LaNi0.3Co0.7O3-δ and SrFe0.2Co0.8O3-δ Ceramic Materials: Structural and Catalytic Reactivity under CO Stream
Abstract
:1. Introduction
2. Results and Discussion
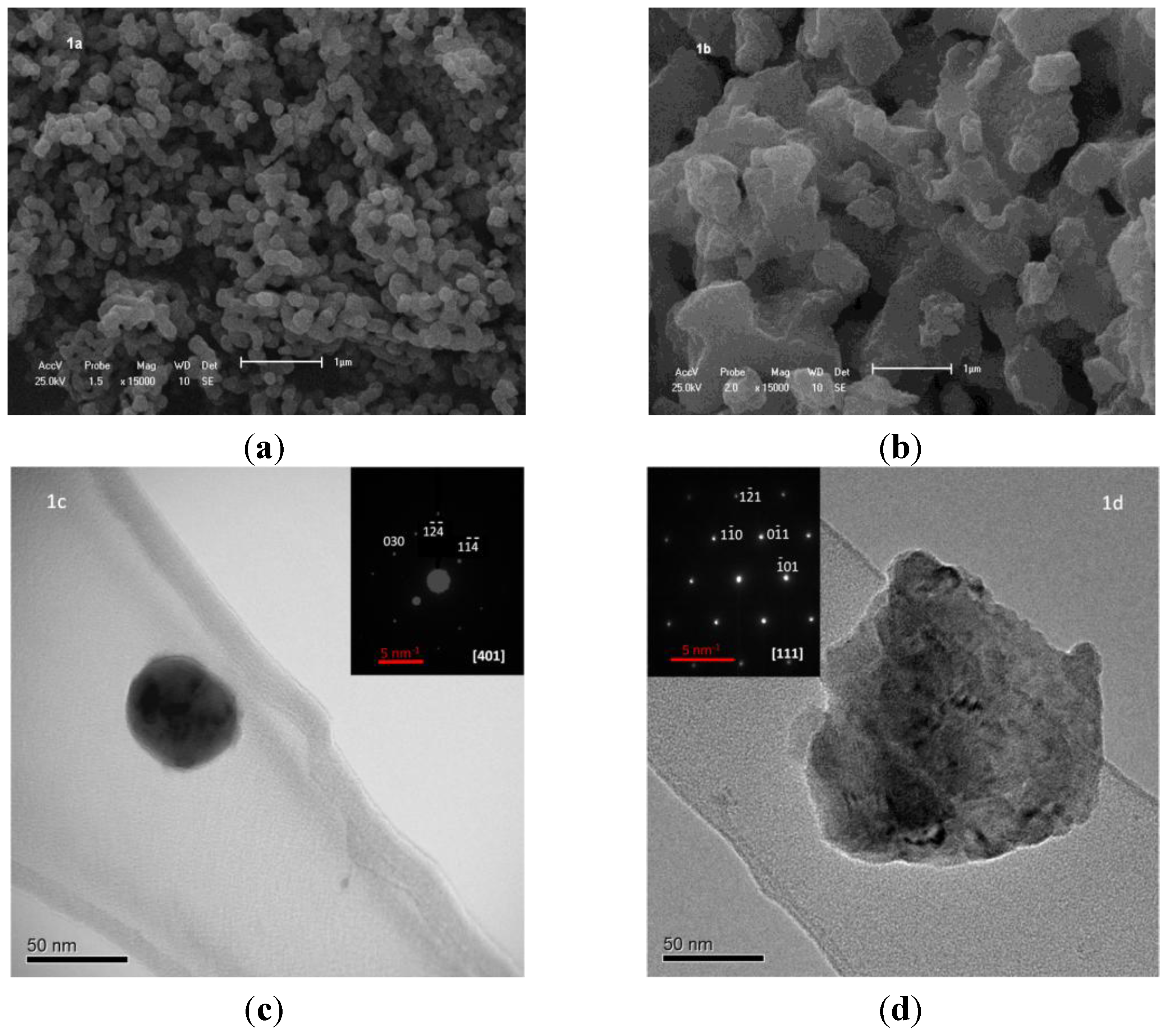
2.1. Structural Analysis: Electron Microscopy and X-ray Diffraction
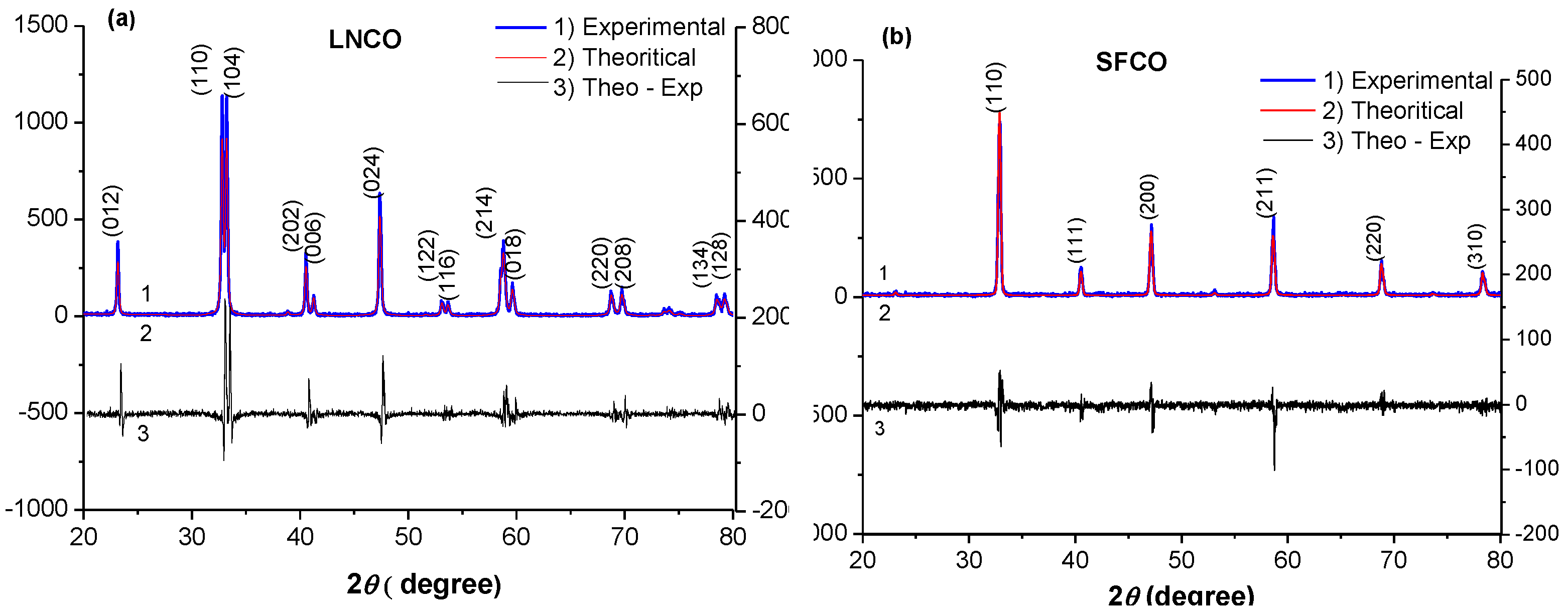
| Samples Crystallographic parameters | LaNi0.3Co0.7O3-δ | SrCo0.8Fe0.2O3-δ |
|---|---|---|
| Crystal structure | Rhombohedral | Cubic |
| Lattice parameters (Å) | ||
| a | 5.4 | 3.8 |
| b | 5.4 | 3.8 |
| c | 13.2 | 3.8 |
| α | 90 | 90 |
| β | 90 | 90 |
| γ | 120 | 90 |
| Volume of cell (Å3) | 340 | 58 |
| Density (gr.cm−3) | 7 | 5 |
| Mean crystallite size (nm) | 37 | 45 |
| Residual parameter S | 1.4 | 1.2 |
| Rp (%) | 13.2 | 12.8 |
| Rwp (%) | 17.3 | 16.7 |
| Rexp (%) | 11.7 | 11.1 |
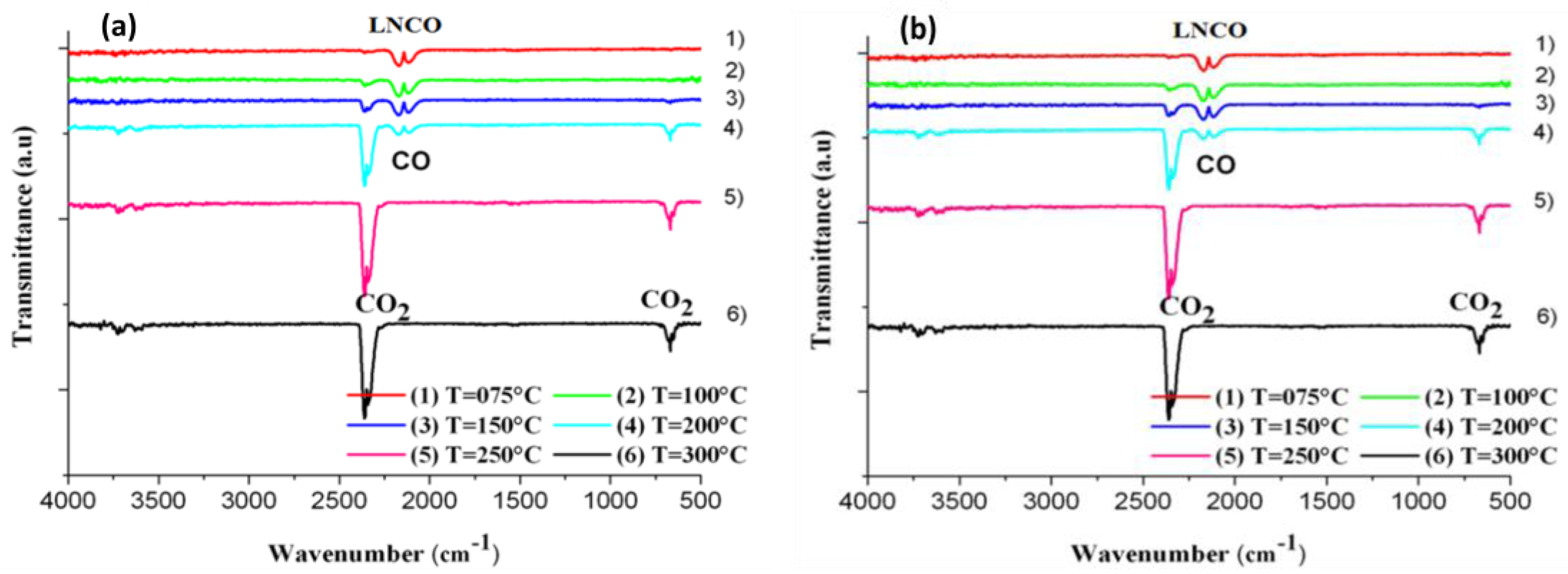
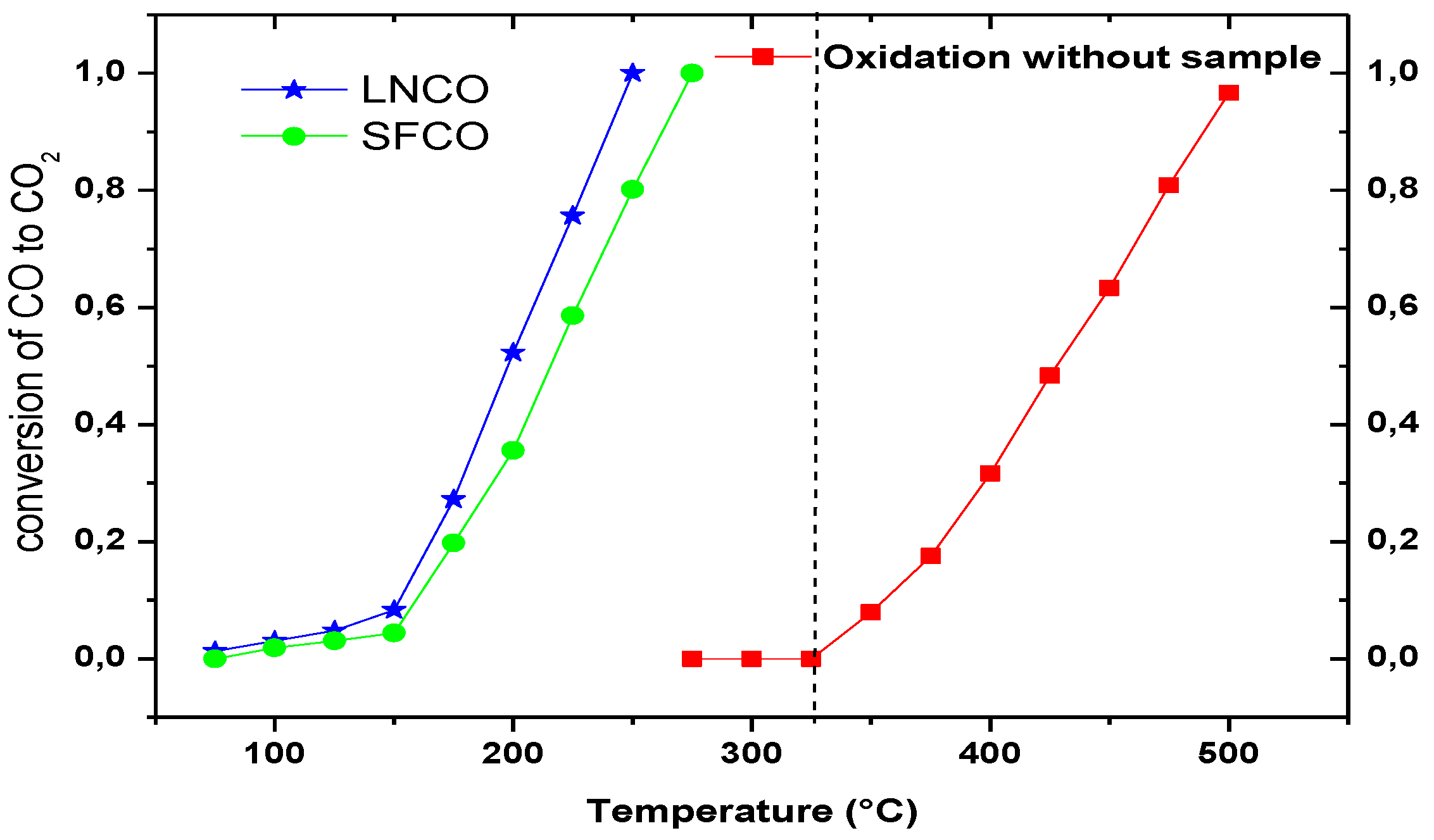
2.2. Catalytic Activity
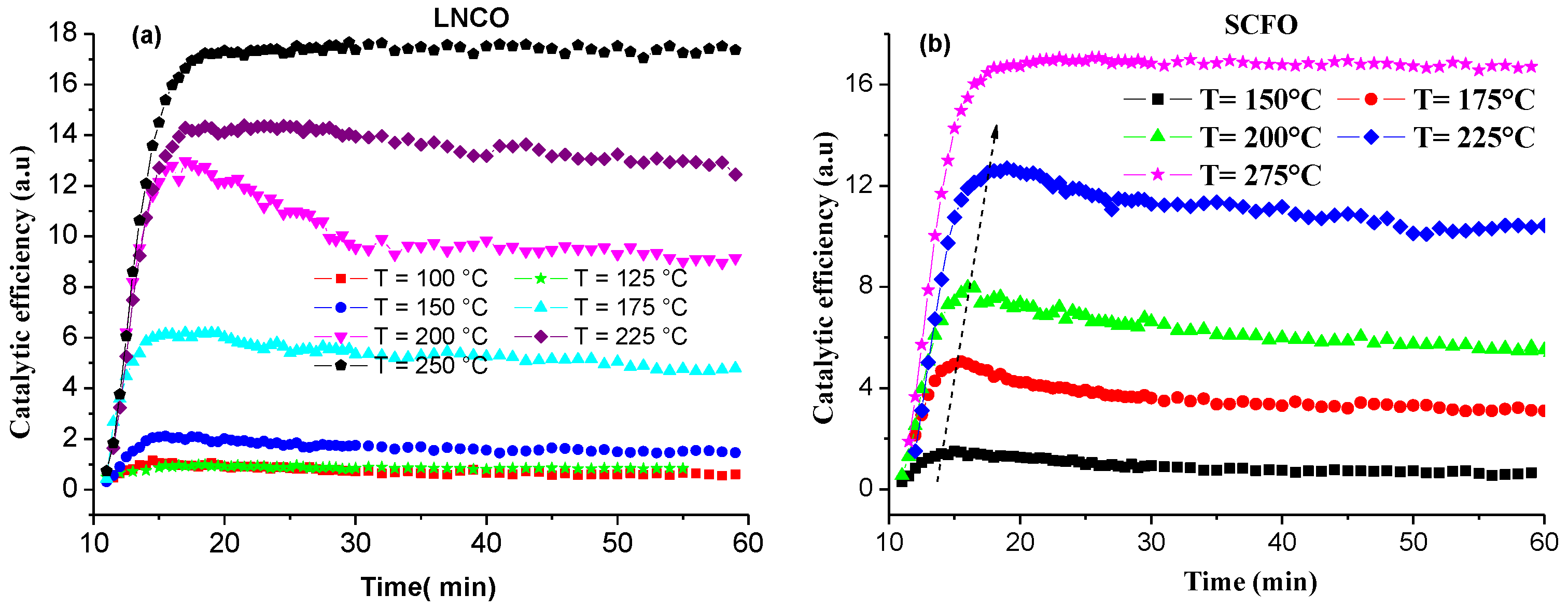
3. Methodology
3.1. Synthesis Method
3.2. Characterization
3.3. Catalytic Activity
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Teraoka, Y.; Zhang, H.-M.; Furukawa, S.; Yamazoi, N. Oxygen permeation through perovskite-type oxides. Chem. Lett. 1985, 14, 1743–1746. [Google Scholar]
