Nitrogen-Doped Carbon Nanotube and Graphene Materials for Oxygen Reduction Reactions
Abstract
:1. Introduction
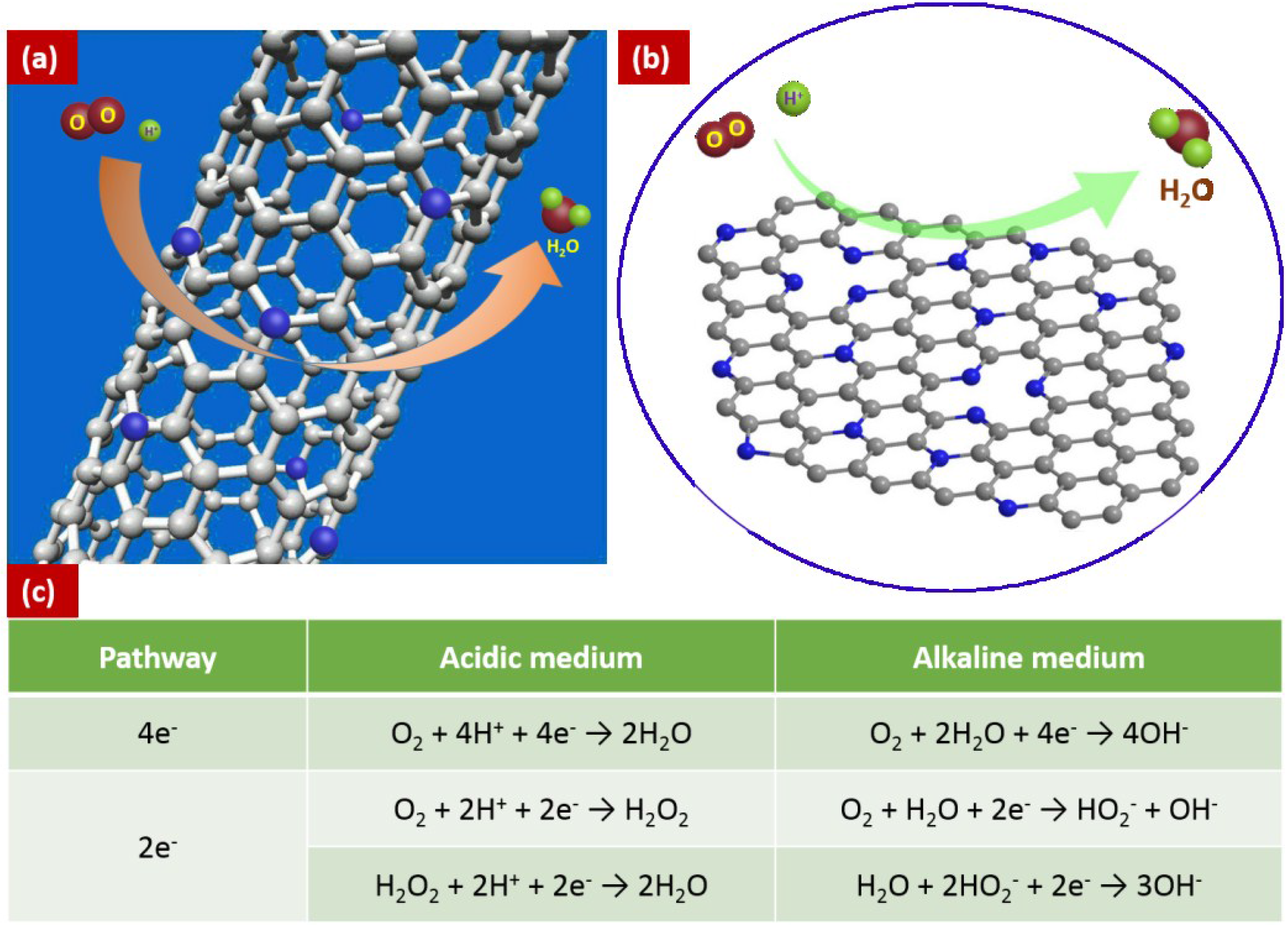
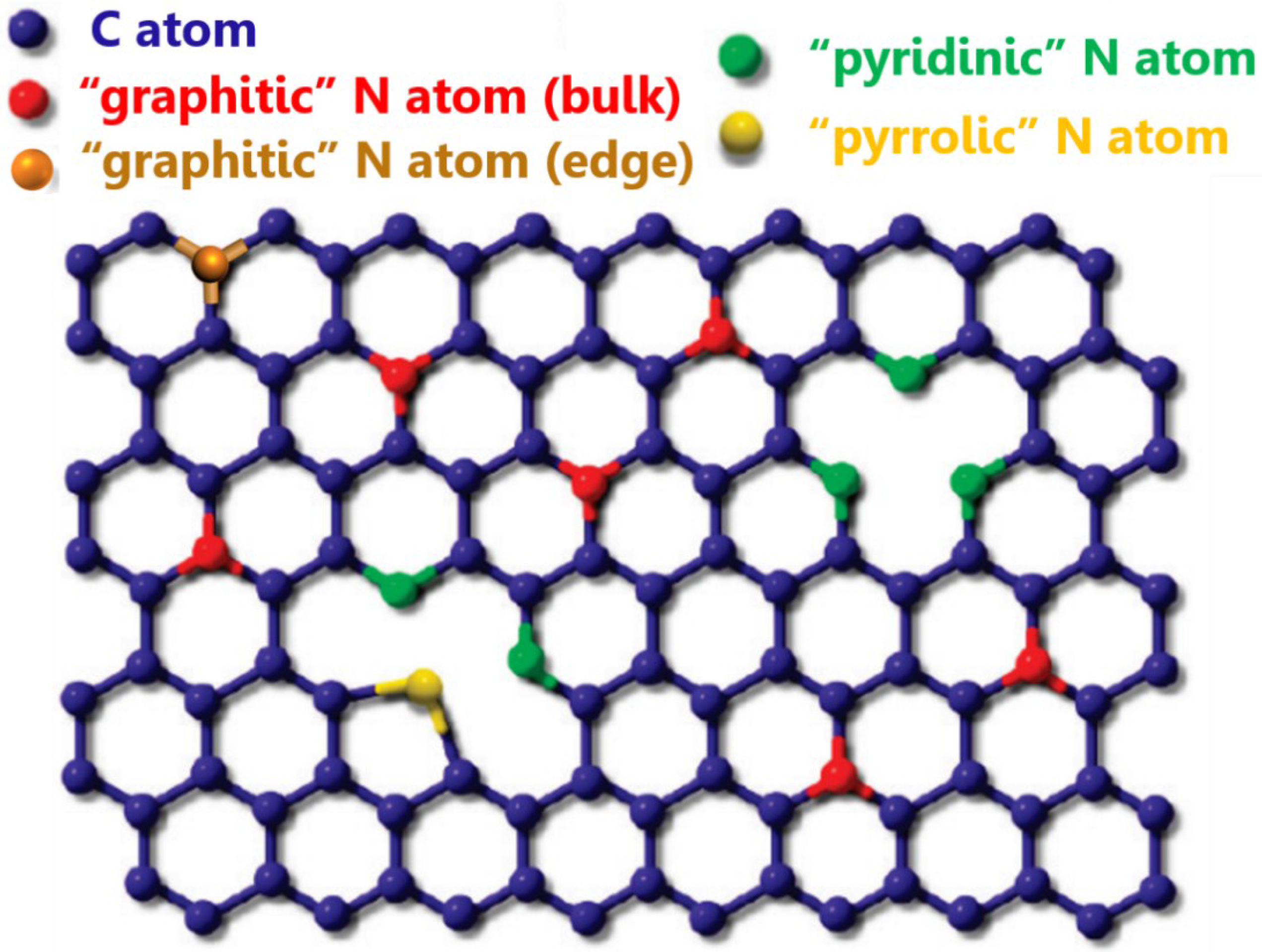
2. Synthesis of Nitrogen-Doped Carbon
2.1. N-Doped Carbon Nanotubes
2.1.1. In Situ Doping
2.1.2. Post-Treatment
2.2. N-Doped Graphene
2.2.1. In Situ Doping
2.2.2. Post-Treatment
3. Nitrogen-Doped Carbon Nanotubes (NCNTs) for Oxygen Reduction Reaction (ORR)
3.1. NCNTs as a Metal-Free Catalyst for ORR
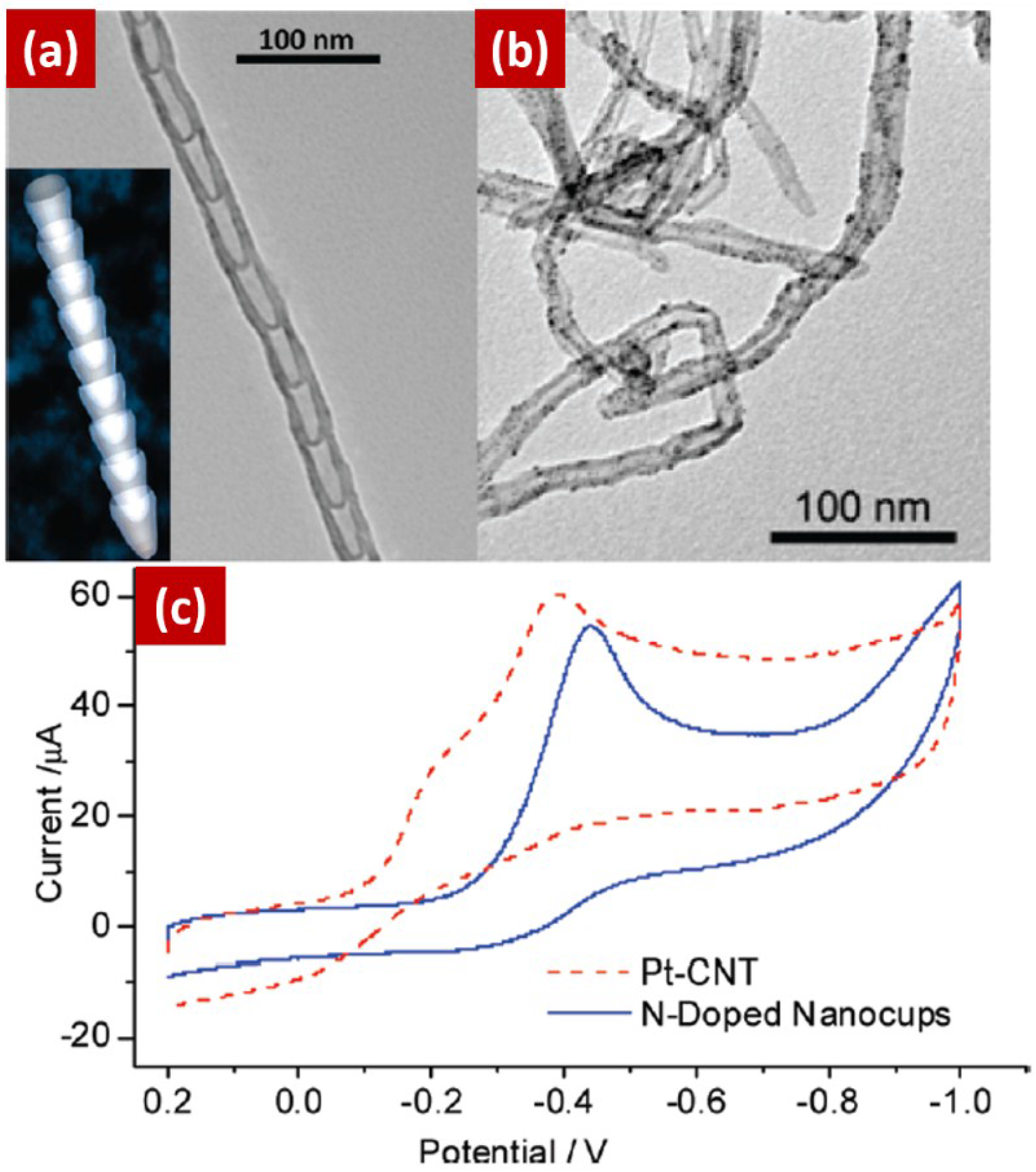
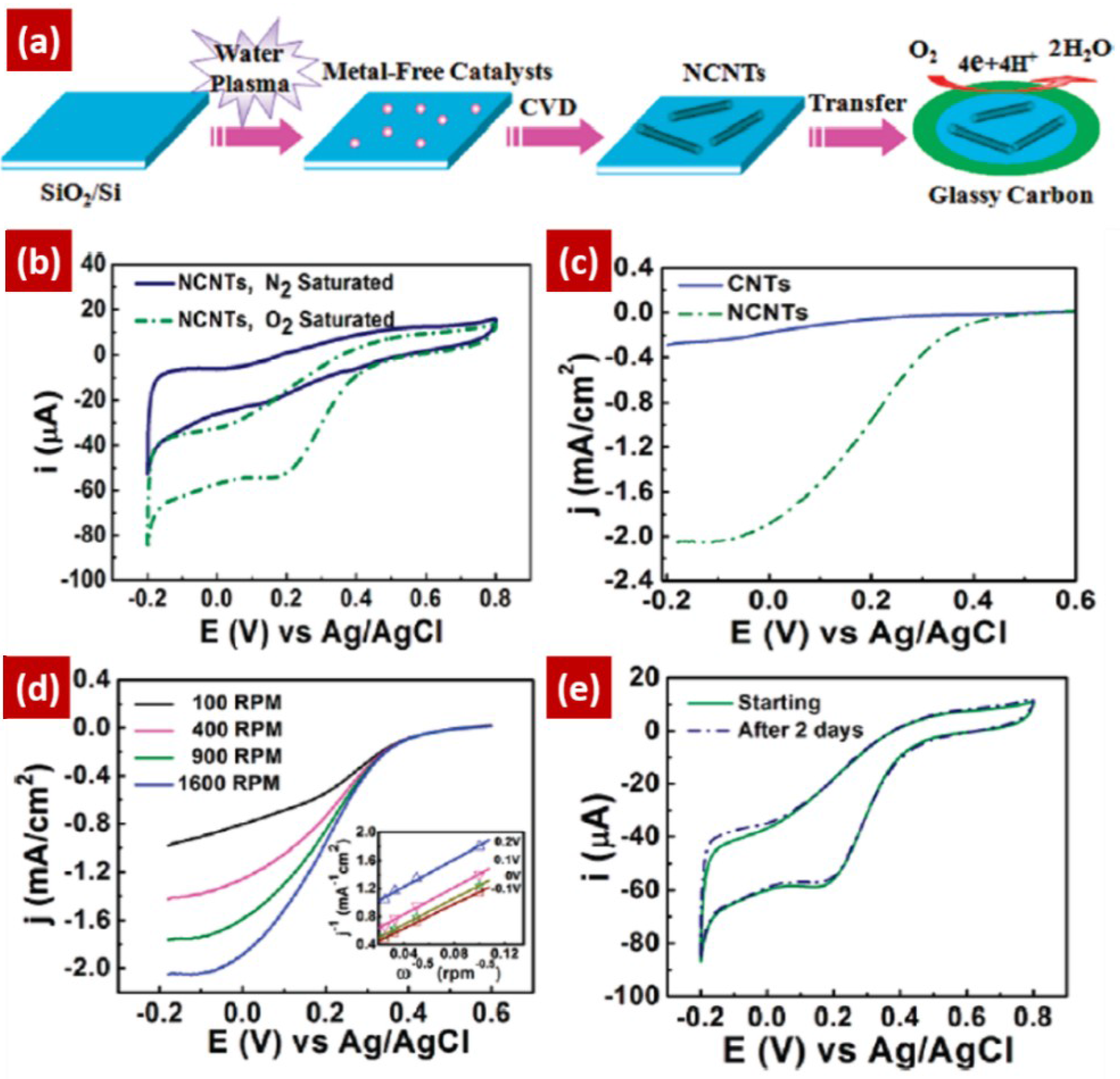
3.2. NCNTs as Catalyst Support Material for ORR

4. Nitrogen-Doped Graphene (NG) for ORR
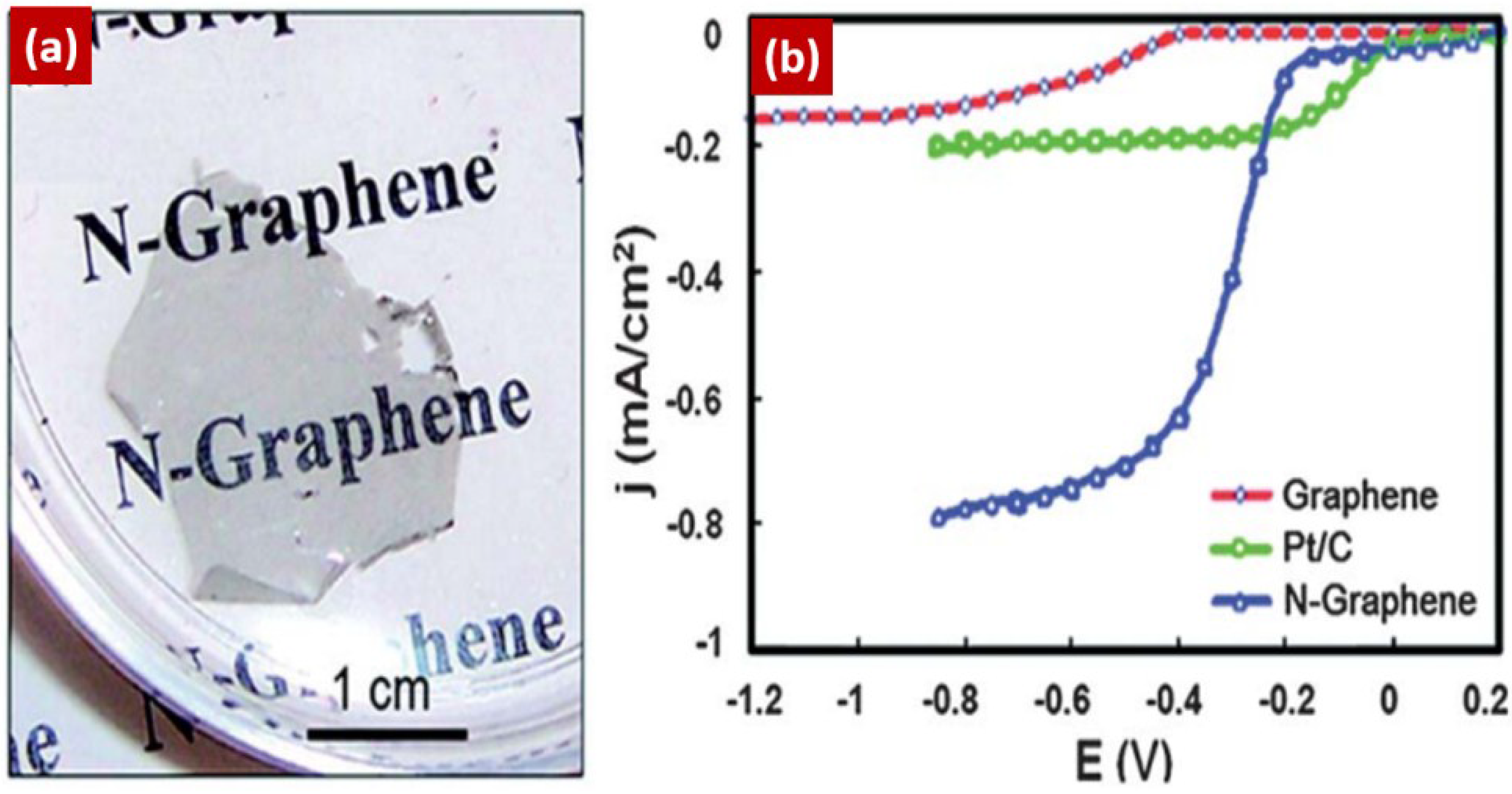
4.1. NG as a Metal-Free Catalyst for ORR
| Synthesis Method and Reactants | N-Content (at.%) | Electrocatalytic Performance | Electron Transfer Number | Ref. | |||
|---|---|---|---|---|---|---|---|
| Thermal treatment of glucose and urea | 33 | NG (25 at.%) shows competitive ORR activities with Pt/C and much better crossover resistance and excellent stability | 3.2–3.7 | [19] | |||
| CVD (C source, ethylene; N source, ammonia; Cu) | up to 16 | Higher onset potential as compared to Pt/C | close to 2 | [49] | |||
| Thermal treatment of GO using melamine | 10.1 | Much higher ORR activity than grapheme | 3.4–3.6 | [57] | |||
| N plasma treatment on graphene | 8.5 | Higher ORR activity than graphene, and higher durability and selectivity than Pt/C | - | [59] | |||
| CVD (C source, methane; N source, ammonia, Cu) | 4 | Higher activity, better stability and tolerance to crossover than Pt | 3.6–4 | [90] | |||
| Detonation technique with cyanuric chloride and trinitrophenol | 12.5 | Comparable to that of Pt, more stable and less expensive | 3.69 | [91] | |||
| A resin-based methodology with N-containing resin and metal ions | 1.8 | The onset potential on the NG electrode is close to that of Pt/C. The current is almost the same for both the Pt/C and NG | 2.1–3.9 | [92] | |||
| Hydrothermal reaction of GO with urea | 6.05–7.65 | The performance of these NG materials towards ORR is still not as good as that of Pt/C in terms of the half-wave potential and current density | ~3 | [93] | |||
| Covalent functionalize GO using organic molecules and thermal treatment | 0.72–4.3 | The NG nanosheet exhibited a good electrocatalytic activity through an efficient one-step, 4e− pathway | 3.63 | [94] | |||
| CVD of N-containing aromatic precursor molecules | 2.0–2.7 | The N dopants in the graphene reduce the ORR overpotential, thereby enhancing the catalytic activity | 3.5–4.0 | [95] | |||
| GO treatment by ammonia hydroxide, heating under ammonia gas, and reaction with melamine | 6.0–6.8 | Pyridinic N plays a vital role in ORR | 3.2–3.7 | [96] | |||
| Annealing of GO with ammonia and N-containing polymers | 2.91–7.56 | The higher limiting current density compared to Pt | 2.85–3.65 | [97] | |||
| Thermal reaction between GO and NH3 | 2.4–4.6 | The onset potential is close to that of Pt/C | ~3.8 | [98] | |||
| Hydrothermal reaction with GO and melamine | 26.08 | It shows lower ORR activity than Pt/C 40 wt.% | 3.2–4.0 | [99] | |||
| Hydrothermal process using urea and holey GO | 8.6 | Superb ORR with 4e− pathway and excellent durability | 3.85 | [100] | |||
| Thermally annealing GO with melamine | 8.05 | The nG-900 exhibits lower activity and onset potential than Pt/C, albeit higher than graphene; excellent stability | 3.3–3.7 | [101] | |||
| Pyrolyzing GO with urea | 7.86 | The NG showed a much-higher activity than glassy carbon (GC) and graphene | 3.6–4.0 | [102] | |||
| Redox GO with pyrrole then thermal treatment | 6 | Shows comparable onset potentials with 40 wt.% Pt/C | 3.3 | [103] | |||
| GO and dicyandiamide under hydrothermal condition | 7.78 | The onset potentials at rGO-N was lower than that at Pt/C | 2.6 | [104] | |||
| Pyrolysis of graphene oxide and polyaniline | 2.4 | High activity toward ORR with a superior long-term stability and tolerance to methanol crossover | 3.8–3.9 | [105] | |||
| Thermally annealing GO 5-aminotetrazole monohydrate | 10.6 | Higher current density than Pt/C. Lower onset potential of ORR than that of the commercial Pt/C | 3.7 | [106] | |||
| Pyrolysis of sugar in the presence of urea | 3.02–11.2 | The NG1000 has comparable ORR half-wave potential to 20 wt.% Pt/C | 3.2–3.8 | [107] | |||
| Hydrothermal reaction of GO with urea | 5.8–6.2 | NG has higher ORR activity than grapheme, but is not yet comparable to the Pt | 3.0–4.0 | [108] | |||
| Pyrolysis of GO and polydopamine | 2.78–3.79 | Much more enhanced ORR activities with positive onset potential and larger current density than graphene | 3.89 | [109] | |||
| Pyrolyzing GO with Melamine, urea and dicyandiamide | 5 | Compared to Pt/C, the half-wave potential of ORR on this NG catalyst was close, wheras the n values are slightly lower | 3.5–4 | [110] | |||
