The New Graphene Family Materials: Synthesis and Applications in Oxygen Reduction Reaction
Abstract
:1. Introduction
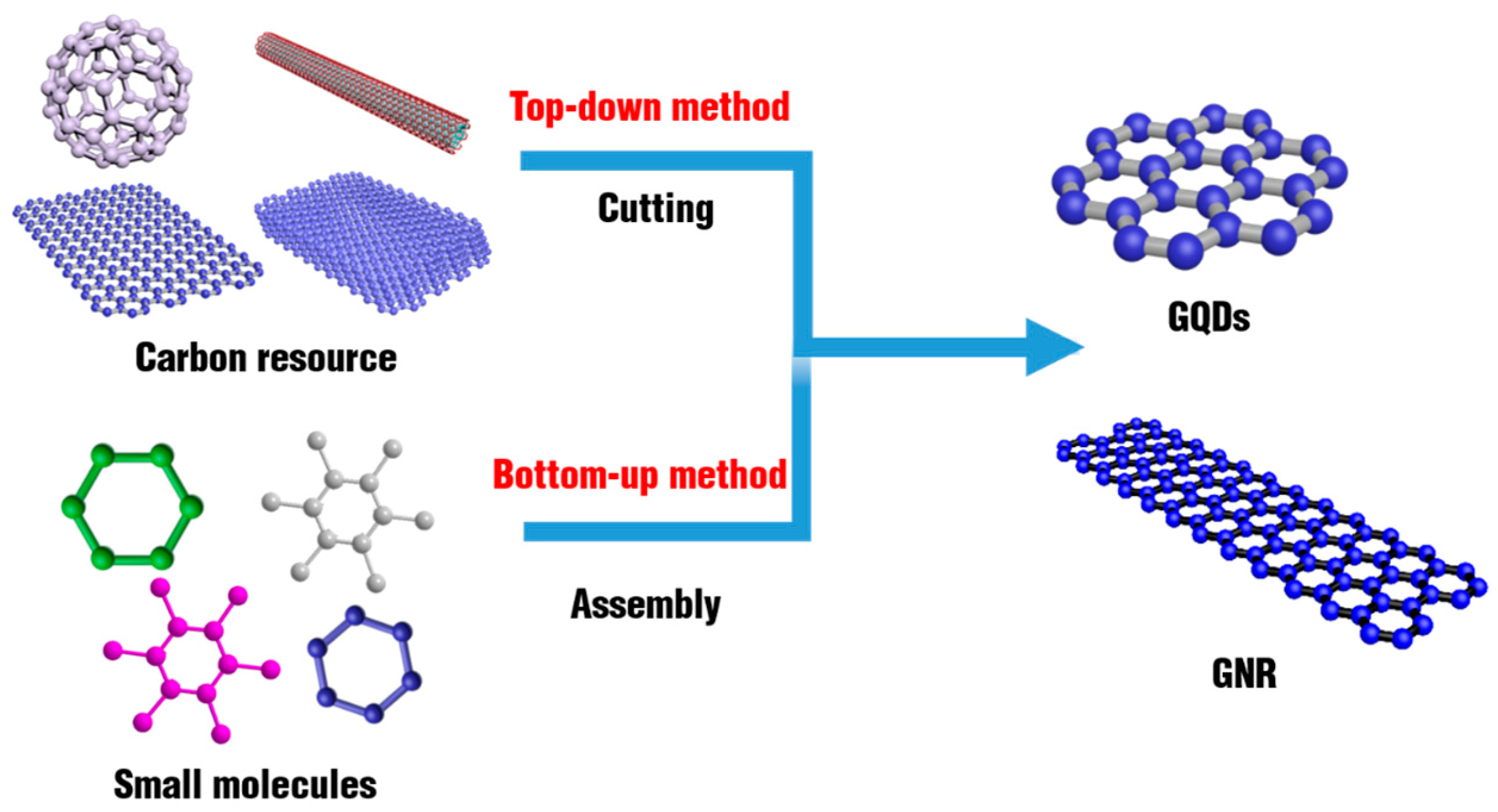
2. The Synthetic Strategy of Graphene Family Materials
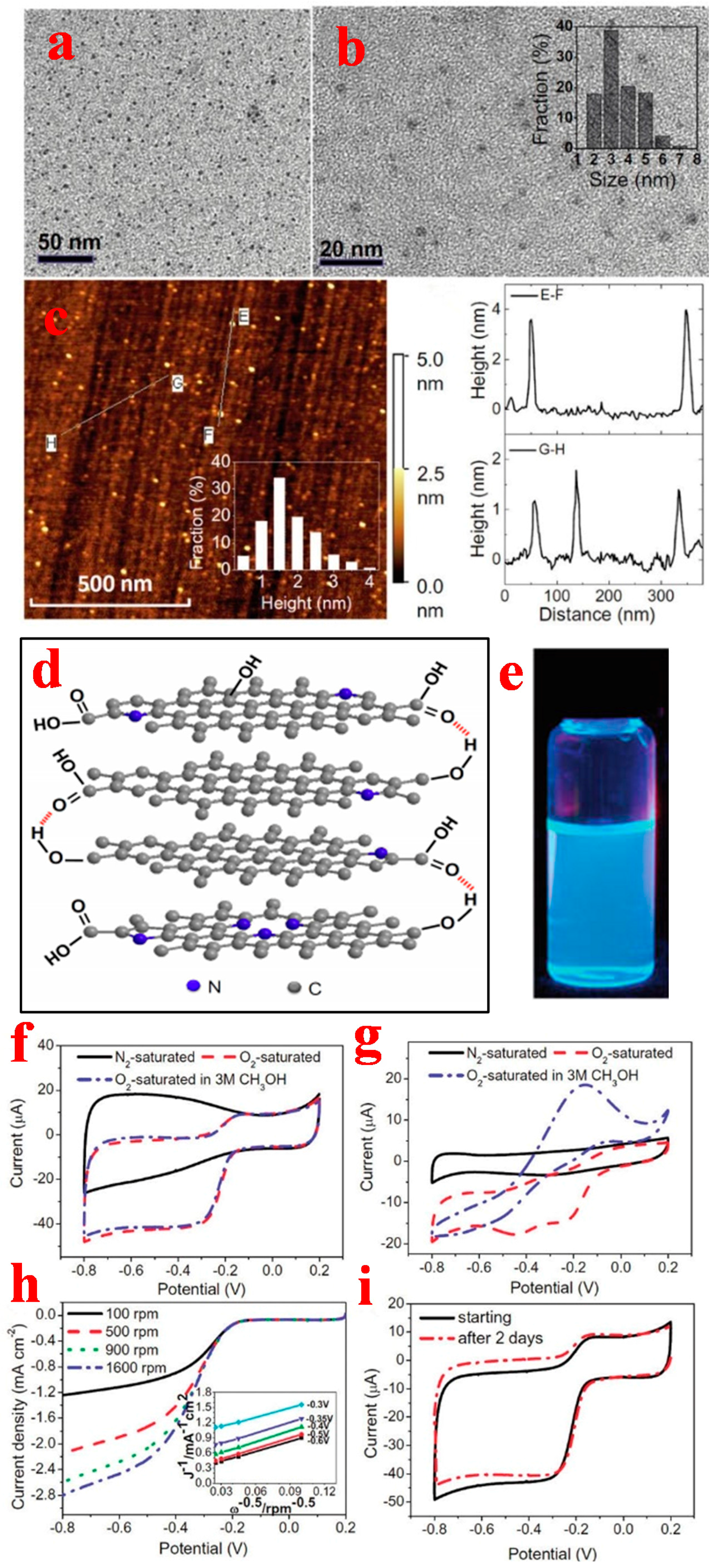
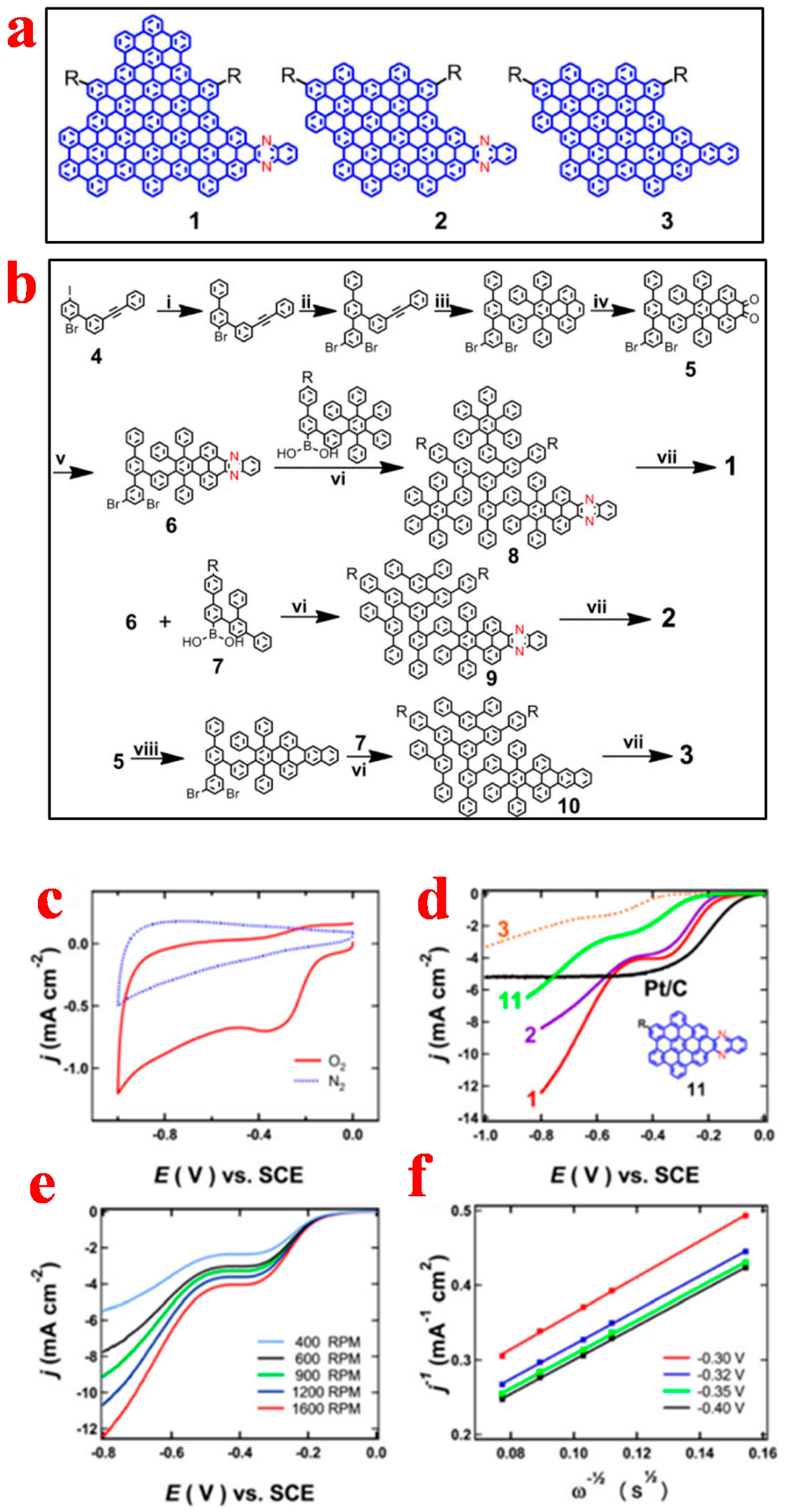
2.1. Quasi-0D Graphene Quantum Dots
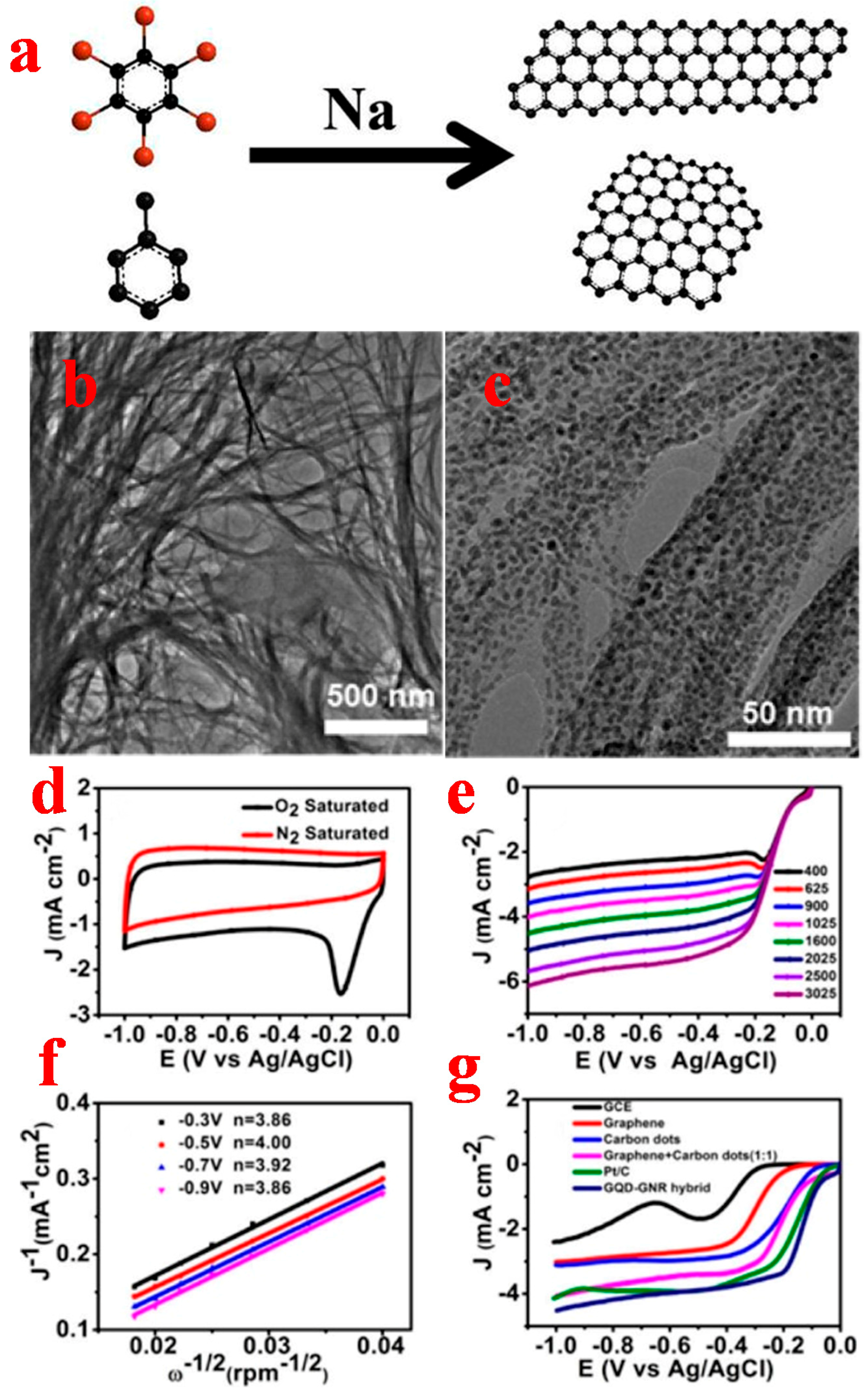
2.1.1. Top-Down Method
2.1.2. Bottom-Up Method
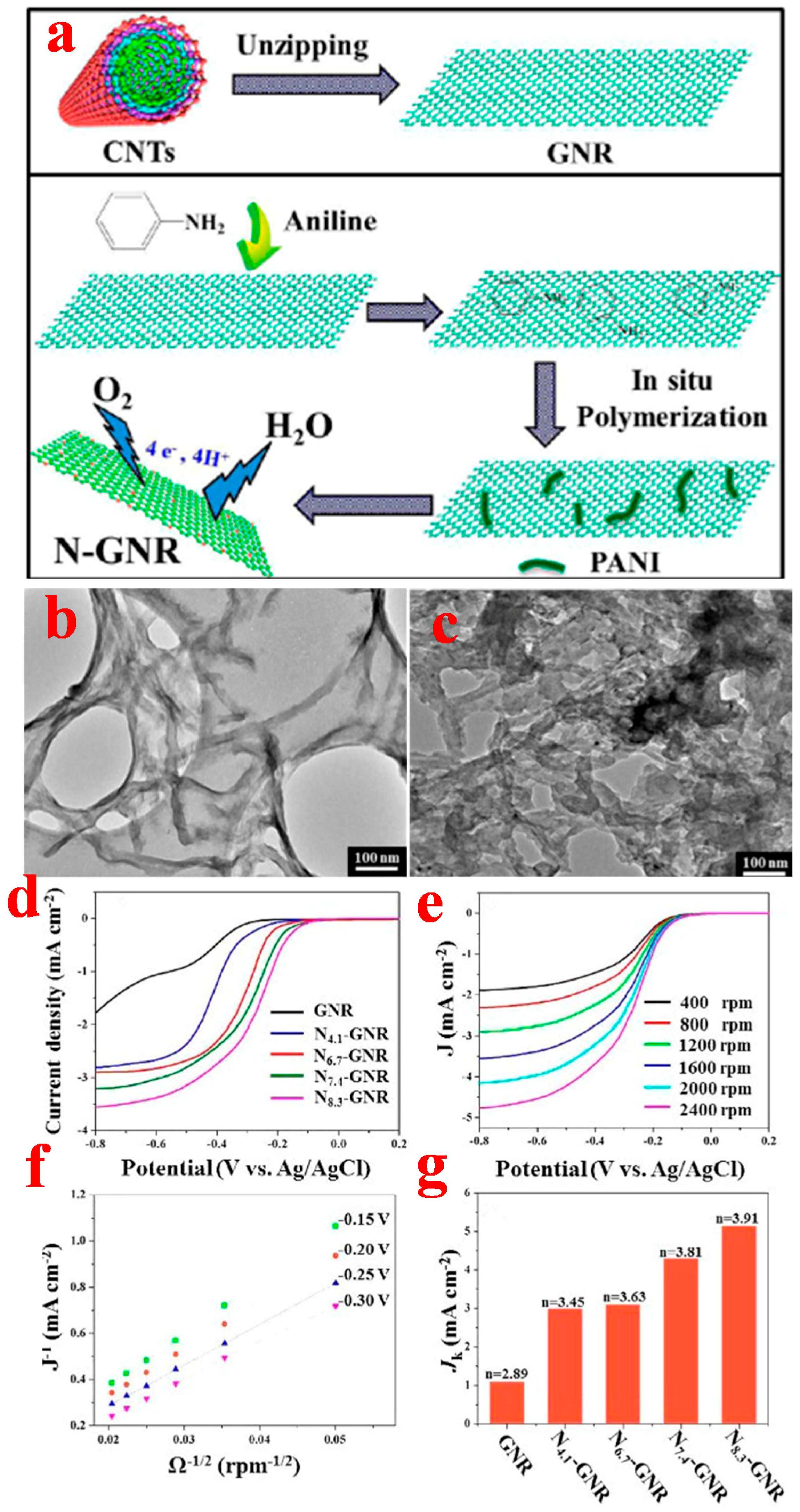
2.2. Quasi-1D Graphene Nanoribbons
2.2.1. Top-Down Method
2.2.2. Bottom-Up Method
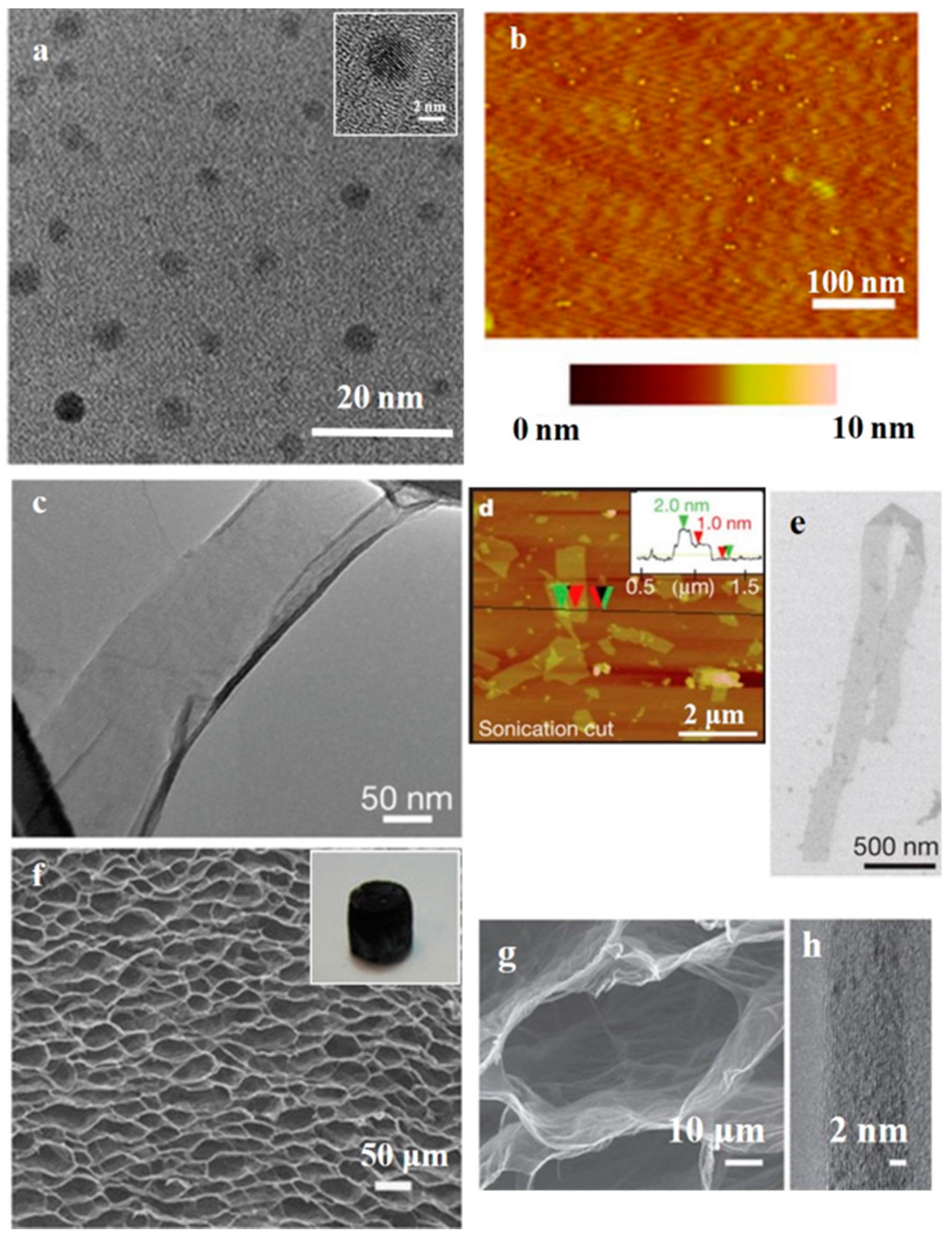
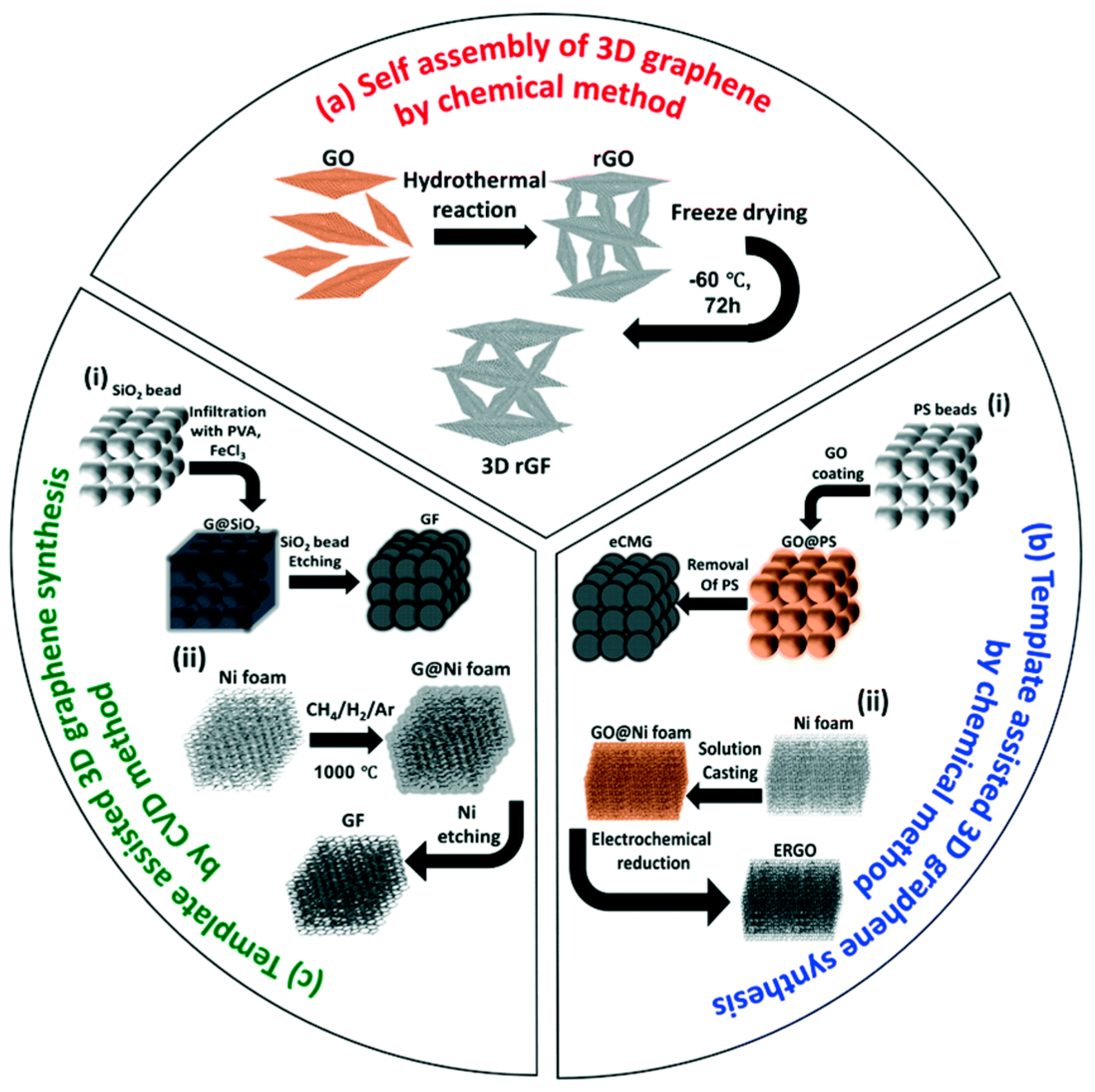
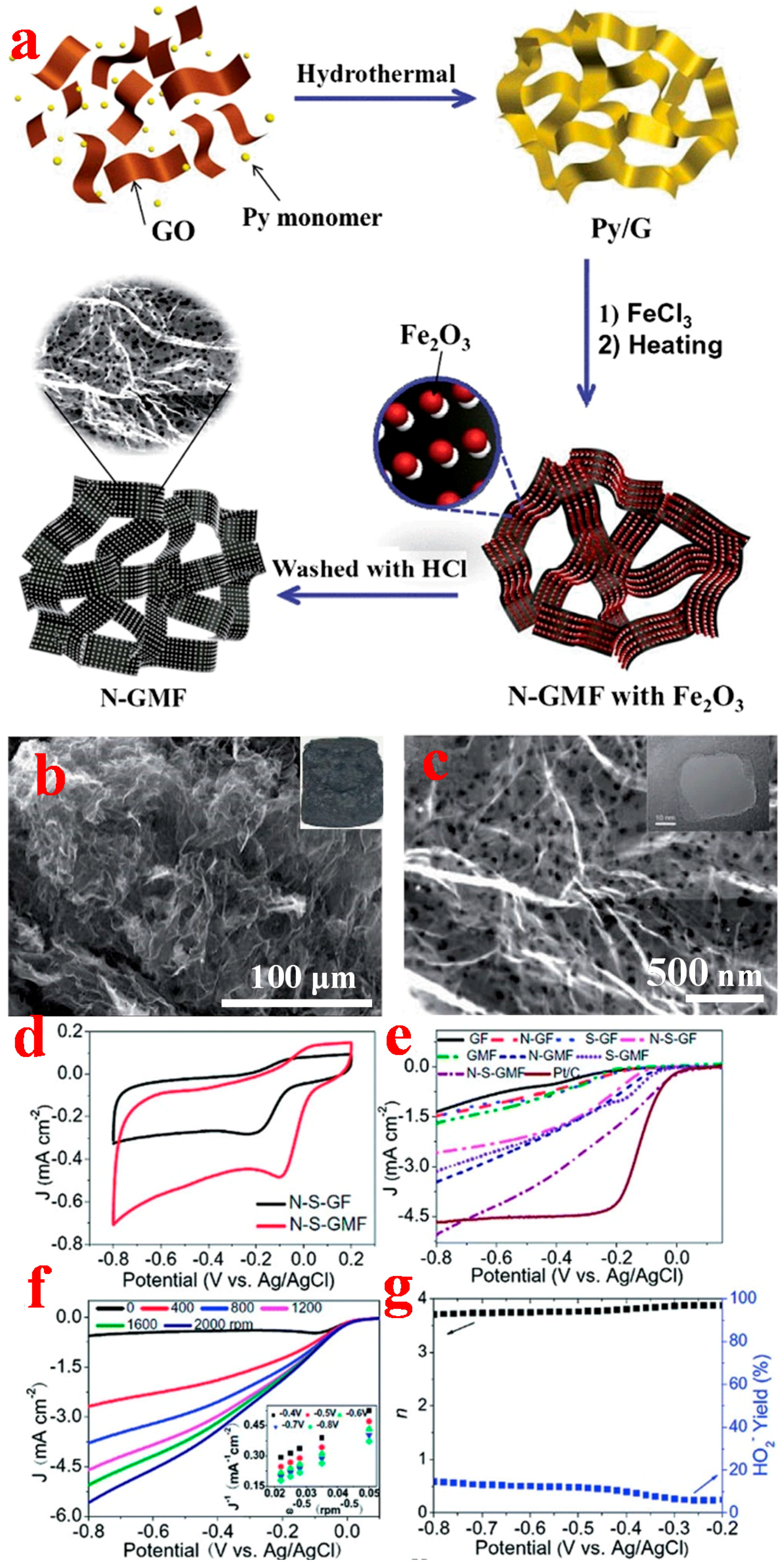
2.3. Macroscopic-3D Graphene
2.3.1. Chemical Assembly Method
2.3.2. Template-Assisted CVD Method
3. Application in Oxygen Reduction Reaction
3.1. Graphene Quantum Dots
3.2. Graphene Nanoribbons
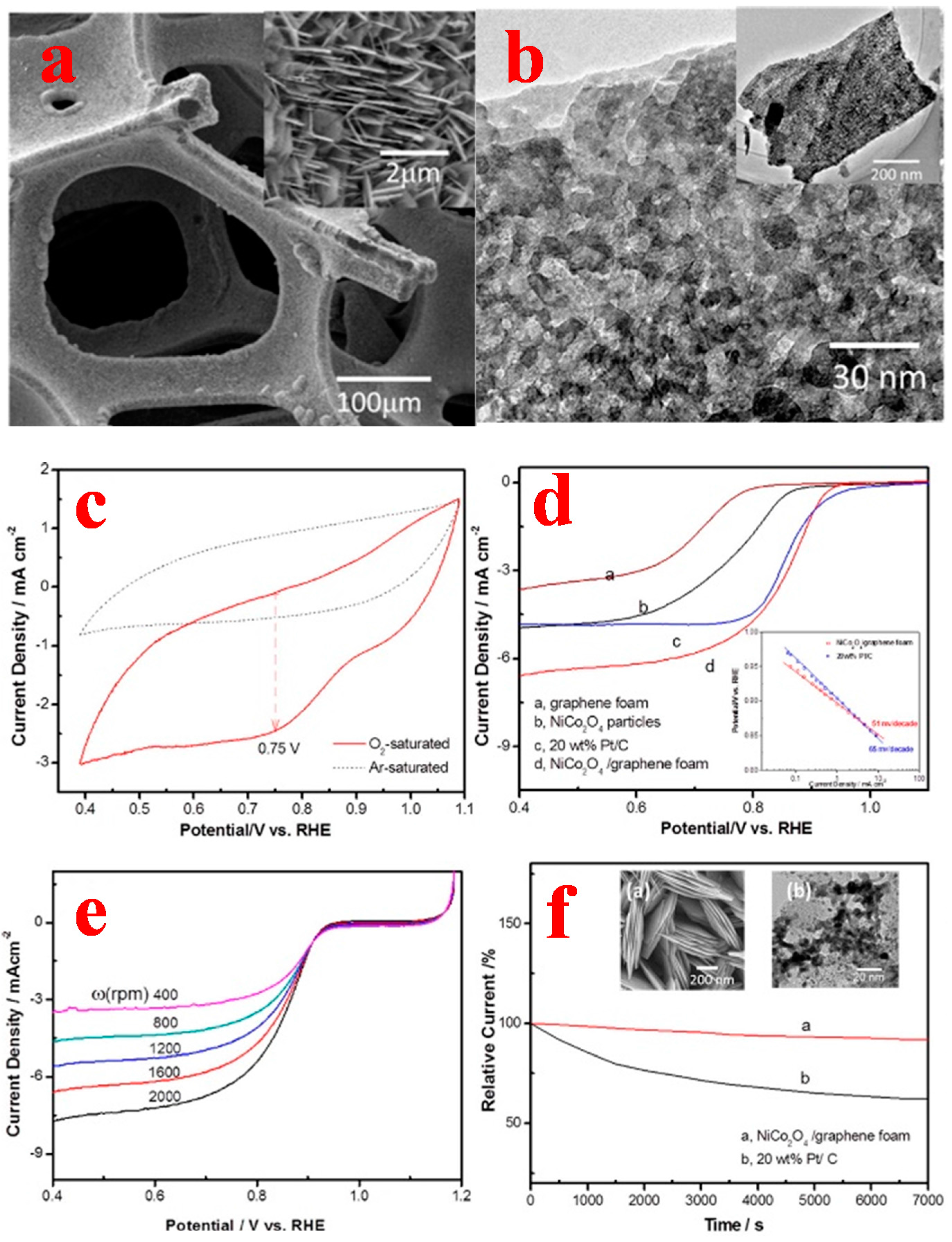
3.3. 3D Graphene
4. Conclusions and Future Outlook
Acknowledgments
Conflicts of Interest
References
- Yang, Z.; Nie, H.; Chen, X.A.; Chen, X.; Huang, S. Recent progress in doped carbon nanomaterials as effective cathode catalysts for fuel cell oxygen reduction reaction. J. Power Sources 2013, 236, 238–249. [Google Scholar] [CrossRef]
- Wu, G.; Zelenay, P. Nanostructured nonprecious metal catalysts for oxygen reduction reaction. Acc. Chem. Res. 2013, 46, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ren, J.; Zhang, Z.; Chen, X.; Guan, G.; Qiu, L.; Zhang, Y.; Peng, H. Recent advancement of nanostructured carbon for energy applications. Chem. Rev. 2015, 115, 5159–5223. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Ding, N.; Andy Hor, T.S.; Liu, Z.; Sun, X.; Zong, Y. Potential of metal-free “graphene alloy” as electrocatalysts for oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 1795–1810. [Google Scholar] [CrossRef]
- Wei, Q.; Tong, X.; Zhang, G.; Qiao, J.; Gong, Q.; Sun, S. Nitrogen-doped carbon nanotube and graphene materials for oxygen reduction reactions. Catalysts 2015, 5, 1574–1602. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, S.; Sun, S. Tuning nanoparticle catalysis for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2013, 52, 8526–8544. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Li, L.; Wei, Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-W.; Su, D. Heterogeneous nanocarbon materials for oxygen reduction reaction. Energy Environ. Sci. 2014, 7, 576–591. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Cheng, N.; Lushington, A.; Sun, X. Recent progress on MOF-derived nanomaterials as advanced electrocatalysts in fuel cells. Catalysts 2016, 6, 116. [Google Scholar] [CrossRef]
- Choi, H.-J.; Jung, S.-M.; Seo, J.-M.; Chang, D.W.; Dai, L.; Baek, J.-B. Graphene for energy conversion and storage in fuel cells and supercapacitors. Nano Energy 2012, 1, 534–551. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, F.; Zhang, Z.; Chen, N.; Qu, L. Dimension-tailored functional graphene structures for energy conversion and storage. Nanoscale 2013, 5, 3112–3126. [Google Scholar] [CrossRef] [PubMed]
- Chabot, V.; Higgins, D.; Yu, A.P.; Xiao, X.C.; Chen, Z.W.; Zhang, J.J. A review of graphene and graphene oxide sponge: Material synthesis and applications to energy and the environment. Energy Environ. Sci. 2014, 7, 1564–1596. [Google Scholar] [CrossRef]
- Holade, Y.; Sahin, N.; Servat, K.; Napporn, T.; Kokoh, K. Recent advances in carbon supported metal nanoparticles preparation for oxygen reduction reaction in low temperature fuel cells. Catalysts 2015, 5, 310–348. [Google Scholar] [CrossRef]
- Ni, J.; Li, Y. Carbon nanomaterials in different dimensions for electrochemical energy storage. Adv. Energy Mater. 2016. [Google Scholar] [CrossRef]
- Liu, J.; Tang, J.; Gooding, J.J. Strategies for chemical modification of graphene and applications of chemically modified graphene. J. Mater. Chem. 2012, 22, 12435–12452. [Google Scholar] [CrossRef]
- Usachov, D.; Vilkov, O.; Grüneis, A.; Haberer, D.; Fedorov, A.; Adamchuk, V.K.; Preobrajenski, A.B.; Dudin, P.; Barinov, A.; Oehzelt, M.; et al. Nitrogen-doped graphene: Efficient growth, structure, and electronic properties. Nano Lett. 2011, 11, 5401–5407. [Google Scholar] [CrossRef] [PubMed]
- Jun, G.H.; Jin, S.H.; Lee, B.; Kim, B.H.; Chae, W.-S.; Hong, S.H.; Jeon, S. Enhanced conduction and charge-selectivity by N-doped graphene flakes in the active layer of bulk-heterojunction organic solar cells. Energy Environ. Sci. 2013, 6, 3000–3006. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.-P.; Chen, J.-F.; Yu, S.-H. Graphene-based macroscopic assemblies and architectures: An emerging material system. Chem. Soc. Rev. 2014, 43, 7295–7325. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, A. Graphene Schottky diodes: An experimental review of the rectifying graphene/semiconductor heterojunction. Phys. Rep. 2016, 606, 1–58. [Google Scholar] [CrossRef]
- Li, X.; Rui, M.; Song, J.; Shen, Z.; Zeng, H. Carbon and graphene quantum dots for optoelectronic and energy devices: A review. Adv. Funct. Mater. 2015, 25, 4929–4947. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Chen, L.; Hernandez, Y.; Feng, X.; Müllen, K. From nanographene and graphene nanoribbons to graphene sheets: Chemical synthesis. Angew. Chem. Int. Ed. 2012, 51, 7640–7654. [Google Scholar] [CrossRef] [PubMed]