- Lu, H.; Cong, Y.; Yang, W.S. Oxygen permeability and stability of Ba0.5Sr0.5Co0.8Fe0.2O3−δ as an oxygen-permeable membrane at high pressures. Solid State Ionics 2006, 177, 595–600. [Google Scholar] [CrossRef]
- Fagg, D.P.; Shaula, A.L.; Kharton, V.V.; Frade, J.R. High oxygen permeability influorite-type Ce0.8Pr0.2O2−δ via the use of sintering aids. J. Membrane Sci. 2007, 299, 1–7. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Pena, M.A.; Yeung, K.L. Hydrogen production from partial oxidation of methane in a membrane reactor. J. Taiwan Inst. Chem. Eng. 2009, 40, 281–288. [Google Scholar] [CrossRef]
- Chen, W.; Chen, C.-H.; Chen, L.W.; Chen, C.; Winnubst, L. Ta-doped SrCo0.8Fe0.2O3−δ membranes: Phase stability and oxygen permeation in CO2 atmosphere. Solid State Ionics 2011, 11, 30–33. [Google Scholar]
- Rui, Z.; Ding, J.; Li, Y.; Lin, S. SrCo0.8Fe0.2O3−δ sorbent for high-temperature production of oxygen-enriched carbon dioxide stream. Fuel 2010, 89, 1429–1434. [Google Scholar] [CrossRef]
- Rajeev, K.P.; Raychaudhuri, A.K. Quantum corrections to theconductivity in a perovskite oxide: A low-temperaturestudy of LaNi1−xCoxO3 (0 ≤ x ≤ 0.75). Phys. Rev. B 1992, 46, 1309–1320. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Sani, N.A.A.; Zein, S.H.S. Synthesis of a TiO2 ceramic membrane containing SrCo0.8Fe0.2O3−δ by the sol-gel method with a wet impregnation process for O2 and N2 permeation. Ceram. Int. 2011, 37, 2981–2989. [Google Scholar] [CrossRef]
