Pt Monolayer Electrocatalyst for Oxygen Reduction Reaction on Pd-Cu Alloy: First-Principles Investigation
Abstract
:1. Introduction
2. Computational Method
3. Results and Discussion
3.1. Structural Properties of PdCu
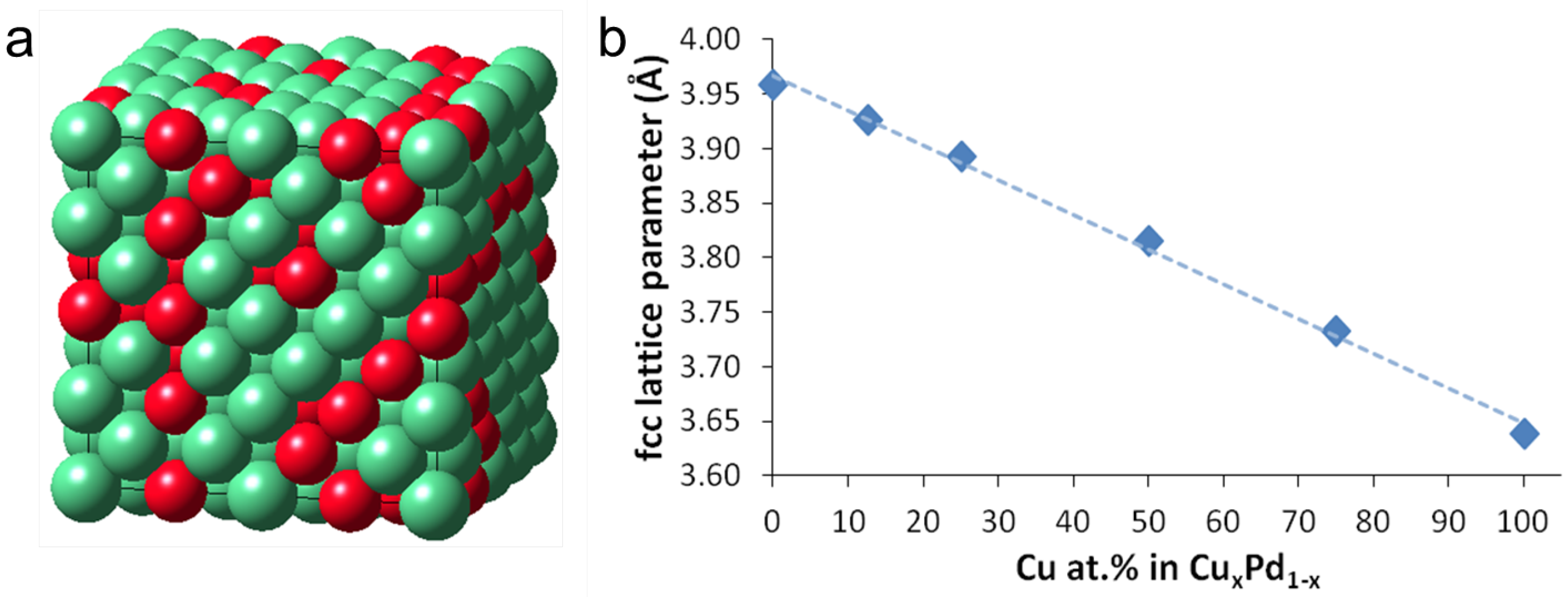
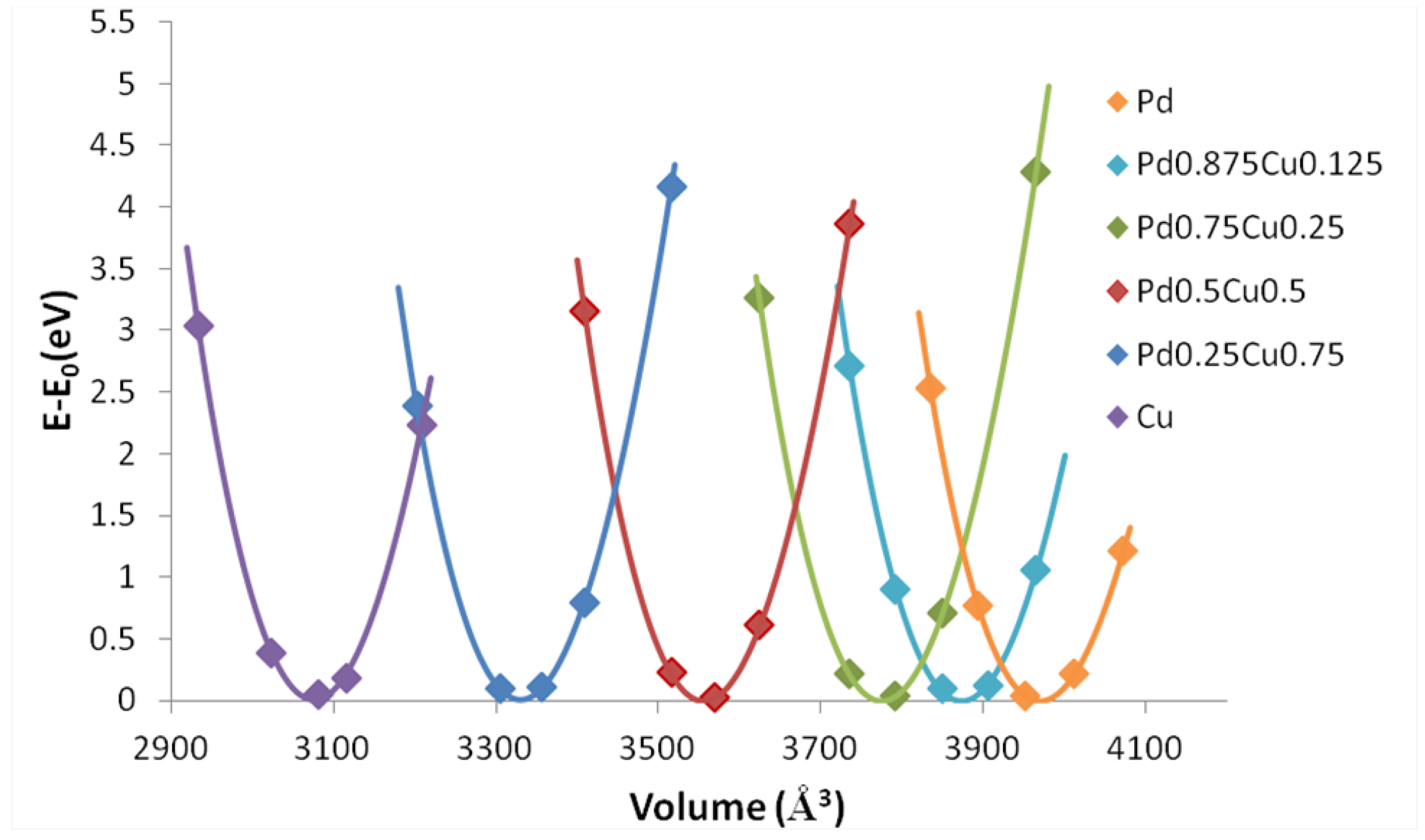
3.2. Chemical Stability and Segregation Profile
| Composition | E in vacuum | E in oxygen | |
|---|---|---|---|
| PdCu | -29.68 | - | - |
| PdCu | -54.84 | 61 | -100 |
| PdCu | -95.47 | 72 | -136 |
| PdCu | -79.88 | 70 | -178 |
3.3. Electronic Structure
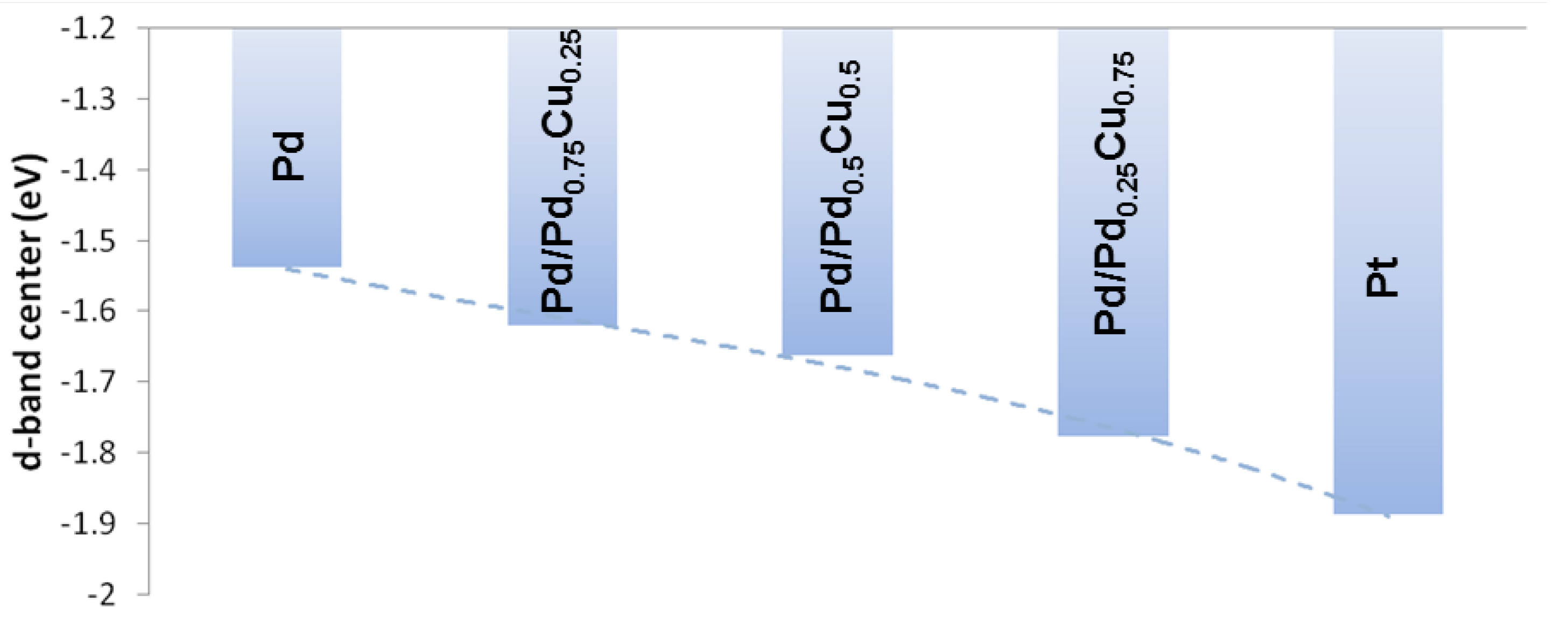
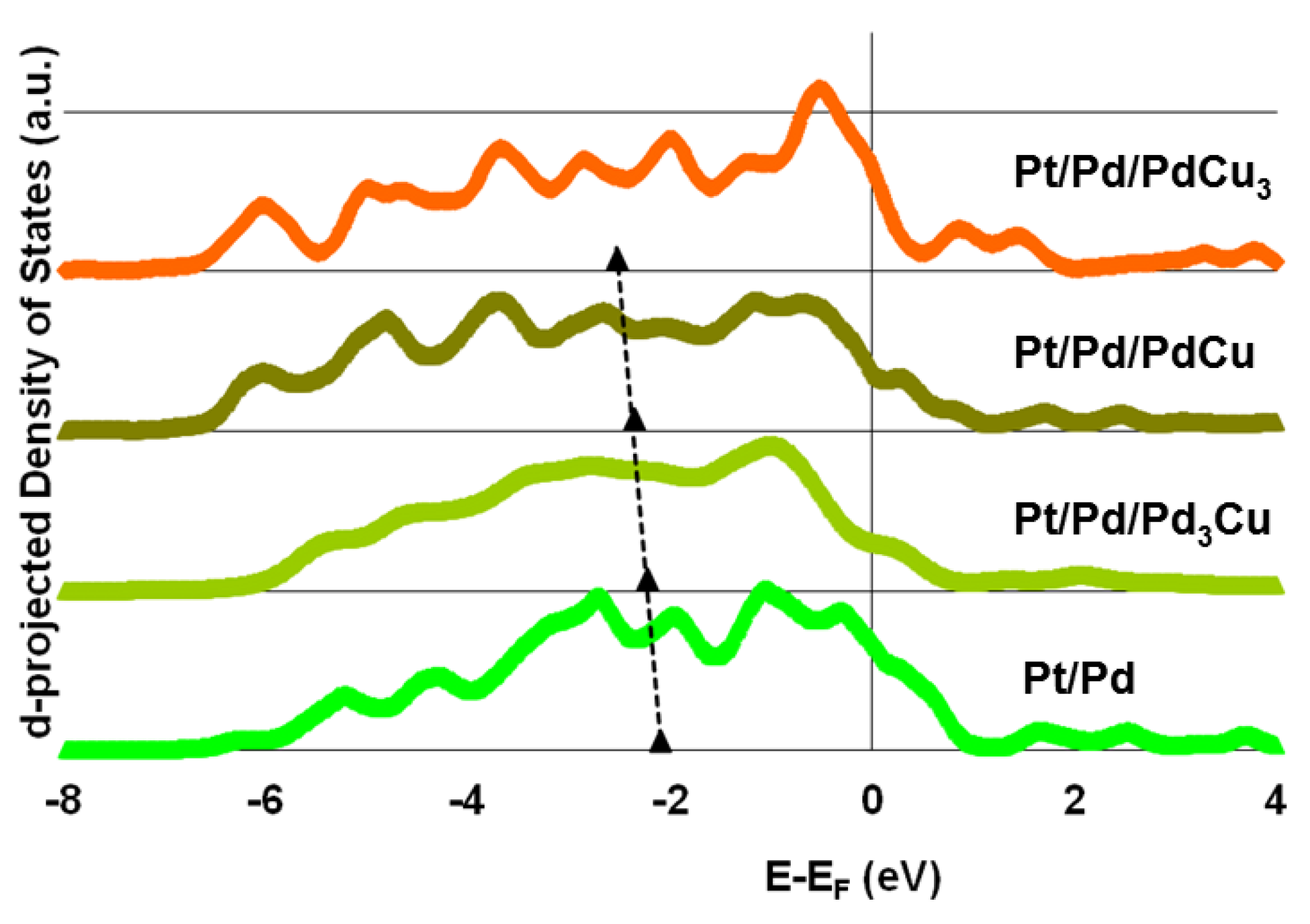

4. Conclusions
Author Contributions
Conflicts of Interest
References
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B 2005, 56, 9. [Google Scholar] [CrossRef]
- Adzic, R.R. Frontiers in electrochemistry. In Electrocatalysis; Lipkowski, J., Ross, P.N., Eds.; Wiley: New York, NY, USA, 1998; p. 197. [Google Scholar]