| PANI acting as a N source were deposited on the surface of GNRs via a layer-by-layer approach | 4.1–8.3 | Very good electrocatalytic activity and stability | 3.91 | [111] | |||
| NG is synthesized by pyrolyzing ion exchange with resin and glycine | 0.98–1.65 | Doping N in graphene is good to improve the activity for ORR, but still lower than Pt/C catalyst | - | [112] | |||
| Microwave heating of graphene under NH3 flow | 4.05–5.47 | The doping of graphite N enhanced the activity of the catalysts in the ORR in alkaline solution | 3.03–3.3 | [113] | |||
| Facile hydrothermal method | 2.8 | Competitive with the commercial Pt/C catalysts in alkaline medium | 3.66–3.92 | [114] | |||
| Gas-phase oxidation strategy using a nitric acid vapor | 0.52 | The onset potential is (0.755 V vs. RHE), comparable to the value of chemically synthesized NG, and the current densities are higher than those demonstrated for NG. | 3.2–3.9 | [115] | |||
| CVD growth of graphene and post-doping with a solid N precursor of graphitic C3N4 | 6.5 | Excellent activity, high stability, and very good crossover resistance for ORR in alkaline medium. | 3.96-4.05 | [116] | |||
| A hard templating approach | 5.07 | Outstanding ORR performance in both acidic and alkaline solutions. | 3.9 | [117] | |||
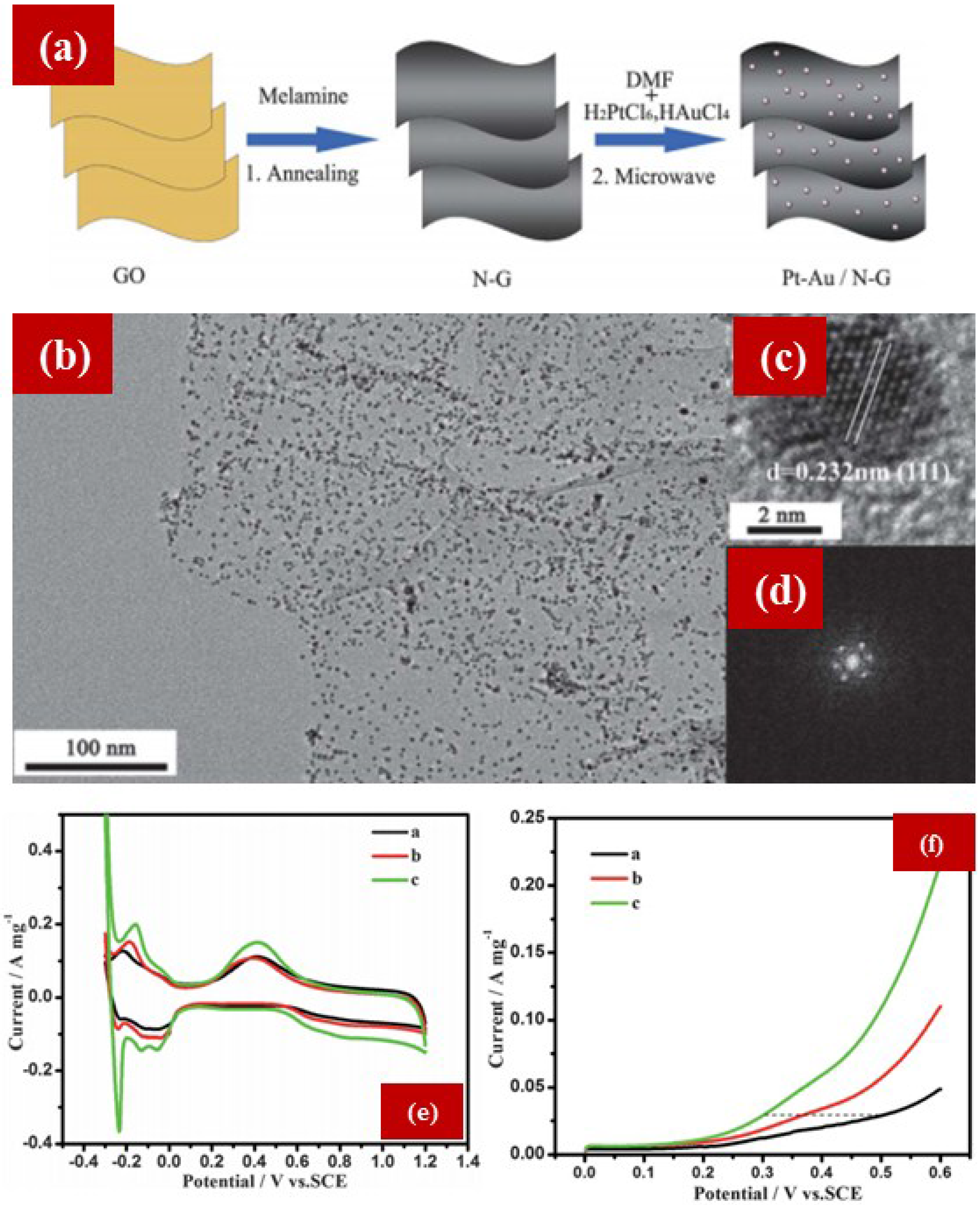
4.2. NG as Support Material for ORR
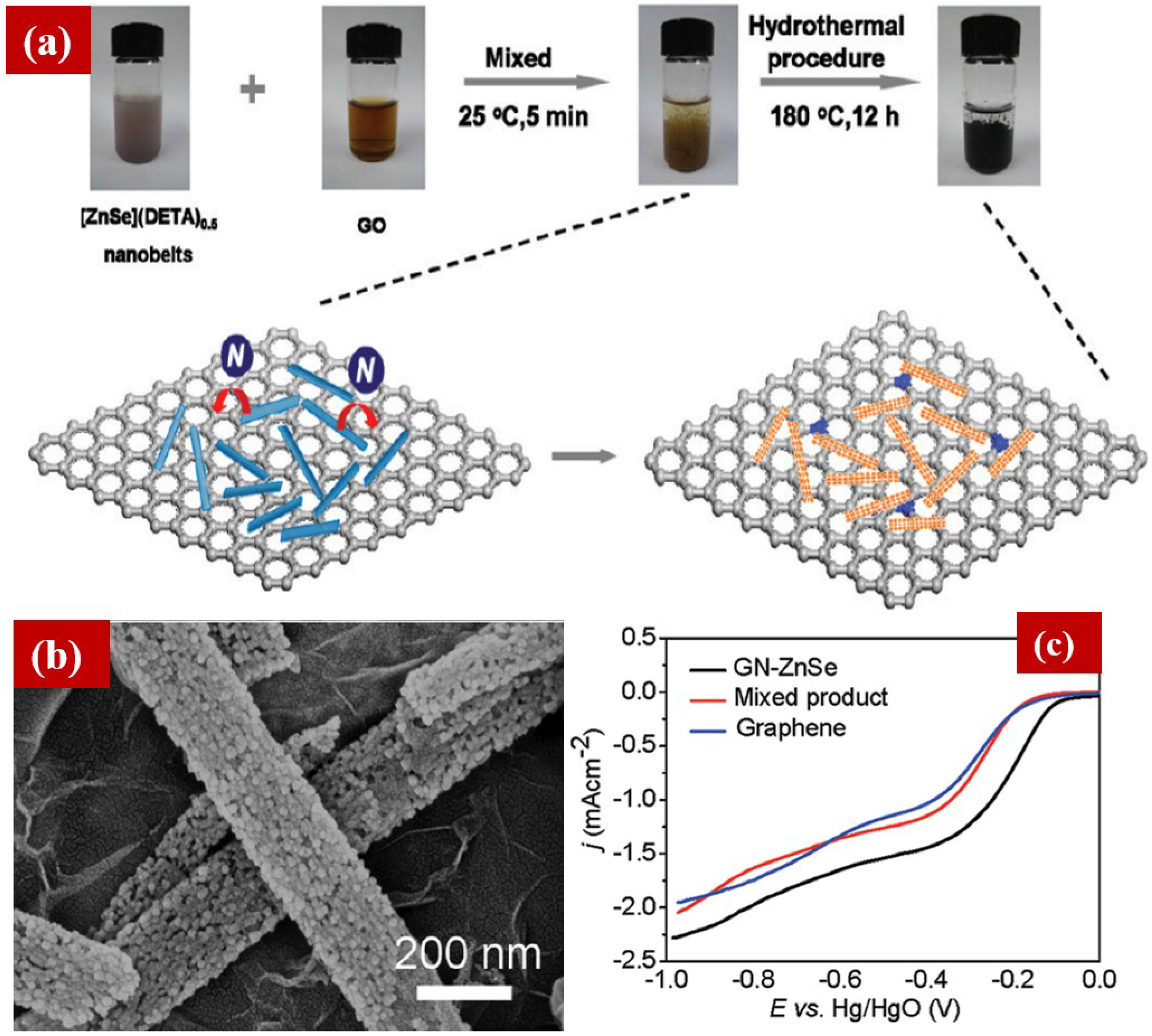
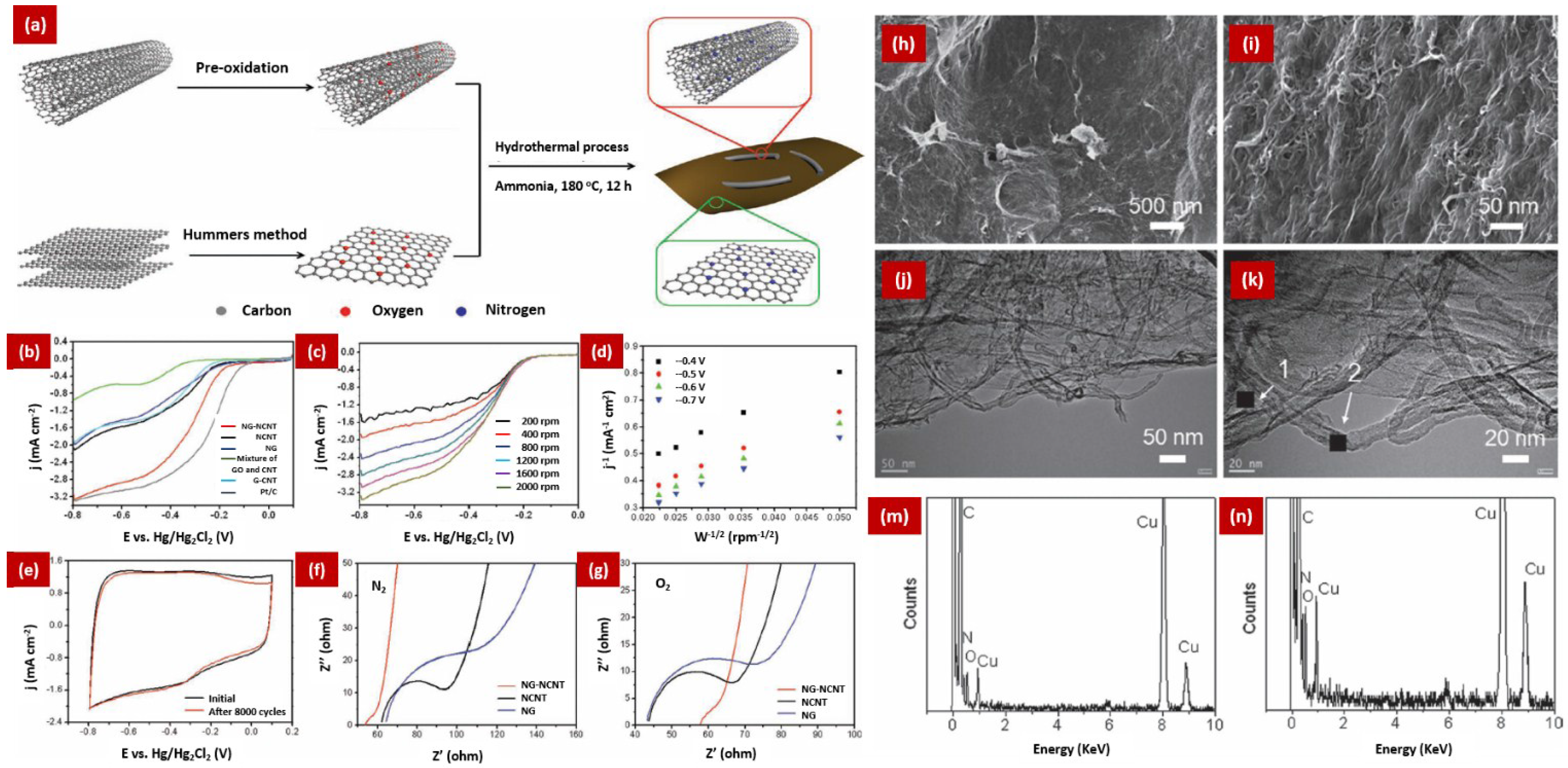
5. The Composites of NCNTs and NG for ORR
6. Conclusion and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Chen, J. Metal-air batteries: From oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172–2192. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-W.; Su, D. Heterogeneous nanocarbon materials for oxygen reduction reaction. Energy Environ. Sci. 2014, 7, 576–591. [Google Scholar] [CrossRef]
- Feng, L.; Yan, Y.; Chen, Y.; Wang, L. Nitrogen-doped carbon nanotubes as efficient and durable metal-free cathodic catalysts for oxygen reduction in microbial fuel cells. Energy Environ. Sci. 2011, 4, 1892–1899. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Liang, H.; Parvez, K.; Zhuang, X.; Feng, X.; Müllen, K. Nitrogen-doped carbon nanosheets with size-defined mesopores as highly efficient metal-free catalyst for the oxygen reduction reaction. Angew. Chem. 2014, 126, 1596–1600. [Google Scholar] [CrossRef]
- Sun, D.M.; Liu, C.; Ren, W.C.; Cheng, H.M. A review of carbon nanotube-and graphene-based flexible thin-film transistors. Small 2013, 9, 1188–1205. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Nagelli, E.; Du, F.; Dai, L. Metal-free carbon nanomaterials become more active than metal catalysts and last longer. J. Phys. Chem. Lett. 2010, 1, 2165–2173. [Google Scholar] [CrossRef]
- Chun, K.-Y.; Lee, H.S.; Lee, C.J. Nitrogen doping effects on the structure behavior and the field emission performance of double-walled carbon nanotubes. Carbon 2009, 47, 169–177. [Google Scholar] [CrossRef]
- Bostwick, A.; Speck, F.; Seyller, T.; Horn, K.; Polini, M.; Asgari, R.; MacDonald, A.H.; Rotenberg, E. Observation of plasmarons in quasi-freestanding doped graphene. Science 2010, 328, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.O.; Park, J.S.; Choi, D.S.; Kim, J.Y.; Lee, S.H.; Lee, K.E.; Kim, Y.-H.; Song, M.H.; Yoo, S.; Kim, S.O. Work function-tunable, N-doped reduced graphene transparent electrodes for high-performance polymer light-emitting diodes. ACS Nano 2011, 6, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Czerw, R.; Terrones, M.; Charlier, J.-C.; Blase, X.; Foley, B.; Kamalakaran, R.; Grobert, N.; Terrones, H.; Tekleab, D.; Ajayan, P. Identification of electron donor states in N-doped carbon nanotubes. Nano Lett. 2001, 1, 457–460. [Google Scholar] [CrossRef]
- Lee, W.J.; Maiti, U.N.; Lee, J.M.; Lim, J.; Han, T.H.; Kim, S.O. Nitrogen-doped carbon nanotubes and graphene composite structures for energy and catalytic applications. Chem. Commun. 2014, 50, 6818–6830. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Y.; Zhu, D. Chemical doping of graphene. J. Mater. Chem. 2011, 21, 3335–3345. [Google Scholar] [CrossRef]
- Yang, Z.; Nie, H.; Chen, X.A.; Chen, X.; Huang, S. Recent progress in doped carbon nanomaterials as effective cathode catalysts for fuel cell oxygen reduction reaction. J. Power Sources 2013, 236, 238–249. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Jin, Y.; Qiao, S.Z. Nanostructured metal-free electrochemical catalysts for highly efficient oxygen reduction. Small 2012, 8, 3550–3566. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Arenas, J.; Higgins, D.; Chen, Z.; Fowler, M.; Chen, Z. Mechanistic analysis of highly active nitrogen-doped carbon nanotubes for the oxygen reduction reaction. J. Power Sources 2012, 205, 215–221. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Ge, J.; Wang, L.; Wang, D.H.; Ding, F.; Tao, X.M.; Chen, W. Manageable N-doped graphene for high performance oxygen reduction reaction. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Kim, M.R.; Jeong, S.H.; Kim, S.U.; Lee, O.J.; Lee, K.H.; Suh, J.H.; Park, C.K. High-yield synthesis of multi-walled carbon nanotubes by arc discharge in liquid nitrogen. Appl. Phys. A 2003, 76, 285–286. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Zhou, Y.; Zhang, X.; Cai, B.; Qiu, J. Flowing nitrogen assisted-arc discharge synthesis of nitrogen-doped single-walled carbon nanohorns. Appl. Surf. Sci. 2013, 277, 88–93. [Google Scholar] [CrossRef]
