How to Determine the Core-Shell Nature in Bimetallic Catalyst Particles?
Abstract
:1. Introduction
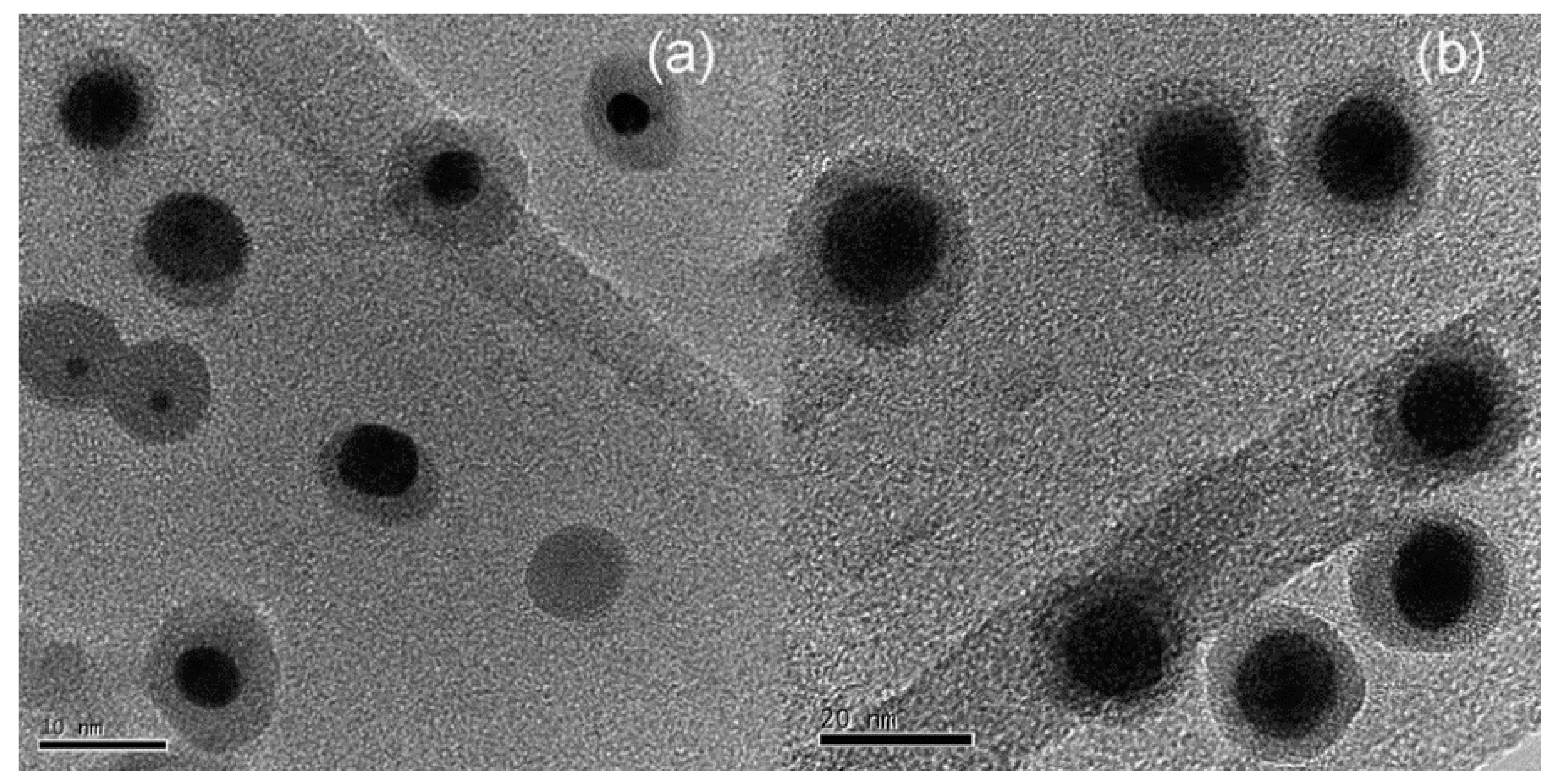
2. Results
| Label used | − − | − | 0 | + | + + |
|---|---|---|---|---|---|
| Surface sensitivity | transmission | > 10 nm | 3–10 nm | 1–2 nm | < 1 nm |
| Spatial resolution | bulk | > 10 nm | 2–10 nm | 0.1–1 nm | < 0.1 nm |
| Composition | Signal strength, sensitivity to atom number, sensitivity to environment/neighboring atoms, oxidation state. | ||||
| Elemental distribution | Ability to determine the distribution of elements across the particle, alloying and crystal structure. | ||||
| Geometry/Porosity | Ability to determine particle size and geometry, particle dispersity and shell porosity. | ||||
| Technique | Type | Technique | Surface sensitivity | Spatial resolution | Composition | Elemental distribution | Geometry/Porosity | Other chemical or physical properties |
|---|---|---|---|---|---|---|---|---|
| Electron Microscopy | e−→e− | SEM, SEI, BEI | SEI + BEI − | ○ | n/a | - | -- | |
| TEM, HRTEM, STEM | -- | ++ | n/a | + | + | |||
| Electron Spectroscopy | e−→e− | EELS | Tr-EELS--Refl-EELS + | + | + | ++ | See TEM | |
| AES | + | ○ | ○ | + | ○ | Adsorption and chemisorption of gases | ||
| HAADF | -- | + | + | + | See TEM | |||
| e−→λ | EDX | - | ○ | + | - | -- | ||
| X-ray Spectroscopy | λ→λ | XRD | - | -- | + | ○ | -- | |
| λ→e− | XPS | + | -- | ++ | ++ | - | Organic surface contaminants | |
| Absorption spectroscopy | λ | XAS | -- | -- | + | + | + | Adsorbates/Ligands |
| λ | UV-vis | + | -- | ○ | - | ○ | SPR | |
| Other | AFM | ++ | + | -- | ○ | + | ||
| Cyclic Voltammetry | ++ | -- | + | - | + | Catalytic activity |
