Response Surface Modeling and Photocatalytic Assessment of CoV2O6 for the Treatment of Organic Dyes
Abstract
1. Introduction
2. Results and Discussion
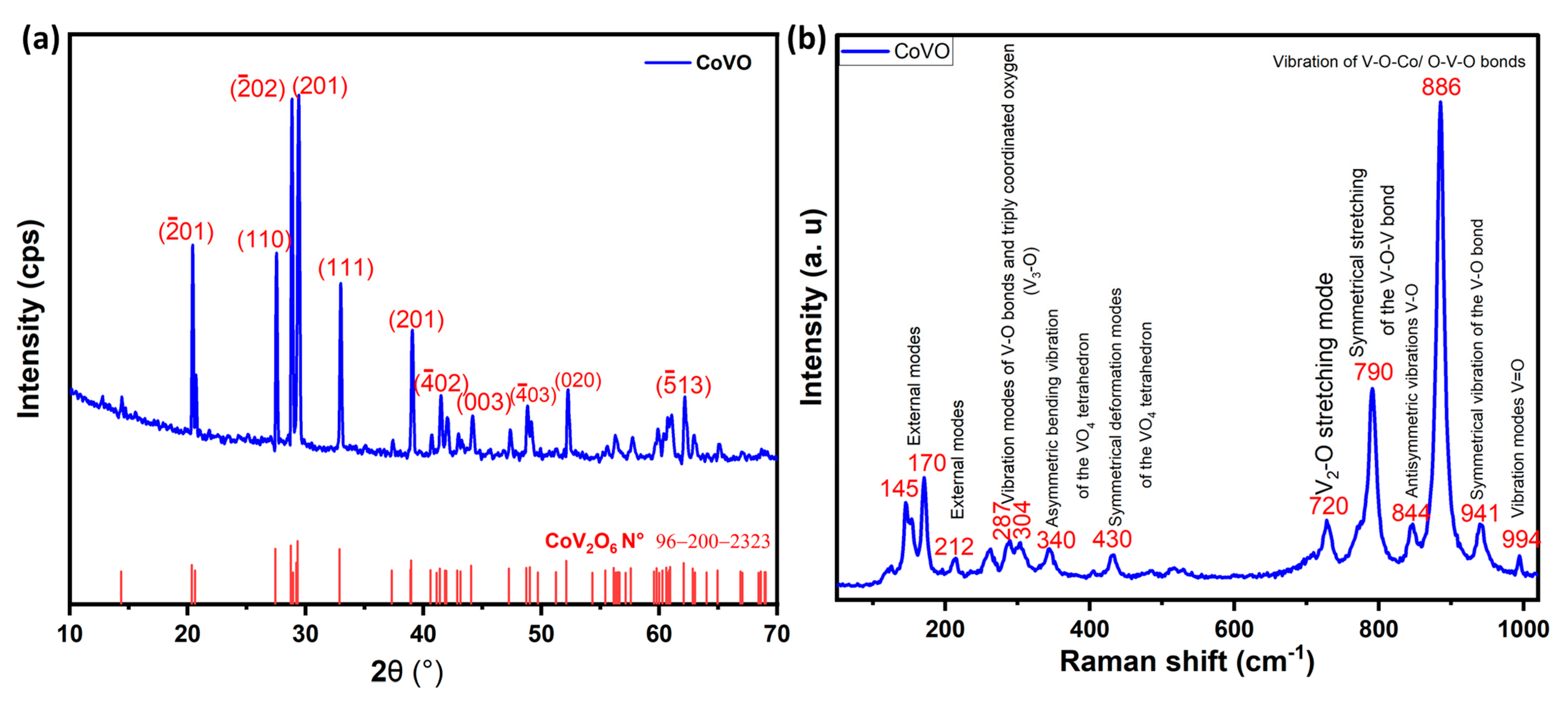
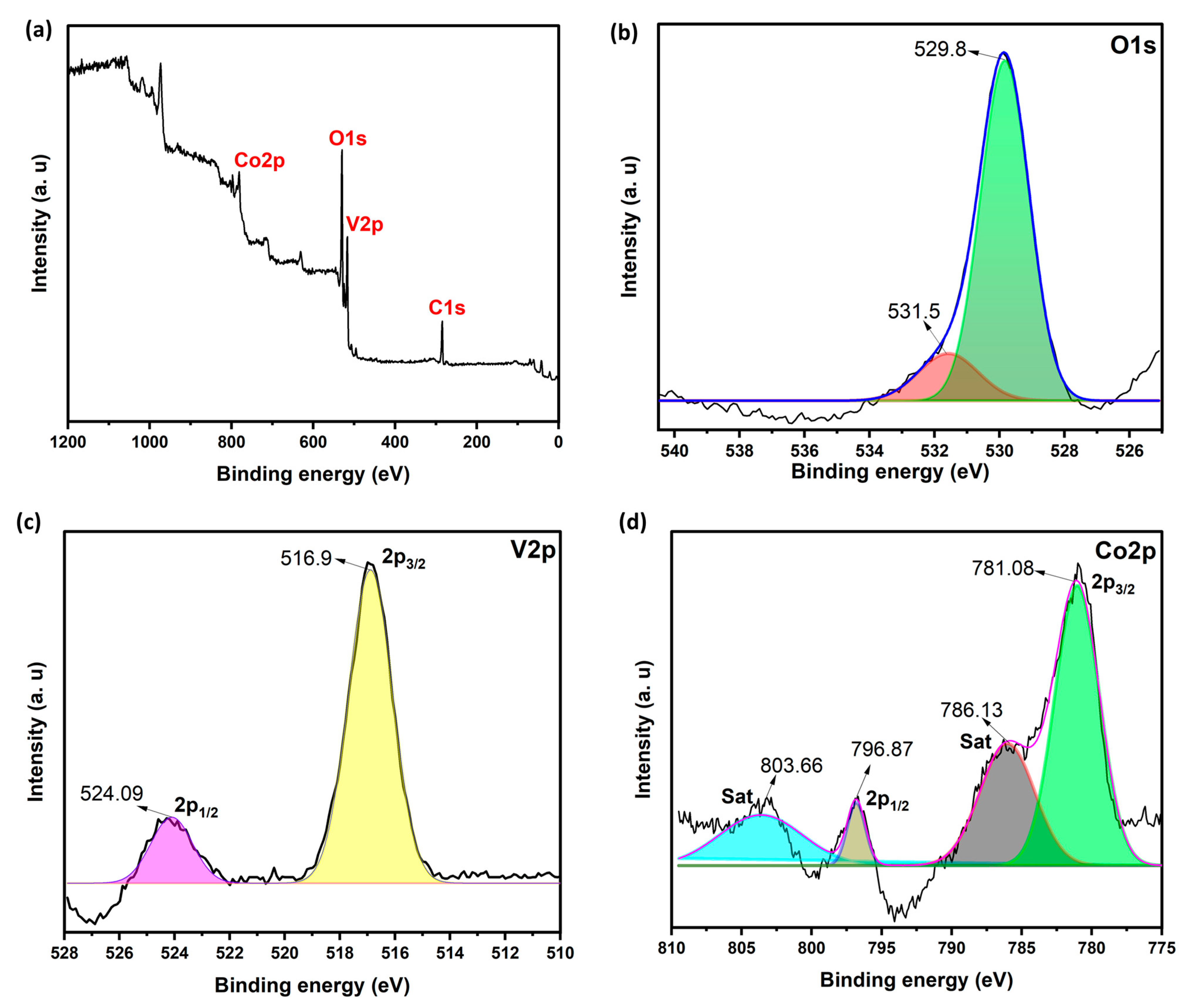
2.1. Structural Analysis
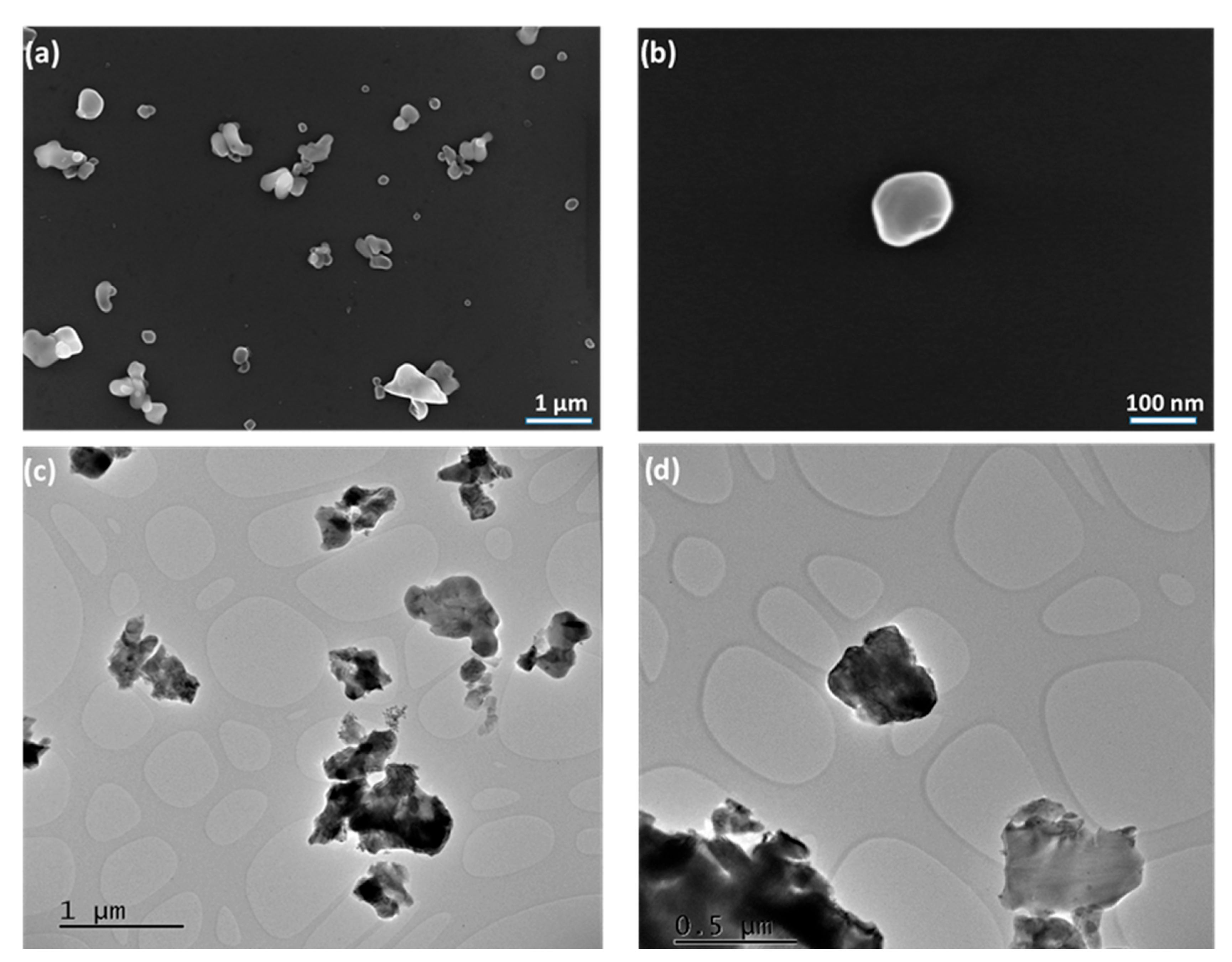
2.2. Morphological Analysis
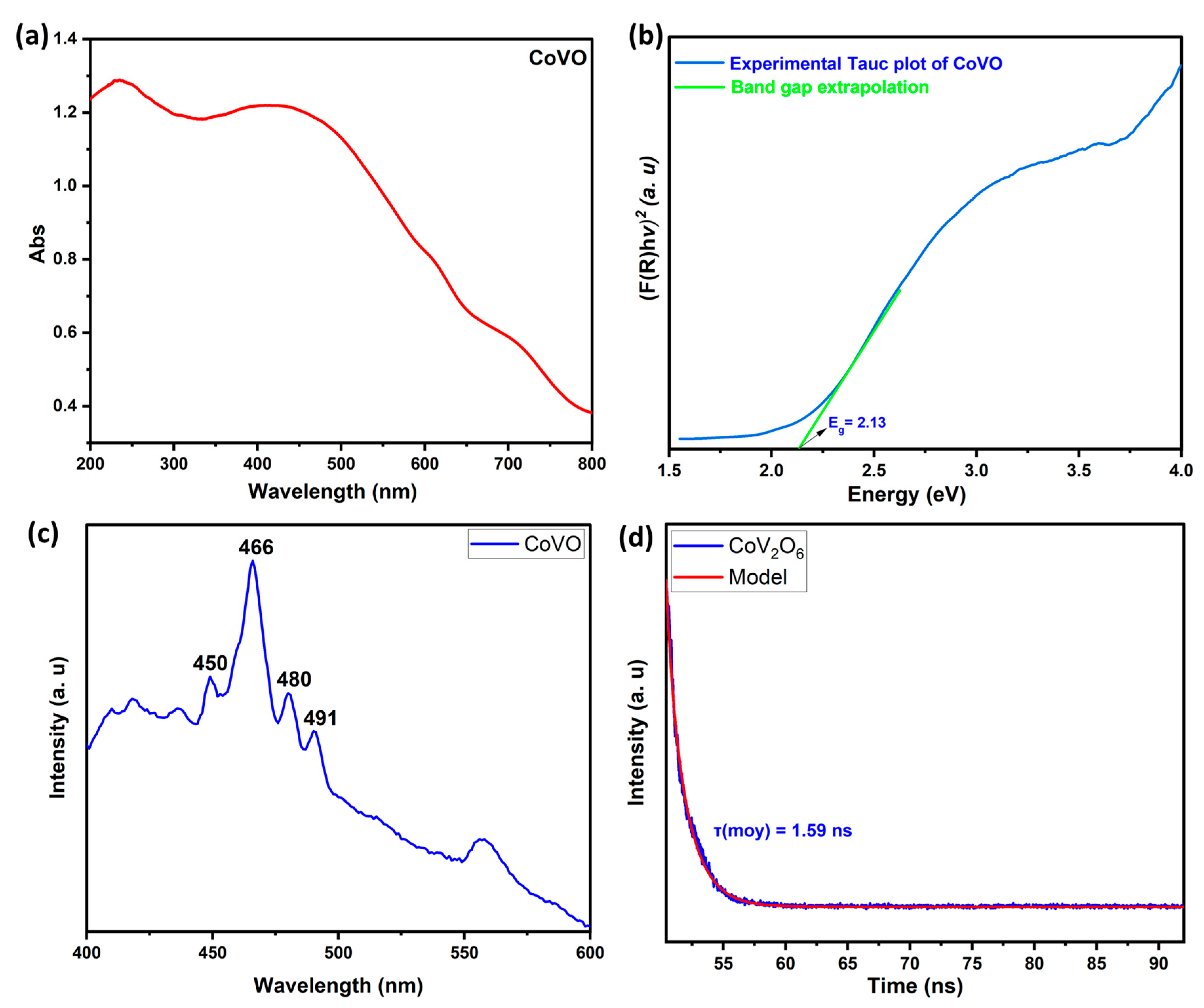
2.3. Optical Characteristics
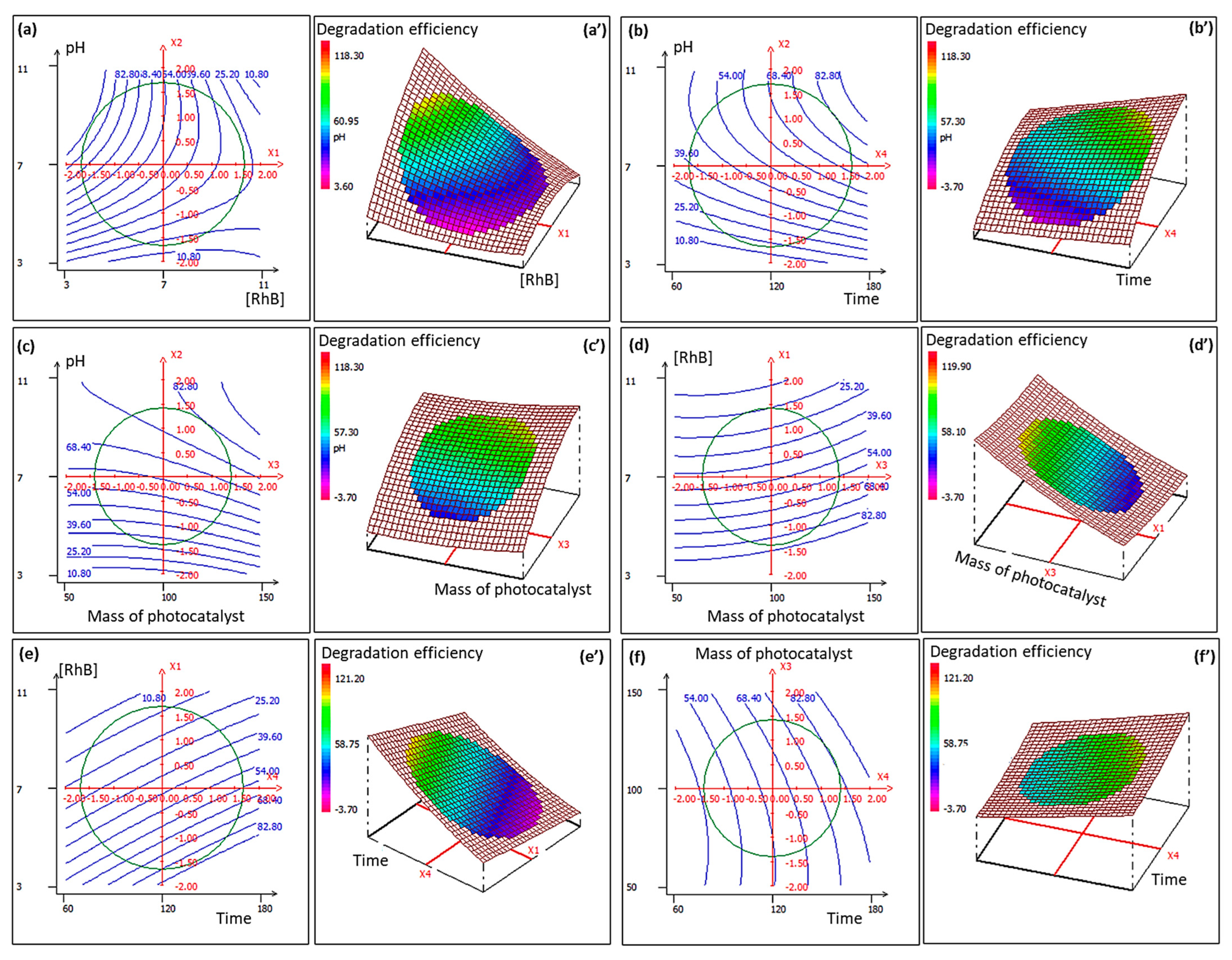
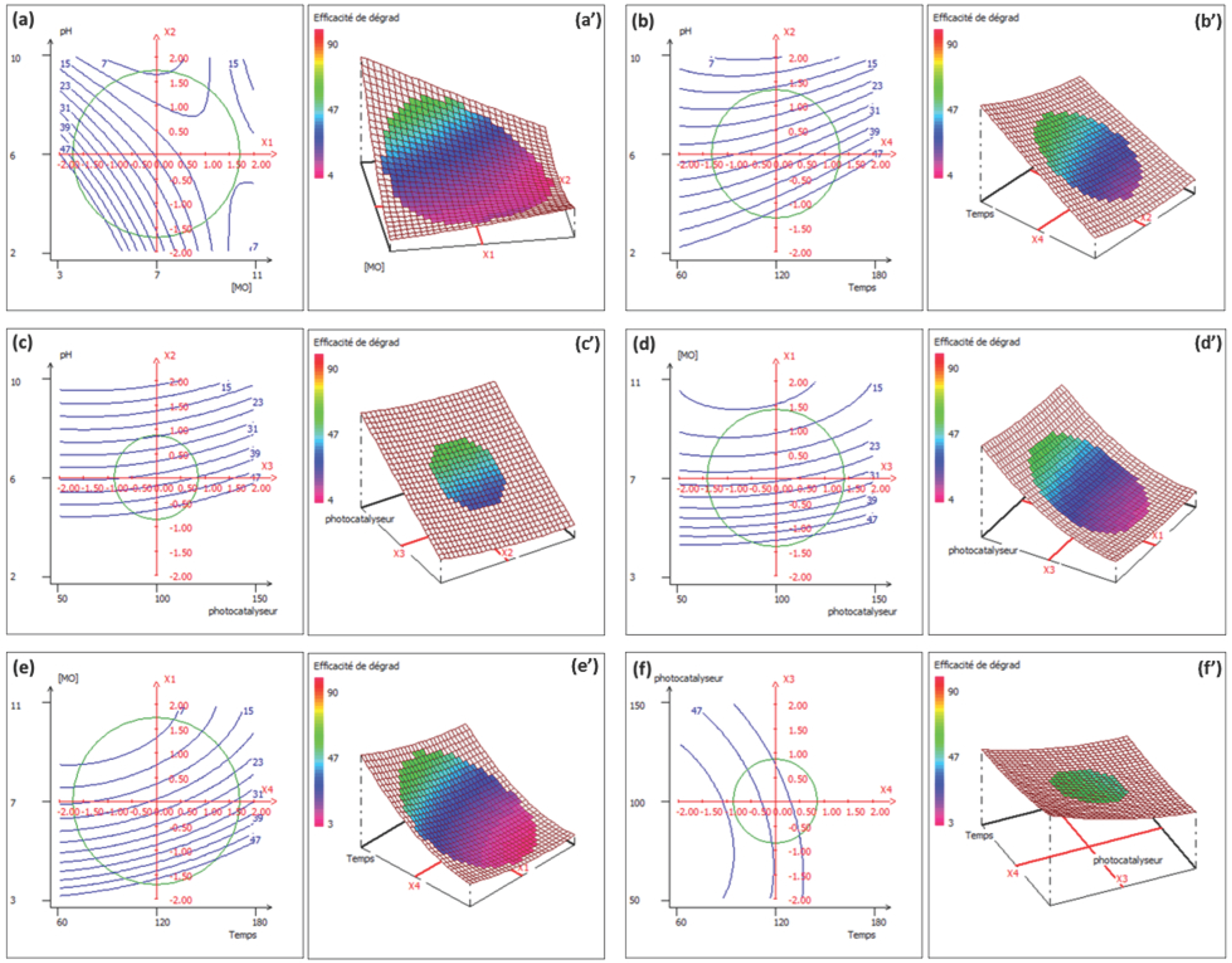
2.4. Photocatalytic Parameter Optimization via Response Surface Methodology
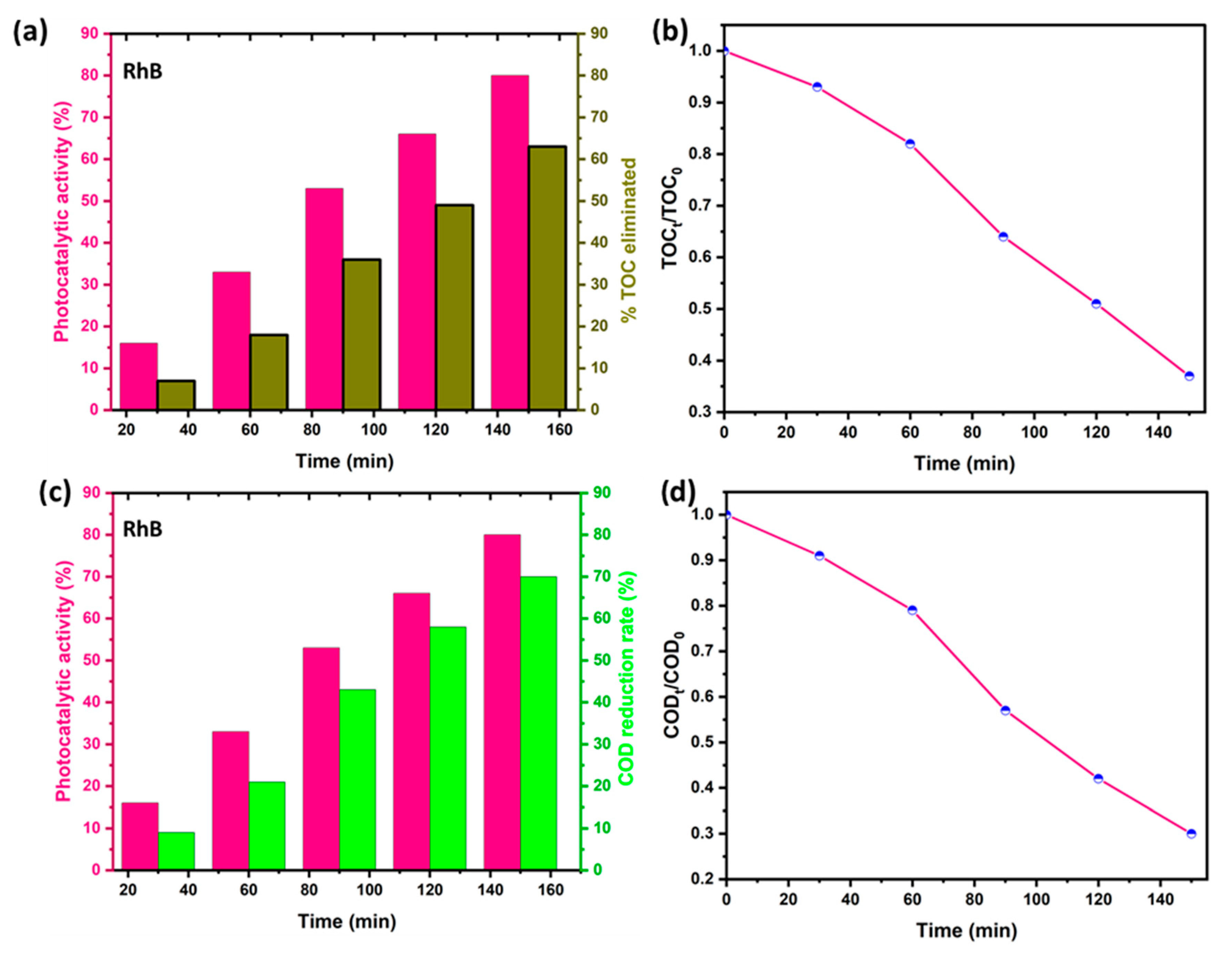
2.5. Kinetic Study of the Photocatalytic Activity of the Compound CoVO
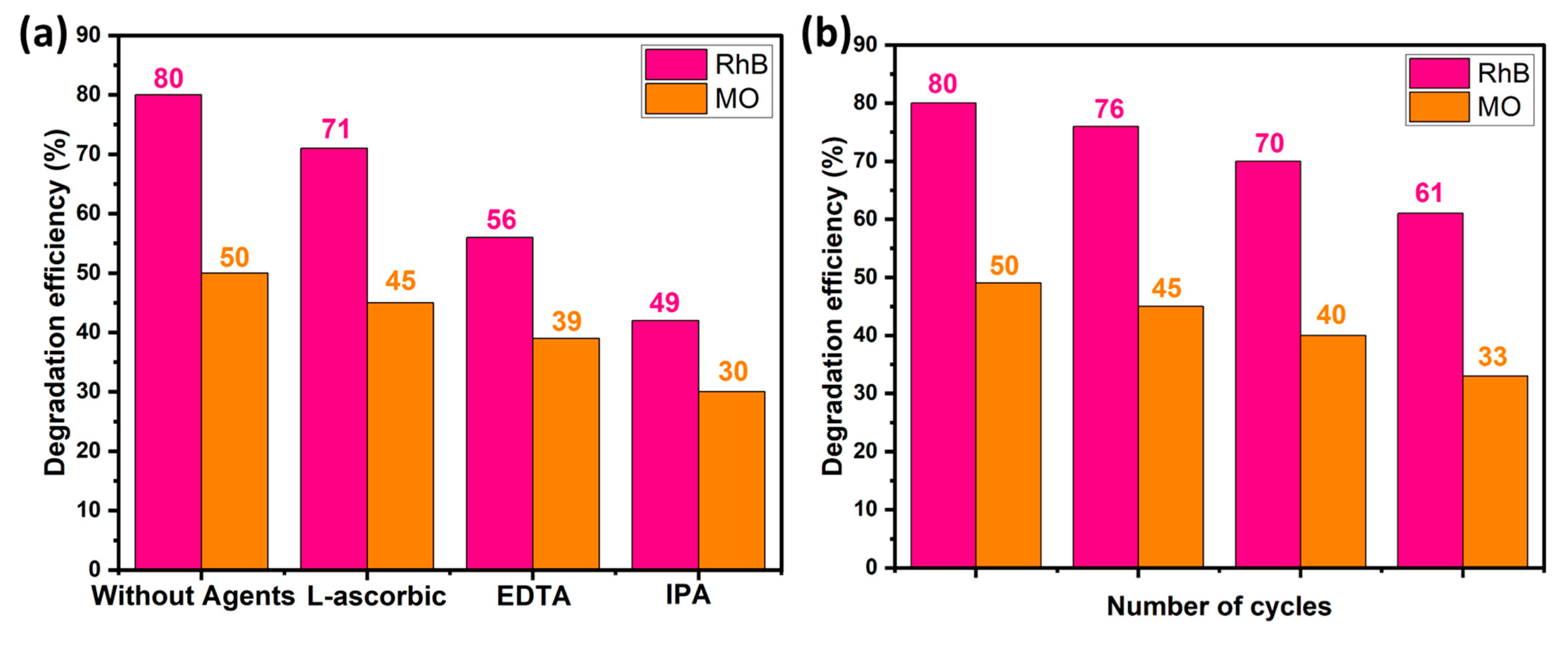
2.6. Trapping Test and Recyclability
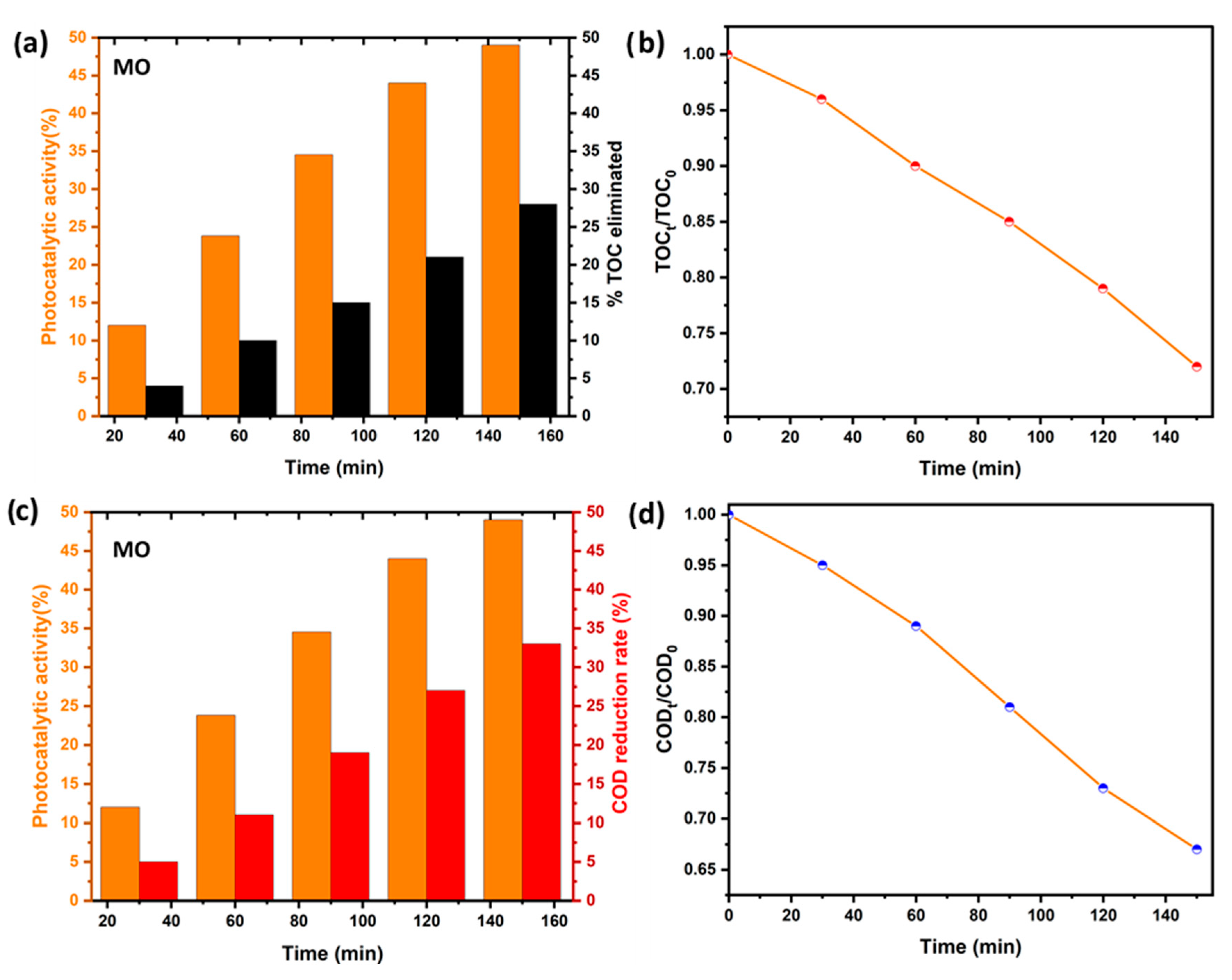
2.7. Breakdown Levels of Dye Decomposition by COD and TOC Analysis
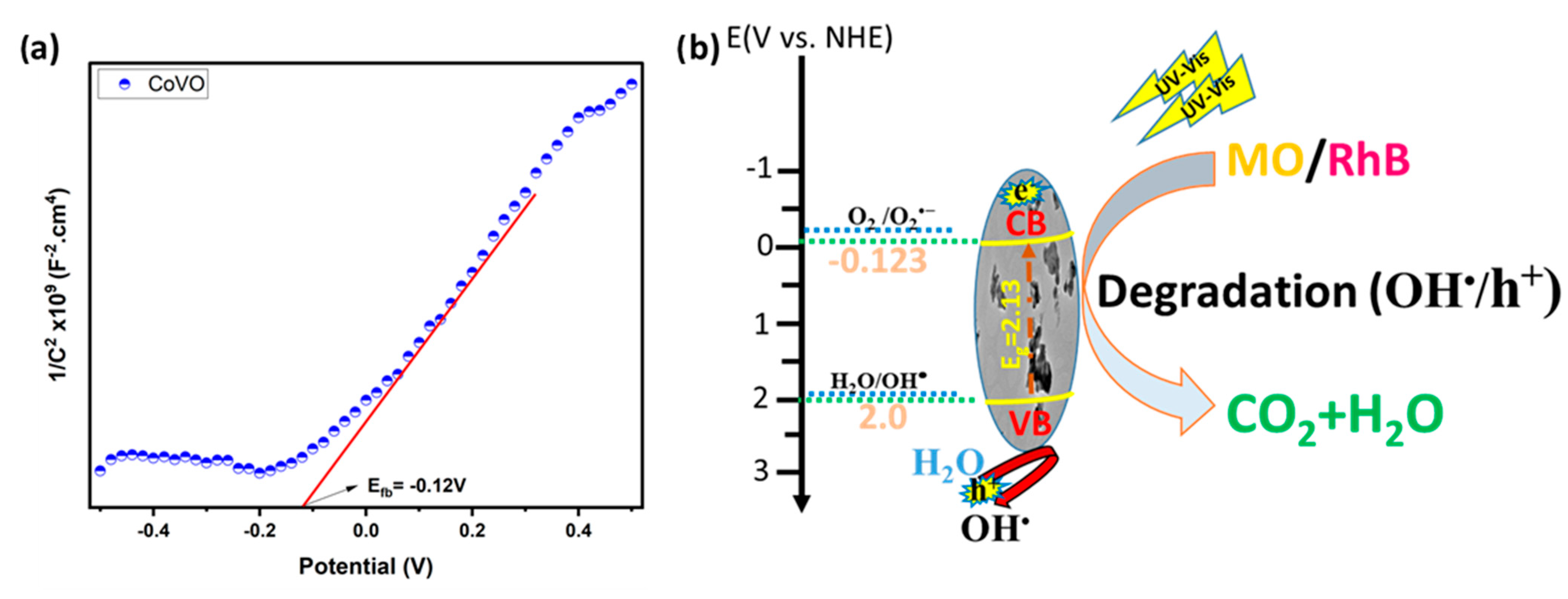
2.8. Proposed Mechanism for Photodegradation of RhB and MO on CoVO
3. Experimental Section
3.1. Materials and Reagents
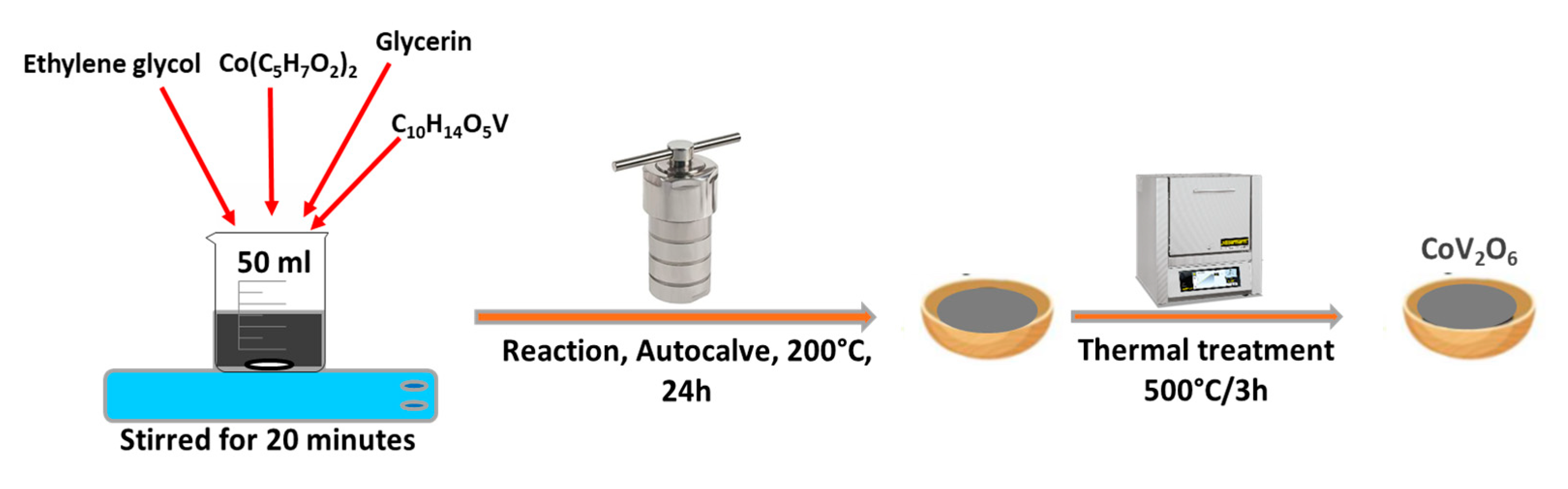
3.2. Synthesis of CoV2O6
3.3. Material Characterizations
3.4. Point of Zero Charge Determination
3.5. CCD-RSM Design and Data Analysis
3.6. Photocatalytic Procedure
3.7. Total Organic Carbon (TOC) and Chemical Oxygen Demand (COD)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- El Allaoui, B.; Benzeid, H.; Zari, N.; el kacem Qaiss, A.; Bouhfid, R. Cellulose Beads Supported CoFe2O4: A Novel Heterogeneous Catalyst for Efficient Rhodamine B Degradation via Advanced Oxidation Processes. Int. J. Biol. Macromol. 2023, 259, 128893. [Google Scholar] [CrossRef] [PubMed]
- El Aouni, A.; El Ouardi, M.; Arab, M.; Saadi, M.; Haspel, H.; Kónya, Z.; Ben Ali, A.; Jada, A.; BaQais, A.; Ait Ahsaine, H. Design of Bismuth Tungstate Bi2WO6 Photocatalyst for Enhanced and Environmentally Friendly Organic Pollutant Degradation. Materials 2024, 17, 1029. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of Coagulation–Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Asghari, A.; Hemati, M.; Ghaedi, M.; Rajabi, M.; Mirtamizdoust, B. Ultrasonic Assisted Adsorption of Basic Dyes from Binary Component Systems onto ZnO Nanoparticles Loaded on Activated Carbon Derived from Almond Shell: Optimization by Central Composite Design. J. Nanostructures 2014, 4, 17–30. [Google Scholar] [CrossRef]
- Dardari, O.; El Idrissi, A.; El Ouardi, M.; Channab, B.-E.; Layachi, O.A.; Farsad, S.; Marrane, S.E.; Mazkad, D.; BaQais, A.; Arab, M.; et al. Polyester Fabric Supported Graphene Oxide/Cu-Ag Bimetallic Nanoparticles as a “Dip Catalyst” for the Reduction of p-Nitrophenol and Organic Dyes. Colloids Surf. A Physicochem. Eng. Asp. 2024, 697, 134314. [Google Scholar] [CrossRef]
