Beyond Fossil Fuels: The Role of V-Doped Hydrotalcites in n-Butane Oxidative Dehydrogenation for a Circular Economy
Abstract
1. Introduction
1.1. Vanadium Catalysts
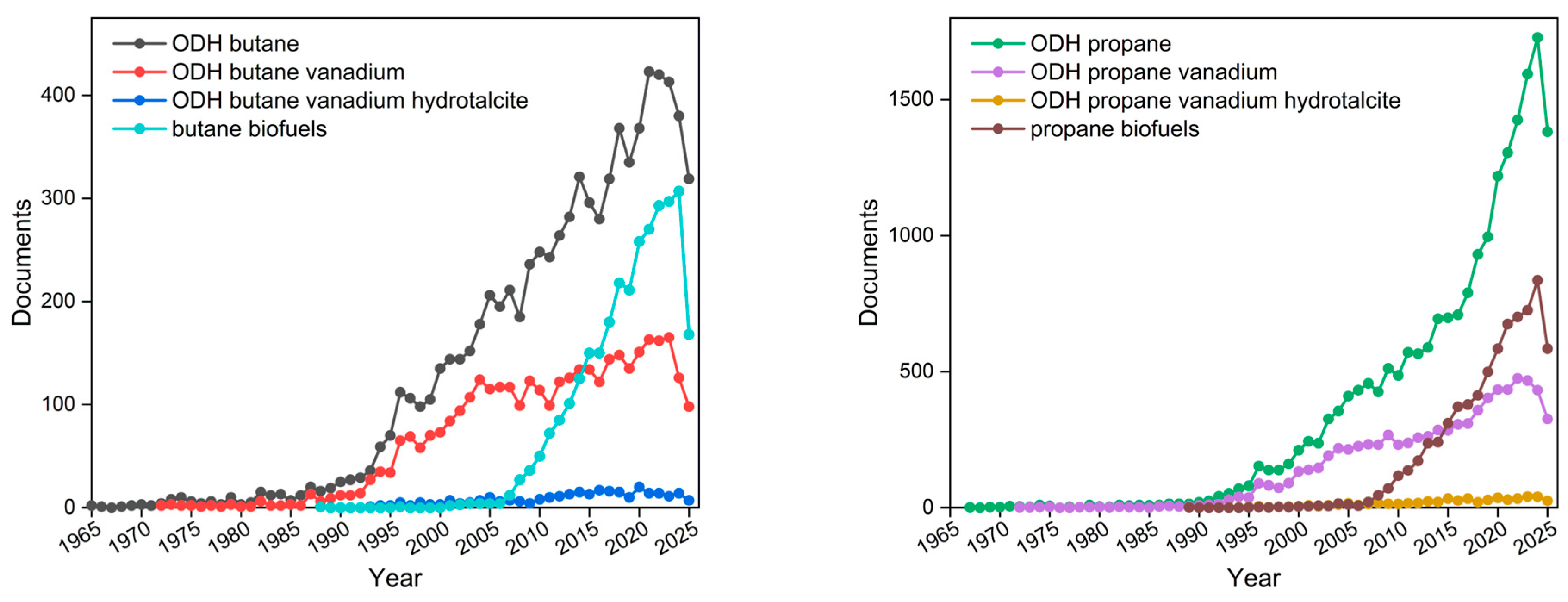
1.2. Beyond Fossil Fuels: Transition into Circular Economy
1.3. The Role of Hydrotalcite Precursors
- Low-cost and relatively simple synthesis;
- The possibility of introducing vanadium in various forms and oxidation states;
- Control over phase composition and active phase dispersion;
- Tunable acidity and basicity (calcined hydrotalcites act as solid bases and basic catalysts);
- The large surface area of hydrotalcite-derived catalysts;
- The potential for recovering components from spent catalysts.
