Selective Ruthenium-Catalysed Functionalisation Reactions and ROMP of exo-Norbornene-Based Organosilicon Boronic Esters
Abstract
1. Introduction
2. Results and Discussion
2.1. Preliminary Studies
2.2. Monomer Synthesis
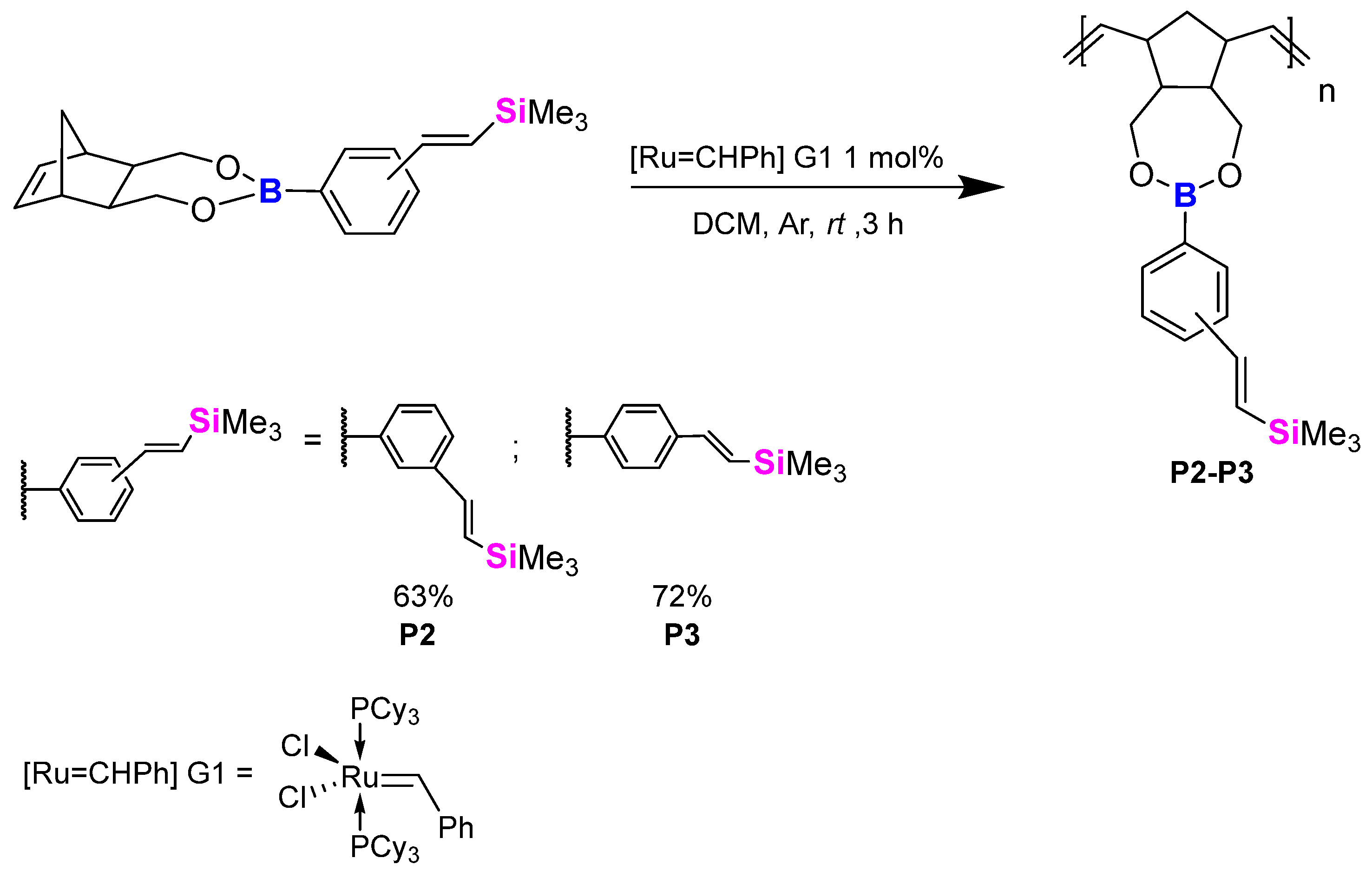
2.3. Polymerisation Studies
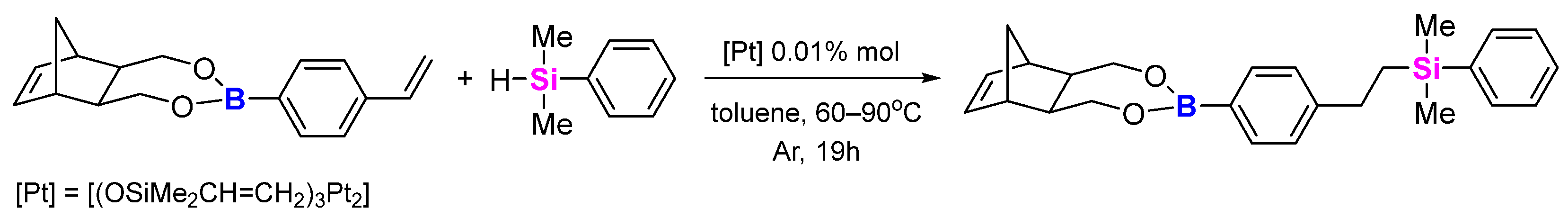
2.4. Hydrosilylation Tests
2.5. GPC Measurements
2.6. TGA Measurements
3. Materials and Methods
3.1. Materials
3.2. Techniques
3.2.1. GC-MS
3.2.2. FT-IR
3.2.3. NMR
3.2.4. GPC
3.2.5. TGA
3.3. Synthesis and Characterisation of Obtained Compounds
3.3.1. Synthesis Procedure of Models 0a and 0b via Condensation Reaction
3.3.2. Ruthenium Hydride Catalyst [Ru(H)Cl(CO)(PCy3)2] Preparation
3.3.3. Synthesis Procedure of Models 0a’ and 0b’ via Silylative Coupling
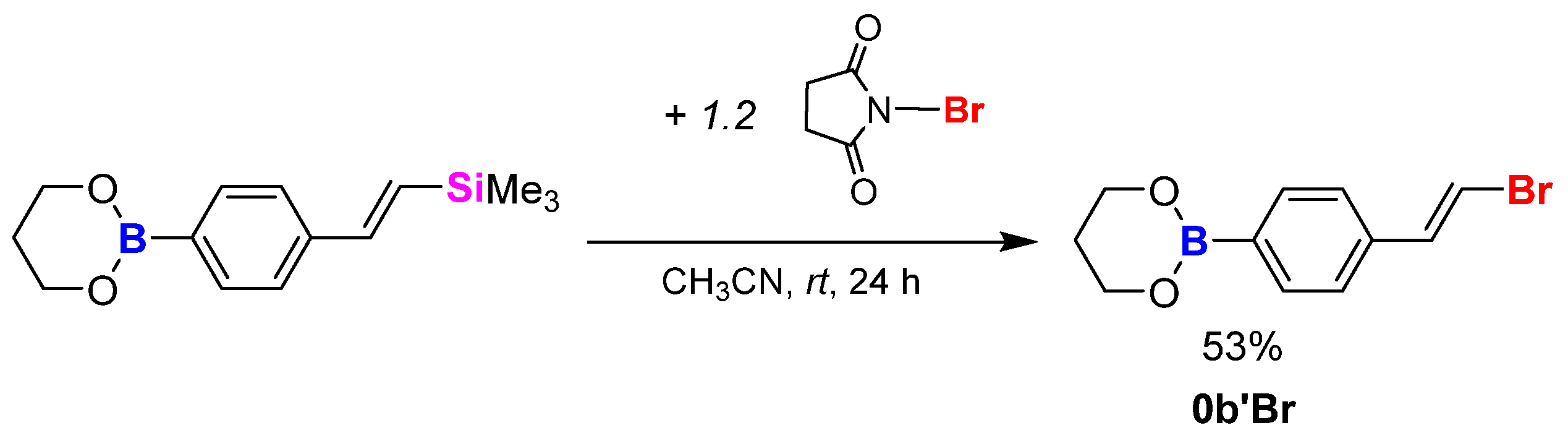
3.3.4. Synthesis Procedure of Model 0b’Br via Bromodesilylation
3.3.5. Synthesis Procedure of Monomers 1 and 2 via Condensation Reaction
3.3.6. Synthesis Procedure of Monomer 3 via Silylative Coupling
3.3.7. Synthesis Procedure of Monomer 4 via Silylative Coupling
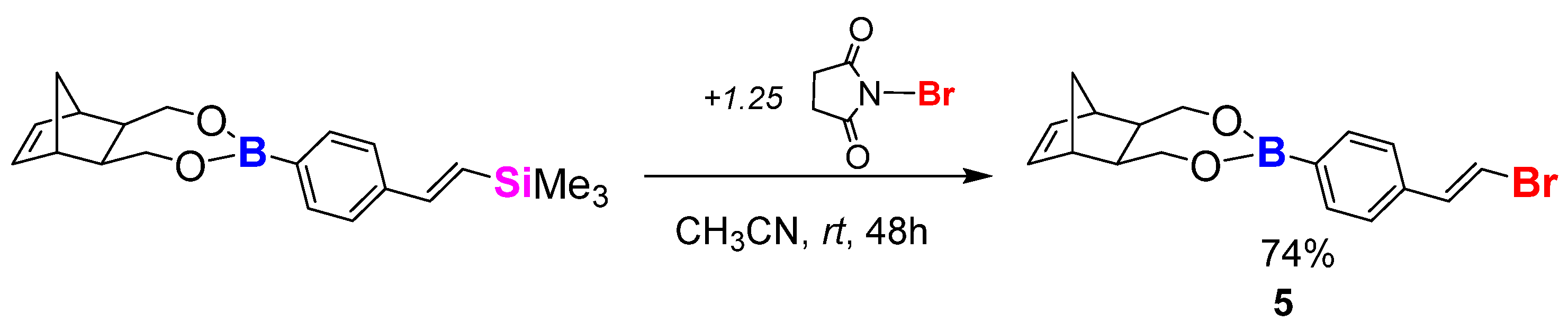
3.3.8. Synthesis Procedure of Monomer 5 via Bromodesilylation
3.3.9. Ring-Opening Metathesis Polymerisation of Monomers 2, 3, 4
3.3.10. Ring-Opening Metathesis Polymerisation of Monomer 5
3.3.11. Hydrosilylation Procedure for Tests 0H1 and 0H2
3.4. General Information on Safe Work Practices and the Schlenk Technique
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROMP | Ring-opening metathesis polymerisation |
| NBE | Norbornene |
| SC | Silylative coupling = silane coupling with olefins |
| NBS | N-bromosuccinimide |
| NMR | Nuclear magnetic resonance |
| GC-MS | Gas chromatography—mass spectrometry |
| ĐM | Polydispersity index |
| DCM | Dichloromethane |
References
- Schrock, R.R. Living Ring-Opening Metathesis Polymerization Catalyzed by Well-Characterized Transition-Metal Alkylidene Complexes. Acc. Chem. Res. 1990, 23, 158–165. [Google Scholar] [CrossRef]
- Feast, W.J.; Gibson, V.C.; Johnson, A.F.; Khosravi, E.; Mohsin, M.A. Tailored Copolymers via Coupled Anionic and Ring Opening Metathesis Polymerization. Synthesis and Polymerization of Bicyclo[2.2.1]hept-5-ene-2,3-trans-bis(Polystyrylcarboxylate)s. Polymer 1994, 35, 3542–3548. [Google Scholar] [CrossRef]
