Enzymes as Catalysts in Industrial Biocatalysis: Advances in Engineering, Applications, and Sustainable Integration
Abstract
1. Introduction
2. Methodology
3. Enzyme Classes in Industrial Biocatalysis
4. Sources of Industrial Enzymes
4.1. Microbial Sources
4.2. Plant-Derived Enzymes
4.3. Animal-Derived Enzymes
4.4. Extremophiles as Enzyme Sources
4.5. Recombinant Enzyme Production
4.6. Metagenomic and Environmental Sources
4.7. Synthetic Biology and Artificial Systems
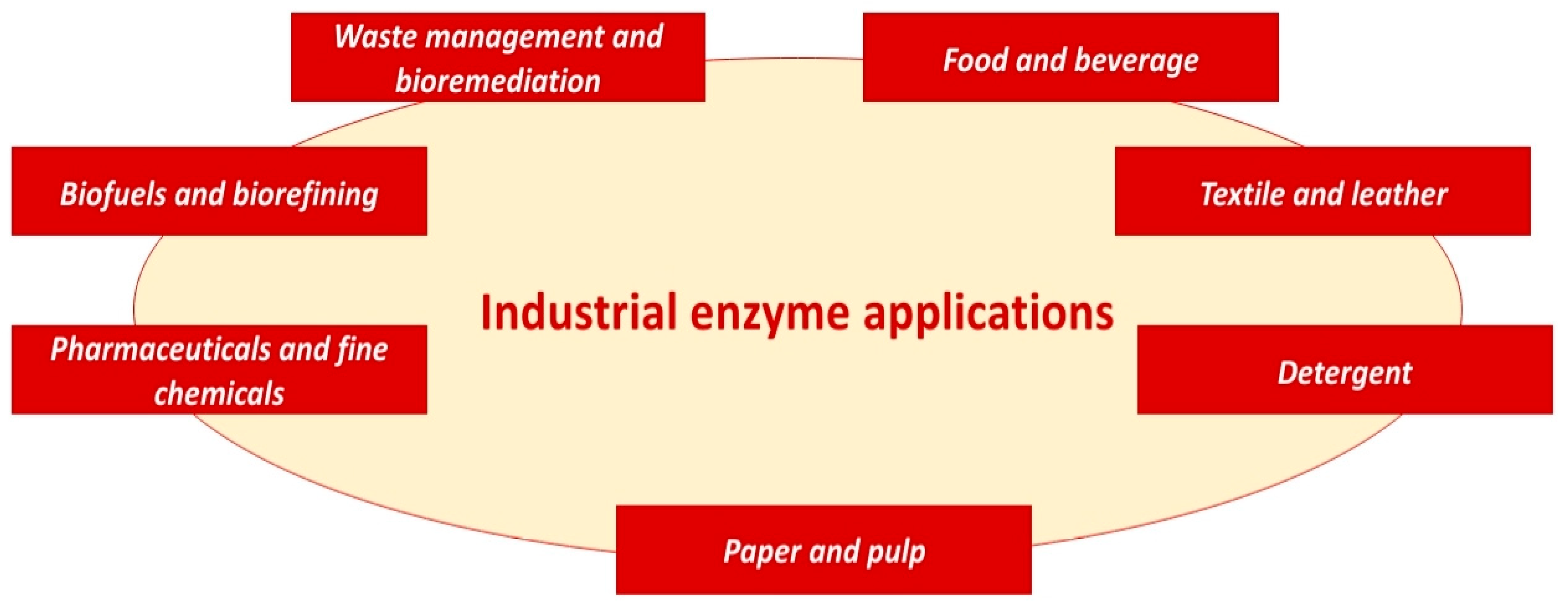
5. Industrial Applications
5.1. Food and Beverage Industry
5.2. Textile and Leather Industry
5.3. Detergent Industry
5.4. Paper and Pulp Industry
5.5. Pharmaceuticals and Fine Chemicals
5.6. Biofuels and Biorefining
5.7. Waste Management and Bioremediation
6. Advances in Enzyme Engineering
6.1. Rational Design
6.2. Directed Evolution
6.3. Computational Modeling and Machine Learning
6.4. Synthetic Biology and CRISPR-Based Engineering
6.5. De Novo Enzyme Design
6.6. Cell-Free Protein Synthesis
7. Limitations of Industrial Enzymes
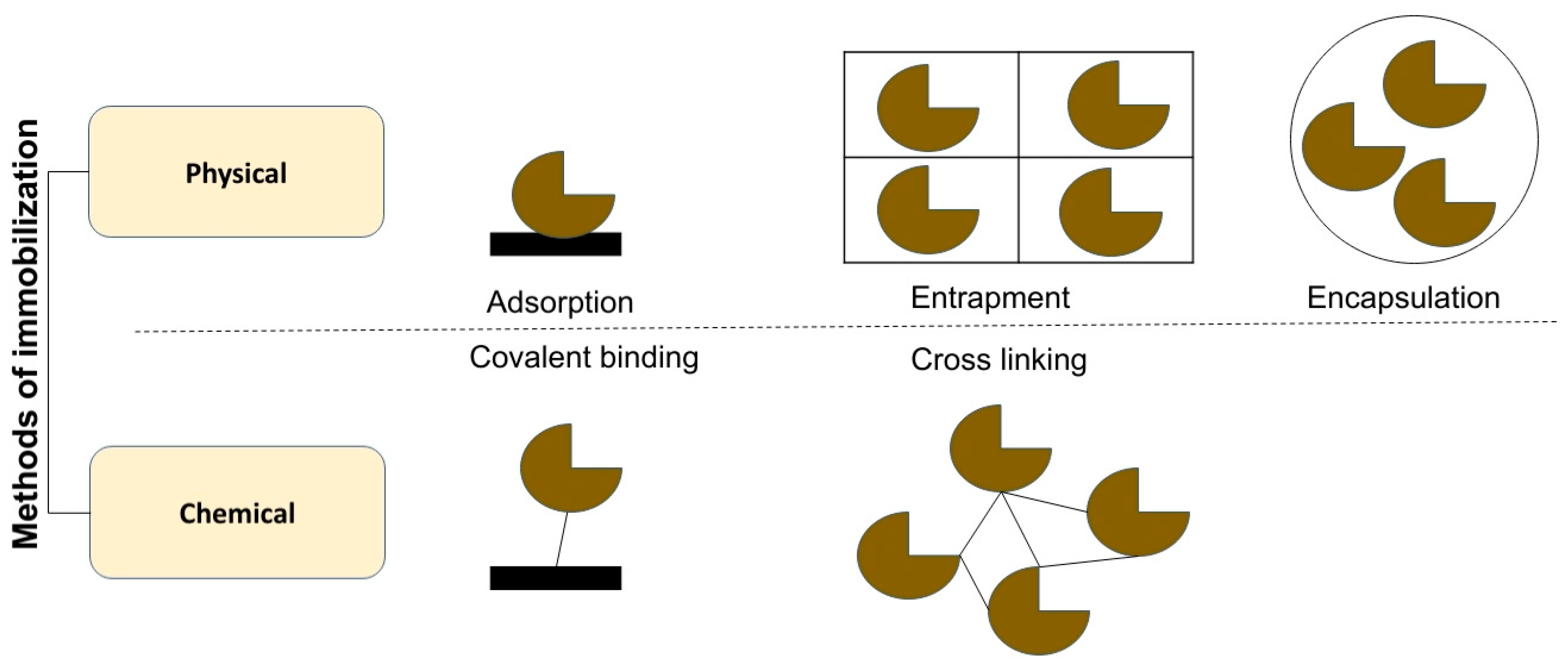
8. Immobilized Enzymes and Reactor Engineering
8.1. Physical Methods
8.1.1. Adsorption
8.1.2. Entrapment
8.1.3. Encapsulation
8.2. Chemical Methods
8.2.1. Covalent Binding
8.2.2. Cross Linking
8.3. Supporting Matrix
- -
- Polymeric materials: Synthetic polymers like polyacrylamide, polyethyleneimine, and polyvinyl alcohol are frequently used due to their structural flexibility, chemical adaptability, and ability to form porous networks that accommodate enzyme molecules while maintaining catalytic activity [115].
