Precursor Engineering of SO42−-Rich CeO2-Pt-TiO2-Fe2O3 Catalyst with Oxygen Vacancy-Mediated Ternary Synergy for Ultralow-Temperature Methane Combustion
Abstract
1. Introduction
2. Experimental Section
2.1. Catalyst Synthesis
- (1)
- Carrier preparation (CeO2-Fe2O3-TiO2): Titanium oxysulfate (TiOSO4·xH2O, ≥99%, Chongqing Boyi Chemical Reagent Co., Ltd., Chongqing, China), iron nitrate (Fe(NO3)3·9H2O, 98%, Chongqing Beibei Chemical Reagent Factory, Chongqing, China), and cerium nitrate (Ce(NO3)3·6H2O, 99%, Chongqing Boyi Chemical Reagent Co., Ltd., Chongqing, China) were dissolved in deionized water at a Fe:Ce:Ti molar ratio of 1:1:8. After 30 min of magnetic stirring, ammonium hydroxide (25 wt%, Chongqing Beibei Chemical Reagent Factory, Chongqing, China) was added dropwise to adjust the pH to 9.0, triggering simultaneous hydrolysis of TiOSO4 and co-precipitation of Fe/Ce hydroxides. The gel was aged at 60 °C for 24 h, centrifuged (8000 rpm, 10 min), washed to neutrality, dried at 110 °C under vacuum, and calcined at 500 °C (2 °C/min, 4 h) in air to obtain the CeO2-Fe2O3-TiO2 carrier (denoted as CFT-TS).
- (2)
- Pt loading: Chloroplatinic acid (H2PtCl6·6H2O, Chongqing Beibei Chemical Reagent Factory, Chongqing, China) solution containing 0.5 wt% Pt was impregnated onto CFT-TS via ultrasonication (30 min), followed by drying (80 °C, 6 h) and calcination (300 °C, 3 °C/min, 2 h in air).
- (3)
