Synergistic Induction by Deep Eutectic Solvent and Carbon Dots for Rapid Construction of FeOOH Electrocatalysts Toward Efficient Oxygen Evolution Reaction
Abstract
1. Introduction
2. Results and Discussion
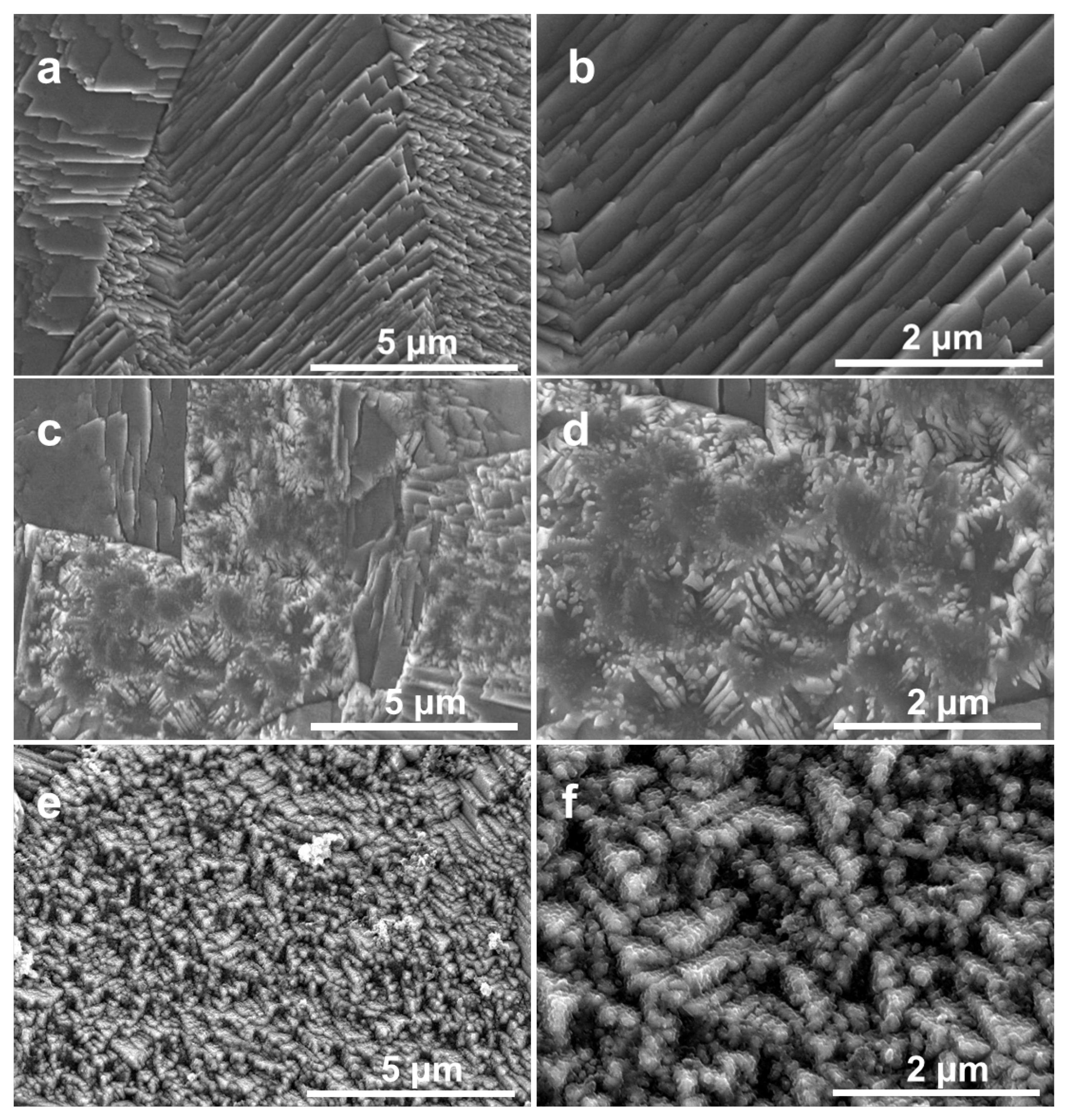
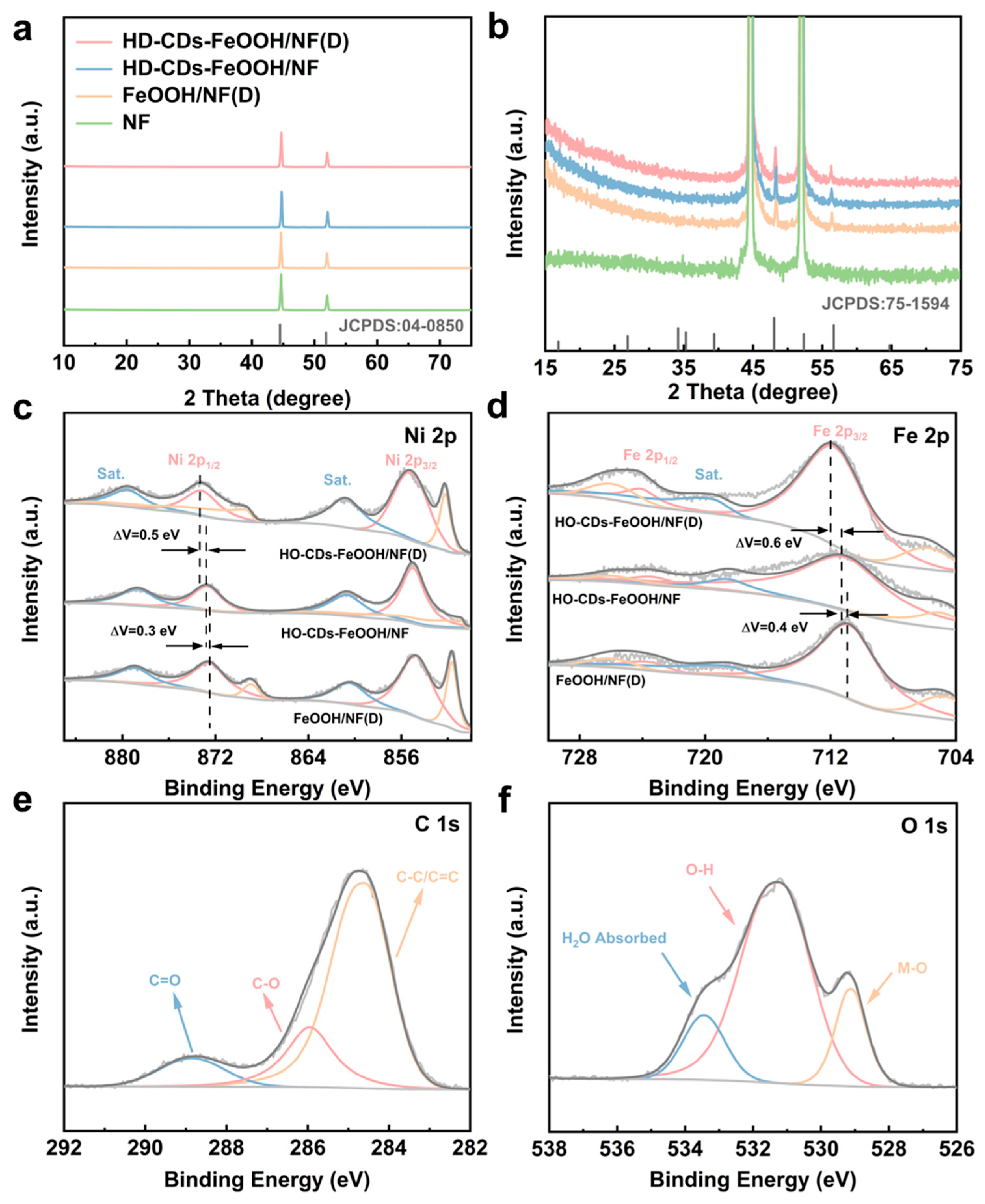
2.1. Morphological Structure Analysis
2.2. Electrocatalytic OER Performance
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Characterization
3.4. Electrochemical Performance Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Weitensfelder, L.; Moshammer, H.; Ataniyazova, O. Energy Consumption, Energy Distribution, and Clean Energy Use Together Affect Life Expectancy. Sustainability 2024, 16, 678. [Google Scholar] [CrossRef]
- Jithin, P.; Renjith, R. Towards sustainable energy access: Investigating the relationship between renewable energy consumption and energy poverty. Energy Policy 2025, 100, 114553. [Google Scholar]
- Bergougui, B.; Ben-Salha, O. The Impact of Environmental Governance on Energy Transitions: Evidence from a Global Perspective. Sustainability 2025, 17, 8759. [Google Scholar] [CrossRef]
- Xu, X.X.; Lieuea, B. Contribution of modern renewables to final energy consumption, energy intensity, and environmental quality: The role of world energy balances, economic performance, and shadow economies. Energy 2025, 332, 137191. [Google Scholar] [CrossRef]
- Ullah, S.; Sohail, S.; Majeed, M.T.; Sohail, M.T. Future of hydrogen energy: The role of environmental policy and political globalization. Int. J. Hydrogen Energy 2025, 122, 12–21. [Google Scholar] [CrossRef]
- Osman, S.H.; Yatim, N.S.M.; Elham, O.S.J.; Shaari, N.; Zakaria, Z. Three decades of hydrogen energy research: A bibliometric analysis on the evolution of green hydrogen technologies. Sustain. Energy Fuels 2025, 9, 3182–3202. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, Y.Q. Risk assessment of zero-carbon hydrogen energy storage systems coupled with renewable energy power generation systems. Int. J. Hydrogen Energy 2025, 149, 150141. [Google Scholar] [CrossRef]
