Recent Advances in Electrocatalytic Ammonia Synthesis: Integrating Electrolyte Effects, Structural Engineering, and Single-Atom Platforms
Abstract
1. Introduction
- (1)
- alloying to tune the d-band center,
- (2)
- heteroatom doping to modify charge localization,
- (3)
- single-atom site creation for high utilization,
- (4)
- morphology and size control,
- (5)
- nanoconfinement to enhance mass transfer,
- (6)
- tandem catalysis to couple sequential reduction steps.
2. Unified Design Framework for Electrocatalytic Ammonia Synthesis
2.1. Electric Double-Layer Field and Solvation Dynamics
2.2. Structural Engineering Strategies for Enhanced Nitrate Electroreduction
- (i)
- defect engineering to regulate electron density and prevent corrosion,
- (ii)
- integration of catalysts into conductive, binder-free electrodes for better electron transport,
- (iii)
- development of standardized eNO3RR testing protocols to ensure cross-study comparability.
2.3. Single-Atom Catalysis Within Adaptive MOFs
3. Outlook and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ju, H.; Yoon, D.; Bong, S.; Lee, J. Challenge and opportunity in scaling-up hydrogen production via electrochemical ammonia electrolysis process. Curr. Opin. Electrochem. 2025, 49, 101609. [Google Scholar]
- Ju, H.; Kaur, G.; Kulkarni, A.P.; Giddey, S. Challenges and trends in developing technology for electrochemically reducing CO2 in solid polymer electrolyte membrane reactors. J. CO2 Util. 2019, 32, 178–186. [Google Scholar] [CrossRef]
- Cho, M.; Ju, H.; Bae, S.; Bong, S.; Lee, J. Scalable ammonia synthesis on the modified crystal structure of Cu3PS4 electrocatalyst. Electrochim. Acta 2024, 507, 145166. [Google Scholar] [CrossRef]
- Chung, S.; Ju, H.; Choi, M.; Yoon, D.; Lee, J. Local Proton Source Enhanced Nitrogen Reduction on a Combined Cobalt-Molybdenum Catalyst for Electrochemical Ammonia Synthesis. Angew. Chem. Int. Ed. 2022, 61, e202212676. [Google Scholar] [CrossRef]
- Ju, H.; Seo, D.H.; Chung, S.; Mao, X.; An, B.-S.; Musameh, M.; Gengenbach, T.R.; Shon, H.; Du, A.; Bendavid, A.; et al. Green ammonia synthesis using CeO2/RuO2 nanolayers on vertical graphene catalyst via electrochemical route in alkaline electrolyte. Nanoscale 2022, 14, 1395–1408. [Google Scholar]