- Control catalysts: CFT-TC, CFT-TB, and CFT-CM carriers were synthesized using TiCl4 (Chongqing Boyi Chemical Reagent Co., Ltd., Chongqing, China), titanium butoxide (Ti(OC4H9)4)(Chongqing Boyi Chemical Reagent Co., Ltd., Chongqing, China), and commercial TiO2 (P25, Chongqing Beibei Chemical Reagent Factory, Chongqing, China), respectively, under identical conditions.
2.2. Characterization
2.3. Catalytic Evaluation
3. Results and Discussion
3.1. Phase and Structural Properties
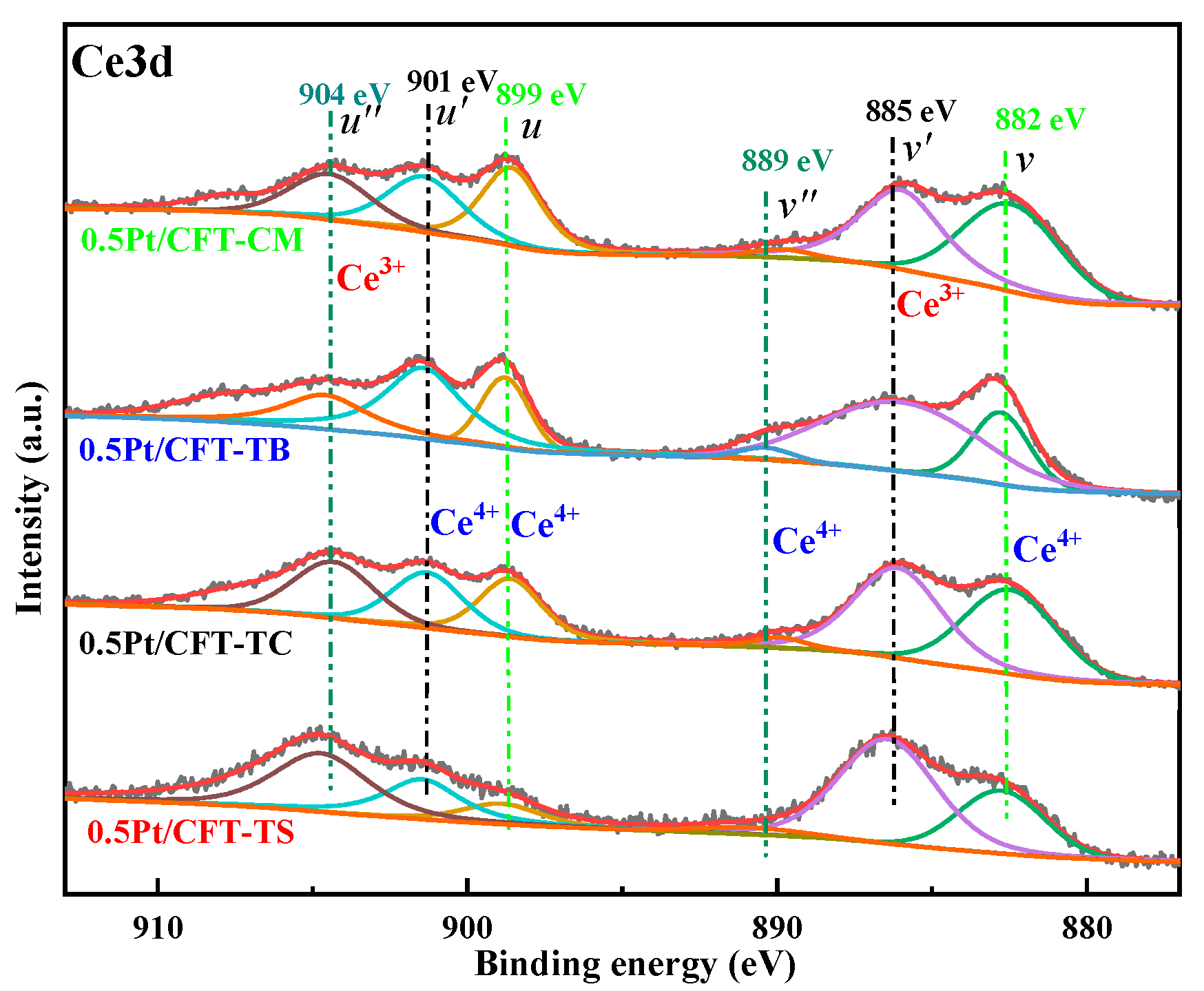
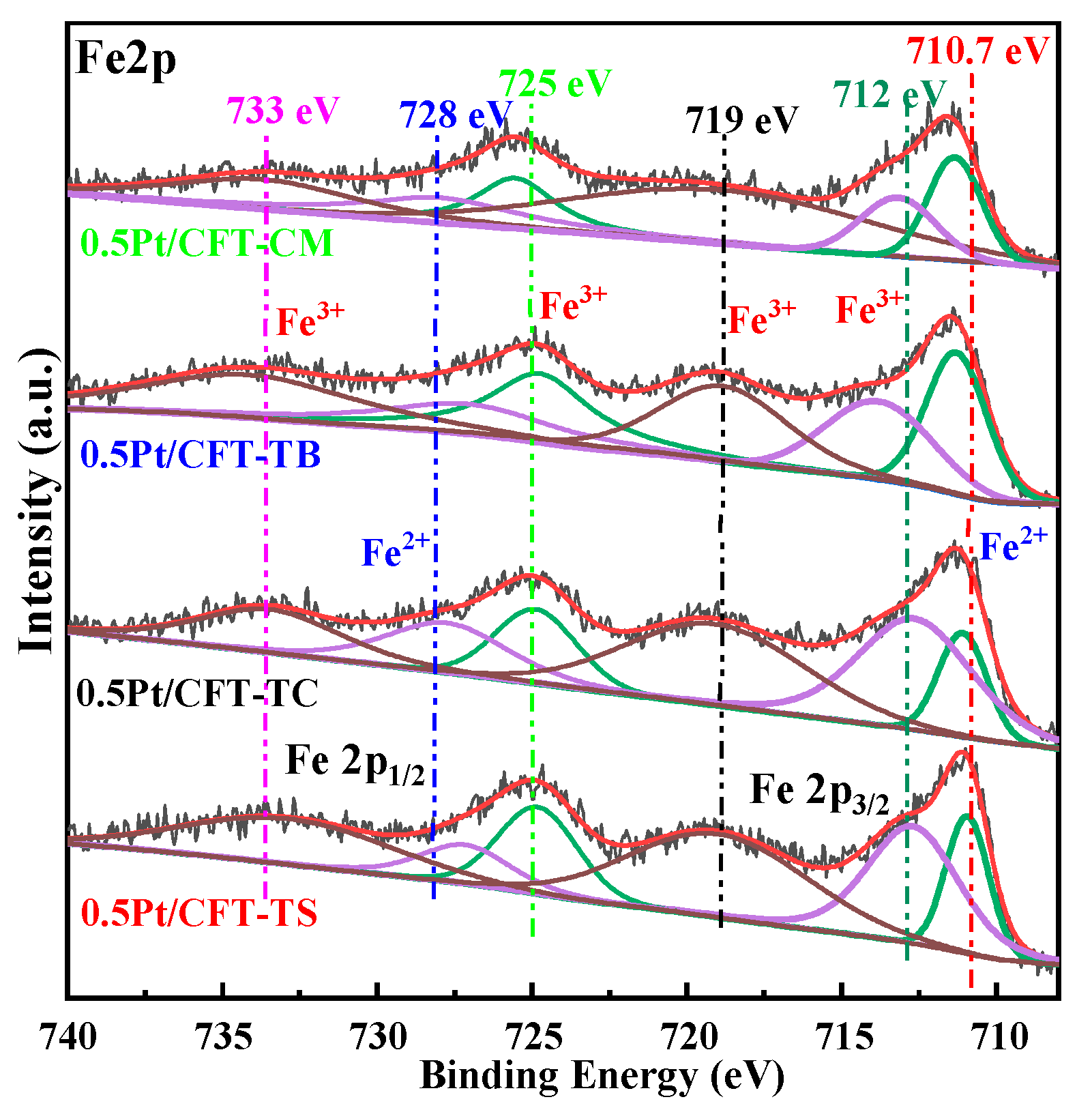
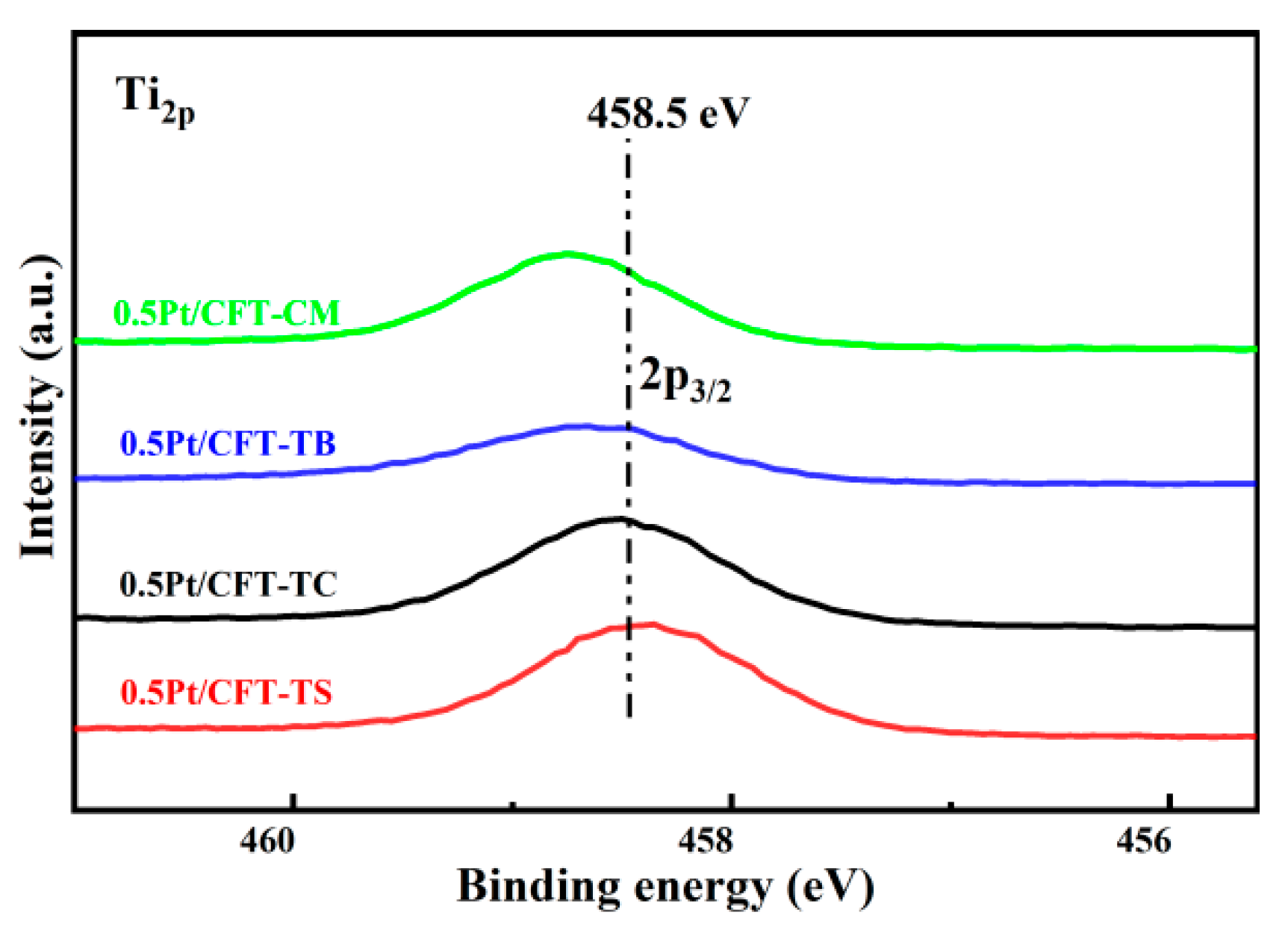
3.2. Surface Chemical States
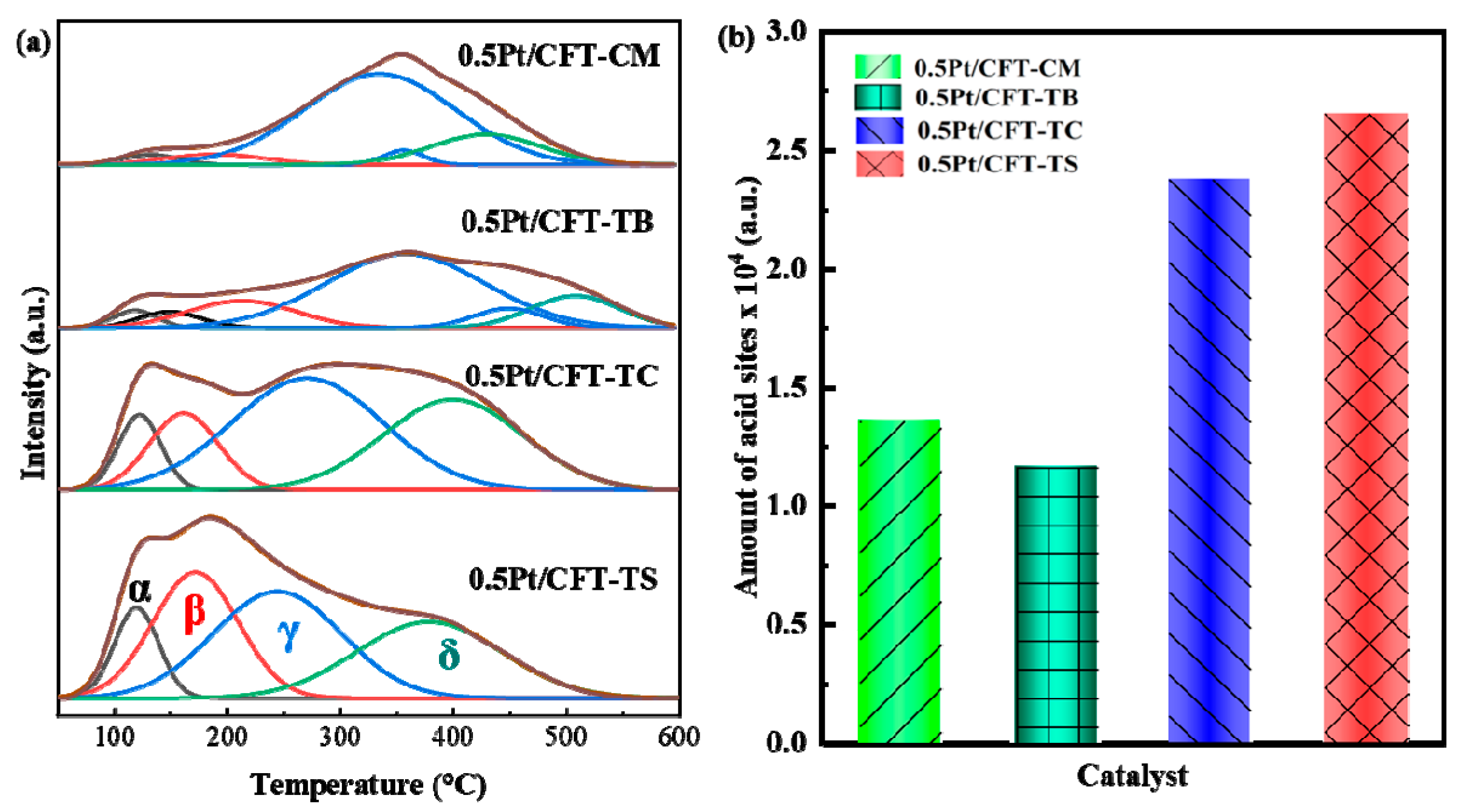
3.3. Redox Behavior and Acidic Properties