- Elaadssi, Y.; Madigou, V.; Arab, M. Cobalt Ferrites CoxFe3-xO4 (x = 1 and x = 1.5) as Photocatalysts under Simulated Sunlight: An Experimental Study Coupled to Predictive RSM Approach. Surf. Interfaces 2025, 68, 106655. [Google Scholar] [CrossRef]
- Chakravorty, A.; Roy, S. A Review of Photocatalysis, Basic Principles, Processes, and Materials. Sustain. Chem. Environ. 2024, 8, 100155. [Google Scholar] [CrossRef]
- Beil, S.B.; Bonnet, S.; Casadevall, C.; Detz, R.J.; Eisenreich, F.; Glover, S.D.; Kerzig, C.; Næsborg, L.; Pullen, S.; Storch, G.; et al. Challenges and Future Perspectives in Photocatalysis: Conclusions from an Interdisciplinary Workshop. JACS Au 2024, 4, 2746–2766. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-E.; Kim, M.-K.; Danish, M.; Jo, W.-K. State-of-the-Art Review on Photocatalysis for Efficient Wastewater Treatment: Attractive Approach in Photocatalyst Design and Parameters Affecting the Photocatalytic Degradation. Catal. Commun. 2023, 183, 106764. [Google Scholar] [CrossRef]
- El Ouardi, M.; Arab, M.; Saadi, M.; BaQais, A.; Ait Ahsaine, H. Vanadate-Based Photocatalytic Materials: A Perspective on Synthesis Approaches and Pollutants Photocatalytic Degradation. Nano Mater. Sci. 2024. [Google Scholar] [CrossRef]
- He, Y.; Cai, J.; Zhang, L.; Wang, X.; Lin, H.; Teng, B.; Zhao, L.; Weng, W.; Wan, H.; Fan, M. Comparing Two New Composite Photocatalysts, t-LaVO4/g-C3N4 and m-LaVO4/g-C3N4, for Their Structures and Performances. Ind. Eng. Chem. Res. 2014, 53, 5905–5915. [Google Scholar] [CrossRef]
- Jenifer, A.; Sriram, S. Enhanced Photocatalytic Organic Dye Degradation Activities of Pristine and Zn-Doped V2O5 Nanoparticles. Appl. Surf. Sci. 2023, 611, 155629. [Google Scholar] [CrossRef]
- Channab, B.-E.; Ouardi, M.E.; Layachi, O.A.; Marrane, S.E.; Idrissi, A.E.; BaQais, A.A.; Ahsaine, H.A. Recent Trends on MIL-Fe Metal-Organic Frameworks: Synthesis Approaches, Structural Insights, and Applications in Organic Pollutant Adsorption and Photocatalytic Degradation: A Review. Environ. Sci. Nano 2023, 10, 2957–2988. [Google Scholar] [CrossRef]
- Elaouni, A.; Ouardi, M.E.; Zbair, M.; BaQais, A.; Saadi, M.; Ahsaine, H.A. ZIF-8 Metal Organic Framework Materials as a Superb Platform for the Removal and Photocatalytic Degradation of Organic Pollutants: A Review. RSC Adv. 2022, 12, 31801–31817. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dhak, P.; Hazra, V.; Goswami, N.; Dhak, D. Synthesis of Mesoporous Fe/Al/La Trimetallic Oxide for Photodegradation of Various Water-Soluble Dyes: Kinetic, Mechanistic, and pH Studies. Environ. Res. 2023, 217, 114862. [Google Scholar] [CrossRef]
