Dual-Function Bare Copper Oxide (Photo)Catalysts for Selective Phenol Production via Benzene Hydroxylation and Low-Temperature Hydrogen Generation from Formic Acid
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalysts Characterization
2.1.1. Selective Photocatalytic Benzene Hydroxylation to Phenol
2.1.2. Photocatalytic Degradation of Phenol Under UV Light Irradiation
2.1.3. Mechanistic Investigation of NCuO (10 min) Through HPLC Monitoring
2.2. Decomposition of Formic Acid to Hydrogen and Carbon Dioxide
3. Materials and Methods
3.1. Materials
3.2. Catalysts Preparation
3.3. Chemical-Physical Characterization Methods
3.4. Photocatalytic Tests
3.5. Catalytic Tests for Formic Acid Decomposition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tahmasbi, M.; Siavashi, M.; Ahmadi, R. A Comprehensive Review of Hydrogen Production and Storage Methods: Fundamentals, Advances, and SWOT Analysis. Energy Convers. Manag. X 2025, 26, 101005. [Google Scholar] [CrossRef]
- Tanksale, A.; Beltramini, J.N.; Lu, G.M. A Review of Catalytic Hydrogen Production Processes from Biomass. Renew. Sustain. Energy Rev. 2010, 14, 166–182. [Google Scholar] [CrossRef]
- Das, R.; Rohit, K.R.; Anilkumar, G. Recent Trends in Non-Noble Metal-Catalyzed Hydroxylation Reactions. J. Organomet. Chem. 2022, 977, 122456. [Google Scholar] [CrossRef]
- Naranov, E.; Ramazanov, D.; Agliullin, M.; Sinyashin, O.; Maximov, A. Recent Advances in Aromatic Hydroxylation to Phenol and Hydroquinone Using H2O2. Catalysts 2024, 14, 930. [Google Scholar] [CrossRef]
- Luo, Y.; Xiong, J.; Pang, C.; Li, G.; Hu, C. Direct Hydroxylation of Benzene to Phenol over TS-1 Catalysts. Catalysts 2018, 8, 49. [Google Scholar] [CrossRef]
- Jeong, D.; Jo, W.; Jeong, J.; Kim, T.; Han, S.; Son, M.-K.; Jung, H. Characterization of Cu2O/CuO Heterostructure Photocathode by Tailoring CuO Thickness for Photoelectrochemical Water Splitting. RSC Adv. 2022, 12, 2632–2640. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, D.; Wu, Q.; Diao, P. Cu2O/CuO Bilayered Composite as a High-Efficiency Photocathode for Photoelectrochemical Hydrogen Evolution Reaction. Sci. Rep. 2016, 6, 35158. [Google Scholar] [CrossRef] [PubMed]
- Bayat, F.; Sheibani, S. Enhancement of Photocatalytic Activity of CuO-Cu2O Heterostructures through the Controlled Content of Cu2O. Mater. Res. Bull. 2022, 145, 111561. [Google Scholar] [CrossRef]
- Dasineh Khiavi, N.; Katal, R.; Kholghi Eshkalak, S.; Masudy-Panah, S.; Ramakrishna, S.; Jiangyong, H. Visible Light Driven Heterojunction Photocatalyst of CuO–Cu2O Thin Films for Photocatalytic Degradation of Organic Pollutants. Nanomaterials 2019, 9, 1011. [Google Scholar] [CrossRef]
- Yashwantrao, G.; Saha, S. Sustainable Strategies of C–N Bond Formation via Ullmann Coupling Employing Earth Abundant Copper Catalyst. Tetrahedron 2021, 97, 132406. [Google Scholar] [CrossRef]
