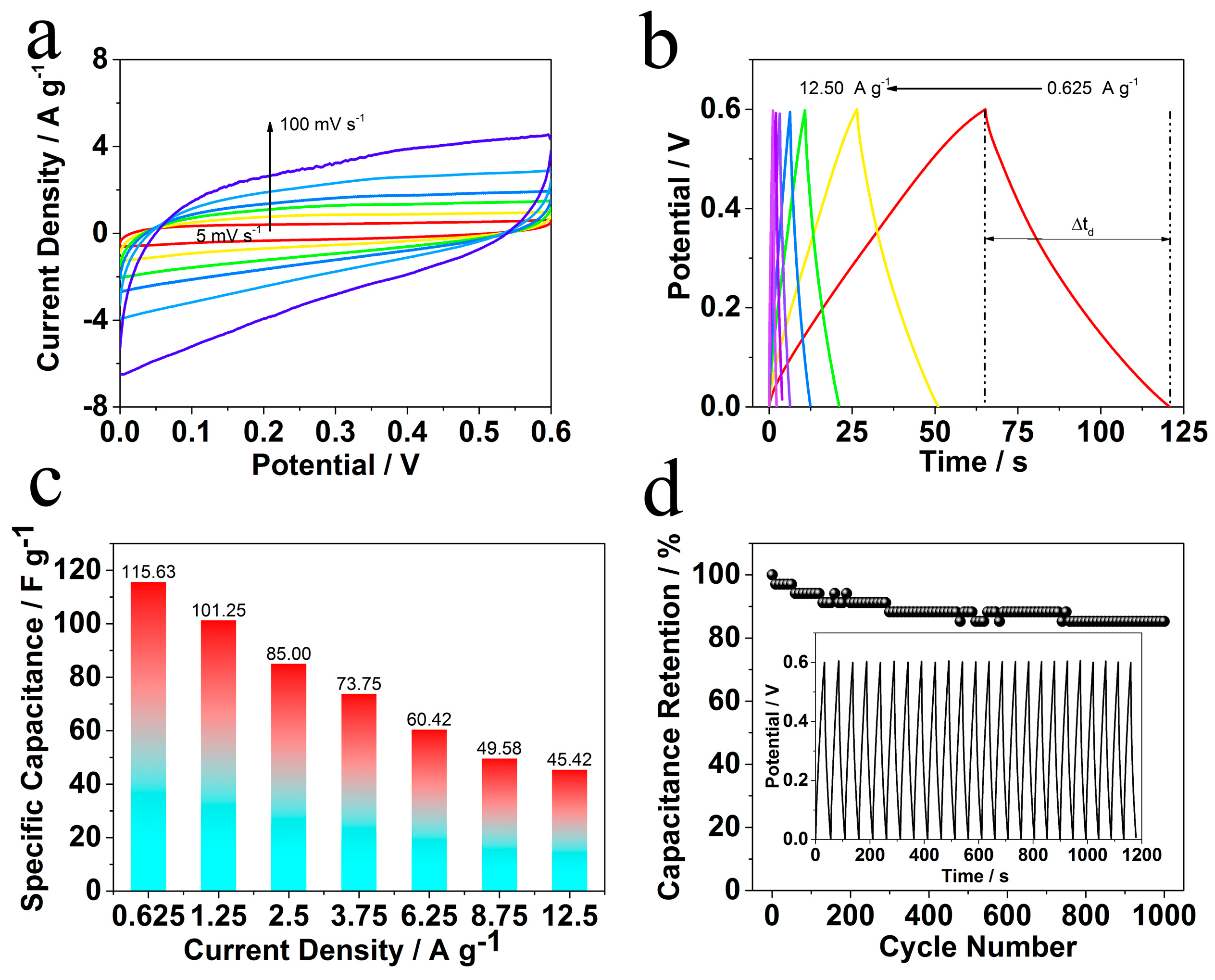
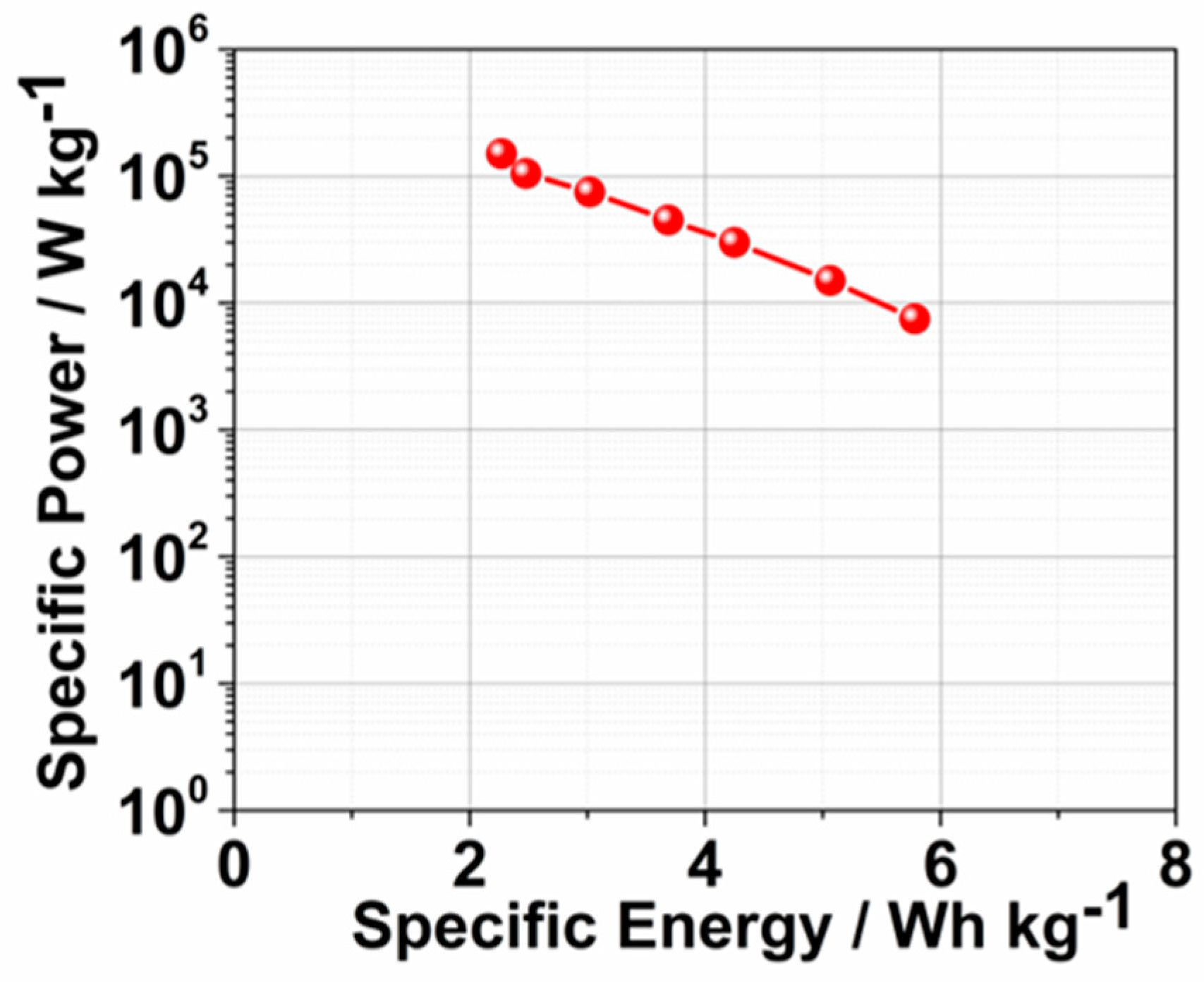
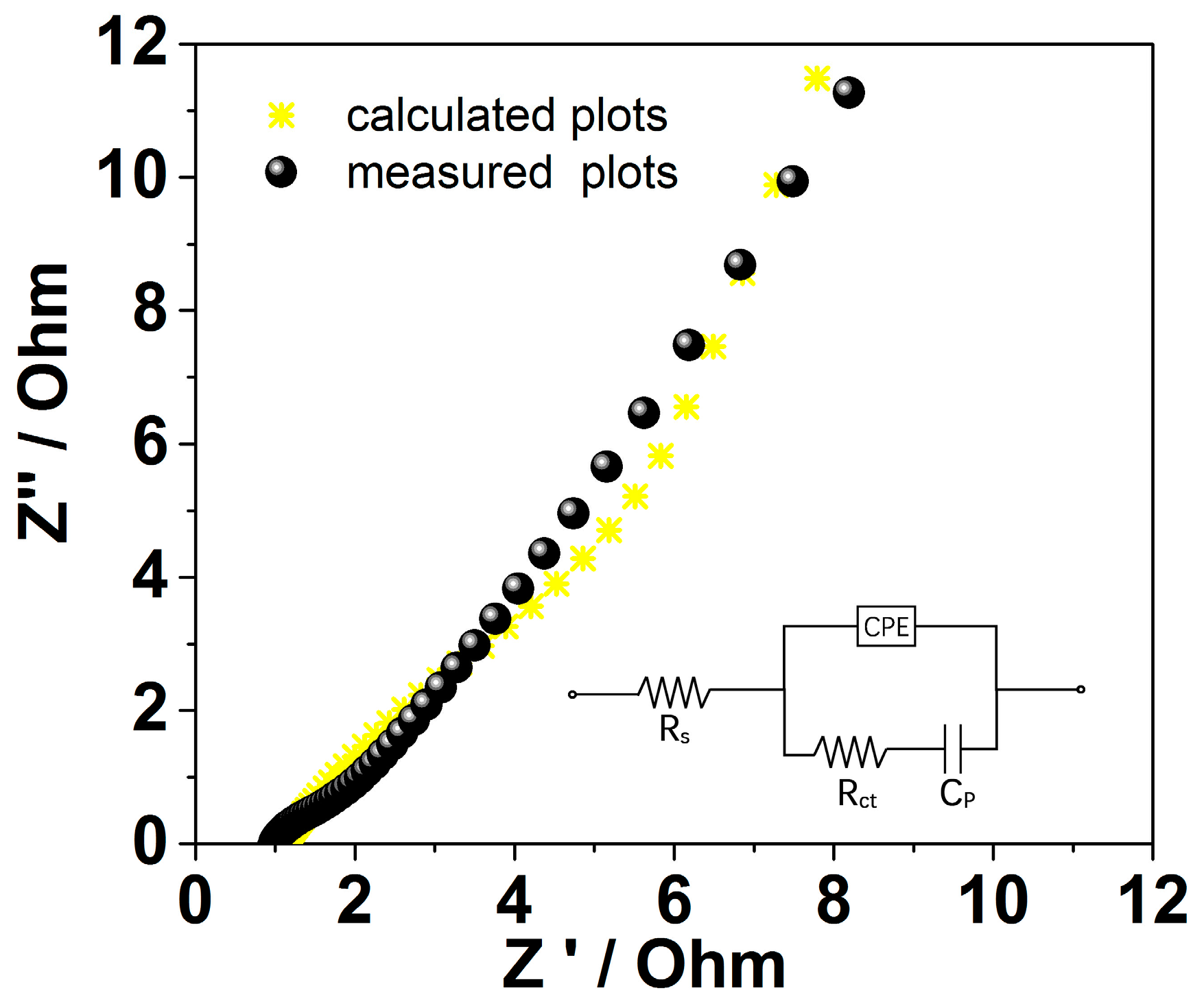
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of MnPO4·H2O Nanowire/GO Composite Material
3.3. Electrochemical Measurement
3.4. Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Conway, B.E. Electrochemical supercapacitors. In Scientific Fundamentals and Technological Applications; Kluwer Academic/Plenum Press: New York, NY, USA, 1999. [Google Scholar]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Brownson, D.A.C.; Kampouris, D.K.; Banks, C.E. An overview of graphene in energy production and storage applications. J. Power Sources 2011, 196, 4873–4885. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, J.; Chen, Y. An overview of the applications of graphene-based materials in supercapacitors. Small 2012, 8, 1805–1834. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhao, Y.; Hu, C.; Cheng, H.; Hu, Y.; Zhang, Z.; Shi, G.; Qu, L. All-graphene core-sheath microfibers for all-solid-state, stretchable fibriform supercapacitors and wearable electronic textiles. Adv. Mater. 2013, 25, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Ren, F.; Wang, C.; Xu, J.; Du, Y. Facile preparation of flower-like graphene-nanosheet clusters with the assistance of copper particles and their application in supercapacitors. RSC Adv. 2014, 4, 500–504. [Google Scholar] [CrossRef]
- Zhao, X.; Zheng, B.; Huang, T.; Gao, C. Graphene-based single fiber supercapacitor with a coaxial structure. Nanoscale 2015, 7, 9399–9404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Song, R.; Wang, M.; Yan, F.; He, L.; Feng, X.; Fang, S.; Zhao, J.; Zhang, H. Manganese(II) phosphate nanoflowers as electrochemical biosensors for the high-sensitivity detection of ractopamine. Sens. Actuators B 2015, 211, 310–317. [Google Scholar] [CrossRef]
- Stanislav, F.A.; Zhi, L.; Rute, A.S.F. New template-free layered manganese(III) phosphate: Hydrothermal synthesis, ab initio structural determination, and magnetic properties. Chem. Mater. 2007, 19, 6025–6029. [Google Scholar]