- Yazyev, O.V. A guide to the design of electronic properties of graphene nanoribbons. Acc. Chem. Res. 2013, 46, 2319–2328. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, J.; Ding, F. Recent progress and challenges in graphene nanoribbon synthesis. Chemphyschem 2013, 14, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yang, S.; Zhou, H.; Lieberwirth, I.; Feng, X.; Müllen, K. 3D graphene foams cross-linked with pre-encapsulated Fe3O4 nanospheres for enhanced lithium storage. Adv. Mater. 2013, 25, 2909–2914. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Tai, N.H.; Lee, S.B.; Kuo, W.S. Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ. Sci. 2012, 5, 7908–7912. [Google Scholar] [CrossRef]
- Worsley, M.A.; Pauzauskie, P.J.; Olson, T.Y.; Biener, J.; Satcher, J.H.; Baumann, T.F. Synthesis of graphene aerogel with high electrical conductivity. J. Am. Chem. Soc. 2010, 132, 14067–14069. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-L.; Xu, D.; Wang, H.-G.; Wu, Z.; Zhang, X.-B. In situ fabrication of porous graphene electrodes for high-performance energy storage. ACS Nano 2013, 7, 2422–2430. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Xiang, C.; Lin, J.; Peng, Z.; Huang, K.; Yan, Z.; Cook, N.P.; Samuel, E.L.G.; Hwang, C.-C.; Ruan, G.; et al. Coal as an abundant source of graphene quantum dots. Nat. Commun 2013, 4, 2943. [Google Scholar] [CrossRef] [PubMed]
- Kosynkin, D.V.; Higginbotham, A.L.; Sinitskii, A.; Lomeda, J.R.; Dimiev, A.; Price, B.K.; Tour, J.M. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 2009, 458, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Liu, J.Z.; Chang, S.L.Y.; Wu, Y.; Li, D. Biomimetic superelastic graphene-based cellular monoliths. Nat. Commun. 2012, 3, 1241. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Wu, G.H.; Yang, G.H.; Peng, J.; Zhao, J.W.; Zhu, J.J. Focusing on luminescent graphene quantum dots: Current status and future perspectives. Nanoscale 2013, 5, 4015–4039. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Wu, L.; Wei, W.L.; Qu, X.G. Recent advances in graphene quantum dots for sensing. Mater. Today 2013, 16, 433–442. [Google Scholar] [CrossRef]
- Shinde, D.B.; Pillai, V.K. Electrochemical preparation of luminescent graphene quantum dots from multiwalled carbon nanotubes. Chem. A Eur. J. 2012, 18, 12522–12528. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhang, S. Creating high yield water soluble luminescent graphene quantum dots via exfoliating and disintegrating carbon nanotubes and graphite flakes. Chem. Commun. 2012, 48, 10177–10179. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene quantum dots derived from carbon fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.-F.; Teng, C.-Y.; Chen, S.-J.; Teng, H. Nitrogen-doped graphene oxide quantum dots as photocatalysts for overall water-splitting under visible light illumination. Adv. Mater. 2014, 26, 3297–3303. [Google Scholar] [CrossRef] [PubMed]
- Song, S.H.; Jang, M.-H.; Chung, J.; Jin, S.H.; Kim, B.H.; Hur, S.-H.; Yoo, S.; Cho, Y.-H.; Jeon, S. Highly efficient light-emitting diode of graphene quantum dots fabricated from graphite intercalation compounds. Adv. Opt. Mater. 2014, 2, 1016–1023. [Google Scholar] [CrossRef]
- Ananthanarayanan, A.; Wang, X.; Routh, P.; Sana, B.; Lim, S.; Kim, D.-H.; Lim, K.-H.; Li, J.; Chen, P. Facile synthesis of graphene quantum dots from 3D graphene and their application for Fe3+ sensing. Adv. Funct. Mater. 2014, 24, 3021–3026. [Google Scholar] [CrossRef]
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H.; et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 4596. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Gong, T.; Jin, Y.; Cho, Y.; Shin, C.; Lee, C.; Kim, T. Near-UV-emitting graphene quantum dots from graphene hydrogels. Carbon 2015, 94, 181–188. [Google Scholar] [CrossRef]
- He, P.; Sun, J.; Tian, S.Y.; Yang, S.W.; Ding, S.J.; Ding, G.Q.; Xie, X.M.; Jiang, M.H. Processable aqueous dispersions of graphene stabilized by graphene quantum dots. Chem. Mater. 2015, 27, 218–226. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, B.; Qiao, Z.; Hu, Y.; Zheng, W.; Zhang, L.; Zhou, Y.; Ji, G.; Yang, G. Gram-scale synthesis of graphene quantum dots from single carbon atoms growth via energetic material deflagration. Chem. Mater. 2015, 27, 4319–4327. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, L.; Shang, W.; Xie, W.; Ma, H.; Fu, Y.; Fang, D.; Sun, H.; Fan, L.; Han, M.; et al. Facile synthesis of water-soluble, highly fluorescent graphene quantum dots as a robust biological label for stem cells. J. Mater. Chem. 2012, 22, 7461–7467. [Google Scholar] [CrossRef]
- Zhu, Z.; Ma, J.; Wang, Z.; Mu, C.; Fan, Z.; Du, L.; Bai, Y.; Fan, L.; Yan, H.; Phillips, D.L.; et al. Efficiency enhancement of perovskite solar cells through fast electron extraction: The role of graphene quantum dots. J. Am. Chem. Soc. 2014, 136, 3760–3763. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Zhang, J.; Li, Z.; Wu, M. Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R.; et al. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Chen, W. In situ growth of surfactant-free gold nanoparticles on nitrogen-doped graphene quantum dots for electrochemical detection of hydrogen peroxide in biological environments. Anal. Chem. 2015, 87, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, L.A.; Schedin, F.; Katsnelson, M.I.; Yang, R.; Hill, E.W.; Novoselov, K.S.; Geim, A.K. Chaotic dirac billiard in graphene quantum dots. Science 2008, 320, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, K.; Park, W.I.; Kim, B.