Abstract
Glycerol hydrodeoxygenation to 1,2-propanediol (1,2-PDO) is a reaction of high interest. However, the need for hydrogen supply is a main drawback of the process. According to the concept investigated here, 1,2-propanediol is efficiently formed using bio-glycerol feedstock with H2 formed in situ via ethanol aqueous phase reforming. Ethanol is thought to be a promising H2 source, as it is alcohol that can be used instead of methanol for transesterification of oils and fats. The H2 generated is consumed in the tandem reaction of glycerol hydrodeoxygenation. The reaction cycle proceeds in liquid phase at 220–250 °C and 1.5–3.5 MPa initial N2 pressure for a 2 and 4-h reaction time. Pt-, Ni- and Cu-based catalysts have been synthesized, characterized and evaluated in the reaction. Among the materials tested, Pt/Fe2O3-Al2O3 exhibited the most promising performance in terms of 1,2-propanediol productivity, while reusability tests showed a stable behavior. Structural integrity and no formation of carbonaceous deposits were verified via Temperature Programmed Desorption of hydrogen (TPD-H2) and thermogravimetric analysis of the fresh and used Pt/FeAl catalyst. A study on the effect of various operating conditions (reaction time, temperature and pressure) indicated that in order to maximize 1,2-propanediol productivity and yield, milder reaction conditions should be applied. The highest 1,2-propanediol yield, 53% (1.1 g1,2-PDO gcat−1·h−1), was achieved at a lower reaction temperature of 220 °C.
1. Introduction
The catalytic conversion of renewable sources to fuels and chemical products has become particularly important, because of the global energy requirements and environmental problems caused by petroleum and its derivatives []. It has been reported that as biomass is the only renewable source of carbon, its upgrading to a value-added chemical product instead of energy, heat and fuels will offer the greatest advantages [].
Among the renewable oxygenates formed from biomass processing, glycerol is identified as a building block intermediate that can be converted to target products. Glycerol is produced as a by-product of various industrial processes, such as transesterification of oils and fats, soap manufacture, fatty acid production, etc. []. Glycerol is now largely available in the market mainly due to the rapid increase in bio-diesel production. For this reason, a great effort has been directed towards the conversion of glycerol to valuable products, such as 1,2- and 1,3-propanediols [,,,,,,], acrolein [,], hydrogen [] and fuel additives [].
Among the various processes studied, the production of 1,2-propanediol (propylene glycol) is one of the most attractive. 1,2-Propanediol, conventionally produced via propylene oxide hydration, is an important chemical that finds applications in antifreeze fluids and in the polymer industry. This process has been already industrialized by companies, such as Archer Daniels Midland (ADM), which has already started the production of 1,2-propanediol from glycerol and announced a 61% reduction in greenhouse gas emissions compared to the conventional method [].
The catalytic glycerol hydrodeoxygenation (HDO, also called hydrogenolysis) to 1,2-propanediol is feasible over metal catalysts and hydrogen pressures up to 8.0 MPa []. The majority of research studies and patents available so far have mainly focused on the development of suitable catalytic systems and on engineering issues [,]. Although this process has been effectively developed, there are still drawbacks that need to be solved. The requirement for an external H2 supply is the main disadvantage of the method, as the cost of hydrogen production, distribution and storage is high and negatively impacts the process economics. In addition, hydrogen is industrially produced using fossil feedstocks, thus rendering the overall reaction petroleum dependent.
Recently, the idea of in situ hydrogen production and consecutive consumption for hydrodeoxygenation has been explored, aiming at overcoming the above-mentioned problems []. Within this concept, two different approaches have been investigated: the first one involves the use of aqueous solutions of glycerol, where hydrogen is formed via aqueous phase reforming (APR) of a part of glycerol [,,,]; and the second, the addition of a hydrogen donor molecule (alcohols, formic acid), through hydrogen transfer reaction. The idea of catalytic transfer hydrogenation (CTH) was firstly realized by Musolino et al. [] for selective transfer hydrogenolysis of glycerol in the presence of palladium catalysts (Pd/Fe2O3) using ethanol and 2-propanol as H2 donor molecules. The pathways proposed include alcohol dehydrogenation, glycerol dehydration to hydroxyacetone and subsequent hydrogenation of the latter, consuming the H2 generated from the alcohols. The reaction sequence was performed in ethanol and 2-propanol under 0.5 MPa inert atmosphere at 150–200 °C. There was no remarkable difference between the different donor alcohols, and the best performance was observed at 180 °C after 24 h, where glycerol was fully converted to a mixture of propylene glycol and ethylene glycol (94 and 6% selectivity, respectively). In a more recent study by Xia et al. [], ethanol was recognized as the most efficient H2 source among various alcohols (methanol, ethanol, 1-propanol, butanol and iso-propanol) tested for their hydrogen donating ability over Cu:Mg:Al catalysts. The latter was attributed to the high dehydrogenation activity shown by copper in ethanol reactions. At 210 °C, 3.0 MPa N2 and 10 h, glycerol was converted by 95% with 92% selectivity to 1,2-propanediol. Gandarias et al. [,,] have significantly contributed to the field of CTH hydrogenation reactions, focusing mainly on 2-propanol and formic acid as hydrogen donor molecules. Formic acid was proven to be an effective donor over Ni-Cu/Al2O3 catalyst. Importantly, the authors proposed a direct mechanism for glycerol conversion to 1,2-propanediol. This mechanism was suggested to occur when hydrogen is generated in the proximity of the active sites and includes an intermediate alkoxide formation.
In our previous studies [,], a different approach of in situ H2 formation has been explored. Specifically, the required hydrogen is formed in situ by the reaction of methanol and water (aqueous phase reforming-APR), which are already components of the crude glycerol stream after transesterification and consumed from glycerol to form 1,2-propanediol (glycerol hydrodeoxygenation). The tandem reaction cycle presents advantages, such as: it is performed in the liquid phase, in the presence of the same catalytic material and reactor set-up, thus allowing the production of 1,2-propanediol under inert atmosphere through a one-step process. A Cu:Zn:Al catalyst prepared by the oxalate gel technique was found to exhibit the best performance with a 45% yield to the target product at 220 °C 3.5 MPa N2 and 4 h. These results correspond to productivity values (g1,2-PDO.gcat−1·h−1) up to five-times higher compared with previous reports [,]. Experiments with labeled 13CH3OH over this catalyst allowed us to quantify the H2 formation origin (methanol and/or glycerol APR) and showed that ~70% of the total H2 is indeed produced from the reformation of methanol.
The objective of the present study is to further develop the innovative process described above using alternative H2 sources, such as bio-ethanol. The idea is based on the fact that bio-ethanol can be used instead of methanol for transesterification during biodiesel production. In addition, in contrast to methanol, bio-ethanol can be derived from renewable resources, such as starch crops and lignocellulosic biomass (see Scheme 1). Within this context, the synthesis, characterization and evaluation of Pt, Ni and Cu catalysts in the ethanol APR-glycerol hydrodeoxygenation reaction cycle under inert atmosphere are investigated. The catalyst formulations evaluated in this study were selected based on their performance in ethanol reforming [] and glycerol hydrodeoxygenation reactions []. Furthermore, the stability of the best performing catalyst was examined for two consecutive reaction tests. In addition, in the presence of the most effective and stable catalyst, the impact of reaction time, reaction temperature and nitrogen initial pressure was examined.