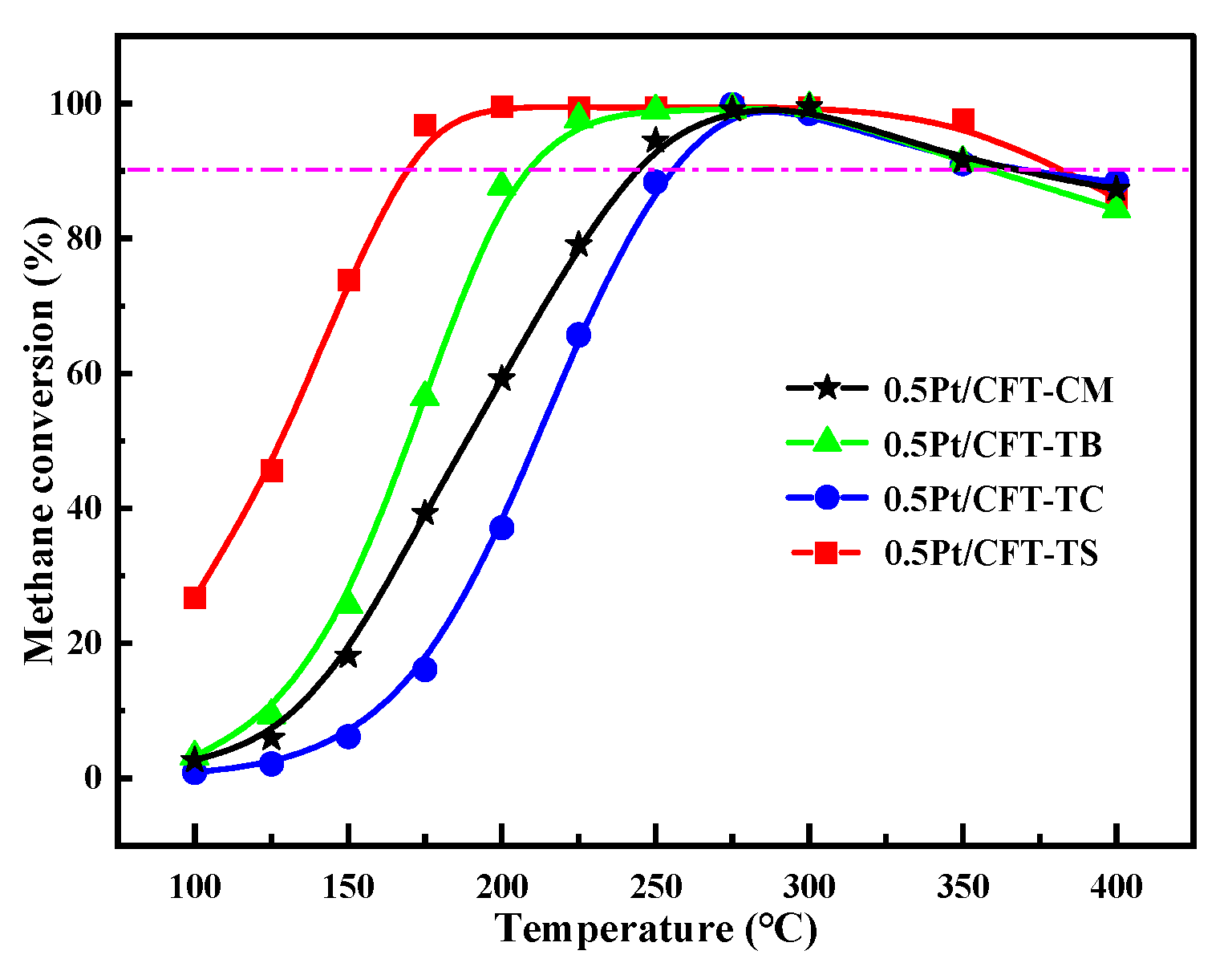
3.4. Catalytic Performance and Kinetics
3.5. Mechanistic Analysis
3.5.1. Pt0-Mediated O2 Activation at Fe3+-Ce3+ Interfaces
3.5.2. Dynamic Oxygen Vacancy Regeneration via Fe3+/Ce4+ Redox Cycling
3.5.3. *O Migration and C–H Bond Cleavage at Ptδ+-Oᵥ-Ce3+ Sites
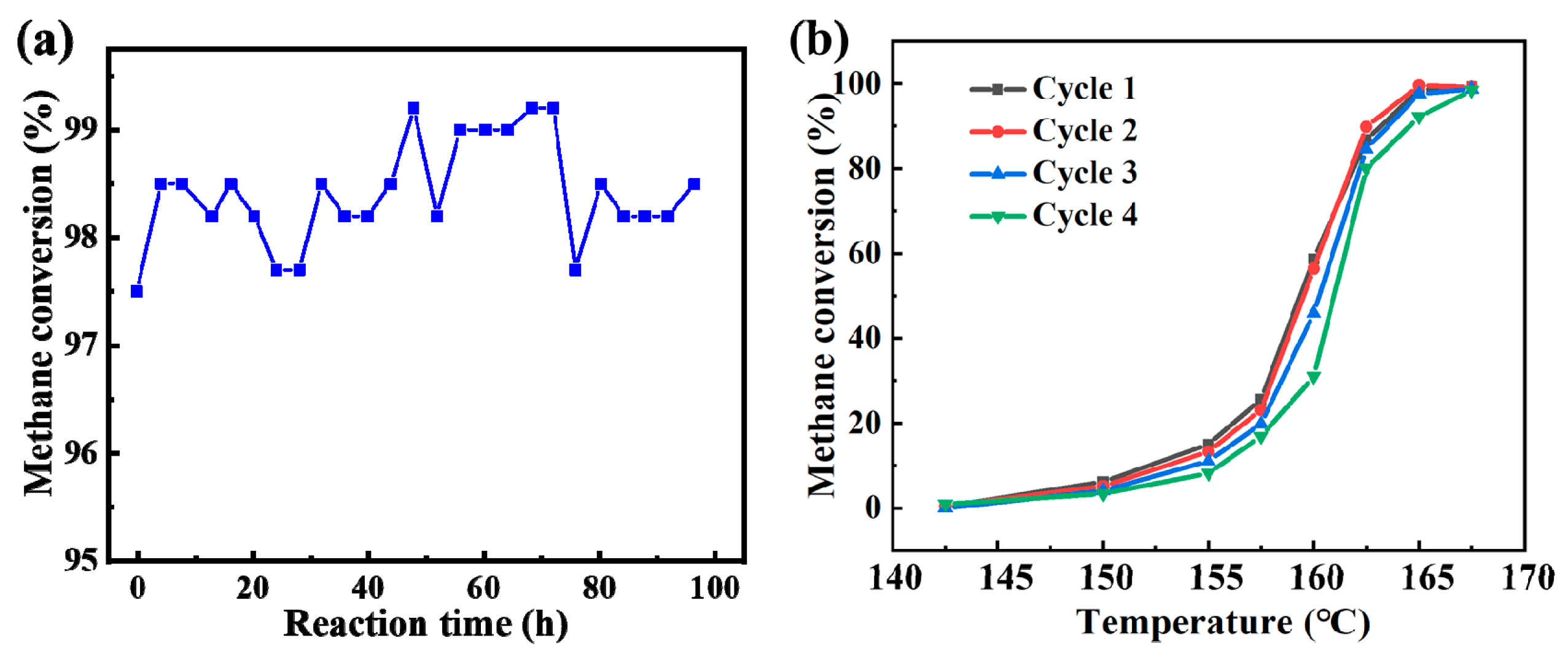
3.5.4. Ternary Synergy and Industrial Viability
4. Conclusions
- (1)
- Synergistic Catalyst Design: The use of TiOSO4 as a precursor enables the formation of a sulfate-rich, mesoporous CeO2–Fe2O3–TiO2 support, which enhances Brønsted acidity (1.23 mmol/g NH3), promotes oxygen vacancy formation (Oα = 51.16%), and facilitates ultrahigh dispersion of Pt nanoparticles (65% dispersion), effectively inhibiting sintering and stabilizing active sites.
- (2)
- Enhanced Redox Dynamics and Mechanism: The catalyst establishes a dynamic Pt0–Fe3+/Ce4+–OV interfacial synergy, enabling efficient redox cycling and lowering the activation energy for methane combustion to 46.77 kJ/mol. This synergy significantly reduces the oxygen vacancy regeneration barrier and enhances lattice oxygen mobility.
- (3)
- Superior Catalytic Performance: The catalyst achieves 90% CH4 conversion at 163 °C under industrial conditions (1% CH4, 4% O2, GHSV = 30,000 h−1), with complete conversion reached at 450 °C. It also exhibits excellent stability over 100 h, highlighting its potential for practical applications.
