Defect Engineering via La Doping and Hydrogenation on Bi4Ti3O12 for Synergistically Enhancing Photocatalytic CO2 to CH3OH
Abstract
1. Introduction
2. Results and Discussion
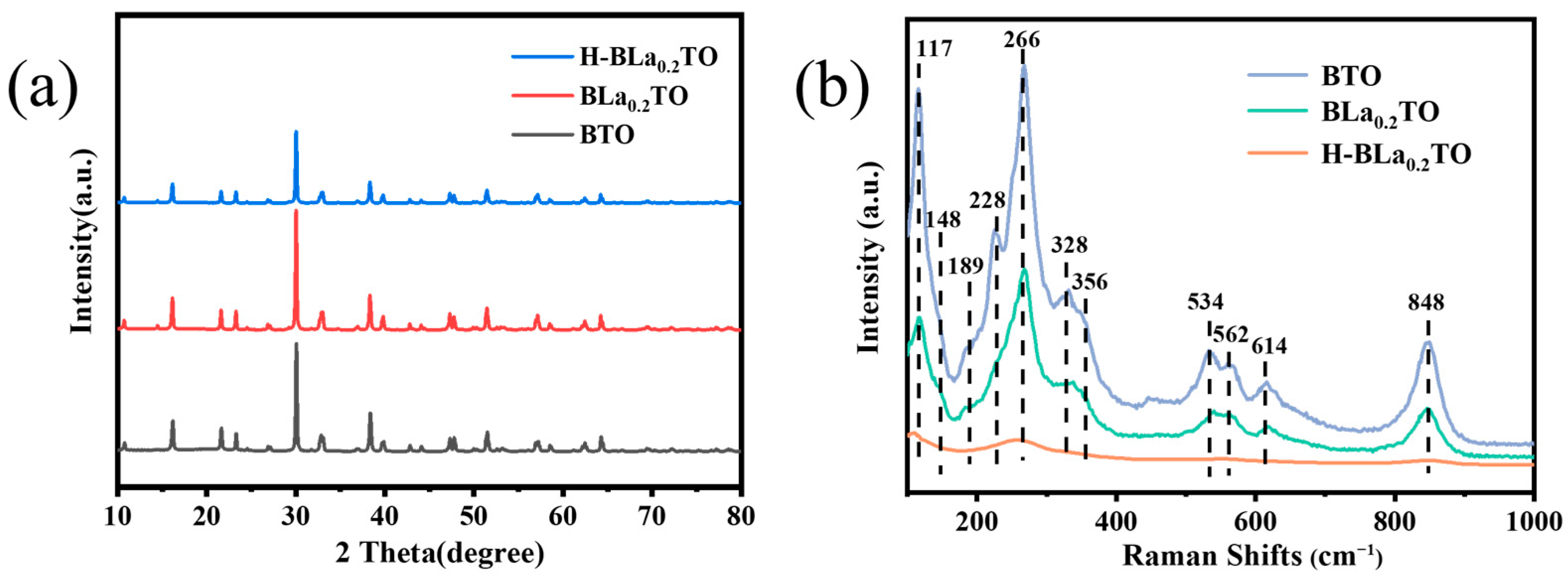
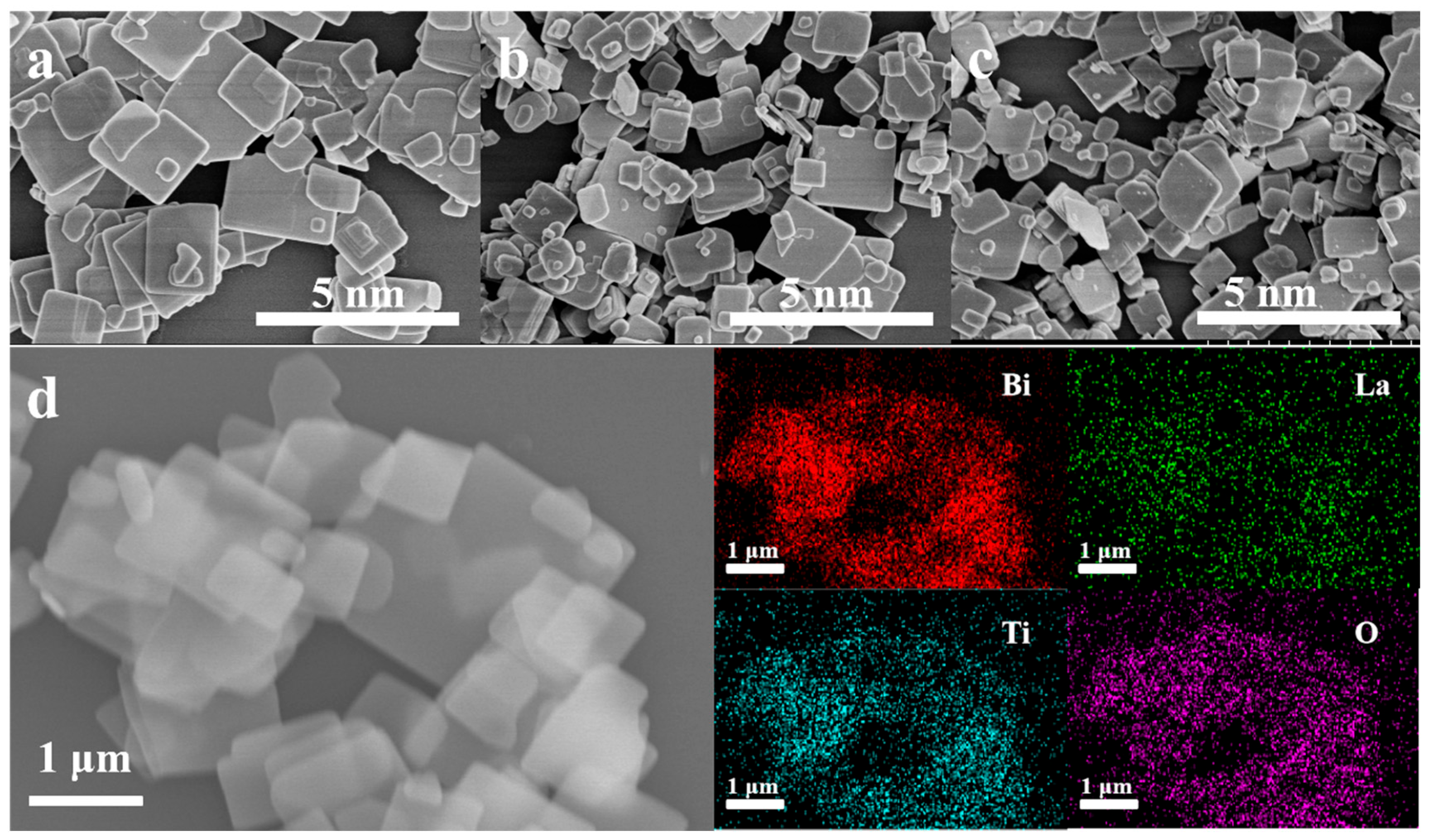
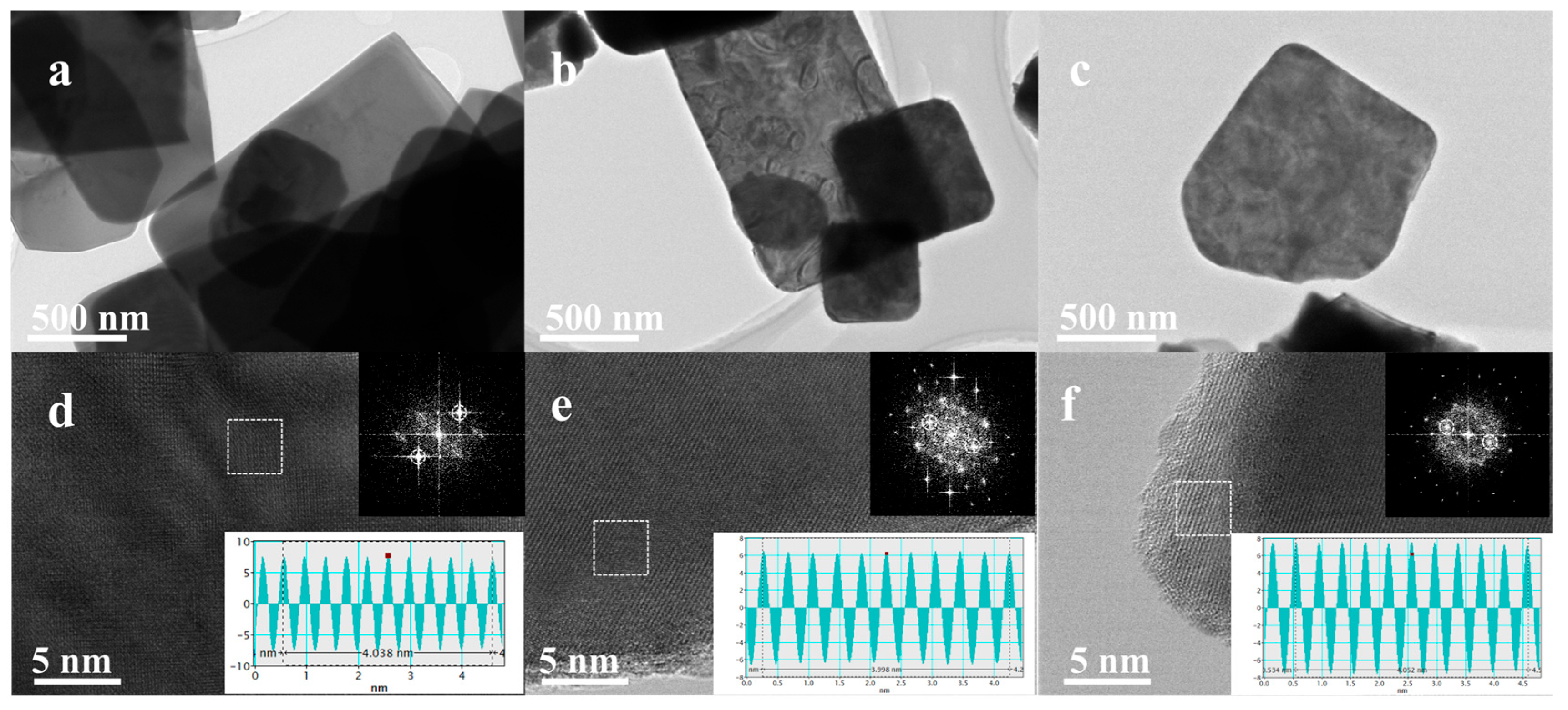
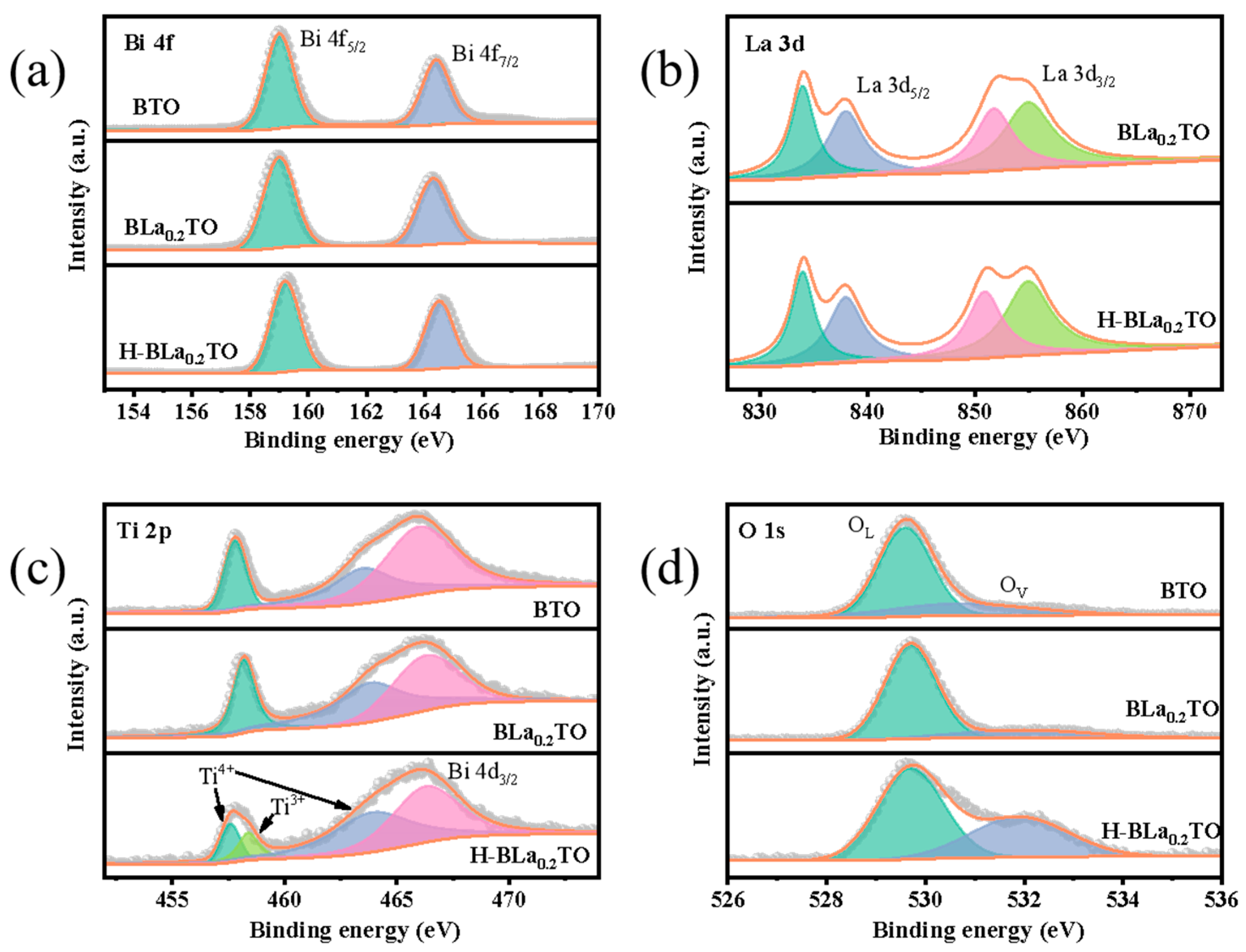
2.1. Structure and Morphology
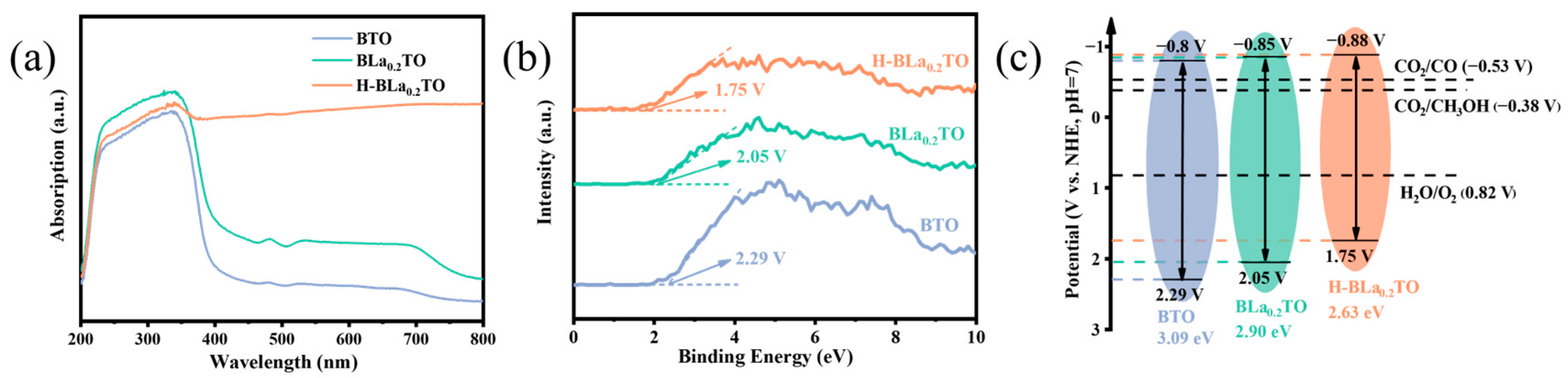
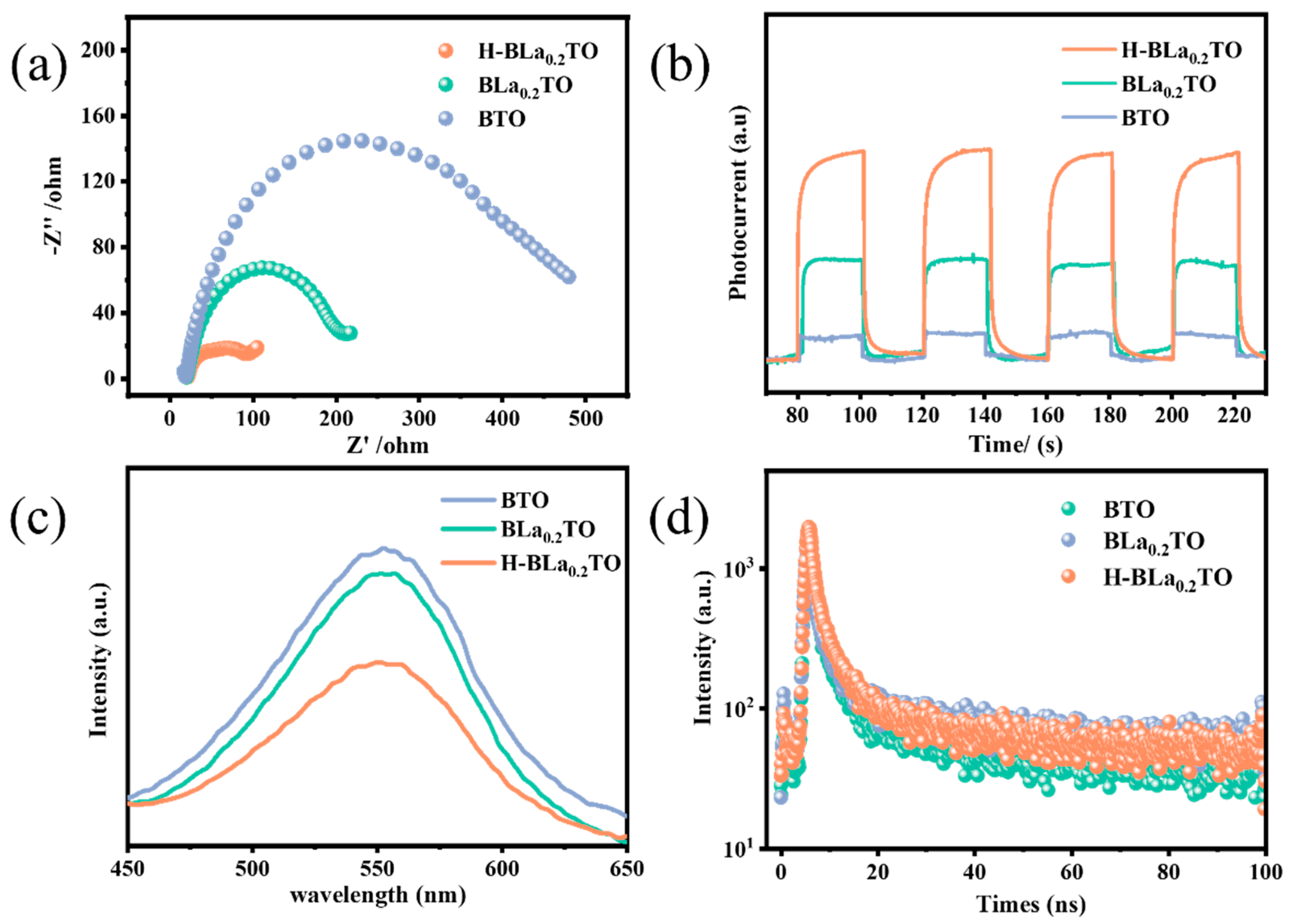
2.2. Photoelectrochemical Properties
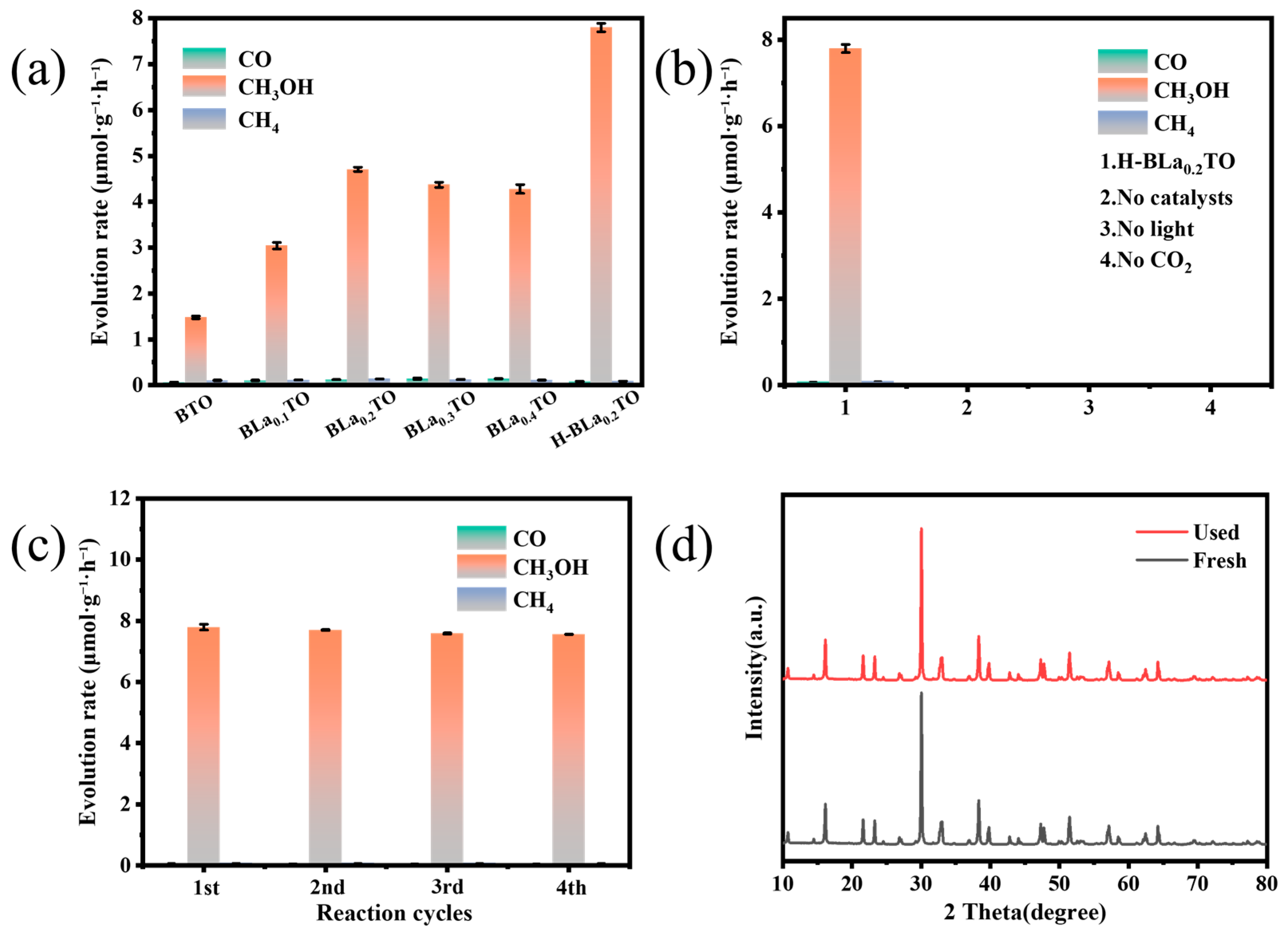
2.3. Photocatalysis Performance
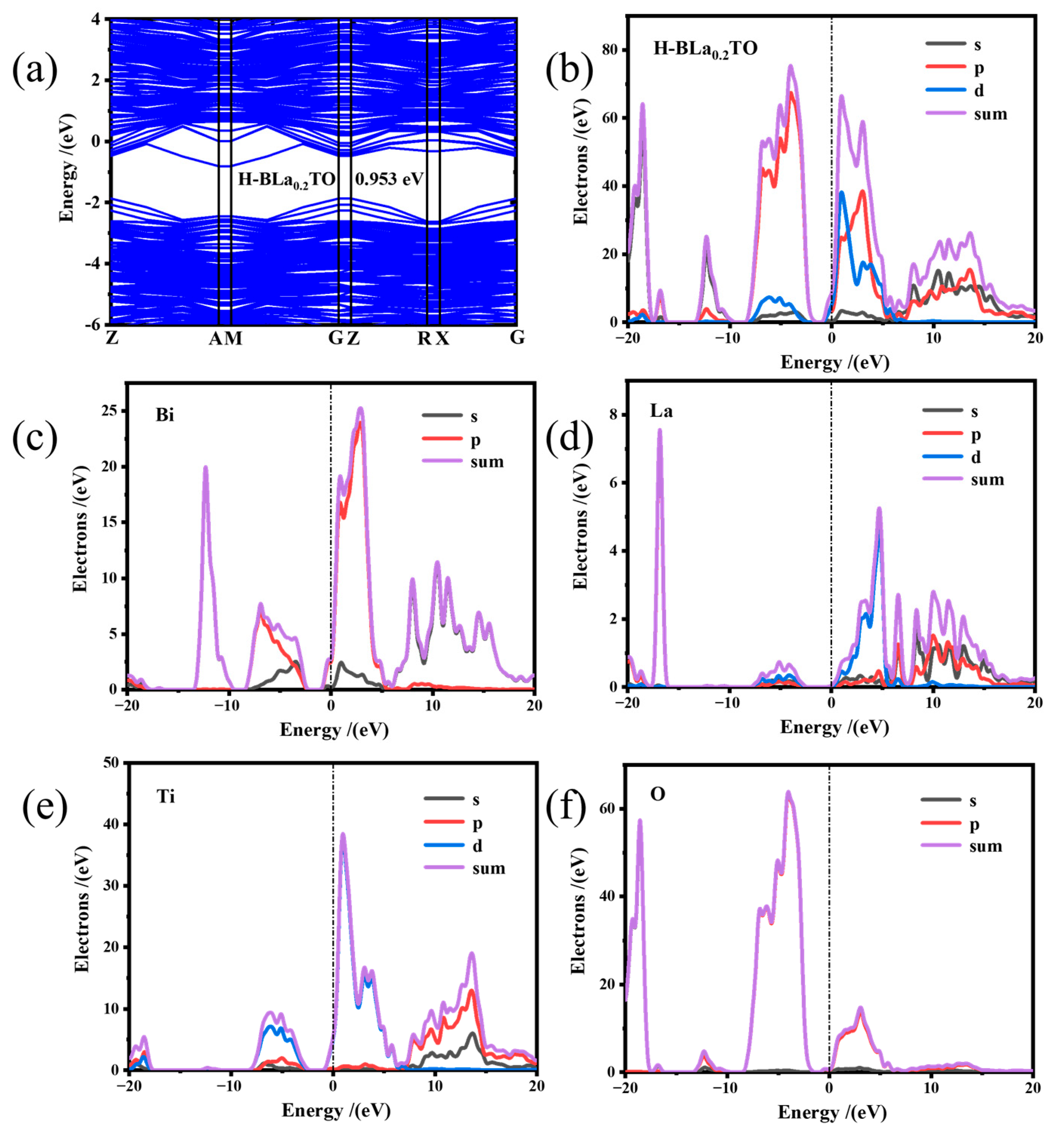
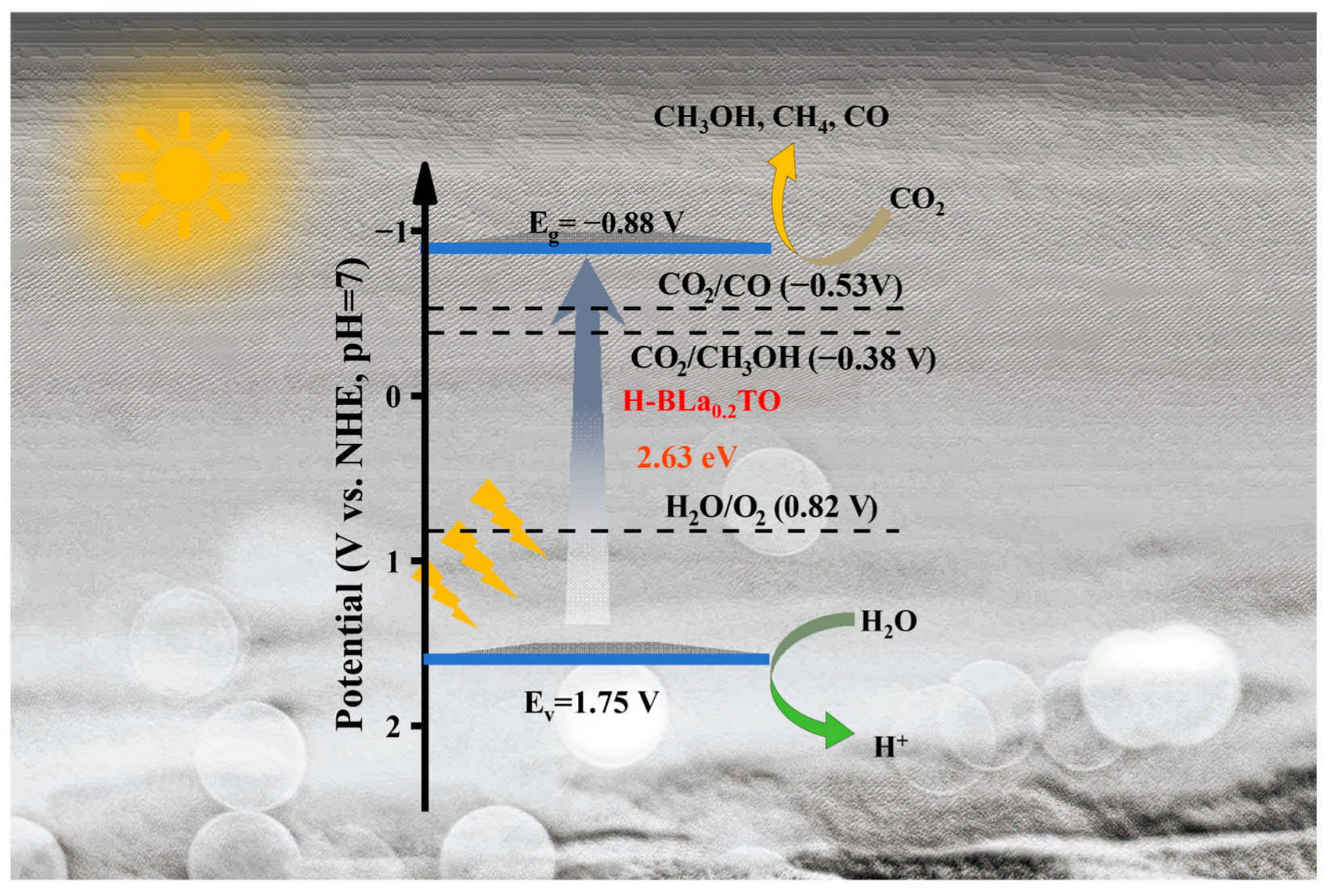
2.4. Photocatalytic Mechanism
3. Experimental Section
3.1. Materials
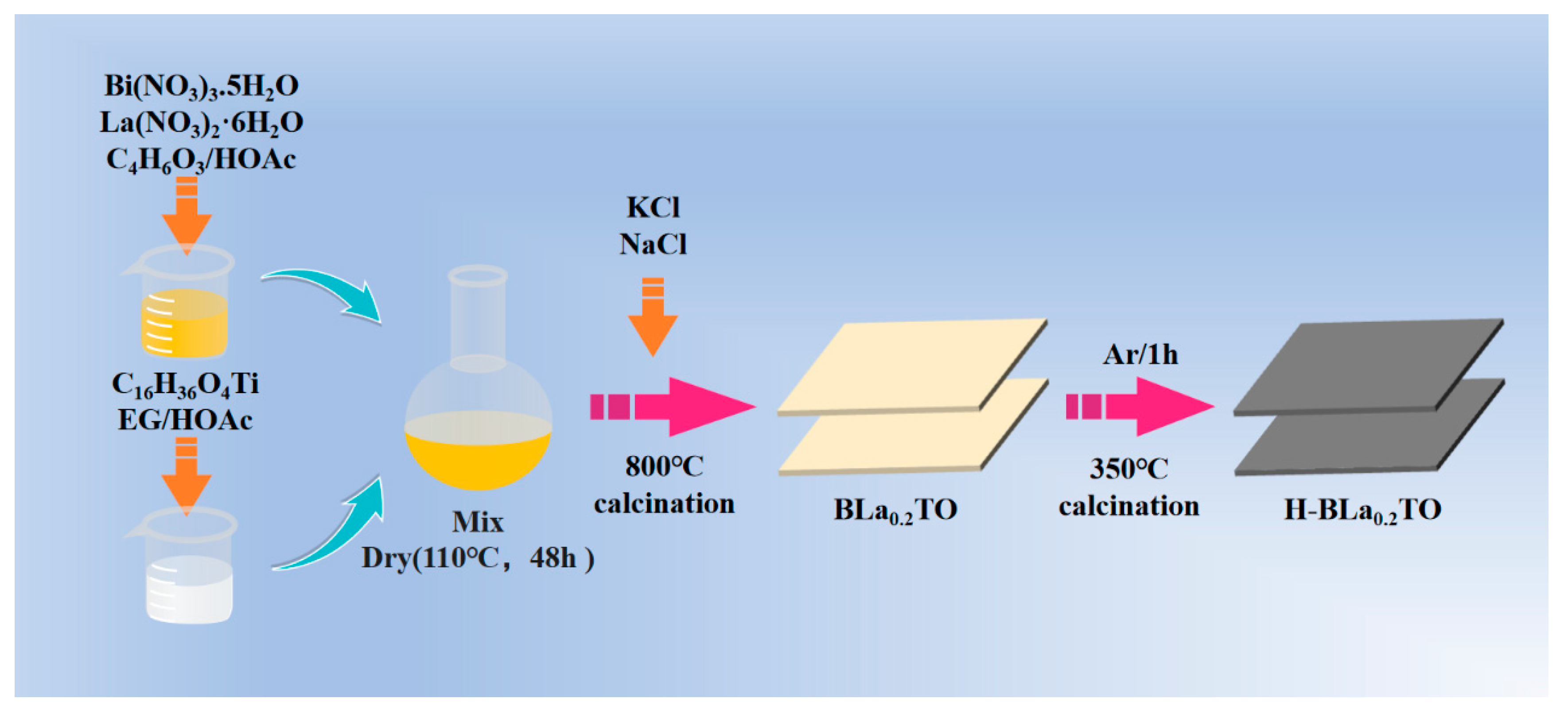
3.2. Fabrication of H-BLa0.2TO Nanosheets
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yergaziyeva, G.; Kuspanov, Z.; Mambetova, M.; Khudaibergenov, N.; Makayeva, N.; Daulbayev, C. Advancements in Catalytic, Photocatalytic, and Electrocatalytic CO2 Conversion Processes: Current Trends and Future Outlook. J. CO2 Util. 2024, 80, 102682. [Google Scholar] [CrossRef]
- Garba, M.D.; Usman, M.; Khan, S.; Shehzad, F.; Galadima, A.; Ehsan, M.F.; Ghanem, A.S.; Humayun, M. CO2 towards Fuels: A Review of Catalytic Conversion of Carbon Dioxide to Hydrocarbons. J. Environ. Chem. Eng. 2021, 9, 104756. [Google Scholar] [CrossRef]