- Meena, P.L.; Poswal, K.; Surela, A.K.; Saini, J.K. Synthesis of Graphitic Carbon Nitride/Zinc Oxide (g-C3N4/ZnO) Hybrid Nanostructures and Investigation of the Effect of ZnO on the Photodegradation Activity of g-C3N4 against the Brilliant Cresyl Blue (BCB) Dye under Visible Light Irradiation. Adv. Compos. Hybrid. Mater. 2022, 6, 16. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Yi, Z.; Wang, S.; Ma, J.; Gao, H.; Wu, X.; Liu, G.; Yang, H. PH-Induced Structural Evolution, Photodegradation Mechanism and Application of Bismuth Molybdate Photocatalyst. Adv. Powder Technol. 2022, 33, 103858. [Google Scholar] [CrossRef]
- Mgidlana, S.; Nyokong, T. Asymmetrical Zinc (II) Phthalocyanines Cobalt Tungstate Nanomaterial Conjugates for Photodegradation of Methylene Blue. J. Photochem. Photobiol. A Chem. 2021, 418, 113421. [Google Scholar] [CrossRef]
- Sasikumar, K.; Rajamanikandan, R.; Ju, H. Z-Scheme Charge Transfer Heterostructure with NiV2O6@gC3N4 Nanocomposite: A Competent Photocatalyst for Boosting the Photodegradation of Antibiotics. J. Taiwan Inst. Chem. Eng. 2025, 168, 105960. [Google Scholar] [CrossRef]
- El Ouardi, M.; Madigou, V.; Chevallier, V.; Merlen, A.; BaQais, A.; Saadi, M.; Ait Ahsaine, H.; Arab, M. Synthesis of ZnV2O6 Nanosheet Photocatalysts for Efficient Photodegradation of Rhodamine B: Experimental and RSM Modeling. J. Environ. Chem. Eng. 2024, 12, 113505. [Google Scholar] [CrossRef]
- Wang, S.; She, L.; Zheng, Q.; Song, Y.; Yang, Y.; Chen, L. Ag-Doped CuV2O6 Nanowires for E hanced Visible-Light Photocatalytic CO2 Reduction. Ind. Eng. Chem. Res. 2023, 62, 455–465. [Google Scholar] [CrossRef]
- Mohamad Yusop, M.F.; Tamar Jaya, M.A.; Idris, I.; Abdullah, A.Z.; Ahmad, M.A. Optimization and Mass Transfer Simulation of Remazol Brilliant Blue R Dye Adsorption onto Meranti Wood Based Activated Carbon. Arab. J. Chem. 2023, 16, 104683. [Google Scholar] [CrossRef]
- Mohammadi, A.; Nemati, S.; Mosaferi, M.; Abdollahnejhad, A.; Almasian, M.; Sheikhmohammadi, A. Predicting the Capability of Carboxymethyl Cellulose-Stabilized Iron Nanoparticles for the Remediation of Arsenite from Water Using the Response Surface Methodology (RSM) Model: Modeling and Optimization. J. Contam. Hydrol. 2017, 203, 85–92. [Google Scholar] [CrossRef]
- Marrane, S.E.; Dänoun, K.; Allouss, D.; Sair, S.; Channab, B.-E.; Rhihil, A.; Zahouily, M. A Novel Approach to Prepare Cellulose-g-Hydroxyapatite Originated from Natural Sources as an Efficient Adsorbent for Heavy Metals: Batch Adsorption Optimization via Response Surface Methodology. ACS Omega 2022, 7, 28076–28092. [Google Scholar] [CrossRef]