- (4)
- Broader Implications: This work provides a scalable and innovative strategy for designing low-noble-metal catalysts via precursor-mediated defect engineering, offering new insights into the role of sulfate in promoting multifunctional synergy for catalytic combustion and other energy-related processes.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feng, X.; Jiang, L.; Li, D.; Tian, S.; Zhu, X.; Wang, H.; He, C.; Li, K. Progress and key challenges in catalytic combustion of lean methane. J. Energy Chem. 2022, 75, 173–215. [Google Scholar] [CrossRef]
- Araújo, J.C.; Oton, L.F.; Oliveira, A.C.; Lang, R.; Otubo, L.; Bueno, J.M. On the role of size controlled Pt particles in nanostructured Pt-containing Al2O3 catalysts for partial oxidation of methane. Int. J. Hydrogen Energy 2019, 44, 27329–27342. [Google Scholar] [CrossRef]
- Ding, S.; Chen, H.-A.; Mekasuwandumrong, O.; Hülsey, M.J.; Fu, X.; He, Q.; Panpranot, J.; Yang, C.-M.; Yan, N. High-temperature flame spray pyrolysis induced stabilization of Pt single-atom catalysts. Appl. Catal. B Environ. 2021, 281, 119471. [Google Scholar]
- Yasumura, S.; Saita, K.; Miyakage, T.; Nagai, K.; Kon, K.; Toyao, T.; Maeno, Z.; Taketsugu, T.; Shimizu, K.I. Designing main-group catalysts for low-temperature methane combustion by ozone. Nat. Commun. 2023, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Li, C.; Zhu, Q.; Wang, H.; Qi, J. Rich Oxygen Vacancies in Bimetallic MnCo2O4.5 Spheres for Enhancing Lean Methane Catalytic Oxidation. Nanomaterials 2025, 15, 524. [Google Scholar] [CrossRef]
- Wu, J.; Gao, J.; Lian, S.; Li, J.; Sun, K.; Zhao, S.; Kim, Y.D.; Ren, Y.; Zhang, M.; Liu, Q.; et al. Engineering the oxygen vacancies enables Ni single-atom catalyst for stable and efficient C–H activation. Appl. Catal. B Environ. 2022, 314, 121516. [Google Scholar] [CrossRef]
- Hantoko, D.; Khan, W.U.; Putra, A.F.P.; Shoaibi, A.A.; Chandrasekar, S.; Hossain, M.M. Fe–Ce–Al Catalysts for Decomposition of Methane to High Purity Hydrogen and High-Value Carbon. Ind. Eng. Chem. Res. 2024, 63, 18869–18878. [Google Scholar]
- Lee, J.; Lim, T.H.; Lee, E.; Kim, D.H. Promoting the Methane Oxidation on Pd/CeO2 Catalyst by Increasing the Surface Oxy-gen Mobility via Defect Engineering. ChemCatChem 2021, 13, 3706–3712. [Google Scholar]
- Pu, C.; Zhang, J.; Chang, G.; Xiao, Y.; Ma, X.; Wu, J.; Luo, T.; Huang, K.; Ke, S.; Li, J.; et al. Nitrogen precursor-mediated construction of N-doped hierarchically porous carbon-supported Pd catalysts with controllable morphology and composition. Carbon 2020, 159, 451–460. [Google Scholar] [CrossRef]
- Senanayake, S.D.; Rodriguez, J.A.; Weaver, J.F. Low Temperature Activation of Methane on Metal-Oxides and Complex Interfaces: Insights from Surface Science. Acc. Chem. Res. 2020, 53, 1488–1497. [Google Scholar] [CrossRef]