-H.; Park, J.H.; Kim, T.-H.; Bong, S.; Kim, C.-H.; Chae, G.; Jun, M.; et al. Uniform graphene quantum dots patterned from self-assembled silica nanodots. Nano Lett. 2012, 12, 6078–6083. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhu, Y.; Chen, C.; Yang, X.; Li, C. Facile preparation and upconversion luminescence of graphene quantum dots. Chem. Commun. 2011, 47, 2580–2582. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Yan, X.; Cui, X.; Li, L.-S. Synthesis of large, stable colloidal graphene quantum dots with tunable size. J. Am. Chem. Soc. 2010, 132, 5944–5945. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Cui, X.; Li, B.; Li, L.-S. Large, solution-processable graphene quantum dots as light absorbers for photovoltaics. Nano Lett. 2010, 10, 1869–1873. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Zhang, L.; Wang, X.; Diankov, G.; Dai, H. Narrow graphene nanoribbons from carbon nanotubes. Nature 2009, 458, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, F.; Compagnini, G.; Patané, G.; Ursini, O.; Angelini, G.; Ribic, P.R.; Margaritondo, G.; Cricenti, A.; Palleschi, G.; Valentini, F. Graphene nanoribbons produced by the oxidative unzipping of single-wall carbon nanotubes. Carbon 2010, 48, 2596–2602. [Google Scholar] [CrossRef]
- Kumar, P.; Panchakarla, L.S.; Rao, C.N.R. Laser-induced unzipping of carbon nanotubes to yield graphene nanoribbons. Nanoscale 2011, 3, 2127–2129. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Martinez, E.; Carretero-Gonzalez, J.; Sovich, J.; Lima, M.D. High temperature structural transformations of few layer graphene nanoribbons obtained by unzipping carbon nanotubes. J. Mater. Chem. A 2014, 2, 221–228. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.-S.; Jiang, J.; Chiang, W.-H. Controllable tailoring graphene nanoribbons with tunable surface functionalities: An effective strategy toward high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 17441–17449. [Google Scholar] [CrossRef] [PubMed]
- Han, M.Y.; Özyilmaz, B.; Zhang, Y.; Kim, P. Energy band-gap engineering of graphene nanoribbons. Phys. Rev. Lett. 2007, 98, 206805. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Duan, X.; Huang, Y. Rational fabrication of graphene nanoribbons using a nanowire etch mask. Nano Lett. 2009, 9, 2083–2087. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.C.; Manfrinato, V.R.; Sanchez-Yamagishi, J.D.; Kong, J.; Jarillo-Herrero, P. Anisotropic etching and nanoribbon formation in single-layer graphene. Nano Lett. 2009, 9, 2600–2604. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Zhang, L.; Lee, S.; Dai, H. Chemically derived, ultrasmooth graphene nanoribbon semiconductors. Science 2008, 319, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, N.; Moore, D.; Xu, Z.; Sreeprasad, T.S.; Nagaraja, A.; Rodriguez, A.A.; Berry, V. Nanotomy-based production of transferable and dispersible graphene nanostructures of controlled shape and size. Nat. Commun. 2012, 3, 844. [Google Scholar] [CrossRef] [PubMed]
- Elías, A.L.; Botello-Méndez, A.R.; Meneses-Rodríguez, D.; Jehová González, V.; Ramírez-González, D.; Ci, L.; Muñoz-Sandoval, E.; Ajayan, P.M.; Terrones, H.; Terrones, M. Longitudinal cutting of pure and doped carbon nanotubes to form graphitic nanoribbons using metal clusters as nanoscalpels. Nano Lett. 2010, 10, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Parashar, U.K.; Bhandari, S.; Srivastava, R.K.; Jariwala, D.; Srivastava, A. Single step synthesis of graphene nanoribbons by catalyst particle size dependent cutting of multiwalled carbon nanotubes. Nanoscale 2011, 3, 3876–3882. [Google Scholar] [CrossRef] [PubMed]
- Cano-Márquez, A.G.; Rodríguez-Macías, F.J.; Campos-Delgado, J.; Espinosa-González, C.G.; Tristán-López, F.; Ramírez-González, D.; Cullen, D.A.; Smith, D.J.; Terrones, M.; Vega-Cantú, Y.I. Ex-MWNTs: Graphene sheets and ribbons produced by lithium intercalation and exfoliation of carbon nanotubes. Nano Lett. 2009, 9, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Kosynkin, D.V.; Lu, W.; Sinitskii, A.; Pera, G.; Sun, Z.; Tour, J.M. Highly conductive graphene nanoribbons by longitudinal splitting of carbon nanotubes using potassium vapor. ACS Nano 2011, 5, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Genorio, B.; Lu, W.; Dimiev, A.M.; Zhu, Y.; Raji, A.-R.O.; Novosel, B.; Alemany, L.B.; Tour, J.M. In situ intercalation replacement and selective functionalization of graphene nanoribbon stacks. ACS Nano 2012, 6, 4231–4240. [Google Scholar] [CrossRef] [PubMed]
- Talirz, L.; Söde, H.; Cai, J.; Ruffieux, P.; Blankenburg, S.; Jafaar, R.; Berger, R.; Feng, X.; Müllen, K.; Passerone, D.; et al. Termini of bottom-up fabricated graphene nanoribbons. J. Am. Chem. Soc. 2013, 135, 2060–2063. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.H.; Shekhirev, M.; Kunkel, D.A.; Morton, M.D.; Berglund, E.; Kong, L.; Wilson, P.M.; Dowben, P.A.; Enders, A.; Sinitskii, A. Large-scale solution synthesis of narrow graphene nanoribbons. Nat. Commun. 2014, 5, 3189. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Liu, Y.; Zhang, H.; Huang, L.; Wu, B.; Chen, J.; Yu, G. Scalable synthesis of few-layer graphene ribbons with controlled morphologies by a template method and their applications in nanoelectromechanical switches. J. Am. Chem. Soc. 2009, 131, 11147–11154. [Google Scholar] [CrossRef] [PubMed]
