2.2. Catalytic Cracking Test
The RFCC catalyst, the *BEA catalysts, and the composite catalysts (RFCC + *BEA, RFCC + MFI) were tested for catalytic cracking of
n-hexadecane. Reaction products were classified into gas (C
1–C
4), gasoline (C
5–216 °C), light cycle oil (LCO, > 216 °C) and coke. Most of the components in LCO fractions were unconverted
n-hexadecane and the yield of other reaction products in LCO fractions was lower than 5%, which suggests that
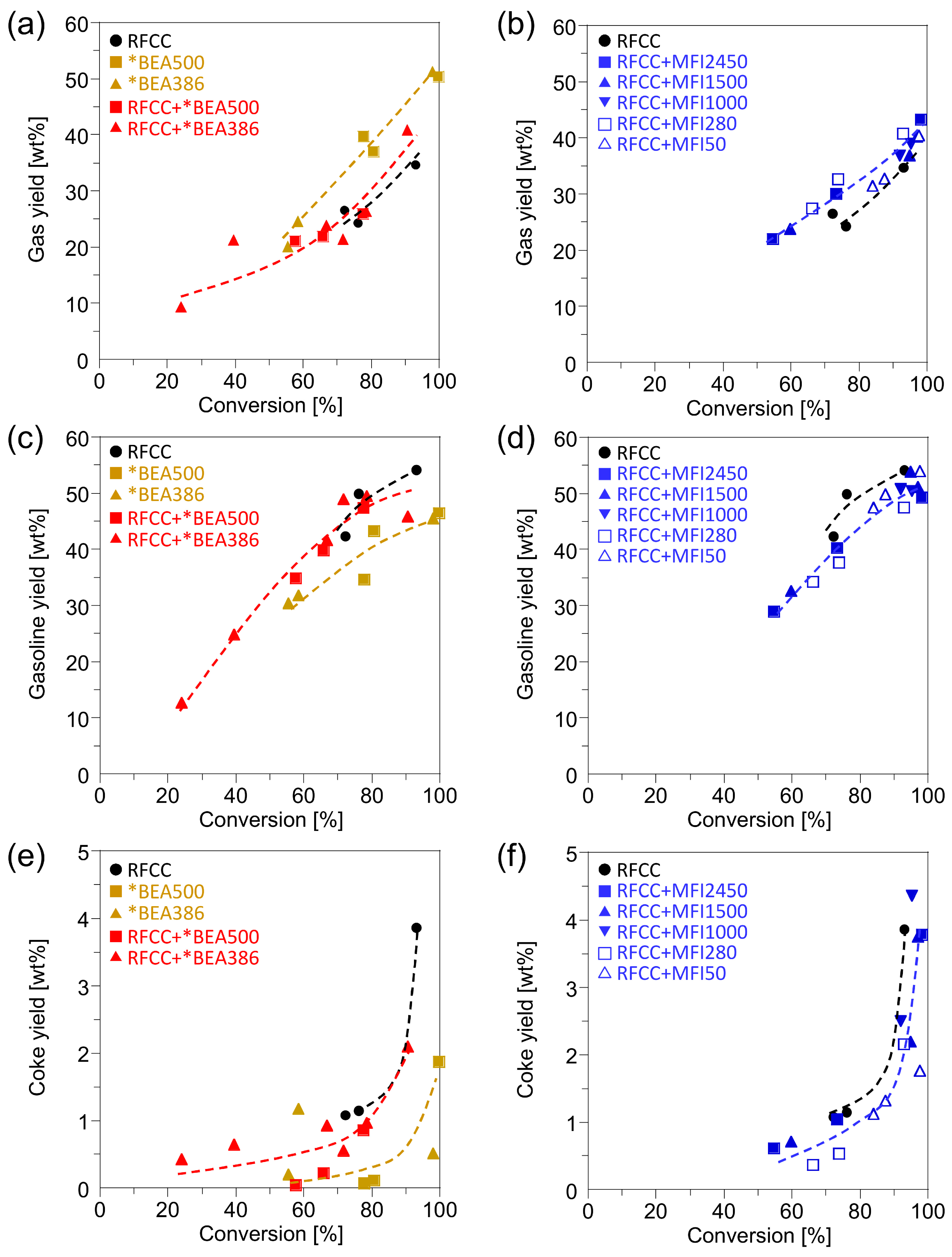
n-hexadecane was mainly cracked into gas, gasoline and coke. The yields of gas, gasoline and coke are shown in
Figure 2. Compared with the RFCC catalyst, the *BEA catalysts showed lower gasoline yield and higher gas and coke yield. However, the yield of gas, gasoline and coke on the composites of the RFCC catalyst and the *BEA zeolite were close to those on the RFCC catalyst, which means that addition of the *BEA zeolite hardly affects the yield of each fraction. On the other hand, the addition of the MFI zeolite to the RFCC catalyst slightly decreased the gasoline yield and increased the gas yield. This result indicates that the gasoline fraction compounds produced by the cracking of
n-hexadecane on the RFCC catalyst were transferred to the MFI zeolite and overcracked into the gas fraction. The coke yield on the RFCC catalyst was hardly affected by the addition of the MFI zeolite.