- Prado, F.; Grunbaum, N.; Caneiro, A.; Manthiram, A. Effect of La3+ doping on the perovskite-to-brownmillerite transformation in Sr1−xLaxCo0.8Fe0.2O3−δ (0 ≤ x ≤ 0.4). Solid State Ionics 2004, 167, 147–154. [Google Scholar] [CrossRef]
- Chang, L.; Sasirekha, N.; Rajesh, B.; Chen, Y. CO oxidation on ceria- and manganese oxide-supported gold catalysts. Sep. Purif. Technol. 2007, 58, 211–218. [Google Scholar] [CrossRef]
- Soares, A.B.; Silva, P.R.N.; Freitas, J.C.C.; Almeida, C.M. Estudo da oxidação total do etanol usando óxidos tipo perovskita (B = Mn, Ni, Fe). Química Nova 2007, 30, 1061–1066. [Google Scholar] [CrossRef]
- Lima, S.M.; Assaf, J.M. Síntese e caracterização de perovsquitas LaNi1−xCoxO3 como precursores de catalisadores para a conversão do metano a gás de síntese pela reforma com CO2. Química Nova 2007, 30, 298–303. [Google Scholar] [CrossRef]
- Worayingyong, A.; Kangvansura, P.; Ausadasuk, S.; Praserthdam, P. The effect of preparation: Pechini and Schiff base methods, on adsorbed oxygen of LaCoO3 perovskite oxidation catalysts. Colloids Surfaces A 2008, 315, 217–225. [Google Scholar] [CrossRef]
- Stathopoulos, V.N.; Belessi, V.C.; Bakas, T.V.; Neophytides, S.G.; Costa, C.N.; Pomonis, P.J.; Efstathiou, A.M. Comparative study of La-Sr-Fe-O perovskite-type oxides prepared by ceramic and surfactant methods over the CH4 and H2 Lean-de NOx. Appl. Catal. 2009, 93, 1–11. [Google Scholar] [CrossRef]