2.1. Electron Microscopy and Spectroscopy
2.2. X-ray Spectroscopies
2.3. Absorption Spectroscopies
2.4. Other
2.5. Core-Shell Particles Made in Microemulsions
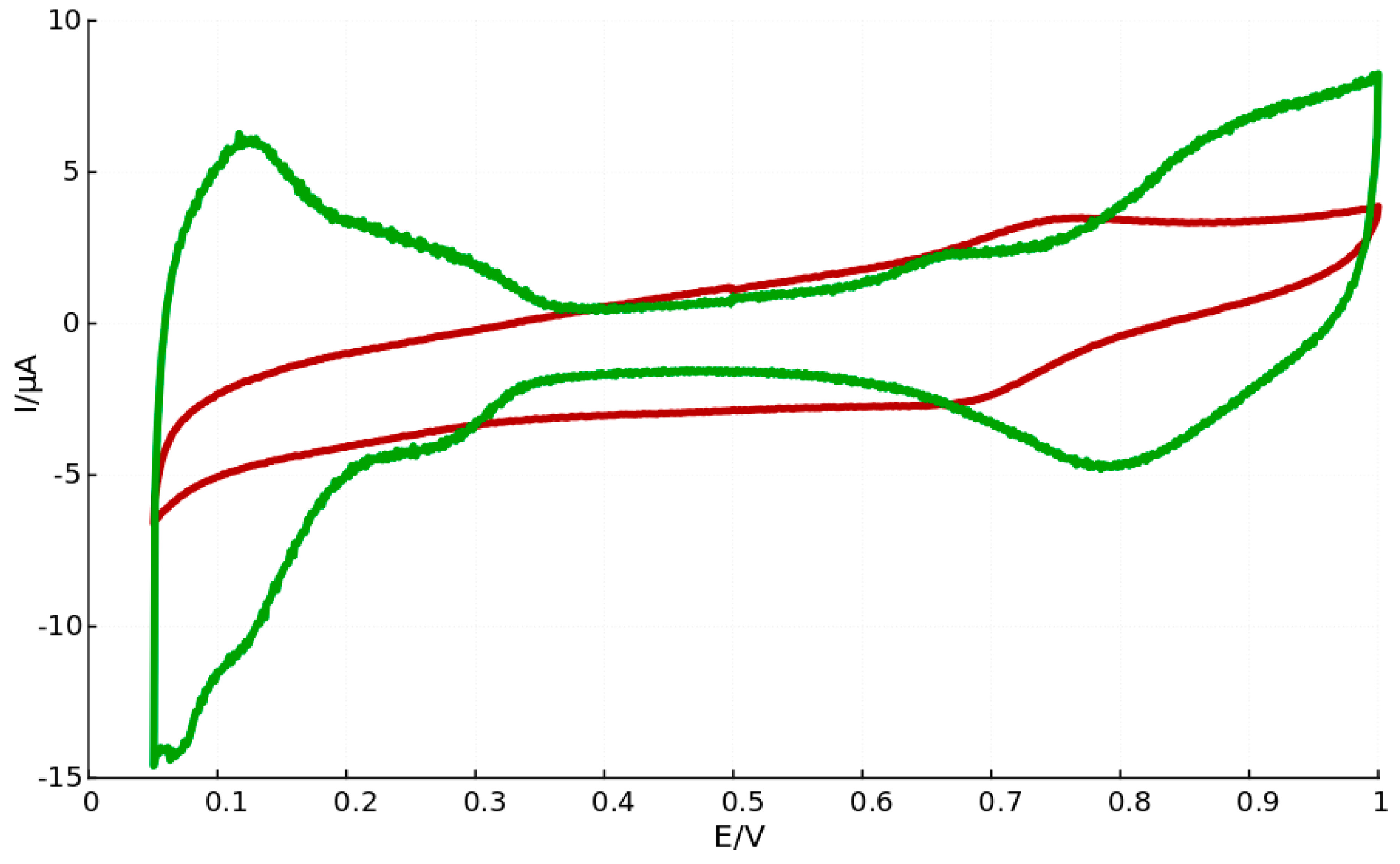
3. Discussion
4. Methods
4.1. Electron Microscopy/Spectroscopy
4.2. X-ray Spectroscopies
4.3. Absorption Spectroscopies
4.4. Other
4.5. Core-Shell Particles Made in Microemulsions
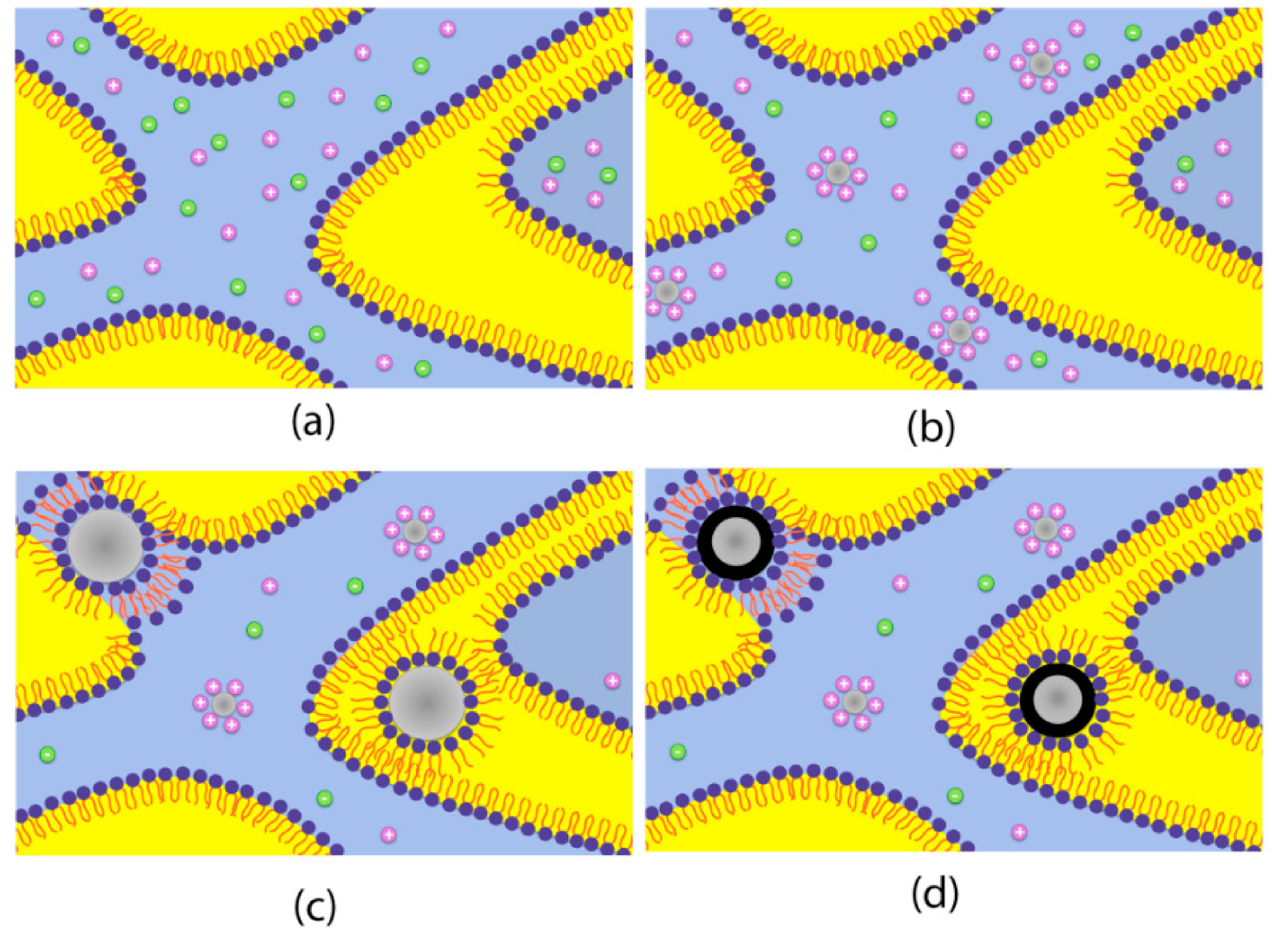
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maillard, F.; Pronkin, S.; Savinova, E.R. Size effects in electrocatalysis of fuel cell reactions on supported metal nanoparticles. 2009. [Google Scholar] [CrossRef]
- Hosokawa, M. Nanoparticle Technology Handbook; Elsevier: Amsterdam, The Netherland, 2007. [Google Scholar]
- Eftekhari, A. Nanostructured Materials in Electrochemistry; Wiley: Manhattan, NY, USA, 2008. [Google Scholar]
- Barcari, G. Metal. Nanoparticles and Nanoalloys; Wilcoxon, J., Johnston, R.L., Eds.; Elsevier: Amsterdam, The Netherland, 2012; pp. 213–247. [Google Scholar]
- Kitchin, J.; Nørskov, J.; Barteau, M.; Chen, J. Role of Strain and Ligand Effects in the Modification of the Electronic and Chemical Properties of Bimetallic Surfaces. Phys. Rev. Lett. 2004, 93. [Google Scholar] [CrossRef] [PubMed]
- Oezaslan, M.; Hasché, F.; Strasser, P. Pt.-Based Core–Shell Catalyst Architectures for Oxygen Fuel Cell Electrodes. J. Phys. Chem. Lett. 2013, 4, 3273–3291. [Google Scholar] [CrossRef]
- Roller, J.; Yu, H.; Vukmirovic, M.; Bliznakov, S.; Kotula, P.; Carter, C.; Adzic, R.; Maric, R. Flame-Based Synthesis of Core-Shell Structures Using Pd-Ru and Pd Cores. Electrochim. Acta 2014, 138, 341–352. [Google Scholar] [CrossRef]