- Rutkowska, A.I.; Wadas, A.; Szaniawska, E.; Chmielnicka, A.; Zlotorowicz, A.; Kulesza, J.P. Elucidation of activity of copper and copper oxide nanomaterials for electrocatalytic and photoelectrochemical reduction of carbon dioxide. Curr. Opin. Electrochem. 2020, 23, 131–138. [Google Scholar] [CrossRef]
- Bonthula, S.; Bonthula, S.R.; Pothu, R.; Srivastava, R.K.; Boddula, R.; Radwan, A.B.; Al-Qahtani, N. Recent Advances in Copper-Based Materials for Sustainable Environmental Applications. Sustain. Chem. 2023, 4, 246–271. [Google Scholar] [CrossRef]
- Ali, S.; Razzaq, A.; Kim, H.; In, S.-I. Activity, Selectivity, and Stability of Earth-Abundant CuO/Cu2O/Cu0-Based Photocatalysts toward CO2 Reduction. Chem. Eng. J. 2022, 429, 131579. [Google Scholar] [CrossRef]
- Impemba, S.; Provinciali, G.; Filippi, J.; Caporali, S.; Muzzi, B.; Casini, A.; Caporali, M. Tightly Interfaced Cu2O with In2O3 to Promote Hydrogen Evolution in Presence of Biomass-Derived Alcohols. ChemNanoMat 2024, 10, e202400459. [Google Scholar] [CrossRef]
- Impemba, S.; Provinciali, G.; Filippi, J.; Salvatici, C.; Berretti, E.; Caporali, S.; Banchelli, M.; Caporali, M. Engineering the Heterojunction between TiO2 and In2O3 for Improving the Solar-Driven Hydrogen Production. Int. J. Hydrogen Energy 2024, 63, 896–904. [Google Scholar] [CrossRef]
- Allen, S.E.; Walvoord, R.R.; Padilla-Salinas, R.; Kozlowski, M.C. Aerobic Copper-Catalyzed Organic Reactions. Chem. Rev. 2013, 113, 6234–6458. [Google Scholar] [CrossRef]
- Marais, L.; Vosloo, H.C.M.; Swarts, A.J. Homogeneous Oxidative Transformations Mediated by Copper Catalyst Systems. Coord. Chem. Rev. 2021, 440, 213958. [Google Scholar] [CrossRef]
- Priya, S.C.; Vijayalakshmi, S.; Raghavendra, S.G.; Yıldızhan, S.; Ranjitha, J. A Critical Review on Efficient Photocatalytic Degradation of Organic Compounds Using Copper-Based Nanoparticles. Mater. Today Proc. 2023, 80, 3075–3081. [Google Scholar] [CrossRef]
- Siavash Moakhar, R.; Hosseini-Hosseinabad, S.M.; Masudy-Panah, S.; Seza, A.; Jalali, M.; Fallah-Arani, H.; Dabir, F.; Gholipour, S.; Abdi, Y.; Bagheri-Hariri, M.; et al. Photoelectrochemical Water-Splitting Using CuO-Based Electrodes for Hydrogen Production: A Review. Adv. Mater. 2021, 33, 2007285. [Google Scholar] [CrossRef]
- Yadav, S.; Jain, A.; Malhotra, P. A review on the sustainable routes for the synthesis and applications of cuprous oxide nanoparticles and their nanocomposites. Green Chem. 2019, 21, 937–955. [Google Scholar] [CrossRef]
- Han, W.; Xiang, W.; Shi, J.; Ji, Y. Recent Advances in the Heterogeneous Photocatalytic Hydroxylation of Benzene to Phenol. Molecules 2022, 27, 5457. [Google Scholar] [CrossRef]
- Mancuso, A.; Sacco, O.; Sannino, D.; Venditto, V.; Vaiano, V. One-Step Catalytic or Photocatalytic Oxidation of Benzene to Phenol: Possible Alternative Routes for Phenol Synthesis? Catalysts 2020, 10, 1424. [Google Scholar] [CrossRef]
- Jia, X.; Wang, F.; Wen, H.; Zhang, L.; Jiao, S.; Wang, X.; Pei, X.; Xing, S. An Efficient Photocatalyst Based on H5PMo10V2O40/UiO-66-NH2 for Direct Hydroxylation of Benzene to Phenol by H2O2. RSC Adv. 2022, 12, 29433–29439. [Google Scholar] [CrossRef]