- Doggali, P.; Kusaba, S.; Teraoka, Y.; Chankapure, P.; Rayalu, S.; Labhsetwar, N. La0.9Ba0.1CoO3 perovskite type catalysts for the control of CO and PM emissions. Catal. Commun. 2010, 11, 665–669. [Google Scholar] [CrossRef]
- Seyfi, B.; Baghalha, M.; Kazemian, H. Modified LaCoO3 nano-perovskite catalysts for the environmental application of automotive CO oxidation. Chem. Eng. J. 2009, 148, 306–311. [Google Scholar] [CrossRef]
- Dreyer, C.; Samanos, B.; Vogt, F. Coke calcination levels and aluminum anode quality. Light Metals (Warrendale) 1996, 4, 535–542. [Google Scholar]
- Garbarino, R.M.; Tonti, R.T. Desulfurization and its effect on calcined cokeproperties. Light Metals (Warrendale) 1993, 4, 517–520. [Google Scholar]
- Harrison, W.T.A.; Lee, T.H.; Yang, Y.L.; Scarfe, D.P.; Liu, L.M.; Jacobson, A.J. Aneutron diffraction study of two strontium cobalt iron oxides. Mater. Res. Bull. 1995, 30, 621–630. [Google Scholar] [CrossRef]
- Liu, L.M.; Lee, T.H.; Yang, Y.L.; Jacobson, A.J.A. themogravimetric study of thephase diagram of strontium cobalt iron oxide, SrCo0.8Fe0.2O3−δ. Mater. Res. Bull. 1996, 31, 29–35. [Google Scholar] [CrossRef]
