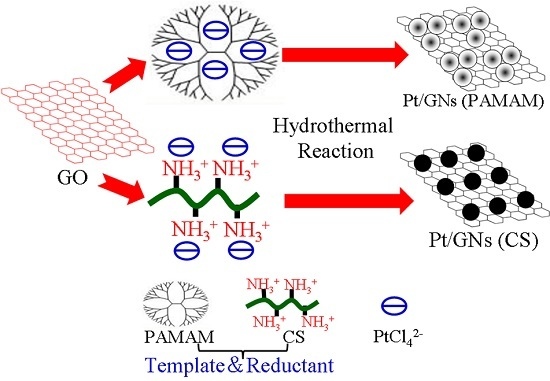
One Pot Synthesis of Pt/Graphene Composite Using Polyamidoamine/Chitosan as a Template and Its Electrocatalysis for Methanol Oxidation
Abstract
:1. Introduction
2. Results and Discussion
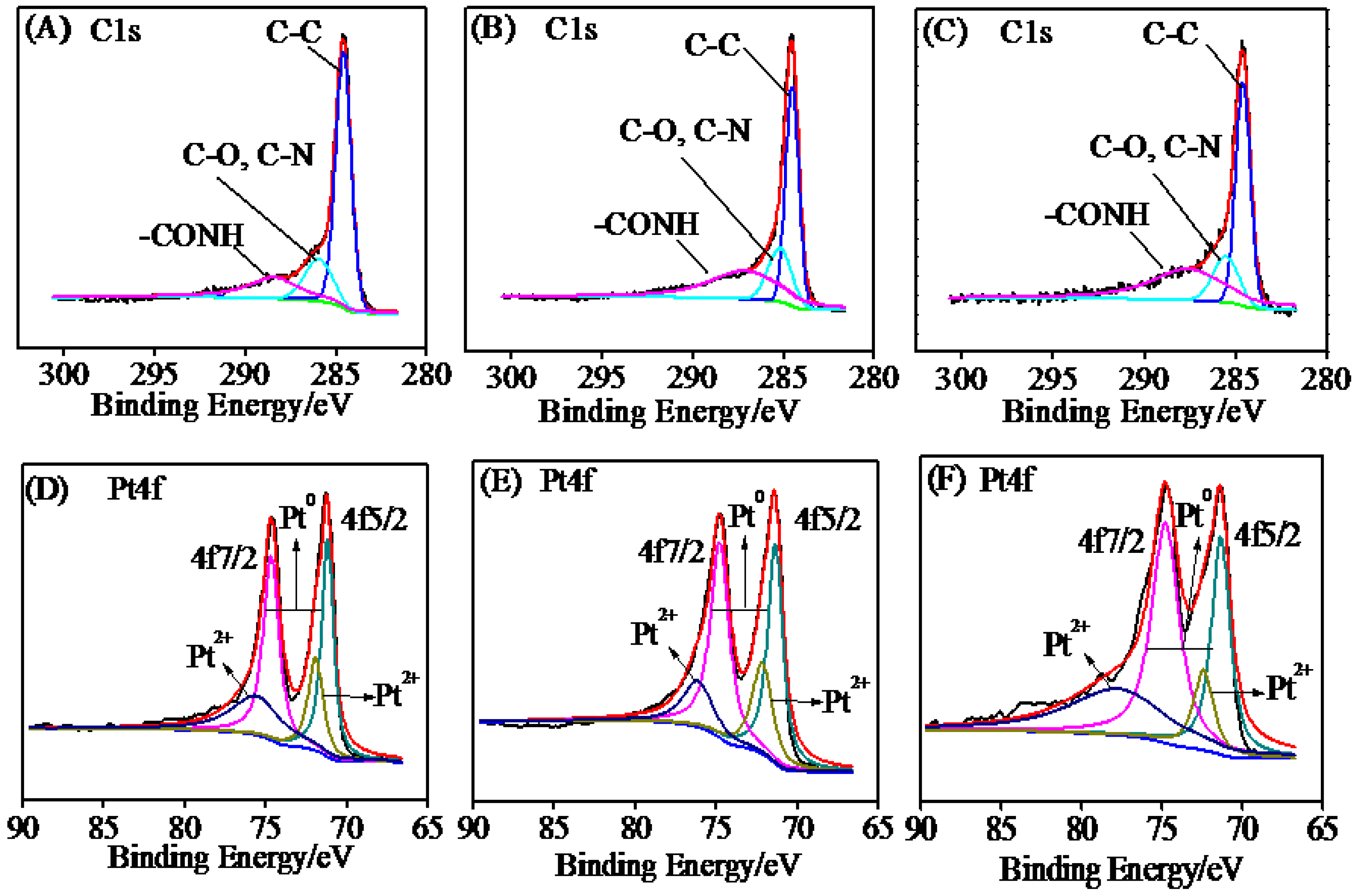
2.1. Characterization
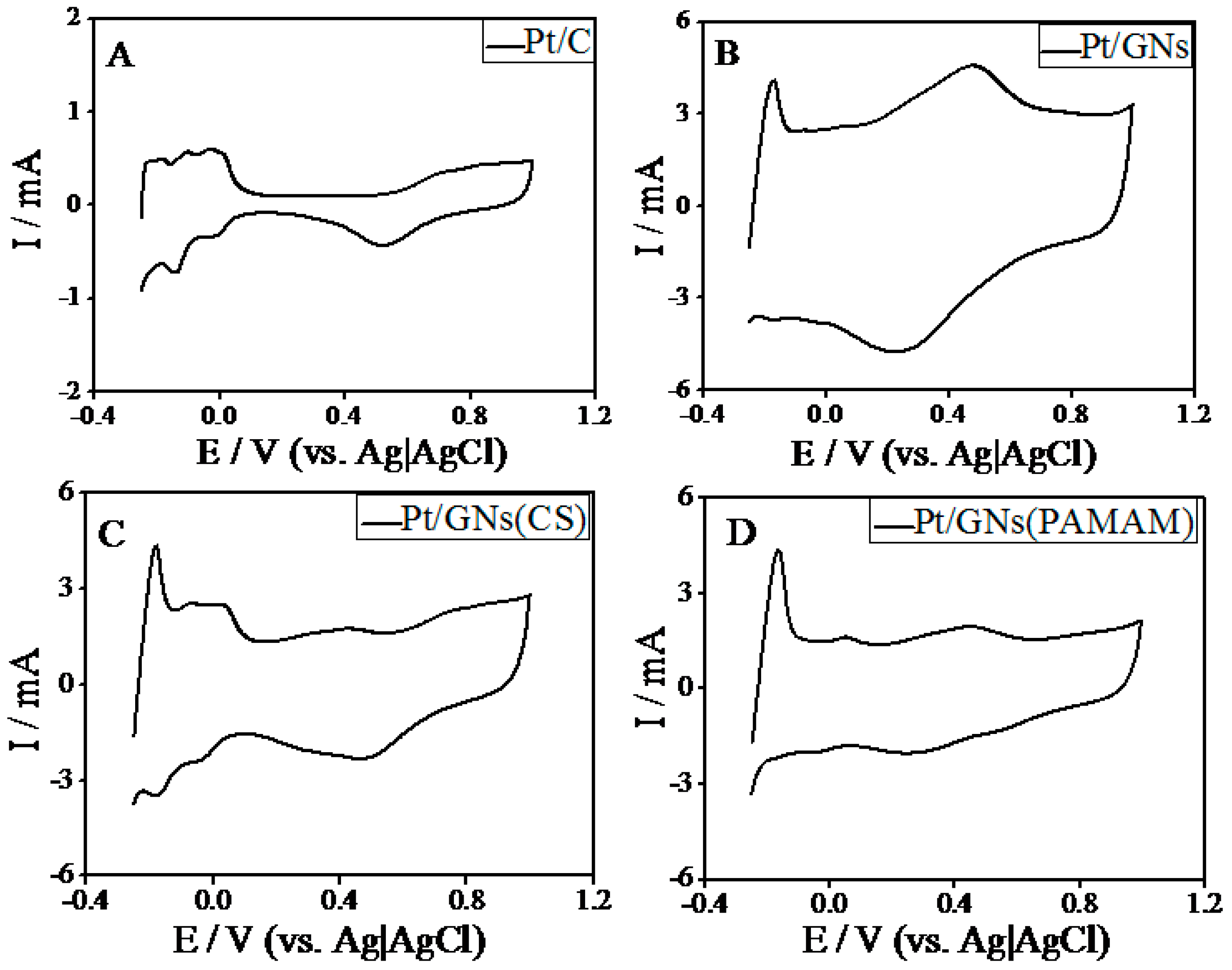
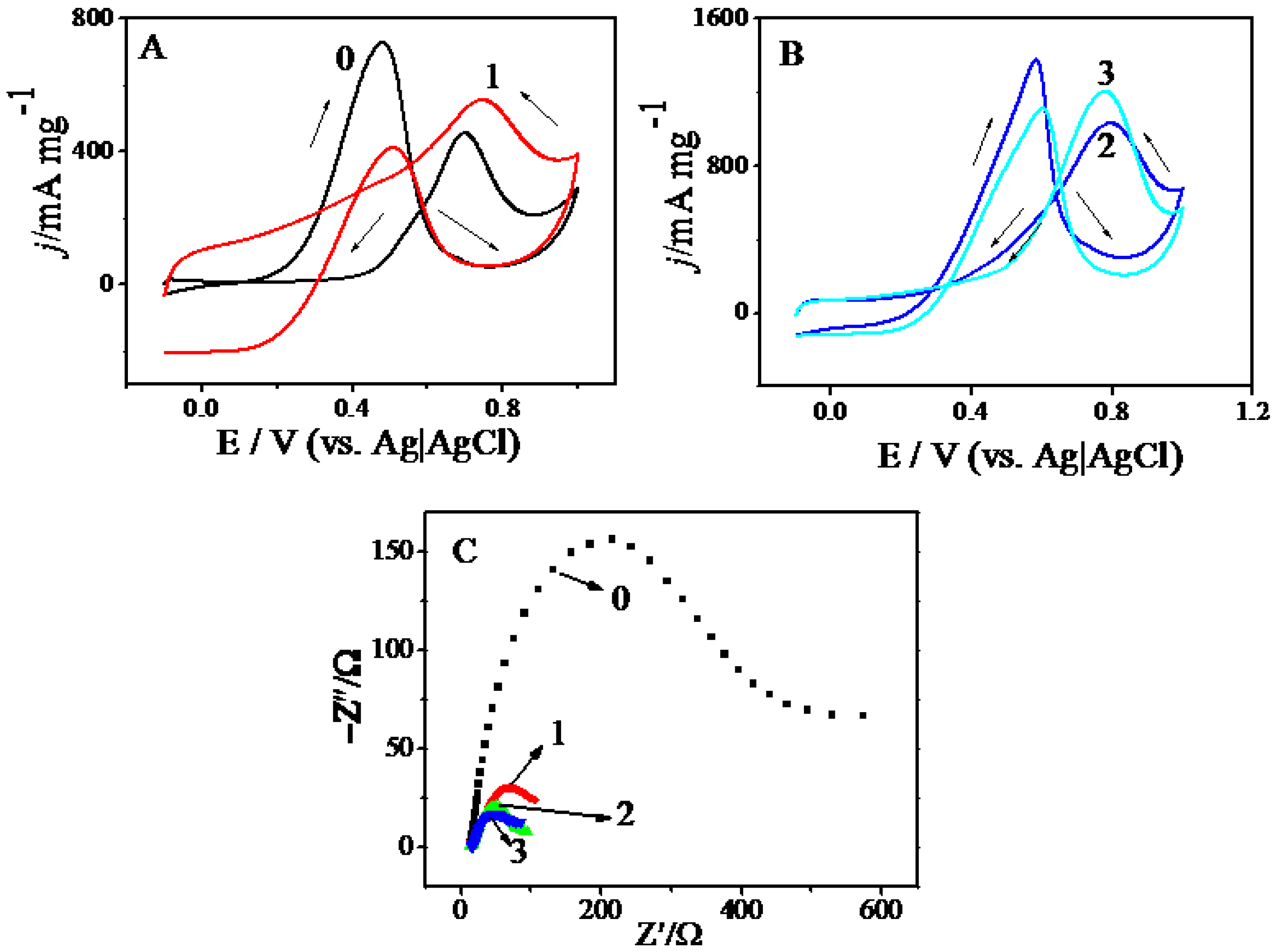
2.2. Electrocatalysis
3. Materials and Methods
3.1. Materials
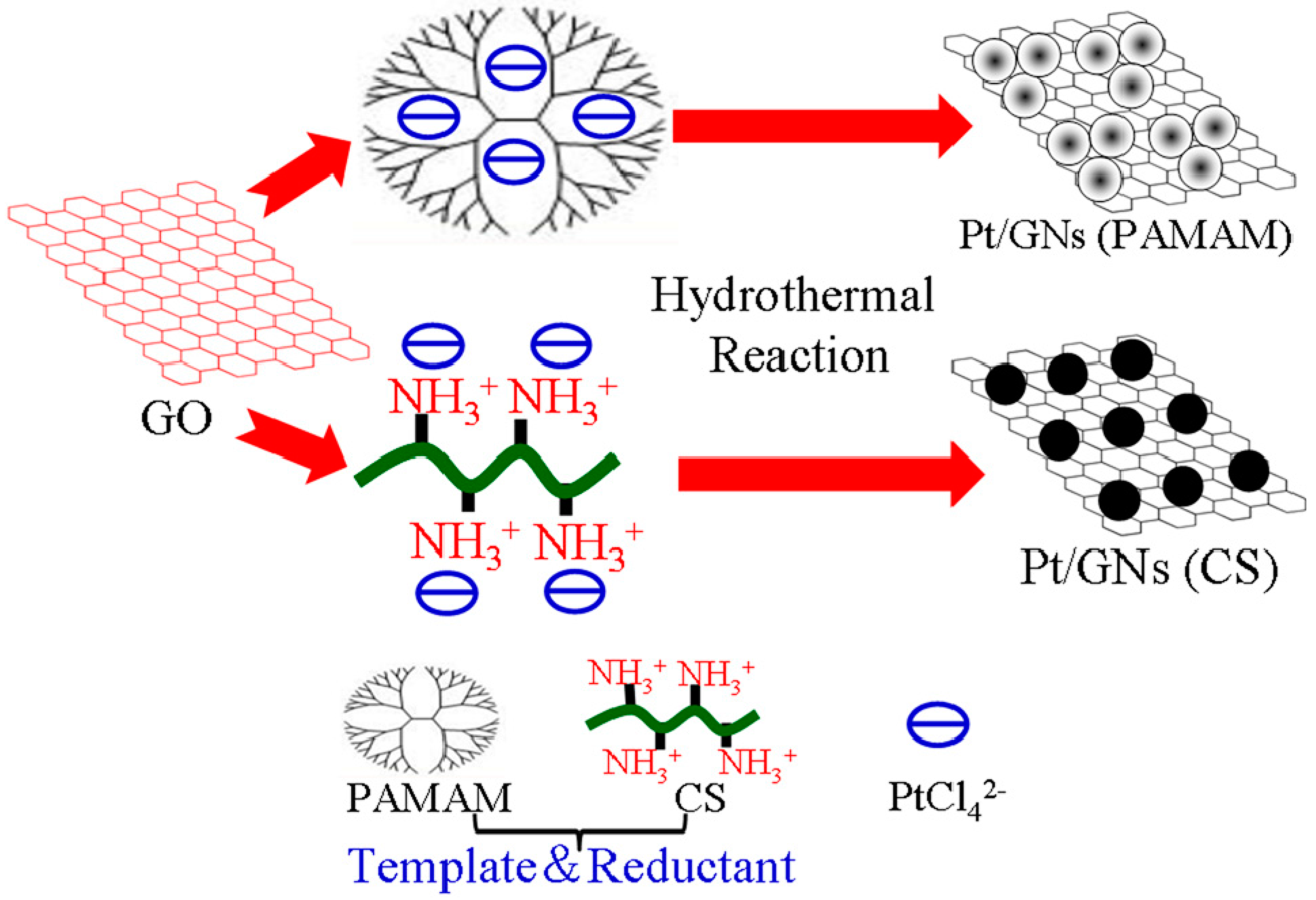
3.2. Synthesis of Pt/GNs (PAMAM) and Pt/GNs (CS)
3.3. Characterization
3.4. Electrochemical Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Boppella, R.; Anjaneyulu, K.; Basak, P.; Manorama, S.V. Facile synthesis of face oriented ZnO crystals: Tunable polar facets and shape induced enhanced photocatalytic performance. J. Phys. Chem. C 2013, 117, 4597–4605. [Google Scholar] [CrossRef]
- Yu, C.L.; Yang, K.; Xie, Y.; Fan, Q.Z.; Yu, J.C.; Shu, Q.; Wang, C.Y. Novel hollow Pt-ZnO nanocomposite microspheres with hierarchical structure and enhanced photocatalytic activity and stability. Nanoscale 2013, 5, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, F.; Lin, C.; Wang, Z.L. Direct growth of TiO2 nanosheet arrays on carbon fibers for highly efficient photocatalytic degradation of methyl orange. Adv. Mater. 2012, 24, 4761–4764. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Kuai, L.; Geng, B.Y. CeO2/rGO/Pt sandwich nanostructure: rGO-enhanced electron transmission between metal oxide and metal nanoparticles for anodic methanol oxidation of direct methanol fuel cells. Nanoscale 2012, 4, 5738–5743. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.L.; Li, Z.S.; Ye, L.T.; Wang, Y.L.; Lin, S. One-pot synthesis of Pt/SnO2/GhTs and its electro-photo-synergistic catalysis for methanol oxidation. Int. J. Hydrog. Energy 2016, 41, 255–264. [Google Scholar] [CrossRef]