- Ribeiro, B.; Baptista, J.; Cerveira, A. Optimizing Renewable Microgrid Performance Through Hydrogen Storage Integration. Algorithms 2025, 18, 656. [Google Scholar] [CrossRef]
- Lu, T.G.; Yi, Y.N.; Li, J.; Wu, S.C. Collaborative planning of integrated hydrogen energy chain multi-energy systems: A review. Appl. Energy. 2025, 393, 126019. [Google Scholar] [CrossRef]
- Yang, J.Z.; Lam, T.Y.; Luo, Z.X.; Cheng, Q.; Wang, G.B.; Yao, H. Renewable energy driven electrolysis of water for hydrogen production, storage, and transportation. Renew. Sustain. Energy Rev. 2025, 218, 115804. [Google Scholar] [CrossRef]
- Iamarino, M.; D’Angola, A. Trends, Challenges, and Viability in Green Hydrogen Initiatives. Energies 2025, 18, 4476. [Google Scholar] [CrossRef]
- Cai, M.X.; Zhang, Y.R.; He, P.L.; Zhang, Z.C. Recent Advances in Revealing the Electrocatalytic Mechanism for Hydrogen Energy Conversion System. Small 2024, 20, 2405008. [Google Scholar] [CrossRef]
- Vadivel, N.; Murthy, A.P. Recent Developments in Membrane-Free Hybrid Water Electrolysis for Low-Cost Hydrogen Production Along with Value-Added Products. Small 2024, 20, 2407845. [Google Scholar]
- Wang, H.J.; Su, R.; Liu, Y.Y.; Kong, Y.; Ren, Z.J.; Jiang, B. Iron-group Metal Compound Electrocatalysts for Efficient Hydrogen Production: Recent Advances and Future Prospects. ChemCatChem 2024, 16, e202301241. [Google Scholar]
- Jiang, Y.W.; Gao, S.S.; Liu, X.J.; Wang, Y.; Zhou, S.X.; Liu, Q.; Abdukayum, A.; Hu, G.Z. Recent achievements in selenium-based transition metal electrocatalysts for pH-universal water splitting. Nano Res. 2024, 17, 5763–5785. [Google Scholar] [CrossRef]
- Song, Y.J.; Du, Z.X.; Sun, C.T.; Qiu, L.K.; Zhang, Y.; Luan, Y.W.; Yang, X.; Ma, J.W.; Gao, H.T. Cobalt-Based Catalysts for Electrocatalytic Water-Splitting Reactions: Mini Review of Material Synthesis, HER, and OER Catalytic Performance. ChemCatChem 2025, 14, e00832. [Google Scholar]
- Han, X.; Guo, C.; Wang, H.; Xu, W.J.; Liu, Q.L.; Zhao, Q.S.; Wu, M.B. Defect-Rich Co(OH)2 Induced by Carbon Dots for Oxygen Evolution Reaction. Catalysts 2025, 15, 219. [Google Scholar]