- Vaez, M.; Zarringhalam Moghaddam, A.; Alijani, S. Optimization and Modeling of Photocatalytic Degradation of Azo Dye Using a Response Surface Methodology (RSM) Based on the Central Composite Design with Immobilized Titania Nanoparticles. Ind. Eng. Chem. Res. 2012, 51, 4199–4207. [Google Scholar] [CrossRef]
- Cho, I.-H.; Zoh, K.-D. Photocatalytic Degradation of Azo Dye (Reactive Red 120) in TiO2/UV System: Optimization and Modeling Using a Response Surface Methodology (RSM) Based on the Central Composite Design. Dye. Pigment. 2007, 75, 533–543. [Google Scholar] [CrossRef]
- Hua, K.; Xu, X.; Fang, D.; Bao, R.; Fu, Z.; Hu, J.; You, X.; Yi, J. In-Situ Synthesis and Room Temperature Magnetic Properties of Cobalt Vanadate Nanowire Array. J. Magn. Magn. Mater. 2019, 473, 435–441. [Google Scholar] [CrossRef]
- von Dreifus, D.; Pereira, R.; Rodrigues, A.D.; Pereira, E.C.; de Oliveira, A.J.A. Sol-Gel Synthesis of Triclinic CoV2O6 Polycrystals. Ceram. Int. 2018, 44, 19397–19401. [Google Scholar] [CrossRef]
- Moschogiannaki, M.; Zouridi, L.; Sukunta, J.; Phanichphant, S.; Gagaoudakis, E.; Liewhiran, C.; Kiriakidis, G.; Binas, V. High Performance Hydrogen Gas Sensors Based on PdO-Decorated p-Type CoV2O6 Nanoparticles. Sens. Actuators B Chem. 2020, 324, 128744. [Google Scholar] [CrossRef]
- Dhananjaya, M.; Guru Prakash, N.; Lakshmi Narayana, A.; Hussain, O.M. Microstructural and supercapacitive properties of one-dimensiona vanadium pentoxide nanowires synthesized by hydrothermal method. APPL PHYS A 2018, 124, 185. [Google Scholar] [CrossRef]
- Helal, A.; El-Sheikh, S.M.; Yu, J.; Eid, A.I.; El-Haka, S.A.; Samra, S.E. Novel Synthesis of BiVO4 Using Homogeneous Precipitation and Its Enhanced Photocatalytic Activity. J. Nanopart Res. 2020, 22, 132. [Google Scholar] [CrossRef]
- Chen, W.; Mai, L.; Peng, J.; Xu, Q.; Zhu, Q. Raman Spectroscopic Study of Vanadium Oxide Nanotubes. J. Solid State Chem. 2004, 177, 377–379. [Google Scholar] [CrossRef]
- He, X.; Zhang, C.; Tian, D. The Structure, Vibrational Spectra, and Thermal Expansion Study of AVO4 (A=Bi, Fe, Cr) and Co2V2O7. Materials 2020, 13, 1628. [Google Scholar] [CrossRef]
- Teng, Y.; Li, Y.; Yu, D.; Meng, Y.; Wu, Y.; Zhao, X.; Liu, X. The Microwave-Assisted Hydrothermal Synthesis of CoV2O6 and Co3V2O8 with Morphology Tuning by pH Adjustments for Supercapacitor Applications. ChemistrySelect 2019, 4, 956–962. [Google Scholar] [CrossRef]
- Chai, H.; Wang, Y.; Fang, Y.; Lv, Y.; Dong, H.; Jia, D.; Zhou, W. Low-Cost Synthesis of Hierarchical Co3V2O8 Microspheres as High-Performance Anode Materials for Lithium-Ion Batteries. Chem. Eng. J. 2017, 326, 587–593. [Google Scholar] [CrossRef]