- Nomura, K.; Abdellatif, M.M. Precise Synthesis of Polymers Containing Functional End Groups by Living Ring-Opening Metathesis Polymerization (ROMP): Efficient Tools for Synthesis of Block/Graft Copolymers. Polymer 2010, 51, 1861–1881. [Google Scholar] [CrossRef]
- Hejl, A.; Scherman, O.A.; Grubbs, R.H. Ring-Opening Metathesis Polymerization of Functionalized Low-Strain Monomers with Ruthenium-Based Catalysts. Macromolecules 2005, 38, 7214–7218. [Google Scholar] [CrossRef]
- Leroux, F.; Pascual, S.; Montembault, V.; Fontaine, L. 1,4-Polybutadienes with Pendant Hydroxyl Functionalities by ROMP: Synthetic and Mechanistic Insights. Macromolecules 2015, 48, 3843–3852. [Google Scholar] [CrossRef]
- Schleyer, P.V.R.; Wiliiams, J.E.; Blanchard, K.R. The Evaluation of Strain in Hydrocarbons. The Strain in Adamantane and Its Origin. J. Am. Chem. Soc. 1970, 92, 2377–2386. [Google Scholar] [CrossRef]
- Sutthasupa, S.; Sanda, F.; Masuda, T. ROMP of Norbornene Monomers Carrying Nonprotected Amino Groups with Ruthenium Catalyst. Macromolecules 2009, 42, 1519–1525. [Google Scholar] [CrossRef]
- Barther, D.; Moatsou, D. Ring-Opening Metathesis Polymerization of Norbornene-Based Monomers Obtained via the Passerini Three Component Reaction. Macromol. Rapid Commun. 2021, 42, 202100027. [Google Scholar] [CrossRef]
- Park, L.Y.; Schrock, R.R.; Stieglitz, S.G.; Crowe, W.E. Preparation of Discrete Polyenes and Norbornene-Polyene Block Copolymers Using Mo(CH-t-Bu)(NAr)(O-t-Bu)2 as the Initiator. Macromolecules 1991, 24, 3489–3495. [Google Scholar] [CrossRef]
- Bazan, G.C.; Khosravi, E.; Schrock, R.R.; Feast, W.J.; Gibson, V.C.; O’Regan, M.B.; Thomas, J.K.; Davis, W.M. Living Ring-Opening Metathesis Polymerization Of 2,3-Difunctionalized Norbornadienes by Mo(CH-t-Bu)(N-2,6-C6H3-/-Pr2)(0-t-Bu)2. J. Am. Chem. Soc. 1990, 112, 8378–8387. [Google Scholar] [CrossRef]
- Flook, M.M.; Gerber, L.C.H.; Debelouchina, G.T.; Schrock, R.R. Z-Selective and Syndioselective Ring-Opening Metathesis Polymerization (ROMP) Initiated by Monoaryloxidepyrrolide (MAP) Catalysts. Macromolecules 2010, 43, 7515–7522. [Google Scholar] [CrossRef] [PubMed]