2. Results and Discussion
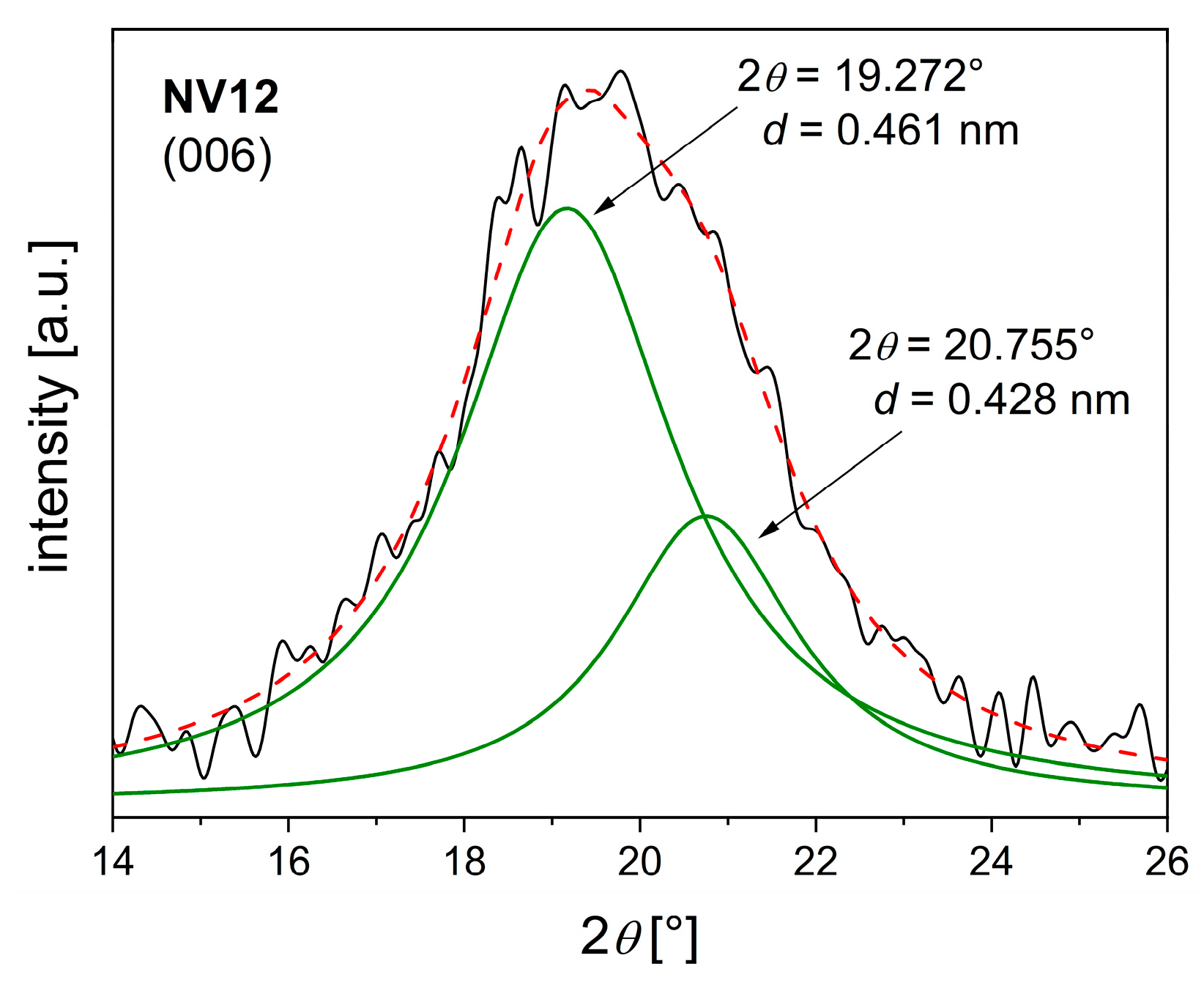
2.1. Characterization of the Hydrotalcite-like Materials
2.2. Characterization of the Catalysts
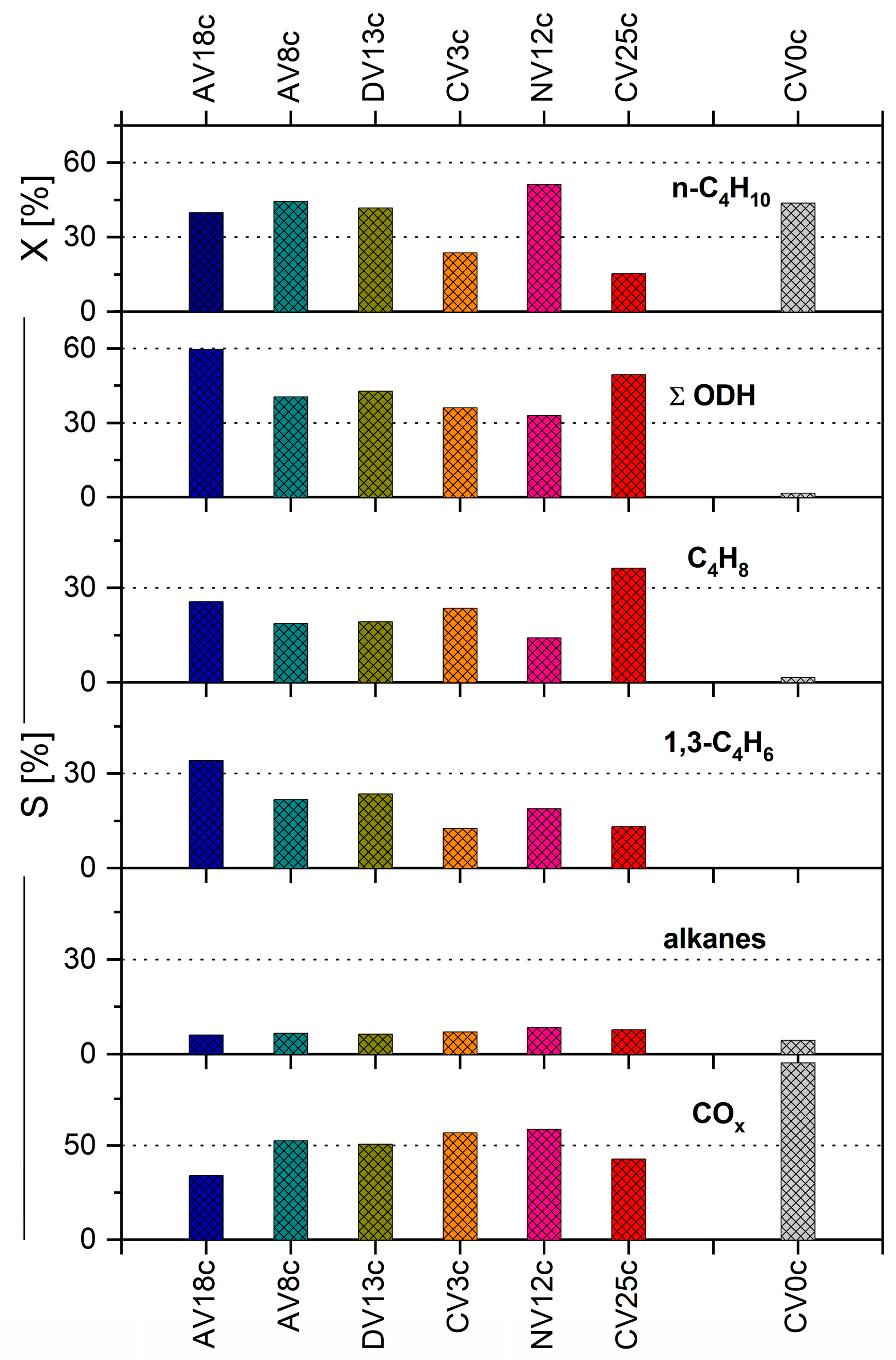
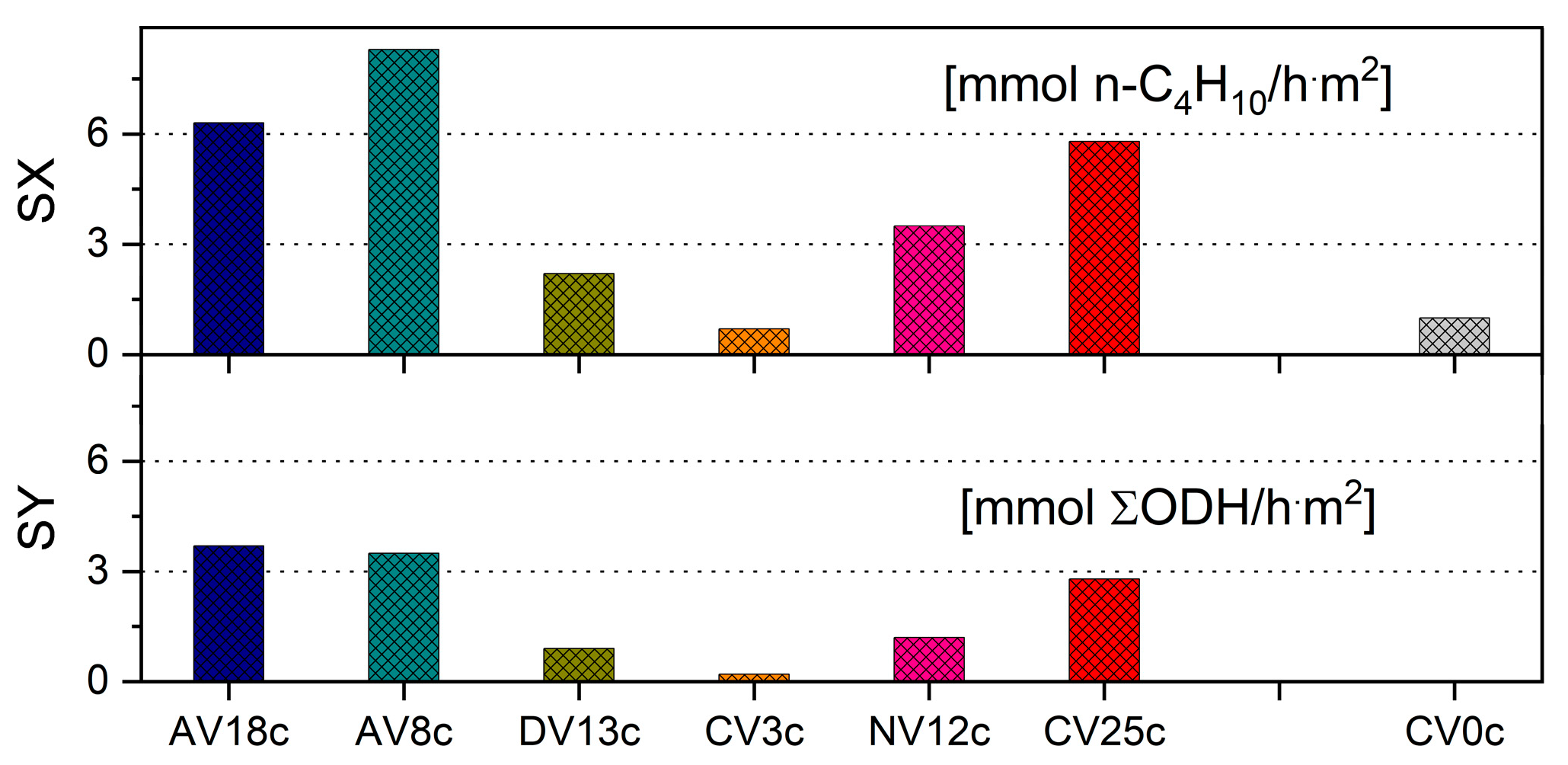
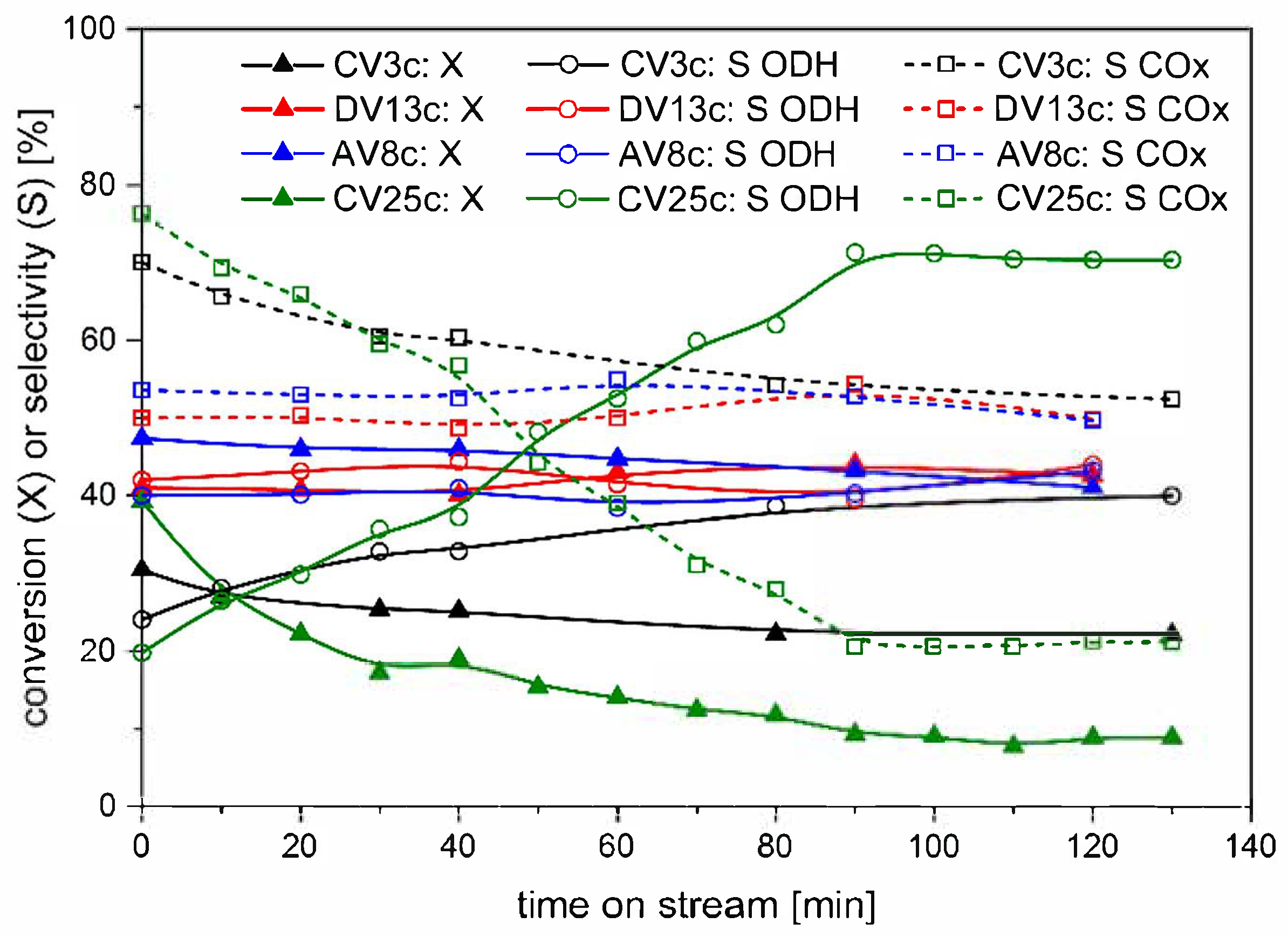
2.3. Catalytic Study
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, D.; Joo, S.-H.; Shin, D.J.; Shin, S.M. Recovery of vanadium and cesium from spent sulfuric acid catalysts by a hydrometallurgical process. Green Chem. 2022, 24, 790–799. [Google Scholar] [CrossRef]
- Mangini, L.F.K.; Valt, R.B.G.; de Santana Ponte, M.J.J.; de Araújo Ponte, H. Vanadium removal from spent catalyst used in the manufacture of sulfuric acid by electrical potential application. Sep. Purif. Technol. 2020, 246, 116854. [Google Scholar] [CrossRef]