- Francis, K.; Kim, H.; Hyeak, C.; Seop, H. Covalent Organic Framework-Based Catalysts for Efficient CO2 Utilization Reactions. Coord. Chem. Rev. 2022, 473, 214835. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.; Wan, S.; Wang, R.; Yu, M.; Song, F.; Zhong, Q. Supramolecular Self-Assembled Deficient Carbon Nitride Nanotubes for Efficient Photocatalytic CO2 Reduction. J. Colloid Interface Sci. 2023, 651, 726–733. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Zhang, J.; Liu, X.; Wang, H.; Zhang, W.; Yang, Q.; Ma, J.; Dong, X.; Yoo, S.J.; et al. Selective Hydrogenation of CO2 to Ethanol over Cobalt Catalysts. Angew. Chem. Int. Ed. 2018, 57, 6104–6108. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Wang, Y.; Sui, X.; Wu, S.; Dai, W.; Zhang, Z.; Ding, Z.; Long, J. Insight into the Selectivity-Determining Step of Various Photocatalytic CO2 Reduction Products by Inorganic Semiconductors. ACS Catal. 2024, 14, 10760–10788. [Google Scholar] [CrossRef]
- Stolarczyk, J.K.; Bhattacharyya, S.; Polavarapu, L.; Feldmann, J. Challenges and Prospects in Solar Water Splitting and CO2 Reduction with Inorganic and Hybrid Nanostructures. ACS Catal. 2018, 8, 3602–3635. [Google Scholar] [CrossRef]
- Ding, J.; He, D.; Du, P.; Wu, J.; Hu, Q.; Chen, Q.; Jiao, X. Design Photocatalysts to Poost Parrier Dynamics in Plastics Photoconversion into Fuels. ACS Appl. Mater. Interfaces 2024, 16, 35865–35873. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, Q.; Wen, D.; Wu, C.; Pan, Y.; Liu, X.; Huang, Z.; Li, N. Particle Size-Dependent Charge Transfer Dynamics for Boosting CO2 Photoreduction Over Ag/TiO2 Heterojunction. ACS Catal. 