Preparation of Pt/xMnO2-CNTs Catalyst and Its Electrooxidation Performance in Methanol
Abstract
1. Introduction
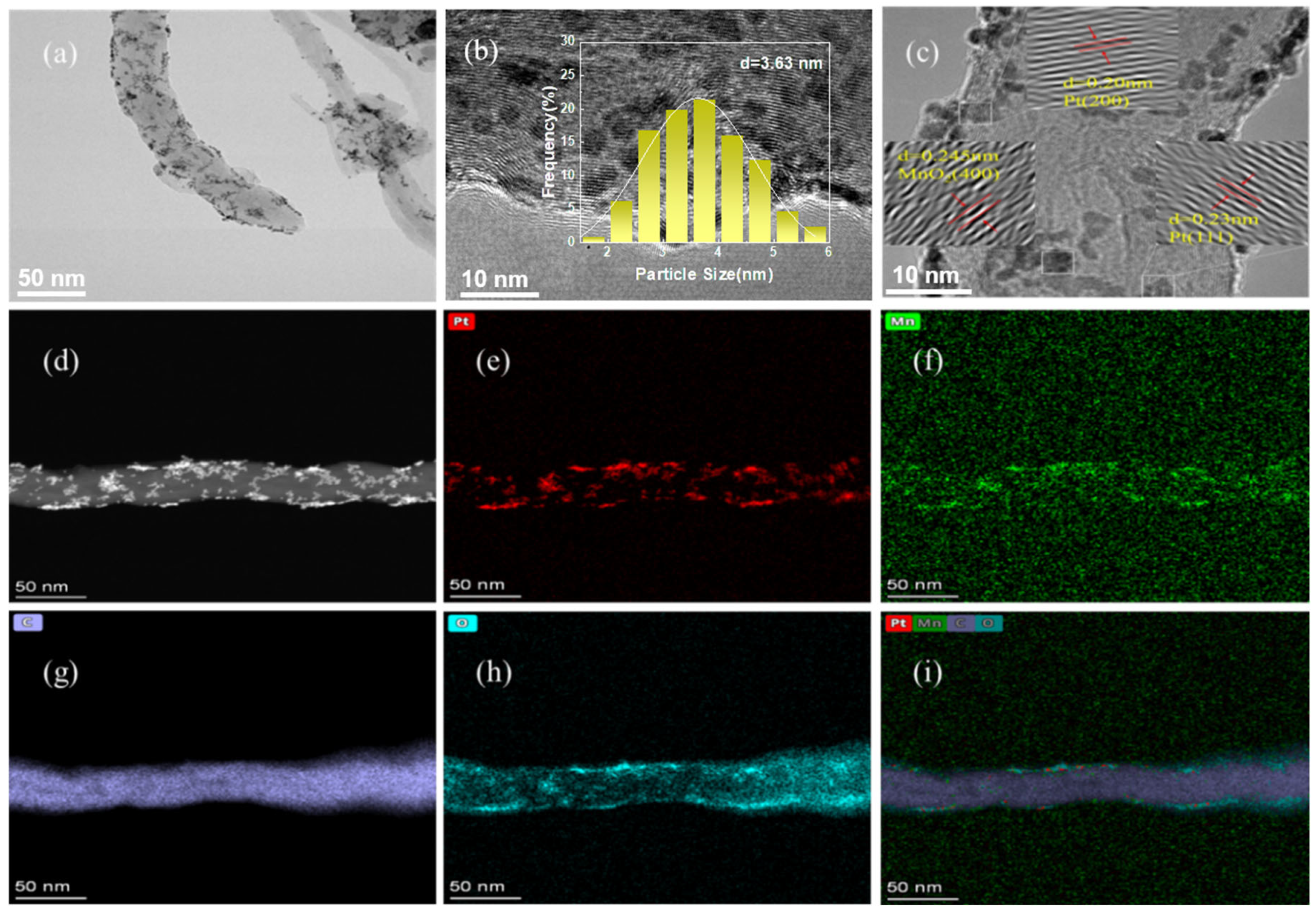
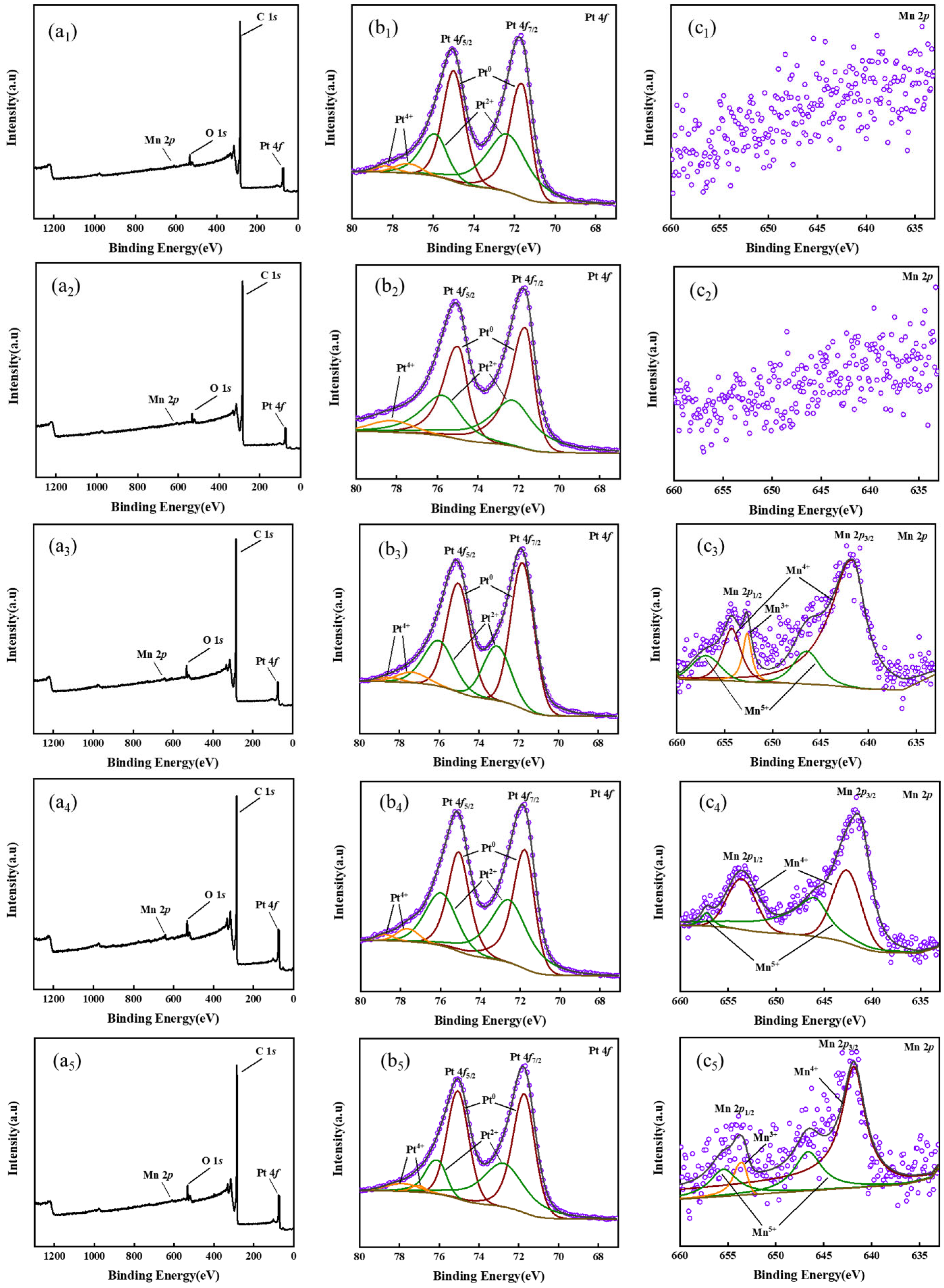
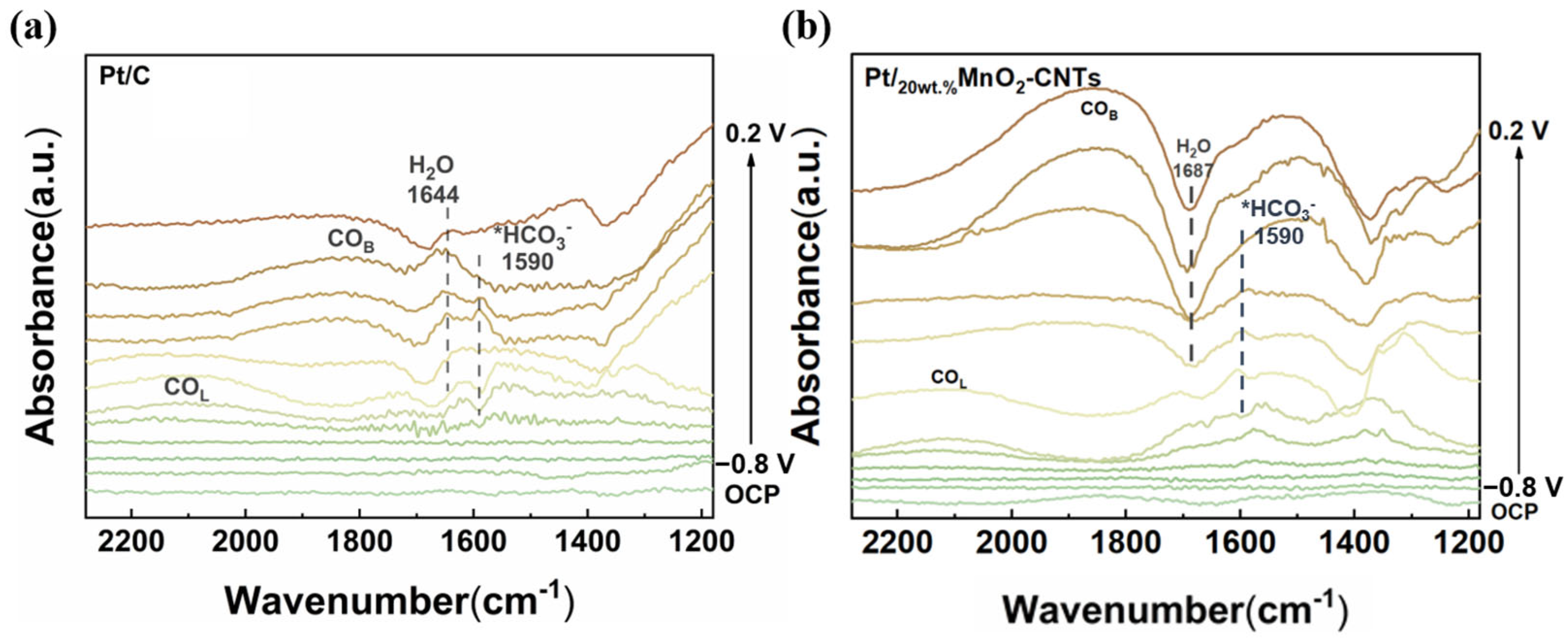
2. Results and Discussion
3. Experiment Section
3.1. Materials
3.2. Synthesis of Electrocatalysts
3.3. Characteristic
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, Z.; Zhang, X.; Sun, H.; Wang, S. Sun, G. Recent advances in multi-scale design and construction of materials for direct methanol fuel cells. Nano Energy 2019, 65, 104048. [Google Scholar] [CrossRef]
- Zhang, Y.; Janyasupab, M.; Liu, C.W.; Li, X.; Xu, J.; Liu, C.C. Three dimensional PtRh alloy porous nanostructures: Tuning the atomic composition and controlling the morphology for the application of direct methanol fuel cells. Adv. Funct. Mater. 2012, 22, 3570–3575. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Singh, G.; Kim, K.S. Recent progress in the development of anode and cathode catalysts for direct methanol fuel cells. Nano Energy 2013, 2, 553–578. [Google Scholar] [CrossRef]
- Rizo, R.; Arán-Ais, R.M.; Padgett, E.; Muller, D.A.; Lázaro, M.J.; Solla-Gullón, J.; Feliu, J.M.; Pastor, E.; Abruña, H.D. Pt-richcore/Sn-richsubsurface/Ptskin nanocubes as highly active and stable electrocatalysts for the ethanol oxidation reaction. J. Am. Chem. Soc. 2018, 140, 3791–3797. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.Y.; Li, H.H.; Hu, B.C.; Cheng, X.; Fu, Q.Q.; Yu, S.H. Synthesis of low Pt-based quaternary PtPdRuTe nanotubes with optimized incorporation of Pd for enhanced electrocatalytic activity. J. Am. Chem. Soc. 2017, 139, 5890–5895. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Bhuvanendran, N.; Liu, H.; Xu, Q.; Hooshyari, K.; Su, H. Ternary PtPdCo mesoporous nanospheres with superior electrocatalytic performance towards methanol oxidation reaction. J. Alloys Compd. 2023, 933, 167706. [Google Scholar] [CrossRef]