- Qiu, L.; Lee, T.H.; Liu, L.M.; Yang, Y.L.; Jacobson, A.J. Oxygen permeation studies of SrCo0.8Fe0.203-. Solid State Ionics 1995, 76, 321–329. [Google Scholar] [CrossRef]
- Gopalakrishnan, J.; Colsmann, G.; Reute, B. A Study of LaNi1−xCoxO3 System. Z. Anorg. Allg. Chem. 1976, 424, 155–161. [Google Scholar] [CrossRef]
- McIntosh, S.; Vente, J.F.; Haije, W.G.; Blank, D.H.A.; Bouwmeester, H.J.M. Structure and oxygen stoichiometry of SrCo0.8Fe0.2O3−δ and Ba0.5Sr0.5Co0.8Fe0.2O3−δ. Solid State Ionics 2006, 177, 1737–1742. [Google Scholar] [CrossRef]
- Ertl, G.; Engel, T. Elementary steps in the catalytic oxidation of carbon monoxideon platinum metals. Adv. Catal. 1979, 28, 1–78. [Google Scholar]
- Nowakowski, P.; Dallas, J.P.; Villain, S.; Kopia, A.; Gavarri, J.R. Structure, microstructure, and size dependent catalytic properties of nanostructured ruthenium dioxide. J. Solid State Chem. 2008, 181, 1005–1016. [Google Scholar] [CrossRef]
- Voorhoeve, R.J.H.; Remeika, J.P.; Gallagher, P.K. Perovskite oxides: Materials science in catalysis. Science 1977, 195, 827–833. [Google Scholar]
- Tascon, J.M.D.; Tejuca, L.G. Catalytic activity of pervoskite-type oxides LaMeO3. React. Kinet. Catal. Lett. 1980, 15, 185–191. [Google Scholar] [CrossRef]
- Vaz, T.; Salker, A.V. Preparation, characterization and catalytic CO oxidationstudies on LaNi1−xCoxO3 system. Mater. Sci. Eng. B 2007, 143, 81–84. [Google Scholar] [CrossRef]