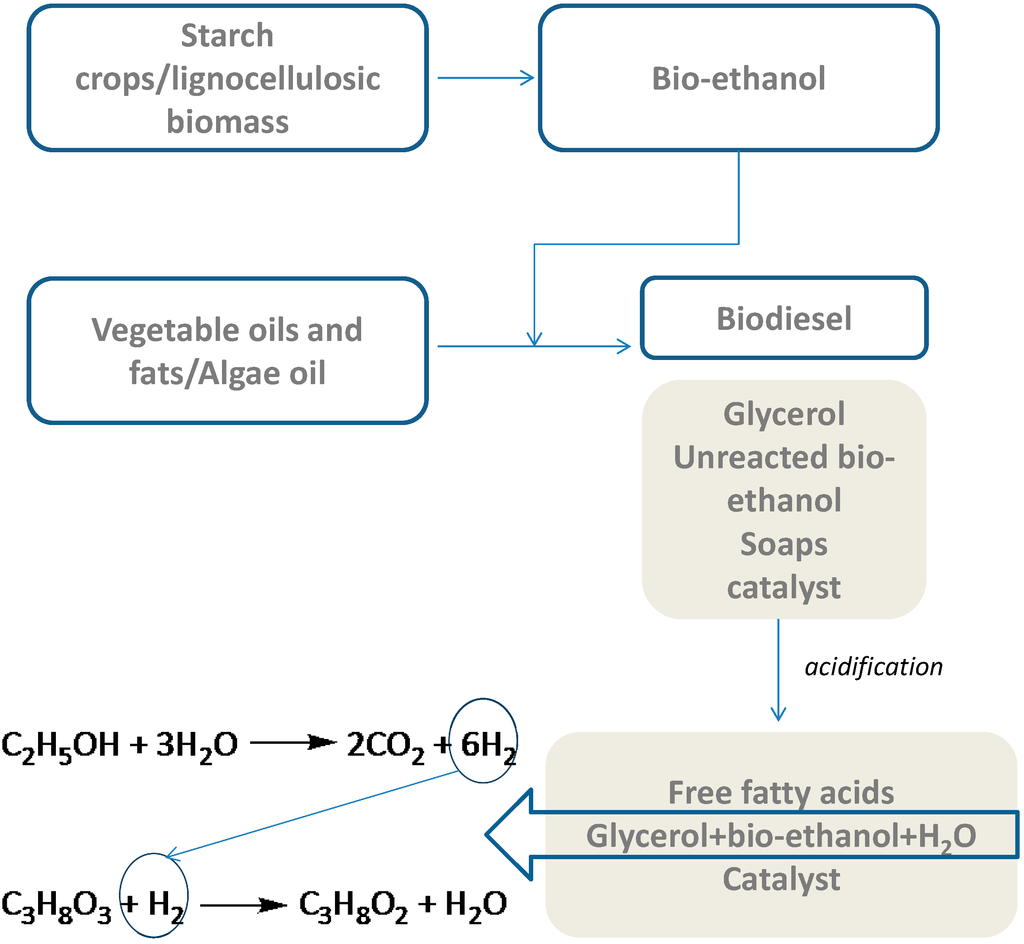
Scheme 1.
Integrated process scheme for 1,2-propanediol production under inert conditions using bio-ethanol as the H2 source.
2. Results and Discussion
2.1. Catalysts Characterization
The composition and the physicochemical properties of the supports and catalysts are presented in Table 1. Pt results in no significant changes when impregnated on Fe2O3-Al2O3 support. The impregnation of Ni on CeZrLa leads to a moderate decrease in the BET surface area. For the bulk Cu:Zn:Al catalyst, a comparison with literature data [,] for the corresponding materials prepared by the conventional carbonate co-precipitation method reveals the structural superiority of the oxalate gel prepared sample possessing a high BET surface area.

Table 1.
Catalyst composition and porous characteristics.
| Support | Composition (wt%) | BET surface area (m2·g−1) |
|---|---|---|
| FeAl | 61 (Fe2O3), 39 (Al2O3) | 80.0 |
| CeZrLa | 78 (ZrO2), 17 (CeO2), 5 (La2O3) | 54.9 |
| Catalyst | ||
| Pt/FeAl | 5.0 (Pt) | 73.6 |
| Ni/CeZrLa | 10.0 (Ni) | 37.2 |
| Cu:Zn:Al | 49.0 (Cu), 26 (Zn), 3.5 (Al) | 71.5 |
The crystalline phases identified over all of the synthesized catalysts were investigated by X-ray diffraction and are presented in Figure 1. The XRD pattern of the Pt/FeAl catalyst is shown in Figure 1a. The dominant crystalline phase present is Fe2O3. The Al2O3 oxide was not detected, probably because of its amorphous nature. Platinum is highly dispersed on the surface, as no characteristic peaks were identified. The diffractogram of the Ni catalyst supported on CeZrLa is illustrated in Figure 1b. The characteristic peaks of the Zr0.84Ce0.16O2 phase were identified, while no peaks corresponding to La2O3 were observed, most probably due to fine dispersion. The NiO phase was also present on the Ni/CeZrLa catalyst. Finally, Figure 1c shows the X-ray diffractogram of the Cu:Zn:Al catalyst. The catalyst exhibits broad CuO and ZnO peaks, which shows highly dispersed Cu and Zn phases, respectively. As previously for the Pt sample, Al2O3 was absent from the diffraction pattern.
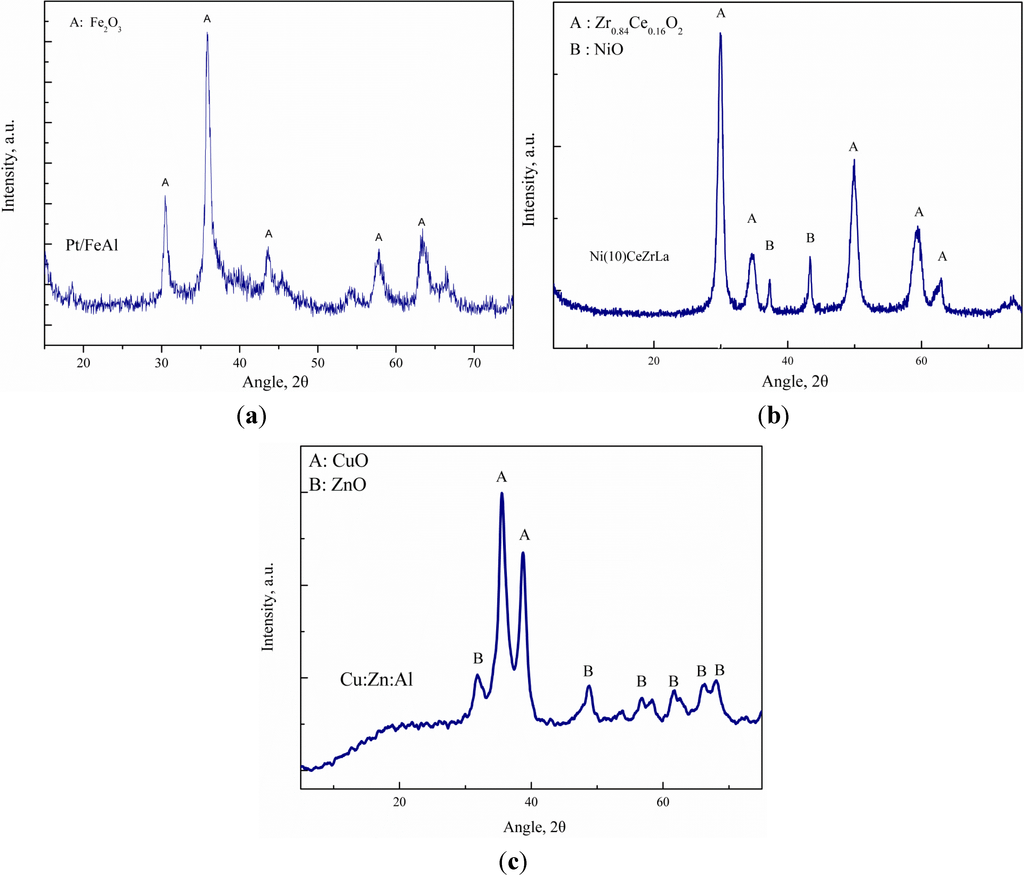
Figure 1.
XRD patterns of (a) Pt/FeAl, (b) Ni/CeZrLa and (c) Cu:Zn:Al catalysts.