- -
- Inorganic substrates: Materials such as silica, alumina, and zeolites serve as excellent immobilization platforms owing to their high surface area, durability, and resistance to harsh chemical environments, making them suitable for industrial biocatalysis [116].
- -
- Membrane-based supports: Membranes made from compounds like cellulose acetate and polyamide act as selective barriers, enabling the compartmentalization of enzymes and substrates. These membranes facilitate controlled and continuous catalytic processes, especially in flow systems.
- -
- Magnetic nanoparticles: Iron oxide-based nanoparticles (e.g., Fe3O4) offer the dual benefit of enzyme immobilization and ease of recovery via magnetic separation. This feature enhances process efficiency and reduces operational costs by allowing enzyme reuse. Nanoparticles (NPs) with magnetic functionality form a unique subclass and will be explored in more detail in the following section [117].
8.4. Nano- and Micromaterials as Enzyme Immobilization Supports
8.4.1. Magnetic Nanoparticles
8.4.2. Non-Magnetic Nanoparticles
8.4.3. Metal-Organic Frameworks
8.4.4. Carbon Nanotubes
| Enzyme | Nanomaterial | Application | Ref. |
|---|---|---|---|
| Urokinase-type plasminogen activator | Magnetic polyelectrolyte-based composites | Thrombolytic and anticoagulant properties | [131] |
| Urokinase-type plasminogen activator | Magnetic NPs | Enhanced thrombolysis rate in a microfluidic channel | [132] |
| Tissue plasminogen activator, streptokinase | Chitosan NPs | Treatment of thrombolytic disorder | [133] |
| Tissue plasminogen activator, streptokinase | Cu NPs | Restores blood flow in arterial thrombosis | [134] |
| Lipase | Zinc Ferrite NPs | Antibacterial activity against E. coli and S. aureas | [135] |
| Streptokinase | Alumina NPs | Thrombolytic colloid with prolonged action | [136] |
| Catalase, SOD, and glutathione peroxidase | Cu5.4O NPs | Cytoprotective effects against ROS-mediated damage | [133] |
| Tissue plasminogen activator | Magnetic iron oxide micro-rods | Enhanced thrombolysis after ischemic stroke | [137] |
| Urokinase | Chitosan NPs | Enhanced thrombolytic activity | [138] |
| Catalase, peroxidase xanthine oxidase | Polyethylene glycol and poly-lactic/polyglycolic acid NPs | Protection and vascular oxidative stress | [137] |
| Catalase, SOD | Polymeric NPs | Protection against inflammation | [132] |
| Catalase | Poly (lactic co-glycolic acid) NPs | Protection of neurons from oxidative damage | [139] |
| Candida antarctica lipase B | Carbon-based magnetic NPs | Biodiesel preparation | [140] |
| Purine nucleoside 2′-deoxyribosyltransferase from Trypanosoma brucei | Glutaraldehyde-activated magnetic microspheres | Pharmaceutical industry | [141] |
| Horseradish peroxidase | Fe3O4–NH2/hNFs | Decolorization of textile dyes | [142] |
| Lipase | ZnFe2O4@Mesoporous silica nano | Food industry | [143] |
8.5. Factors Influencing the Enzyme Immobilization
9. Multi-Enzymatic Nano Biocatalyst
10. Limitations of Nanomaterials During Enzyme Immobilization
11. Some Examples Where Immobilization Can Hardly Improve Enzyme Stability
12. Logistical Considerations for Industrial-Scale Immobilized Enzyme Applications
13. Enzyme Immobilization Cost
14. Industrial and Economic Perspectives
15. Future Trends and Perspectives
16. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Tawfik, D.S. Accuracy-rate tradeoffs: How do enzymes meet demands of selectivity and catalytic efficiency? Curr. Opin. Chem. Biol. 2014, 21, 73–80. [Google Scholar] [CrossRef]
- Leveson-Gower, R.B.; Mayer, C.; Roelfes, G. The importance of catalytic promiscuity for enzyme design and evolution. Nat. Rev. Chem. 2019, 3, 687–705. [Google Scholar] [CrossRef]
- Mesbah, N.M. Industrial biotechnology based on enzymes from extreme environments. Front. Bioeng. Biotechnol. 2022, 10, 870083. [Google Scholar] [CrossRef]
- Mazzei, R.; Gebreyohannes, A.Y.; Papaioannou, E.; Nunes, S.P.; Vankelecom, I.F.J.; Giorno, L. Enzyme catalysis coupled with artificial membranes towards process intensification in biorefinery-a review. Bioresour. Technol. 2021, 335, 125248. [Google Scholar] [CrossRef]
- Seth, R.; Meena, A. Enzymes-based nanomaterial synthesis: An eco-friendly and green synthesis approach. Clean Technol. Environ. Policy 2024, 1–24. [Google Scholar] [CrossRef]
- de Souza Vandenberghe, L.P.; Karp, S.G.; Pagnoncelli, M.G.B.; von Linsingen Tavares, M.; Junior, N.L.; Diestra, K.V.; Viesser, J.A.; Soccol, C.R. Classification of enzymes and catalytic properties. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 11–30. [Google Scholar] [CrossRef]
- Pagar, A.D.; Patil, M.D.; Flood, D.T.; Yoo, T.H.; Dawson, P.E.; Yun, H. Recent advances in biocatalysis with chemical modification and expanded amino acid alphabet. Chem. Rev. 2021, 121, 6173–6245. [Google Scholar] [CrossRef] [PubMed]
- Fasim, A.; More, V.S.; More, S.S. Large-scale production of enzymes for biotechnology uses. Curr. Opin. Biotechnol. 2021, 69, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, K.; Bornscheuer, U.T. Enzyme technology: History and current trends. In Applied Bioengineering: Innovations and Future Directions; Wiley: Hoboken, NJ, USA, 2017; pp. 11–46. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S. Directed evolution: Tailoring biocatalysts for industrial applications. Crit. Rev. Biotechnol. 2013, 33, 365–378. [Google Scholar] [CrossRef]
- Becker, M.; Lütz, S.; Rosenthal, K. Environmental assessment of enzyme production and purification. Molecules 2021, 26, 573. [Google Scholar] [CrossRef]
- Sampaio, P.S.; Fernandes, P. Machine learning: A suitable method for biocatalysis. Catalysts 2023, 13, 961. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Concu, R.; Cordeiro, M.N.D.S. Alignment-free method to predict enzyme classes and subclasses. Int. J. Mol. Sci. 2019, 20, 5389. [Google Scholar] [CrossRef] [PubMed]
- Horn, P.J. Where do the electrons go? How numerous redox processes drive phytochemical diversity: Redox processes in phytochemistry. Phytochem. Rev. 2021, 20, 367–407. [Google Scholar]
- Rostami, A.; Abdelrasoul, A.; Shokri, Z.; Shirvandi, Z. Applications and mechanisms of free and immobilized laccase in detoxification of phenolic compounds—A review. Korean J. Chem. Eng. 2022, 39, 821–832. [Google Scholar] [CrossRef]