- Hammer, B.M.; Morikawa, Y.; Norskov, J.K. CO Chemisorption at Metal. Surfaces and Overlayers. Phys. Rev. Lett. 1996, 76. [Google Scholar] [CrossRef]
- Holmblad, P.M.L.; Larsen, J.H.; Chorkendorff, I.; Nielsen, L.P.; Besenbacher, F.S.I.; Laegsgaard, E.; Kratzer, P.; Hammer, B.N.; NØrskov, J.K. Designing surface alloys with specific active sites. Catal. Lett. 1996, 40, 131–135. [Google Scholar] [CrossRef]
- Teng, X.; Yang, H. Synthesis of magnetic nanocomposites and alloys from platinum-iron oxide core-shell nanoparticles. Nanotechnology 2005, 16, S554–S561. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-T.; Dutta, P. Synthesis of Au/SnO2 core–shell structure nanoparticles by a microwave-assisted method and their optical properties. J. Solid State 2011, 184, 312–316. [Google Scholar] [CrossRef]
- Liu, Y.T.; Yuan, Q.B.; Duan, D.H.; Zhang, Z.L.; Hao, X.G.; Wei, G.Q.; Liu, S.B. Electrochemical activity and stability of core–shell Fe2O3/Pt nanoparticles for methanol oxidation. J. Power Sources 2013, 243, 622–629. [Google Scholar] [CrossRef]
- Geboes, B.; Mintsouli, I.; Wouters, B.; Georgieva, J.; Kakaroglou, A.; Sotiropoulos, S.; Valova, E.; Armyanov, S.; Hubin, A.; Breugelmans, T. Surface and Electrochemical Characterisation of a Pt-Cu/C Nano-structured Electrocatalyst, Prepared by Galvanic Displacement. Appl. Catal. B 2013. [Google Scholar] [CrossRef]
- Xia, L.; Hua, X.; Kanga, X.; Zhao, H.; Sunb, M.; Cihen, X. A one-step facile synthesis of Ag–Ni core–shell nanoparticles in water-in-oil microemulsions. Colloids Surf. 2010, 367, 96–101. [Google Scholar] [CrossRef]
- Balerna, A.; Evangelisti, C.; Schiavi, E.; Vitulli, G.; Bertinetti, L.; Martra, G.; Mobilio, S. EXAFS and XANES structural characterization of bimetallic AuPd vapor derived catalysts. J. Phys. 2013, 430. [Google Scholar] [CrossRef]