The reduction characteristics of the catalysts were studied by temperature-programmed reduction (TPR). The TPR profiles of all catalysts are illustrated in Figure 2. The TPR profiles of the Pt catalyst and the support are depicted in Figure 2a. The Fe2O3-Al2O3 support shows a H2 consumption peak at 500 °C. This peak corresponds to Fe2O3 reduction to Fe3O4. The low temperature peak (T < 100 °C) that appears in the TPR profile of Pt/FeAl is attributed to Pt oxide reduction to metallic Pt°. The impregnation of platinum leads to a shift at lower temperature (380 °C) of the peak observed for Fe2O3-Al2O3, as Pt facilitates the diffusion of hydrogen []. As seen from the H2 consumption profile of the La2O3-doped CeO2-ZrO2 support (Figure 2b), a peak attributed to the partial reduction of CeO2 appears in the temperature range between 300–400 °C []. In the TPR profile of the corresponding catalyst (Figure 2b), a main reduction peak is observed with maximum H2 consumption at 445 °C, which corresponds to the reduction of NiO to metallic Ni0. Moreover, a shoulder is also obvious in the temperature range of 300–400 °C, probably due to the reduction of the support. Figure 2c shows the reduction curves of unsupported CuO and the Cu:Zn:Al catalyst. The TPR profile of the catalyst exhibits a broad reduction peak at a temperature range of 200–300 °C, which suggests that the reduction of CuO to metallic Cu0 proceeds through consecutive steps []. Compared with the unsupported CuO, it seems that the Cu:Zn:Al catalyst is more easily reduced.
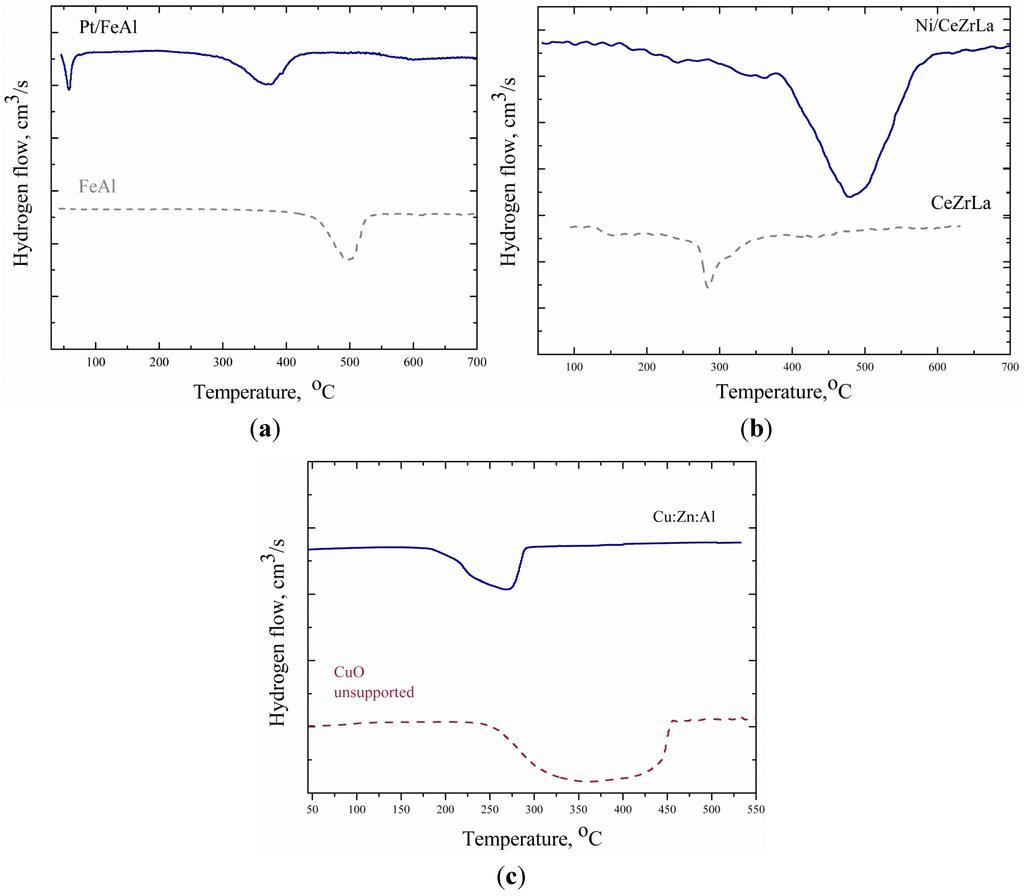
Figure 2.
Reduction profiles of: (a) FeAl support, Pt/FeAl; (b) CeZrLa support, Ni/CeZrLa; and (c) CuO unsupported and Cu:Zn:Al.
2.2. Catalytic Performance in the Ethanol APR-Glycerol Hydrodeoxygenation Reaction Cycle
In order for the catalytic results to be clearly discussed, the possible reactions for individual compounds (glycerol and ethanol) are given below. The overall stoichiometric reaction leading to hydrogen formation via ethanol reforming is as follows:
Apart from the reforming route, which requires a sufficient H2O to ethanol ratio, ethanol dehydrogenation to acetaldehyde, dehydration to ethylene (the proposed precursor of coke formation), decomposition and subsequent reactions of decomposition products, as well as the water gas shift reaction may also take place []. Glycerol can undergo hydrodeoxygenation (Reaction 2), leading to 1,2-propanediol formation, while glycerol reforming (Reaction 3) is also an option under the present conditions.
The three series of catalytic materials synthesized were tested under the standard reaction conditions. The catalytic results obtained over the different catalysts tested are summarized in Table 2. The experimental data show that 1,2-propanediol formation is possible under inert conditions with H2 formed in situ from ethanol APR. Among the catalysts tested, the Pt-based sample showed the most promising performance in the tandem reaction cycle of ethanol APR and glycerol hydrodeoxygenation. Glycerol was almost fully converted in the presence of this catalyst, while for Ni and Cu, the conversion was somewhat lower. Of interest is the superior mass-specific rate exhibited by the Pt catalyst, which is over four-times higher compared with the Ni and Cu samples. Moreover, hydrogen conversion (defined as H2 consumed for glycerol conversion to products that consume hydrogen/total H2 formed) ranges between 75% and 85%, proving the potentiality of this cascade system. A comparison with our previous study [], where H2 is generated via methanol APR in the presence of the same Cu:Zn:Al catalyst, shows that methanol is a more efficient H2 donor (see Table 2). The same performance was observed for the Pt/FeAl (Table 2), suggesting that methanol is much more reactive than ethanol under these conditions. It should be highlighted here that in this study, the ethanol/glycerol molar ratio corresponds to values lower than that of transesterification conditions (1.25 and 9.0, respectively []), which means that a part of the unreacted ethanol can be still recycled to the biodiesel reactor. This is very important, as in previous studies, ethanol/glycerol molar ratios up to 46.0 have been used [].

Table 2.
Catalyst screening results under inert atmosphere, standard conditions: 3.5 MPa·N2 initial pressure, 4 h, 250 °C, 7.1 wt% EtOH, 11.3 wt% glycerol (ethanol/glycerol molar ratio = 1.25) and water, catalyst/glycerol + ethanol weight ratio = 0.06 (for Pt) and 0.25 (for non-noble metal catalysts, i.e., Ni and Cu).
| Catalyst | Conversion (%) | Mass-specific rate (Integral) (MSR) (mmoles gcat−1·h−1) | Selectivity (%) | 1,2-PDO productivity (g1,2-PDO gcat−1·h−1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Glycerol | Ethanol/Methanol | Glycerol | Ethanol/Methanol | 1,2-PDO | EG | AC | 1-PrOH | ||
| Pt/FeAl | 97.4 | 9.5 | 26.4 | 3.6 | 32.2 | 2.1 | 4.5 | 7.3 | 0.65 |
| Pt/FeAl a | 96.4 | 14.4 | 26.2 | 12.2 | 41.7 | 2.6 | 5.3 | 5.4 | 0.83 |
| Ni/CeZrLa | 82.3 | 41.0 | 5.4 | 3.2 | 21.6 | 3.9 | 8.4 | 8.8 | 0.09 |
| Cu:Zn:Al | 87.7 | 3.6 | 5.8 | 0.3 | 32.9 | 1.9 | 7.5 | 5.6 | 0.15 |
| Cu:Zn:Al b | 88.8 | 14.1 | 6.7 | 1.9 | 39.2 | 1.9 | 5.4 | 6.1 | 0.20 |
a With methanol as the H2 source; b with methanol as the H2 source, from [].
Under these conditions, glycerol was also subjected to reforming to some extent, generating additional hydrogen. Nevertheless, glycerol APR was not significant, especially over the Pt and Cu catalysts, while in the presence of Ni, glycerol reforming became more pronounced. The latter was expected, as Ni catalysts are among the most efficient materials for glycerol APR, aiming at renewable hydrogen production [].