2024, 14, 8105–8115. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Y.; Tian, J.; Li, J.; Chen, G.; Zhou, L.; Sun, Y.; Qiu, Y. Selective Photocatalytic Reduction of CO2 to Syngas over Tunable Metal-Perovskite Interface. ChemSusChem 2022, 15, e202102729. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Sun, F.; Zhou, T.; Wang, Y.; Cui, X.; Guo, Z.; Fan, F.; Yu, J.C. Charge Redistribution of a Spatially Differentiated Ferroelectric Bi4Ti3O12 Single Crystal for Photocatalytic Overall Water Splitting. Nat. Commun. 2024, 15, 4746. [Google Scholar] [CrossRef]
- Shen, M.; Shi, Y.; Wang, Z.; Wu, T.; Hu, L.; Wu, L. Enhanced Photocatalytic Benzyl Alcohol Oxidation over Bi4Ti3O12 Ultrathin Nanosheets. J. Colloid Interface Sci. 2022, 608, 2529–2538. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, D.; Zheng, X.; Han, W. Oxygen Vacancies and Electrical Properties of Different Valence Ions (W6+, Nb5+, Zr4+, Cr3+) Doped Bi4Ti3O12 Ceramics. Ceram. Int. 2024, 50, 2778–2787. [Google Scholar] [CrossRef]
- Liu, L.; Huang, H.; Chen, F.; Yu, H.; Tian, N.; Zhang, Y.; Zhang, T. Cooperation of Oxygen Vacancies and 2D Ultrathin Structure Promoting CO2 Photoreduction Performance of Bi4Ti3O12. Sci. Bull. 2020, 65, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, W.; Duan, W.; Liao, G.; Wang, C. Improved Solar-Powered Water-Splitting Performance of Bi4Ti3O12/TiO2 Composite with Synergistically Interacted Heterointerfaces Under Platinum Cocatalysis. Small 2025, 21, 2503677. [Google Scholar] [CrossRef]