- Jia, H.-P.; Quadrelli, E.A. Mechanistic aspects of dinitrogen cleavage and hydrogenation to produce ammonia in catalysis and organometallic chemistry: Relevance of metal hydride bonds and dihydrogen. Chem. Soc. Rev. 2013, 43, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Hyung Kim, J.; Cha, J.-E.; Ju, H.; Choi, Y.-W.; Baek, J.; Georg Albers, J.; Shim, J.; Hyung Kim, S.; Lee, K.; Chul Yoon, H. Utilizing water as a proton source for sustainable Li-mediated electrochemical ammonia synthesis. Chem. Eng. J. 2024, 497, 154644. [Google Scholar] [CrossRef]
- Mao, X.; Bai, X.; Wu, G.; Qin, Q.; O’Mullane, A.P.; Jiao, Y.; Du, A. Electrochemical Reduction of N2 to Ammonia Promoted by Hydrated Cation Ions: Mechanistic Insights from a Combined Computational and Experimental Study. J. Am. Chem. Soc. 2024, 146, 18743–18752. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, Y.; Xie, M.; Jin, Z.; Li, P.; Yu, G. Structural engineering of catalysts for ammonia electrosynthesis from nitrate: Recent advances and challenges. EES Catal. 2024, 2, 202–219. [Google Scholar] [CrossRef]
- Niu, Z.; Wang, G. Rational electrocatalyst design for selective nitrate reduction to ammonia. Chem. Phys. Rev. 2025, 6, 011307. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Chen, A.; Yang, S.; Jiang, L.; Sui, Y. 99.7% Faradaic Efficiency in Nitrate Reduction Enabled by Defect-Rich Copper Nanoparticles. ACS Appl. Nano Mater. 2025, 8, 17518–17526. [Google Scholar] [CrossRef]
- Jang, D.; Maeng, J.; Kim, J.; Han, H.; Park, G.H.; Ha, J.; Shin, D.; Hwang, Y.J.; Kim, W.B. Boosting electrocatalytic nitrate reduction reaction for ammonia synthesis by plasma-induced oxygen vacancies over MnCuOx. Appl. Surf. Sci. 2023, 610, 155521. [Google Scholar] [CrossRef]
- Shan, L.; Ma, Y.; Xu, S.; Zhou, M.; He, M.; Sheveleva, A.M.; Cai, R.; Lee, D.; Cheng, Y.; Tang, B.; et al. Efficient electrochemical reduction of nitrate to ammonia over metal-organic framework single-atom catalysts. Commun. Mater. 2024, 5, 104. [Google Scholar]
- Zhang, K.; Liu, Y.; Pan, Z.; Xia, Q.; Huo, X.; Christopher Esan, O.; Zhang, X.; An, L. Cu-based catalysts for electrocatalytic nitrate reduction to ammonia: Fundamentals and recent advances. EES Catal. 2024, 2, 727–752. [Google Scholar] [CrossRef]
- Cao, Y.; Yuan, S.; Meng, L.; Wang, Y.; Hai, Y.; Su, S.; Ding, W.; Liu, Z.; Li, X.; Luo, M. Recent Advances in Electrocatalytic Nitrate Reduction to Ammonia: Mechanism Insight and Catalyst Design. ACS Sustain. Chem. Eng. 2023, 11, 7965–7985. [Google Scholar] [CrossRef]
- Zhang, K.; Zou, X.; Liu, Y.; Zhang, X.; An, L. Progress and perspectives in the electroreduction of low-concentration nitrate for wastewater management. iScience 2025, 28, 111687. [Google Scholar]