- Wang, C.; Wang, G.; van der Vliet, D.; Chang, K.C.; Markovic, N.M.; Stamenkovic, V.R. Monodisperse Pt(3)Co nanoparticles as electrocatalyst: the effects of particle size and pretreatment on electrocatalytic reduction of oxygen. Phys. Chem. Chem. Phys. 2010, 12, 6933–6939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lima, F.H.B.; Shao, M.H.; Sasaki, K.; Wang, J.X.; Hanson, J.; Adzic, R.R. Platinum monolayer on nonnoble metal-noble metal core-shell nanoparticle electrocatalysts for O2 reduction. J. Phys. Chem. B 2005, 109, 22701–22704. [Google Scholar] [CrossRef] [PubMed]
- Mayrhofer, K.J.; Juhart, V.; Hartl, K.; Hanzlik, M.; Arenz, M. Adsorbate-induced surface segregation for core-shell nanocatalysts. Angew. Chem. Int. Ed. 2009, 48, 3529–3531. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, C.; Zhu, Y.; Si, R.; Cai, Y.; Wang, J.X.; Adzic, R.R. Hollow core supported Pt monolayer catalysts for oxygen reduction. Catal. Today 2013, 202, 50–54. [Google Scholar] [CrossRef]
- Pigozzi, G.; Mukherji, D.; Elerman, Y.; Strunz, P.; Gilles, R.; Hoelzel, M.; Barbier, B.; Schmutz, P. Effects of size reduction on the structure and magnetic properties of core–shell Ni3Si/silica nanoparticles prepared by electrochemical synthesis. J. Alloys Compd. 2014, 584, 119–127. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, J.; Kawamura, G.; Nogami, M. Preparation of Au–Ag, Ag–Au core–shell bimetallic nanoparticles for surface-enhanced Raman scattering. Scripta Mater. 2008, 58, 862–865. [Google Scholar] [CrossRef]
- Steinbrück, A.; Csáki, A.; Festag, G.; Fritzsche, W. Preparation and Optical Characterization of Core–Shell Bimetal Nanoparticles. Plasmonics 2006, 1, 79–85. [Google Scholar] [CrossRef]