- Li, F.M.; Gao, X.Q.; Xue, Q.; Li, S.N.; Chen, Y.; Lee, J.M. Reduced graphene oxide supported platinum nanocubes composites: One-pot hydrothermal synthesis and enhanced catalytic activity. Nanotechnology 2015, 26, 065603. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Lee, S.; Shin, C.; Park, S.; Kwon, S.J.; Park, H.S. One-pot self-assembled, reduced graphene oxide/palladium nanoparticle hybrid aerogels for electrocatalytic applications. Electrochim. Acta 2015, 180, 902–908. [Google Scholar] [CrossRef]
- Alvarez, A.; Guzman, C.; Rivas, S.; Godinez, L.A.; Sacca, A.; Carbone, A.; Passalacqua, E.; Arriaga, L.G.; Ledesma-Garcia, J. Composites membranes based on nafion and PAMAM dendrimers for PEMFC applications. Int. J. Hydrog. Energy 2014, 39, 16686–16693. [Google Scholar] [CrossRef]
- Maiyalagan, T. Pt-Ru nanoparticles supported PAMAM dendrimer functionalized carbon nanofiber composite catalysts and their application to methanol oxidation. J. Solid State Electrochem. 2009, 13, 1561–1566. [Google Scholar] [CrossRef]
- Crespilho, F.N.; Huguenin, F.; Zucolotto, V.; Olivi, P.; Nart, F.C.; Oliveira, O.N. Dendrimers as nanoreactors to produce platinum nanoparticles embedded in layer-by-layer films for methanol-tolerant cathodes. Electrochem. Commun. 2006, 8, 348–352. [Google Scholar] [CrossRef]
- Sun, L.S.; Ca, D.V.; Cox, J.A. Electrocatalysis of the hydrogen evolution reaction by nanocomposites of poly(amidoamine)-encapsulated platinum nanoparticles and phosphotungstic acid. J. Solid State Electrochem. 2005, 9, 816–822. [Google Scholar] [CrossRef]
- Noroozifar, M.; Khorasani-Motlagh, M.; Ekrami-Kakhki, M.-S.; Khaleghian-Moghadam, R. Enhanced electrocatalytic properties of Pt-chitosan nanocomposite for direct methanol fuel cell by LaFeO3 and carbon nanotube. J. Power Sources 2014, 248, 130–139. [Google Scholar] [CrossRef]
- Cogo, L.C.; Batisti, M.V.; Pereira-da-Silva, M.A.; Oliveira, O.N., Jr.; Nart, F.C.; Huguenin, F. Layer-by-layer films of chitosan, poly(vinyl sulfonic acid), and platinum for methanol electrooxidation and oxygen electroreduction. J. Power Sources 2006, 158, 160–163. [Google Scholar] [CrossRef]