- Ju, H.; Sanglay, G.D.; Bae, S.; Cho, M.; Ocon, J.D.; Jeong, B.; Bong, S.; Lee, J. Synthesis of 2D Nanosheet FePS3 Electrocatalyst via Salt-Template Method for Electrochemical Green Ammonia Production. J. Electrochem. Sci. Technol. 2025, 16, 180–187. [Google Scholar]
- Hao, Y.-C.; Guo, Y.; Chen, L.-W.; Shu, M.; Wang, X.-Y.; Bu, T.-A.; Gao, W.-Y.; Zhang, N.; Su, X.; Feng, X.; et al. Promoting nitrogen electroreduction to ammonia with bismuth nanocrystals and potassium cations in water. Nat. Catal. 2019, 2, 448–456. [Google Scholar] [CrossRef]
- Duan, F.; Chen, J.; Zhang, M.; Liu, Y.; Xue, H.; Sun, Y.; Li, Q.; Zhang, X.; Gao, Z.; Lu, Z.; et al. Lithium-Mediated Ammonia Electrosynthesis over Orderly Arranged Dipoles Regulated Solid-Electrolyte Interphase. J. Am. Chem. Soc. 2025, 147, 24317–24325. [Google Scholar]
- Paliwal, A.; Bandas, C.D.; Thornburg, E.S.; Haasch, R.T.; Gewirth, A.A. Enhanced Nitrate Reduction Activity from Cu-Alloy Electrodes in an Alkaline Electrolyte. ACS Catal. 2023, 13, 6754–6762. [Google Scholar] [CrossRef]
- Yu, S.; Levell, Z.; Jiang, Z.; Zhao, X.; Liu, Y. What Is the Rate-Limiting Step of Oxygen Reduction Reaction on Fe–N–C Catalysts? J. Am. Chem. Soc. 2023, 145, 25352–25356. [Google Scholar] [CrossRef]
- Bai, X.; He, M.; Xu, Y.; Xu, B.; Lu, Q.; Wang, J.; Ling, C. New Mechanistic Insights into CO2/CO Electroreduction to Acetate by Combining Computations and Experiments. ACS Catal. 2024, 14, 3171–3180. [Google Scholar] [CrossRef]
- Bai, X.; Zhao, X.; Zhang, Y.; Ling, C.; Zhou, Y.; Wang, J.; Liu, Y. Dynamic Stability of Copper Single-Atom Catalysts under Working Conditions. J. Am. Chem. Soc. 2022, 144, 17140–17148. [Google Scholar] [CrossRef] [PubMed]
- Mähler, J.; Persson, I. A Study of the Hydration of the Alkali Metal Ions in Aqueous Solution. Inorg. Chem. 2012, 51, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Jiao, Y.; Huang, J.; Chen, S. Electric Double Layer Effects in Electrocatalysis: Insights from Ab Initio Simulation and Hierarchical Continuum Modeling. JACS Au 2023, 3, 2640–2659. [Google Scholar] [CrossRef]
- Rana, R.; Ali, S.M.; Maity, D.K. Structure and dynamics of the Li+ ion in water, methanol and acetonitrile solvents: Ab initio molecular dynamics simulations. Phys. Chem. Chem. Phys. 2023, 25, 31382–31395. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, M.; Eslamibidgoli, M.J.; Eikerling, M.; Groß, A. Cation Overcrowding Effect on the Oxygen Evolution Reaction. JACS Au 2021, 1, 1752–1765. [Google Scholar] [CrossRef]