- Bochkarev, L.N.; Begantsova, Y.E.; Iĺichev, V.A.; Abakumov, G.A. Electroluminescent Platinum-Containing Polymers Based on Functionalized Norbornenes. Russ. Chem. Bull. 2014, 63, 2534–2540. [Google Scholar] [CrossRef]
- Miao, Y.; Bazan, C.G. Paracyclophene Route to Poly(p-Phenylenevinylene). J. Am. Chem. Soc. Am. 1994, 116, 9379–9380. [Google Scholar] [CrossRef]
- Spring, A.M.; Yu, C.Y.; Horie, M.; Turner, M.L. MEH-PPV by Microwave Assisted Ring-Opening Metathesis Polymerisation. Chem. Commun. 2009, 2676–2678. [Google Scholar] [CrossRef]
- Lidster, B.J.; Behrendt, J.M.; Turner, M.L. Monotelechelic Poly(p-Phenylenevinylene)s by Ring Opening Metathesis Polymerisation. Chem. Commun. 2014, 50, 11867–11870. [Google Scholar] [CrossRef]
- Dias, E.L.; Nguyen, S.B.T.; Grubbs, R.H. Well-Defined Ruthenium Olefin Metathesis Catalysts: Mechanism and Activity. J. Am. Chem. Soc. 1997, 119, 3887–3897. [Google Scholar] [CrossRef]
- Courchay, F.C.; Sworen, J.C.; Wagener, K.B. Metathesis Activity and Stability of New Generation Ruthenium Polymerization Catalysts. Macromolecules 2003, 36, 8231–8239. [Google Scholar] [CrossRef]
- Ritter, T.; Hejl, A.; Wenzel, A.G.; Funk, T.W.; Grubbs, R.H. A Standard System of Characterization for Olefin Metathesis Catalysts. Organometallics 2006, 25, 5740–5745. [Google Scholar] [CrossRef]
- Rule, J.D.; Moore, J.S. ROMP Reactivity of Endo- and Exo-Dicyclopentadiene. Macromolecules 2002, 35, 7878–7882. [Google Scholar] [CrossRef]
- Garbarek, J.; Majchrzak, M.; Wałęsa-Chorab, M.; Kubicki, M. Synthesis and Electrochemical Properties of Well-defined Norbornene-B-Ferrocene-dioxaborolane Hybrid Polymeric Material. Inorg. Chem. 2025, 64, 21368–21378. [Google Scholar] [CrossRef]
- Ghose, B.N. Synthesis of Some Carbon-Functional Organosilicon Compounds. J. Organomet. Chem. 1979, 164, 11–18. [Google Scholar] [CrossRef]
- Marciniec, B. Catalysis by Transition Metal Complexes of Alkene Silylation—Recent Progress and Mechanistic Implications. Coord. Chem. Rev. 2005, 249, 2374–2390. [Google Scholar] [CrossRef]
- Marciniec, B.; Guliński, J. Metathesis of Vinyltrialkoxysilanes. J. Organomet. Chem. 1984, 266, 19–21. [Google Scholar] [CrossRef]