- Osifo, P.O.; Masala, A. The influence of chitosan membrane properties for direct methanol fuel cell applications. J. Fuel Cell Sci. Technol. 2011, 9, 011003. [Google Scholar] [CrossRef]
- Pan, Y.; Bao, H.; Li, L. Noncovalently functionalized multiwalled carbon nanotubes by chitosan-grafted reduced graphene oxide and their synergistic reinforcing effects in chitosan films. ACS Appl. Mater. Interfaces 2011, 3, 4819–4830. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Pan, Y.; Ping, Y.; Sahoo, N.G.; Wu, T.; Li, L.; Li, J.; Gan, L.H. Chitosan-functionalized graphene oxide as a nanocarrier for drug and gene delivery. Small 2011, 7, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Lei, F.L.; Ye, L.T.; Zhang, X.F.; Lin, S. Controlled synthesis of Pt/CS/PW12-GNs composite as an anodic electrocatalyst for direct methanol fuel cells. J. Nanopart. Res. 2015, 17, 192. [Google Scholar] [CrossRef]
- Yu, D.; Dai, L. Self-assembled graphene/carbon nanotube hybrid films for supercapacitors. J. Phys. Chem. Lett. 2010, 1, 467–470. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; He, X.; Ma, A.; Le, L.; Lin, S. Facile electrodeposition of flower-like PMo12-Pt/rGO composite with enhanced electrocatalytic activity towards methanol oxidation. Catalysts 2015, 5, 1275–1288. [Google Scholar] [CrossRef]
- Huang, H.; Liu, Y.; Gao, Q.; Ruan, W.; Lin, X.; Li, X. Rational construction of strongly coupled metal-metal oxide-graphene nanostructure with excellent electrocatalytic activity and durability. ACS Appl. Mater. Interfaces 2014, 6, 10258–10264. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Huang, X.M.; Zhang, X.F.; Zhang, L.; Lin, S. The synergistic effect of graphene and polyoxometalates enhanced electrocatalytic activities of Pt-{PEI-GNs/[PMo12O40]3−}n composite films regarding methanol oxidation. J. Mater. Chem. 2012, 22, 23602–23607. [Google Scholar] [CrossRef]