- Popov, I.; Zhu, Z.; Young-Gonzales, A.R.; Sacci, R.L.; Mamontov, E.; Gainaru, C.; Paddison, S.J.; Sokolov, A.P. Search for a Grotthuss mechanism through the observation of proton transfer. Commun. Chem. 2023, 6, 77. [Google Scholar] [CrossRef]
- Cioncoloni, G.; Roger, I.; Wheatley, P.S.; Wilson, C.; Morris, R.E.; Sproules, S.; Symes, M.D. Proton-Coupled Electron Transfer Enhances the Electrocatalytic Reduction of Nitrite to NO in a Bioinspired Copper Complex. ACS Catal. 2018, 8, 5070–5084. [Google Scholar] [CrossRef]
- Li, P.; Jiang, Y.-L.; Men, Y.; Jiao, Y.-Z.; Chen, S. Kinetic cation effect in alkaline hydrogen electrocatalysis and double layer proton transfer. Nat. Commun. 2025, 16, 1844. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Ruan, Y.; Iftikhar, R.; Khan, F.Z.; Li, W.; Hao, L.; Robertson, A.W.; Percoco, G.; Sun, Z. Lithium-mediated electrochemical dinitrogen reduction reaction. Ind. Chem. Mater. 2023, 1, 563–581. [Google Scholar] [CrossRef]
- Xie, W.; Zhong, L.; Zeng, H.; Wang, X.; Xiao, Y.; Cheng, B.; Lei, S. MIL-88-derived porous carbon rod-supported FePS3 nanosheet arrays for robust sodium storage. J. Energy Storage 2025, 105, 114799. [Google Scholar] [CrossRef]
- Huang, H.; Song, J.; Yu, D.; Hao, Y.; Wang, Y.; Peng, S. Few-layer FePS3 decorated with thin MoS2 nanosheets for efficient hydrogen evolution reaction in alkaline and acidic media. Appl. Surf. Sci. 2020, 525, 146623. [Google Scholar] [CrossRef]
- Aissa, M.A.B.; Tremblay, B.; Andrieux-Ledier, A.; Maisonhaute, E.; Raouafi, N.; Courty, A. Copper nanoparticles of well-controlled size and shape: A new advance in synthesis and self-organization. Nanoscale 2015, 7, 3189–3195. [Google Scholar] [CrossRef] [PubMed]
- Devautour-Vinot, S.; Maurin, G.; Serre, C.; Horcajada, P.; Paula da Cunha, D.; Guillerm, V.; de Souza Costa, E.; Taulelle, F.; Martineau, C. Structure and Dynamics of the Functionalized MOF Type UiO-66(Zr): NMR and Dielectric Relaxation Spectroscopies Coupled with DFT Calculations. Chem. Mater. 2012, 24, 2168–2177. [Google Scholar] [CrossRef]
- Fujita, S.; Yamaguchi, S.; Yamasaki, J.; Nakajima, K.; Yamazoe, S.; Mizugaki, T.; Mitsudome, T. Ni2P Nanoalloy as an Air-Stable and Versatile Hydrogenation Catalyst in Water: P-Alloying Strategy for Designing Smart Catalysts. Chem.–A Eur. J. 2021, 27, 4439–4446. [Google Scholar] [CrossRef]
- Tan, W.; Zhao, H.; Ding, L.; Ren, N.; Yu, X.; Wang, A.; Zhao, M. Advancements in Electrocatalytic Nitrogen Reduction Reaction: A Review on the Role of Catalyst Electronic Structure and Design Strategies. ACS Appl. Nano Mater. 2025, 8, 2632–2651. [Google Scholar] [CrossRef]