- Ren, Y.; Küngas, R.; Gorte, R.J.; Deng, C. The effect of A-site cation (Ln = La, Pr, Sm) on the crystal structure, conductivity and oxygen reduction properties of Sr-doped ferrite perovskites. Solid State Ionics 2012, 212, 47–54. [Google Scholar] [CrossRef]
- Voorhoeve, R.J.H. Advance Materials in Catalysis; Plenum Press: New York, NJ, USA, 1975; Chapter 5. [Google Scholar]
- Montini, T.; Bevilacqua, M.; Fonda, E.; Casula, M.F.; Lee, S.; Tavagnacco, C.; Gorte, R.J.; Fornasiero, P. Relationship between electrical behavior and structural characterization in Sr-doped LaNi0.6Fe0.4O3-δ mixed oxides. Chem. Mater. 2009, 21, 1768–1774. [Google Scholar]
- Shao, Z.; Dong, H.; Xiong, G.; Cong, Y.; Yang, W. Performance of a mixed-conductingceramic membrane reactor with high oxygen permeability for methane conversion. J. Membrane Sci. 2001, 183, 181–192. [Google Scholar] [CrossRef]
- Ghadimi, A.; Alaee, M.A.; Behrouzifar, A.; Asadi, A.A.; Mohammadi, T. Oxygen permeation of BaxSr1−xCo0.8Fe0.2O3−δ perovskite-type membrane: Experimental and modeling. Desalination 2011, 270, 64–75. [Google Scholar]
- Ikeguchi, M.; Yoshino, Y.; Kanie, K.; Nomura, M.; Kikuchi, E.; Matsukata, M. Effects ofpreparation method on oxygen permeation properties of SrFeCo0.5Ox membrane. Sep. Purif. Tecnol. 2003, 32, 313–318. [Google Scholar] [CrossRef]