- Marciniec, B.; Majchrzak, M.; Prukała, W.; Kubicki, M.; Chadyniak, D. Highly Stereoselective Synthesis, Structure, and Application of (E)-9-[2-(Silyl)Ethenyl]-9H-Carbazoles. J. Org. Chem. 2005, 70, 8550–8555. [Google Scholar] [CrossRef]
- Marciniec, B.; Pietraszuk, C. Silylation of Styrene with Vinylsilanes Catalyzed by RuCl(SiR3)(CO)(PPh3)2 and Ru(H)Cl(CO)(PPh3)3. Organometallics 1997, 16, 4320–4326. [Google Scholar] [CrossRef]
- Majchrzak, M.; Hybsz, M.; Kostera, S.; Kubicki, M.; Marciniec, B. A highly stereoselective synthesis of new styryl-π-conjugate organosilicon compounds. Tetrahedron Lett. 2014, 55, 3055–3058. [Google Scholar] [CrossRef]
- Marciniec, B.; Chadyniak, D.; Krompiec, S. Stereoselective Synthesis of Amides Possessing a Vinylsilicon Functionality via a Ruthenium Catalyzed Silylative Coupling Reaction. Tetrahedron Lett. 2004, 45, 4065–4068. [Google Scholar] [CrossRef]
- Majchrzak, M.; Marciniec, B.; Itami, Y. Highly Stereoselective Synthesis of Arylene-Silylene-Vinylene Polymers. Adv. Synth. Catal. 2005, 347, 1285–1294. [Google Scholar] [CrossRef]
- Itami, Y.; Marciniec, B.; Kubicki, M. Functionalization of Octavinylsilsesquioxane by Ruthenium-Catalyzed Silylative Coupling versus Cross-Metathesis. Chem. Eur. J. 2004, 10, 1239–1248. [Google Scholar] [CrossRef]
- Pawluć, P.; Prukala, W.; Marciniec, B. Silylative Coupling of Olefins with Vinylsilanes in the Synthesis of π-Conjugated Double Bond Systems. Eur. J. Org. Chem. 2010, 2010, 219–229. [Google Scholar] [CrossRef]
- Hatanaka, Y.; Hiyama, T. Cross-Coupling of Organosilanes with Organic Halides Mediated by Palladium Catalyst and Tris(Diethylamino)Sulfonium Difluorotrimethylsilicate. J. Org. Chem. 1988, 53, 918–920. [Google Scholar] [CrossRef]
- Prukała, W.; Majchrzak, M.; Pietraszuk, C.; Marciniec, B. Highly Stereoselective Synthesis of E-4-Chlorostilbene and Its Derivatives via Tandem Cross-Metathesis (or Silylative Coupling) and Hiyama Coupling. J. Mol. Catal. A Chem. 2006, 254, 58–63. [Google Scholar] [CrossRef]
- Prukała, W.; Marciniec, B.; Majchrzak, M.; Kubicki, M. Highly Stereoselective Synthesis of Para-Substituted (E)-N-Styrylcarbazoles via Sequential Silylative Coupling-Hiyama Coupling Reaction. Tetrahedron 2007, 63, 1107–1115. [Google Scholar] [CrossRef]