- Freire, C.; Pereira, C.; Jarrais, B.; Fernandes, D.; Peixoto, A.; Cordeiro, N.; Teixeira, F. Supported Vanadium Catalysts: Heterogeneous Molecular Complexes, Electrocatalysis and Biomass Transformation. In Vanadium Catalysis; The Royal Society of Chemistry: London, UK, 2020; pp. 241–284. [Google Scholar]
- Domingues, C.; Correia, M.J.N.; Carvalho, R.; Henriques, C.; Bordado, J.; Dias, A.P.S. Vanadium phosphate catalysts for biodiesel production from acid industrial by-products. J. Biotechnol. 2013, 164, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Luo, J.; Paul, S.; Liu, Y.; Khodakov, A.; Bordes, E. Synthesis and performance of vanadium-based catalysts for the selective oxidation of light alkanes. Catal. Today 2017, 298, 145–157. [Google Scholar] [CrossRef]
- Tian, Y.-P.; Liu, X.-M.; Ma, W.-S.; Cheng, S.-X.; Zhang, L.-L. Boosting activity of γ-alumina-supported vanadium catalyst for isobutane non-oxidative dehydrogenation via pure V3+. J. Colloid Interface Sci. 2023, 652, 508–517. [Google Scholar] [CrossRef]
- Marx, R.; Wölk, H.-J.; Mestl, G.; Turek, T. Reaction scheme of oxylene oxidation on vanadia catalyst. Appl. Catal. A Gen. 2011, 398, 37–43. [Google Scholar] [CrossRef]
- Rezaei, M.; Chermahini, A.N.; Dabbagh, H.A. Green and selective oxidation of cyclohexane over vanadium pyrophosphate supported on mesoporous KIT-6. Chem. Eng. J. 2017, 314, 515–525. [Google Scholar] [CrossRef]
- Ye, B.; Jeong, B.; Lee, M.-J.; Kim, T.H.; Park, S.-S.; Jung, J.; Lee, S.; Kim, H.-D. Recent trends in vanadium-based SCR catalysts for NOx reduction in industrial applications: Stationary sources. Nano Converg. 2022, 9, 51. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Chen, P.; Zhu, B. Research Status and Prospect on Vanadium-Based Catalysts for NH3-SCR Denitration. Materials 2018, 11, 1632. [Google Scholar] [CrossRef]
- Kamika, I.; Momba, M.N. Effect of vanadium toxicity at its different oxidation states on selected bacterial and protozoan isolates in wastewater systems. Environ. Technol. 2014, 35, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- CFR Part 1065 Subpart L-Vanadium Sublimation in SCR Catalysts. Available online: https://www.ecfr.gov/current/title-40/part-1065/subject-group-ECFR402811cb661d755 (accessed on 7 August 2025).