- Lopes, F.W.B.; Arab, M.; Macedo, H.P.; de Souza, C.P.; de Souza, J.F.; Gavarri, J.R. High temperature conduction and methane conversion capability of BaCeO3 perovskite. Powder Technol. 2012, 219, 186–192. [Google Scholar] [CrossRef]
- Azároff, L.V. Elementsof X-ray Crystallography; McGraw-Hill Book Company: New York, NY, USA, 1968. [Google Scholar]
- Young, R.A. The Rietveld Method, International Union of Crystallography (IUCr); Oxford University Press Inc.: New York, NY, USA, 1995. [Google Scholar]
- Jenkins, R.; Synder, L. Introduction to X-ray Powder Diffractometry; Wiley-Interscience: New York, NY, USA, 1996. [Google Scholar]
- Gualtieri, M.L.; Prudenziati, M.; Gualtieri, A.F. Quantitative determination of the amorphous phase in plasma sprayed alumina coatings using the rietveld method. Surface Coatings Technol. 2006, 201, 2984–2989. [Google Scholar] [CrossRef]
- Martinez, J.R.; Palomares-Sánches, S.; Ortega-Zarzosa, G.; Ruiz, F.; Chumakov, Y. Rietveld refinement of amorphous SiO2 prepared via sol-gel method. Mater. Lett. 2006, 60, 3526–3529. [Google Scholar] [CrossRef]
- Rasberry, S.; Hubbard, C.; Zhang, Y.; McKenzie, R. Certificate of Analysis, Standard Reference Material 660. Instrument Line Position and Profile Shape Standard for X- ray Powder Diffraction; National Institute of Standards Technology Center for Neutron Research: St Louis, MO, USA, 1989. [Google Scholar]