- Zhou, P.; Niu, P.; Liu, J.; Zhang, N.; Bai, H.; Chen, M.; Feng, J.; Liu, D.; Wang, L.; Chen, S.; et al. Anodized Steel: The Most Promising Bifunctional Electrocatalyst for Alkaline Water Electrolysis in Industry. Adv. Funct. Mater. 2022, 32, 2202068. [Google Scholar] [CrossRef]
- Yu, W.; Yu, J.; Huang, M.; Wang, Y.; Wang, Y.; Li, J.; Liu, H.; Zhou, W. Laser-controlled tandem catalytic sites of CuNi alloys with ampere-level electrocatalytic nitrate-to-ammonia reduction activities for Zn–nitrate batteries. Energy Environ. Sci. 2023, 16, 2991–3001. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Jia, R.; Yu, Y.; Zhang, B. Unveiling the Activity Origin of a Copper-based Electrocatalyst for Selective Nitrate Reduction to Ammonia. Angew. Chem. Int. Ed. 2020, 59, 5350–5354. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S.Z. Heteroatom-Doped Graphene-Based Materials for Energy-Relevant Electrocatalytic Processes. ACS Catal. 2015, 5, 5207–5234. [Google Scholar] [CrossRef]
- Chen, W.; Ge, C.; Li, J.T.; Beckham, J.L.; Yuan, Z.; Wyss, K.M.; Advincula, P.A.; Eddy, L.; Kittrell, C.; Chen, J.; et al. Heteroatom-Doped Flash Graphene. ACS Nano 2022, 16, 6646–6656. [Google Scholar] [CrossRef]
- Li, C.; Zhu, Q.; Song, C.; Zeng, Y.; Zheng, Y. Electrocatalysts for Urea Synthesis from CO2 and Nitrogenous Species: From CO2 and N2/NOxReduction to urea synthesis. ChemSusChem 2024, 17, e202401333. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Mao, J. Effect of N-doping on graphene: NRR activity and N-source. Diam. Relat. Mater. 2021, 118, 108494. [Google Scholar] [CrossRef]
- Zhu, C.; Fu, S.; Shi, Q.; Du, D.; Lin, Y. Single-Atom Electrocatalysts. Angew. Chem. Int. Ed. 2017, 56, 13944–13960. [Google Scholar]
- Yang, F.; Song, P.; Ge, X.; Wang, Y.; Gunji, T.; Zhang, W.; Zhao, X.; Xu, W. Operando analysis reveals potential-driven in situ formation of single-Fe-atom electrocatalysts for green production of ammonia. Proc. Natl. Acad. Sci. USA 2023, 120, e2301011120. [Google Scholar] [PubMed]
- Zhang, P.; Chen, H.-C.; Zhu, H.; Chen, K.; Li, T.; Zhao, Y.; Li, J.; Hu, R.; Huang, S.; Zhu, W.; et al. Inter-site structural heterogeneity induction of single atom Fe catalysts for robust oxygen reduction. Nat. Commun. 2024, 15, 2062. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, J.; Zheng, M.; Jin, X.; Shen, Z.; Li, Z.; Wang, Y.; Wang, Q.; Wang, X.; Wei, H.; et al. Fe/Cu diatomic catalysts for electrochemical nitrate reduction to ammonia. Nat. Commun. 2023, 14, 3634. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.; Luo, D.; Zhang, S.; Kong, L.; Xia, H.; Xie, Q.; Xu, G.; Chen, Z.; Sun, Z. Stabilizing Solid Electrolyte Interphase on Liquid Metal Via Dynamic Hydrogel-Derived Carbon Framework Encapsulation. Adv. Mater. 2024, 36, 2401234. [Google Scholar]