- Xu, G.; Wu, Z.; Xie, Z.; Wei, Z.; Li, J.; Qu, K.; Li, Y.; Cai, W. Graphene quantum dot reinforced hyperbranched polyamide proton exchange membrane for direct methanol fuel cell. Int. J. Hydrogen Energy 2021, 46, 9782–9789. [Google Scholar] [CrossRef]
- Zhao, F.; Zhu, B.; Li, S.; Kong, X.; Liu, Q. Bismuth-doped cobaltosic oxide as a noble-metal free electrocatalyst for the efficient methanol oxidation reaction. J. Taiwan Inst. Chem. Eng. 2022, 131, 104182. [Google Scholar] [CrossRef]
- Yang, F.; Yang, B.; Rani, K.K.; Wei, Y.; Peng, L.; Wang, L.; Liu, X.; Chen, D.H.; Fan, Y.; Chen, W. Revealing the role of Ni2+ ions in inducing the synthesis of porous carbon balls: A novel substrate to enhance the Pt catalytic activity towards methanol-oxidation. Int. J. Hydrogen Energy 2022, 47, 23583–23592. [Google Scholar] [CrossRef]
- Chiang, T.H.; Hsu, J.W. Improved the methanol electro-oxidation and carbon monoxide tolerance for direct methanol fuel cells using strontium molybdate. Catalysts 2022, 12, 676. [Google Scholar] [CrossRef]
- Poerwoprajitno, A.R.; Gloag, L.; Watt, J.; Cheong, S.; Tan, X.; Lei, H.; Tahini, H.A.; Henson, A.; Subhash, B.; Bedford, N.M.; et al. A single-Pt-atom-on-Ru-nanoparticle electrocatalyst for CO-resilient methanol oxidation. Nat. Catal. 2022, 5, 231–237. [Google Scholar] [CrossRef]
- Liang, Z.; Hong, Z.; Xie, M.; Gu, D. Recent progress of mesoporous carbons applied in electrochemical catalysis. New Carbon Mater. 2022, 37, 152–179. [Google Scholar] [CrossRef]
- Huang, H.; Wang, X. Recent progress on carbon-based support materials for electrocatalysts of direct methanol fuel cells. J. Mater. Chem. A 2014, 2, 6266–6291. [Google Scholar] [CrossRef]
- El-Khatib, K.M.; Hameed, R.M.A.; Amin, R.S.; Fetohi, A.E. Core-shell structured Pt-transition metals nanoparticles supported on activated carbon for direct methanol fuel cells. Microchem. J. 2019, 145, 566–577. [Google Scholar] [CrossRef]