- Kumar, S.; Zou, S. Electrooxidation of Carbon Monoxide and Methanol on Platinum-Overlayer-Coated Gold Nanoparticles: Effects of Film Thickness. Langmuir 2007, 23, 7365–7371. [Google Scholar] [CrossRef] [PubMed]
- Kuttiyiel, K.A.; Sasaki, K.; Su, D.; Vukmirovic, M.B.; Marinkovic, N.S.; Adzic, R.R. Pt monolayer on Au-stabilized PdNi core–shell nanoparticles for oxygen reduction reaction. Electrochim. Acta 2013, 110, 267–272. [Google Scholar] [CrossRef]
- Najjar, R. Microemulsions—An Introduction to Properties and Applications; InTech Publisher: Rijeka, Croatia, 2012. [Google Scholar]
- Negro, E.; Latsuzbaia, R.; Koper, G.J. Bicontinuous microemulsions for high yield wet synthesis of ultrafine platinum nanoparticles: Effect of precursors and kinetics. Langmuir 2014, 30, 8300–8307. [Google Scholar] [CrossRef] [PubMed]
- Sieben, J.M.; Comignani, V.; Alvarez, A.E.; Duarte, M.M. Synthesis and characterization of Cu core Pt–Ru shell nanoparticles for the electro-oxidation of alcohols. Int. J. Hydrogen Energ. 2014, 39, 8667–8674. [Google Scholar] [CrossRef]
- Tsai, C.W.; Chen, H.M.; Liu, R.S.; Lee, J.F.; Chang, S.M.; Weng, B.J. Magnetically recyclable Fe@Co core-shell catalysts for dehydrogenation of sodium borohydride in fuel cells. Int. J. Hydrogen Energ. 2012, 37, 3338–3343. [Google Scholar] [CrossRef]
- Wojtysiak, S.; Solla-Gullón, J.; Dłużewski, P.; Kudelski, A. Synthesis of core–shell silver–platinum nanoparticles, improving shell integrity. Colloids Surf. A 2014, 441, 178–183. [Google Scholar] [CrossRef]