The target product, i.e., 1,2-propanediol, was the main product formed in all cases, though in relatively low overall selectivity values of 21%–33%. It should be underlined that selectivity was based on the total C moles of glycerol reacted (see Section 3.3). Pt and Cu catalysts proved to be equally selective, while Ni, known for its C–C scission ability, also resulted in higher amounts of ethylene glycol and methanol (methanol selectivity Pt, 3.2%; Ni, 6.3%; and Cu, 2.1%) in the liquid phase. The undesirable sequential hydrodeoxygenation of 1,2-propanediol to propanols seems not to be promoted over the catalysts evaluated, as the selectivity to these mono-alcohols ranges between 5.5% and 9.0%. It should be underlined that the intermediate glycerol dehydration product, hydroxyacetone (acetol), was always detected in the product mixture at selectivity values of 4.5%–8.5%, supporting the proposed two-step dehydration-hydrogenation mechanism for the glycerol hydrodeoxygenation reaction [,].
For the present tandem process, ethanol APR is used as the hydrogen donor reaction. The activity order with respect to ethanol conversion was as follows: Ni > Pt > Cu (see Table 2). However, in terms of the mass-specific rate, the Pt-based catalyst shows better performance. Moreover, although Cu was the less active catalyst for ethanol APR, its high hydrogenation activity towards glycerol hydrodeoxygenation to 1,2-propanediol resulted in 1,2-PDO productivity higher than the Ni catalyst. The gas phase analysis showed CO2 as the main product, while in the presence of Ni catalyst, CH4 was also produced in considerable amounts (~48% selectivity based on gas phase products). Ethane originating from ethylene (an ethanol dehydration product) hydrogenation was additionally detected, though at very low concentrations (0.6%–2.9% selectivity in the gas phase). Due to the low reaction temperature, which favors the water gas shift reaction, CO formation is limited to selectivity values up to 0.8%–1.4%.
Comparing the performance of the catalysts on the tandem cycle of ethanol APR-glycerol HDO in terms of integral mass-specific rates, interesting observations can be obtained. The ratio between the two rates can be used as an indication for assessing the cascade nature of the system. For the noble metal catalyst, Pt/FeAl, this ratio equals 7.3, which means that the rate of glycerol conversion exceeds that of ethanol APR; however, the values are of the same order of magnitude. In the case of Cu catalyst, the mass-specific rates (MSRs) of glycerol conversion prevail over the ethanol rates (glycerol to ethanol MSR ratio = 19.3), as copper shows superior performance for glycerol hydrodeoxygenation and not for ethanol APR. The performance of the Ni/CeZeLa is clearly different, as glycerol and ethanol conversion proceeded in parallel (glycerol to ethanol MSR ratio = 1.7). However, Ni favors undesirable pathways, which include C–C bond scission reactions, leading to degradation product formation, like ethylene glycol, methanol and methane. Based on the above, it can be deduced that Pt-based catalysts are potential candidates for the ethanol APR-glycerol hydrodeoxygenation cycle.
2.3. Catalyst Stability
As deactivation phenomena are common in liquid phase reactions, the best performing Pt/FeAl catalyst was subjected to four reaction cycles without any pre-treatment between the tests. After every reaction cycle, the catalyst was collected by filtration and dried overnight. The performance upon reuse is illustrated in Figure 3. Our stability tests showed that there was no deactivation of the catalyst, as glycerol conversion is practically the same over the four catalytic tests. Moreover, hydrogen conversion was also the same, i.e., 76%. Furthermore, 1,2-propanediol selectivity and yield are somewhat improved compared with the first reaction cycle and stabilized at the initial performance during the third and fourth reaction cycles.
Based on previous reported studies on Pt/FeAl catalysts, we propose that the presence of iron is crucial for the catalyst’s stable performance []. For this type of catalyst, Pt/FeAl, Pt sintering is the usual parameter causing deactivation. Iron addition improves the structural integrity, forming Pt-Fe alloy particles on Al2O3 under a reductive atmosphere. These particles have been found to segregate into Pt and Fe2O3 and form a Fe2O3 layer on the Pt particles, thus preventing them from sintering.
Measurement of Pt dispersion and particle size using the H2-TPD technique (Table 3) showed a moderate dispersion decrease and particle size increment from 1.4 to 1.6 nm. However, this result seems not to significantly affect the performance upon reuse, as Pt still remains highly dispersed on the surface. Moreover, thermogravimetric analysis (Figure 4) of the used catalyst (after the fourth time) presents weight losses at low temperatures <480 °C, attributed to the loss of water and strongly adsorbed reactant or products onto the catalytic surface. From the thermogravimetric profile at higher temperatures, >480 °C, it is obvious that no carbonaceous products were formed, as no weight loss was observed.
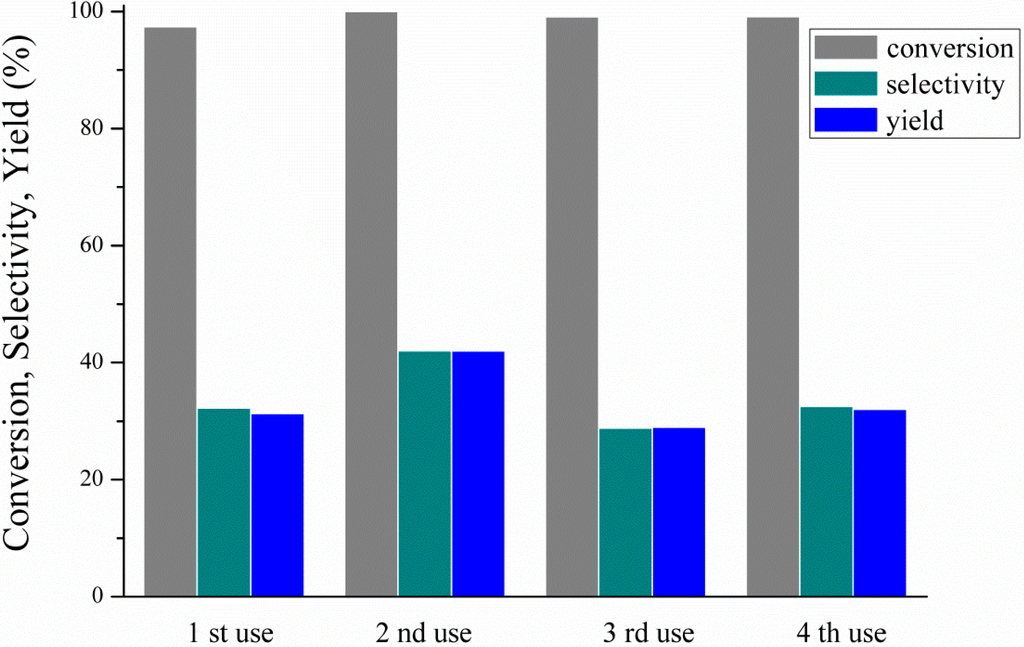
Figure 3.
Reusability tests of the Pt/Fe-Al catalysts: 250 °C, 4 h, 7.1 wt% EtOH, 11.3 wt% glycerol (ethanol/glycerol molar ratio = 1.25) and water, catalyst/glycerol + ethanol weight ratio = 0.06.

Table 3.