- Mo, Z.; Liao, S.; Zheng, Y.; Fu, Z. Preparation of nitrogen-doped carbon nanotube arrays and their catalysis towards cathodic oxygen reduction in acidic and alkaline media. Carbon 2012, 50, 2620–2627. [Google Scholar] [CrossRef]
- Sharifi, T.; Nitze, F.; Barzegar, H.R.; Tai, C.-W.; Mazurkiewicz, M.; Malolepszy, A.; Stobinski, L.; Wågberg, T. Nitrogen doped multi walled carbon nanotubes produced by CVD-correlating xps and raman spectroscopy for the study of nitrogen inclusion. Carbon 2012, 50, 3535–3541. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, D.; Liu, S.; Chen, S.; Hanif, M.; Hou, H. Free-standing nitrogen-doped carbon nanotubes at electrospun carbon nanofibers composite as an efficient electrocatalyst for oxygen reduction. Electrochim. Acta 2014, 138, 318–324. [Google Scholar] [CrossRef]
- Tao, X.Y.; Zhang, X.B.; Sun, F.Y.; Cheng, J.P.; Liu, F.; Luo, Z.Q. Large-scale CVD synthesis of nitrogen-doped multi-walled carbon nanotubes with controllable nitrogen content on a CoxMg1−xMoO4 catalyst. Diamond Relat. Mater. 2007, 16, 425–430. [Google Scholar] [CrossRef]
- She, X.; Yang, D.; Jing, D.; Yuan, F.; Yang, W.; Guo, L.; Che, Y. Nitrogen-doped one-dimensional (1D) macroporous carbonaceous nanotube arrays and their application in electrocatalytic oxygen reduction reactions. Nanoscale 2014, 6, 11057–11061. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xia, K.; Huang, L.; Li, L.; Pei, L.; Fei, S. Facile synthesis and hydrogen storage application of nitrogen-doped carbon nanotubes with bamboo-like structure. Int. J. Hydrogen Energy 2013, 38, 3297–3303. [Google Scholar] [CrossRef]
- Shi, W.; Venkatachalam, K.; Gavalas, V.; Qian, D.; Andrews, R.; Bachas, L.G.; Chopra, N. The role of plasma treatment on electrochemical capacitance of undoped and nitrogen doped carbon nanotubes. Nanomater. Energy 2013, 2, 71–81. [Google Scholar] [CrossRef]
- Du, Z.; Wang, S.; Kong, C.; Deng, Q.; Wang, G.; Liang, C.; Tang, H. Microwave plasma synthesized nitrogen-doped carbon nanotubes for oxygen reduction. J. Solid State Electrochem. 2015, 19, 1541–1549. [Google Scholar] [CrossRef]
- Magrez, A.; Seo, J.W.; Smajda, R.; Mionić, M.; Forró, L. Catalytic CVD synthesis of carbon nanotubes: Towards high yield and low temperature growth. Materials 2010, 3, 4871–4891. [Google Scholar] [CrossRef]
- Donato, M.G.; Galvagno, S.; Lanza, M.; Messina, G.; Milone, C.; Piperopoulos, E.; Pistone, A.; Santangelo, S. Influence of carbon source and Fe-catalyst support on the growth of multi-walled carbon nanotubes. J. Nanosci. Nanotechnol. 2009, 9, 3815–3823. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Papadopoulos, C.; Xu, J.M.; Moskovits, M. Highly-ordered carbon nanotube arrays for electronics applications. Appl. Phys. Lett. 1999, 75, 367–369. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Li, Y.; Chen, L.; Chen, H.; Zhang, L.; Shi, J. A co-pyrolysis route to synthesize nitrogen doped multiwall carbon nanotubes for oxygen reduction reaction. Carbon 2014, 68, 232–239. [Google Scholar] [CrossRef]
- Ayala, P.; Grüneis, A.; Gemming, T.; Grimm, D.; Kramberger, C.; Rümmeli, M.H.; Freire, F.L.; Kuzmany, H.; Pfeiffer, R.; Barreiro, A. Tailoring N-doped single and double wall carbon nanotubes from a nondiluted carbon/nitrogen feedstock. J. Phys. Chem. C 2007, 111, 2879–2884. [Google Scholar] [CrossRef]
- Tang, C.; Golberg, D.; Bando, Y.; Xu, F.; Liu, B. Synthesis and field emission of carbon nanotubular fibers doped with high nitrogen content. Chem. Commun. 2003, 3050–3051. [Google Scholar] [CrossRef]
- Rao, C.V.; Cabrera, C.R.; Ishikawa, Y. In search of the active site in nitrogen-doped carbon nanotube electrodes for the oxygen reduction reaction. J. Phys. Chem. Lett. 2010, 1, 2622–2627. [Google Scholar] [CrossRef]
- Rao, C.V.; Ishikawa, Y. Activity, selectivity, and anion-exchange membrane fuel cell performance of virtually metal-free nitrogen-doped carbon nanotube electrodes for oxygen reduction reaction. J. Phys. Chem. C 2012, 116, 4340–4346. [Google Scholar]
- Guo, Q.; Xie, Y.; Wang, X.; Zhang, S.; Hou, T.; Lv, S. Synthesis of carbon nitride nanotubes with the C3N4 stoichiometry via a benzene-thermal process at low temperatures. Chem. Commun. 2004, 26–27. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Huang, F.; Cao, C.; Li, J.; Zhu, H. Synthesis of carbon nitride nanotubes via a catalytic-assembly solvothermal route. Chem. Mater. 2004, 16, 5213–5215. [Google Scholar] [CrossRef]