- Sprinkle, M.; Ruan, M.; Hu, Y.; Hankinson, J.; Rubio Roy, M.; Zhang, B.; Wu, X.; Berger, C.; de Heer, W.A. Scalable templated growth of graphene nanoribbons on sic. Nat. Nano 2010, 5, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Patil, U.; Lee, S.C.; Kulkarni, S.; Sohn, J.S.; Nam, M.S.; Han, S.; Jun, S.C. Nanostructured pseudocapacitive materials decorated 3D graphene foam electrodes for next generation supercapacitors. Nanoscale 2015, 7, 6999–7021. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Shen, Y.; Chen, B. Synthesis, decoration and properties of three-dimensional graphene-based macrostructures: A review. Chem. Eng. J. 2015, 264, 753–771. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y. Three-dimensional graphene networks: Synthesis, properties and applications. Natl. Sci. Rev. 2015, 2, 40–53. [Google Scholar] [CrossRef]
- Mao, S.; Lu, G.; Chen, J. Three-dimensional graphene-based composites for energy applications. Nanoscale 2015, 7, 6924–6943. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shi, G.; Duan, X. Self-assembled three-dimensional graphene macrostructures: Synthesis and applications in supercapacitors. Acc. Chem. Res. 2015, 48, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yao, W.; Hu, L.; Liu, D.; Wang, C.; Lee, C.-S. Progress in the preparation and application of three-dimensional graphene-based porous nanocomposites. Nanoscale 2015, 7, 5563–5577. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, S.; Chen, C.; Yan, L. Self-assembly and embedding of nanoparticles by in situ reduced graphene for preparation of a 3D graphene/nanoparticle aerogel. Adv. Mater. 2011, 23, 5679–5683. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.; Sun, Y.; Li, C.; Yuan, W.; Shi, G. Ultrahigh-rate supercapacitors based on eletrochemically reduced graphene oxide for ac line-filtering. Sci. Rep. 2012, 2, 247. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.-P.; Ren, X.-C.; Wang, P.; Yu, S.-H. Macroscopic multifunctional graphene-based hydrogels and aerogels by a metal ion induced self-assembly process. ACS Nano 2012, 6, 2693–2703. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Seo, T.S. A controllable self-assembly method for large-scale synthesis of graphene sponges and free-standing graphene films. Adv. Funct. Mater. 2010, 20, 1930–1936. [Google Scholar] [CrossRef]
- Choi, B.G.; Yang, M.; Hong, W.H.; Choi, J.W.; Huh, Y.S. 3D macroporous graphene frameworks for supercapacitors with high energy and power densities. ACS Nano 2012, 6, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, G.; Ling, Y.; Qian, F.; Song, Y.; Lu, X.; Chen, S.; Tong, Y.; Li, Y. High power density microbial fuel cell with flexible 3D graphene-nickel foam as anode. Nanoscale 2013, 5, 10283–10290. [Google Scholar] [CrossRef] [PubMed]
- Song, W.L.; Song, K.; Fan, L.Z. A versatile strategy toward binary three-dimensional architectures based on engineering graphene aerogels with porous carbon fabrics for supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 4257–4264. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Shi, Y.; Shi, W.; Lu, G.; Huang, X.; Yan, Q.; Zhang, Q.; Zhang, H. Preparation of novel 3D graphene networks for supercapacitor applications. Small 2011, 7, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.-M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, X.; Wang, J.; Song, H.; Li, X.; Wang, L.; Chan-Park, M.B.; Li, C.M.; Chen, P. Synthesis of a MnO2–graphene foam hybrid with controlled MnO2 particle shape and its use as a supercapacitor electrode. Carbon 2012, 50, 4865–4870. [Google Scholar] [CrossRef]
- Ito, Y.; Tanabe, Y.; Qiu, H.J.; Sugawara, K.; Heguri, S.; Tu, N.H.; Huynh, K.K.; Fujita, T.; Takahashi, T.; Tanigaki, K. High-quality three-dimensional nanoporous graphene. Angew. Chem. Int. Ed. 2014, 53, 4822–4826. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gao, S.; Wu, L.; Qiu, S.; Guo, Y.; Geng, X.; Chen, M.; Liao, S.; Zhu, C.; Gong, Y.; et al. High-density three-dimension graphene macroscopic objects for high-capacity removal of heavy metal ions. Sci. Rep. 2013, 3, 2125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Lin, T.; Huang, F.; Zhong, Y.; Wang, Z.; Tang, Y.; Bi, H.; Wan, D.; Lin, J. Highly conductive porous graphene/ceramic composites for heat transfer and thermal energy storage. Adv. Funct. Mater. 2013, 23, 2263–2269. [Google Scholar] [CrossRef]
- Mecklenburg, M.; Schuchardt, A.; Mishra, Y.K.; Kaps, S.; Adelung, R.; Lotnyk, A.; Kienle, L.; Schulte, K. Aerographite: Ultra lightweight, flexible nanowall, carbon microtube material with outstanding mechanical performance. Adv. Mater. 2012, 24, 3486–3490. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Beechem, T.E.; Brumbach, M.T.; Lambert, T.N.; Davis, D.J.; Michael, J.R.; Washburn, C.M.; Wang, J.; Brozik, S.M.; Wheeler, D.R.; et al. Lithographically defined three-dimensional graphene structures. ACS Nano 2012, 6, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-C.; Lee, J.-S.; Kim, S.-I.; Kim, K.-H.; Jang, J.-H. Three-dimensional graphene nano-networks with high quality and mass production capability via precursor-assisted chemical vapor deposition. Sci. Rep. 2013, 3, 1788. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-doped graphene quantum dots with oxygen-rich functional groups. J. Am. Chem. Soc. 2012, 134, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Deming, C.P.; Mercado, R.; Gadiraju, V.; Sweeney, S.W.; Khan, M.; Chen, S. Graphene quantum dots-supported palladium nanoparticles for efficient electrocatalytic reduction of oxygen in alkaline media. ACS Sustain. Chem. Eng. 2015, 3, 3315–3323. [Google Scholar] [CrossRef]