- Nam, K.M.; Cheon, E.A.; Shin, W.J.; Bard, A.J. Improved photoelectrochemical water oxidation by the WO3/CuWO4 composite with a manganese phosphate electrocatalyst. Langmuir 2015, 31, 10897–10903. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wu, C.; Liao, L.; Xie, J.; Zhang, S.; Zhu, P.; Cao, G.; Zhao, X. Performance improvement of lithium manganese phosphate by controllable morphology tailoring with acid-engaged nano engineering. Inorg. Chem. 2015, 54, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Minakshi, S.M.; Biswal, A.; Mitchell, D.; Jones, R.; Fernandez, C. Correlation among physical and electrochemical behaviour of nanostructured electrolytic manganese dioxide from leach liquor and synthetic for aqueous asymmetric capacitor. Phys. Chem. Chem. Phys. 2016, 18, 4711–4720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Han, X.; Hu, Z.; Zhang, X.; Tao, Z.; Chen, J. Nanostructured Mn-based oxides for electrochemical energy storage and conversion. Chem. Soc. Rev. 2015, 44, 699–728. [Google Scholar] [CrossRef] [PubMed]
- Di, L.D.; Hu, T.; Hassoun, J. Electrochemical features of LiMnPO4 olivine prepared by sol-gel pathway. J. Alloys Compd. 2017, 693, 730–737. [Google Scholar]
- Huang, Y.; Chernova, N.A.; Yin, Q.; Wang, Q.; Quackenbush, N.F.; Leskes, M. What Happens to LiMnPO4 upon Chemical Delithiation? Inorg. Chem. 2016, 55, 4335–4343. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, V.; Gnanaraj, J.; Lee, Y.S.; Madhavi, S. LiMnPO4—A next generation cathode material for lithium-ion batteries. J. Mater. Chem. A 2013, 1, 3518–3539. [Google Scholar] [CrossRef]
- Su, J.; Liu, Z.Z.; Long, Y.F.; Yao, H.; Lv, X.Y.; Wen, Y.X. Enhanced electrochemical performance of LiMnPO4/C prepared by microwave-assisted solvothermal method. Electrochim. Acta 2015, 173, 559–565. [Google Scholar] [CrossRef]