- Gasteiger, H.A.; Markovic, N.M. Just a dream-or future reality? Science 2009, 324, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Bashyam, R.; Zelenay, P. A class of non-precious metal composite catalysts for fuel cells. Nature 2006, 443, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Adzic, R.R.; Zhang, J.; Sasaki, K.; Vukmirovic, M.B.; Shao, M.; Wang, J.X.; Nilekar, A.U.; Mavrikakis, M.; Valerio, J.A.; Uribe, F. Platinum monolayer fuel cell electrocatalysts. Top. Catal. 2007, 46, 249–262. [Google Scholar]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Markovic, N.M. Improved oxygen reduction activity on Pt3Ni (111) via increased surface site availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.; Nørskov, J.K. Electronic factors determining the reactivity of metal surfaces. Surf. Sci. 1995, 343, 211. [Google Scholar] [CrossRef]
- Greeley, J.; Mavrikakis, M. Alloy catalysts designed from first principles. Nat. Mater. 2004, 3, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Wakisaka, M.; Yano, H.; Uchida, H. Analyses of oxygen reduction reaction at Pt-based electrocatalysts. ECS Trans. 2008, 16, 199. [Google Scholar]
- Shao, M.; Shoemaker, K.; Peles, A.; Kaneko, K.; Protsailo, L. Pt monolayer on porous Pd-Cu alloys as oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2010, 132, 9253–9255. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.; Strasser, P. Electrocatalysis on bimetallic surfaces: Modifying catalytic reactivity for oxygen reduction by voltammetric surface dealloying. J. Am. Chem. Soc. 2007, 129, 12624–12625. [Google Scholar] [CrossRef] [PubMed]
- Zeis, R.; Mathur, A.; Fritz, G.; Lee, J.; Erlebacher, J. Platinum-plated nanoporous gold: An efficient, low Pt loading electrocatalyst for PEM fuel cells. J. Power Sour. 2007, 165, 65. [Google Scholar] [CrossRef]
- Pedrew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244. [Google Scholar]
- Blöch, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Subramanian, P.R.; Laughlin, D.E. Cu-Pd (Copper-Palladium), Binary Alloy Phase Diagrams, 2nd ed.; Massalski, T.B., Ed.; Springer-Verlag: Berlin, Germany, 1990; Volume 2, pp. 1454–1456. [Google Scholar]