- Brundiek, H.; Höhne, M. Transaminases—A Biosynthetic Route for Chiral Amines. In Applied Biocatalysis: From Fundamental Science to Industrial Applications: From Fundamental Science to Industrial Applications; Wiley: Hoboken, NJ, USA, 2016; pp. 199–218. [Google Scholar] [CrossRef]
- López-Iglesias, M.; Gotor-Fernández, V. Recent advances in biocatalytic promiscuity: Hydrolase-catalyzed reactions for nonconventional transformations. Chem. Rec. 2015, 15, 743–759. [Google Scholar] [CrossRef]
- Hasan, M.J.; Haque, P.; Rahman, M.M. Protease enzyme based cleaner leather processing: A review. J. Clean. Prod. 2022, 365, 132826. [Google Scholar] [CrossRef]
- Niyonzima, F.N.; More, S. Detergent-compatible proteases: Microbial production, properties, and stain removal analysis. Prep. Biochem. Biotechnol. 2015, 45, 233–258. [Google Scholar] [CrossRef]
- Fahim, Y.A.; El-Khawaga, A.M.; Sallam, R.M.; Elsayed, M.A.; Assar, M.F.A. A review on Lipases: Sources, assays, immobilization techniques on nanomaterials and applications. BioNanoScience 2024, 14, 1780–1797. [Google Scholar] [CrossRef]
- SÁ, A.G.A.; de Meneses, A.C.; de Araújo, P.H.H.; de Oliveira, D. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci. Technol. 2017, 69, 95–105. [Google Scholar] [CrossRef]
- Chen, P.; Shrotri, A.; Fukuoka, A. Unraveling the hydrolysis of β-1, 4-glycosidic bonds in cello-oligosaccharides over carbon catalysts. Catal. Sci. Technol. 2020, 10, 4593–4601. [Google Scholar] [CrossRef]
- Zhu, B.; Yin, H. Alginate lyase: Review of major sources and classification, properties, structure-function analysis and applications. Bioengineered 2015, 6, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Yeom, S.-J.; Kim, S.-E.; Oh, D.-K. Development of aldolase-based catalysts for the synthesis of organic chemicals. Trends Biotechnol. 2022, 40, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, R.; Kangeswaren, M.; Perumal, V.; Asiedu, S.K. Tapping into bioplastic potential with glucose isomerase from Priestia megaterium for enhanced degradation and mechanical strength. Chem. Eng. J. 2024, 496, 153679. [Google Scholar] [CrossRef]
- Sharma, J. Evolution and Evaluation of Engineered DNA Ligases for Improved Blunt-End Ligation. Master’s Thesis, Open Access Te Herenga Waka-Victoria University of Wellington, Wellington, New Zealand, 2020. [Google Scholar]
- Regassa, H.; Bose, D.; Mukherjee, A. Review of microorganisms and their enzymatic products for industrial bioprocesses. Ind. Biotechnol. 2021, 17, 214–226. [Google Scholar] [CrossRef]
- Ngalimat, M.S.; Yahaya, R.S.R.; Baharudin, M.M.A.-a.; Yaminudin, S.M.; Karim, M.; Ahmad, S.A.; Sabri, S. A review on the biotechnological applications of the operational group Bacillus amyloliquefaciens. Microorganisms 2021, 9, 614. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, T.; Hasunuma, T.; Kondo, A.; Zhao, X.-Q.; Feng, J.-X. Every road leads to Rome: Diverse biosynthetic regulation of plant cell wall-degrading enzymes in filamentous fungi Penicillium oxalicum and Trichoderma reesei. Crit. Rev. Biotechnol. 2024, 44, 1241–1261. [Google Scholar] [CrossRef]
- Manoochehri, H.; Hosseini, N.F.; Saidijam, M.; Taheri, M.; Rezaee, H.; Nouri, F. A review on invertase: Its potentials and applications. Biocatal. Agric. Biotechnol. 2020, 25, 101599. [Google Scholar] [CrossRef]
- Thapa, S.; Li, H.; Ohair, J.; Bhatti, S.; Chen, F.-C.; Nasr, K.A.; Johnson, T.; Zhou, S. Biochemical characteristics of microbial enzymes and their significance from industrial perspectives. Mol. Biotechnol. 2019, 61, 579–601. [Google Scholar] [CrossRef]
- Omar, R.M.; Galala, A.A.; Badria, F. Medicinal Plants: Dual Source Enzym. Enzym. modulators. Polymorph 2019, 3, 15–31. [Google Scholar]
- Meshram, A.; Singhal, G.; Bhagyawant, S.S.; Srivastava, N. Plant-derived enzymes: A treasure for food biotechnology. In Enzymes in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 483–502. [Google Scholar]
- Guzmán-Ortiz, F.A.; Castro-Rosas, J.; Gómez-Aldapa, C.A.; Mora-Escobedo, R.; Rojas-León, A.; Rodríguez-Marín, M.L.; Falfán-Cortés, R.N.; Román-Gutiérrez, A.D. Enzyme activity during germination of different cereals: A review. Food Rev. Int. 2019, 35, 177–200. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Ora, A.; Häkkinen, S.T.; Ritala, A.; Räisänen, R.; Kallioinen-Mänttäri, M.; Melin, K. Innovative extraction technologies of bioactive compounds from plant by-products for textile colorants and antimicrobial agents. Biomass Convers. Biorefinery 2024, 14, 24973–25002. [Google Scholar] [CrossRef]
- Bedford, M.R. The evolution and application of enzymes in the animal feed industry: The role of data interpretation. Br. Poult. Sci. 2018, 59, 486–493. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Lee, C.C.; Ashaolu, J.O.; Tarhan, O.; Pourjafar, H.; Jafari, S.M. Pepsin: An excellent proteolytic enzyme for the production of bioactive peptides. Food Rev. Int. 2024, 40, 1875–1912. [Google Scholar] [CrossRef]
- Zhang, X.; Tao, L.; Wei, G.; Yang, M.; Wang, Z.; Shi, C.; Shi, Y.; Huang, A. Plant-derived rennet: Research progress, novel strategies for their isolation, identification, mechanism, bioactive peptide generation, and application in cheese manufacturing. Crit. Rev. Food Sci. Nutr. 2025, 65, 444–456. [Google Scholar] [CrossRef]
- Hassan, Y.I.; Lahaye, L.; Gong, M.M.; Peng, J.; Gong, J.; Liu, S.; Gay, C.G.; Yang, C. Innovative drugs, chemicals, and enzymes within the animal production chain. Vet. Res. 2018, 49, 71. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Merugu, R.; Mohapatra, S.; Sharma, S. Extremophiles life of microorganisms in extreme environments. In Extremophiles; CRC Press: Boca Raton, FL, USA, 2022; pp. 43–66. [Google Scholar]
- Akram, F.; Shah, F.I.; Ibrar, R.; Fatima, T.; ul Haq, I.; Naseem, W.; Gul, M.A.; Tehreem, L.; Haider, G. Bacterial thermophilic DNA polymerases: A focus on prominent biotechnological applications. Anal. Biochem. 2023, 671, 115150. [Google Scholar] [CrossRef] [PubMed]
- Salas-Bruggink, D.I.J.; Sánchez-San Martín, J.; Leiva, G.; Blamey, J.M. Extremozymes: Challenges and Opportunities on the Road to Novel Enzymes Production. Process Biochem. 2024, 143, 323–336. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, M.W.; Siddique, R.; Nabi, G.; Manan, S.; Yousaf, M.; Hou, H. Role of recombinant DNA technology to improve life. Int. J. Genom. 2016, 2016, 2405954. [Google Scholar] [CrossRef]