- Chun, K.-Y.; Lee, H.S.; Lee, C.J. Nitrogen doping effects on the structure behavior and the field emission performance of double-walled carbon nanotubes. Carbon 2009, 47, 169–177. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Dai, L.; Li, L.-S. Nitrogen-doped colloidal graphene quantum dots and their size-dependent electrocatalytic activity for the oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 18932–18935. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, P. Graphene quantum dot hybrids as efficient metal-free electrocatalyst for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 2013, 5, 3362–3369. [Google Scholar] [CrossRef] [PubMed]
- Saidi, W.A. Oxygen reduction electrocatalysis using N-doped graphene quantum-dots. J. Phys. Chem. Lett. 2013, 4, 4160–4165. [Google Scholar] [CrossRef]
- Fei, H.; Ye, R.; Ye, G.; Gong, Y.; Peng, Z.; Fan, X.; Samuel, E.L.G.; Ajayan, P.M.; Tour, J.M. Boron- and nitrogen-doped graphene quantum dots/graphene hybrid nanoplatelets as efficient electrocatalysts for oxygen reduction. ACS Nano 2014, 8, 10837–10843. [Google Scholar] [CrossRef] [PubMed]
- Favaro, M.; Ferrighi, L.; Fazio, G.; Colazzo, L.; Di Valentin, C.; Durante, C.; Sedona, F.; Gennaro, A.; Agnoli, S.; Granozzi, G. Single and multiple doping in graphene quantum dots: Unraveling the origin of selectivity in the oxygen reduction reaction. ACS Catal. 2015, 5, 129–144. [Google Scholar] [CrossRef]
- Fan, M.; Zhu, C.; Yang, J.; Sun, D. Facile self-assembly N-doped graphene quantum dots/graphene for oxygen reduction reaction. Electrochim. Acta 2016, 216, 102–109. [Google Scholar] [CrossRef]
- Wang, M.; Fang, Z.; Zhang, K.; Fang, J.; Qin, F.; Zhang, Z.; Li, J.; Liu, Y.; Lai, Y. Synergistically enhanced activity of graphene quantum dots/graphene hydrogel composites: A novel all-carbon hybrid electrocatalyst for metal/air batteries. Nanoscale 2016, 8, 11398–11402. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Song, Y.; He, S.; Tjiu, W.W.; Pan, J.; Xia, Y.-Y.; Liu, T. Nitrogen-doped graphene nanoribbons as efficient metal-free electrocatalysts for oxygen reduction. ACS Appl. Mater. Interfaces 2014, 6, 4214–4222. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, K.; Woo, S.I.; Jung, Y. On the mechanism of enhanced oxygen reduction reaction in nitrogen-doped graphene nanoribbons. Phys. Chem. Chem. Phys. 2011, 13, 17505–17510. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, H.; Zhao, J.; Zhu, Y.; Yuan, W.Z.; Zhang, Y. Graphene nanoribbons as a novel support material for high performance fuel cell electrocatalysts. Int. J. Hydrog. Energy 2013, 38, 13230–13237. [Google Scholar] [CrossRef]
- Davis, D.J.; Raji, A.-R.O.; Lambert, T.N.; Vigil, J.A.; Li, L.; Nan, K.; Tour, J.M. Silver-graphene nanoribbon composite catalyst for the oxygen reduction reaction in alkaline electrolyte. Electroanal 2014, 26, 164–170. [Google Scholar] [CrossRef]
- Xiao, B.B.; Lang, X.Y.; Jiang, Q. Pt monatomic wire supported on graphene nanoribbon for oxygen reduction reaction. RSC. Adv. 2014, 4, 28400–28408. [Google Scholar] [CrossRef]
- Fan, X.; Peng, Z.; Ye, R.; Zhou, H.; Guo, X. M3C (M: Fe, Co, Ni) nanocrystals encased in graphene nanoribbons: An active and stable bifunctional electrocatalyst for oxygen reduction and hydrogen evolution reactions. ACS Nano 2015, 9, 7407–7418. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Fei, H.; Zou, X.; Zhou, W.; Yang, S.; Ye, G.; Liu, Z.; Peng, Z.; Lou, J.; Vajtai, R.; et al. Boron- and nitrogen-substituted graphene nanoribbons as efficient catalysts for oxygen reduction reaction. Chem. Mater. 2015, 27, 1181–1186. [Google Scholar] [CrossRef]
- Jin, H.; Huang, H.; He, Y.; Feng, X.; Wang, S.; Dai, L.; Wang, J. Graphene quantum dots supported by graphene nanoribbons with ultrahigh electrocatalytic performance for oxygen reduction. J. Am. Chem. Soc. 2015, 137, 7588–7591. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chan, H.M.; Sun, C.-L.; Tseng, C.-M.; Zhao, C. Interconnected core-shell carbon nanotube-graphene nanoribbon scaffolds for anchoring cobalt oxides as bifunctional electrocatalysts for oxygen evolution and reduction. J. Mater. Chem. A 2015, 3, 13371–13376. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhang, L.; Yoon, Y.; Weber, P.K.; Wang, H.; Guo, J.; Dai, H. N-Doping of graphene through electrothermal reactions with ammonia. Science 2009, 324, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, G.; Chen, P. Three-dimensional porous architectures of carbon nanotubes and graphene sheets for energy applications. Front. Energy Res. 2014, 2. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Zeng, G.; Wu, Y.; Liu, Y.; Jiang, Q.; Gu, S. Three dimensional graphene based materials: Synthesis and applications from energy storage and conversion to electrochemical sensor and environmental remediation. Adv. Colloid Interfaces 2015, 221, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.-J.; Liu, L.; Wang, Y. Template-directed fabrication of 3D graphene-based composite and their electrochemical energy-related applications. Sci. Bull. 2016, 61, 443–450. [Google Scholar] [CrossRef]
- Wang, L.; Sofer, Z.; Ambrosi, A.; Šimek, P.; Pumera, M. 3D-graphene for electrocatalysis of oxygen reduction reaction: Increasing number of layers increases the catalytic effect. Electrochem. Commun. 2014, 46, 148–151. [Google Scholar] [CrossRef]