- Zhu, B.X.; Liu, Y.C.; Yan, Y.; Wang, H.; Zhang, Y.; Xin, Y.; Xu, W.J.; Zhao, Q.S. DES-Mediated Mild Synthesis of Synergistically Engineered 3D FeOOH-Co2(OH)3Cl/NF for Enhanced Oxygen. Evolution Reaction. Catalysts 2025, 15, 725. [Google Scholar]
- Zeng, Q.; Tang, J.; Ji, Y.; Jiang, Q.; Xia, C. Recent Developments in Single-Atom Engineering Ir/Ru-Based Catalysts for the Oxygen Evolution Reaction in Acidic Media. Adv. Energy Mater. 2025, e04414. [Google Scholar] [CrossRef]
- Zhang, S.L.; Shi, M.X.; Wang, F.Y.; Wu, L.; Hua, Q.; Xia, M.Z. Research progress on non-precious-metal-based transition metal alkaline OER electrocatalysts. J. Environ. Chem. Eng. 2025, 13, 120378. [Google Scholar]
- Li, Y.X.; Zhang, Z.; Yang, Y.L.; Li, C.G.; Shi, Z.; Feng, S.H. Manipulation of Electrochemical Surface Reconstruction on Spinel Oxides for Boosted Water Oxidation Reaction. ACS Catal. 2025, 15, 8361–8389. [Google Scholar] [CrossRef]
- Yang, K.; Ren, W.J.; Li, L.W.; Ye, B.J.; Li, W.; Fang, W.H.; Liu, S.X. Constructing heterojunction interface between Co-Fe layered double hydroxide and Ni-Fe metal–organic framework as efficient oxygen evolution electrocatalyst: Mechanism insights into CoOOH-FeOOH-NiOOH ternary system. J. Colloid Interface Sci. 2025, 698, 137991. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.L.; Jiang, Z.G.; Wang, C.; Yang, S.H.; Liu, H.Z.; Xing, B.; Cheng, H.H.; Wang, K.K. Unveiling the Synergistic Effect of Two-Dimensional Heterostructure NiFeP@FeOOH as Stable Electrocatalyst for Oxygen Evolution Reaction. Catalysts 2024, 14, 511. [Google Scholar] [CrossRef]
- Sun, M.H.; Zhang, Q.C.; Chen, Q.M.; Hou, X.H.; Peng, W.C.; Li, Y.; Zhang, F.B.; Xia, Q.; Fan, X.B. Coupling LaNiO3 Nanorods with FeOOH Nanosheets for Oxygen Evolution Reaction. Catalysts 2022, 12, 594. [Google Scholar] [CrossRef]
- Selvaraju, N.; Sasi, S.; Sivalingam, Y.; Venugopal, G. Enhanced oxygen evolution reaction performance of nitrogen-doped carbon dots sensitized with rare-earth metal nanorods. Diamond Relat. Mater. 2024, 148, 111362. [Google Scholar] [CrossRef]