- He, J.; Zhang, M.; Primo, A.; García, H.; Li, Z. Selective Photocatalytic Benzene Hydroxylation to Phenol Using Surface-Modified Cu2O Supported on Graphene. J. Mater. Chem. A 2018, 6, 19782–19787. [Google Scholar] [CrossRef]
- Mancuso, A.; Sacco, O.; Vaiano, V.; Venditto, V. Simultaneous Boosting Visible Light-Driven Benzene Hydroxylation Reaction Rate and Phenol Selectivity via a Novel Monolithic Polymer Photoreactive Composite Based on N-Doped TiO2 Supported CuO. J. Catal. 2025, 447, 116116. [Google Scholar] [CrossRef]
- Mancuso, A.; Picca, R.A.; Izzi, M.; Di Franco, C.; Sacco, O.; Vaiano, V.; Venditto, V. An Innovative Biphasic Reaction System for Highly Selective Benzene Hydroxylation Based on Photocatalytic Polymer Composites. ChemPhotoChem 2024, 8, e202400203. [Google Scholar] [CrossRef]
- Mancuso, A.; Vaiano, V.; Antico, P.; Sacco, O.; Venditto, V. Photoreactive Polymer Composite for Selective Oxidation of Benzene to Phenol. Catal. Today 2023, 413–415, 113914. [Google Scholar] [CrossRef]
- Basyach, P.; Guha, A.K.; Borthakur, S.; Kalita, L.; Chetia, P.; Sonowal, K.; Saikia, L. Efficient Hydroxylation of Benzene to Phenol by H2O2 Using Ni-Doped CuWO4 on Carbon Nitride as a Catalyst under Solar Irradiation and Its Structure–Activity Correlation. J. Mater. Chem. A 2020, 8, 12774–12789. [Google Scholar] [CrossRef]
- Mancuso, A.; Gottuso, A.; Parrino, F.; Picca, R.A.; Venditto, V.; Sacco, O.; Vaiano, V. Tuning the Selectivity of Visible Light-Driven Hydroxylation of Benzene to Phenol by Using Cu, Fe and V Oxides Supported on N-Doped TiO2. Green Chem. 2023, 25, 10664–10677. [Google Scholar] [CrossRef]
- Eppinger, J.; Huang, K.-W. Formic Acid as a Hydrogen Energy Carrier. ACS Energy Lett. 2017, 2, 188–195. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Xu, Q. Liquid Organic and Inorganic Chemical Hydrides for High-Capacity Hydrogen Storage. Energy Environ. Sci. 2015, 8, 478–512. [Google Scholar] [CrossRef]
- Voskresenskaya, E.N.; Kirilets, V.M.; Taran, O.P.; Kuznetsov, B.N. Hydrogen Production by the Heterogeneous Catalytic Dehydrogenation of Formic Acid: A Review. Catal. Ind. 2024, 16, 339–349. [Google Scholar] [CrossRef]
- Li, H.; Song, D.; Wang, X.; Li, X.; Lei, G. Recent Progress on Heterogeneous Catalytic Formic Acid Decomposition for Hydrogen Production. Fuel 2025, 383, 133824. [Google Scholar] [CrossRef]
- Wen, H.; Liu, Y.; Liu, S.; Peng, Z.; Wu, X.; Yuan, H.; Jiang, J.; Li, B. Heterogeneous Catalysis in Production and Utilization of Formic Acid for Renewable Energy. Small 2024, 20, 2305405. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, S.; Kumar, A. Hydrogen Energy Future with Formic Acid: A Renewable Chemical Hydrogen Storage System. Catal. Sci. Technol. 2016, 6, 12–40. [Google Scholar] [CrossRef]
- Zhong, H.; Iguchi, M.; Chatterjee, M.; Himeda, Y.; Xu, Q.; Kawanami, H. Formic Acid-Based Liquid Organic Hydrogen Carrier System with Heterogeneous Catalysts. Adv. Sustainable Syst. 2018, 2, 1700161. [Google Scholar] [CrossRef]