- Moreau, P.; Valero, P.; Tschamber, V.; Brillard, A.; Brilhac, J.-F.; Hohl, Y.; Vonarb, R. Investigation of vanadium sublimation from SCR catalysts. SAE Tech. Pap. Ser. 2015, 1, 2503. [Google Scholar] [CrossRef]
- Hu, S.; Herner, J.D.; Shafer, M.; Robertson, W.; Schauer, J.J.; Dwyer, H.; Collins, J.; Huai, T.; Ayala, A. Metals emitted from heavy-duty diesel vehicles equipped with advanced PM and NOX emission controls. Atmos. Environ. 2009, 43, 2950–2959. [Google Scholar] [CrossRef]
- Chapman, D.M. Behavior of titania-supported vanadia and tungsta SCR catalysts at high temperatures in reactant streams: Tungsten and vanadium oxide and hydroxide vapor pressure reduction by surficial stabilization. Appl. Catal. A Gen. 2011, 392, 143–150. [Google Scholar] [CrossRef]
- Liu, Z.G.; Ottinger, N.A.; Cremeens, C.M. Vanadium and tungsten release from V-based selective catalytic reduction diesel aftertreatment. Atmos. Environ. 2015, 104, 154–161. [Google Scholar] [CrossRef]
- Rostom, S.; de Lasa, H. Propane Oxidative Dehydrogenation on Vanadium-Based Catalysts under Oxygen-Free Atmospheres. Catalysts 2020, 10, 418. [Google Scholar] [CrossRef]
- Madeira, L.M.; Portela, M.F. Catalytic oxidative dehydrogenation of n-butane. Catal. Rev. 2002, 44, 247–286. [Google Scholar] [CrossRef]
- Chaar, M. Selective oxidative dehydrogenation of butane over V Mg O catalysts. J. Catal. 1987, 105, 483–498. [Google Scholar] [CrossRef]
- Kung, H.; Kung, M. Oxidative dehydrogenation of alkanes over vanadium-magnesium-oxides. Appl. Catal. A 1997, 157, 105–116. [Google Scholar] [CrossRef]
- Blasco, T.; Nieto, J.; Dejoz, A.; Vazquez, M. Influence of the Acid-Base Character of Supported Vanadium Catalysts on Their Catalytic Properties for the Oxidative Dehydrogenation of n-Butane. J. Catal. 1995, 157, 271–282. [Google Scholar] [CrossRef]
- Sam, D.S.H.; Soenen, V.; Volta, J. Oxidative dehydrogenation of propane over V Mg O catalysts. J. Catal. 1990, 123, 417–435. [Google Scholar] [CrossRef]
- Carja, G.; Nakamura, R.; Aida, T.; Niiyama, H. Mg–V–Al mixed oxides with mesoporous properties using layered double hydroxides as precursors: Catalytic behavior for the process of ethylbenzene dehydrogenation to styrene under a carbon dioxide flow. J. Catal. 2003, 218, 104–110. [Google Scholar] [CrossRef]
- Bahranowski, K.; Bueno, G.; Corberán, V.C.; Kooli, F.; Serwicka, E.; Valenzuela, R.; Wcisło, K. Oxidative dehydrogenation of propane over calcined vanadate-exchanged Mg,Al-layered double hydroxides. Appl. Catal. A 1999, 185, 65–73. [Google Scholar] [CrossRef]
- Dula, R.; Wcisło, K.; Stoch, J.; Grzybowska, B.; Serwicka, E.; Kooli, F.; Bahranowski, K.; Gaweł, A. Layered double hydroxide-derived vanadium catalysts for oxidative dehydrogenation of propane. Appl. Catal. A 2002, 230, 281–291. [Google Scholar] [CrossRef]
- Holgado, M.; Labajos, F.; Montero, M.; Rives, V. Thermal decomposition of Mg/V hydrotalcites and catalytic performance of the products in oxidative dehydrogenation reactions. Mater. Res. Bull. 2003, 38, 1879–1891. [Google Scholar] [CrossRef]
- Nieto, J.L.; Concepción, P.; Dejoz, A.; Knözinger, H.; Melo, F.; Vázquez, M. Selective Oxidation of n-Butane and Butenes over Vanadium-Containing Catalysts. J. Catal. 2000, 189, 147–157. [Google Scholar] [CrossRef]