- Nagaiah, T.C.; Kundu, S.; Bron, M.; Muhler, M.; Schuhmann, W. Nitrogen-doped carbon nanotubes as a cathode catalyst for the oxygen reduction reaction in alkaline medium. Electrochem. Commun. 2010, 12, 338–341. [Google Scholar] [CrossRef]
- Chan, L.H.; Hong, K.H.; Xiao, D.Q.; Lin, T.C.; Lai, S.H.; Hsieh, W.J.; Shih, H.C. Resolution of the binding configuration in nitrogen-doped carbon nanotubes. Phys. Rev. B 2004, 70, 125408. [Google Scholar] [CrossRef]
- Vikkisk, M.; Kruusenberg, I.; Ratso, S.; Joost, U.; Shulga, E.; Kink, I.; Rauwel, P.; Tammeveski, K. Enhanced electrocatalytic activity of nitrogen-doped multi-walled carbon nanotubes towards the oxygen reduction reaction in alkaline media. RSC Adv. 2015, 5, 59495–59505. [Google Scholar] [CrossRef]
- Chen, P.; Xiao, T.Y.; Qian, Y.H.; Li, S.S.; Yu, S.H. A nitrogen-doped graphene/carbon nanotube nanocomposite with synergistically enhanced electrochemical activity. Adv. Mater. 2013, 25, 3192–3196. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, T.; Hu, G.; Jia, X.; Wågberg, T. Formation of active sites for oxygen reduction reactions by transformation of nitrogen functionalities in nitrogen-doped carbon nanotubes. ACS Nano 2012, 6, 8904–8912. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.N.; O'Hayre, R.; Pylypenko, S. Recent progress on nitrogen/carbon structures designed for use in energy and sustainability applications. Energy Environ. Sci. 2014, 7, 1212–1249. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Srivastava, A.; Gowda, S.R.; Gullapalli, H.; Dubey, M.; Ajayan, P.M. Synthesis of nitrogen-doped graphene films for lithium battery application. ACS Nano 2010, 4, 6337–6342. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yao, J.; Kittrell, C.; Tour, J.M. Large-scale growth and characterizations of nitrogen-doped monolayer graphene sheets. ACS Nano 2011, 5, 4112–4117. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Lim, S.; Tian, Z.; Shang, J.; Lai, L.; MacDonald, B.; Fu, C.; Shen, Z.; Yu, T.; Lin, J. Pyridinic N doped graphene: Synthesis, electronic structure, and electrocatalytic property. J. Mater. Chem. 2011, 21, 8038–8044. [Google Scholar] [CrossRef]
- Deng, D.; Pan, X.; Yu, L.; Cui, Y.; Jiang, Y.; Qi, J.; Li, W.-X.; Fu, Q.; Ma, X.; Xue, Q.; et al. Toward N-doped graphene via solvothermal synthesis. Chem. Mater. 2011, 23, 1188–1193. [Google Scholar] [CrossRef]
- Ghosh, A.; Late, D.J.; Panchakarla, L.S.; Govindaraj, A.; Rao, C.N.R. NO2 and humidity sensing characteristics of few-layer graphenes. J. Exp. Nanosci. 2009, 4, 313–322. [Google Scholar] [CrossRef]
- Panchakarla, L.S.; Subrahmanyam, K.S.; Saha, S.K.; Govindaraj, A.; Krishnamurthy, H.R.; Waghmare, U.V.; Rao, C.N.R. Synthesis, structure, and properties of boron-and nitrogen-doped graphene. Adv. Mater. 2009, 21, 4726–4730. [Google Scholar] [CrossRef]
- Subrahmanyam, K.S.; Panchakarla, L.S.; Govindaraj, A.; Rao, C.N.R. Simple method of preparing graphene flakes by an arc-discharge method. J. Phys. Chem. C 2009, 113, 4257–4259. [Google Scholar] [CrossRef]
- Geng, D.; Chen, Y.; Chen, Y.; Li, Y.; Li, R.; Sun, X.; Ye, S.; Knights, S. High oxygen-reduction activity and durability of nitrogen-doped graphene. Energy Environ. Sci. 2011, 4, 760–764. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhang, L.; Yoon, Y.; Weber, P.K.; Wang, H.; Guo, J.; Dai, H. N-doping of graphene through electrothermal reactions with ammonia. Science 2009, 324, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Robinson, J.T.; Sanchez, H.; Diankov, G.; Dai, H. Simultaneous nitrogen doping and reduction of graphene oxide. J. Am. Chem. Soc. 2009, 131, 15939–15944. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.-H.; Shao, L.; Chen, J.-J.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and its potential applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, S.; Engelhard, M.H.; Li, G.; Shao, G.; Wang, Y.; Liu, J.; Aksay, I.A.; Lin, Y. Nitrogen-doped graphene and its electrochemical applications. J. Mater. Chem. 2010, 20, 7491–7496. [Google Scholar] [CrossRef]
- Guo, B.; Liu, Q.; Chen, E.; Zhu, H.; Fang, L.; Gong, J.R. Controllable N-doping of graphene. Nano Lett. 2010, 10, 4975–4980. [Google Scholar] [CrossRef] [PubMed]
- Long, D.; Li, W.; Ling, L.; Miyawaki, J.; Mochida, I.; Yoon, S.-H. Preparation of nitrogen-doped graphene sheets by a combined chemical and hydrothermal reduction of graphene oxide. Langmuir 2010, 26, 16096–16102. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, L.; Tian, C.; Tan, T.; Xie, Y.; Shi, K.; Li, M.; Fu, H. Nitrogen-doped graphene with high nitrogen level via a one-step hydrothermal reaction of graphene oxide with urea for superior capacitive energy storage. RSC Adv. 2012, 2, 4498–4506. [Google Scholar] [CrossRef]