- Chen, L.; Dilena, E.; Paolella, A.; Bertoni, G.; Ansaldo, A.; Colombo, M. Relevance of LiPF6 as Etching Agent of LiMnPO4 Colloidal Nanocrystals for High Rate Performing Li-ion Battery Cathodes. ACS Appl. Mater Interfaces 2016, 8, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Jung, G.; Yoo, S.; Suh, K.S.; Ruoff, R.S. Activated graphene-based carbons as supercapacitor electrodes with macro- and mesopores. ACS Nano 2013, 7, 6899–6905. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; El-Kady, M.F.; Wang, L.J.; Zhang, Q.; Li, Y.; Wang, H. Graphene-based materials for flexible supercapacitors. Chem. Soc. Rev. 2015, 44, 3639–3665. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Wang, H.; Zhai, C.; Zhu, M.; Yue, R.; Du, Y. Clean method for the synthesis of reduced graphene oxide-supported PtPd alloys with high electrocatalytic activity for ethanol oxidation in alkaline medium. ACS Appl. Mater Interfaces 2014, 6, 3607–3614. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Yang, B.; Bin, D.; Wang, J.; Li, S.; Yang, P. Electrochemical synthesis of gold nanoparticles decorated flower-like graphene for high sensitivity detection of nitrite. J. Colloid Interface Sci. 2017, 488, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Liu, Y.; Li, J.; Ma, Y.; Li, G.; Ai, Y.; Chen, J.; Zhang, J.; Zheng, H. Cobalt phosphite microarchitectures assembled by ultralong nanoribbons and their application as effective electrochemical capacitor electrode materials. Nanoscale 2013, 5, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Miguel, A.G.A.; Attfield, J.P.; Bruquea, S.; Fernando, P. Magnetic structures of MnPO4·D2O and MnAsO4·D2O from time-of-flight neutron powder diffraction data. J. Mater. Chem. 1992, 2, 501–505. [Google Scholar]
- Cote, L.J.; Kim, J.; Zhang, Z.; Sun, C.; Huang, J. Tunable assembly of graphene oxide surfactant sheets: Wrinkles, overlaps and impacts on thin film properties. Soft Matter 2010, 6, 6096–6101. [Google Scholar] [CrossRef]
- Kazi, S.N.; Badarudin, A.; Zubir, M.N.; Ming, H.N.; Misran, M.; Sadeghinezhad, E.; Mehrali, M.; Syuhada, N.I. Investigation on the use of graphene oxide as novel surfactant to stabilize weakly charged graphene nanoplatelets. Nanoscale Res. Lett. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.; Cote, L.J.; Huang, J. Graphene oxide: Surface activity and two-dimensional assembly. Adv. Mater. 