- Chen, D.; Liu, S.; Li, J.; Zhao, N.; Shi, C.; Du, X.; Sheng, J. Nanometre Ni and core/shell Ni/Au nanoparticles with controllable dimensions synthesized in reverse microemulsion. J. Alloys Compd. 2009, 475, 494–500. [Google Scholar] [CrossRef]
- Tojo, C.; Dios, M.; Barroso, F. Surfactant Effects on Microemulsion-Based. Nanoparticle Synthesis. Materials 2010, 4, 55–72. [Google Scholar]
- Lee, J.P.; Chen, D.; Li, X.; Yoo, S.; Bottomley, L.A.; El-Sayed, M.A.; Park, S.; Liu, M. Well-organized raspberry-like Ag@Cu bimetal nanoparticles for highly reliable and reproducible surface-enhanced Raman scattering. Nanoscale 2013, 5, 11620–11624. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yamauchi, Y. Facile solution synthesis of Ag@Pt core-shell nanoparticles with dendritic Pt shells. Phys. Chem. Chem. Phys. 2013, 15, 3490–3496. [Google Scholar] [CrossRef] [PubMed]
- Merzlikin, S. Depth Profiling by X-ray Photoelectron Spectroscopy. In Fakultät für Chemie; Ruhr-Universität Bochum: Bochum, Germany, 2007. [Google Scholar]
- Mendis, B.G.; Craven, A.J. Characterising the surface and interior chemistry of core-shell nanoparticles using scanning transmission electron microscopy. Ultramicroscopy 2011, 111, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. Scanning Transmission Electron Microscopy Studies of Mono- and Bimetallic. Nanoclusters 2012, 3, 213–247. [Google Scholar]
- Vogt, T.; Dahmen, W.; Binev, P. Modeling Nanoscale Imaging in Electron; Springer: Manhattan, NY, USA, 2012. [Google Scholar]
- Zhou, W.; Wang, Z.L. Scanning Microscopy for Nanotechnology; Springer: Manhattan, NY, USA, 2007. [Google Scholar]
- Tilinin, I.S. Qualitative Surface Analysis by Auger and X-Ray Photoelectron Spectroscopy. Available online: http://www.surfaceanalysis.org/BriggsGrant.html (accessed on 19 November 2014).