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Sulfur and nitrogen dual-doped mesoporous graphene electrocatalyst for oxygen reduction with synergistically enhanced performance. Angew. Chem. Int. Ed. 2012, 51, 11496–11500. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, C.; Song, L.; Wang, L.; Shi, G.; Dai, L.; Qu, L. Functional graphene nanomesh foam. Energy Environ. Sci. 2014, 7, 1913–1918. [Google Scholar] [CrossRef]
- Tong, X.; Chen, S.; Guo, C.; Xia, X.; Guo, X.-Y. Mesoporous NiCo2O4 nanoplates on three-dimensional graphene foam as an efficient electrocatalyst for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 2016, 8, 28274–28282. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Nie, H.; Yang, Z.; Zhang, J.; Liu, Z.; Xu, X.; Huang, S. Metal-free selenium doped carbon nanotube/graphene networks as a synergistically improved cathode catalyst for oxygen reduction reaction. Nanoscale 2012, 4, 6455–6460. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-S.; Yang, S.; Sun, Y.; Parvez, K.; Feng, X.; Müllen, K. 3D nitrogen-doped graphene aerogel-supported Fe3O4 nanoparticles as efficient electrocatalysts for the oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 9082–9085. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, C.; Hu, Y.; Cheng, H.; Shi, G.; Qu, L. A versatile, ultralight, nitrogen-doped graphene framework. Angew. Chem. 2012, 124, 11533–11537. [Google Scholar] [CrossRef]
- Fu, X.; Jin, J.; Liu, Y.; Liu, Q.; Niu, K.; Zhang, J.; Cao, X. Graphene-xerogel-based non-precious metal catalyst for oxygen reduction reaction. Electrochem. Commun. 2013, 28, 5–8. [Google Scholar] [CrossRef]
- Liang, J.; Du, X.; Gibson, C.; Du, X.W.; Qiao, S.Z. N-Doped graphene natively grown on hierarchical ordered porous carbon for enhanced oxygen reduction. Adv. Mater. 2013, 25, 6226–6231. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Waller, G.H.; Liu, Y.; Liu, M.; Wong, C.-P. 3D nitrogen-doped graphene prepared by pyrolysis of graphene oxide with polypyrrole for electrocatalysis of oxygen reduction reaction. Nano Energy 2013, 2, 241–248. [Google Scholar] [CrossRef]
- Lin, Z.; Waller, G.H.; Liu, Y.; Liu, M.; Wong, C.-P. Simple preparation of nanoporous few-layer nitrogen-doped graphene for use as an efficient electrocatalyst for oxygen reduction and oxygen evolution reactions. Carbon 2013, 53, 130–136. [Google Scholar] [CrossRef]
- Xue, Y.; Yu, D.; Dai, L.; Wang, R.; Li, D.; Roy, A.; Lu, F.; Chen, H.; Liu, Y.; Qu, J. Three-dimensional B,N-doped graphene foam as a metal-free catalyst for oxygen reduction reaction. Phys. Chem. Chem. Phys. 2013, 15, 12220–12226. [Google Scholar] [CrossRef] [PubMed]
- Bikkarolla, S.K.; Yu, F.; Zhou, W.; Joseph, P.; Cumpson, P.; Papakonstantinou, P. A three-dimensional Mn3O4 network supported on a nitrogenated graphene electrocatalyst for efficient oxygen reduction reaction in alkaline media. J. Mater. Chem. A 2014, 2, 14493–14501. [Google Scholar] [CrossRef]
- Chen, L.; Cui, X.; Wang, Y.; Wang, M.; Qiu, R.; Shu, Z.; Zhang, L.; Hua, Z.; Cui, F.; Wei, C.; et al. One-step synthesis of sulfur doped graphene foam for oxygen reduction reactions. Dalton Trans. 2014, 43, 3420–3423. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Takeshi, D.; Orejon, D.; Sasaki, K.; Lyth, S.M. Defective nitrogen-doped graphene foam: A metal-free, non-precious electrocatalyst for the oxygen reduction reaction in acid. J. Electrochem. Soc. 2014, 161, F544–F550. [Google Scholar] [CrossRef]
- Liu, J.; Takeshi, D.; Sasaki, K.; Lyth, S.M. Platinum-decorated nitrogen-doped graphene foam electrocatalysts. Fuel Cells 2014, 14, 728–734. [Google Scholar] [CrossRef]
- Qin, Y.; Li, J.; Yuan, J.; Kong, Y.; Tao, Y.; Lin, F.; Li, S. Hollow mesoporous carbon nitride nanosphere/three-dimensional graphene composite as high efficient electrocatalyst for oxygen reduction reaction. J. Power Sources 2014, 272, 696–702. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, C.; Liu, F.; Hou, Y. Hybrid of iron nitride and nitrogen-doped graphene aerogel as synergistic catalyst for oxygen reduction reaction. Adv. Funct. Mater. 2014, 24, 2930–2937. [Google Scholar] [CrossRef]
- Yuan, W.; Li, J.; Wang, L.; Chen, P.; Xie, A.; Shen, Y. Nanocomposite of N-doped TiO2 nanorods and graphene as an effective electrocatalyst for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 2014, 6, 21978–21985. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yan, J.; Liu, Y.; Li, J. Three-dimensional nitrogen-doped graphene/MnO nanoparticle hybrids as a high-performance catalyst for oxygen reduction reaction. J. Phys. Chem. C 2015, 119, 8032–8037. [Google Scholar] [CrossRef]
- Cheng, J.; Li, Y.; Huang, X.; Wang, Q.; Mei, A.; Shen, P.K. Highly stable electrocatalysts supported on nitrogen-self-doped three-dimensional graphene-like networks with hierarchical porous structures. J. Mater. Chem. A 2015, 3, 1492–1497. [Google Scholar] [CrossRef]