- Gu, X.; Lu, Z.-H.; Jiang, H.-L.; Akita, T.; Xu, Q. Synergistic Catalysis of Metal–Organic Framework-Immobilized Au–Pd Nanoparticles in Dehydrogenation of Formic Acid for Chemical Hydrogen Storage. J. Am. Chem. Soc. 2011, 133, 11822–11825. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.-Y.; Lin, J.-D.; Liu, Y.-M.; He, H.-Y.; Huang, F.-Q.; Cao, Y. Gold Supported on Zirconia Polymorphs for Hydrogen Generation from Formic Acid in Base-Free Aqueous Medium. J. Power Sources 2016, 328, 463–471. [Google Scholar] [CrossRef]
- Bi, Q.-Y.; Du, X.-L.; Liu, Y.-M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Efficient Subnanometric Gold-Catalyzed Hydrogen Generation via Formic Acid Decomposition under Ambient Conditions. J. Am. Chem. Soc. 2012, 134, 8926–8933. [Google Scholar] [CrossRef]
- Diglio, M.; Contento, I.; Impemba, S.; Berretti, E.; Della Sala, P.; Oliva, G.; Naddeo, V.; Caporali, S.; Primo, A.; Talotta, C.; et al. Hydrogen Production from Formic Acid Decomposition Promoted by Gold Nanoparticles Supported on a Porous Polymer Matrix. Energy Fuels 2025, 39, 14320–14329. [Google Scholar] [CrossRef]
- Barlocco, I.; Bellomi, S.; Delgado, J.J.; Chen, X.; Prati, L.; Dimitratos, N.; Roldan, A.; Villa, A. Enhancing Activity, Selectivity and Stability of Palladium Catalysts in Formic Acid Decomposition: Effect of Support Functionalization. Catal. Today 2021, 382, 61–70. [Google Scholar] [CrossRef]
- Barlocco, I.; Capelli, S.; Lu, X.; Bellomi, S.; Huang, X.; Wang, D.; Prati, L.; Dimitratos, N.; Roldan, A.; Villa, A. Disclosing the Role of Gold on Palladium—Gold Alloyed Supported Catalysts in Formic Acid Decomposition. ChemCatChem 2021, 13, 4210–4222. [Google Scholar] [CrossRef]
- Chen, T.; Chen, J.; Wu, J.; Song, W.; Hu, S.; Feng, X.; Chen, Z.; Yuan, E.; Ji, W.; Au, C.-T. Atomic-Layer-Deposition Derived Pt Subnano Clusters on the (110) Facet of Hexagonal Al2O3 Plates: Efficient for Formic Acid Decomposition and Water Gas Shift. ACS Catal. 2023, 13, 887–901. [Google Scholar] [CrossRef]
- Cai, L.; Zhou, J.; Chen, X.; Huang, B.; Hu, W.; Yuan, D. Pt-Based Intermetallic Compounds with Tunable Activity and Selectivity toward Hydrogen Production from Formic Acid. Appl. Surf. Sci. 2022, 597, 153530. [Google Scholar] [CrossRef]
- Iglesias, M.; Fernández-Alvarez, F.J. Advances in Nonprecious Metal Homogeneously Catalyzed Formic Acid Dehydrogenation. Catalysts 2021, 11, 1288. [Google Scholar] [CrossRef]
- Wang, A.; He, P.; Wu, J.; Chen, N.; Pan, C.; Shi, E.; Jia, H.; Hu, T.; He, K.; Cai, Q.; et al. Reviews on Homogeneous and Heterogeneous Catalysts for Dehydrogenation and Recycling of Formic Acid: Progress and Perspectives. Energy Fuels 2023, 37, 17075–17093. [Google Scholar] [CrossRef]
- Guo, J.; Yin, C.K.; Zhong, D.L.; Wang, Y.L.; Qi, T.; Liu, G.H.; Shen, L.T.; Zhou, Q.S.; Peng, Z.H.; Yao, H.; et al. Formic Acid as a Potential On-Board Hydrogen Storage Method: Development of Homogeneous Noble Metal Catalysts for Dehydrogenation Reactions. ChemSusChem 2021, 14, 2655–2681. [Google Scholar] [CrossRef] [PubMed]