- Egerton, R.F. Electron Energy-Loss Spectroscopy. In Electron Microscope; Springer: Manhattan, NY, USA, 2011. [Google Scholar]
- Liu, J.; Hembree, G.G.; Spinnler, G.E.; Venables, J.A. High-Resolution Auger-Electron Spectroscopy and Microscopy of a Supported Metal Catalyst. Surf. Sci. 1992, 262, L111–L117. [Google Scholar] [CrossRef]
- Lukasczyk, T. Generation of Pure Iron Nanostructures via Electron-beam Induced Deposition in UHV. In Der Naturwissenschaftlichen Fakultät; Friedrich-Alexander-Universität: Erlangen-Nürnberg, Germany, 2010. [Google Scholar]
- Camardese, J.; McCalla, E.; Abarbanel, D.W.; Dahn, J.R. Determination of Shell Thickness of Spherical Core-Shell NixMn1-x(OH)2 Particles via Absorption Calculations of X-ray Diffraction Patterns. J. Electrochem. Soc. 2014, 161, A814–A820. [Google Scholar] [CrossRef]
- Zolotoyabko, E. Basic Concepts of X-ray Diffraction; Wiley: Manhattan, NY, USA, 2014. [Google Scholar]
- Merzlikin, S.V.; Tolkachev, N.N.; Strunskus, T.; Witte, G.; Glogowski, T.; Wöll, C.; Grünert, W. Resolving the depth coordinate in photoelectron spectroscopy—Comparison of excitation energy variation vs. angular-resolved XPS for the analysis of a self-assembled monolayer model system. Surf. Sci. 2008, 602, 755–767. [Google Scholar]
- Silva, D.O.; Luza, L.; Gual, A.; Daniel, B.L.; Bernardi, F.; Zapata, M.J.; Dupont, J. Straightforward synthesis of bimetallic Co/Pt nanoparticles in ionic liquid: Atomic rearrangement driven by reduction-sulfidation processes and Fischer-Tropsch catalysis. Nanoscale 2014, 6, 9085–9092. [Google Scholar] [CrossRef] [PubMed]
- Prabhuram, J.; Wang, X.; Hui, C.L.; Hsing, I.M. Synthesis and Characterization of Surfactant-Stabilized Pt/C Nanocatalysts for Fuel Cell Applications. J. Phys. Chem. B 2003, 107, 11057–11064. [Google Scholar] [CrossRef]
- Kendick, I.; Smotkin, E.S. Infrared and X-ray Absorption Spectroscopy of Operating Fuel Cells. Available online: http://nuvant.com/pdfs/Kendrick%20PCCP_Review_cut.pdf (accessed on 19 November 2014).
- Russell, A.E.; Rose, A. X-ray absorption spectroscopy of low temperature fuel cell catalysts. Chem. Rev. 2004, 104, 4613–4635. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hu, J.E.; Wang, Q.; Gaskell, K.; Frenkel, A.I.; Jackson, G.S.; Eichhorn, B. PtMo Alloy and MoOx@Pt Core-Shell Nanoparticles as Highly CO-Tolerant Electrocatalysts. J. Am. Chem. Soc. 2009, 131, 6924–6925. [Google Scholar] [CrossRef] [PubMed]
- Alayoglu, S.; Zavalij, P.; Eichhorn, B.; Wang, Q.; Frenkel, A.I.; Chupas, P. Structural and Architectural Evaluation of Bimetallic Nanoparticles: A Case Study of PtRu Core-Shell and Alloy Nanoparticles. ACS Nano 2009, 3, 3127–3137. [Google Scholar] [CrossRef] [PubMed]
- Ochal, P.; de la Fuente, J.; Tsypkin, M.; Seland, F.; Sunde, S.; Muthuswamy, N.; Rønning, M.; Chen, D.; Garcia, S.; Alayoglu, S.; et al. CO stripping as an electrochemical tool for characterization of Ru@Pt core-shell catalysts. J. Electroanal. Chem. 2011, 655, 140–146. [Google Scholar] [CrossRef]
- Cookson, N.J. Preparation and Characterisation of Bimetallic Core-Shell Particles. Master Thesis, University of Birmingham, Birmingham, UK, September 2009. [Google Scholar]
- Henglein, A. Preparation and Optical Aborption Spectra of AucorePtshell and PtcoreAushell Colloidal Nanoparticles in Aqueous Solution. J. Phys. Chem. B 2000, 104, 2201–2203. [Google Scholar] [CrossRef]