- Cheng, X.; Cao, T.; Li, X.; Luo, G.; Cao, S.; Wang, C.; Guo, D. Enhanced Visible-Light Photocatalytic Performance of Hollow Bi/Bi4Ti3O12 Microspheres Assembled with Bi4Ti3O12 Nanosheets and Bi Nanodots Synthesized by Hydrothermal Method. J. Alloys Compd. 2024, 1010, 177483. [Google Scholar] [CrossRef]
- Liu, L.; Hu, J.; Sheng, Y.; Akhoundzadeh, H.; Tu, W.; Siow, W.J.S.; Ong, J.H.; Huang, H.; Xu, R. Ru Single Atom Dispersed Cu Nanoparticle with Dual Sites Enables Outstanding Photocatalytic CO2 Reduction. ACS Nano 2024, 18, 26271–26280. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, K.; Peng, R.; Zhou, J.; Li, N. Interface Charge Separation in Fe-Doped Bi4Ti3O12/ZnIn2S4 Heterostructure for Enhancing Photocatalytic CO2 Reduction. J. Alloys Compd. 2025, 1022, 180167. [Google Scholar] [CrossRef]
- He, W.; Jing, L.; Zhang, W.; Dong, X.; Zou, Y.; Liu, X.; Lv, X.; Chen, P.; Liao, J.; Zhang, X.; et al. Plasmonic Metallic Bi-Modified Defective Bi4Ti3O12 Nanosheets with Upward Migrating Electrons for Efficient Photocatalytic NO Removal and NO2 Inhibition. Chin. Chem. Lett. 2025, 111357. [Google Scholar] [CrossRef]
- Geng, S.; Sui, X.; Li, Y.; Wang, H.; Zhao, X.; Chang, L.; Duan, X. Reinforcing Piezo-Photocatalytic Properties and Enhancing RhB Degradation Efficiency of Ce-Doped Bi4Ti3O12: Mechanistic Insights. J. Mater. Chem. A 2025, 13, 23838–23852. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, C.; Xie, Y.; Wang, L.; Ding, J.; Zhang, J.; Wan, H.; Guan, G. Intentionally Introducing Oxygen Vacancies and Ti3+ Defects on the Surface of Bi4Ti3O12 Nanosheets for Promoting the Photoreduction of CO2 to CH3OH. ACS Appl. Nano Mater. 2024, 7, 3012–3023. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, L.; Chen, R.; Zhang, J.; Ding, J.; Zhang, J.; Wan, H.; Guan, G. Insight of the state for deliberately introduced A-site defect in nanofibrous LaFeO3 for boosting artificial photosynthesis of CH3OH. ACS Appl. Mater. Interfaces 2023, 15, 56945–56956. [Google Scholar] [CrossRef]
- Arif, M.; Zhang, M.; Mao, Y.; Bu, Q.; Ali, A.; Qin, Z.; Muhmood, T.; Shahnoor; Liu, X.; Zhou, B.; et al. Oxygen Vacancy Mediated Single Unit Cell Bi2WO6 by Ti Doping for Ameliorated Photocatalytic Performance. J. Colloid Interface Sci. 2021, 581, 276–291. [Google Scholar] [CrossRef]
- Jia, P.; Li, Y.; Zheng, Z.; Wang, Y.; Liu, T. Ferroelectric Polarization Promotes the Excellent CO2 Photoreduction Performance of Bi4Ti3O12 Synthesized by Molten Salt Method. J. Alloys Compd. 2022, 920, 165880. [Google Scholar] [CrossRef]
- Tech, S.J.; Choong, C.E.; Choi, E.H.; Yoon, Y.; Jang, M. Ru-doped Aurivillius phase perovskites Bi4Ti3O12 for boosting photo-redox of N2 in photocatalytic nitrogen fixation. Appl. Surf. Sci. 2025, 684, 161890. [Google Scholar]