- Soundharrajan, V.; Sambandam, B.; Song, J.; Kim, S.; Jo, J.; Kim, S.; Lee, S.; Mathew, V.; Kim, J. Co3V2O8 Sponge Network Morphology Derived from Metal–Organic Framework as an Excellent Lithium Storage Anode Material. ACS Appl. Mater. Interfaces 2016, 8, 8546–8553. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Teng, Y.; Zhang, Z.; Feng, Y.; Xue, P.; Tong, W.; Liu, X. Microwave-Assisted Synthesis of Novel Nanostructured Zn3(OH)2V2O7·2H2O and Zn2V2O7 as Electrode Materials for Supercapacitors. New J. Chem. 2017, 41, 15298–15304. [Google Scholar] [CrossRef]
- Wu, F.; Yu, C.; Liu, W.; Wang, T.; Feng, J.; Xiong, S. Large-Scale Synthesis of Co2V2O7 Hexagonal Microplatelets under Ambient Conditions for Highly Reversible Lithium Storage. J. Mater. Chem. A 2015, 3, 16728–16736. [Google Scholar] [CrossRef]
- Maitra, A.; Das, A.K.; Karan, S.K.; Paria, S.; Bera, R.; Khatua, B.B. A Mesoporous High-Performance Supercapacitor Electrode Based on Polypyrrole Wrapped Iron Oxide Decorated Nanostructured Cobalt Vanadium Oxide Hydrate with Enhanced Electrochemical Capacitance. Ind. Eng. Chem. Res. 2017, 56, 2444–2457. [Google Scholar] [CrossRef]
- Desseigne, M.; Madigou, V.; Coulet, M.-V.; Heintz, O.; Chevallier, V.; Arab, M. Au/WO3 Nanocomposite Based Photocatalyst for Enhanced Solar Photocatalytic Activity. J. Photochem. Photobiol. A Chem. 2023, 437, 114427. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Shawabkeh, R.; Al-Harahsheh, A.; Tarawneh, K.; Batiha, M.M. Surface Modification and Characterization of Jordanian Kaolinite: Application for Lead Removal from Aqueous Solutions. Appl. Surf. Sci. 2009, 255, 8098–8103. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, X.; Yang, F.; Ning, X.; Zhan, L.; Wu, Z.; Zhou, X. Generating a Captivating S-Scheme CuBi2O4/CoV2O6 Heterojunction with Boosted Charge Spatial Separation for Efficiently Removing Tetracycline Antibiotic from Wastewater. J. Clean. Prod. 2022, 357, 131992. [Google Scholar] [CrossRef]
- Hossain, M.K.; Sotelo, P.; Sarker, H.P.; Galante, M.T.; Kormányos, A.; Longo, C.; Macaluso, R.T.; Huda, M.N.; Janáky, C.; Rajeshwar, K. Rapid One-Pot Synthesis and Photoelectrochemical Properties of Copper Vanadates. ACS Appl. Energy Mater. 2019, 2, 2837–2847. [Google Scholar] [CrossRef]
- Yang, A.; Luo, J.; Xie, Z.; Chen, Q. Photocatalytic Activity of V2O5/ZnV2O6 Catalysts and Its Origin: Insights into Enhanced Photocatalytic Mechanisms via DFT Study. Appl. Surf. Sci. 2022, 599, 153894. [Google Scholar] [CrossRef]