- Lopeznieto, J.; Dejoz, A.; Vazquez, M. Preparation, characterization and catalytic properties of vanadium oxides supported on calcined Mg/Al-hydrotalcite. Appl. Catal. A 1995, 132, 41–59. [Google Scholar] [CrossRef]
- Huang, X.; Wang, D.; Yang, Q.; Peng, Y.; Li, J. Multi-pollutant control (MPC) of NO and chlorobenzene from industrial furnaces using a vanadia-based SCR catalyst. Appl. Catal. B Environ. 2021, 285, 119835. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Z.; Wang, D.; Peng, Y.; Li, J. The effect of additives and intermediates on vanadia-based catalyst for multi-pollutant control. Catal. Sci. Technol. 2010, 10, 323–326. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Yang, F.; Ai, Y.; Chen, Y.; Pan, D. Strategy and Technical Progress of Recycling of Spent Vanadium–Titanium-Based Selective Catalytic Reduction Catalysts. ACS Omega 2024, 9, 6036–6058. [Google Scholar] [CrossRef]
- Ferella, F. A review on management and recycling of spent selective catalytic reduction catalysts. J. Clean. Prod. 2020, 246, 118990. [Google Scholar] [CrossRef]
- Amato, A.; Ippolito, N.M.; D’ARcangelo, M.; Becci, A.; Innocenzi, V.; Ferella, F. Vanadium, molybdenum and nickel: A sustainability analysis of the extraction from ores versus recovery from spent catalysts. J. Clean. Prod. 2025, 515, 145817. [Google Scholar] [CrossRef]
- Kurniawan, A.; Zhong, J.Q.; Wang, Z.Z.; Zhou, C.H. Catalytic Glycerol Conversion over Bifunctional Montmorillonite-Supported Mo−V Oxide Catalysts: Insights into the Dehydration-Hydrogen Transfer Reaction Pathway Toward Allyl Alcohol. Ind. Eng. Chem. Res. 2025, 64, 1994–2005. [Google Scholar] [CrossRef]
- Kim, Y.; Jabed, M.A.; Price, D.M.; Kilin, D.; Kim, S. Toward rational design of supported vanadia catalysts of lignin conversion to phenol. Chem. Eng. J. 2022, 446, 136965. [Google Scholar] [CrossRef]
- Hou, S.; Xie, W. Exploring hierarchical porous γ-Al2O3 modified with MoV metal oxides used as a high-efficiency solid catalyst for sustainable biodiesel production from low-cost acidic oils. Renew. Energy 2025, 250, 123303. [Google Scholar] [CrossRef]
- Gucbilmez, Y.; Dogu, T.; Balci, S. Ethylene and Acetaldehyde Production by Selective Oxidation of Ethanol Using Mesoporous V−MCM-41 Catalysts. Ind. Eng. Chem. Res. 2006, 45, 3496–3502. [Google Scholar] [CrossRef]
- Gutiérrez-Antonio, C. Circular Economy Principles in Renewable Fuels. In Hydrogen and Low-Carbon Fuels in Circular Bio-Economy; Springer: Berlin/Heidelberg, Germany, 2025; pp. 27–49. [Google Scholar]
- Tsolakis, N.; Bam, W.; Srai, J.S.; Kumar, M. Renewable chemical feedstock supply network design: The case of terpenes. J. Clean. Prod. 2019, 222, 802–822. [Google Scholar] [CrossRef]
- Kumagai, S.; Fujiwara, K.; Nishiyama, T.; Saito, Y.; Yoshioka, T. Chemical Feedstock Recovery Through Plastic Pyrolysis: Challenges and Perspectives Toward a Circular Economy. ChemSusChem 2025, 18, e202500210. [Google Scholar] [CrossRef]
- Palkovits, R.; Delidovich, I. Efficient utilization of renewable feedstocks: The role of catalysis and process design. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 376, 20170064. [Google Scholar] [CrossRef] [PubMed]