- Liu, X.; Du, H.; Sun, X.W. High-performance photoresponse of carbon-doped ZnO/reduced graphene oxide hybrid nanocomposites under UV and visible illumination. RSC Adv. 2014, 4, 5136–5140. [Google Scholar] [CrossRef]
- You, H.J.; Zhang, F.L.; Liu, Z.; Fang, J.X. Free-standing Pt-Au hollow nanourchins with enhanced activity and stability for catalytic methanol oxidation. ACS Catal. 2014, 4, 2829–2835. [Google Scholar] [CrossRef]
- Wang, H.; Wang, R.F.; Li, H.; Wang, Q.F.; Kang, J.A.; Lei, Z.Q. Facile synthesis of carbon-supported pseudo-core@shell PdCu@Pt nanoparticles for direct methanol fuel cells. Int. J. Hydrog. Energy 2011, 36, 839–848. [Google Scholar] [CrossRef]
- Park, K.W.; Choi, J.H.; Kwon, B.K.; Lee, S.A.; Sung, Y.E.; Ha, H.Y.; Hong, S.A.; Kim, H.; Wieckowski, A. Chemical and electronic effects of Ni in Pt/Ni and Pt/Ru/Ni alloy nanoparticles in methanol electrooxidation. J. Phys. Chem. B 2002, 106, 1869–1877. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Cui, Z.M.; Jiang, S.P.; Li, C.M. Highly dispersed MoOx on carbon nanotube as support for high performance Pt catalyst towards methanol oxidation. Chem. Commun. 2011, 47, 8418–8420. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, Q.; He, M.; Sun, X.; Wang, X. A ternary Pt/MnO2/graphene nanohybrid with an ultrahigh electrocatalytic activity toward methanol oxidation. J. Power Sources 2013, 239, 189–195. [Google Scholar] [CrossRef]
- Yu, S.; Liu, Q.; Yang, W.; Han, K.; Wang, Z.; Zhu, H. Graphene-CeO2 hybrid support for Pt nanoparticles as potential electrocatalyst for direct methanol fuel cells. Electrochim. Acta 2013, 94, 245–251. [Google Scholar] [CrossRef]
- Wietecha, M.S.; Zhu, J.; Gao, G.; Wang, N.; Feng, H.; Gorring, M.L.; Kasner, M.L.; Hou, S. Platinum nanoparticles anchored on chelating group-modified graphene for methanol oxidation. J. Power Sources 2012, 198, 30–35. [Google Scholar] [CrossRef]