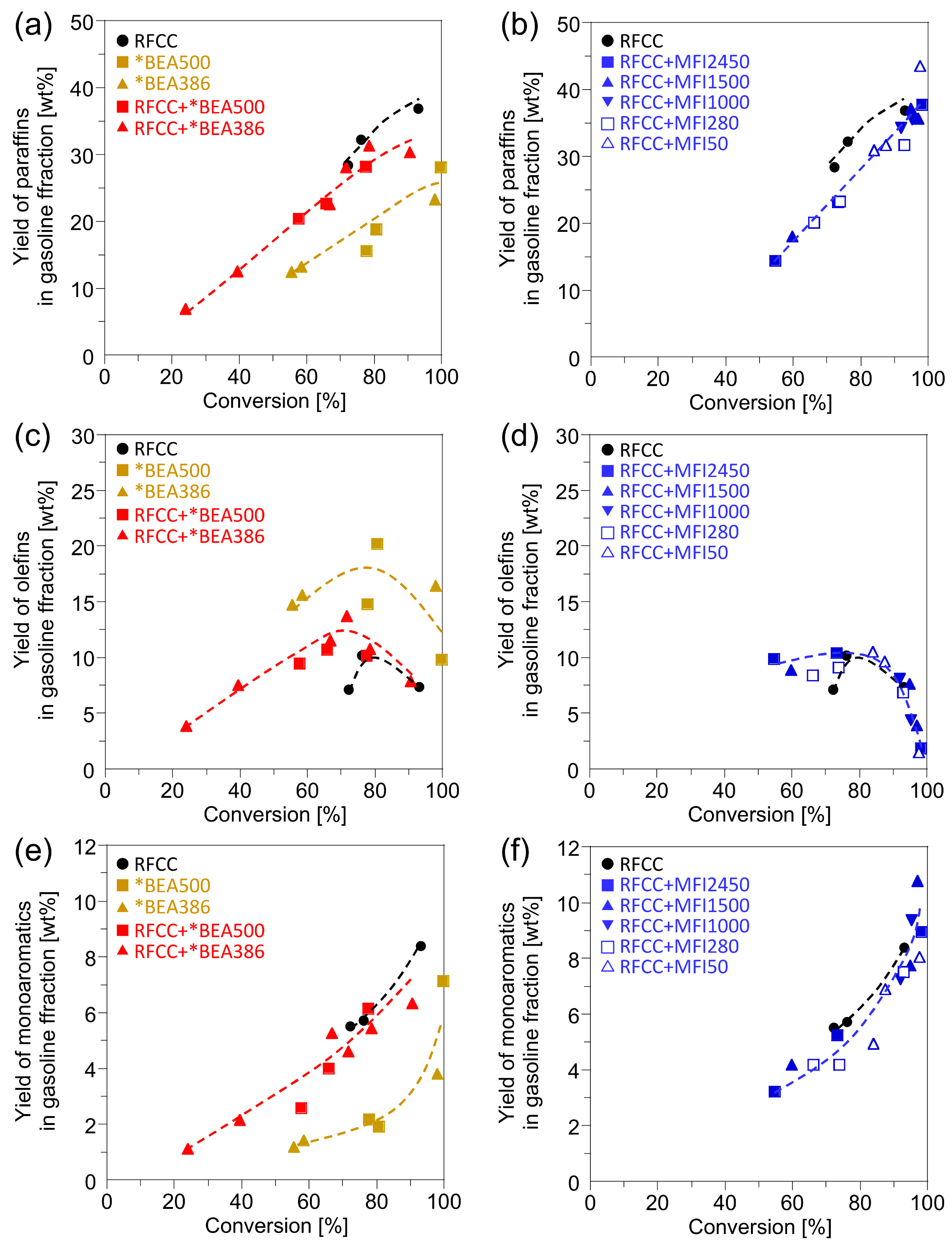
Figure 3 shows the yield of the products in the gasoline fraction obtained from the
n-hexadecane cracking on different catalysts. On all the catalysts, the paraffins and aromatics increased with the increase in the
n-hexadecane conversion, while the olefins decreased drastically at high
n-hexadecane conversion. This is because the hydrogen transfer reaction is activated under the severe reaction condition, which results in the conversion of olefins into paraffins and aromatics by the following reaction.