- Montiel, G.; Fuentes-Quezada, E.; Bruno, M.M.; Corti, H.R.; Viva, F.A. Effect of bimodal mesoporous carbon as PtRu catalyst support for direct methanol fuel cells. RSC Adv. 2020, 10, 30631–30639. [Google Scholar] [CrossRef]
- Ke, Y.; Li, J.; Yuan, W.; Chen, Y.; Zhao, B.; Tang, Z.; Wu, X.; Zhang, S.; Tang, Y. Mangrove root-inspired carbon nanotube film for micro-direct methanol fuel cells. ACS Appl. Mater. Interfaces 2022, 14, 19897–19906. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jang, J.S.; Peck, D.H.; Lee, Y.; Yoon, S.H.; Jung, D.H. Methanol-tolerant platinum-palladium catalyst supported on nitrogen-doped carbon nanofiber for high concentration direct methanol fuel cells. Nanomaterials 2016, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.H.; Wu, R.; Xu, J.B.; Luo, Z.; Zhao, T. A monolayer graphene—Nafion sandwich membrane for direct methanol fuel cells. J. Power Sources 2016, 311, 188–194. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Zhao, Y.; Ji, D.; Guo, P.; Li, G.; Zhao, X. Insights on the roles of nitrogen configuration in enhancing the performance of electrocatalytic methanol oxidation over Pt nanoparticles. Small 2023, 19, 2303065. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Grinter, D.C.; Liu, Z.; Palomino, R.M.; Senanayake, S.D. Ceria-based model catalysts: Fundamental studies on the importance of the metal–ceria interface in CO oxidation, the water–gas shift, CO2 hydrogenation, and methane and alcohol reforming. Chem. Soc. Rev. 2017, 46, 1824–1841. [Google Scholar] [CrossRef]
- van Deelen, T.W.; Hernández Mejía, C.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Kumar, G.; Tibbitts, L.; Newell, J.; Panthi, B.; Mukhopadhyay, A.; Rioux, R.M.; Pursell, C.J.; Janik, M.; Chandler, B.D. Evaluating differences in the active-site electronics of supported Au nanoparticle catalysts using Hammett and DFT studies. Nat. Chem. 2018, 10, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Vayssilov, G.N.; Lykhach, Y.; Migani, A.; Staudt, T.; Petrova, G.P.; Tsud, N.; Skála, T.; Bruix, A.; Illas, F.; Prince, K.C.; et al. Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles. Nat. Mater. 2011, 10, 310–315. [Google Scholar] [CrossRef]