2010, 22, 1954–1958. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Han, X.; Li, L.; Zhu, Z.; Cheng, F.; Srinivansan, M.; Adam, S.; Ramakrishna, S. Unique cobalt sulfide/reduced graphene oxide composite as an anode for sodium-ion batteries with superior rate capability and long cycling stability. Small 2016, 12, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O. Bacteriorhodopsin as a superior substitute for hydrazine in chemical reduction of single-layer graphene oxide sheets. Carbon 2015, 81, 158–166. [Google Scholar] [CrossRef]
- Govindhan, M.; Chen, A. Simultaneous synthesis of gold nanoparticle/graphene nanocomposite for enhanced oxygen reduction reaction. J. Power Sources 2015, 274, 928–936. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surface Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Huang, Y.; Fang, J.; Omenya, F.; O’Shea, M.; Chernova, N.A.; Zhang, R.; Wang, Q.; Quackenbush, N.F.; Piper, L.F.J.; Scanlon, D.O.; et al. Understanding the stability of MnPO4. J. Mater. Chem. A 2014, 2, 12827–12834. [Google Scholar] [CrossRef]
- Yang, B.; Wang, J.; Bin, D.; Zhu, M.; Yang, P.; Du, Y. A three dimensional Pt nanodendrite/graphene/MnO2 nanoflower modified electrode for the sensitive and selective detection of dopamine. J. Mater. Chem. B 2015, 3, 7440–7448. [Google Scholar] [CrossRef]
- Heller, E.J.; Yang, Y.; Kocia, L.; Chen, W.; Fang, S.; Borunda, M.; Kaxiras, E. Theory of graphene raman scattering. ACS Nano 2016, 10, 2803–2818. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Du, J.; Wang, H.; Zou, C.; Jiang, F.; Yang, P.; Du, Y. A facile electrochemical sensor based on reduced graphene oxide and Au nanoplates modified glassy carbon electrode for simultaneous detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B 2014, 204, 302–309. [Google Scholar] [CrossRef]
- Zhai, C.; Zhu, M.; Bin, D.; Ren, F.; Wang, C.; Yang, P. Two dimensional MoS2/graphene composites as promising supports for Pt electrocatalysts towards methanol oxidation. J. Power Sources 2015, 275, 483–488. [Google Scholar] [CrossRef]