- Rehman, M.Y.U.; Khan, M.M.; Nayer, S.; Shah, S.I.A.; Alsaiari, N.S.; Ehsan, M.F.; Chughtai, A.H.; Ashiq, M.N. Development of carbon dots supported on Zr-MOFs nano-composites for effective oxygen evolution reaction. Diamond Relat. Mater. 2024, 149, 111559. [Google Scholar] [CrossRef]
- Rashid, A.R.; Fatima, S.; Siddique, Z.; Tighezza, A.M.; Abid, A.G.; Shah, J.H.; Qin, H.L. Development of a Durable and Robust Co3O4@Carbon Dot Heterostructure Electrocatalyst for Clean Hydrogen Production. Energy Fuels 2025, 39, 9056–9065. [Google Scholar] [CrossRef]
- Wu, L.; Qin, H.; Ji, Z.Y.; Zhou, H.; Shen, X.P.; Zhu, G.X.; Yuan, A.H. Nitrogen-Doped Carbon Dots Modified Fe–Co Sulfide Nanosheets as High-Efficiency Electrocatalysts toward Oxygen Evolution Reaction. Small 2023, 20, 2305965. [Google Scholar] [CrossRef]
- Ding, P.; Song, H.Q.; Chang, J.W.; Lu, S.Y. N-doped carbon dots coupled NiFe-LDH hybrids for robust electrocatalytic alkaline water and seawater oxidation. Nano Res. 2022, 15, 7063–7070. [Google Scholar] [CrossRef]
- Khan, M.J.; Sawatdee, S.; Suksaard, M.; Nantarattikul, Y.; Botalo, A.; Juntree, N.; Pongchaikul, P.; Arjfuk, P.; Khemthong, P.; Wanmolee, W.; et al. Ultrafast magneto-inductive synthesis of carbon dots from plant-based precursors using deep eutectic solvents: A comparative study with traditional hydrothermal methods. Process Saf. Environ. Prot. 2025, 195, 106752. [Google Scholar] [CrossRef]
- Guan, S.Q.; Xu, B.E.; Wu, J.C.; Han, J.; Guan, T.T.; Yang, Y.; Li, K.X.; Wang, J.L. High-entropy materials based on deep eutectic solvent for boosting oxygen evolution reaction. Fuel 2024, 358, 130315. [Google Scholar] [CrossRef]
- Yang, H.Y.; Cheng, Z.F.; Wu, P.C.; Wei, Y.H.; Jiang, J.Y.; Xu, Q. Deep eutectic solvents regulation synthesis of multi-metal oxalate for electrocatalytic oxygen evolution reaction and supercapacitor applications. Electrochim. Acta 2022, 427, 140879. [Google Scholar] [CrossRef]