- Green, I.X.; Tang, W.; Neurock, M.; John, T.Y. Spectroscopic observation of dual catalytic sites during oxidation of CO on a Au/TiO2 catalyst. Science 2011, 333, 736–739. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Shao, Z. Perovskite/carbon composites: Applications in oxygen electrocatalysis. Small 2017, 13, 1603793. [Google Scholar] [CrossRef]
- Luo, W.; Zafeiratos, S. A brief review of the synthesis and catalytic applications of graphene-coated oxides. ChemCatChem 2017, 9, 2432–2442. [Google Scholar] [CrossRef]
- Xiong, H.; Pham, H.N.; Datye, A.K. Hydrothermally stable heterogeneous catalysts for conversion of biorenewables. Green Chem. 2014, 16, 4627–4643. [Google Scholar] [CrossRef]
- Zhou, M.J.; Wang, H.J.; Peng, F.; Liang, J.; Yu, H.; Yang, J. MnO2/CNT Supported Pt and PtRu Nanocatalysts for Direct Methanol Fuel Cells. Langmuir 2009, 25, 7711–7717. [Google Scholar] [CrossRef] [PubMed]
- Berretti, E.; Osmieri, L.; Baglio, V.; Miller, H.A.; Filippi, J.; Vizza, F.; Santamaria, M.; Specchia, S.; Santoro, C.; Lavacchi, A. Direct Alcohol Fuel Cells: A Comparative Review of Acidic and Alkaline Systems. Electrochem. Energy Rev. 2023, 6, 30. [Google Scholar] [CrossRef]
- Strandberg, L.; Shokhen, V.; Luneau, M.; Lindbergh, J.; Lagergren, C.; Wickman, B. Comparison of Oxygen Adsorption and Platinum Dissolution in Acid and Alkaline Solutions Using Electrochemical Quartz Crystal Microbalance. ChemElectroChem 2022, 9, e202200591. [Google Scholar] [CrossRef]
- Gao, P.; Huang, S.; Tao, K.; Li, Z.; Feng, L.; Liu, Y.; Zhang, L. Synthesis of adjustable {312}/{004} facet heterojunction MWCNTs/Bi5O7I photocatalyst for ofloxacin degradation: Novel insights into the charge carriers transport. J. Hazard. Mater. 2022, 437, 129374. [Google Scholar] [CrossRef]