As shown in
Figure 3, the addition of the *BEA or MFI zeolites to the RFCC catalyst increased the olefin yields while it decreased the paraffin yields. Among them, the increase in the olefin yields by the addition of *BEA386 was remarkable. These results can be ascribed to the lower activity of the *BEA and MFI zeolites for the hydrogen transfer reaction than those of the RFCC catalyst. Especially, the *BEA386 contains very few acid sites on the outer shell of the particle because it was synthesized by a two-step crystallization method in which the aluminum source was not added at the second crystallization step as shown in the
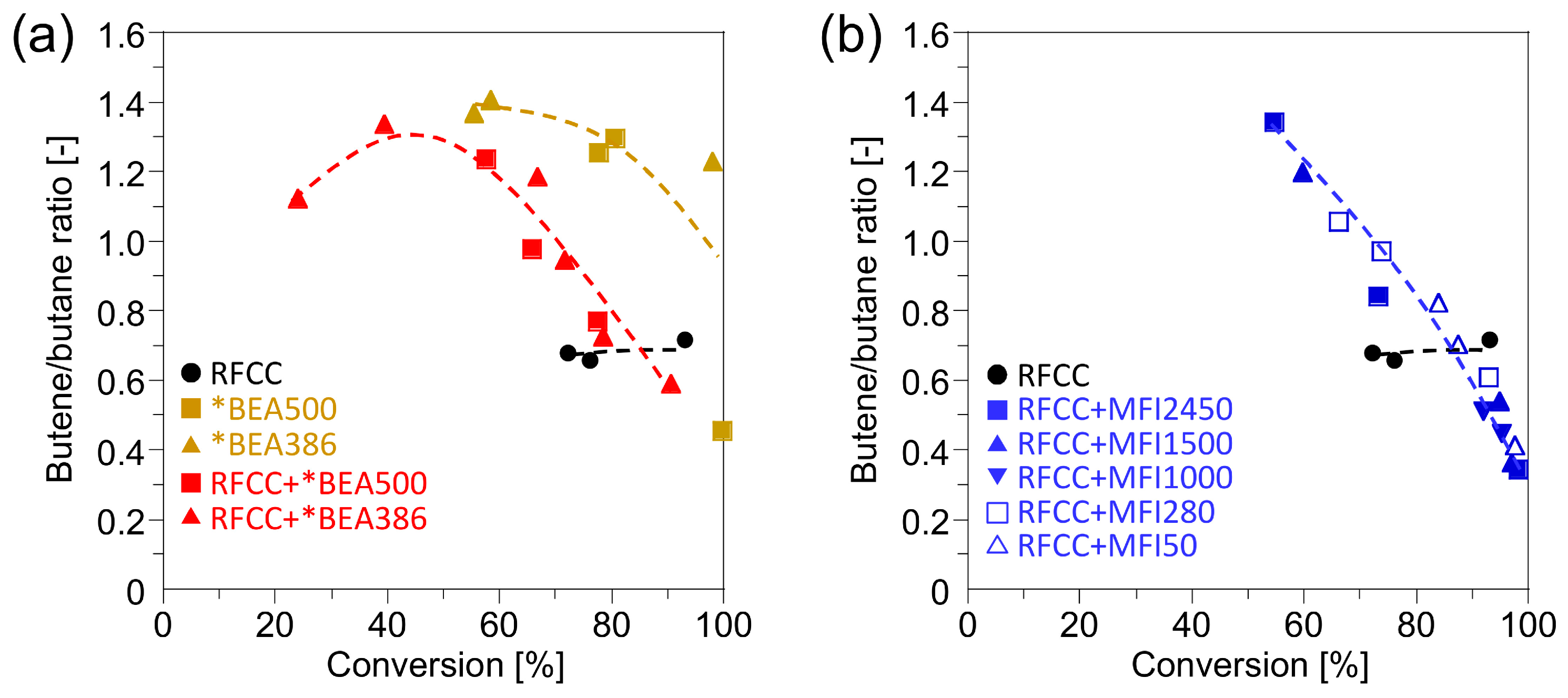
experimental section. Therefore, it can be assumed that the *BEA386 zeolite produces a low level of activity in the hydrogen transfer reaction which results in the high olefin yield. To confirm this assumption, the butene/butane ratio obtained from the
n-hexadecane cracking, which is an index representing the hydrogen transfer activity [
20], were compared in
Figure 4. The butene/butane ratios on the composite catalysts were higher than those on the RFCC catalyst, especially at a lower conversion, which indicates the lower hydrogen transfer reaction activity.