- Durga, J.; Rajaganapathy, K.; Srinivasan, R.; Saravanan, R. Potential of Probiotic Recombinant Microbial Enzymes: Overview of Expression, Purification, Characterization and Its Application in Various Diseases. J. Adv. Zool. 2023, 44, 884–901. [Google Scholar]
- Liu, M.; Xiao, R.; Li, X.; Zhao, Y.; Huang, J. A comprehensive review of recombinant technology in the food industry: Exploring expression systems, application, and future challenges. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70078. [Google Scholar] [CrossRef] [PubMed]
- Prayogo, F.A.; Budiharjo, A.; Kusumaningrum, H.P.; Wijanarka, W.; Suprihadi, A.; Nurhayati, N. Metagenomic applications in exploration and development of novel enzymes from nature: A review. J. Genet. Eng. Biotechnol. 2020, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- DeCastro, M.-E.; Rodríguez-Belmonte, E.; González-Siso, M.-I. Metagenomics of thermophiles with a focus on discovery of novel thermozymes. Front. Microbiol. 2016, 7, 1521. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.L.; Piel, J.; Sunagawa, S. A roadmap for metagenomic enzyme discovery. Nat. Prod. Rep. 2021, 38, 1994–2023. [Google Scholar] [CrossRef]
- Shanmugam, S.; Ngo, H.-H.; Wu, Y.-R. Advanced CRISPR/Cas-based genome editing tools for microbial biofuels production: A review. Renew. Energy 2020, 149, 1107–1119. [Google Scholar] [CrossRef]
- Karthika, V.; Sridharan, B.; Nam, J.W.; Kim, D.; Gyun Lim, H. Neuromodulation by nanozymes and ultrasound during Alzheimer’s disease management. J. Nanobiotechnol. 2024, 22, 139. [Google Scholar] [CrossRef]
- Silverman, A.D.; Karim, A.S.; Jewett, M.C. Cell-free gene expression: An expanded repertoire of applications. Nat. Rev. Genet. 2020, 21, 151–170. [Google Scholar] [CrossRef]
- Ben Amira, A.; Besbes, S.; Attia, H.; Blecker, C. Milk-clotting properties of plant rennets and their enzymatic, rheological, and sensory role in cheese making: A review. Int. J. Food Prop. 2017, 20, S76–S93. [Google Scholar] [CrossRef]
- Bulgaru, V.; Popescu, L.; Siminiuc, R. Lactose intolerance and the importance of lactose-free dairy products in this condition. J. Soc. Sci. 2021, 4, 119–133. [Google Scholar]
- Kendirci, P.; Salum, P.; Bas, D.; Erbay, Z. Production of enzyme-modified cheese (EMC) with ripened white cheese flavour: II-effects of lipases. Food Bioprod. Process. 2020, 122, 230–244. [Google Scholar] [CrossRef]
- Dura, A.; Rosell, C.M. Enzymes in baking. In Microbial Enzyme Technology in Food Applications; CRC Press: Boca Raton, FL, USA, 2017; pp. 295–314. [Google Scholar]
- Fox, G.P.; Bettenhausen, H.M. Variation in quality of grains used in malting and brewing. Front. Plant Sci. 2023, 14, 1172028. [Google Scholar] [CrossRef]
- Patel, V.B.; Chatterjee, S.; Dhoble, A.S. A review on pectinase properties, application in juice clarification, and membranes as immobilization support. J. Food Sci. 2022, 87, 3338–3354. [Google Scholar] [CrossRef] [PubMed]
- de Souza, T.S.P.; Kawaguti, H.Y. Cellulases, hemicellulases, and pectinases: Applications in the food and beverage industry. Food Bioprocess Technol. 2021, 14, 1446–1477. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Choudhury, A.K.R. Enzyme applications in textile chemical processing. In Sustainable Technologies for Fashion and Textiles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 91–115. [Google Scholar]
- Madhu, A.; Chakraborty, J.N. Recovery and reuse of immobilized α-amylase during desizing of cotton fabric. Res. J. Text. Appar. 2018, 22, 271–290. [Google Scholar] [CrossRef]
- Gao, M.; Song, J.; Zhang, X.; Zhang, C.; Peng, B.; Chattha, S.A. Key mechanism of enzymatic dehairing technology for leather-making: Permeation behaviors of protease into animal hide and the mechanism of charge regulation. Collagen Leather 2023, 5, 9. [Google Scholar] [CrossRef]
- Liaqat, I.; Naseem, S.; Ali, S.; Aftab, M.N.; Arshad, M.; Yang, G. Perspectives Applications of Protease in Textile Industries. In Enzymes in Textile Processing: A Climate Changes Mitigation Approach: Textile Industry, Enzymes, and SDGs; Springer: Berlin/Heidelberg, Germany, 2025; pp. 273–302. [Google Scholar]
- Jegannathan, K.R.; Nielsen, P.H. Environmental assessment of enzyme use in industrial production—A literature review. J. Clean. Prod. 2013, 42, 228–240. [Google Scholar] [CrossRef]
- Nyanhongo, G.; Herrero Acero, E.; Matuchaki, M.; Rau, M.; Guebitz, G.; Andreaus, J. Microbial Applications for Fabric and Textile Industries; Cambridge University Library: Cambridge, UK, 2022. [Google Scholar]
- Al-Ghanayem, A.A.; Joseph, B. Current prospective in using cold-active enzymes as eco-friendly detergent additive. Appl. Microbiol. Biotechnol. 2020, 104, 2871–2882. [Google Scholar] [CrossRef]
- Abdella, M.A.A.; Ahmed, S.A. Stable protease from Bacillus licheniformis-MA1 strain: Statistical production optimization, kinetic and thermodynamic characterization, and application in silver recovery from used X-ray films. Microb. Cell Factories 2025, 24, 98. [Google Scholar] [CrossRef]
- Gupta, G.K.; Dixit, M.; Kapoor, R.K.; Shukla, P. Xylanolytic enzymes in pulp and paper industry: New technologies and perspectives. Mol. Biotechnol. 2022, 64, 130–143. [Google Scholar] [CrossRef]
- Kumar, V.; Pathak, P.; Harsh, N.S.K.; Bhardwaj, N.K. Biodeinking: An eco-friendly alternative for chemicals based recycled fiber processing. Phys. Sci. Rev. 2023, 8, 1941–1965. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, M.; Tiruneh, W. Debarking, pitch removal and retting: Role of microbes and their enzymes. Phys. Sci. Rev. 2020, 5, 20190048. [Google Scholar] [CrossRef]
- Meghwanshi, G.K.; Kaur, N.; Verma, S.; Dabi, N.K.; Vashishtha, A.; Charan, P.D.; Purohit, P.; Bhandari, H.S.; Bhojak, N.; Kumar, R. Enzymes for pharmaceutical and therapeutic applications. Biotechnol. Appl. Biochem. 2020, 67, 586–601. [Google Scholar] [CrossRef]
- de Gonzalo, G.; Alcántara, A.R.; Domínguez de María, P.; Sánchez-Montero, J.M. Biocatalysis for the asymmetric synthesis of Active Pharmaceutical Ingredients (APIs): This time is for real. Expert Opin. Drug Discov. 2022, 17, 1159–1171. [Google Scholar] [CrossRef]
- Bhalla, T.C.; Kumar, V.; Kumar, V.; Thakur, N.; Savitri. Nitrile metabolizing enzymes in biocatalysis and biotransformation. Appl. Biochem. Biotechnol. 2018, 185, 925–946. [Google Scholar] [CrossRef] [PubMed]