- Kundu, S.; Nagaiah, T.C.; Xia, W.; Wang, Y.; Dommele, S.V.; Bitter, J.H.; Santa, M.; Grundmeier, G.; Bron, M.; Schuhmann, W. Electrocatalytic activity and stability of nitrogen-containing carbon nanotubes in the oxygen reduction reaction. J. Phys. Chem. C 2009, 113, 14302–14310. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, R.; Zheng, J.; Zhao, J.; Li, L.; Song, J.; Zhu, Z. Nitrogen-promoted self-assembly of N-doped carbon nanotubes and their intrinsic catalysis for oxygen reduction in fuel cells. ACS Nano 2011, 5, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, H.; Jong, Z.; Qu, W.; Geng, D.; Sun, X.; Wang, H. Nitrogen-doped carbon nanotubes with high activity for oxygen reduction in alkaline media. Int. J. Hydrogen Energy 2011, 36, 2258–2265. [Google Scholar] [CrossRef]
- Qiu, Y.; Yin, J.; Hou, H.; Yu, J.; Zuo, X. Preparation of nitrogen-doped carbon submicrotubes by coaxial electrospinning and their electrocatalytic activity for oxygen reduction reaction in acid media. Electrochim. Acta 2013, 96, 225–229. [Google Scholar] [CrossRef]
- Wiggins-Camacho, J.D.; Stevenson, K.J. Mechanistic discussion of the oxygen reduction reaction at nitrogen-doped carbon nanotubes. J. Phys. Chem. C 2011, 115, 20002–20010. [Google Scholar] [CrossRef]
- Okamoto, Y. First-principles molecular dynamics simulation of O2 reduction on nitrogen-doped carbon. Appl. Surf. Sci. 2009, 256, 335–341. [Google Scholar] [CrossRef]
- Hu, X.; Wu, Y.; Li, H.; Zhang, Z. Adsorption and activation of O2 on nitrogen-doped carbon nanotubes. J. Phys. Chem. C 2010, 114, 9603–9607. [Google Scholar] [CrossRef]
- Barone, V.; Peralta, J.E.; Wert, M.; Heyd, J.; Scuseria, G.E. Density functional theory study of optical transitions in semiconducting single-walled carbon nanotubes. Nano Lett. 2005, 5, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, H.; Yamada, T.; Cao, B.; Mizorogi, N.; Slanina, Z.; Tsuchiya, T.; Akasaka, T.; Yoza, K.; Nagase, S. Missing metallofullerene with C80 cage. J. Am. Chem. Soc. 2009, 131, 10950–10954. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Allen, B.L.; Kauffman, D.R.; Star, A. Electrocatalytic activity of nitrogen-doped carbon nanotube cups. J. Am. Chem. Soc. 2009, 131, 13200–13201. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, D.; Chen, H.; Gao, Y.; Li, H. Nitrogen-doped carbon nanotubes as catalysts for the oxygen reduction reaction in alkaline medium. J. Power Sources 2015, 279, 28–35. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Tao, H.; Hsu, R.S.; Chen, Z. Highly active nitrogen-doped carbon nanotubes for oxygen reduction reaction in fuel cell applications. J. Phys. Chem. C 2009, 113, 21008–21013. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Chen, Z. Nitrogen doped carbon nanotubes and their impact on the oxygen reduction reaction in fuel cells. Carbon 2010, 48, 3057–3065. [Google Scholar] [CrossRef]
- Higgins, D.; Chen, Z.; Chen, Z. Nitrogen doped carbon nanotubes synthesized from aliphatic diamines for oxygen reduction reaction. Electrochim. Acta 2011, 56, 1570–1575. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Chen, Z. Electrocatalytic activity of nitrogen doped carbon nanotubes with different morphologies for oxygen reduction reaction. Electrochim. Acta 2010, 55, 4799–4804. [Google Scholar] [CrossRef]
- Geng, D.; Liu, H.; Chen, Y.; Li, R.; Sun, X.; Ye, S.; Knights, S. Non-noble metal oxygen reduction electrocatalysts based on carbon nanotubes with controlled nitrogen contents. J. Power Sources 2011, 196, 1795–1801. [Google Scholar] [CrossRef]
- Borghei, M.; Kanninen, P.; Lundahl, M.; Susi, T.; Sainio, J.; Anoshkin, I.; Nasibulin, A.; Kallio, T.; Tammeveski, K.; Kauppinen, E. High oxygen reduction activity of few-walled carbon nanotubes with low nitrogen content. Appl. Catal. B 2014, 158, 233–241. [Google Scholar] [CrossRef]
- Tuci, G.; Zafferoni, C.; D’Ambrosio, P.; Caporali, S.; Ceppatelli, M.; Rossin, A.; Tsoufis, T.; Innocenti, M.; Giambastiani, G. Tailoring carbon nanotube N-dopants while designing metal-free electrocatalysts for the oxygen reduction reaction in alkaline medium. ACS Catal. 2013, 3, 2108–2111. [Google Scholar] [CrossRef]
- Tuci, G.; Zafferoni, C.; Rossin, A.; Milella, A.; Luconi, L.; Innocenti, M.; Truong Phuoc, L.; Duong-Viet, C.; Pham-Huu, C.; Giambastiani, G. Chemically functionalized carbon nanotubes with pyridine groups as easily tunable N-decorated nanomaterials for the oxygen reduction reaction in alkaline medium. Chem. Mater. 2014, 26, 3460–3470. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, Q.; Dai, L. Highly efficient metal-free growth of nitrogen-doped single-walled carbon nanotubes on plasma-etched substrates for oxygen reduction. J. Am. Chem. Soc. 2010, 132, 15127–15129. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, G.; Zhong, Y.; Liu, H.; Li, R.; Zhou, X.; Sun, X. Ultrathin single crystal Pt nanowires grown on N-doped carbon nanotubes. Chem. Commun. 2009, 7048–7050. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, G.; Stevenson, K.J. Synergistic assembly of dendrimer-templated platinum catalysts on nitrogen-doped carbon nanotube electrodes for oxygen reduction. Langmuir 2007, 23, 5279–5282. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Liu, H.; Banis, M.N.; Li, R.; Sun, X.; Sham, T.-K.; Ye, S.; Knights, S. Nitrogen doping effects on carbon nanotubes and the origin of the enhanced electrocatalytic activity of supported Pt for proton-exchange membrane fuel cells. J. Phys. Chem. C 2011, 115, 3769–3776. [Google Scholar] [CrossRef]