- David, M.; Arab, M.; Guinneton, F.; Flahaut, E.; Bakiz, B.; Nowakowski, P.; Gavarri, J.R. Electrical properties and reactivity under air–CO flows of composite systems based on ceria coated carbon nanotubes. Chem. Eng. J. 2011, 171, 272–278. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dos Santos, A.G.; Arab, M.; Patout, L.; De Souza, C.P. LaNi0.3Co0.7O3-δ and SrFe0.2Co0.8O3-δ Ceramic Materials: Structural and Catalytic Reactivity under CO Stream. Catalysts 2014, 4, 77-88. https://doi.org/10.3390/catal4020077
Dos Santos AG, Arab M, Patout L, De Souza CP. LaNi0.3Co0.7O3-δ and SrFe0.2Co0.8O3-δ Ceramic Materials: Structural and Catalytic Reactivity under CO Stream. Catalysts. 2014; 4(2):77-88. https://doi.org/10.3390/catal4020077
Chicago/Turabian StyleDos Santos, Andarair Gomes, Madjid Arab, Loic Patout, and Carlson Periera De Souza. 2014. "LaNi0.3Co0.7O3-δ and SrFe0.2Co0.8O3-δ Ceramic Materials: Structural and Catalytic Reactivity under CO Stream" Catalysts 4, no. 2: 77-88. https://doi.org/10.3390/catal4020077
APA StyleDos Santos, A. G., Arab, M., Patout, L., & De Souza, C. P. (2014). LaNi0.3Co0.7O3-δ and SrFe0.2Co0.8O3-δ Ceramic Materials: Structural and Catalytic Reactivity under CO Stream. Catalysts, 4(2), 77-88. https://doi.org/10.3390/catal4020077