- Huang, Q.; Tao, F.; Zou, L.; Yuan, T.; Zou, Z.; Zhang, H.; Zhang, X.; Yang, H. One-step synthesis of Pt nanoparticles highly loaded on graphene aerogel as durable oxygen reduction electrocatalyst. Electrochim. Acta 2015, 152, 140–145. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Hou, Y.; Shi, D.; Wexler, D.; Poynton, S.D.; Slade, R.C.T.; Zhang, W.; Liu, H.; Chen, J. N-Doped crumpled graphene derived from vapor phase deposition of PPy on graphene aerogel as an efficient oxygen reduction reaction electrocatalyst. ACS Appl. Mater. Interfaces 2015, 7, 7066–7072. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cao, X.; Ping, J.; Wang, Y.; Lin, T.; Huang, X.; Ma, Q.; Wang, F.; He, C.; Zhang, H. Electrochemical doping of three-dimensional graphene networks used as efficient electrocatalysts for oxygen reduction reaction. Nanoscale 2015, 7, 9394–9398. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Su, Y.; Liu, D.; He, X. Three-dimensional N,B-doped graphene aerogel as a synergistically enhanced metal-free catalyst for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2015, 17, 25440–25448. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Wang, L.; Zhang, R.; Liu, B.; Wang, Y.; Kong, J. Facile preparation of N-doped mesocellular graphene foam from sludge flocs for highly efficient oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 15171–15176. [Google Scholar] [CrossRef]
- Yun, S.; Lee, S.; Shin, C.; Park, S.; Kwon, S.J.; Park, H.S. One-pot self-assembled, reduced graphene oxide/palladium nanoparticle hybrid aerogels for electrocatalytic applications. Electrochim. Acta 2015, 180, 902–908. [Google Scholar] [CrossRef]
- Zhou, X.; Bai, Z.; Wu, M.; Qiao, J.; Chen, Z. 3-Dimensional porous N-doped graphene foam as a non-precious catalyst for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 3343–3350. [Google Scholar] [CrossRef]
- Zhou, Y.; Yen, C.H.; Fu, S.; Yang, G.; Zhu, C.; Du, D.; Wo, P.C.; Cheng, X.; Yang, J.; Wai, C.M.; et al. One-pot synthesis of B-doped three-dimensional reduced graphene oxide via supercritical fluid for oxygen reduction reaction. Green Chem. 2015, 17, 3552–3560. [Google Scholar] [CrossRef]
- Fu, X.; Choi, J.-Y.; Zamani, P.; Jiang, G.; Hoque, M.A.; Hassan, F.M.; Chen, Z. Co–n decorated hierarchically porous graphene aerogel for efficient oxygen reduction reaction in acid. ACS Appl. Mater. Interfaces 2016, 8, 6488–6495. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ma, X.; Zhang, X.; Zhang, Y.; Yu, D.; He, X. Spinel CoMn2O4 nanoparticles supported on nitrogen and phosphorus dual doped graphene aerogel as efficient electrocatalysts for the oxygen reduction reaction. RSC Adv. 2016, 6, 96436–96444. [Google Scholar] [CrossRef]
- Tang, S.; Zhou, X.; Xu, N.; Bai, Z.; Qiao, J.; Zhang, J. Template-free synthesis of three-dimensional nanoporous n-doped graphene for high performance fuel cell oxygen reduction reaction in alkaline media. Appl. Energy 2016, 175, 405–413. [Google Scholar] [CrossRef]
- Wang, M.; Hou, Y.; Slade, R.C.T.; Wang, J.; Shi, D.; Wexler, D.; Liu, H.; Chen, J. Core-shell Co/CoO integrated on 3D nitrogen doped reduced graphene oxide aerogel as an enhanced electrocatalyst for the oxygen reduction reaction. Front. Chem. 2016, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Dou, Z.; Chang, J.; Cui, L. Nitrogen and sulfur Co-doped graphene aerogels as an efficient metal-free catalyst for oxygen reduction reaction in an alkaline solution. RSC Adv. 2016, 6, 22781–22790. [Google Scholar] [CrossRef]
- Yan, W.; Cao, X.; Tian, J.; Jin, C.; Ke, K.; Yang, R. Nitrogen/sulfur dual-doped 3D reduced graphene oxide networks-supported CoFe2O4 with enhanced electrocatalytic activities for oxygen reduction and evolution reactions. Carbon 2016, 99, 195–202. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, Y.; Guan, Y.; Xue, J.; Cui, L. Construction of a cobalt-embedded nitrogen-doped carbon material with the desired porosity derived from the confined growth of MOFs within graphene aerogels as a superior catalyst towards HER and ORR. J. Mater. Chem. A 2016, 4, 15536–15545. [Google Scholar] [CrossRef]

| The Catalyst | The Types of the Catalysts | Electro-Catalytic Performance | Onset Potential | Electron Transfer Number | Ref. |
|---|---|---|---|---|---|
| 3D-Se-CNTs/G 1 | Metal NPs, supported | Excellent catalytic activity, long-term stability and a high methanol tolerance | - | 3.95 | [127] |
| 3D-S,N-G | Mixed doped | Excellent catalytic activity, including highly positive onset potential and high kinetic limiting current | −0.06 V vs. Ag/AgCl | 3.3–3.6 | [124] |
| Fe3O4/3D-N-G 2 | Metal oxide, supported | More positive onset potential, higher cathodic density, lower H2O2 yield | −0.19 V vs. Ag/AgCl | 3.72–3.95 | [128] |
| 3D-N-G | N-doped | Lower onset potential than that of Pt/C, higher diffusion current density | 0.18 V vs. Ag/AgCl | 3.7 | [129] |
| Co-3D-N-G | Metal oxide, supported | Excellent electrocatalytic activity for the ORR in alkaline electrolyte | −0.05 V vs. Ag/AgCl | 3.97 | [130] |
| N-macro-mesoporous carbon/3D-G | Nanomaterial, supported | Excellent ORR activity, a complete tolerance to methanol cross-over effect and excellent long-term durability | −0.05 V vs. Ag/AgCl | - | [131] |
| 3D-N-G | N-doped | One of the best performing NGs for ORR electrocatalysis and superior to other scalable preparation methods | −0.2 V vs. Ag/AgCl | 3.8–3.9 | [132] |
| 3D-N-G | N-doped | Large kinetic-limiting current density, long-term stability and good tolerance to methanol crossover | −0.2 V vs. Ag/AgCl | 3.8–3.9 | [133] |
| 3D-B,N-G | Mixed doped | A higher current generation capability, better stability and superior tolerance to the methanol crossover effect | −0.16 V vs. SCE | 3.4–3.8 | [134] |
| Mn3O4/3D-N-G | Metal oxide supported | Improved durability and methanol tolerance, far exceeding the commercial Pt/C | −0.1 V vs. Ag/AgCl | 3.7 | [135] |