- Pawluć, P.; Szudkowska, J.; Hreczycho, G.; Marciniec, B. One-Pot Synthesis of (E)-Styryl Ketones from Styrenes. J. Org. Chem. 2011, 76, 6438–6441. [Google Scholar] [CrossRef]
- Miller, R.B.; Mcgarvey, G. A Highly Stereoselective Synthesis of Vinyl Bromides and Chlorides via Disubstituted Vinylsilanes. J. Org. Chem. 1978, 43, 4424–4431. [Google Scholar] [CrossRef]
- Brook, M.A.; Neuy, A. The β-effect: Changing the Ligands on Silicon. J. Org. Chem. 1990, 55, 3609–3616. [Google Scholar] [CrossRef]
- Tamao, K.; Akita, M.; Maeda, K.; Kumada, M. Silafunctional Compounds in Organic Synthesis. 32. Stereoselective Halogenolysis of Alkenylsilanes: Stereochemical Dependence on the Coordination State of the Leaving Silyl Groups. J. Org. Chem. 1987, 52, 1100–1106. [Google Scholar] [CrossRef]
- Stamos, D.P.; Taylor, A.G.; Kishi, Y. A Mild Preparation of Vinyliodides from Vinylsilanes. Tetrahedron Lett. 1996, 37, 8647–8650. [Google Scholar] [CrossRef]
- Nagao, M.; Asano, K.; Umeda, K.; Katayama, H.; Ozawa, F. Highly (Z)-Selective Hydrosilylation of Terminal Alkynes Catalyzed by a Diphosphinidenecyclobutene-Coordinated Ruthenium Complex: Application to the Synthesis of (Z,Z)-bis(2-Bromoethenyl)Arenes. J. Org. Chem. 2005, 70, 10511–10514. [Google Scholar] [CrossRef]
- Pawluć, P.; Hreczycho, G.; Szudkowska, J.; Kubicki, M.; Marciniec, B. New One-Pot Synthesis of (E)-β-Aryl Vinyl Halides from Styrenes. Org. Lett. 2009, 11, 3390–3393. [Google Scholar] [CrossRef]
- Pawluć, P.; Franczyk, A.; Walkowiak, J.; Hreczycho, G.; Kubicki, M.; Marciniec, M. (E)-9-(2-Iodovinyl)-9H-Carbazole-a-New-Coupling-Reagent-for-the-Synthesis-of-π-Conjugated-Carbazoles. Org. Lett. 2011, 13, 1976–1979. [Google Scholar] [CrossRef] [PubMed]
- Pawluć, P.; Franczyk, A.; Walkowiak, J.; Hreczycho, G.; Kubicki, M.; Marciniec, B. Highly Stereoselective Synthesis of N-Substituted π-Conjugated Phthalimides. Tetrahedron 2012, 68, 3545–3551. [Google Scholar] [CrossRef]
- Adachi, C.; Tsutsui, T.; Saito, S. Blue Light-Emitting Organic Electroluminescent Devices. Appl. Phys. Lett. 1990, 56, 799–801. [Google Scholar] [CrossRef]
- Singh, A.K.; Darshi, M.; Kanvah, S. α-ω-Diphenylpolyenes-Cabable-of-Exhibiting-Twisted-Intramolecular-Charge-Transfer- Fluorescence: A Fluorescence and Fluorescence Probe Study of Nitro- and Nitrocyano-Substituted 1,4-Diphenylbutadienes. J. Phys. Chem. A 2000, 104, 464–471. [Google Scholar] [CrossRef]