- Bera, P.; Priolkar, K.R.; Gayen, A.; Sarode, P.R.; Hegde, M.S.; Emura, S.; Kumashiro, R.; Jayaram, V.; Subbanna, G.N. Ionic dispersion of Pt over CeO2 by the combustion method: Structural investigation by XRD, TEM, XPS, and EXAFS. Chem. Mater. 2003, 15, 2049–2060. [Google Scholar] [CrossRef]
- Tan, X.; Liu, S.; Guo, Q.; Zhang, J.; Liang, S.; He, M.; Luo, J. Synthesis and characterization of amorphous MnO2/CNT via solid-state microwave for high-performance supercapacitors. Int. J. Energy Res. 2020, 44, 4556–4567. [Google Scholar] [CrossRef]
- Lim, J.S.; Yam, F.K. Structural parameters of CVD synthesized Ga2O3 nanostructures from X-ray diffraction analysis derived by Scherrer, Williamson-Hall, Size-Strain Plot and Halder-Wagner methods—A comparative study. Phys. B Condens. Matter 2025, 699, 416798. [Google Scholar] [CrossRef]
- Hossain, S.; Ahmed, S. Easy and green synthesis of TiO2 (Anatase and Rutile): Estimation of crystallite size using Scherrer equation, Williamson-Hall plot, Monshi-Scherrer Model, size-strain plot, Halder- Wagner Model. Results Mater. 2023, 20, 100492. [Google Scholar] [CrossRef]
- Yu, Y.Q.; Wang, T.; Shokhtukhbk, A.; Hu, C.; Chen, K.; Wu, Q.; Zhang, Y.; Shi, D.; Li, H. Triple-junction interfacial engineering Pt–CeO2/three-dimensional nitrogen-doped carbon frameworks electrocatalysts for methanol oxidation reaction. Int. J. Hydrogen Energy 2024, 73, 407–418. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, M.; Li, J.; Zhang, T.; Wang, X.; Cheng, L.; Sun, H. Mass transfer analysis of boron-doped carbon nanotube cathodes for dual-electrolyte lithium—Air batteries. Phys. Chem. Chem. Phys. 2022, 24, 5604–5609. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, J.; Luo, X.; Tu, Z. Comparison of the performance and degradation mechanism of PEMFC with Pt/C and Pt black catalyst. Int. J. Hydrogen Energy 2022, 47, 5418–5428. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, X.; Liu, S.; Yang, H.; Tao, Z.; Liang, J. A highly efficient cathode catalyst γ-MnO2@ CNT composite for sodium-air batteries. Sci. China Chem. 2019, 62, 727–731. [Google Scholar] [CrossRef]