- Viyanni, P.M.; Sethuraman, M.G. Influence of diverse hydrogen bond donors in deep eutectic solvents on the electrochemical deposition of NiCu alloy on stainless steel mesh: Exploring NiCu efficacy in water splitting and methanol oxidation reactions. Int. J. Hydrogen Energy 2025, 130, 615–630. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Zhang, B.H.; Li, Z.H.; Hao, J.C. Deep Eutectic Solvent-Mediated Hierarchically Structured Fe-Based Organic−Inorganic Hybrid Catalyst for Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2019, 2, 3343–3351. [Google Scholar]
- Čižmar, T.; Kojić, V.; Rukavina, M.; Brkljačić, L.; Salamon, K.; Grčić, L.; Radetić, L.; Gajović, A. Hydrothermal Synthesis of FeOOH and Fe2O3 Modified Self-Organizing Immobilized TiO2 Nanotubes for Photocatalytic Degradation of 1H-Benzotriazole. Catalysts 2020, 10, 1371. [Google Scholar] [CrossRef]
- Shi, H.C.; Lv, J.; Feng, Y.C.; Zhang, H.R.; Xiong, Z.B.; Lu, S.J.; Jin, J.; Tontiwachwuthikul, P. Catalytic CO2 Desorption from MEA Solution of Al-FeOOH Composite Catalysts’ Desorption Performance, Structure–Activity Relationship, and New Mechanism. Catalysts 2024, 14, 779. [Google Scholar] [CrossRef]
- Wang, H.; Xu, W.J.; Han, X.; Yan, Y.; Zhu, B.X.; Wang, Z.Y.; Wang, L.B.; Zhao, Q.S.; Wu, M.B. One-Pot Hydrothermal Oxidation Enables In Situ Construction of CDs/Ni(OH)2 Composite for Electrocatalytic Oxygen Evolution. Front. Chem. 2025, 13, 1656451. [Google Scholar] [CrossRef]
- Wang, J.; Lian, Y.F. The Self-Supporting NiMn-LDHs/rGO/NF Composite Electrode Showing Much Enhanced Electrocatalytic Performance for Oxygen Evolution Reaction. Catalysts 2023, 13, 1012. [Google Scholar] [CrossRef]
- Dutta, D.P.; Abraham, S. Composite of α-FeOOH and Mesoporous Carbon Derived from Indian Blackberry Seeds as Low-Cost and Recyclable Photocatalyst for Degradation of Ciprofloxacin. Catalysts 2023, 13, 191. [Google Scholar]
- Lu, M.J.; Cheng, R.; Wang, L.; Liang, D.D.; Qin, M.; Wang, B.L.; Song, R.; Chen, D. One-Step Scalable Synthesis of 3D Self-Supported Superaerophobic Ce-Coupled Ni3S2/NiS@NF Nanobud Catalyst for Efficient Oxygen Evolution Reaction. Catalysts 2024, 14, 752. [Google Scholar] [CrossRef]