- Diemer, V.; Chaumeil, H.; Defoin, A.; Carré, C. Synthesis of Alkoxynitrostilbenes as Chromophores for Nonlinear Optical Materials. Synthesis 2007, 2007, 3333–3338. [Google Scholar] [CrossRef]
- Yoshiyuki, K.; Hiromichi, O.; Shigeru, A. Effects of Stilbene Derivatives on Arachidonate Metabolism in Leukocytes. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1985, 837, 209–212. [Google Scholar] [CrossRef]
- Cushman, M.; Nagarathnam, D.; Gopal, D.; Chakraborti, A.K.; Lin, C.M.; Hamel, E. Synthesis and Evaluation of Stilbene and Dihydrostilbene Derivatives as Potential Anticancer Agents That Inhibit Tubulin Polymerization. J. Med. Chem. 1991, 34, 2579–2588. [Google Scholar] [CrossRef]
- Jang, D.S.; Kang, B.S.; Ryu, S.Y.; Chang, I.M.; Min, K.R.; Kim, Y. Inhibitory Effects of Resveratrol Analogs on Unopsonized Zymosan-Induced Oxygen Radical Production. Biochem. Pharmacol. 1999, 57, 705–712. [Google Scholar] [CrossRef]
- Gupta, Y.K.; Chaudhary, G.; Srivastava, A.K. Protective Effect of Resveratrol against Pentylenetetrazole-Induced Seizures and Its Modulation by an Adenosinergic System. Pharmacology 2002, 65, 170–174. [Google Scholar] [CrossRef]
- Wong, K.T.; Chien, Y.Y.; Liao, Y.L.; Lin, C.C.; Chou, M.Y.; Leung, M.K. Efficient and Convenient Nonaqueous Workup Procedure for the Preparation of Arylboronic Esters. J. Org. Chem. 2002, 67, 1041–1044. [Google Scholar] [CrossRef]
- Alver, Ö. DFT, FT-Raman, FT-IR, Solution and Solid State NMR Studies of 2,4-Dimethoxyphenylboronic Acid. Comptes Rendus Chim. 2011, 14, 446–455. [Google Scholar] [CrossRef]
- Oh, S.W.; Weiss, J.W.E.; Kerneghan, P.A.; Korobkov, I.; Maly, K.E.; Bryce, D.L. Solid-State 11B and 13C NMR, IR, and X-Ray Crystallographic Characterization of Selected Arylboronic Acids and Their Catechol Cyclic Esters. Magn. Reson. Chem. 2012, 50, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Wrackmeyer, B. Organoboranes and Tetraorganoborates Studied by 11B and 13C NMR Spectroscopy and DFT Calculations. Z. Naturforsch. Sect. B J. Chem. Sci. 2015, 70, 421–424. [Google Scholar] [CrossRef]