- Carrales-Alvarado, D.H.; López-Olmos, C.; Dongil, A.B.; Kubacka, A.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Effect of N-Doping and Carbon Nanostructures on NiCu Particles for Hydrogen Production from Formic Acid. Appl. Catal. B Environ. 2021, 298, 120604. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Chuvilin, A.L.; Sobolev, V.I.; Stolyarova, S.G.; Shubin, Y.V.; Asanov, I.P.; Ishchenko, A.V.; Magnani, G.; Riccò, M.; Okotrub, A.V.; et al. Copper on Carbon Materials: Stabilization by Nitrogen Doping. J. Mater. Chem. A 2017, 5, 10574–10583. [Google Scholar] [CrossRef]
- Pechenkin, A.; Badmaev, S.; Belyaev, V.; Sobyanin, V. Production of Hydrogen-Rich Gas by Formic Acid Decomposition over CuO-CeO2/γ-Al2O3 Catalyst. Energies 2019, 12, 3577. [Google Scholar] [CrossRef]
- Esper Neto, M.; Britt, D.W.; Jackson, K.A.; Braccini, A.L.; Inoue, T.T.; Batista, M.A. Early Development of Corn Seedlings Primed with Synthetic Tenorite Nanofertilizer. J. Seed Sci. 2020, 42, e202042040. [Google Scholar] [CrossRef]
- Montgomery, M.J.; Sugak, N.V.; Yang, K.R.; Rogers, J.M.; Kube, S.A.; Ratinov, A.C.; Schroers, J.; Batista, V.S.; Pfefferle, L.D. Semiconductor-to-Conductor Transition in 2D Copper(II) Oxide Nanosheets through Surface Sulfur-Functionalization. Nanoscale 2020, 12, 14549–14559. [Google Scholar] [CrossRef]
- Kooti, M. Fabrication of Nanosized Cuprous Oxide Using Fehling’s Solution. Trans. F Nanotechnol. 2010, 17, 73. [Google Scholar]
- Murthy, P.S.; Venugopalan, V.P.; Das, D.A.; Dhara, S.; Pandiyan, R.; Tyagi, A.K. Antibiofilm Activity of Nano Sized CuO. In International Conference on Nanoscience, Engineering and Technology (ICONSET 2011); IEEE: Chennai, India, 2011; pp. 580–583. [Google Scholar] [CrossRef]
- Mo, X.; Hu, J.; Shen, H.; Shui, L.; Shu, D.; He, J.; He, G.; Wang, Y.; Li, W.; He, Q. Surface Modification of Micro-Sized CuO by in Situ-Growing Heterojunctions CuO/Cu2O and CuO/Cu2O/Cu: Effect on Surface Charges and Photogenerated Carrier Lifetime. Appl. Phys. A 2018, 124, 719. [Google Scholar] [CrossRef]
- Kumar, N.; Limbu, S.; Chauhan, R.N. Correlation between Different Characterizations and Improved Electrochemical Performances of Cuprous Oxide-Based Nanocrystals. Bull. Mater. Sci. 2023, 46, 141. [Google Scholar] [CrossRef]
- Li, H.; Ban, L.; Niu, Z.; Huang, X.; Meng, P.; Han, X.; Zhang, Y.; Zhang, H.; Zhao, Y. Application of CuxO-FeyOz Nanocatalysts in Ethynylation of Formaldehyde. Nanomaterials 2019, 9, 1301. [Google Scholar] [CrossRef]
- Zheng, S.; Duley, W.W.; Peng, P.; Norman Zhou, Y. Engineering Intrinsic Defects in CuO NWs through Laser Irradiation: Oxygen vs Copper Vacancies. Appl. Surf. Sci. 2024, 642, 158630. [Google Scholar] [CrossRef]
- Yang, C.P.; Wu, Q.; Jiang, Z.W.; Wang, X.; Huang, C.Z.; Li, Y.F. Cu Vacancies Enhanced Photoelectrochemical Activity of Metal-Organic Gel-Derived CuO for the Detection of l-Cysteine. Talanta 2021, 228, 122261. [Google Scholar] [CrossRef]