- Saha, M.S.; Li, R.; Sun, X.; Ye, S. 3-d composite electrodes for high performance pem fuel cells composed of Pt supported on nitrogen-doped carbon nanotubes grown on carbon paper. Electrochem. Commun. 2009, 11, 438–441. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Liu, H.; Li, R.; Sun, X.; Ye, S.; Knights, S. Enhanced stability of Pt electrocatalysts by nitrogen doping in CNTs for PEM fuel cells. Electrochem. Commun. 2009, 11, 2071–2076. [Google Scholar] [CrossRef]
- Banis, M.N.; Sun, S.; Meng, X.; Zhang, Y.; Wang, Z.; Li, R.; Cai, M.; Sham, T.-K.; Sun, X. TiSi2Ox coated N-doped carbon nanotubes as Pt catalyst support for the oxygen reduction reaction in PEMFCs. J. Phys. Chem. C 2013, 117, 15457–15467. [Google Scholar] [CrossRef]
- Higgins, D.C.; Meza, D.; Chen, Z. Nitrogen-doped carbon nanotubes as platinum catalyst supports for oxygen reduction reaction in proton exchange membrane fuel cells. J. Phys. Chem. C 2010, 114, 21982–21988. [Google Scholar] [CrossRef]
- Qu, L.; Liu, Y.; Baek, J.-B.; Dai, L. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.Y.; Chen, Y.G.; Chen, L. Easy-to-operate and low-temperature synthesis of gram-scale nitrogen-doped graphene and its application as cathode catalyst in microbial fuel cells. ACS Nano 2011, 5, 9611–9618. [Google Scholar] [CrossRef] [PubMed]
- He, C.Y.; Li, Z.S.; Cai, M.L.; Cai, M.; Wang, J.Q.; Tian, Z.Q.; Zhang, X.; Shen, P.K. A strategy for mass production of self-assembled nitrogen-doped graphene as catalytic materials. J. Mater. Chem. A 2013, 1, 1401–1406. [Google Scholar] [CrossRef]
- Wu, J.J.; Zhang, D.; Wang, Y.; Hou, B.R. Electrocatalytic activity of nitrogen-doped graphene synthesized via a one-pot hydrothermal process towards oxygen reduction reaction. J. Power Sources 2013, 227, 185–190. [Google Scholar] [CrossRef]
- Park, M.; Lee, T.; Kim, B.-S. Covalent functionalization based heteroatom doped graphene nanosheet as a metal-free electrocatalyst for oxygen reduction reaction. Nanoscale 2013, 5, 12255–12260. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Yu, L.; Kim, J.; Murakoshi, K. Selective nitrogen doping in graphene for oxygen reduction reactions. Chem. Commun. 2013, 49, 9627–9629. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Zheng, Y.; Li, L.H.; Cowie, B.C.C.; Gunzelmann, D.; Qiao, S.Z.; Huang, S.M.; Chen, Y. Observation of active sites for oxygen reduction reaction on nitrogen-doped multilayer graphene. ACS Nano 2014, 8, 6856–6862. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.; Gong, H.; Shen, Z.; Lin, J.; Ruoff, R.S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Yang, S.; Zhi, L.; Tang, K.; Feng, X.; Maier, J.; Muellen, K. Efficient synthesis of heteroatom (N or S)-doped graphene based on ultrathin graphene oxide-porous silica sheets for oxygen reduction reactions. Adv. Funct. Mater. 2012, 22, 3634–3640. [Google Scholar] [CrossRef]
- Jiang, Z.J.; Jiang, Z.Q.; Chen, W.H. The role of holes in improving the performance of nitrogen-doped holey graphene as an active electrode material for supercapacitor and oxygen reduction reaction. J. Power Sources 2014, 251, 55–65. [Google Scholar] [CrossRef]
- Yu, D.S.; Wei, L.; Jiang, W.C.; Wang, H.; Sun, B.; Zhang, Q.; Goh, K.L.; Si, R.M.; Chen, Y. Nitrogen doped holey graphene as an efficient metal-free multifunctional electrochemical catalyst for hydrazine oxidation and oxygen reduction. Nanoscale 2013, 5, 3457–3464. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.Y.; Song, M.K.; Ding, Y.; Liu, Y.; Liu, M.L.; Wong, C.P. Facile preparation of nitrogen-doped graphene as a metal-free catalyst for oxygen reduction reaction. Phys. Chem. Chem. Phys. 2012, 14, 3381–3387. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.Y.; Waller, G.; Liu, Y.; Liu, M.L.; Wong, C.P. Facile synthesis of nitrogen-doped graphene via pyrolysis of graphene oxide and urea, and its electrocatalytic activity toward the oxygen-reduction reaction. Adv. Energy Mater. 2012, 2, 884–888. [Google Scholar] [CrossRef]
- Unni, S.M.; Devulapally, S.; Karjule, N.; Kurungot, S. Graphene enriched with pyrrolic coordination of the doped nitrogen as an efficient metal-free electrocatalyst for oxygen reduction. J. Mater. Chem. 2012, 22, 23506–23513. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Fugane, K.; Mori, T.; Niu, L.; Ye, J.H. Wet chemical synthesis of nitrogen-doped graphene towards oxygen reduction electrocatalysts without high-temperateure pyrolysis. J. Mater. Chem. 2012, 22, 6575–6580. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Waller, G.H.; Liu, Y.; Liu, M.L.; Wong, C.P. Simple preparation of nanoporous few-layer nitrogen-doped graphene for use as an efficient electrocatalyst for oxygen reduction and oxygen evolution reactions. Carbon 2013, 53, 130–136. [Google Scholar] [CrossRef]