- Zang, S.; Cai, X.; Chen, M.; Teng, D.; Jing, F.; Leng, Z.; Zhou, Y.; Lin, F. Tunable Carrier Transfer of Polymeric Carbon Nitride with Charge-Conducting CoV2O6∙2H2O for Photocatalytic O2 Evolution. Nanomaterials 2022, 12, 1931. [Google Scholar] [CrossRef]
- Lin, X.; Yu, L.-L.; Yan, L.-N.; Guan, Q.-F.; Yan, Y.-S.; Zhao, H. Controllable Synthesis and Photocatalytic Activity of Spherical, Flowerlike and Threadlike Bismuth Vanadates. Acta Phys.-Chim. Sin. 2013, 29, 1771–1777. [Google Scholar] [CrossRef]
- Haleem, A.; Ullah, M.; ur Rehman, S.; Shah, A.; Farooq, M.; Saeed, T.; Ullah, I.; Li, H. In-Depth Photocatalytic Degradation Mechanism of the Extensively Used Dyes Malachite Green, Methylene Blue, Congo Red, and Rhodamine B via Covalent Organic Framework-Based Photocatalysts. Water 2024, 16, 1588. [Google Scholar] [CrossRef]
- Oon, Y.-L.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Dahalan, F.A.; Oon, Y.-S.; Teoh, T.-P.; Lehl, H.K.; Thung, W.-E. Constructed Wetland–Microbial Fuel Cell for Azo Dyes Degradation and Energy Recovery: Influence of Molecular Structure, Kinetics, Mechanisms and Degradation Pathways. Sci. Total Environ. 2020, 720, 137370. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, R.; Suresh, R.; Jayaprakash, N. One-Step Formation of CoV2O6 Nanostructure and Its Photocatalytic Activity. AJC 2025, 37, 2237–2243. [Google Scholar] [CrossRef]
- Ghiyasiyan-Arani, M.; Masjedi-Arani, M.; salavati-Niasari, M. Size Controllable Synthesis of Cobalt Vanadate Nanostructures with Enhanced Photocatalytic Activity for the Degradation of Organic Dyes. J. Mol. Catal. A Chem. 2016, 425, 31–42. [Google Scholar] [CrossRef]
- Matsumoto, Y. Energy positions of oxide semiconductors and photocatalysis with iron complex oxides. J. Solid. State Chem 1996, 126, 227–234. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Lin, C.K.; Lin, J. Core–Shell Structured SiO2@YVO4:Dy3+/Sm3+ Phosphor Particles: Sol–Gel Preparation and Characterization. J. Colloid Interface Sci. 2006, 300, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, Q.; Chen, G.; Yan, B.; Yao, J.; Wang, H.; Wang, R.; Zhang, Y. Application of S-Type Heterojunction CaTiO3/rGO-g-C3N4 Photocatalyst in the Conversion of Waste Cooking Oil to Biodiesel: Optimization via RSM and Performance Evaluation. Sep. Purif. Technol. 2025, 362, 131922. [Google Scholar] [CrossRef]
- Sulaiman, N.F.; Mubin, M.H.A.; Gunasekaran, S.S.; Sofiah, A.G.N.; Chaudhary, K.T.; Mohamad, S.N.; Ali, J. Optimization Using RSM-CCD in the Fabrication of Magnesium-Doped Bismuth Ferrite Nanoparticles via Sol–Gel Auto-Combustion Method for Enhanced Photocatalytic Performance. Results Phys. 2025, 69, 108114. [Google Scholar] [CrossRef]