- Zhakypov, A.S.; Nemkayeva, R.R.; Yerlanuly, Y.; Tulegenova, M.A.; Kurbanov, B.Y.; Aitzhanov, M.B.; Markhabayeva, A.A.; Gabdullin, M.T. Synthesis and in Situ Oxidation of Copper Micro- and Nanoparticles by Arc Discharge Plasma in Liquid. Sci. Rep. 2023, 13, 15714. [Google Scholar] [CrossRef]
- Deng, Y.; Handoko, A.D.; Du, Y.; Xi, S.; Yeo, B.S. In Situ Raman Spectroscopy of Copper and Copper Oxide Surfaces during Electrochemical Oxygen Evolution Reaction: Identification of CuIII Oxides as Catalytically Active Species. ACS Catal. 2016, 6, 2473–2481. [Google Scholar] [CrossRef]
- Anuar, N.A.S.K.; Sheng, C.K. Structural and Morphological Characterization of CuO Nanostructure Precipitated by Water-Soluble Copper (II) Nitrate Hemi(Pentahydrate) and NaOH as Reactants. J. Nano-Electron. Phys. 2021, 13, 05015-1–05015-4. [Google Scholar] [CrossRef]
- Rahnama, A.; Gharagozlou, M.; Gardeshzadeh, A.R. Comparative study of copper precursors for synthesis of CuO nanoparticles by ultrasonic-assisted thermal decomposition method. J. Indian Chem. Soc. 2013, 90, 271–277. [Google Scholar]
- Mansour, S.A.A. Thermoanalytical Investigations of the Decomposition Course of Copper Oxysalts: III. Copper(II) Acetate Monohydrate. J. Therm. Anal. 1996, 46, 263–274. [Google Scholar] [CrossRef]
- Iwamoto, H.; Kameoka, S.; Xu, Y.; Nishimura, C.; Tsai, A.P. Effects of Cu Oxidation States on the Catalysis of NO + CO and N2O + CO Reactions. J. Phys. Chem. Solids 2019, 125, 64–73. [Google Scholar] [CrossRef]
- Youssef, I.; Sall, S.; Dintzer, T.; Labidi, S.; Petit, C. Forward Looking Analysis Approach to Assess Copper Acetate Thermal Decomposition Reaction Mechanism. AJAC 2019, 10, 153–170. [Google Scholar] [CrossRef]
- Živković, A.; Mallia, G.; King, H.E.; De Leeuw, N.H.; Harrison, N.M. Mind the Interface Gap: Exposing Hidden Interface Defects at the Epitaxial Heterostructure between CuO and Cu2O. ACS Appl. Mater. Interfaces 2022, 14, 56331–56343. [Google Scholar] [CrossRef] [PubMed]
- Valvo, M.; Thyr, J.; Edvinsson, T. Defect-Induced Raman Scattering in Cu2O Nanostructures and Their Photocatalytic Performance. ChemElectroChem 2023, 10, e202300376. [Google Scholar] [CrossRef]
- Xie, C.; Wang, W.; Yang, Y.; Jiang, L.; Chen, Y.; He, J.; Wang, J. Enhanced Stability and Activity for Solvent-Free Selective Oxidation of Cyclohexane over Cu2O/CuO Fabricated by Facile Alkali Etching Method. Mol. Catal. 2020, 495, 111134. [Google Scholar] [CrossRef]
- Xie, J.; Li, X.; Guo, J.; Luo, L.; Delgado, J.J.; Martsinovich, N.; Tang, J. Highly Selective Oxidation of Benzene to Phenol with Air at Room Temperature Promoted by Water. Nat. Commun. 2023, 14, 4431. [Google Scholar] [CrossRef]
- Park, H.; Choi, W. Photocatalytic Conversion of Benzene to Phenol Using Modified TiO2 and Polyoxometalates. Catal. Today 2005, 101, 291–297. [Google Scholar] [CrossRef]