- Anwar, K.; Naqvi, F.K.; Beg, S. Facile Hydrothermal Synthesis of Yb Doped Bi4V2O11 Nanoparticles for the Improvement of Photocatalytic and AC Impedance Performance: Investigation of Polymorphism, Adsorption Isotherm, Optical Properties and Possible Mechanism with the Help of GCMS. J. Alloys Compd. 2023, 938, 168483. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Zhong, D.; Ma, Y.; Ma, Q.; Wang, Z.; Pan, J. Fabrication of Bismuth Titanate Nanosheets with Tunable Crystal Facets for Photocatalytic Degradation of Antibiotic. J. Mater. Sci. 2019, 54, 13740–13752. [Google Scholar] [CrossRef]
- Sun, S.; Dong, Q.; Chen, Z. Macroscopic Polarization Enhancement Boosting Piezo-Photocatalytic Performance of Bi4Ti3O12 via La-doping. Ceram. Int. 2025, 51, 3846–3856. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, L.; Kaneko, H.; Gu, R.; Su, G.; Li, L.; Zhang, J.; Song, H.; Zhu, F.; Yamaguchi, A.; et al. Light-driven CO2 methanation over Au-grafted Ce0.95Ru0.05O2 solid-solution catalysts with activities approaching the thermodynamic limit. Nat. Catal. 2013, 6, 519–530. [Google Scholar] [CrossRef]
- Fang, H.; Kang, Y.; Yuan, S.; Zhang, M.; Rui, Z. One step synthesis and interfacial properties of black Ag/TiO2-x for enhancing sunlight absorption with application to photothermocatalytic VOCs degradation. Appl. Surf. Sci. 2024, 655, 159519. [Google Scholar] [CrossRef]
- Tang, Q.; Wu, J.; Chen, X.-Z.; Sanchis-Gual, R.; Veciana, A.; Franco, C.; Kim, D.; Surin, I.; Pérez-Ramírez, J.; Mattera, M.; et al. Tuning Oxygen Vacancies in Bi4Ti3O12 Nanosheets to Boost Piezo-Photocatalytic Activity. Nano Energy 2023, 108, 108202. [Google Scholar] [CrossRef]