Dispersion and particle size of Pt/FeAl fresh and used samples.
| Catalysts | Dispersion, % | Pt particle size, nm |
|---|---|---|
| Pt/FeAl-fresh | 81 | 1.4 |
| Pt/FeAl-used after 4th cycle | 72 | 1.6 |
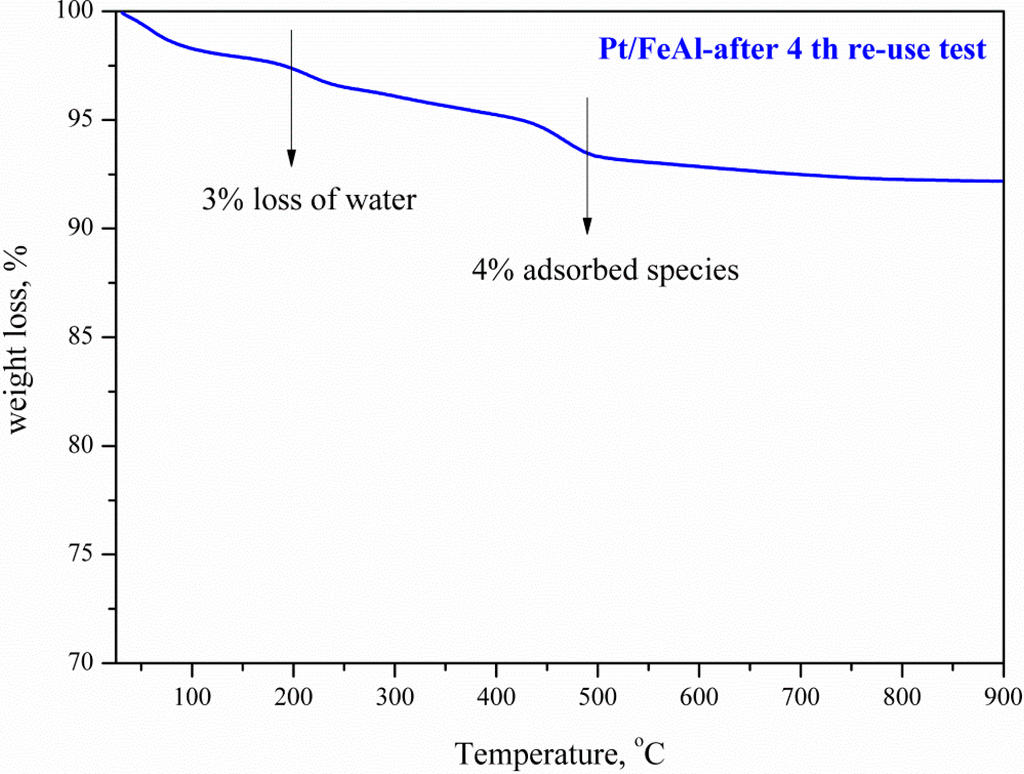
Figure 4.
Thermogravimetric analysis in oxidative atmosphere of the fourth use of Pt/FeAl catalyst.
2.4. Effect of Various Operating Conditions
For the best performing catalyst, Pt/FeAl, the effects of reaction time (2 and 4 h), reaction temperature (220 and 250 °C) and system pressure (1.5 and 3.5 MPa N2) were studied. Figure 5 illustrates the results obtained with reaction time variation. The increase of reaction time had no substantial effect on glycerol conversion, while the selectivity to 1,2-PDO was somewhat lower, due to sequential hydrodeoxygenation to 1-propanol. The above results demonstrate that the glycerol hydrodeoxygenation reaction is already completed at very short reaction times. This result enables efficient operation, as in such liquid phase reaction systems, prolonged reaction times are usually applied (>10 h) [].
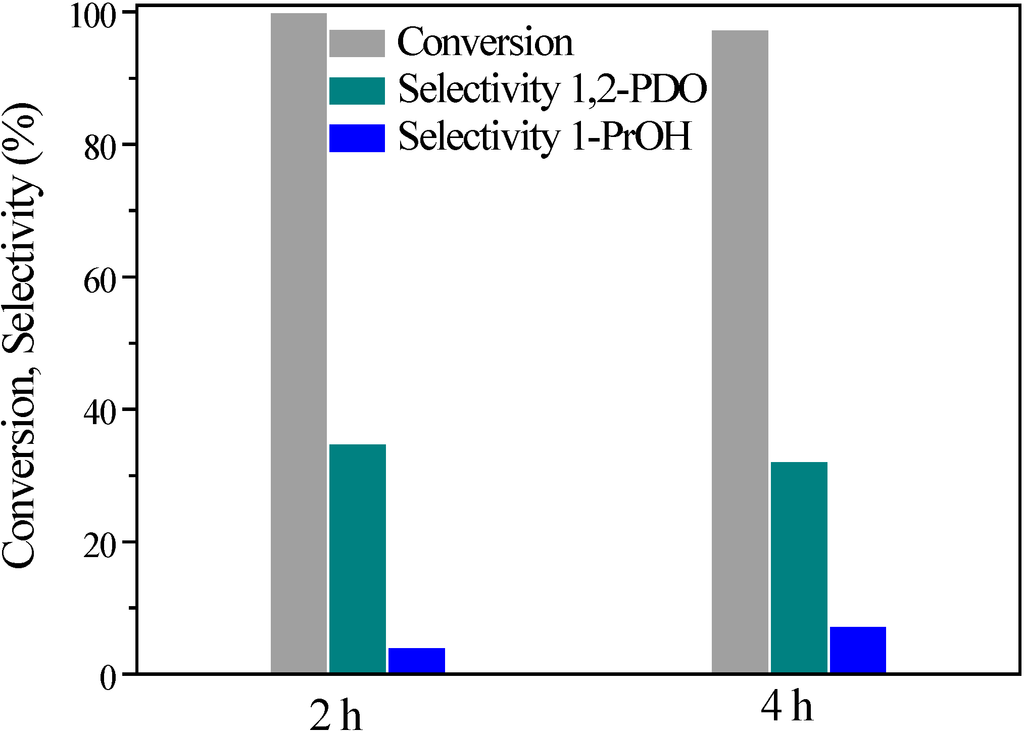
Figure 5.
Effect of reaction time on glycerol conversion, 1,2-PDO selectivity and yield: 3.5 MPa N2 initial pressure, 250 °C, 7.1 wt% EtOH, 11.3 wt% glycerol (ethanol/glycerol molar ratio = 1.25) and water, catalyst/glycerol + ethanol weight ratio = 0.06.
The influence of the reaction temperature on glycerol conversion, 1,2-PDO selectivity and yield is presented in Figure 6. The decrease of reaction temperature from 250 to 220 °C results in a decrease of glycerol conversion from 97.4% to 80.6%, as expected. The opposite trend is, however, observed for 1,2-PDO selectivity (from 32.2 at 250 °C to 66.0% at 220 °C), as it seems to be favored at the low temperature. The increased 1,2-PDO selectivity at 220 °C is mainly associated with the limitation of unidentified side product formation and secondarily with the decreased extent of its sequential hydrodeoxygenation to propanols (3% at 220 °C and 7.3% at 250 °C). The latter has been previously reported and is proposed to be favored under excess hydrogen and higher temperatures []. The operation at lower temperatures leads to a significant increase of the target product yield, from 31.3% at 250 °C to 53.3% at 220 °C, the highest yield obtained in this study. It is worth noticing here that at 220 °C, 1,2-PDO productivity equals 1.1 g1,2-PDO gcat−1·h−1, a value significantly higher compared with previous works using ethanol as the hydrogen donor (0.13 g1,2-PDO gcat−1·h−1 at 180 °C and 0.62 g1,2-PDO gcat−1·h−1 at 210 °C, both at almost complete glycerol conversion levels [,]).
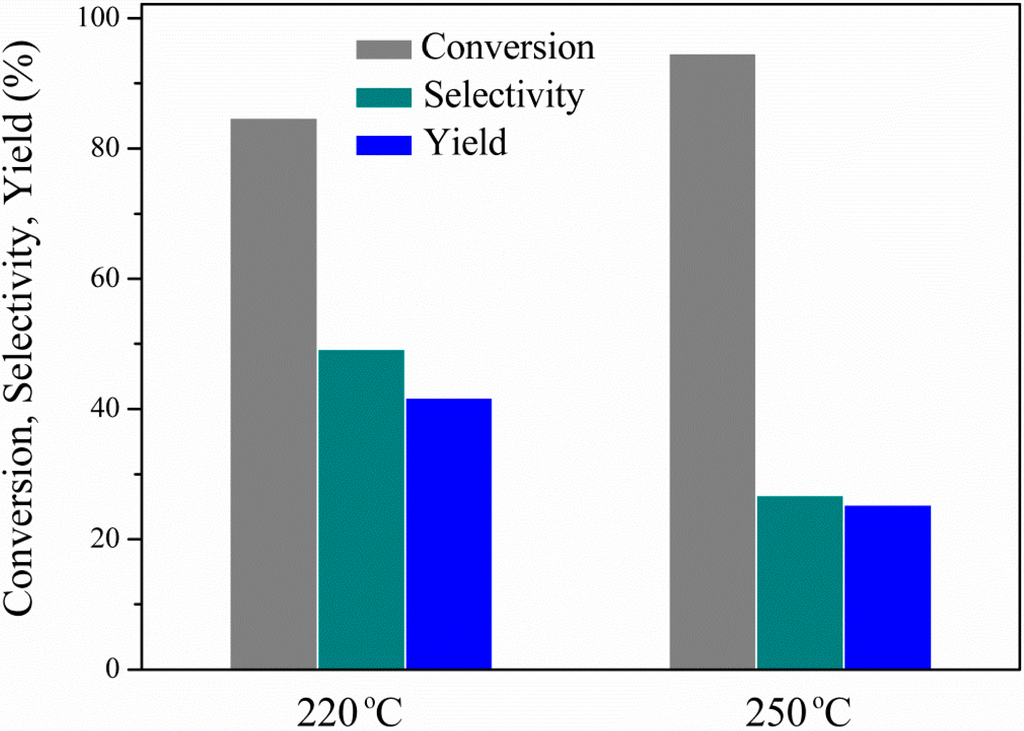
Figure 6.