- Han, W.; Xiang, W.; Meng, Z.; Dong, S.; Lv, Y. A Novel Cr-Doped CdS/ZnO Nanocomposite for Efficient Photocatalytic Hydroxylation of Benzene to Phenol. Colloids Surf. A Physicochem. Eng. Asp. 2023, 670, 131529. [Google Scholar] [CrossRef]
- Wang, Z.; Hisahiro, E. Recent Trends in Phenol Synthesis by Photocatalytic Oxidation of Benzene. Dalton Trans. 2023, 52, 9525–9540. [Google Scholar] [CrossRef]
- Gupta, N.; Bansal, P.; Pal, B. Metal Ion-TiO2 Nanocomposites for the Selective Photooxidation of Benzene to Phenol and Cycloalkanol to Cycloalkanone. J. Exp. Nanosci. 2015, 10, 148–160. [Google Scholar] [CrossRef]
- Yu, H.; Ming, H.; Gong, J.; Li, H.; Huang, H.; Pan, K.; Liu, Y.; Kang, Z.; Wei, J.; Wang, D. Facile Synthesis of Au/ZnO Nanoparticles and Their Enhanced Photocatalytic Activity for Hydroxylation of Benzene. Bull. Mater. Sci. 2013, 36, 367–372. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium Dioxide Photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous Photocatalysis: Fundamentals and Applications to the Removal of Various Types of Aqueous Pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Zinc Oxide Based Photocatalysis: Tailoring Surface-Bulk Structure and Related Interfacial Charge Carrier Dynamics for Better Environmental Applications. RSC Adv. 2015, 5, 3306–3351. [Google Scholar] [CrossRef]
- Tuama, A.N.; Alzubaidi, L.H.; Jameel, M.H.; Abass, K.H.; Bin Mayzan, M.Z.H.; Salman, Z.N. Impact of Electron–Hole Recombination Mechanism on the Photocatalytic Performance of ZnO in Water Treatment: A Review. J. Sol-Gel Sci. Technol. 2024, 110, 792–806. [Google Scholar] [CrossRef]
- Bulut, A.; Yurderi, M.; Karatas, Y.; Zahmakiran, M.; Kivrak, H.; Gulcan, M.; Kaya, M. Pd-MnO Nanoparticles Dispersed on Amine-Grafted Silica: Highly Efficient Nanocatalyst for Hydrogen Production from Additive-Free Dehydrogenation of Formic Acid under Mild Conditions. Appl. Catal. B Environ. 2015, 164, 324–333. [Google Scholar] [CrossRef]
- Loges, B.; Boddien, A.; Junge, H.; Beller, M. Kontrollierte Wasserstofferzeugung aus Ameisensäure-Amin-Addukten bei Raumtemperatur und direkte Nutzung in H2/O2-Brennstoffzellen. Angew. Chem. 2008, 120, 4026–4029. [Google Scholar] [CrossRef]
- Mosleh, S.; Rahimi, M.R.; Ghaedi, M.; Dashtian, K.; Hajati, S. Sonochemical-Assisted Synthesis of CuO/Cu2O/Cu Nanoparticles as Efficient Photocatalyst for Simultaneous Degradation of Pollutant Dyes in Rotating Packed Bed Reactor: LED Illumination and Central Composite Design Optimization. Ultrason. Sonochem. 2018, 40, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Canton, P.; Fazzini, P.F.; Meneghini, C.; Benedetti, A.; Pozzi, G. Nanoscale Characterization of Metal Nanoclusters by Means of X-Ray Diffraction (XRD) and Transmission Electron Microscopy (TEM) Techniques. In Metal Nanoclusters in Catalysis and Materials Science; Elsevier: Amsterdam, The Netherlands, 2008; pp. 129–147. [Google Scholar] [CrossRef]
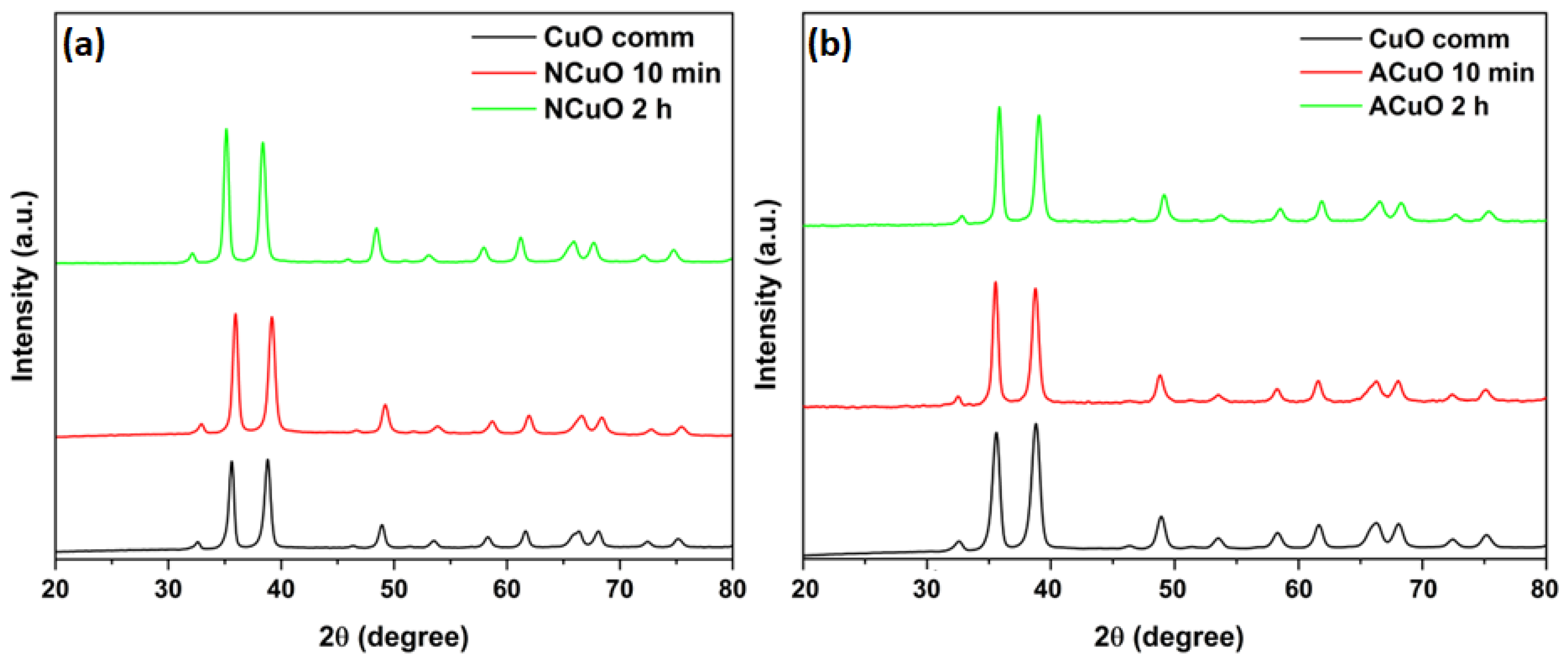
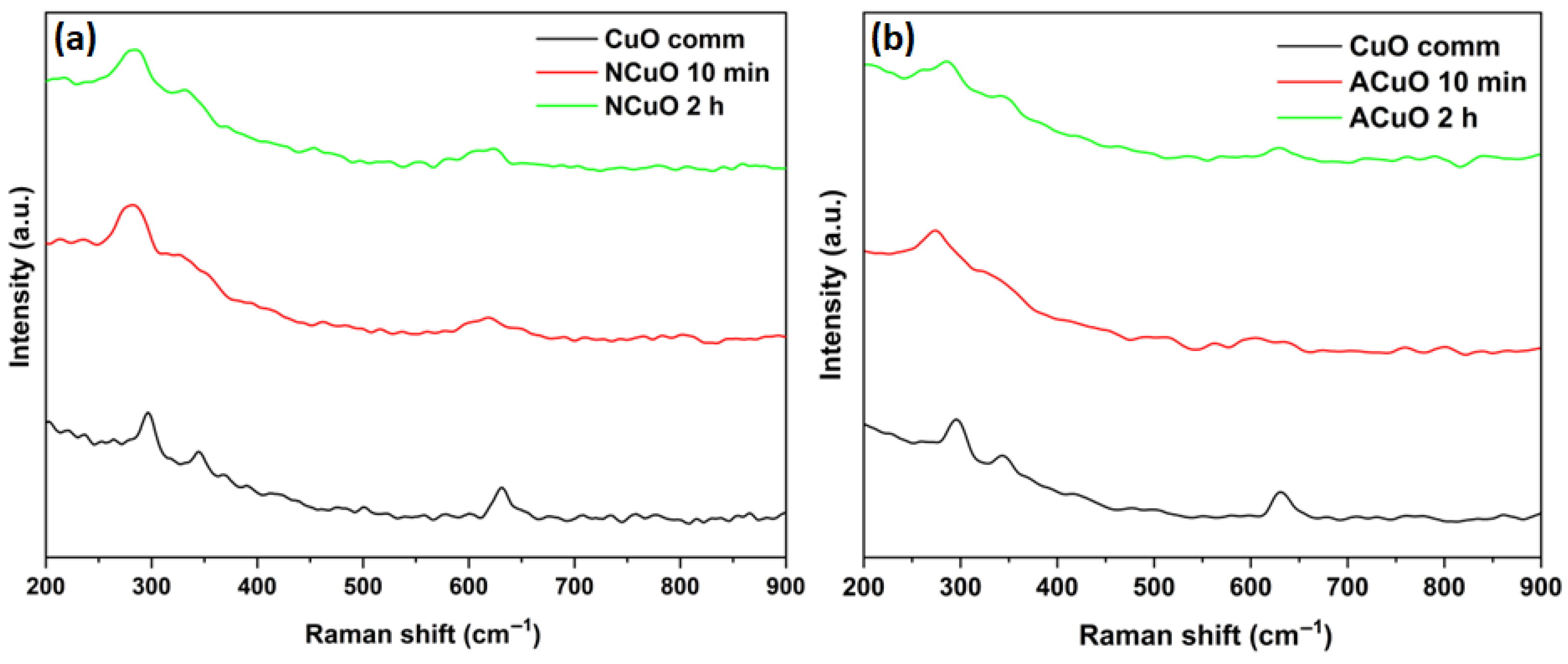