| 3D-S-G | S-doped | A much enhanced ORR catalytic activity, as well as an especially high electrochemical stability | - | - | [136] |
| 3D-N-G | N-doped | A truly metal-free electrocatalyst material for the oxygen reduction reaction in acid medium | 0.83 V vs. RHE | 2.6–3.5 | [137] |
| Pt/3D-G | Metal NPs, supported | Much better durability, out-performing the undoped sample after 6000 start-stop cycles | 1.05 V vs. RHE | - | [138] |
| C3N4-3D-G | NPs, supported | Significantly enhanced electrocatalytic activity in terms of the electron-transfer number, current density and onset potential | −0.3 V vs. Ag/AgCl | 3.7 | [139] |
| 3D-G | pristine | Multilayer 3D graphene exhibits higher electrocatalytic activity | −0.252 V vs. Ag/AgCl | - | [123] |
| FexN/3D-G | NPs, supported | Comparable catalytic activity as commercial Pt/C, while its stability and resistance to methanol crossover are superior | 0.00 V vs. Ag/AgCl | 4.0 | [140] |
| TiO2/3D-N-G | Metal oxide supported | Great ORR electrocatalytic performance and long durability and methanol tolerance than that of 20% Pt/C | 0.005 V vs. Hg/HgO | 3.85 | [141] |
| N-S-3D-graphene nano-mesh | Mixed doped | Excellent electrocatalytic activity for ORR, better than most of the graphene-based catalysts reported | 0.04 V vs. Ag/AgCl | 3.6 | [125] |
| MnO/3D-G | Metal oxide, supported | Enhanced catalytic current, more positive potential, excellent methanol tolerance and long-term stability | −0.22 V vs. Ag/AgCl | 3.03 | [142] |
| Pt/N-3D-G | Metal NPs, supported | Improvement in the support can reach 2.6-times the catalytic activity and almost no degradation after 5000 cycles | 1.05 V vs. RHE | - | [143] |
| Pt/3D-G | Metal NPs, supported | Enhanced electrocatalytic activity and improved durability | 0.95 V vs. RHE | - | [144] |
| 3D-N-G | N-doped | Comparable electrocatalytic performance with the commercial Pt/C in alkaline | −0.05 V vs. Ag/AgCl | 3.79 | [145] |
| N-B-3D-G | Dual doped | The onset potential and current density of N and B co-doped 3D-G are comparable to those of the commercial Pt (30%)/C catalyst | −0.04 V vs. Ag/AgCl | 3.8 | [146] |
| 3D-N-B-G | Dual doped | The onset potential, half-wave potential and limiting current density were comparable to or even better than those in previous reports | −0.07 V vs. SCE | 3.9 | [147] |
| N-3D-G | N-doped | A high diffusion-limited current, superior durability and better immunity towards methanol crossover in alkaline solution | 0.9 V vs. RHE | 4.2–4.6 | [148] |
| Pd/3D-G | Metal NPs, supported | Enhancement in the reduction current, lower stability than Pt/C, but free from the catalytic poisoning | −0.65 V vs. NHE | - | [149] |
| 3D-N-G | N-doped | Remarkable ORR activity and long-term stability in both alkaline and acidic solutions | 0.83 V vs. RHE | 3.9 | [150] |
| B-3D-G | B-doped | Comparable to Pt/C (20 wt %) catalyst, in addition to their superior durability and resistance to the crossover effect | −0.05 V vs. Ag/AgCl | 4.16 | [151] |
| Co-N-3D-G | Metal NPs, supported | Significant catalytic activity with positive onset and half-wave potentials, low hydrogen peroxide yield, high resistance to methanol crossover and remarkable stability | 0.99 V vs. RHE | 3.94–3.97 | [152] |
| CoMn2O4/N-P-3D-G | Metal oxide, dual doped, supported | More positive onset potential and amazingly high current density towards the ORR | −0.094 V vs. SCE | 3.64–3.70 | [153] |
| Co3O4/N-S-3D-G | Metal oxide, dual doped, supported | Favored a 4e− pathway in catalyzing ORR and exhibited intrinsic long-term durability | −0.05 V vs. SCE | 3.7 | [154] |
| NiCo2O4/3D-G | Metal oxide, supported | Outstanding ORR performance with the four-electron reduction of O2 to H2O in alkaline media | 0.95 V vs. RHE | 4.0 | [126] |
| GQDs/3D-G | Nanomaterial, supported | Enhanced electrocatalytic activity, good durability in alkaline solution | −0.13 V vs. Ag/AgCl | 3.2–4.0 | [109] |
| Co/CoO/3D-G | Metal oxide, supported | Comparable oxygen reduction performance with excellent methanol resistance and better durability | −0.06 V vs. Ag/AgCl | 3.5 | [155] |
| N-S-3D-G | Dual doped | Higher electrocatalytic activity and diffusion-limiting current density, better stability and methanol tolerance | −0.12 V vs. SCE | 3.5 | [156] |
| CoFe2O4/3D-N-S-G | Metal oxide, dual doped, supported | A pronounced ORR activity (4-electron pathway) and high durability | −0.10 V 3D-N-G | 3.85–3.95 | [157] |
| Co/3D-G | Metal NPs, supported | Extraordinarily superior activity along with better stability | 0.9 V vs. RHE | 3.5 | [158] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, X.; Wei, Q.; Zhan, X.; Zhang, G.; Sun, S. The New Graphene Family Materials: Synthesis and Applications in Oxygen Reduction Reaction. Catalysts 2017, 7, 1. https://doi.org/10.3390/catal7010001
Tong X, Wei Q, Zhan X, Zhang G, Sun S. The New Graphene Family Materials: Synthesis and Applications in Oxygen Reduction Reaction. Catalysts. 2017; 7(1):1. https://doi.org/10.3390/catal7010001
Chicago/Turabian StyleTong, Xin, Qiliang Wei, Xinxing Zhan, Gaixia Zhang, and Shuhui Sun. 2017. "The New Graphene Family Materials: Synthesis and Applications in Oxygen Reduction Reaction" Catalysts 7, no. 1: 1. https://doi.org/10.3390/catal7010001
APA StyleTong, X., Wei, Q., Zhan, X., Zhang, G., & Sun, S. (2017). The New Graphene Family Materials: Synthesis and Applications in Oxygen Reduction Reaction. Catalysts, 7(1), 1. https://doi.org/10.3390/catal7010001