- Creighton, J.A.; Eadon, D.G. Ultraviolet Visible Absorption-Spectra of the Colloidal Metallic Elements. J. Chem. Soc. 1991, 87, 3881–3891. [Google Scholar]
- Rao, A.; Schoenenberger, M.; Gnecco, E.; Glatzel, T.; Meyer, E.; Brändlin, D.; Scandella, L. Characterization of nanoparticles using Atomic Force Microscopy. J. Phys. 2007, 61, 971–976. [Google Scholar]
- Newton, J.E.; Preece, J.A.; Rees, N.V.; Horswell, S.L. Nanoparticle catalysts for proton exchange membrane fuel cells: Can surfactant effects be beneficial for electrocatalysis? Phys. Chem. Chem. Phys. 2014, 16, 11435–11446. [Google Scholar] [CrossRef] [PubMed]
- Crespo, P.; Litrán, R.; Rojas, T.C.; Multigner, M.; De la Fuente, J.M.; Sánchez-López, J.C.; Garcia, M.A.; Hernando, A.; Penadés, S.; Fernández, A. Permanent Magnetism, Magnetic Anisotropy, and Hysteresis of Thiol-Capped Gold Nanoparticles. Phys. Rev. Lett. 2004, 93. [Google Scholar] [CrossRef] [PubMed]
- Vesperinas, A.; Eastoe, J.; Jackson, S.; Wyatt, P. Light-induced flocculation of gold nanoparticles. Chem. Commun. 2007, 38, 3912–3914. [Google Scholar] [CrossRef]
- Eastoe, J. Photo-destructible Surfactants in Microemulsions. In Progress in Colloid and Polymer Science; Springer: Berlin, Germany, 2006; Volume 133, pp. 106–110. [Google Scholar]
- Lim, J.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res. Lett. 2013, 8. [Google Scholar] [CrossRef]
- Pecora, R. Dynamic Light Acattering; Plenum Press: New York, NY, USA, 1985. [Google Scholar]
- Goldstein, J. Scanning Electron Microscopy and X-ray Microanalysis; Springer: Manhattan, NY, USA, 2003. [Google Scholar]
- Crewe, A.V. The use of backscattered electrons for imaging purposes in a scanning electron microscope. Ultramicroscopy 1976, 1, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.W. Backscattered Electron Imaging: Theory and Applications. Micron 1980, 11, 259–260. [Google Scholar]
- Vogt, T.; Dahmen, W.; Binev, P. Modeling Nanoscale Imaging in Electron; Springer: Manhattan, NY, USA, 2012. [Google Scholar]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths. IX. Data for 41 elemental solids over the 50 eV to 30 keV range. Surf. Interface Anal. 2011, 43, 689–713. [Google Scholar] [CrossRef]
- Hergert, W.; Wriedt, T. The Mie Theory; Springer: Manhattan, NY, USA, 2012; Volume 169. [Google Scholar]
- Eccles, J.W.L.; Bangert, U.; Bromfield, M.; Christian, P.; Harvey, A.J.; Thomas, P. UV-Vis. plasmon studies of metal nanoparticles. J. Phys. 2010, 241. [Google Scholar] [CrossRef]
- Pletcher, D. Instrumental Methods in Electrochemistry; Horwood Publishing: Cambridge, UK, 2001. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westsson, E.; Koper, G.J.M. How to Determine the Core-Shell Nature in Bimetallic Catalyst Particles? Catalysts 2014, 4, 375-396. https://doi.org/10.3390/catal4040375
Westsson E, Koper GJM. How to Determine the Core-Shell Nature in Bimetallic Catalyst Particles? Catalysts. 2014; 4(4):375-396. https://doi.org/10.3390/catal4040375
Chicago/Turabian StyleWestsson, Emma, and Ger J.M. Koper. 2014. "How to Determine the Core-Shell Nature in Bimetallic Catalyst Particles?" Catalysts 4, no. 4: 375-396. https://doi.org/10.3390/catal4040375
APA StyleWestsson, E., & Koper, G. J. M. (2014). How to Determine the Core-Shell Nature in Bimetallic Catalyst Particles? Catalysts, 4(4), 375-396. https://doi.org/10.3390/catal4040375