Effect of reaction temperature on glycerol conversion, 1,2-PDO selectivity and yield: 3.5 MPa N2 initial pressure, 4 h, 7.1 wt% EtOH, 11.3 wt% glycerol (ethanol/glycerol molar ratio = 1.25) and water, catalyst/glycerol + ethanol weight ratio = 0.06.
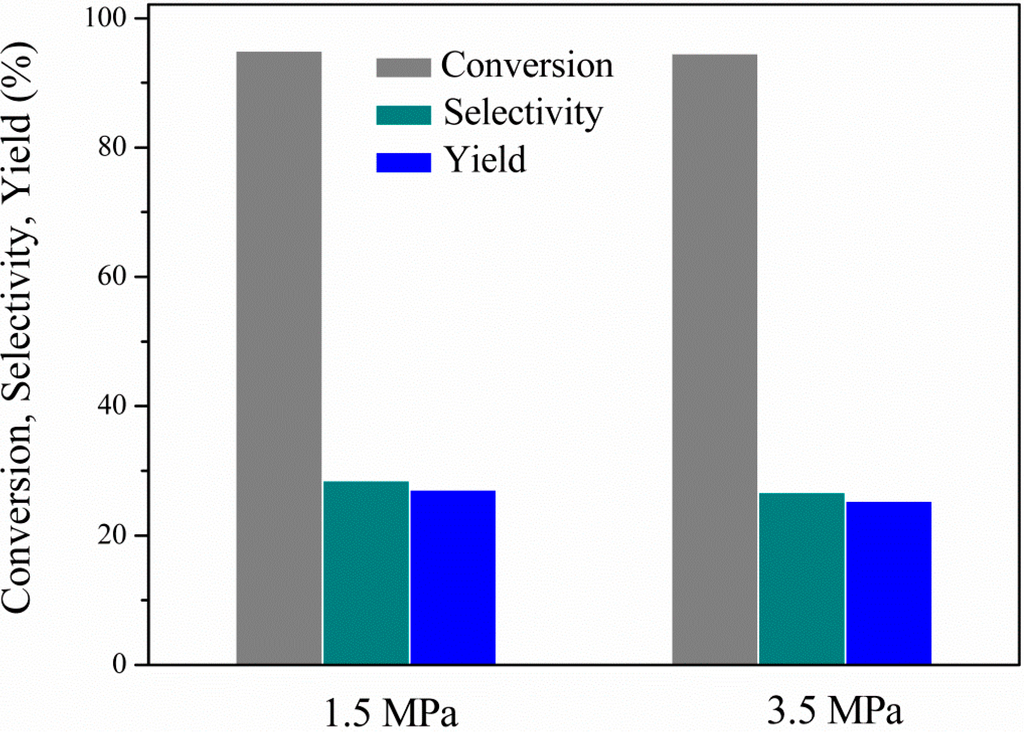
Figure 7.
Effect of system pressure on glycerol conversion, 1,2-PDO selectivity and yield: 250 °C, 4 h, 7.1 wt% EtOH, 11.3 wt% glycerol (ethanol/glycerol molar ratio = 1.25) and water, catalyst/glycerol + ethanol weight ratio = 0.06.
The effect of the initial nitrogen pressure on the conversion, 1,2-propanediol selectivity and yield is shown in Figure 7. It is quite clear that the variation of the N2 system pressure between the range tested had no significant influence on the performance. This indicates that active hydrogen formed nearby the catalytic active sites readily reacts with glycerol, thus avoiding its escape to the gas phase. Moreover, it is also evident that hydrogen generation from ethanol APR and its consumption from glycerol proceed at comparable rates, in accordance with previous reported studies [].
Taking into account the catalytic results discussed above, it is clear that the application of milder conditions is more effective. Short reaction times <2 h, low temperature (220 °C) and nitrogen pressure (1.5 MPa) positively affect the performance in terms of 1,2-propanediol selectivity and yield, exhibiting a maximum value of 53%. Therefore, operation at these conditions is beneficial as a means of reducing the energy requirements of the system.
3. Experimental Section
3.1. Catalyst Synthesis
3.1.1. Platinum Catalyst
The sol-gel technique was used to firstly prepare Fe2O3-Al2O3. The initial step of the sol-gel method was the preparation of a colloidal suspension (sol) of boehmite (γ-AlOOH), which was obtained by the hydrolysis of aluminum alkoxide (aluminum tri-sec-butylate, C12H27AlO3) and stabilized in an acidic environment. The procedure was as follows:
- (1)
- C12H27AlO3 was dropwise and under vigorous stirring added into deionized water while heating at 80 °C.
- (2)
- After the addition of the alkoxide, the solution was maintained under stirring and heating for 30 min, so as to evaporate butanol (C4H9OH); then, Fe(NO3)3·9H2O was added, and the solution was subjected to reflux conditions for 17 h at 80 °C.
- (3)
- The final sample was obtained after calcination at 600 °C for 3 h.
The supported platinum catalyst with 5 wt% Pt was prepared using the wet impregnation method. Platinum was deposited on Fe2O3-Al2O3 using an aqueous solution of H2PtCl6·6H2O. The impregnation was performed in a rotary evaporator at 70 °C for 1 h, followed by solvent removal drying at 120 °C for 17 h. The catalyst was then calcined under synthetic air at 450 °C for 3 h. Before the catalytic tests, the catalyst pre-reduced under continuous flow 10 v/v% H2/N2 for 2 h, at a temperature 90 °C.
3.1.2. Nickel Catalyst
The supported 10 wt% Ni catalyst was also prepared by the wet impregnation method. The support used was commercially available (CeZrLa-Mel Chemicals), and its composition is shown in Table 1. Ni was deposited on the carrier using a metal precursor compound, Ni(NO3)2·6H2O, in aqueous solution form. The impregnation took place in a rotary evaporator under stirring at 70 °C for 1 h, followed by removal of the solvent at 80 °C under vacuum and then drying at 120 °C for 17 h. The catalyst was then calcined in the presence of synthetic air at 450 °C for 3 h. The last step is the reduction in a specially-designed streaming unit. The reduction of the catalyst samples was carried out under continuous flow of 10 v/v% H2/N2 at a temperature of 500 °C for a period of 2 h.
3.1.3. Copper Catalyst
The synthesis method used was the oxalate gel co-precipitation. Details about the preparation and activation of this catalyst can be found in our previous publication [].
3.2. Catalyst Characterization
Surface areas of the samples were determined by N2 adsorption at −196 °C, using the multipoint BET analysis method, with an Autosorb-1 Quantachrome flow apparatus (Quantachrome Instruments, Boynton Beach, FL, USA). Prior to the measurements, the samples were dehydrated in a vacuum at 250 °C overnight.
X-ray diffraction (XRD) patterns were obtained using a Siemens (Munich, Germany) D500 diffractometer, with Cu-Kα radiation.
The reduction characteristics of the catalysts were studied by temperature-programmed reduction (TPR). These experiments were performed in a gas flow system equipped with a quadrupole mass analyzer (OMNIStar™, PFEIFFER, Asslar, Germany). Typically, the catalyst sample (100 mg) was placed in a U-shaped quartz reactor and pretreated in flowing He (30 cm3/min) for 0.5 h at 250 °C, followed by cooling at room temperature. After pretreatment, the temperature was raised from room temperature up to 900 °C at a rate of 10 °C/min in a 10% H2/He flow (30 cm3/min).