- Lu, Z.J.; Bao, S.J.; Gou, Y.T.; Cai, C.J.; Ji, C.C.; Xu, M.W.; Song, J.; Wang, R.Y. Nitrogen-doped reduced-graphene oxide as an efficient metal-free electrocatalyst for oxygen reduction in fuel cells. RSC Adv. 2013, 3, 3990–3995. [Google Scholar] [CrossRef]
- Pan, F.P.; Jin, J.T.; Fu, X.G.; Liu, Q.; Zhang, J.Y. Advanced oxygen reduction electrocatalyst based on nitrogen-doped graphene derived from edible sugar and urea. ACS Appl. Mater. Interfaces 2013, 5, 11108–11114. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Wang, J.; Wang, F.B.; Xia, X.H. Synthesis of nitrogen doped graphene with high electrocatalytic activity toward oxygen reduction reaction. Electrochem. Commun. 2013, 28, 24–26. [Google Scholar] [CrossRef]
- Cong, H.P.; Wang, P.; Gong, M.; Yu, S.H. Facile synthesis of mesoporous nitrogen-doped graphene: An efficient methanol-tolerant cathodic catalyst for oxygen reduction reaction. Nano Energy 2014, 3, 55–63. [Google Scholar] [CrossRef]
- Vikkisk, M.; Kruusenberg, I.; Joost, U.; Shulga, E.; Kink, I.; Tammeveski, K. Electrocatalytic oxygen reduction on nitrogen-doped graphene in alkaline media. Appl. Catal. B 2014, 147, 369–376. [Google Scholar] [CrossRef]
- Liu, M.K.; Song, Y.F.; He, S.X.; Tjiu, W.W.; Pan, J.S.; Xia, Y.Y.; Liu, T.X. Nitrogen-doped graphene nanoribbons as efficient metal-free electrocatalysts for oxygen reduction. ACS Appl. Mater. Interfaces 2014, 6, 4214–4222. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Zeng, D.; Yu, X.; Xie, F.; Zhang, W.; Chen, J.; Yan, J.; Xie, F.; Wang, L.; Meng, H.; et al. Exploring the active sites of nitrogen-doped graphene as catalysts for the oxygen reduction reaction. Int. J. Hydrogen Energy 2014, 39, 15996–16005. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Xin, Y.; Liu, J.; Yao, Y.; Zou, Z. Rapid synthesis of nitrogen-doped graphene by microwave heating for oxygen reduction reactions in alkaline electrolyte. Chin. J. Catal. 2014, 35, 509–513. [Google Scholar] [CrossRef]
- Chen, L.; Du, R.; Zhu, J.; Mao, Y.; Xue, C.; Zhang, N.; Hou, Y.; Zhang, J.; Yi, T. Three-dimensional nitrogen-doped graphene nanoribbons aerogel as a highly efficient catalyst for the oxygen reduction reaction. Small 2015, 11, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Masa, J.; Weide, P.; Fairclough, S.M.; Robertson, A.W.; Ebbinghaus, P.; Warner, J.H.; Tsang, S.C.E.; Muhler, M.; Schuhmann, W. High-quality functionalized few-layer graphene: Facile fabrication and doping with nitrogen as a metal-free catalyst for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 15444–15450. [Google Scholar] [CrossRef]
- Wu, J.; Ma, L.; Yadav, R.M.; Yang, Y.; Zhang, X.; Vajtai, R.; Lou, J.; Ajayan, P.M. Nitrogen-doped graphene with pyridinic dominance as a highly active and stable electrocatalyst for oxygen reduction. ACS Appl. Mater. Interfaces 2015, 7, 14763–14769. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Bai, Z.; Wu, M.; Qiao, J.; Chen, Z. 3-dimensional porous N-doped graphene foam as a non-precious catalyst for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 3343–3350. [Google Scholar] [CrossRef]
- Kong, X.-K.; Chen, C.-L.; Chen, Q.-W. Doped graphene for metal-free catalysis. Chem. Soc. Rev. 2014, 43, 2841–2857. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Pan, X.; Cao, X.; Hu, P.; Bao, X. Oxygen reduction reaction mechanism on nitrogen-doped graphene: A density functional theory study. J. Catal. 2011, 282, 183–190. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, Z. Mechanisms of oxygen reduction reaction on nitrogen-doped graphene for fuel cells. J. Phys. Chem. C 2011, 115, 11170–11176. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.; Woo, S.I.; Jung, Y. On the mechanism of enhanced oxygen reduction reaction in nitrogen-doped graphene nanoribbons. Phys. Chem. Chem. Phys. 2011, 13, 17505–17510. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Origin of the electrocatalytic oxygen reduction activity of graphene-based catalysts: A roadmap to achieve the best performance. J. Am. Chem. Soc. 2014, 136, 4394–4403. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, H.; Liu, Y.; Qu, J.; Li, J. Graphene-based transition metal oxide nanocomposites for the oxygen reduction reaction. Nanoscale 2015, 7, 1250–1269. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.-H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Qiao, J.; Yang, L.; Zhang, J. A review of graphene-based nanostructural materials for both catalyst supports and metal-free catalysts in pem fuel cell oxygen reduction reactions. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Li, Q.; Pan, H.; Higgins, D.; Cao, R.; Zhang, G.; Lv, H.; Wu, K.; Cho, J.; Wu, G. Metal-organic framework-derived bamboo-like nitrogen-doped graphene tubes as an active matrix for hybrid oxygen-reduction electrocatalysts. Small 2015, 11, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.H.; Li, Y.J.; Rana, R.K.; Zhu, J.J. Pt-Au/nitrogen-doped graphene nanocomposites for enhanced electrochemical activities. J. Mater. Chem. A 2013, 1, 1754–1762. [Google Scholar] [CrossRef]