- Saidi, W.A.; Feng, H.J.; Fichthorn, K.A. Binding of polyvinylpyrrolidone to Ag surfaces: Insight into a structure-directing agent from dispersion-corrected density functional theory. J. Phys. Chem. C 2013, 117, 1163–1171. [Google Scholar] [CrossRef]
- Al-Saidi, W.A.; Feng, H.J.; Fichthorn, K.A. Adsorption of polyvinylpyrrolidone on Ag surfaces: Insight into a structure-directing agent. Nano Lett. 2012, 12, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Balankura, T.; Zhou, Y.; Fichthorn, K.A. How structure-directing agents control nanocrystal shape: Polyvinylpyrrolidone-mediated growth of Ag nanocubes. Nano Lett. 2015, 15, 7711–7717. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Zhang, L.; Huang, X.M.; Ye, L.T.; Lin, S. Shape-controlled synthesis of Pt nanoparticles via integration of graphene and beta-cyclodextrin and using as a noval electrocatalyst for methanol oxidation. Electrochim. Acta 2014, 121, 215–222. [Google Scholar] [CrossRef]
- Wu, T.; Wang, X.; Qiu, H.; Gao, J.; Wang, W.; Liu, Y. Graphene oxide reduced and modified by soft nanoparticles and its catalysis of the Knoevenagel condensation. J. Mater. Chem. 2012, 22, 4772–4779. [Google Scholar] [CrossRef]
- Kim, N.H.; Kuila, T.; Lee, J.H. Simultaneous reduction, functionalization and stitching of graphene oxide with ethylenediamine for composites application. J. Mater. Chem. A 2013, 1, 1349–1358. [Google Scholar] [CrossRef]
- Qiu, J.-D.; Wang, G.-C.; Liang, R.-P.; Xia, X.-H.; Yu, H.-W. Controllable deposition of platinum nanoparticles on graphene as an electrocatalyst for direct methanol fuel cells. J. Phys. Chem. C 2011, 115, 15639–15645. [Google Scholar] [CrossRef]
- Guo, Q.; Zheng, Z.; Gao, H.L.; Ma, J.; Qin, X. SnO2/graphene composite as highly reversible anode materials for lithium ion batteries. J. Power Sources 2013, 240, 149–154. [Google Scholar] [CrossRef]