Figure 2.
Reaction product yields of n-hexadecane cracking on the RFCC and the composite catalysts. (a)(b) Gas yield. (c)(d) Gasoline yield. (e)(f) Coke yield. (a)(c)(e) Reaction product on the *BEA catalyst and the composite catalyst with the *BEA zeolite. (b)(d)(f) Reaction product on the composite catalyst with the MFI zeolite.
Figure 2.
Reaction product yields of n-hexadecane cracking on the RFCC and the composite catalysts. (a)(b) Gas yield. (c)(d) Gasoline yield. (e)(f) Coke yield. (a)(c)(e) Reaction product on the *BEA catalyst and the composite catalyst with the *BEA zeolite. (b)(d)(f) Reaction product on the composite catalyst with the MFI zeolite.
Figure 3.
Yields of the products in the gasoline fractions. (a)(b) Paraffin yield. (c)(d) Olefins yield. (e)(f)Monoaromatics yield. (a)(c)(e) Reaction product on the *BEA catalyst and the composite catalyst with the *BEA zeolite. (b)(d)(f) Reaction product on the composite catalyst with the MFI zeolite.
Figure 3.
Yields of the products in the gasoline fractions. (a)(b) Paraffin yield. (c)(d) Olefins yield. (e)(f)Monoaromatics yield. (a)(c)(e) Reaction product on the *BEA catalyst and the composite catalyst with the *BEA zeolite. (b)(d)(f) Reaction product on the composite catalyst with the MFI zeolite.
Figure 4.
Butene/butane ratio of the reaction product on the composite catalysts. (a) Reaction product on the *BEA catalyst and the composite catalyst with the *BEA zeolite. (b) Reaction product on the composite catalyst with the MFI zeolite.
Figure 4.
Butene/butane ratio of the reaction product on the composite catalysts. (a) Reaction product on the *BEA catalyst and the composite catalyst with the *BEA zeolite. (b) Reaction product on the composite catalyst with the MFI zeolite.
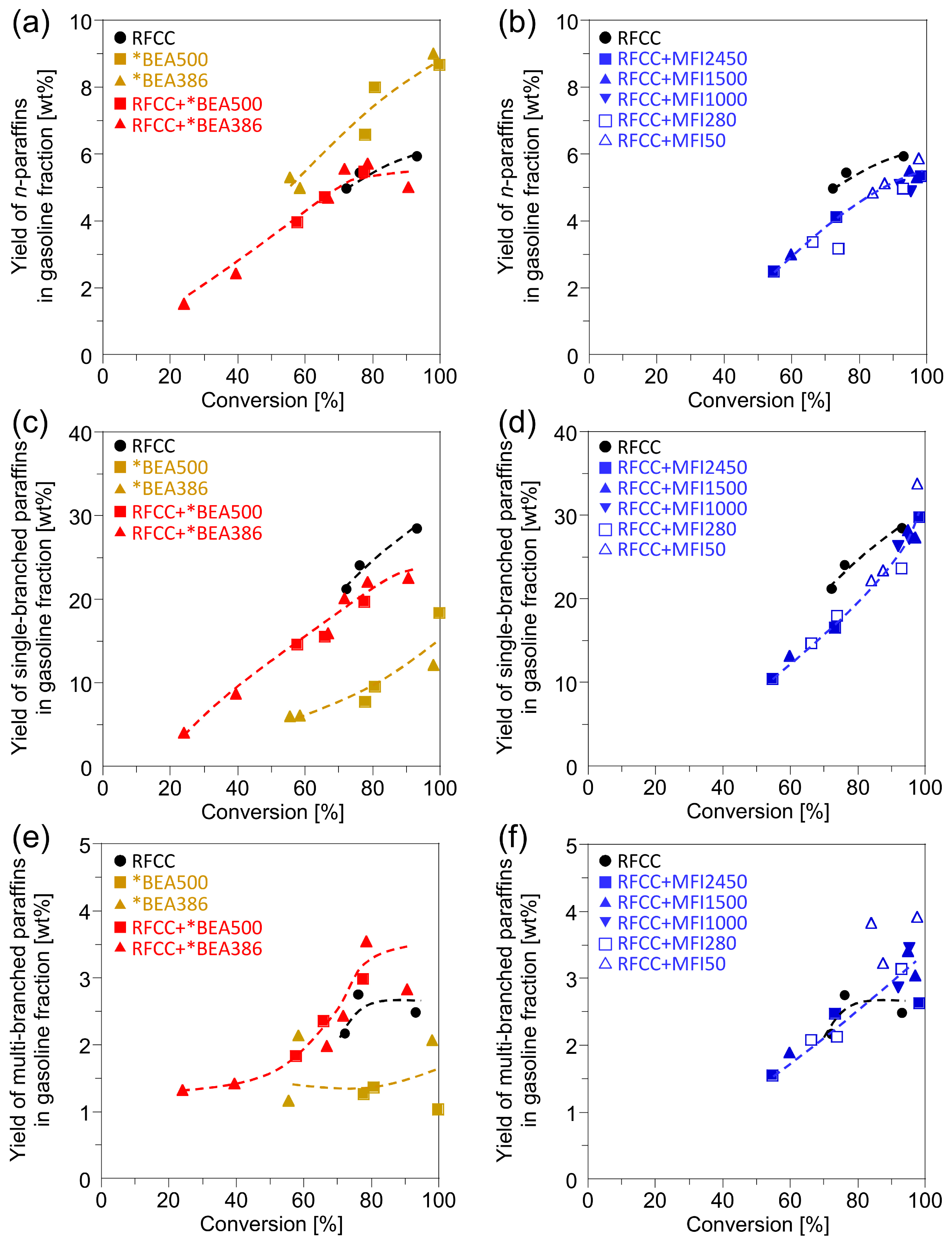