- Wakatsuki, Y.; Yamazaki, H.; Nakano, M.; Yamamoto, Y. Ruthenium-catalysed Disproportionation between Vinylsilanes and Mono-substituted Alkenes via Silyl Group Transfer. J. Chem. Soc. Chem. Commun. 1991, 703–704. [Google Scholar] [CrossRef]
- Sellmann, D.; Ruf, R.; Knoch, F.; Moll, M. Transition-Metal Complexes with Sulfur Ligands. 112. Synthesis and Characterization of Ruthenium Complexes with [RuPS2N2] Cores. Substitution, Redox and Acid-Base Reactions of [RuII(L)(PR3)(‘S2N2H2’)], (L = CO, PPr3, R = Pr or Cy) and Five-Coordinate [RuIV(PCy3)(‘S2N2’)]. Inorg. Chem. 1995, 34, 4745–4755. [Google Scholar] [CrossRef]
- Dinger, B.M.; Mol, C.J. Degradation of the First-Generation Grubbs Metathesis Catalyst with Primary Alcohols, Water, and Oxygen. Formation and Catalytic Activity of Ruthenium(II) Monocarbonyl Species. Organometallics 2003, 22, 1089–1095. [Google Scholar] [CrossRef]
- Qu, F.; Park, S.; Martinez, K.; Gray, J.L.; Thowfeik, F.S.; Lundeen, J.A.; Kuhn, A.E.; Charboneau, D.J.; Gerlach, D.L.; Lockart, M.M.; et al. Ruthenium Complexes are pH-Activated Metallo Prodrugs (pHAMPs) with Light-Triggered Selective Toxicity Toward Cancer Cells. Inorg. Chem. 2017, 56, 7519–7532. [Google Scholar] [CrossRef]
- Zak, P.; Pietraszuk, C.; Marciniec, B. Cross-Metathesis of Vinyl-Substituted Linear and Cyclic Siloxanes with Olefins in the Presence of Grubbs Catalysts. J. Mol. Catal. A Chem. 2008, 289, 1–8. [Google Scholar] [CrossRef]
- Grachek, V.I.; Lukashik, A.N. Boric Acid Esters as Thermal Stabilizers and Fungicidal Additives for Elastomers from Natural Rubber. Russ. J. Appl. Chem. 2006, 79, 819–822. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J. Synthesis and Characterization of a New Thermosetting Boric Acid Ester. Adv. Mater. Res. 2011, 239–242, 1907–1910. [Google Scholar] [CrossRef]
- Grachek, V.I.; Krut’Ko, E.T.; Osmolovskaya, L.Y.; Globa, A.I. Thermal Stabilization of Polyimides with Boric Acid Esters. Russ. J. Appl. Chem. 2011, 84, 1582–1586. [Google Scholar] [CrossRef]