- Li, L.; Jiang, Y.-F.; Zhang, T.; Cai, H.; Zhou, Y.; Lin, B.; Lin, X.; Zheng, Y.; Zheng, L.; Wang, X.; et al. Size sensitivity of supported Ru catalysts for ammonia synthesis: From nanoparticles to subnanometric clusters and atomic clusters. Chem 2022, 8, 749–768. [Google Scholar] [CrossRef]
- Peng, J. Toward data-driven predictive modeling of electrocatalyst stability and surface reconstruction. J. Chem. Phys. 2025, 163, 040902. [Google Scholar] [CrossRef] [PubMed]
- Zybert, M.; Tarka, A.; Patkowski, W.; Ronduda, H.; Mierzwa, B.; Kępiński, L.; Raróg-Pilecka, W. Structure Sensitivity of Ammonia Synthesis on Cobalt: Effect of the Cobalt Particle Size on the Activity of Promoted Cobalt Catalysts Supported on Carbon. Catalysts 2022, 12, 1285. [Google Scholar] [CrossRef]
- Duca, M.; Weeks, J.R.; Fedor, J.G.; Weiner, J.H.; Vincent, K.A. Combining Noble Metals and Enzymes for Relay Cascade Electrocatalysis of Nitrate Reduction to Ammonia at Neutral pH. ChemElectroChem 2015, 2, 1086–1089. [Google Scholar] [CrossRef]
- Milton, R.D.; Minteer, S.D. Enzymatic Bioelectrosynthetic Ammonia Production: Recent Electrochemistry of Nitrogenase, Nitrate Reductase, and Nitrite Reductase. ChemPlusChem 2017, 82, 513–521. [Google Scholar] [CrossRef]
- Cook, A.R.; Dimitrijevic, N.; Dreyfus, B.W.; Meisel, D.; Curtiss, L.A.; Camaioni, D.M. Reducing Radicals in Nitrate Solutions. The NO32− System Revisited. J. Phys. Chem. A 2001, 105, 3658–3666. [Google Scholar]
- Zhang, R.; Guo, Y.; Zhang, S.; Chen, D.; Zhao, Y.; Huang, Z.; Ma, L.; Li, P.; Yang, Q.; Liang, G.; et al. Efficient Ammonia Electrosynthesis and Energy Conversion through a Zn-Nitrate Battery by Iron Doping Engineered Nickel Phosphide Catalyst. Adv. Energy Mater. 2022, 12, 2103872. [Google Scholar]
- Ma, Y.; Han, X.; Xu, S.; Wang, Z.; Li, W.; da Silva, I.; Chansai, S.; Lee, D.; Zou, Y.; Nikiel, M.; et al. Atomically Dispersed Copper Sites in a Metal–Organic Framework for Reduction of Nitrogen Dioxide. J. Am. Chem. Soc. 2021, 143, 10977–10985. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Ren, J.; Xue, Y.; Tian, J. In Situ Construction of Cu1+/Cu0 and Cu2+/Cu0 Pairs of Cu-Based Catalysts for Electrocatalytic Nitrate Reduction. Adv. Sci. 2025, e17773. [Google Scholar] [CrossRef]