Figure 5 shows the branching degree of the paraffins in the gasoline fractions. Compared with the RFCC catalyst, the *BEA catalysts were less active for isomerization of paraffins in gasoline fractions. However, the change of product distribution by the addition of the *BEA zeolites to the RFCC catalyst was different form the product distribution predicted by weighted average of the reaction products on the RFCC catalyst and the *BEA catalysts. Addition of the *BEA zeolite to the RFCC catalysts decreased the single-branched paraffins slightly and increased the multi-branched paraffins, while the
n-paraffin yield was not affected. This result suggests that the *BEA zeolites catalyze the isomerization of single-branched paraffins produced on the RFCC catalyst into multi-branched paraffins. The addition of *BEA386, which contains less acid site on the outer shell of the particle, was especially effective for increasing the yield of multi-branched paraffins. On the other hand, the addition of the MFI zeolites to the RFCC catalyst led to different changes of product distribution. The yield of
n-paraffins and single-branched paraffins decreased with the MFI zeolite addition, while that of multi-branched paraffins was not affected. This may be because
n-olefins and single-branched olefins produced on the FAU zeolites were transferred to the MFI zeolites and overcracked into gas fraction, while multi-branched olefins were not able to diffuse into the MFI zeolite pores because of steric hindrance. Therefore, few of the
n-olefins and single-branched olefins were available for conversion to
n-paraffins and single-branched paraffins by hydrogen transfer reaction on the FAU zeolites. From the results of
Figure 2,
Figure 3, and
Figure 5, it is concluded that the *BEA zeolites are active for the isomerization reaction while the MFI zeolites are active for the cracking reaction. Both zeolites are less active for the hydrogen transfer reaction than the FAU zeolite.