- Estudillo-Wong, L.A.; Vargas-Gomez, A.M.; Arce-Estrada, E.M.; Manzo-Robledo, A. TiO2/c composite as a support for Pd-nanoparticles toward the electrocatalytic oxidation of methanol in alkaline media. Electrochim. Acta 2013, 112, 164–170. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, J.; Sun, Q.; Yan, Z.; Chen, M.; Jing, J. Enhanced electrocatalytic activity of high Pt-loadings on surface functionalized graphene nanosheets for methanol oxidation. Int. J. Hydrog. Energy 2013, 38, 16402–16409. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, Z.; Huang, Y.; Yu, Y.; Feng, Q.; Li, Q. Fabrication of Pt nanoparticles on ethylene diamine functionalized graphene for formic acid electrooxidation. Int. J. Hydrog. Energy 2014, 39, 15920–15927. [Google Scholar] [CrossRef]
- Luo, Z.; Yuwen, L.; Bao, B.; Tian, J.; Zhu, X.; Weng, L.; Wang, L. One-pot, low-temperature synthesis of branched platinum nanowires/reduced graphene oxide (BPtNW/RGO) hybrids for fuel cells. J. Mater. Chem. 2012, 22, 7791–7796. [Google Scholar] [CrossRef]
- Liu, R.; Li, S.; Yu, X.; Zhang, G.; Zhang, S.; Yao, J.; Keita, B.; Nadjo, L.; Zhi, L. Facile synthesis of Au-nanoparticle/polyoxometalate/graphene tricomponent nanohybrids: An enzyme-free electrochemical biosensor for hydrogen peroxide. Small 2012, 8, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-J.; Yang, G.-H.; Zhu, J.-J. Sonoelectrochemical fabrication of PDDA-RGO-PdPt nanocomposites as electrocatalyst for DAFCs. J. Mater. Chem. 2011, 21, 7343–7349. [Google Scholar] [CrossRef]
- Luo, B.; Yan, X.; Xu, S.; Xue, Q. Polyelectrolyte functionalization of graphene nanosheets as support for platinum nanoparticles and their applications to methanol oxidation. Electrochim. Acta 2012, 59, 429–434. [Google Scholar] [CrossRef]
- Mayavan, S.; Jang, H.-S.; Lee, M.-J.; Choi, S.H.; Choi, S.-M. Enhancing the catalytic activity of Pt nanoparticles using poly sodium styrene sulfonate stabilized graphene supports for methanol oxidation. J. Mater. Chem. A 2013, 1, 3489–3494. [Google Scholar] [CrossRef]