| Parameters Levels | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pollutants | RhB | MO | ||||||||
| CCD levels | −α | −1 | 0 | +1 | +α | −α | −1 | 0 | +1 | +α |
| [Pollutants] (mg L−1): A | 3 | 5 | 7 | 9 | 11 | 3 | 5 | 7 | 9 | 11 |
| pH: B | 3 | 5 | 7 | 9 | 11 | 2 | 4 | 6 | 8 | 10 |
| mass of photocatalyst (mg): C | 50 | 75 | 100 | 125 | 150 | 50 | 75 | 100 | 125 | 150 |
| Irradiation time (min): D | 60 | 90 | 120 | 150 | 180 | 60 | 90 | 120 | 150 | 180 |
| RhB | MO | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Test | A | C | D | B | Y Experience (%) | Y Predicted (%) | B | Y Experience (%) | Y Predicted (%) |
| 1 | 5 | 75 | 90 | 5 | 23.87 | 24.836 | 4 | 35.2 | 31.8 |
| 2 | 9 | 75 | 90 | 5 | 13.32 | 17.53 | 4 | 11.3 | 6.4 |
| 3 | 5 | 75 | 90 | 9 | 50.61 | 50.34 | 8 | 13.8 | 12.7 |
| 4 | 9 | 75 | 90 | 9 | 22.24 | 16.46 | 8 | 8.2 | 10.1 |
| 5 | 5 | 125 | 90 | 5 | 29.19 | 29.6 | 4 | 37.6 | 34.7 |
| 6 | 9 | 125 | 90 | 5 | 19.33 | 20.73 | 4 | 11.5 | 7.3 |
| 7 | 5 | 125 | 90 | 9 | 58.9 | 57.41 | 8 | 15.9 | 15.0 |
| 8 | 9 | 125 | 90 | 9 | 29.98 | 21.96 | 8 | 10.3 | 10.4 |
| 9 | 5 | 75 | 150 | 5 | 32.41 | 37.08 | 4 | 46.6 | 43.0 |
| 10 | 9 | 75 | 150 | 5 | 21.17 | 20.71 | 4 | 17.9 | 13.7 |
| 11 | 5 | 75 | 150 | 9 | 75.82 | 72.47 | 8 | 18.1 | 17.1 |
| 12 | 9 | 75 | 150 | 9 | 33.29 | 29.53 | 8 | 11.2 | 10.6 |
| 13 | 5 | 125 | 150 | 5 | 41.10 | 44.93 | 4 | 55.3 | 48.2 |
| 14 | 9 | 125 | 150 | 5 | 30.07 | 26.99 | 4 | 19.2 | 16.8 |
| 15 | 5 | 125 | 150 | 9 | 90.19 | 82.63 | 8 | 20.2 | 21.6 |
| 16 | 9 | 125 | 150 | 9 | 41.03 | 38.12 | 8 | 14.8 | 13.1 |
| 17 | 3 | 100 | 120 | 7 | 72.69 | 71.43 | 6 | 39.6 | 44.5 |
| 18 | 11 | 100 | 120 | 7 | 13.08 | 19.62 | 6 | 6.8 | 10.6 |
| 19 | 7 | 100 | 120 | 3 | 10.78 | 2.15 | 2 | 14.2 | 26.2 |
| 20 | 7 | 100 | 120 | 11 | 24.88 | 38.78 | 10 | 6.7 | 3.4 |
| 21 | 7 | 50 | 120 | 7 | 37.19 | 36.42 | 6 | 10.2 | 14.4 |
| 22 | 7 | 150 | 120 | 7 | 43.72 | 49.77 | 6 | 15.2 | 19.8 |
| 23 | 7 | 100 | 60 | 7 | 22.92 | 24.54 | 6 | 8.2 | 11.7 |
| 24 | 7 | 100 | 180 | 7 | 49.29 | 52.94 | 6 | 20.3 | 25.6 |
| 25 | 7 | 100 | 120 | 7 | 38.12 | 37.93 | 6 | 13.3 | 13.7 |
| 26 | 7 | 100 | 120 | 7 | 37.88 | 37.93 | 6 | 13.8 | 13.7 |
| 27 | 7 | 100 | 120 | 7 | 37.79 | 37.93 | 6 | 14.0 | 13.7 |
| RhB | MO | |||
|---|---|---|---|---|
| Source | Coefficients | p-Value | Coefficients | p-Value |
| Model | - | <0.01 | - | <0.01 |
| b0 | 37.93 | <0.01 | 13.7 | 0.0199 |
| bA | −12.95 | <0.01 | −8.5 | <0.01 |
| bB | 9.15 | <0.01 | −5.7 | 0.0143 |
| bC | 3.33 | 0.0109 | 1.4 | 0.250 |
| bD | 7.09 | <0.01 | 3.5 | 0.0384 |
| bA-A | 1.89 | 0.0378 | 3.5 | 0.0438 |
| bB-B | −4.362 | <0.01 | 0.3 | 6.2 |