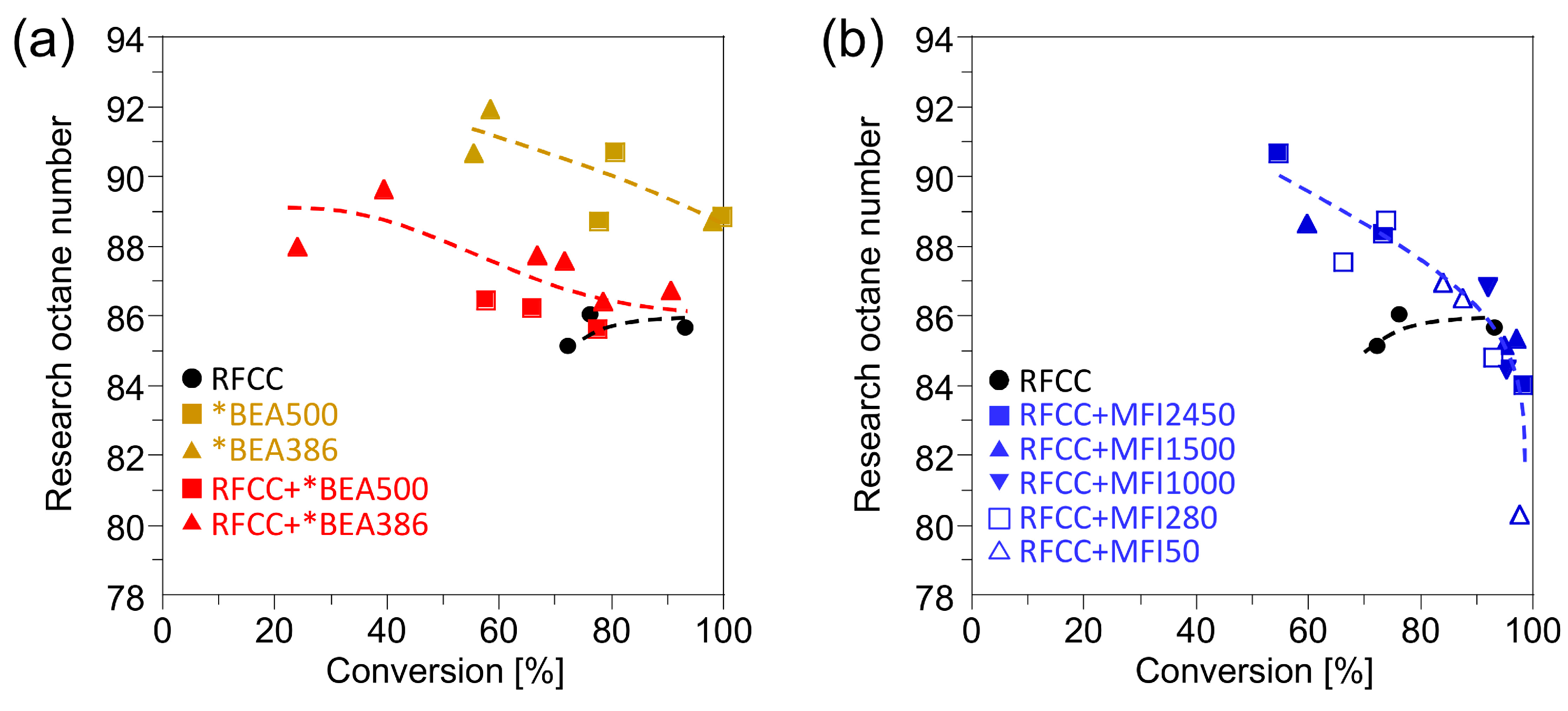
Figure 6 shows the research octane number (RON) of the gasoline fraction obtained with the RFCC and the composite catalysts. The composite catalysts showed a higher RON value than the RFCC catalysts. However, factors that contribute to the octane boosting are different between the additives. Enhancement of RON value by the addition of *BEA zeolite is attributed to preventing the loss of olefins by a strong hydrogen transfer reaction on FAU zeolites and accelerating the isomerization of single-branched molecules into multi-branched molecules. This result suggests the possibility for increasing the octane value of the gasoline produced from the catalytic cracking of heavy oils without sacrificing the gasoline fraction yields, even under the reaction conditions with high hydrogen transfer activity. In contrast, RON increase by the addition of MFI zeolites can be explained by a decrease of
n-paraffins and single-branched paraffins which have low octane value as shown in
Figure 5. In this mechanism of octane boosting, the simultaneous loss of gasoline yield is unavoidable.
Figure 5.
Yields of the paraffin products in the gasoline fraction. (a)(b) Linear paraffin yield. (c)(d) Single-branched paraffin yield. (e)(f)Multi-branched paraffin yield. (a)(c)(e) Reaction product on the *BEA catalyst and the composite catalyst with the *BEA zeolite. (b)(d)(f) Reaction product on the composite catalyst with the MFI zeolite.
Figure 5.
Yields of the paraffin products in the gasoline fraction. (a)(b) Linear paraffin yield. (c)(d) Single-branched paraffin yield. (e)(f)Multi-branched paraffin yield. (a)(c)(e) Reaction product on the *BEA catalyst and the composite catalyst with the *BEA zeolite. (b)(d)(f) Reaction product on the composite catalyst with the MFI zeolite.
Figure 6.
Research octane number of the gasoline fraction. (a) Reaction product on the *BEA catalyst and the composite catalyst with the *BEA zeolite. (b) Reaction product on the composite catalyst with the MFI zeolite.
Figure 6.
Research octane number of the gasoline fraction. (a) Reaction product on the *BEA catalyst and the composite catalyst with the *BEA zeolite. (b) Reaction product on the composite catalyst with the MFI zeolite.