- Chen, G.-F.; Yuan, Y.; Jiang, H.; Ren, S.-Y.; Ding, L.-X.; Ma, L.; Wu, T.; Lu, J.; Wang, H. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 2020, 5, 605–613. [Google Scholar]
- Jiao, L.; Wan, G.; Zhang, R.; Zhou, H.; Yu, S.-H.; Jiang, H.-L. From Metal–Organic Frameworks to Single-Atom Fe Implanted N-doped Porous Carbons: Efficient Oxygen Reduction in Both Alkaline and Acidic Media. Angew. Chem. Int. Ed. 2018, 57, 8525–8529. [Google Scholar]
- Yin, P.; Yao, T.; Wu, Y.; Zheng, L.; Lin, Y.; Liu, W.; Ju, H.; Zhu, J.; Hong, X.; Deng, Z.; et al. Single Cobalt Atoms with Precise N-Coordination as Superior Oxygen Reduction Reaction Catalysts. Angew. Chem. Int. Ed. 2016, 55, 10800–10805. [Google Scholar] [CrossRef] [PubMed]
- Kottwitz, M.; Li, Y.; Wang, H.; Frenkel, A.I.; Nuzzo, R.G. Single Atom Catalysts: A Review of Characterization Methods. Chem.-Methods 2021, 1, 278–294. [Google Scholar] [CrossRef]
- Sarma, B.B.; Maurer, F.; Doronkin, D.E.; Grunwaldt, J.-D. Design of Single-Atom Catalysts and Tracking Their Fate Using Operando and Advanced X-ray Spectroscopic Tools. Chem. Rev. 2023, 123, 379–444. [Google Scholar] [CrossRef]
- Dang, S.; Zhu, Q.-L.; Xu, Q. Nanomaterials derived from metal–organic frameworks. Nat. Rev. Mater. 2017, 3, 17075. [Google Scholar] [CrossRef]
- Tomboc, G.M.; Kim, T.; Jung, S.; Yoon, H.J.; Lee, K. Modulating the Local Coordination Environment of Single-Atom Catalysts for Enhanced Catalytic Performance in Hydrogen/Oxygen Evolution Reaction. Small 2022, 18, 2105680. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, L.; Jiang, H.; Zhang, Y.; Cao, D.; Wu, C.; Zhang, G.; Jiang, J.; Song, L. Boosted Reactivity of Ammonia Borane Dehydrogenation over Ni/Ni2P Heterostructure. J. Phys. Chem. Lett. 2019, 10, 1048–1054. [Google Scholar] [CrossRef]
- Lou, Y.; He, J.; Liu, G.; Qi, S.; Cheng, L.; Chen, J.; Zhao, Y.; Zhu, J.-J. Efficient hydrogen evolution from the hydrolysis of ammonia borane using bilateral-like WO3−x nanorods coupled with Ni2P nanoparticles. Chem. Commun. 2018, 54, 6188–6191. [Google Scholar]
- Gomes, K.L.; Winkler, M.E.G.; Sales, M.P.; Souza Junior, J.B.; Bonacin, J.A.; Nagao, R. Electrocatalytic Nitrate Reduction to Ammonia Using Co3O4 Nanowires Supported on TiO2/Ti. ACS Appl. Energy Mater. 2025, 8, 15993–16001. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, Y.; Gao, Y.; Sun, M.; Yao, Y.; Yu, H.; Xu, Y.; Wang, X.; Yang, Y. Electroreduction of nitrate into ammonia on Co3O4: Mechanistic insights into Co2+-promoted NO3RR performance. Chem. Eng. J. 2025, 512, 162506. [Google Scholar] [CrossRef]