- Ren, F.; Zhai, C.; Zhu, M.; Wang, C.; Wang, H.; Bin, D. Facile synthesis of PtAu nanoparticles supported on polydopamine reduced and modified graphene oxide as a highly active catalyst for methanol oxidation. Electrochim. Acta 2015, 153, 175–183. [Google Scholar] [CrossRef]
- Oraon, R.; De, A.A.; Tiwari, S.K.; Nayak, G.C. Enhanced specific capacitance of self-assembled three-dimensional carbon nanotube/layered silicate/polyaniline hybrid sandwiched nanocomposite for supercapacitor applications. ACS Sustain. Chem. Eng. 2016, 4, 1392–1403. [Google Scholar] [CrossRef]
- Younis, A.; Chu, D.; Li, S. Ethanol-directed morphological evolution of hierarchical CeOx architectures as advanced electrochemical capacitors. J. Mater. Chem. A 2015, 3, 13970–13977. [Google Scholar] [CrossRef]
- Wan, H.; Jiang, J.; Yu, J.; Xu, K.; Miao, L.; Zhang, L.; Chen, H.; Ruan, Y. NiCo2S4 porous nanotubes synthesis via sacrificial templates: High-performance electrode materials of supercapacitors. CrystEngComm 2013, 15, 7649–7651. [Google Scholar] [CrossRef]
- Qiu, K.; Lu, Y.; Zhang, D.; Cheng, J.; Yan, H.; Xu, J.; Liu, X.; Kim, J.; Luo, Y. Mesoporous, hierarchical core/shell structured ZnCo2O4/MnO2 nanocone forests for high-performance supercapacitors. Nano Energy 2015, 11, 687–696. [Google Scholar] [CrossRef]
- Cui, X.; Lv, R.; Sagar, R.U.R.; Liu, C.; Zhang, Z. Reduced graphene oxide/carbon nanotube hybrid film as high performance negative electrode for supercapacitor. Electrochim. Acta 2015, 169, 342–350. [Google Scholar] [CrossRef]
- Zhu, Y.; Cui, H.; Meng, X.; Zheng, J.; Yang, P.; Li, L.; Wang, Z.; Jia, S.; Zhu, P. Chlorine-induced in situ regulation to synthesize graphene frameworks with large specific area for excellent supercapacitor performance. ACS Appl. Mater. Interfaces 2016, 8, 6481–6487. [Google Scholar] [CrossRef] [PubMed]
- Portet, C.; Taberna, P.L.; Simon, P.; Flahaut, E.; Laberty-Robert, C. High power density electrodes for carbon supercapacitor applications. Electrochim. Acta 2005, 50, 4174–4181. [Google Scholar] [CrossRef] [Green Version]