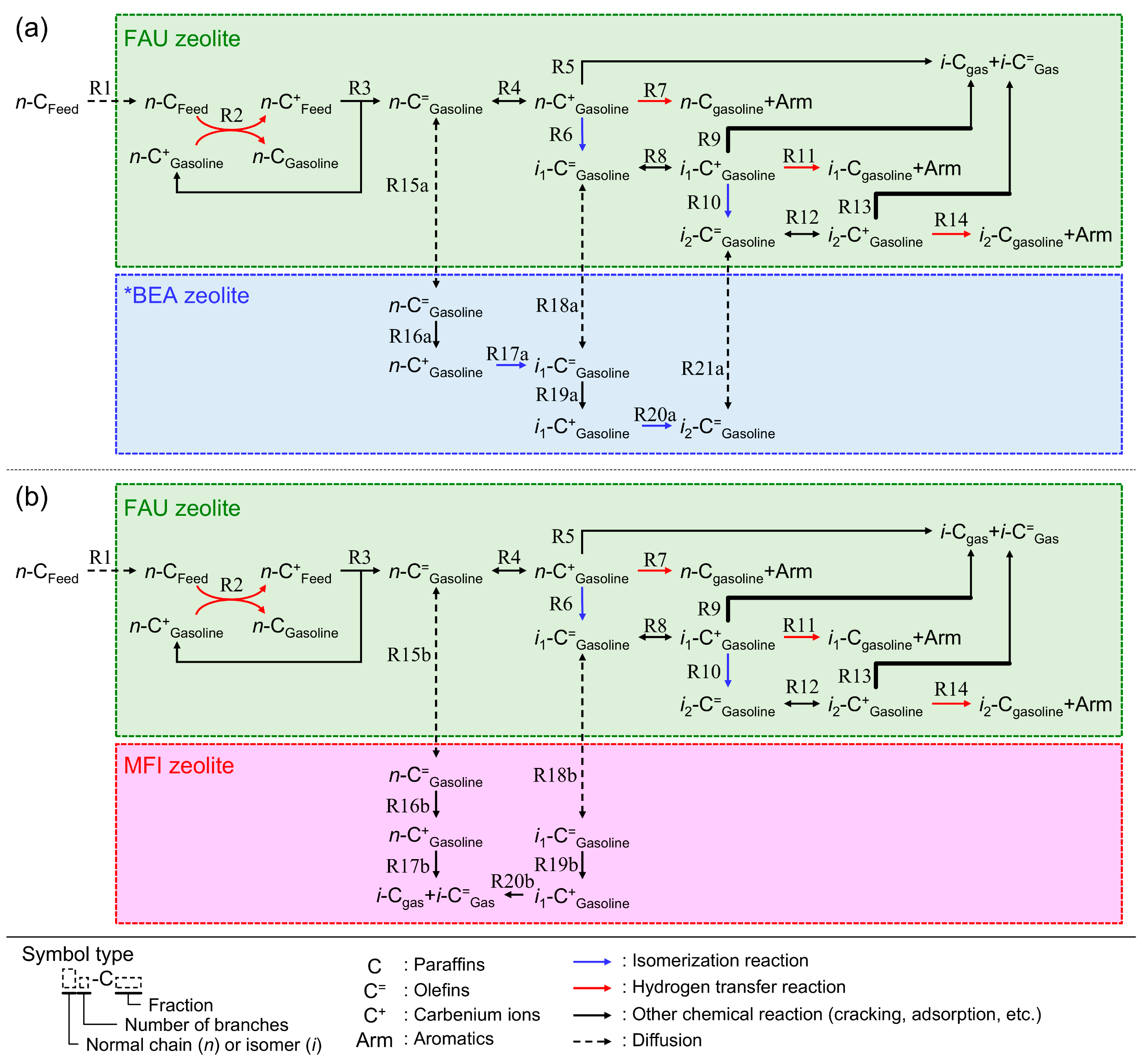
2.3. Reaction Mechanism on the Composite Catalyst
In this section, we discuss the reaction scheme for the catalytic cracking of
n-hexadecane on the RFCC and the composite catalysts. As shown above, the addition of the *BEA or MFI zeolites to the RFCC catalyst caused the change of the product distribution of the
n-hexadecane cracking such as (i) an increase in the yield of olefins in the gasoline fraction on both additives, and (ii) an acceleration of isomerization which resulted in the increase in the yield of multi-branched paraffins on the *BEA zeolites, while the cracking of linear and single-branched olefins were accelerated on the MFI zeolites. Considering these results, a schematic reaction mechanism on the composite catalysts can be proposed as shown in
Figure 7.