- Mao, C.; Cheng, H.; Tian, H.; Li, H.; Xiao, W.-J.; Xu, H.; Zhao, J.; Zhang, L. Visible Light Driven Selective Oxidation of Amines to Imines with BiOCl: Does Oxygen Vacancy Concentration Matter. Appl. Catal. B-Environ. 2018, 228, 87–96. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, J.; Xiang, L.; Chen, C.; Feng, N.; Lv, Y.; Chang, K.; Huang, J. Ordering bimetallic Cu-Pd catalysts onto orderly mesoporous SrTiO3-crystal nanotubular networks for efficient carbon dioxide photoreduction. Angew. Chem. Int. Ed. 2024, 63, e202405807. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, Z.; Wu, J.; Chen, X.; Wang, L.; Yin, H.; Li, H.; Ding, J.; Wan, H.; Guan, G. “Carbon diffusion” engineered carbon nitride nanosheets for high-efficiency photocatalytic solar-to-fuels conversion. Renew. Energy 2022, 197, 943–952. [Google Scholar] [CrossRef]
- Feng, X.; Sun, T.; Feng, X.; Chen, L.; Yang, Y.; Zhang, F. Engineering the Near-Surface Structure of WO3 by an Amorphous Layer with Trivalent Ni and Self-Adapting Oxygen Vacancies for Efficient Photocatalytic and Photoelectrochemical Acidic Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2022, 14, 54769–54780. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, S.; Wu, B.; Wei, Y.; Yu, X.; Gan, Y.; Lin, T.; Yu, F.; Sun, F.; Jiang, Z.; et al. Selective Photocatalytic Oxidation of Methane to Oxygenates over Cu–W–TiO2 with Significant Carrier Traps. ACS Catal. 2022, 12, 9515–9525. [Google Scholar] [CrossRef]