- Lu, L.; Xu, H.; Shi, J.; Zhu, S.; Zhao, H.; Wang, G. Pt-supported C–MnO2 as a catalyst for polymer electrolyte membrane fuel cells. J. Appl. Electrochem. 2018, 48, 801–810. [Google Scholar] [CrossRef]
- Xie, J.; Wang, S.; Zhao, K.; Wu, M.; Wang, F. Regulating the Pt–MnO2 interaction and interface for room temperature formaldehyde oxidation. Inorg. Chem. 2023, 62, 904–915. [Google Scholar] [CrossRef]
- Vovk, E.I.; Kalinkin, A.V.; Smirnov, M.Y.; Klembovskii, I.O.; Bukhtiyarov, V.I. XPS study of stability and reactivity of oxidized Pt nanoparticles supported on TiO2. J. Phys. Chem. C 2017, 121, 17297–17304. [Google Scholar] [CrossRef]
- Hu, J.; Gao, X.; Fan, Q.; Gao, X. Facial controlled synthesis of Pt/MnO2 catalysts with high efficiency for VOCs combustion. RSC Adv. 2021, 11, 16547–16556. [Google Scholar] [CrossRef]
- Jiang, Q.; Jiang, L.; Hou, H.; Qi, J.; Wang, S.; Sun, G. Promoting effect of Ni in PtNi bimetallic electrocatalysts for the methanol oxidation reaction in alkaline media: Experimental and density functional theory studies. J. Phys. Chem. C 2010, 114, 19714–19722. [Google Scholar] [CrossRef]
- Zhan, F.; Bian, T.; Zhao, W.; Zhang, H.; Jin, M.; Yang, D. Facile synthesis of Pd–Pt alloy concave nanocubes with high-index facets as electrocatalysts for methanol oxidation. CrystEngComm 2014, 16, 2411–2416. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, P.; Yin, Y.; Zhang, H.; Cai, H. Effects of structure, composition, and carbon support properties on the electrocatalytic activity of Pt-Ni-graphene nanocatalysts for the methanol oxidation. Appl. Catal. B Environ. 2012, 111, 208–217. [Google Scholar] [CrossRef]
- Li, H.W.; Li, Y.R.; Zhao, Y.; Jing, D.; Li, G.; Zhao, X. Insights into the roles of nitrogen and phosphorus co-doping for efficient methanol electrooxidation. J. Colloid Interface Sci. 2025, 677, 331–341. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, W.H.; Chen, C. Highly active and stable surface defects on Pt-based nanoshell/carbon nanotubes composite for alcohol oxidation catalysis. Carbon 2025, 238, 120215. [Google Scholar] [CrossRef]