- Thorwart, T.; Greb, L. Reversible C–H bond silylation with a neutral silicon Lewis acid. Chem. Sci. 2023, 14, 11237–11242. [Google Scholar] [CrossRef]
- Niyitanga, T.; Kim, H. Synergistic effect of trimetallic-based CuxMox/Co1−xO nanoparticles/reduced graphene oxide as high efficient electrocatalyst for oxygen evolution reaction. Chem. Eng. J. 2023, 451, 138649. [Google Scholar] [CrossRef]
- He, D.; Ding, X.; Li, S.; Liu, Y.; Zhao, M.; Wang, J.; Chen, Y. Pt/doped mullite catalysts with abundant oxygen vacancies and enhanced oxygen migration capacity for NO oxidation. J. Catal. 2023, 423, 62–80. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, X.; Lian, H.; Liu, J.; Li, X. Plasma catalytic steam methane reforming for distributed hydrogen production. Catal. Today 2019, 337, 69–75. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, K.; Yang, J.; Liu, P.; Song, L.; Zhang, J.; Fu, M.; Chen, L.; Huang, H.; Wu, J.; et al. The effect of existence states of PdOx supported by Co3O4 nanoplatelets on catalytic oxidation of methane. Appl. Surf. Sci. 2021, 539, 148211. [Google Scholar] [CrossRef]
- Yan, S.; Yang, L.; Ning, J.; Liu, Y.; Cao, Y.; Liu, F. Catalytic combustion of low-concentration methane over transition metal oxides supported on open cell foams. J. Environ. Chem. Eng. 2025, 13, 115041. [Google Scholar] [CrossRef]
- Guntida, A.; Rattanachartnarong, T.; Jongsomjit, B.; Sooknoi, T.; Weerachawanasak, P.; Praserthdam, S.; Praserthdam, P. Determining the role of oxygen vacancies in palmitone selectivity and coke formation over acid metal oxide catalysts for the ketonization of methyl palmitate. Appl. Catal. A Gen. 2021, 628, 118405. [Google Scholar] [CrossRef]
- Li, X.; Hu, R.; Zhou, X.; Liu, Z.; Zhao, S.; Khan, A.; Li, W.; Xiong, Z.; Xu, A. Influence of surface properties of transition metal oxides for permanganate activation: Key role of Lewis acid sites. Surf. Interfaces 2024, 55, 105368–105375. [Google Scholar] [CrossRef]
- Shen, D.; Li, Z.; Shan, J.; Yu, G.; Wang, X.; Zhang, Y.; Liu, C.; Lyu, S.; Li, J.; Li, L. Synergistic Pt-CeO2 interface boosting low temperature dry reforming of methane. Appl. Catal. B Environ. 2022, 318, 121809. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Wang, X.; Zhang, Z.; Zhao, J.; Yan, J.; Du, Y.; Zhang, H.; Ma, D. Complete CO oxidation by O2 and H2O over Pt–CeO2−δ/MgO following lang-muir–hinshelwood and Mars–van Krevelen Mechanisms, respectively. ACS Catal. 2021, 11, 11820–11830. [Google Scholar] [CrossRef]
- Jayachandran, V.; Palanisami, S.; Paneerselvam, J.; Elango, M.; Chakrabortty, S.; Ghosh, S.; Albaqami, M.D.; Mohammad, S.; Sangaraju, S. A new insight on surface chemistry and redox species of transition metal (Fe, Mn) doped CeO2-SnO2/Al2O3 nanocomposites for automobile emission control. J. Environ. Chem. Eng. 2024, 12, 113896. [Google Scholar] [CrossRef]
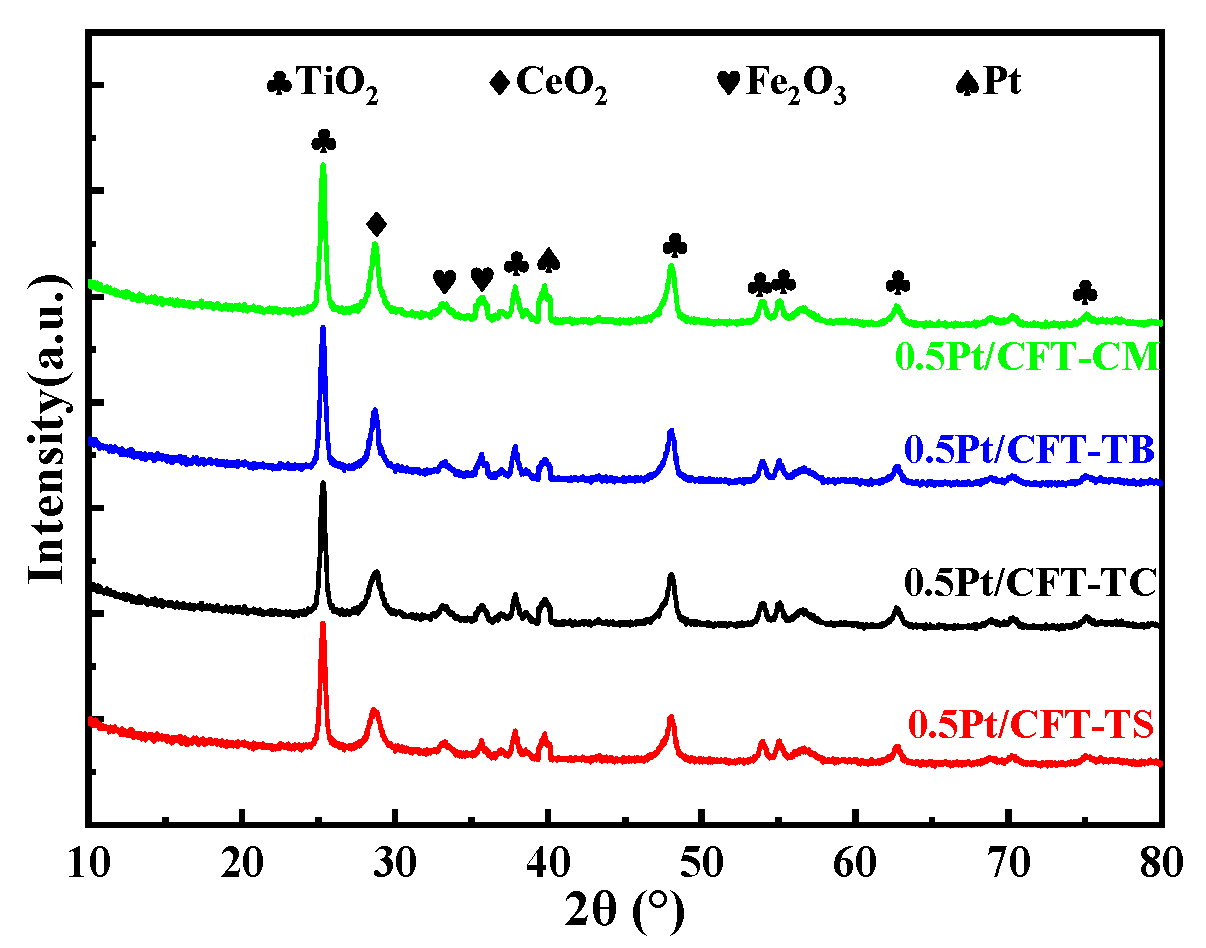
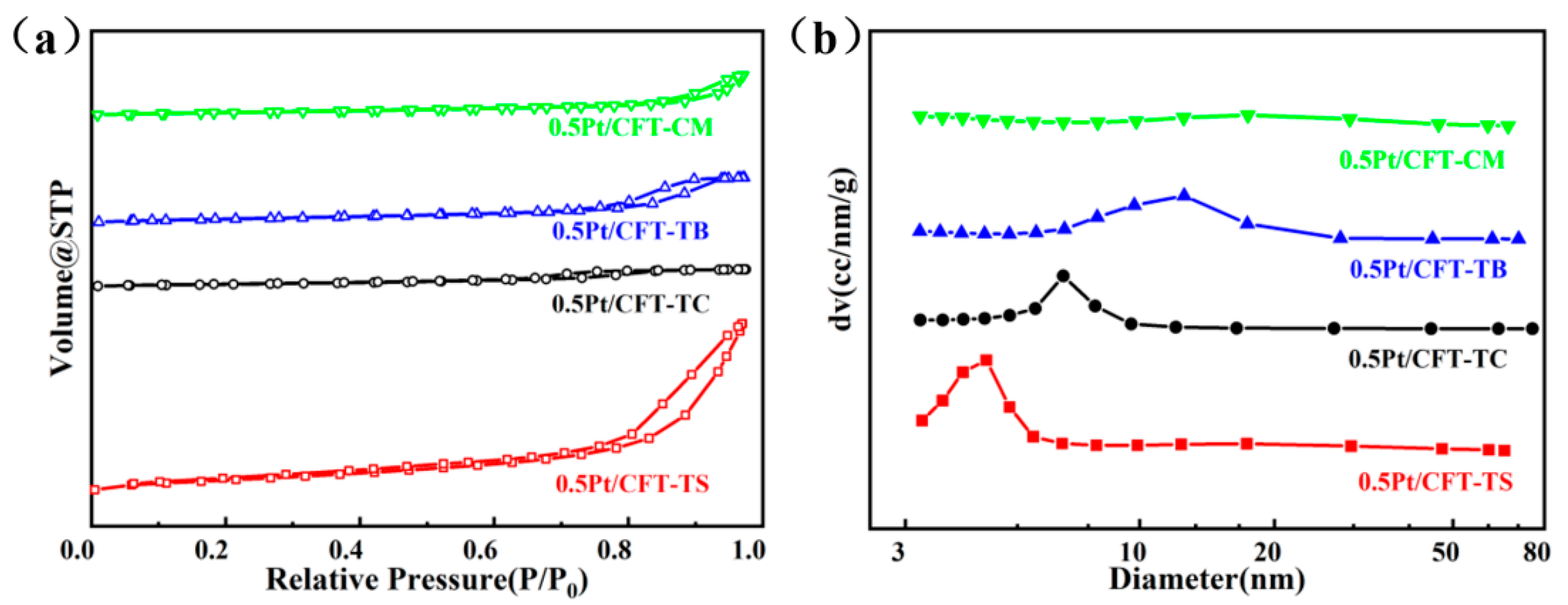