- Li, G.; Lian, Z.; Wan, Z.; Liu, Z.; Qian, J.; Deng, Y.; Zhang, S.; Zhong, Q. Efficient Photothermal-Assisted Photocatalytic NO Removal on Molecular Cobalt Phthalocyanine/Bi2WO6 Z-Scheme Heterojunctions by Promoting Charge Transfer and Oxygen Activation. Appl. Catal. B 2022, 317, 121787. [Google Scholar] [CrossRef]
- Xia, P.; Cao, S.; Zhu, B.; Liu, M.; Shi, M.; Yu, J.; Zhang, Y. Designing a 0D/2D S-Scheme Heterojunction over Polymeric Carbon Nitride for Visible-Light Photocatalytic Inactivation of Bacteria. Angew. Chem. Int. Ed. 2020, 59, 5218–5225. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, L.; Wang, Y.; Qiu, C.; Wan, H. Defect Engineering via La Doping and Hydrogenation on Bi4Ti3O12 for Synergistically Enhancing Photocatalytic CO2 to CH3OH. Catalysts 2025, 15, 889. https://doi.org/10.3390/catal15090889
Xue L, Wang Y, Qiu C, Wan H. Defect Engineering via La Doping and Hydrogenation on Bi4Ti3O12 for Synergistically Enhancing Photocatalytic CO2 to CH3OH. Catalysts. 2025; 15(9):889. https://doi.org/10.3390/catal15090889
Chicago/Turabian StyleXue, Lijun, Yuxuan Wang, Chenhui Qiu, and Hui Wan. 2025. "Defect Engineering via La Doping and Hydrogenation on Bi4Ti3O12 for Synergistically Enhancing Photocatalytic CO2 to CH3OH" Catalysts 15, no. 9: 889. https://doi.org/10.3390/catal15090889
APA StyleXue, L., Wang, Y., Qiu, C., & Wan, H. (2025). Defect Engineering via La Doping and Hydrogenation on Bi4Ti3O12 for Synergistically Enhancing Photocatalytic CO2 to CH3OH. Catalysts, 15(9), 889. https://doi.org/10.3390/catal15090889