- Mukerjee, S.; Srinivasan, S.; Soriaga, M.P.; McBreen, J. Effect of Preparation Conditions of Pt Alloys on Their Electronic, Structural, and Electrocatalytic Activities for Oxygen Reduction-XRD, XAS, and Electrochemical Studies. J. Phys. Chem. 1995, 99, 4577–4589. [Google Scholar] [CrossRef]
- Fan, A.; Qin, C.; Zhao, R.; Sun, H.; Dai, X.; Ye, J.Y.; Sun, S.G.; Lu, Y.; Zhang, X. Phosphorus-doping-tuned PtNi concave nanocubes with high-index facets for enhanced methanol oxidation reaction. Nano Res. 2022, 15, 6961–6968. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, Z.W.; He, H.Y.; Ying, G.; Huang, H. Immobilizing ultrafine PtRu alloy nanoparticles onto 3D interconnected MXene-graphene frameworks for highly efficient methanol oxidation. Ceram. Int. 2024, 50, 16443–16451. [Google Scholar] [CrossRef]
- Hu, M.M.; Chen, L.H.; Wang, Y.; Dai, J.; Bi, Y.; Li, Y.; Zhao, J.; Li, G.; Wang, L.; Meng, A.; et al. Boosting electrochemical activity via manipulating the d-band center of CoNi2Se4/MXene heterostructure for supercapacitor application. Chem. Eng. J. 2025, 513, 162785. [Google Scholar] [CrossRef]
- Sharma, R.; Gyergyek, S.; Andersen, S.M. Critical thinking on baseline corrections for electrochemical surface area (ECSA) determination of Pt/C through H-adsorption/H-desorption regions of a cyclic voltammogram. Appl. Catal. B Environ. 2022, 311, 121351. [Google Scholar] [CrossRef]
- Li, H.W.; Xu, H.Q.; Qi, J.J.; Da, H.; Ji, D.; Zhao, X.; Li, G. Preparation of Pt-Based Bimetallic Catalysts and Electrocatalytic Performance for Methanol Oxidation. J. Phys. Chem. C 2024, 128, 14989–14999. [Google Scholar] [CrossRef]