Structural characterization and dispersion measurements of the Pt/FeAl catalyst were performed with hydrogen chemisorption and subsequent temperature-programmed desorption of the chemisorbed hydrogen. These experiments were performed in a gas flow system equipped with a quadrupole mass analyzer (OMNIStar™, PFEIFFER, Asslar, Germany). Typically, the fresh catalyst sample (100 mg) was placed in a U-shaped quartz reactor and pre-treated in flowing He (30 cm3/min) for 0.5 h at 250 °C, followed by cooling at 90 °C (Pt/FeAl catalyst reduction temperature; see Figure 2a). The fresh sample was isothermally reduced at 90 °C with a mixture of 10% v/v H2/He flow (30 cm3/min flow). After reduction, H2 was flushed with a 60 cm3/min He flow, and then hydrogen chemisorption was performed at 90 °C with the same gas mixture as used for the reduction for 1 h. The sample was then cooled to room temperature under He flow and was kept at that temperature for 2 h in order to remove physisorbed H2. The TPD experiment was performed by heating the sample at a rate of 10 °C/min from room temperature to 800 °C, under a He flow of 30 cm3/min. The same procedure was followed for the used catalyst (after the 4th reuse test), but without the reduction step at 90 °C as described above for the fresh catalyst. Dispersion values were calculated from the quantification of the desorbed hydrogen and the assumption that the stoichiometry factor between chemisorbed hydrogen and surface Pt equals H:Pt = 1:1.
3.3. Catalyst Evaluation
The reaction tests were carried out in a 100-mL monel batch reactor (Parr Instrument Company, Moline, IL, USA) equipped with an electronic temperature controller and a mechanical stirrer (Scheme 2).
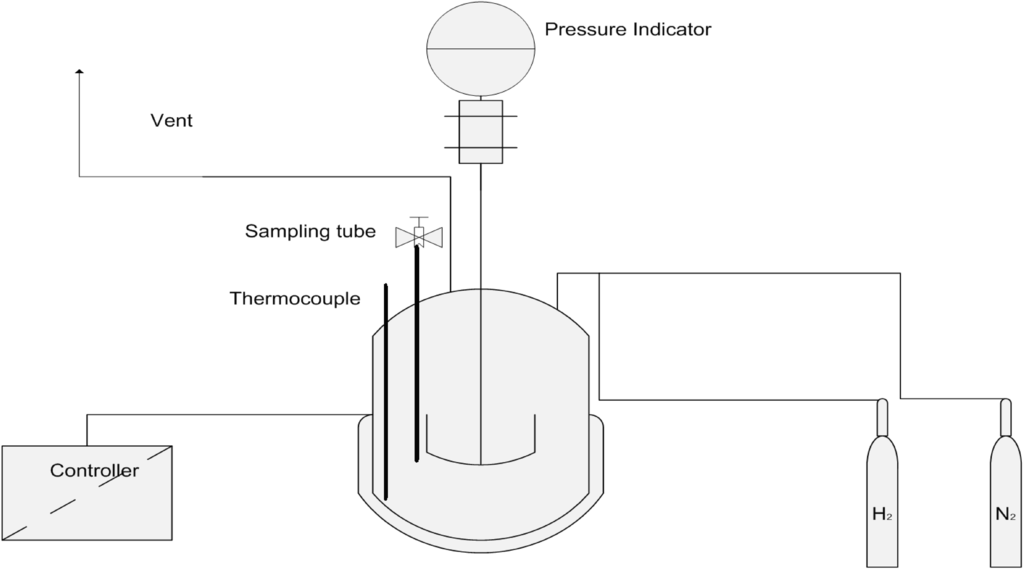
Scheme 2.
Schematic representation of the experimental unit.
The reaction was typically conducted under the following standard conditions: 250 °C, 3.5 MPa initial N2 pressure, 0.06–0.25 catalyst/glycerol + ethanol weight ratio, a feedstock mixture of: 7.1 wt% EtOH, 11.3 wt% glycerol (ethanol/glycerol molar ratio = 1.25) and water, 4-h reaction time and a 1000 rpm stirring rate. The effects of reaction time, temperature and nitrogen pressure were studied by varying the parameters, such as: 2 and 4 h, 220 and 250 °C and 1.5 and 3.5 MPa, respectively. The reactant conversion was calculated as follows:
The product selectivity and yield were calculated using the equations:
Liquid samples were analyzed by GC (Agilent 7890A, Santa Clara, CA, USA, FID, DB-Wax 30 m × 0.53 mm × 1.0 μm). Acetonitrile was used as a solvent for the GC analysis. The multiple point internal standard method was used for the quantification of the results. The liquid compounds detected were: 1,2-propanediol-1,2-PDO, ethylene glycol-EG, hydroxyacetone-AC (acetol), 1-propanol-1-PrOH, 2-propanol-2-PrOH and ethanol-EtOH. Unidentified side liquid products were also detected by GC analysis. Gas analysis was performed in an Agilent GC (Santa Clara, CA, USA) 7890A, Molecular Sieve and Poraplot) equipped with a thermal conductivity detector. The gaseous products were: CO2, H2, CO, CH4 and C2H6.
4. Conclusions
In this study, the formation of 1,2-propanediol using a glycerol by-product as a raw material without the need of external H2 addition was realized. The required hydrogen is formed via the ethanol aqueous phase reforming over Pt, Ni and Cu catalysts. Ethanol was selected as a hydrogen source, because this alcohol is used instead of methanol for biodiesel production. All of the catalysts tested are able to catalyze the tandem reaction sequence (aqueous phase reforming and hydrodeoxygenation), producing 1,2-propanediol under initial N2 pressure. Among them, the Pt-based catalyst (Pt/Fe2O3-Al2O3) exhibited the best results concerning 1,2-propanediol productivity and stability under reaction conditions. Moreover, it was found that milder operating conditions (reaction times <2 h, temperature 220 °C and nitrogen pressure 1.5 MPa) are essential for maximizing 1,2-propanediol productivity and yield.
Acknowledgments
This work was funded by the Aristotle University of Thessaloniki Research Committee in the frame of the Scholarships for Post-Doctoral Researchers 2013. Stylliani Sklari is acknowledged for the synthesis of the Fe2O3/Al2O3 support. Sofia Angeli and Vasileia-Loukia Yfanti are acknowledged for TPR experiments of the Ni and Pt catalysts, respectively.
Author Contributions
Efterpi S. Vasiliadou did the preparational work and wrote the paper; Angeliki A. Lemonidou supervised all the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ruppert, A.M.; Weinberg, K.; Palkovits, R. Hydrogenolysis goes bio: From carbohydrates and sugar alcohols to platform chemicals. Angew. Chem. Int. Ed. Engl. 2012, 51, 2564–2601. [Google Scholar] [CrossRef] [PubMed]
- Vennestrøm, P.N.R.; Osmundsen, C.M.; Christensen, C.H.; Taarning, E. Beyond petrochemicals: the renewable chemicals industry. Angew. Chem. Int. Ed. Engl. 2011, 50, 10502–10509. [Google Scholar] [CrossRef] [PubMed]
- Bauer, F.; Hulteberg, C. Is there a future in glycerol as a feedstock in the production of biofuels and biochemicals? Biofuels Bioprod. Biorefin. 2013, 7, 43–51. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Heracleous, E.; Vasalos, I.A.; Lemonidou, A.A. Ru-based catalysts for glycerol hydrogenolysis—Effect of support and metal precursor. Appl. Catal. B 2009, 92, 90–99. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Lemonidou, A.A. Parameters Affecting the Formation of 1,2-Propanediol from Glycerol over Ru/SiO2 Catalyst. Org. Process. Res. Dev. 2011, 15, 925–931. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Lemonidou, A.A. Investigating the performance and deactivation behaviour of silica-supported copper catalysts in glycerol hydrogenolysis. Appl. Catal. A 2011, 396, 177–185. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Eggenhuisen, T.M.; Munnik, P.; de Jongh, P.E.; de Jong, K.P.; Lemonidou, A.A. Synthesis and performance of highly dispersed Cu/SiO2 catalysts for the hydrogenolysis of glycerol. Appl. Catal. B 2014, 145, 108–119. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Lemonidou, A.A. Kinetic study of liquid-phase glycerol hydrogenolysis over Cu/SiO2 catalyst. Chem. Eng. J. 2013, 231, 103–112. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Shinmi, Y.; Koso, S.; Tomishige, K. Direct hydrogenolysis of glycerol into 1,3-propanediol over rhenium-modified iridium catalyst. J. Catal. 2010, 272, 191–194. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, Y.; Zheng, H.; Ding, G.; Li, Y. Direct Conversion of Glycerol into 1,3-Propanediol over Cu-H4SiW12O40/SiO2 in Vapor Phase. Catal. Lett. 2009, 131, 312–320. [Google Scholar] [CrossRef]
- Martin, A.; Armbruster, U.; Atia, H. Recent developments in dehydration of glycerol toward acrolein over heteropolyacids. Eur. J. Lipid Sci. Technol. 2012, 114, 10–23. [Google Scholar] [CrossRef]
- Liu, L.; Ye, X.P.; Bozell, J.J. A comparative review of petroleum-based and bio-based acrolein production. ChemSusChem 2012, 5, 1162–1180. [Google Scholar] [CrossRef] [PubMed]
- Dou, B.; Song, Y.; Wang, C.; Chen, H.; Xu, Y. Hydrogen production from catalytic steam reforming of biodiesel byproduct glycerol: Issues and challenges. Renew. Sustain. Energy Rev. 2014, 30, 950–960. [Google Scholar] [CrossRef]
- Serafim, H.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Valorization of glycerol into fuel additives over zeolites as catalysts. Chem. Eng. J. 2011, 178, 291–296. [Google Scholar] [CrossRef]
- ADM Propylene Glycol—Life Cycle Analysis. Available online: http://www.adm.com (accessed on 30 September 2014).