- Rakhi, R.B.; Chen, W.; Cha, D.; Alshareef, H.N. High performance supercapacitors using metal oxide anchored graphene nanosheet electrodes. J. Mater. Chem. 2011, 21, 16197–16204. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Ramaprabhu, S. Nanocrystalline metal oxides dispersed multiwalled carbon nanotubes as supercapacitor electrodes. J. Phys. Chem. C 2007, 111, 7727–7734. [Google Scholar] [CrossRef]
- Minakshi, M.; Meyrick, D.; Appadoo, D. Maricite (NaMn1/3Ni1/3Co1/3PO4)/activated carbon: Hybrid capacitor. Energy Fuels 2013, 27, 3516–3522. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.; Jones, R.; Alenazey, F.; Watcharatharapong, T.; Chakraborty, S.; Ahuja, R. Synthesis, structural and electrochemical properties of sodium nickel phosphate for energy storage devices. Nanoscale 2016, 8, 11291–11305. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, B.; Bin, D.; Ren, F.; Xiong, Z.; Zhang, K.; Wang, C.; Du, Y. Facile Synthesis of MnPO4·H2O Nanowire/Graphene Oxide Composite Material and Its Application as Electrode Material for High Performance Supercapacitors. Catalysts 2016, 6, 198. https://doi.org/10.3390/catal6120198
Yan B, Bin D, Ren F, Xiong Z, Zhang K, Wang C, Du Y. Facile Synthesis of MnPO4·H2O Nanowire/Graphene Oxide Composite Material and Its Application as Electrode Material for High Performance Supercapacitors. Catalysts. 2016; 6(12):198. https://doi.org/10.3390/catal6120198
Chicago/Turabian StyleYan, Bo, Duan Bin, Fangfang Ren, Zhiping Xiong, Ke Zhang, Caiqin Wang, and Yukou Du. 2016. "Facile Synthesis of MnPO4·H2O Nanowire/Graphene Oxide Composite Material and Its Application as Electrode Material for High Performance Supercapacitors" Catalysts 6, no. 12: 198. https://doi.org/10.3390/catal6120198
APA StyleYan, B., Bin, D., Ren, F., Xiong, Z., Zhang, K., Wang, C., & Du, Y. (2016). Facile Synthesis of MnPO4·H2O Nanowire/Graphene Oxide Composite Material and Its Application as Electrode Material for High Performance Supercapacitors. Catalysts, 6(12), 198. https://doi.org/10.3390/catal6120198