- Straumanis, M.E.; Yu, L.S. Lattice parameters, densities, expansion coefficients and perfection of structure of Cu and of Cu-In (α) phase. Acta Crystallogr. 1969, 25, 676. [Google Scholar] [CrossRef]
- Rao, C.N.; Rao, K.K. Effect of temperature on the lattice parameters of some silver-palladium alloys. Can. J. Phys. 1964, 42, 1336–1342. [Google Scholar]
- Alex Zunger, A.; Wei, S.H.; Ferreira, L.G.; Bernard, J.E. Special quasirandom structures. Phys. Rev. Lett. 1995, 65, 353–356. [Google Scholar] [CrossRef]
- Stamenkovic, V.; Moon, B.S.; Mayrhofer, K.J.J.; Ross, P.N.; Markovic, N.M.; Rossmeisl, J.; Greeley, J.; Nørskov, J.K. Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure. Angew. Chem. 2006, 45, 2897–2901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Vukmirovic, M.B.; Xu, Y.; Mavrikakis, M.; Adzic, R.R. Controlling the catalytic activity of platinum-monolayer electrocatalysts for oxygen reduction with different substrates. Angew. Chem. 2005, 44, 2132–2125. [Google Scholar] [CrossRef] [PubMed]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peles, A.; Shao, M.; Protsailo, L. Pt Monolayer Electrocatalyst for Oxygen Reduction Reaction on Pd-Cu Alloy: First-Principles Investigation. Catalysts 2015, 5, 1193-1201. https://doi.org/10.3390/catal5031193
Peles A, Shao M, Protsailo L. Pt Monolayer Electrocatalyst for Oxygen Reduction Reaction on Pd-Cu Alloy: First-Principles Investigation. Catalysts. 2015; 5(3):1193-1201. https://doi.org/10.3390/catal5031193
Chicago/Turabian StylePeles, Amra, Minhua Shao, and Lesia Protsailo. 2015. "Pt Monolayer Electrocatalyst for Oxygen Reduction Reaction on Pd-Cu Alloy: First-Principles Investigation" Catalysts 5, no. 3: 1193-1201. https://doi.org/10.3390/catal5031193
APA StylePeles, A., Shao, M., & Protsailo, L. (2015). Pt Monolayer Electrocatalyst for Oxygen Reduction Reaction on Pd-Cu Alloy: First-Principles Investigation. Catalysts, 5(3), 1193-1201. https://doi.org/10.3390/catal5031193