- Rodionova, M.V.; Bozieva, A.M.; Zharmukhamedov, S.K.; Leong, Y.K.; Lan, J.C.-W.; Veziroglu, A.; Veziroglu, T.N.; Tomo, T.; Chang, J.-S.; Allakhverdiev, S.I. A comprehensive review on lignocellulosic biomass biorefinery for sustainable biofuel production. Int. J. Hydrogen Energy 2022, 47, 1481–1498. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Martin-Martinez, F.J. Biorefineries: Achievements and challenges for a bio-based economy. Front. Chem. 2022, 10, 973417. [Google Scholar] [CrossRef]
- Sasso, F.; Natalello, A.; Castoldi, S.; Lotti, M.; Santambrogio, C.; Grandori, R. Burkholderia cepacia lipase is a promising biocatalyst for biofuel production. Biotechnol. J. 2016, 11, 954–960. [Google Scholar] [CrossRef]
- Nunes, C.S.; Malmlöf, K. Enzymatic decontamination of antimicrobials, phenols, heavy metals, pesticides, polycyclic aromatic hydrocarbons, dyes, and animal waste. In Enzymes in Human and Animal Nutrition; Elsevier: Amsterdam, The Netherlands, 2018; pp. 331–359. [Google Scholar]
- Kuthiala, T.; Thakur, K.; Sharma, D.; Singh, G.; Khatri, M.; Arya, S.K. The eco-friendly approach of cocktail enzyme in agricultural waste treatment: A comprehensive review. Int. J. Biol. Macromol. 2022, 209, 1956–1974. [Google Scholar] [CrossRef]
- Thulasisingh, A.; Ananthakrishnan, K.; Raja, A.; Kannaiyan, S. Bioprospecting of novel and industrially appropriate enzymes: A review. Water Air Soil Pollut. 2024, 235, 12. [Google Scholar] [CrossRef]
- Kim, S.; Ga, S.; Bae, H.; Sluyter, R.; Konstantinov, K.; Shrestha, L.K.; Kim, Y.H.; Kim, J.H.; Ariga, K. Multidisciplinary approaches for enzyme biocatalysis in pharmaceuticals: Protein engineering, computational biology, and nanoarchitectonics. EES Catal. 2024, 2, 14–48. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, H.; Yu, H.; Pu, Z.; Xu, J.; Zhang, H.; Wu, J.; Yang, L. Computational redesign of the substrate binding pocket of glutamate dehydrogenase for efficient synthesis of noncanonical L-amino acids. ACS Catal. 2022, 12, 13619–13629. [Google Scholar] [CrossRef]
- Xu, Z.; Cen, Y.-K.; Zou, S.-P.; Xue, Y.-P.; Zheng, Y.-G. Recent advances in the improvement of enzyme thermostability by structure modification. Crit. Rev. Biotechnol. 2020, 40, 83–98. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, P.; Cao, M.; Yu, T.; Lane, S.T.; Zhao, H. Directed evolution: Methodologies and applications. Chem. Rev. 2021, 121, 12384–12444. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.L.; Rusli, R.A.; Ollis, D.L. Directed evolution of enzymes for industrial biocatalysis. ChemBioChem 2016, 17, 197–203. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. New Directions for Chemical Engineering; National Academy of Sciences: Washington, DC, USA, 2022. [Google Scholar]
- Ebert, M.C.; Pelletier, J.N. Computational tools for enzyme improvement: Why everyone can–and should–use them. Curr. Opin. Chem. Biol. 2017, 37, 89–96. [Google Scholar] [CrossRef]
- Parise, A.; Cresca, S.; Magistrato, A. Molecular dynamics simulations for the structure-based drug design: Targeting small-GTPases proteins. Expert Opin. Drug Discov. 2024, 19, 1259–1279. [Google Scholar] [CrossRef]
- Helm, J.M.; Swiergosz, A.M.; Haeberle, H.S.; Karnuta, J.M.; Schaffer, J.L.; Krebs, V.E.; Spitzer, A.I.; Ramkumar, P.N. Machine learning and artificial intelligence: Definitions, applications, and future directions. Curr. Rev. Musculoskelet. Med. 2020, 13, 69–76. [Google Scholar] [CrossRef]
- Chandra, A.; Tünnermann, L.; Löfstedt, T.; Gratz, R. Transformer-based deep learning for predicting protein properties in the life sciences. eLife 2023, 12, e82819. [Google Scholar] [CrossRef]
- Buecherl, L.; Myers, C.J. Engineering genetic circuits: Advancements in genetic design automation tools and standards for synthetic biology. Curr. Opin. Microbiol. 2022, 68, 102155. [Google Scholar] [CrossRef]
- Erb, T.J.; Jones, P.R.; Bar-Even, A. Synthetic metabolism: Metabolic engineering meets enzyme design. Curr. Opin. Chem. Biol. 2017, 37, 56–62. [Google Scholar] [CrossRef]
- Zhang, F. Development of CRISPR-Cas systems for genome editing and beyond. Q. Rev. Biophys. 2019, 52, e6. [Google Scholar] [CrossRef]
- Morgado, G.; Gerngross, D.; Roberts, T.M.; Panke, S. Synthetic biology for cell-free biosynthesis: Fundamentals of designing novel in vitro multi-enzyme reaction networks. In Synthetic Biology—Metabolic Engineering; Springer International Publishing: Cham, Switzerland, 2018; pp. 117–146. [Google Scholar] [CrossRef]
- Zhou, L.; Tao, C.; Shen, X.; Sun, X.; Wang, J.; Yuan, Q. Unlocking the potential of enzyme engineering via rational computational design strategies. Biotechnol. Adv. 2024, 73, 108376. [Google Scholar] [CrossRef]
- Benítez-Mateos, A.I.; Roura Padrosa, D.; Paradisi, F. Multistep enzyme cascades as a route towards green and sustainable pharmaceutical syntheses. Nat. Chem. 2022, 14, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.W.; Manan, S.; Ul-Islam, M.; Khattak, W.A.; Khan, K.A.; Liu, J.; Yang, G.; Sun, J. Cell-free systems for biosynthesis: Towards a sustainable and economical approach. Green Chem. 2023, 25, 4912–4940. [Google Scholar] [CrossRef]
- Guan, A.; He, Z.; Wang, X.; Jia, Z.-J.; Qin, J. Protein engineering for the synthetic cell factory: Recent advance and perspective. Biotechnol. Adv. 2024, 73, 108366. [Google Scholar] [CrossRef] [PubMed]
- Kathirvel, I.; Gayathri Ganesan, N. Computational Strategies to Enhance Cell-Free Protein Synthesis Efficiency. BioMedInformatics 2024, 4, 2022–2042. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.-S. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef]
- Khafaga, D.S.R.; Muteeb, G.; Elgarawany, A.; Aatif, M.; Farhan, M.; Allam, S.; Almatar, B.A.; Radwan, M.G. Green nanobiocatalysts: Enhancing enzyme immobilization for industrial and biomedical applications. PeerJ 2024, 12, e17589. [Google Scholar] [CrossRef]
- Khafaga, D.S.R.; Radwan, M.G.; Muteeb, G.; Aatif, M.; Farhan, M. Green synthesis of biocatalysts based on nanocarriers promises an effective role in pharmaceutical and biomedical fields. Catalysts 2023, 13, 1448. [Google Scholar] [CrossRef]
- Hoarau, M.; Badieyan, S.; Marsh, E.N.G. Immobilized enzymes: Understanding enzyme–surface interactions at the molecular level. Org. Biomol. Chem. 2017, 15, 9539–9551. [Google Scholar] [CrossRef]