- Imran Jafri, R.; Rajalakshmi, N.; Ramaprabhu, S. Nitrogen doped graphene nanoplatelets as catalyst support for oxygen reduction reaction in proton exchange membrane fuel cell. J. Mater. Chem. 2010, 20, 7114–7117. [Google Scholar] [CrossRef]
- Vinayan, B.P.; Nagar, R.; Rajalakshmi, N.; Ramaprabhu, S. Novel platinum–cobalt alloy nanoparticles dispersed on nitrogen-doped graphene as a cathode electrocatalyst for PEMFC applications. Adv. Funct. Mater. 2012, 22, 3519–3526. [Google Scholar] [CrossRef]
- Xiong, B.; Zhou, Y.; Zhao, Y.; Wang, J.; Chen, X.; O’Hayre, R.; Shao, Z. The use of nitrogen-doped graphene supporting Pt nanoparticles as a catalyst for methanol electrocatalytic oxidation. Carbon 2013, 52, 181–192. [Google Scholar] [CrossRef]
- Chen, P.; Xiao, T.-Y.; Li, H.-H.; Yang, J.-J.; Wang, Z.; Yao, H.-B.; Yu, S.-H. Nitrogen-doped graphene/ZnSe nanocomposites: Hydrothermal synthesis and their enhanced electrochemical and photocatalytic activities. ACS Nano 2012, 6, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Parvez, K.; Yang, S.B.; Hernandez, Y.; Winter, A.; Turchanin, A.; Feng, X.L.; Mullen, K. Nitrogen-doped graphene and its iron-based composite as efficient electrocatalysts for oxygen reduction reaction. ACS Nano 2012, 6, 9541–9550. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.C.; Zhu, Q.Q.; Lv, Z.X.; Dong, H.Z.; Yu, J.H.; Dong, L.F. Nitrogen-doped graphene as catalysts and catalyst supports for oxygen reduction in both acidic and alkaline solutions. Int. J. Hydrogen Energy 2013, 38, 1413–1418. [Google Scholar] [CrossRef]
- Huang, T.; Mao, S.; Pu, H.; Wen, Z.; Huang, X.; Ci, S.; Chen, J. Nitrogen-doped graphene-vanadium carbide hybrids as a high-performance oxygen reduction reaction electrocatalyst support in alkaline media. J. Mater. Chem. A 2013, 1, 13404–13410. [Google Scholar] [CrossRef]
- Park, H.W.; Lee, D.U.; Nazar, L.F.; Chen, Z.W. Oxygen reduction reaction using MnO2 nanotubes/nitrogen-doped exfoliated graphene hybrid catalyst for Li-O2 battery applications. J. Electrochem. Soc. 2013, 160, A344–A350. [Google Scholar] [CrossRef]
- Xiao, J.; Bian, X.; Liao, L.; Zhang, S.; Ji, C.; Liu, B. Nitrogen-doped mesoporous graphene as a synergistic electrocatalyst matrix for high-performance oxygen reduction reaction. ACS Appl. Mater. Interfaces 2014, 6, 17654–17660. [Google Scholar] [CrossRef] [PubMed]
- Brownson, D.A.C.; Munro, L.J.; Kampouris, D.K.; Banks, C.E. Electrochemistry of graphene: Not such a beneficial electrode material? RSC Adv. 2011, 1, 978–988. [Google Scholar] [CrossRef]
- Choi, C.H.; Chung, M.W.; Kwon, H.C.; Chung, J.H.; Woo, S.I. Nitrogen-doped graphene/carbon nanotube self-assembly for efficient oxygen reduction reaction in acid media. Appl. Catal. B 2014, 144, 760–766. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Wang, H.; Xie, L.; Liang, Y.; Wei, F.; Idrobo, J.-C.; Pennycook, S.J.; Dai, H. An oxygen reduction electrocatalyst based on carbon nanotube-graphene complexes. Nat. Nanotechnol. 2012, 7, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sun, L.; Huang, W.; Zhang, L.; Zhao, J.; Fan, Q.; Huang, W. Three-dimensional nitrogen-doped carbon nanotubes/graphene structure used as a metal-free electrocatalyst for the oxygen reduction reaction. J. Phys. Chem. C 2011, 115, 24592–24597. [Google Scholar] [CrossRef]
- Ratso, S.; Kruusenberg, I.; Vikkisk, M.; Joost, U.; Shulga, E.; Kink, I.; Kallio, T.; Tammeveski, K. Highly active nitrogen-doped few-layer graphene/carbon nanotube composite electrocatalyst for oxygen reduction reaction in alkaline media. Carbon 2014, 73, 361–370. [Google Scholar] [CrossRef]
- Liu, J.Y.; Wang, Z.; Chen, J.Y.; Wang, X. Nitrogen-doped carbon nanotubes and graphene nanohybrid for oxygen reduction reaction in acidic, alkaline and neutral solutions. J. Nano Res. 2015, 30, 50–58. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Q.; Tong, X.; Zhang, G.; Qiao, J.; Gong, Q.; Sun, S. Nitrogen-Doped Carbon Nanotube and Graphene Materials for Oxygen Reduction Reactions. Catalysts 2015, 5, 1574-1602. https://doi.org/10.3390/catal5031574
Wei Q, Tong X, Zhang G, Qiao J, Gong Q, Sun S. Nitrogen-Doped Carbon Nanotube and Graphene Materials for Oxygen Reduction Reactions. Catalysts. 2015; 5(3):1574-1602. https://doi.org/10.3390/catal5031574
Chicago/Turabian StyleWei, Qiliang, Xin Tong, Gaixia Zhang, Jinli Qiao, Qiaojuan Gong, and Shuhui Sun. 2015. "Nitrogen-Doped Carbon Nanotube and Graphene Materials for Oxygen Reduction Reactions" Catalysts 5, no. 3: 1574-1602. https://doi.org/10.3390/catal5031574
APA StyleWei, Q., Tong, X., Zhang, G., Qiao, J., Gong, Q., & Sun, S. (2015). Nitrogen-Doped Carbon Nanotube and Graphene Materials for Oxygen Reduction Reactions. Catalysts, 5(3), 1574-1602. https://doi.org/10.3390/catal5031574