According to the carbenium ion mechanism, which is a generally accepted theory of the acid-catalyzed cracking [
21], the reaction route of the
n-hexadecane cracking on the FAU zeolites is as follows. The feedstock paraffin molecules diffuse into the zeolite pores (R1) and react with the carbenium ions adsorbed on the acid site (R2). Hydride ions are transferred from the paraffins to the carbenium ions and produce small paraffins and large, new carbenium ions. Then, the β-scission of the large carbenium ions proceeds and form olefins and small carbenium ions (R3). These small carbenium ions are consumed in the hydride-transfer reactions of the next feedstock molecules (R2), and therefore, the cracking of the feedstock continues. The produced olefins adsorb again on the acid sites and forms carbenium ions (R4), which are further cracked into gas fractions (R5) or isomerized into branched olefins (R6, R10). In addition, if the two carbenium ions adsorb on the adjacent acid site, a hydrogen transfer reaction proceeds and the active olefins are converted into the stable paraffins and aromatics, which prohibits the overcracking of the gasoline fractions (R7, R11, R14). Because the FAU zeolites have high hydrogen transfer activity, the gasoline fraction on the RFCC catalyst includes a large amount of paraffins as shown in
Figure 3.
Figure 7.
Reaction scheme of the n-hexadecane cracking on the composite catalyst. (a) Composite with *BEA zeolite. (b) Composite with MFI zeolite.
Figure 7.
Reaction scheme of the n-hexadecane cracking on the composite catalyst. (a) Composite with *BEA zeolite. (b) Composite with MFI zeolite.
In the case of the composite catalysts, however, the cracking of the feedstock molecule proceeds on the two parts of the catalyst that have different catalytic activities. As mentioned above, the *BEA zeolites have lower hydrogen transfer activity than the FAU catalysts in the RFCC catalyst. Therefore, it is more difficult to initiate the cracking of feedstock paraffins on *BEA zeolites than on FAU zeolites, even if
n-hexadecane can diffuse into the pores, because the bimolecular cracking hardly proceeds on the *BEA zeolites. The reason for the higher yield of olefins on the composite catalysts shown in
Figure 3 is assumed to be that the gasoline fraction olefins produced from the β-scission on the acid site of the FAU zeolites diffuse into the *BEA zeolites and become stable by being isolated from the strong catalytic activity for cracking and the hydrogen transfer reaction on the FAU zeolites (R15a, R18a, R21a). This is confirmed by the remarkable increase in the yield of olefins by the addition of *BEA386 zeolite, even though it contains very few acid sites on the outer shells of the particle. In addition, the isomerization of the olefins transferred from the FAU zeolites to the *BEA zeolites proceeds on the *BEA zeolites with high Si/Al ratios and the multi-branched olefins are produced (R17a, R20a) [
14]. These multi-branched olefins are transferred into the FAU zeolites again (R21a) and converted to the multi-branched paraffins via hydrogen transfer reaction (R14). This scheme can explain the increase in the yield of the multi-branched paraffins by the addition of the *BEA zeolites to the RFCC catalyst. The effect could be especially enhanced for the *BEA386. Here, the ramification of carbenium ions is thermodynamically favorable, while the cracking of ramified carbenium ions also proceeds more rapidly than that of linear ions [
22]. Therefore, increasing the yield of multi-branched molecule requires reaction control, which only enhances isomerization reactions and suppress cracking. From the results shown in
Figure 5, the *BEA386 with less acidity is suitable for the reaction control because the isomerization requires far less catalyst acidity than cracking.
On the other hand, the reaction on the additives is different in the case of the addition of MFI zeolites. Firstly, the MFI zeolites are not active for hydrogen transfer reaction, which is in common with the *BEA zeolites. Therefore, olefins become stable by the addition of the MFI zeolites to the RFCC catalyst as shown in
Figure 3. The difference between *BEA and MFI zeolites is their pore size and selectivity of the reactions. Because of the small pore size of MFI zeolites, only linear and single-branched molecules can diffuse into the active site of the additives (R15b, R18b) [
4]. In the pore of MFI zeolites, cracking of the olefins into gas fractions is more active than isomerization because the formation of multi-branched molecules is prohibited by steric hindrance. Therefore, the
n-paraffins and single-branched paraffins in gasoline fractions are decreased by the MFI zeolite additions as shown in
Figure 5. Consequently, as the additive to the RFCC catalyst, *BEA zeolites are active in the isomerization of the gasoline range molecules, while the MFI zeolites are active in the cracking of linear and single-branched molecules in gasoline fractions.