- Sigurdardóttir, S.B.; Lehmann, J.; Ovtar, S.; Grivel, J.C.; Negra, M.D.; Kaiser, A.; Pinelo, M. Enzyme immobilization on inorganic surfaces for membrane reactor applications: Mass transfer challenges, enzyme leakage and reuse of materials. Adv. Synth. Catal. 2018, 360, 2578–2607. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme entrapment, biocatalyst immobilization without covalent attachment. Green Chem. 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Naseem, K.; Arif, M.; Ahmad Haral, A.; Tahir, M.H.; Khurshid, A.; Ahmed, K.; Majeed, H.; Haider, S.; Khan, S.U.-D.; Nazar, M.F. Enzymes encapsulated smart polymer micro assemblies and their tuned multi-functionalities: A critical review. Int. J. Polym. Mater. Polym. Biomater. 2024, 73, 785–816. [Google Scholar] [CrossRef]
- Gaur, D.; Dubey, N.C.; Tripathi, B.P. Biocatalytic self-assembled synthetic vesicles and coacervates: From single compartment to artificial cells. Adv. Colloid Interface Sci. 2022, 299, 102566. [Google Scholar] [CrossRef]
- Prakash, O.; Verma, D.; Singh, P.C. Exploring the potential of enzyme-immobilized MOFs: Biosensing, biocatalysis, targeted drug delivery and cancer therapy. J. Mater. Chem. B 2024, 12, 10198–10214. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Xu, K.; Chen, X.; Zheng, R.; Zheng, Y. Immobilization of multi-enzymes on support materials for efficient biocatalysis. Front. Bioeng. Biotechnol. 2020, 8, 660. [Google Scholar] [CrossRef]
- Chen, N.; Chang, B.; Shi, N.; Yan, W.; Lu, F.; Liu, F. Cross-linked enzyme aggregates immobilization: Preparation, characterization, and applications. Crit. Rev. Biotechnol. 2023, 43, 369–383. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; Cavalcante, A.L.G.; de Sousa, I.G.; Neto, F.S.; dos Santos, J.C.S. Current status and future perspectives of supports and protocols for enzyme immobilization. Catalysts 2021, 11, 1222. [Google Scholar] [CrossRef]
- Virgen-Ortiz, J.J.; Dos Santos, J.C.S.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Polyethylenimine: A very useful ionic polymer in the design of immobilized enzyme biocatalysts. J. Mater. Chem. B 2017, 5, 7461–7490. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Noreen, S.; Shah, S.Z.H.; Bharagava, R.N.; Iqbal, H.M.N. Modifying bio-catalytic properties of enzymes for efficient biocatalysis: A review from immobilization strategies viewpoint. Biocatal. Biotransform. 2019, 37, 159–182. [Google Scholar] [CrossRef]
- Cavalcante, A.L.G.; Dari, D.N.; da Silva Aires, F.I.; de Castro, E.C.; Dos Santos, K.M.; Dos Santos, J.C.S. Advancements in enzyme immobilization on magnetic nanomaterials: Toward sustainable industrial applications. RSC Adv. 2024, 14, 17946–17988. [Google Scholar] [CrossRef]
- Holyavka, M.G.; Goncharova, S.S.; Redko, Y.A.; Lavlinskaya, M.S.; Sorokin, A.V.; Artyukhov, V.G. Novel biocatalysts based on enzymes in complexes with nano-and micromaterials. Biophys. Rev. 2023, 15, 1127–1158. [Google Scholar] [CrossRef]
- Fahim, Y.A.; Hasani, I.W.; Mahmoud Ragab, W. Promising biomedical applications using superparamagnetic nanoparticles. Eur. J. Med. Res. 2025, 30, 441. [Google Scholar] [CrossRef]
- Hassan, A.A.; Fahim, Y.A.; Ali, M.E.M. Efficient removal of Cr (VI) and As (V) from aqueous solution using magnetically separable nickel ferrite nanoparticles. J. Clust. Sci. 2025, 36, 4. [Google Scholar] [CrossRef]
- Hassan, A.A.; Ali, M.E.M.; Abdel-Latif, S.A.; Hasani, I.W.; Fahim, Y.A. Efficient removal of Remazol Red dye from aqueous solution using magnetic nickel ferrite nanoparticles synthesized via aqueous reflux. Sci. Rep. 2025, 15, 17527. [Google Scholar] [CrossRef] [PubMed]
- Fahim, Y.A.; Ragab, W.M.; Hasani, I.W.; El-Khawaga, A.M. Biomedical and environmental applications via nanobiocatalysts and enzyme immobilization. Eur. J. Med. Res. 2025, 30, 505. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M.N. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef] [PubMed]
- Kazi, R.N.A.; Hasani, I.W.; Khafaga, D.S.R.; Kabba, S.; Farhan, M.; Aatif, M.; Muteeb, G.; Fahim, Y.A. Nanomedicine: The Effective Role of Nanomaterials in Healthcare from Diagnosis to Therapy. Pharmaceutics 2025, 17, 987. [Google Scholar] [CrossRef]
- Matveeva, V.G.; Bronstein, L.M. Magnetic nanoparticle-containing supports as carriers of immobilized enzymes: Key factors influencing the biocatalyst performance. Nanomaterials 2021, 11, 2257. [Google Scholar] [CrossRef] [PubMed]
- Eleraky, M.I.; Razek, T.M.A.; Hasani, I.W.; Fahim, Y.A. Adsorptive removal of lead, copper, and nickel using natural and activated Egyptian calcium bentonite clay. Sci. Rep. 2025, 15, 13050. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhou, Y.-L.; Zeng, M.-H.; Kurmoo, M. The concept of mixed organic ligands in metal–organic frameworks: Design, tuning and functions. Dalton Trans. 2015, 44, 5258–5275. [Google Scholar] [CrossRef]
- Wang, K.-Y.; Zhang, J.; Hsu, Y.-C.; Lin, H.; Han, Z.; Pang, J.; Yang, Z.; Liang, R.-R.; Shi, W.; Zhou, H.-C. Bioinspired framework catalysts: From enzyme immobilization to biomimetic catalysis. Chem. Rev. 2023, 123, 5347–5420. [Google Scholar] [CrossRef]
- Rahman, G.; Najaf, Z.; Mehmood, A.; Bilal, S.; Shah, A.u.H.A.; Mian, S.A.; Ali, G. An overview of the recent progress in the synthesis and applications of carbon nanotubes. C 2019, 5, 3. [Google Scholar] [CrossRef]
- Ye, J.; Lu, J.; Wen, D. Engineering carbon nanomaterials toward high-efficiency bioelectrocatalysis for enzymatic biofuel cells: A review. Mater. Chem. Front. 2023, 7, 5806–5825. [Google Scholar] [CrossRef]
- Drozdov, A.S.; Prilepskii, A.Y.; Koltsova, E.M.; Anastasova, E.I.; Vinogradov, V.V. Magnetic polyelectrolyte-based composites with dual anticoagulant and thrombolytic properties: Towards optimal composition. J. Sol-Gel Sci. Technol. 2020, 95, 771–782. [Google Scholar] [CrossRef]
- Hood, E.D.; Chorny, M.; Greineder, C.F.; Alferiev, I.S.; Levy, R.J.; Muzykantov, V.R. Endothelial targeting of nanocarriers loaded with antioxidant enzymes for protection against vascular oxidative stress and inflammation. Biomaterials 2014, 35, 3708–3715. [Google Scholar] [CrossRef]
- Liu, T.; Xiao, B.; Xiang, F.; Tan, J.; Chen, Z.; Zhang, X.; Wu, C.; Mao, Z.; Luo, G.; Chen, X. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat. Commun. 2020, 11, 2788. [Google Scholar] [CrossRef]