| bC-C | 1.29 | 0.0816 | 0.8 | 0.729 |
| bD-D | 0.204 | 3.11 | 1.2 | 0.344 |
| bA-B | −6.64 | <0.01 | 5.7 | 0.0215 |
| bA-C | −0.39 | 1.16 | −0.5 | 2.58 |
| bB-C | 0.576 | 0.543 | −0.2 | 19.4 |
| bA-D | −2.26 | 0.0354 | −1 | 0.705 |
| bB-D | 2.47 | 0.0297 | −1.7 | 0.241 |
| bC-D | 0.771 | 0.304 | 0.6 | 2.17 |
| R2 | 0.937 | 0.89 | ||
| Adj, R2 | 0.862 | 0.77 | ||
| RhB | MO | |||||
|---|---|---|---|---|---|---|
| Factor | Optimum Values | Predicted Rate (%) | Exp Rate (%) | Optimum Values | Predicted Rate (%) | Exp Rate (%) |
| [RhB] (mg/L): A | 5 | 85% | 80% | 4 | 55% | 50% |
| pH: B | 9 | 4 | ||||
| Mass of photocatalyst (mg): C | 110 | 100 | ||||
| Time (min): D | 150 | 150 | ||||
| Photocatalyst | Pollutant Studied | Synthesis Procedure | Conditions (Concentration, Light Source) | Decomposition Efficacy | Reference |
|---|---|---|---|---|---|
| CoV2O6 | Methylene Blue, Sunset Yellow, Brilliant Blue | Thermal decomposition | 1 × 10–5 M; Visible light + H2O2 | MB: ~92.8% in 30 min; SY: 72% in 135 min; BB: 12.5% in 150 min | [49] |
| CuBi2O4/CoV2O6 | Tetracycline antibiotics | In situ precipitation-calcination | 20 mg L−1; Visible light | 93.4% in 180 min | [42] |
| CoV2O6 | Methylene Blue | Solid-state | UV and visible source | 39% in 120 min | [50] |
| CoV2O6 | Rhodamine B (RhB) and Methyl Orange | Solvothermal | 5 mg L−1 for RhB and 4 mg L−1 for MO and visible source | RhB: 80% in 150 min; MO: 50% in 150 min | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Ouardi, M.; Madigou, V.; Chevallier, V.; Haspel, H.; BaQais, A.; Saadi, M.; Ahsaine, H.A.; Arab, M. Response Surface Modeling and Photocatalytic Assessment of CoV2O6 for the Treatment of Organic Dyes. Catalysts 2025, 15, 908. https://doi.org/10.3390/catal15090908
El Ouardi M, Madigou V, Chevallier V, Haspel H, BaQais A, Saadi M, Ahsaine HA, Arab M. Response Surface Modeling and Photocatalytic Assessment of CoV2O6 for the Treatment of Organic Dyes. Catalysts. 2025; 15(9):908. https://doi.org/10.3390/catal15090908
Chicago/Turabian StyleEl Ouardi, Mohamed, Véronique Madigou, Virginie Chevallier, Henrik Haspel, Amal BaQais, Mohamed Saadi, Hassan Ait Ahsaine, and Madjid Arab. 2025. "Response Surface Modeling and Photocatalytic Assessment of CoV2O6 for the Treatment of Organic Dyes" Catalysts 15, no. 9: 908. https://doi.org/10.3390/catal15090908
APA StyleEl Ouardi, M., Madigou, V., Chevallier, V., Haspel, H., BaQais, A., Saadi, M., Ahsaine, H. A., & Arab, M. (2025). Response Surface Modeling and Photocatalytic Assessment of CoV2O6 for the Treatment of Organic Dyes. Catalysts, 15(9), 908. https://doi.org/10.3390/catal15090908