| Catalyst | Specific Surface Area m2/g | Pore Volume cc/g | Pore Size nm | Pt Size XRD (nm) |
|---|---|---|---|---|
| 0.5Pt/CFT-CM | 72.3 | 0.26 | 15.45 | 2.1 |
| 0.5Pt/CFT-TB | 53.2 | 0.16 | 9.31 | 3.6 |
| 0.5Pt/CFT-TC | 112.3 | 0.36 | 13.41 | 2.7 |
| 0.5Pt/CFT-TS | 156.2 | 0.46 | 4.59 | 1.2 |
| Catalyst | u″ | u′ | u | v″ | v′ | v | Ce3+ Fraction/% |
|---|---|---|---|---|---|---|---|
| 0.5Pt/CFT-CM | 3797.31 | 4469.31 | 5492.37 | 687.01 | 6863.26 | 8897.21 | 35.29 |
| 0.5Pt/CFT-TB | 2144.13 | 5566.79 | 4106.08 | 800.87 | 8473.56 | 5531.19 | 39.88 |
| 0.5Pt/CFT-TC | 4708.74 | 4126.31 | 4460.63 | 758.85 | 7688.45 | 7341.62 | 42.62 |
| 0.5Pt/CFT-TS | 4894.31 | 3229.06 | 2151.25 | 884.53 | 8006.18 | 6921.54 | 49.45 |
| Catalyst | 733 eV | 728 eV | 725 eV | 719 eV | 712 eV | 710.7 eV | Fe3+ Content (%) |
|---|---|---|---|---|---|---|---|
| 0.5Pt/CFT-CM | 694.31 | 729.06 | 751.25 | 1384.53 | 1841.01 | 1622.34 | 66.52 |
| 0.5Pt/CFT-TB | 628.74 | 826.31 | 760.63 | 1358.85 | 2170.58 | 1677.61 | 66.27 |
| 0.5Pt/CFT-TC | 744.13 | 566.79 | 746.08 | 1280.87 | 1397.72 | 1935.97 | 62.49 |
| 0.5Pt/CFT-TS | 597.31 | 469.31 | 692.37 | 1987.01 | 1059.84 | 1590.37 | 67.80 |
| Catalyst | Oα | Oβ | Oα Fraction % |
|---|---|---|---|
| 0.5Pt/CFT-CM | 12287.26 | 26072.63 | 32.03 |
| 0.5Pt/CFT-TB | 21858.29 | 22856.77 | 48.88 |
| 0.5Pt/CFT-TC | 19556.85 | 20745.00 | 48.53 |
| 0.5Pt/CFT-TS | 11203.98 | 10693.81 | 51.16 |
| Catalyst | T90 °C | T90′ °C |
|---|---|---|
| 0.5Pt/CFT-CM | 256 | 370 |
| 0.5Pt/CFT-TB | 246 | 400 |
| 0.5Pt/CFT-TC | 198 | 359 |
| 0.5Pt/CFT-TS | 163 | 383 |
| Catalyst | Normalized Activation Energy kJ/mol | Correlation Coefficient R2 |
|---|---|---|
| 0.5Pt/CFT-CM | 66.35 | 0.99 |
| 0.5Pt/CFT-TB | 58.31 | 0.99 |
| 0.5Pt/CFT-TC | 50.26 | 0.99 |
| 0.5Pt/CFT-TS | 46.77 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Zhang, R.; Xiang, X.; Fang, X. Precursor Engineering of SO42−-Rich CeO2-Pt-TiO2-Fe2O3 Catalyst with Oxygen Vacancy-Mediated Ternary Synergy for Ultralow-Temperature Methane Combustion. Catalysts 2025, 15, 896. https://doi.org/10.3390/catal15090896
Zeng X, Zhang R, Xiang X, Fang X. Precursor Engineering of SO42−-Rich CeO2-Pt-TiO2-Fe2O3 Catalyst with Oxygen Vacancy-Mediated Ternary Synergy for Ultralow-Temperature Methane Combustion. Catalysts. 2025; 15(9):896. https://doi.org/10.3390/catal15090896
Chicago/Turabian StyleZeng, Xiaoyi, Ruikun Zhang, Xianbing Xiang, and Xianghong Fang. 2025. "Precursor Engineering of SO42−-Rich CeO2-Pt-TiO2-Fe2O3 Catalyst with Oxygen Vacancy-Mediated Ternary Synergy for Ultralow-Temperature Methane Combustion" Catalysts 15, no. 9: 896. https://doi.org/10.3390/catal15090896
APA StyleZeng, X., Zhang, R., Xiang, X., & Fang, X. (2025). Precursor Engineering of SO42−-Rich CeO2-Pt-TiO2-Fe2O3 Catalyst with Oxygen Vacancy-Mediated Ternary Synergy for Ultralow-Temperature Methane Combustion. Catalysts, 15(9), 896. https://doi.org/10.3390/catal15090896