- Tadayon, A.; Jamshidi, R.; Esmaeili, A. Targeted thrombolysis of tissue plasminogen activator and streptokinase with extracellular biosynthesis nanoparticles using optimized Streptococcus equi supernatant. Int. J. Pharm. 2016, 501, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Fahim, Y.A.; El-Khawaga, A.M.; Sallam, R.M.; Elsayed, M.A.; Assar, M.F.A. Immobilized lipase enzyme on green synthesized magnetic nanoparticles using Psidium guava leaves for dye degradation and antimicrobial activities. Sci. Rep. 2024, 14, 8820. [Google Scholar] [CrossRef] [PubMed]
- Chapurina, Y.E.; Drozdov, A.S.; Popov, I.; Vinogradov, V.V.; Dudanov, I.P.; Vinogradov, V.V. Streptokinase@ alumina nanoparticles as a promising thrombolytic colloid with prolonged action. J. Mater. Chem. B 2016, 4, 5921–5928. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Huang, S.; Zhu, L.; Huang, W.; Zhao, Y.; Jin, K.; ZhuGe, Q. Tissue plasminogen activator-porous magnetic microrods for targeted thrombolytic therapy after ischemic stroke. ACS Appl. Mater. Interfaces 2018, 10, 32988–32997. [Google Scholar] [CrossRef]
- Jin, H.-j.; Zhang, H.; Sun, M.-l.; Zhang, B.-g.; Zhang, J.-w. Urokinase-coated chitosan nanoparticles for thrombolytic therapy: Preparation and pharmacodynamics in vivo. J. Thromb. Thrombolysis 2013, 36, 458–468. [Google Scholar] [CrossRef]
- Singhal, A.; Morris, V.B.; Labhasetwar, V.; Ghorpade, A. Nanoparticle-mediated catalase delivery protects human neurons from oxidative stress. Cell Death Dis. 2013, 4, e903. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, G.; Ma, X.; Ge, F.; Zhao, Y.; Li, A.; Hu, L.; Ren, S.; Zhu, C.; You, Q. Carbon-based magnetic nano-particle utilizing nano-biochar as core and its immobilizing lipase for biodiesel preparation. Ind. Crops Prod. 2024, 222, 119693. [Google Scholar] [CrossRef]
- Pérez, E.; Sánchez-Murcia, P.A.; Jordaan, J.; Blanco, M.D.; Mancheño, J.M.; Gago, F.; Fernández-Lucas, J. Enzymatic Synthesis of Therapeutic Nucleosides using a Highly Versatile Purine Nucleoside 2′-DeoxyribosylTransferase from Trypanosoma brucei. ChemCatChem 2018, 10, 4406–4416. [Google Scholar] [CrossRef]
- Bakar, B.; Akbulut, M.; Ulusal, F.; Ulu, A.; Ozdemir, N.; Ates, B. Horseradish peroxidase immobilized onto mesoporous magnetic hybrid nanoflowers for enzymatic decolorization of textile dyes: A highly robust bioreactor and boosted enzyme stability. ACS Omega 2024, 9, 24558–24573. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Oveisi, H.; Meshkini, A. Functionalized ZnFe2O4@ Mesoporous silica nano-support for lipase enzyme immobilization: Enhanced biocatalysis and antibacterial activity for food industry applications. Food Biosci. 2024, 61, 104985. [Google Scholar] [CrossRef]
- Onyeogaziri, F.C.; Papaneophytou, C. A general guide for the optimization of enzyme assay conditions using the design of experiments approach. SLAS Discov. Adv. Life Sci. RD 2019, 24, 587–596. [Google Scholar] [CrossRef]
- Santos, J.C.S.d.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the support properties for immobilization or purification of enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef]
- Hassan, M.E.; Yang, Q.; Xiao, Z.; Liu, L.; Wang, N.; Cui, X.; Yang, L. Impact of immobilization technology in industrial and pharmaceutical applications. 3 Biotech 2019, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Taheri-Kafrani, A.; Kharazmi, S.; Nasrollahzadeh, M.; Soozanipour, A.; Ejeian, F.; Etedali, P.; Mansouri-Tehrani, H.-A.; Razmjou, A.; Yek, S.M.-G.; Varma, R.S. Recent developments in enzyme immobilization technology for high-throughput processing in food industries. Crit. Rev. Food Sci. Nutr. 2021, 61, 3160–3196. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Sardar, M. Enzyme immobilization: An overview on nanoparticles as immobilization matrix. Biochem. Anal. Biochem. 2015, 4, 1. [Google Scholar]
- Ren, S.; Li, C.; Jiao, X.; Jia, S.; Jiang, Y.; Bilal, M.; Cui, J. Recent progress in multienzymes co-immobilization and multienzyme system applications. Chem. Eng. J. 2019, 373, 1254–1278. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Yang, C.; Ma, C.; Tang, J. Enzyme-inorganic hybrid nanoflowers: Classification, synthesis, functionalization and potential applications. Chem. Eng. J. 2021, 415, 129075. [Google Scholar] [CrossRef]
- Zdarta, J.; Jankowska, K.; Wyszowska, M.; Kijeńska-Gawrońska, E.; Zgoła-Grześkowiak, A.; Pinelo, M.; Meyer, A.S.; Moszyński, D.; Jesionowski, T. Robust biodegradation of naproxen and diclofenac by laccase immobilized using electrospun nanofibers with enhanced stability and reusability. Mater. Sci. Eng. C 2019, 103, 109789. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Sirohi, R.; Tarafdar, A.; Arun, K.B.; Madhavan, A.; Binod, P.; Kumar Awasthi, M.; Varjani, S.; Szakacs, G.; et al. Nanobiocatalysts: Advancements and applications in enzyme technology. Bioresour. Technol. 2021, 337, 125491. [Google Scholar] [CrossRef]
- Mazzei, L.; Cianci, M.; Contaldo, U.; Musiani, F.; Ciurli, S. Urease inhibition in the presence of N-(n-butyl) thiophosphoric triamide, a suicide substrate: Structure and kinetics. Biochemistry 2017, 56, 5391–5404. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.-X.; Liu, Y.; Wang, Y. Suicide inhibition of cytochrome P450 enzymes by cyclopropylamines via a ring-opening mechanism: Proton-coupled electron transfer makes a difference. Front. Chem. 2017, 5, 3. [Google Scholar] [CrossRef]
- Betancor, L.; Hidalgo, A.; Fernández-Lorente, G.; Mateo, C.; Rodríguez, V.; Fuentes, M.; López-Gallego, F.; Fernández-Lafuente, R.; Guisan, J.M. Use of physicochemical tools to determine the choice of optimal enzyme: Stabilization of d-amino acid oxidase. Biotechnol. Prog. 2003, 19, 784–788. [Google Scholar] [CrossRef]
- Kaddour, S.; Lopez-Gallego, F.; Sadoun, T.; Fernandez-Lafuente, R.; Guisan, J.M. Preparation of an immobilized–stabilized catalase derivative from Aspergillus niger having its multimeric structure stabilized: The effect of Zn2+ on enzyme stability. J. Mol. Catal. B Enzym. 2008, 55, 142–145. [Google Scholar] [CrossRef]