- Cui, X.; Wu, S.N.; Jungwirth, S.; Chen, Z.B.; Wang, Z.H.; Wang, L.; Li, Y.X. The deposition of Au-Pt core-shell nanoparticles on reduced graphene oxide and their catalytic activity. Nanotechnology 2013, 24, 295402. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Yin, L. Well-dispersed and size-tuned bimetallic PtFex nanoparticle catalysts supported on ordered mesoporous carbon for enhanced electrocatalytic activity in direct methanol fuel cells. J. Mater. Chem. 2012, 22, 9584–9593. [Google Scholar] [CrossRef]
- Yang, S.D.; Shen, C.M.; Lu, X.J.; Tong, H.; Zhu, J.J.; Zhang, X.G.; Gao, H.J. Preparation and electrochemistry of graphene nanosheets-multiwalled carbon nanotubes hybrid nanomaterials as Pd electrocatalyst support for formic acid oxidation. Electrochim. Acta 2012, 62, 242–249. [Google Scholar] [CrossRef]
- Huang, H.; Chen, H.; Sun, D.; Wang, X. Graphene nanoplate-Pt composite as a high performance electrocatalyst for direct methanol fuel cells. J. Power Sources 2012, 204, 46–52. [Google Scholar] [CrossRef]
- Qian, L.; Yang, X. Dendrimer films as matrices for electrochemical fabrication of novel gold/palladium bimetallic nanostructures. Talanta 2008, 74, 1649–1653. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Xu, L.; Li, F.; Xu, B.; Yang, Y.; Liu, S.; Sun, Z. Chitosan-assisted fabrication and electrocatalytic activity of the composite film electrode of heteropolytungstate/carbon nanotubes. Electrochim. Acta 2010, 55, 1523–1527. [Google Scholar] [CrossRef]
- Qian, L.; Yang, X. Polyamidoamine dendrimers-assisted electrodeposition of gold-platinum bimetallic nanoflowers. J. Phys. Chem. B 2006, 110, 16672–16678. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dong, S.; Wang, E. Three-dimensional Pt-on-Pd bimetallic nanodendrites supported on graphene nanosheet: Facile synthesis and used as an advanced nanoelectrocatalyst for methanol oxidation. ACS Nano 2010, 4, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Feng, W.; Feng, Y.; Liu, Q.; Xu, X.; Sekino, T.; Fujii, A.; Ozaki, M. Preparation and characterization of chitosan-grafted multiwalled carbon nanotubes and their electrochemical properties. Carbon 2007, 45, 1212–1218. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Zeng, Q.O.; Cheng, J.S.; Tang, L.H.; Liu, X.F.; Liu, Y.Z.; Li, J.H.; Jiang, J.H. Self-assembled graphene-enzyme hierarchical nanostructures for electrochemical biosensing. Adv. Funct. Mater. 2010, 20, 3366–3372. [Google Scholar] [CrossRef]


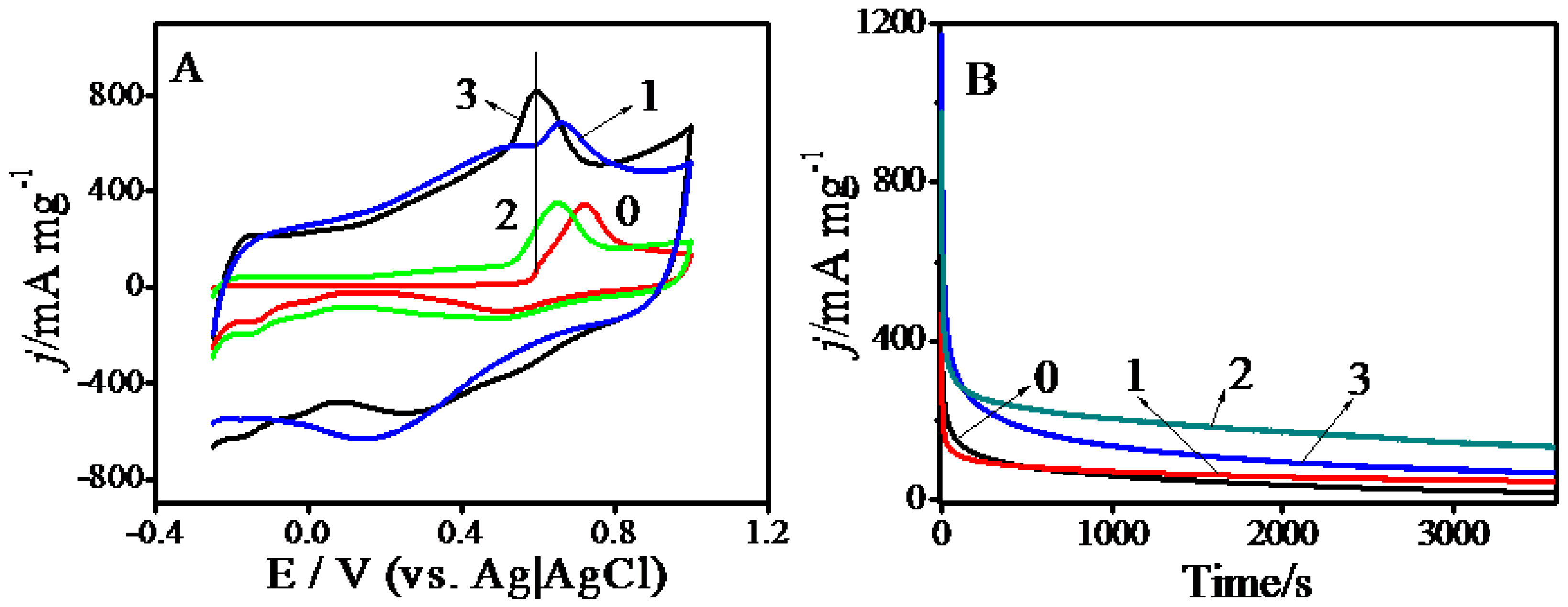
| Composites | ECSA/m2·g−1 (Electrochemical Surface Area) | Onset Potential b/V | Massactivity/mA·mg−1 |
|---|---|---|---|
| Pt/C-JM | 75.1 | 0.45 | 455 |
| Pt/GNs | 47.7 | 0.40 | 556 |
| Pt/GNs (CS) | 117.8 | 0.36 | 1031 |
| Pt/GNs (PAMAM- polyamidoamine) | 103.7 | 0.35 | 1203 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, Z.; Xu, S.; Lei, F.; Lin, S. One Pot Synthesis of Pt/Graphene Composite Using Polyamidoamine/Chitosan as a Template and Its Electrocatalysis for Methanol Oxidation. Catalysts 2016, 6, 165. https://doi.org/10.3390/catal6100165
Wang Y, Li Z, Xu S, Lei F, Lin S. One Pot Synthesis of Pt/Graphene Composite Using Polyamidoamine/Chitosan as a Template and Its Electrocatalysis for Methanol Oxidation. Catalysts. 2016; 6(10):165. https://doi.org/10.3390/catal6100165
Chicago/Turabian StyleWang, Yanli, Zhongshui Li, Shuhong Xu, Fengling Lei, and Shen Lin. 2016. "One Pot Synthesis of Pt/Graphene Composite Using Polyamidoamine/Chitosan as a Template and Its Electrocatalysis for Methanol Oxidation" Catalysts 6, no. 10: 165. https://doi.org/10.3390/catal6100165
APA StyleWang, Y., Li, Z., Xu, S., Lei, F., & Lin, S. (2016). One Pot Synthesis of Pt/Graphene Composite Using Polyamidoamine/Chitosan as a Template and Its Electrocatalysis for Methanol Oxidation. Catalysts, 6(10), 165. https://doi.org/10.3390/catal6100165