- Grinberg, V.A.; Emets, V.V.; Modestov, A.D.; Averin, A.A.; Shiryaev, A.A.; Botryakova, L.G.; Shapagin, A.V. Sn-Doped Hematite Films as Photoanodes for Photoelectrochemical Alcohol Oxidation. Catalysts 2023, 13, 1397. [Google Scholar] [CrossRef]
- Radinović, K.; Samancı, M.; Bayrakçeken, A.; Bajuk-Bogdanović, D.; Gavrilov, N.; Santos, D.M.F.; Šljukić, B. Maximizing electrochemical energy conversion and storage performance of carbon aerogel with Co-Fe by tuning the synthesis method. Mater. Chem. Phys. 2026, 348, 131614. [Google Scholar]
- Sun, Q.M.; Zhong, W.G.; Wang, H.; Ao, X.; Zhai, Y.L.; Zhai, J.S.; Wu, B.; Romero-Salguero, F.J.; Qiao, J.L.; Li, Z.S.; et al. Unveiling the role of FeOOH on catalyst evolution for advanced water oxidation. Rare Met. 2025, 44, 5462–5474. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhu, Q.X.; Zhang, P.; Liu, S.L.; Wang, J.K. Boosting the electrocatalytic activity and stability of Ni/NiO toward the oxygen evolution reaction by coupling FeOOH nanosheets. New J. Chem. 2024, 48, 9954. [Google Scholar] [CrossRef]
- Huang, X.R.; Xiao, L.Y.; Han, C.Y.; Zhang, W.; Liu, Y.; Zhang, J.T.; Tan, H.W.; Zhang, R.; Yin, P.F.; Dong, C.K.; et al. Impact of electron modulation on V-doped NiFeOOH: Insights into enhanced OER activity and stabilization of vanadium incorporation. Int. J. Hydrogen Energy 2025, 140, 187–193. [Google Scholar]
- Sun, H.Y.; Gu, X.Y.; Yu, J.; Zhang, N.N.; Wu, Z.Y.; Qiang, J.W.; Song, Z.G.; Du, Y.K. Self-supported Fe, Mn-CoCH/NF electrocatalyst for oxygen evolution reaction. J. Colloid Interface Sci. 2026, 703, 139149. [Google Scholar]
- Yuan, Y.D.; Wang, J.W.; Gu, Z.L.; Huang, Y.B.; Gong, H.Y.; Zhao, Z.Q.; Sun, C.G.; Li, Y.S.; Bao, F.X. Construction of FeS/NiCo-LDH heterostructure for enhancing oxygen evolution reaction kinetics. Colloids Surf. A Physicochem. Eng. Asp. 2025, 727, 138215. [Google Scholar]
- Yang, Y.Y.; Chen, Y.Z.; Xiong, Y.L.; He, Y.L.; Sun, Q.N.; Xu, D.Y.; Hu, Z.A. Self-supported monometallic FeS2/FeOOH-ZnO@NF with abundant oxygen vacancies as efficient and stable electrocatalysts for the OER and HER. J. Alloys Compd. 2024, 991, 174525. [Google Scholar]