- Murphy, E.; Sun, B.; Rüscher, M.; Liu, Y.; Zang, W.; Guo, S.; Chen, Y.-H.; Hejral, U.; Huang, Y.; Ly, A.; et al. Synergizing Fe2O3 Nanoparticles on Single Atom Fe-N-C for Nitrate Reduction to Ammonia at Industrial Current Densities. Adv. Mater. 2024, 36, 2401133. [Google Scholar] [CrossRef]
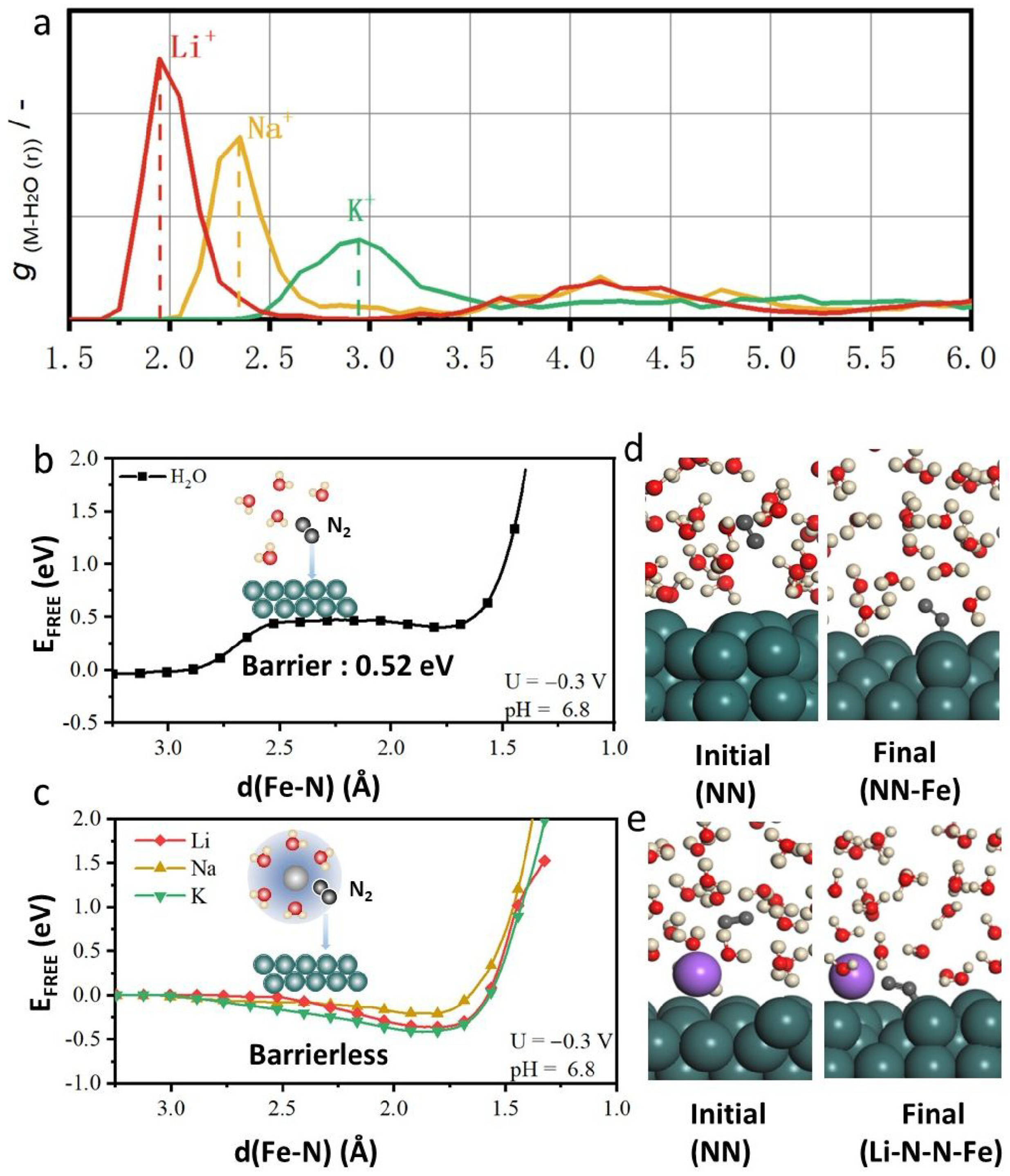
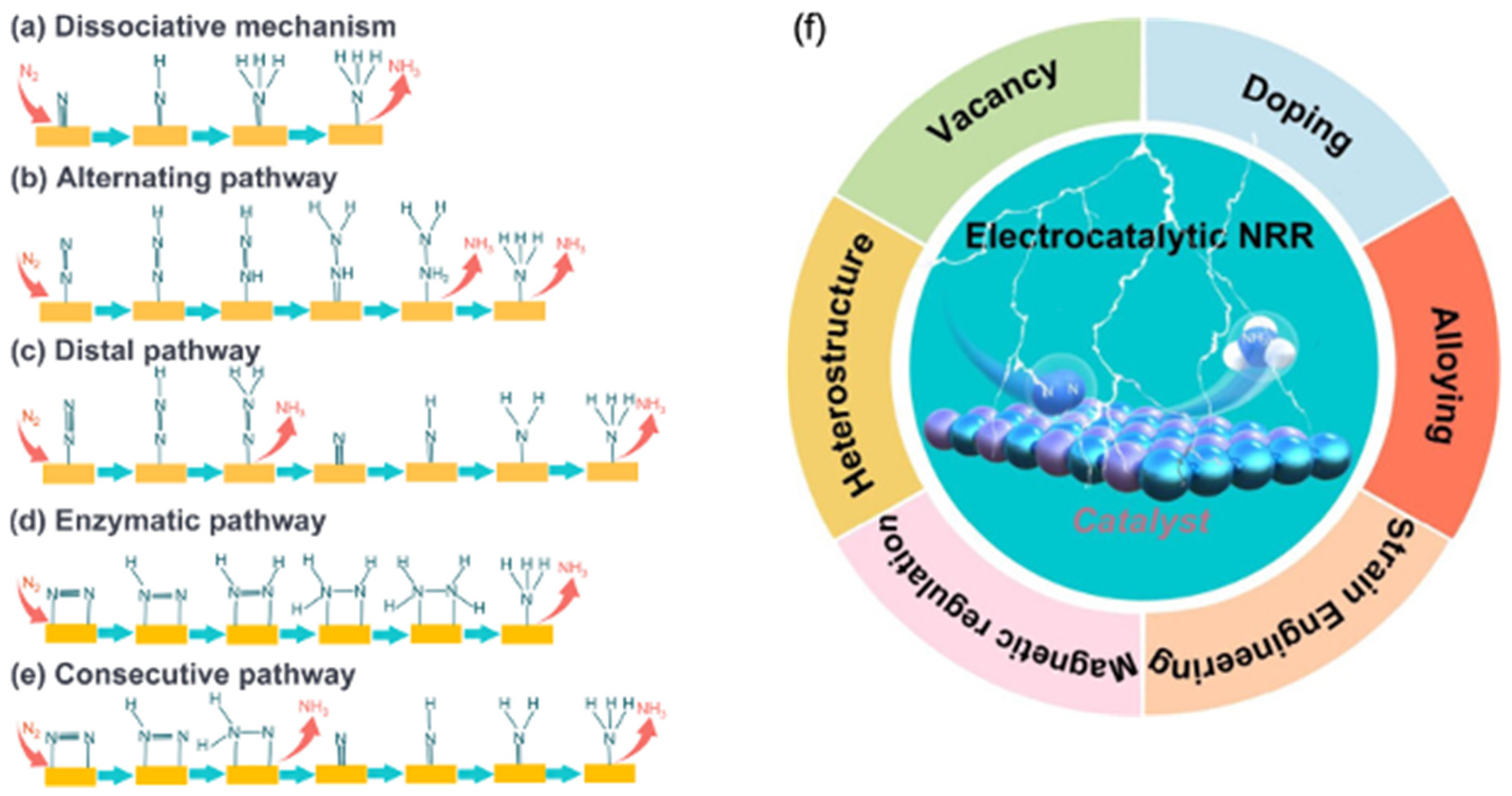
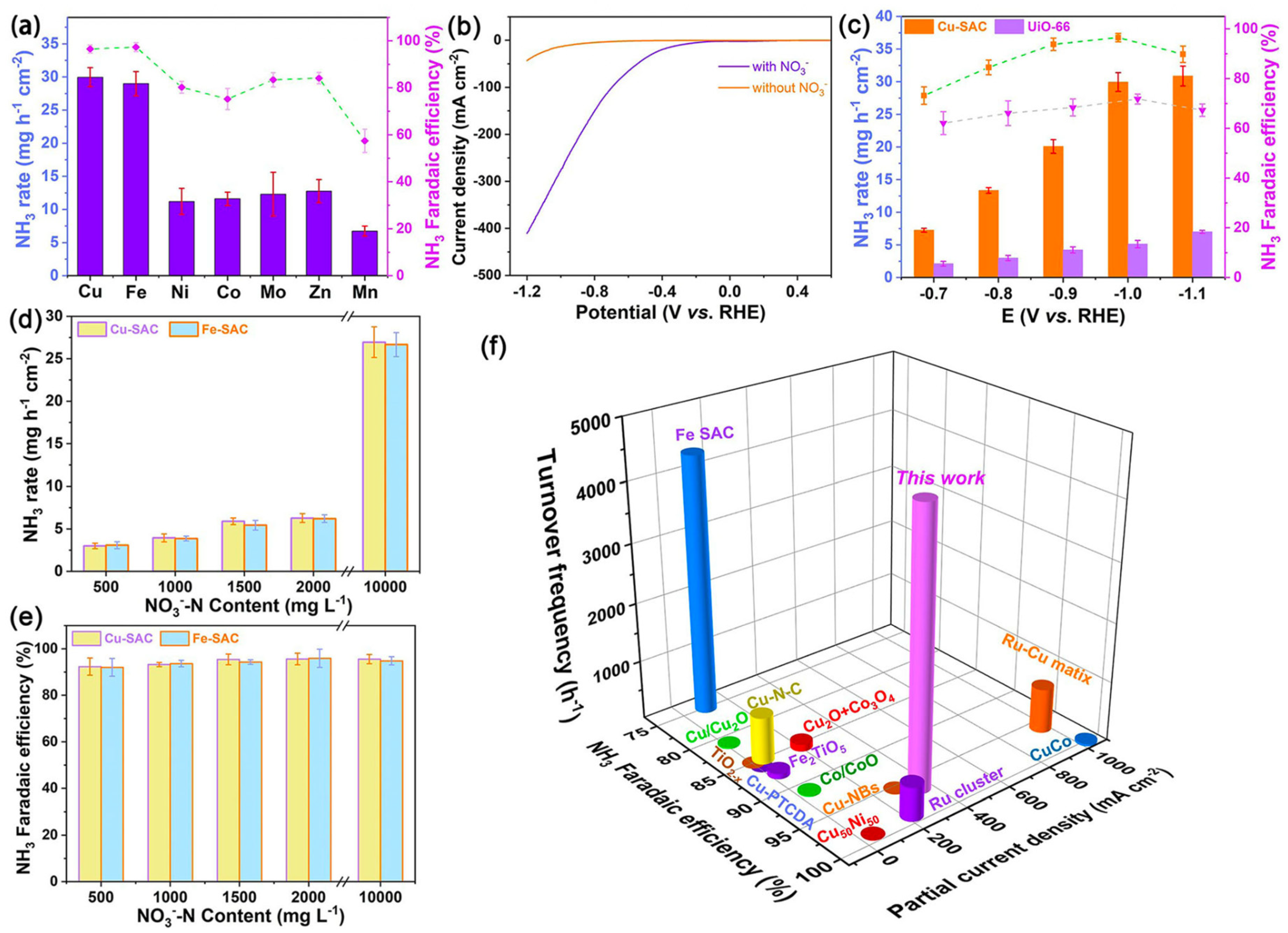
| No. | Pathway | Catalyst | Electrolyte/Cell | Current Density (mA cm−2) | Faradaic Efficiency (%) | NH3 Yield Rate (µg h−1 mg−1) | Key Feature | Verifiable Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | eNRR | Fe electrode (Li+-mediated) | Li2SO4 (aq) | ~20 | 27.9 | ~23 | Hydrated Li+ EDL effect | [8] |
| 2 | eNRR | RuO2/CeO2 on graphene | KOH (aq) | ~35 | 22–25 | ~30 | Oxide interface synergy | [5] |
| 3 | eNRR | Cu3PS4 | KOH (aq) | ~40 | ~18 | ~35 | Crystal-structure tuning | [3] |
| 4 | eNRR | FePS3 nanosheets | KOH (aq) | ~25 | ~21 | ~28 | 2D confinement | [17] |
| 5 | eNRR | Bi nanocrystals | KOH (aq) | ~15 | ~10 | ~12 | Cation-assisted N2 activation | [18] |
| 6 | eNRR | Li-mediated NRR (SEI-controlled) | Organic electrolyte | ~50 | ~60 | ~40 | SEI-regulated pathway | [19] |
| 7 | eNRR | Co–Mo catalyst | Neutral electrolyte | ~18 | ~25 | ~20 | Local proton source | [4] |
| 8 | eNRR | Cu–Ag alloy | KOH (aq) | ~30 | ~20 | ~26 | Alloy electronic tuning | [20] |
| 9 | eNRR | Fe–N–C SAC | Alkaline | ~22 | ~24 | ~24 | Single-atom Fe sites | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Ju, H.; Lee, H.J.; Bong, S. Recent Advances in Electrocatalytic Ammonia Synthesis: Integrating Electrolyte Effects, Structural Engineering, and Single-Atom Platforms. Catalysts 2026, 16, 149. https://doi.org/10.3390/catal16020149
Ju H, Lee HJ, Bong S. Recent Advances in Electrocatalytic Ammonia Synthesis: Integrating Electrolyte Effects, Structural Engineering, and Single-Atom Platforms. Catalysts. 2026; 16(2):149. https://doi.org/10.3390/catal16020149
Chicago/Turabian StyleJu, HyungKuk, Hyuck Jin Lee, and Sungyool Bong. 2026. "Recent Advances in Electrocatalytic Ammonia Synthesis: Integrating Electrolyte Effects, Structural Engineering, and Single-Atom Platforms" Catalysts 16, no. 2: 149. https://doi.org/10.3390/catal16020149
APA StyleJu, H., Lee, H. J., & Bong, S. (2026). Recent Advances in Electrocatalytic Ammonia Synthesis: Integrating Electrolyte Effects, Structural Engineering, and Single-Atom Platforms. Catalysts, 16(2), 149. https://doi.org/10.3390/catal16020149