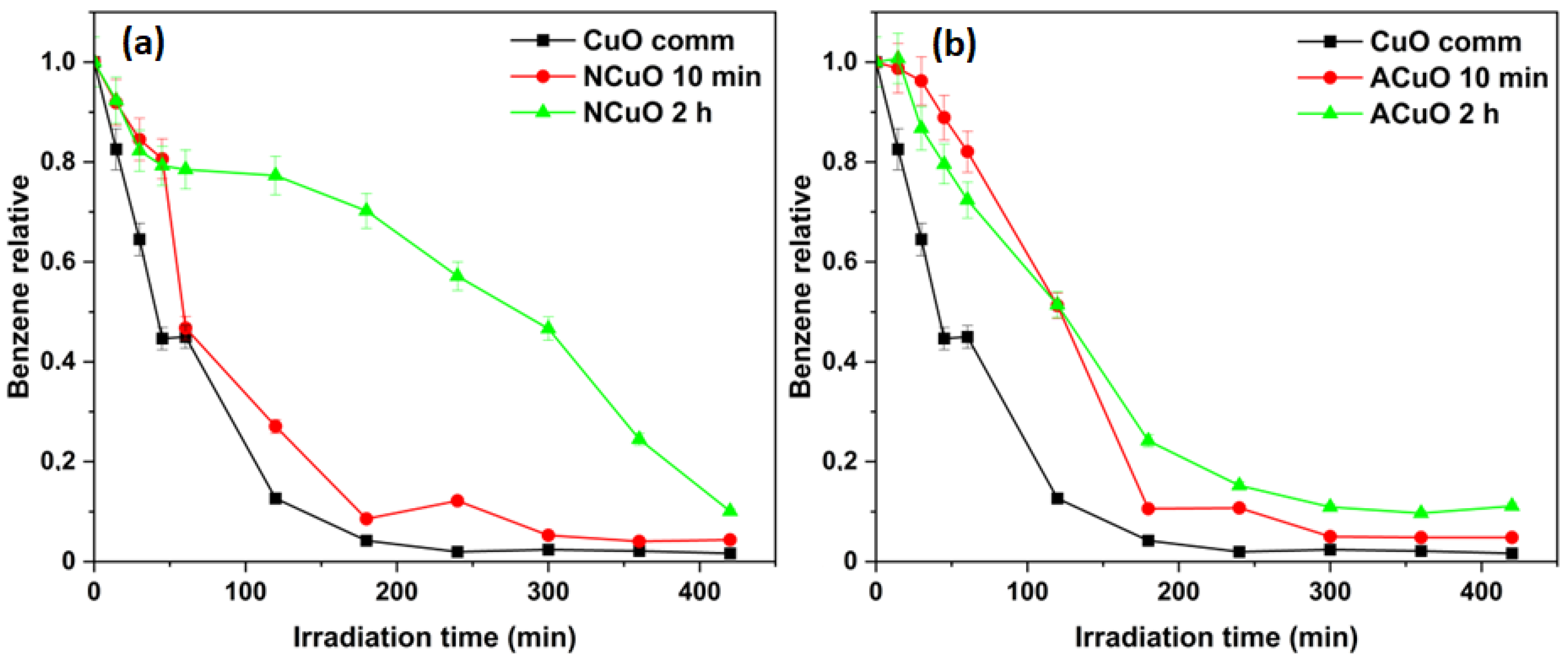
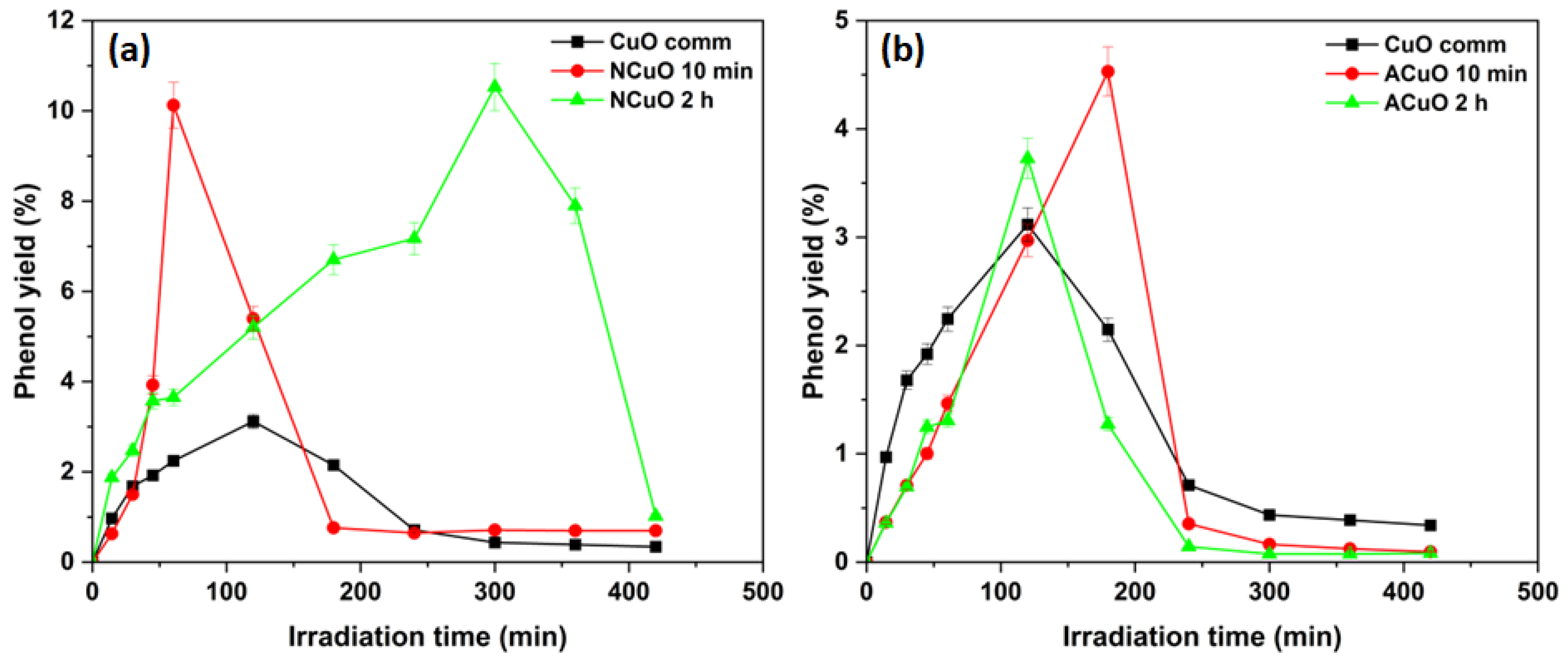

| Samples | CuXO Precursor | Calcination Time | Crystallite Size, nm | SSA, m2/g |
|---|---|---|---|---|
| NCuO 10 min | Copper nitrate | 10 min | 16.9 | 6 |
| NCuO 2 h | Copper nitrate | 2 h | 18.1 | 6 |
| ACuO 10 min | Copper acetate | 10 min | 18.6 | 5 |
| ACuO 2 h | Copper acetate | 2 h | 19.9 | 4 |
| Commercial CuO | - | - | 16.7 | 2 |
| Samples | Time to Maximum Phenol Yield | X Bz | Yphl | Sphl | SDeg |
|---|---|---|---|---|---|
| NCuO 10 min | 60 min | 55.5% | 10% | 18.0% | 76.9% |
| NCuO 2 h | 300 min | 55% | 10.5% | 19.0% | 75% |
| ACuO 10 min | 180 min | 80% | 4.5% | 5.6% | 87.2% |
| ACuO 2 h | 120 min | 51.8% | 3.7% | 7.1% | 90.3% |
| CuO | 120 min | 89% | 2.8% | 3.2% | 94.5% |
| Photocatalyst | Irradiation Time (min) | Phenol Degradation (%) |
|---|---|---|
| NCuO 10 min | 180 | ~2 |
| NCuO 2 h | 180 | ~1 |
| ACuO 10 min | 180 | ~19 |
| ACuO 2 h | 180 | ~18 |
| CuO | 180 | ~3 |
| Entry a | Catalyst (g) | T (°C) | Time (h) | Conversion (%) b |
|---|---|---|---|---|
| 1 | NCuO 10 min (0.1) | 100 | 1 | >99 |
| 2 | NcuO 2 h (0.1) | 100 | 1 | >99 |
| 3 | AcuO 10 min (0.1) | 100 | 1 | >99 |
| 4 | AcuO 2 h (0.1) | 100 | 1 | >99 |
| 5 | NcuO 10 min (0.1) | 25 | 1 | >99 |
| 6 | NcuO 2 h (0.1) | 25 | 1 | >99 |
| 7 | AcuO 10 min (0.1) | 25 | 1 | >99 |
| 8 | AcuO 2 h (0.1) | 25 | 1 | >99 |
| 9 | NcuO 10 min (0.05) | 25 | 0.25 | >99 |
| 10 | NcuO 2 h (0.05) | 25 | 0.25 | >99 |
| 11 | AcuO 10 min (0.05) | 25 | 0.25 | >99 |
| 12 | AcuO 2 h (0.05) | 25 | 0.25 | >99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancuso, A.; Diglio, M.; Impemba, S.; Venditto, V.; Vaiano, V.; Buonerba, A.; Sacco, O. Dual-Function Bare Copper Oxide (Photo)Catalysts for Selective Phenol Production via Benzene Hydroxylation and Low-Temperature Hydrogen Generation from Formic Acid. Catalysts 2025, 15, 866. https://doi.org/10.3390/catal15090866
Mancuso A, Diglio M, Impemba S, Venditto V, Vaiano V, Buonerba A, Sacco O. Dual-Function Bare Copper Oxide (Photo)Catalysts for Selective Phenol Production via Benzene Hydroxylation and Low-Temperature Hydrogen Generation from Formic Acid. Catalysts. 2025; 15(9):866. https://doi.org/10.3390/catal15090866
Chicago/Turabian StyleMancuso, Antonietta, Matteo Diglio, Salvatore Impemba, Vincenzo Venditto, Vincenzo Vaiano, Antonio Buonerba, and Olga Sacco. 2025. "Dual-Function Bare Copper Oxide (Photo)Catalysts for Selective Phenol Production via Benzene Hydroxylation and Low-Temperature Hydrogen Generation from Formic Acid" Catalysts 15, no. 9: 866. https://doi.org/10.3390/catal15090866
APA StyleMancuso, A., Diglio, M., Impemba, S., Venditto, V., Vaiano, V., Buonerba, A., & Sacco, O. (2025). Dual-Function Bare Copper Oxide (Photo)Catalysts for Selective Phenol Production via Benzene Hydroxylation and Low-Temperature Hydrogen Generation from Formic Acid. Catalysts, 15(9), 866. https://doi.org/10.3390/catal15090866