- Zhou, H.B.; Hao, X.; Guan, J.X.; Deng, Y.L.; Wei, Z.; Liu, Y.S.; Zhu, G.X. Coordination tuning of Ni/Fe complex-based electrocatalysts for enhanced oxygen evolution. Inorg. Chem. Front. 2024, 11, 8110. [Google Scholar] [CrossRef]
- Zhu, L.Q.; Wang, R.Z.; Liu, X.H.; Yang, S.H.; Liu, H.Z.; Xing, B.; Wang, K.K. Three-dimensional heterostructured MnNiCoP/FeOOH cross-linked nano-flake supported by Ni Foam as a bifunctional electrocatalysts for overall water splitting. Appl. Surf. Sci. 2024, 656, 159711. [Google Scholar]
- Yin, X.Y.; Mu, X.; Qi, S.N.; Li, H.; Qi, M.L.; Yusuf, A. Se-doped FeS2/CoS nanosphere catalysts bifunctional electrocatalyst for superior OER and HER activity. Res. Chem. Intermed. 2025, 51, 3229–3242. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Xu, W.; Wang, H.; Han, X.; Qu, S.; Yan, Y.; Zhu, B.; Zhang, H.; Zhao, Q. Synergistic Induction by Deep Eutectic Solvent and Carbon Dots for Rapid Construction of FeOOH Electrocatalysts Toward Efficient Oxygen Evolution Reaction. Catalysts 2026, 16, 73. https://doi.org/10.3390/catal16010073
Xu W, Wang H, Han X, Qu S, Yan Y, Zhu B, Zhang H, Zhao Q. Synergistic Induction by Deep Eutectic Solvent and Carbon Dots for Rapid Construction of FeOOH Electrocatalysts Toward Efficient Oxygen Evolution Reaction. Catalysts. 2026; 16(1):73. https://doi.org/10.3390/catal16010073
Chicago/Turabian StyleXu, Weijuan, Hui Wang, Xuan Han, Shuzheng Qu, Yue Yan, Bingxian Zhu, Haipeng Zhang, and Qingshan Zhao. 2026. "Synergistic Induction by Deep Eutectic Solvent and Carbon Dots for Rapid Construction of FeOOH Electrocatalysts Toward Efficient Oxygen Evolution Reaction" Catalysts 16, no. 1: 73. https://doi.org/10.3390/catal16010073
APA StyleXu, W., Wang, H., Han, X., Qu, S., Yan, Y., Zhu, B., Zhang, H., & Zhao, Q. (2026). Synergistic Induction by Deep Eutectic Solvent and Carbon Dots for Rapid Construction of FeOOH Electrocatalysts Toward Efficient Oxygen Evolution Reaction. Catalysts, 16(1), 73. https://doi.org/10.3390/catal16010073