- Nakagawa, Y.; Tomishige, K. Heterogeneous catalysis of the glycerol hydrogenolysis. Catal. Sci. Technol. 2011, 1, 179–190. [Google Scholar] [CrossRef]
- Mizugaki, T.; Arundhathi, R.; Mitsudome, T.; Jitsukawa, K.; Kaneda, K. Selective Hydrogenolysis of Glycerol to 1,2-Propanediol Using Heterogeneous Copper Nanoparticle Catalyst Derived from Cu–Al Hydrotalcite. Chem. Lett. 2013, 42, 729–731. [Google Scholar] [CrossRef]
- Akiyama, M.; Sato, S.; Takahashi, R.; Inui, K.; Yokota, M. Dehydration–hydrogenation of glycerol into 1,2-propanediol at ambient hydrogen pressure. Appl. Catal. A 2009, 371, 60–66. [Google Scholar] [CrossRef]
- Martin, A.; Armbruster, U.; Gandarias, I.; Arias, P.L. Glycerol hydrogenolysis into propanediols using in situ generated hydrogen—A critical review. Eur. J. Lipid Sci. Technol. 2013, 115, 9–27. [Google Scholar] [CrossRef]
- D’Hondt, E.; van de Vyver, S.; Sels, B.F.; Jacobs, P. Catalytic glycerol conversion into 1,2-propanediol in absence of added hydrogen. Chem. Commun. 2008, 45, 6011–6012. [Google Scholar] [CrossRef]
- Dasari, M.A.; Kiatsimkul, P.P.; Sutterlin, W.R.; Suppes, G.J. Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl. Catal. A 2005, 281, 225–231. [Google Scholar] [CrossRef]
- Miyazawa, T.; Kusunoki, Y.; Kunimori, K.; Tomishige, K. Glycerol conversion in the aqueous solution under hydrogen over Ru/C + an ion-exchange resin and its reaction mechanism. J. Catal. 2006, 240, 213–221. [Google Scholar] [CrossRef]
- Mane, R.B.; Rode, C.V. Simultaneous glycerol dehydration and in situ hydrogenolysis over Cu–Al oxide under an inert atmosphere. Green Chem. 2012, 14, 2780–2789. [Google Scholar] [CrossRef]
- Musolino, M.G.; Scarpino, L.A.; Mauriello, F.; Pietropaolo, R. Selective transfer hydrogenolysis of glycerol promoted by palladium catalysts in absence of hydrogen. Green Chem. 2009, 11, 1511–1513. [Google Scholar] [CrossRef]
- Xia, S.; Zheng, L.; Wang, L.; Chen, P.; Hou, Z. Hydrogen-free synthesis of 1,2-propanediol from glycerol over Cu-Mg-Al catalysts. RSC Adv. 2013, 3, 16569–16576. [Google Scholar] [CrossRef]
- Gandarias, I.; Arias, P.L.; Requies, J.; El Doukkali, M.; Güemez, M.B. Liquid-phase glycerol hydrogenolysis to 1,2-propanediol under nitrogen pressure using 2-propanol as hydrogen source. J. Catal. 2011, 282, 237–247. [Google Scholar] [CrossRef]
- Gandarias, I.; Requies, J.; Arias, P.L.; Armbruster, U.; Martin, A. Liquid-phase glycerol hydrogenolysis by formic acid over Ni-Cu/Al2O3 catalysts. J. Catal. 2012, 290, 79–89. [Google Scholar] [CrossRef]
- Gandarias, I.; Fernández, S.G.; El Doukkali, M.; Requies, J.; Arias, P.L. Physicochemical Study of Glycerol Hydrogenolysis Over a Ni–Cu/Al2O3 Catalyst Using Formic Acid as the Hydrogen Source. Top. Catal. 2013, 56, 995–1007. [Google Scholar] [CrossRef]
- Vasileiadou, E.S.; Lemonidou, A.A. Catalytic process for the production of 1,2-propanediol from crude glycerol stream. Patent EP 2565175 A1, 31 August 2011. [Google Scholar]
- Vasiliadou, E.S.; Yfanti, V.-L.; Lemonidou, A.A. One-pot tandem processing of glycerol stream to 1,2-propanediol with methanol reforming as hydrogen donor reaction. Appl. Catal. B 2014, 163, 258–266. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. A review on reforming bio-ethanol for hydrogen production. Int. J. Hydrogen Energy 2007, 32, 3238–3247. [Google Scholar] [CrossRef]
- Zhang, X.-R.; Wang, L.-C.; Yao, C.-Z.; Cao, Y.; Dai, W.-L.; He, H.-Y.; Fan, K.-N. A highly efficient Cu/ZnO/Al2O3 catalyst via gel-coprecipitation of oxalate precursors for low-temperature steam reforming of methanol. Catal. Lett. 2005, 102, 183–190. [Google Scholar] [CrossRef]
- Pendem, C.; Gupta, P.; Chaudhary, N.; Singh, S.; Kumar, J.; Sasaki, T.; Datta, A.; Bal, R. Aqueous phase reforming of glycerol to 1,2-propanediol over Pt-nanoparticles supported on hydrotalcite in the absence of hydrogen. Green Chem. 2012, 14, 3107–3113. [Google Scholar] [CrossRef]
- Angeli, S.D.; Monteleone, G.; Giaconia, A.; Angeliki, A. Low Temperature Methane Steam Reforming: Catalytic Activity and Coke Deposition Study. Chem. Eng. Trans. 2013, 35, 1201–1206. [Google Scholar]
- Guerreiro, E.D.; Gorriz, O.F.; Rivarola, J.B.; Arrfia, L.A. Characterization of Cu/SiO2 catalysts prepared by ion exchange for methanol dehydrogenation. Appl. Catal. A 1997, 165, 259–271. [Google Scholar] [CrossRef]
- Sales, A. Production of Biodiesel from Sunflower Oil and Ethanol by Base Catalyzed Transesterification. Master Thesis, Department of Chemical Engineering, Royal Institute of Technology (KTH), Stockholm, Sweden, June 2011. [Google Scholar]
- Vaidya, P.D.; Rodrigues, A.E. Glycerol Reforming for Hydrogen Production: A Review. Chem. Eng. Technol. 2009, 32, 1463–1469. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Higuchi, K.; Takahashi, N.; Yokota, K.; Doi, H.; Sugiura, M. Effect of the addition of Fe on catalytic activities of Pt/Fe/γ-Al2O3 catalyst. Appl. Catal. B 1999, 23, 159–167. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).