- Yu-Ran, L. “Chemical Bond Energies” Comprehensive Handbook of Chemical Bond Energies, 1st ed.; Taylor & Francis Group, CRC Press: Abingdon, UK, 2007. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Vazquez, A.; Wang, D.; Li, S. Mechanism of thermal decomposition of tetramethylsilane: A flash pyrolysis vacuum ultraviolet photoionization time-of-flight mass spectrometry and density functional theory study. Phys. Chem. Chem. Phys. 2018, 20, 18782–18789. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Prudent Practices in the Laboratory. Prudent Practices in The Laboratory: Handling and Management of Chemical Hazards; The National Academies Press: Washington, DC, USA, 2011; ISBN 0-309-13865-5. [Google Scholar] [CrossRef]











| Polymer | Mn (105) | Mw (105) | ĐM |
|---|---|---|---|
| P2 [a] | 0.08 | 0.14 | 1.67 |
| P3 [a] | 0.11 | 0.19 | 1.70 |
| P4 [b] | 0.09 | 0.15 | 1.78 |
| Polymer | Td5 (°C) | Td10 (°C) | 1st Step of Thermal Decomp. (Weight Resid. %) Top Temp. (°C) | 2nd Step of Thermal Decomp. (Weight Resid. %) Top Temp. (°C) | 3rd Step of Thermal Decomp. (Weight Resid. %) Top Temp. (°C) | 4th Step of Thermal Decomp. (Weight Resid. %) Top Temp. (°C) | Total Weight Loss [%] |
|---|---|---|---|---|---|---|---|
| P2 [a] | 349.9 | 401.5 | 32.3–133.2 (0.7) | 133.2–268.4 (2.2) Max. 147.1 | 268.4–529.2 (70.8) Max. 422.4 | ---- | 80 |
| P3 [a] | 346.1 | 394.8 | 25.4–273.1 (2.4) | 273.1–534.0 (72.4) Max. 418.7 | ---- | ---- | 82 |
| P4 [b] | 248.4 | 270.2 | 78.1–201.1 (2.3) | 201.1–357.1 (31.3) Max. 283.1 | 357.1–495.0 (29.4) Max. 395.7 | 495.0–736.3 (17.7) Max. 590.7 | 85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Garbarek, J.; Majchrzak, M. Selective Ruthenium-Catalysed Functionalisation Reactions and ROMP of exo-Norbornene-Based Organosilicon Boronic Esters. Catalysts 2026, 16, 45. https://doi.org/10.3390/catal16010045
Garbarek J, Majchrzak M. Selective Ruthenium-Catalysed Functionalisation Reactions and ROMP of exo-Norbornene-Based Organosilicon Boronic Esters. Catalysts. 2026; 16(1):45. https://doi.org/10.3390/catal16010045
Chicago/Turabian StyleGarbarek, Jerzy, and Mariusz Majchrzak. 2026. "Selective Ruthenium-Catalysed Functionalisation Reactions and ROMP of exo-Norbornene-Based Organosilicon Boronic Esters" Catalysts 16, no. 1: 45. https://doi.org/10.3390/catal16010045
APA StyleGarbarek, J., & Majchrzak, M. (2026). Selective Ruthenium-Catalysed Functionalisation Reactions and ROMP of exo-Norbornene-Based Organosilicon Boronic Esters. Catalysts, 16(1), 45. https://doi.org/10.3390/catal16010045