- Siar, E.-H.; Zaak, H.; Kornecki, J.F.; Zidoune, M.N.; Barbosa, O.; Fernandez-Lafuente, R. Stabilization of ficin extract by immobilization on glyoxyl agarose. Preliminary characterization of the biocatalyst performance in hydrolysis of proteins. Process Biochem. 2017, 58, 98–104. [Google Scholar]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of enzymes via immobilization: Multipoint covalent attachment and other stabilization strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef] [PubMed]
- Siar, E.-H.; Arana-Peña, S.; Barbosa, O.; Zidoune, M.N.; Fernandez-Lafuente, R. Immobilization/stabilization of ficin extract on glutaraldehyde-activated agarose beads. Variables that control the final stability and activity in protein hydrolyses. Catalysts 2018, 8, 149. [Google Scholar]
- García-García, P.; Guisan, J.M.; Fernandez-Lorente, G. A mild intensity of the enzyme-support multi-point attachment promotes the optimal stabilization of mesophilic multimeric enzymes: Amine oxidase from Pisum sativum. J. Biotechnol. 2020, 318, 39–44. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef]
- Ferreira, R.d.G.; Azzoni, A.R.; Freitas, S. Techno-economic analysis of the industrial production of a low-cost enzyme using E. coli: The case of recombinant β-glucosidase. Biotechnol. Biofuels 2018, 11, 81. [Google Scholar]
- Liese, A.; Hilterhaus, L. Evaluation of immobilized enzymes for industrial applications. Chem. Soc. Rev. 2013, 42, 6236–6249. [Google Scholar] [CrossRef]
- de Sousa, R.R.; Pinto, M.C.C.; Aguieiras, E.C.G.; Cipolatti, E.P.; Manoel, E.A.; da Silva, A.S.A.; Pinto, J.C.; Freire, D.M.G.; Ferreira-Leitão, V.S. Comparative performance and reusability studies of lipases on syntheses of octyl esters with an economic approach. Bioprocess Biosyst. Eng. 2022, 45, 131–145. [Google Scholar] [CrossRef]
- Aguieiras, E.C.G.; Cavalcanti-Oliveira, E.D.; de Castro, A.M.; Langone, M.A.P.; Freire, D.M.G. Biodiesel production from Acrocomia aculeata acid oil by (enzyme/enzyme) hydroesterification process: Use of vegetable lipase and fermented solid as low-cost biocatalysts. Fuel 2014, 135, 315–321. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Pinto, M.C.C.; Robert, J.d.M.; da Silva, T.P.; Beralto, T.d.C.; Santos, J.G.F., Jr.; de Castro, R.d.P.V.; Fernandez-Lafuente, R.; Manoel, E.A.; Pinto, J.C. Pilot-scale development of core–shell polymer supports for the immobilization of recombinant lipase B from Candida antarctica and their application in the production of ethyl esters from residual fatty acids. J. Appl. Polym. Sci. 2018, 135, 46727. [Google Scholar] [CrossRef]
- Pasha, M.K.; Dai, L.; Liu, D.; Guo, M.; Du, W. An overview to process design, simulation and sustainability evaluation of biodiesel production. Biotechnol. Biofuels 2021, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Taher, H.; Giwa, A.; Abusabiekeh, H.; Al-Zuhair, S. Biodiesel production from Nannochloropsis gaditana using supercritical CO2 for lipid extraction and immobilized lipase transesterification: Economic and environmental impact assessments. Fuel Process. Technol. 2020, 198, 106249. [Google Scholar] [CrossRef]
- O’Connell, A.; Barry, A.; Burke, A.J.; Hutton, A.E.; Bell, E.L.; Green, A.P.; O’Reilly, E. Biocatalysis: Landmark discoveries and applications in chemical synthesis. Chem. Soc. Rev. 2024, 53, 2828–2850. [Google Scholar] [CrossRef]


| Source | Advantages | Limitations |
|---|---|---|
| Microorganisms | High yield, scalable, easy genetic manipulation, and cost-effective | Some enzymes may lack post-translational modifications |
| Plants | Naturally occurring, and used in food and traditional medicine | Seasonal availability, low yield, and complex purification |
| Animals | High substrate specificity, and historically used | Ethical concerns, and lower sustainability |
| Extremophiles | Exceptional stability (high temp, pH, salinity), and ideal for extreme conditions | Harder to culture, and lower expression yields |
| Recombinant Systems | High yield, customizable, consistent quality, and scalable production | Requires infrastructure and expertise in genetic engineering |
| Metagenomics | Access to uncultured organisms, high biodiversity, and novel functions | Complex screening and expression optimization |
| Synthetic Biology | Precision design, multifunctionality, and creation of new-to-nature enzymes | Expensive, still emerging, requires computational and molecular expertise |
| Immobilization Methods | Advantages | Disadvantages |
|---|---|---|
| Adsorption | Simple preparation and operation, cost-effective, and capable of regeneration | Weak bonding, limited activity and selectivity |
| Entrapment | High activity, robust bonds, and low costs | The preparation and operation are difficult and irreversible |
| Encapsulation | Simple and efficient preparation, cost-effective, highly active, and capable of regeneration | Lack of specificity and the presence of weak bonding |
| Covalent Binding | Strong binding with a high level of activity and specificity | Preparation is a challenging and expensive process that cannot be regenerated |
| Crosslinking | Intense activity, robust binding, and cost-effectiveness | Strong bonds and affordable; the process of preparation and operation is difficult and irreversible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhan, M.; Hasani, I.W.; Khafaga, D.S.R.; Ragab, W.M.; Ahmed Kazi, R.N.; Aatif, M.; Muteeb, G.; Fahim, Y.A. Enzymes as Catalysts in Industrial Biocatalysis: Advances in Engineering, Applications, and Sustainable Integration. Catalysts 2025, 15, 891. https://doi.org/10.3390/catal15090891
Farhan M, Hasani IW, Khafaga DSR, Ragab WM, Ahmed Kazi RN, Aatif M, Muteeb G, Fahim YA. Enzymes as Catalysts in Industrial Biocatalysis: Advances in Engineering, Applications, and Sustainable Integration. Catalysts. 2025; 15(9):891. https://doi.org/10.3390/catal15090891
Chicago/Turabian StyleFarhan, Mohd, Ibrahim W. Hasani, Doaa S. R. Khafaga, Waleed Mahmoud Ragab, Raisa Nazir Ahmed Kazi, Mohammad Aatif, Ghazala Muteeb, and Yosri A. Fahim. 2025. "Enzymes as Catalysts in Industrial Biocatalysis: Advances in Engineering, Applications, and Sustainable Integration" Catalysts 15, no. 9: 891. https://doi.org/10.3390/catal15090891
APA StyleFarhan, M., Hasani, I. W., Khafaga, D. S. R., Ragab, W. M., Ahmed Kazi, R. N., Aatif, M., Muteeb, G., & Fahim, Y. A. (2025). Enzymes as Catalysts in Industrial Biocatalysis: Advances in Engineering, Applications, and Sustainable Integration. Catalysts, 15(9), 891. https://doi.org/10.3390/catal15090891