- Young, S.C.; Nguyen, H.H.; Nguyen, H.T.T. Modeling of slurry-type photocatalytic reactors containing core-shell particles for predicting transient behaviours based on Langmuir-Hinshelwood kinetics. Catal. Today 2023, 411–412, 113909. [Google Scholar]

| Samples | Theoretical Metal Load (wt.%) | Actual Metal Load (wt.%) | Actual Load (wt.%) | Effective Load Rate (%) | ||
|---|---|---|---|---|---|---|
| Pt | Mn | Pt | Mn | |||
| Pt/10wt.%MnO2-CNTs | 20 | 10 | 14.63 | 8.36 | 22.99 | 76.63 |
| Pt/15wt.%MnO2-CNTs | 20 | 15 | 15.16 | 14.57 | 29.73 | 84.94 |
| Pt/20wt.%MnO2-CNTs | 20 | 20 | 15.62 | 18.65 | 34.27 | 85.68 |
| Pt/25wt.%MnO2-CNTs | 20 | 25 | 14.36 | 23.56 | 37.92 | 84.27 |
| Pt/30wt.%MnO2-CNTs | 20 | 30 | 15.10 | 27.13 | 42.23 | 84.46 |
| Samples | Pt | Mn | ||||
|---|---|---|---|---|---|---|
| Pt0 Content (%) | Pt2+ Content (%) | Pt4+ Content (%) | Mn3+ Content (%) | Mn4+ Content (%) | Mn5+ Content (%) | |
| Pt/10wt.%MnO2-CNTs | 51.26 | 36.5 | 12.24 | / | / | / |
| Pt/15wt.%MnO2-CNTs | 56.19 | 36.45 | 7.36 | / | / | / |
| Pt/20wt.%MnO2-CNTs | 62.38 | 31.26 | 6.36 | 6.87 | 70.36 | 22.77 |
| Pt/25wt.%MnO2-CNTs | 61.89 | 30.54 | 7.57 | / | 62.95 | 37.05 |
| Pt/30wt.%MnO2-CNTs | 60.59 | 28.23 | 11.18 | 4.45 | 60.64 | 34.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Teng, Z.; Xu, H.; Li, H. Preparation of Pt/xMnO2-CNTs Catalyst and Its Electrooxidation Performance in Methanol. Catalysts 2025, 15, 864. https://doi.org/10.3390/catal15090864
Chen G, Teng Z, Xu H, Li H. Preparation of Pt/xMnO2-CNTs Catalyst and Its Electrooxidation Performance in Methanol. Catalysts. 2025; 15(9):864. https://doi.org/10.3390/catal15090864
Chicago/Turabian StyleChen, Guang, Zhijun Teng, Hanqiao Xu, and Hongwei Li. 2025. "Preparation of Pt/xMnO2-CNTs Catalyst and Its Electrooxidation Performance in Methanol" Catalysts 15, no. 9: 864. https://doi.org/10.3390/catal15090864
APA StyleChen, G., Teng, Z., Xu, H., & Li, H. (2025). Preparation of Pt/xMnO2-CNTs Catalyst and Its Electrooxidation Performance in Methanol. Catalysts, 15(9), 864. https://doi.org/10.3390/catal15090864