- Ewing, T.A.; Nouse, N.; van Lint, M.; van Haveren, J.; Hugenholtz, J.; van Es, D.S. Fermentation for the production of biobased chemicals in a circular economy: A perspective for the period 2022–2050. Green Chem. 2022, 24, 6373–6405. [Google Scholar] [CrossRef]
- Chen, T.-L.; Kim, H.; Pan, S.-Y.; Tseng, P.-C.; Lin, Y.-P.; Chiang, P.-C. Implementation of green chemistry principles in circular economy system towards sustainable development goals: Challenges and perspectives. Sci. Total. Environ. 2020, 716, 136998. [Google Scholar] [CrossRef] [PubMed]
- Guarieiro, L.; Rezende, M.; Barbosa, W.; da Rocha, G.; Pereira, P.A.; Fernandes, D.; Lopes, W.; Mota, C.; de Andrade, J. Reaching Circular Economy through Circular Chemistry: The Basis for Sustainable Development. J. Braz. Chem. Soc. 2022, 33, 1353–1374. [Google Scholar] [CrossRef]
- Linder, M. Ripe for disruption: Reimagining the role of green chemistry in a circular economy. Green Chem. Lett. Rev. 2017, 10, 428–435. [Google Scholar] [CrossRef]
- Amer, M.; Toogood, H.; Scrutton, N.S. Engineering nature for gaseous hydrocarbon production. Microb. Cell Factories 2020, 19, 209. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.; Hoeven, R.; Kelly, P.; Faulkner, M.; Smith, M.H.; Toogood, H.S.; Scrutton, N.S. Renewable and tuneable bio-LPG blends derived from amino acids. Biotechnol. Biofuels 2020, 13, 125. [Google Scholar] [CrossRef]
- Johnson, E. Process Technologies and Projects for BioLPG. Energies 2019, 12, 250. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, K. Microbial production of alka(e)ne biofuels. Curr. Opin. Biotechnol. 2018, 50, 11–18. [Google Scholar] [CrossRef]
- Liu, Y.; Khusnutdinova, A.; Chen, J.; Crisante, D.; Batyrova, K.; Raj, K.; Feigis, M.; Shirzadi, E.; Wang, X.; Dorakhan, R.; et al. Systems engineering of Escherichia coli for n-butane production. Metab. Eng. 2022, 74, 98–107. [Google Scholar] [CrossRef]
- Jiang, J.; Li, T.; Huang, K.; Sun, G.; Zheng, J.; Chen, J.; Yang, W. Efficient Preparation of Bio-based n-Butane Directly from Levulinic Acid over Pt/C. Ind. Eng. Chem. Res. 2020, 59, 5736–5744. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Drezdzon, M.A. Synthesis of isopolymetalate-pillared hydrotalcite via organic-anion-pillared precursors. Inorg. Chem. 1988, 27, 4628–4632. [Google Scholar] [CrossRef]
- Gardner, E.; Pinnavaia, T.J. On the nature of selective olefin oxidation catalysts derived from molybdate- and tungstate-intercalated layered double hydroxides. Appl. Catal. A 1998, 167, 65–74. [Google Scholar] [CrossRef]
- Wang, Q.; O’hAre, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Newman, S.P.; Jones, W.; O’Connor, P.; Stamires, D.N. Synthesis of the 3R2 polytype of a hydrotalcite-like mineral. J. Mater. Chem. 2002, 12, 153–155. [Google Scholar] [CrossRef]
- Labajos, F.M.; Rives, V.; Malet, P.; Centeno, M.A.; Ulibarri, M.A. Synthesis and Characterization of Hydrotalcite-like Compounds Containing V3+ in the Layers and of Their Calcination Products. Inorg. Chem. 1996, 35, 1154–1160. [Google Scholar] [CrossRef]
- Kwon, T.; Tsigdinos, G.A.; Pinnavaia, T.J. Pillaring of layered double hydroxides (LDH’s) by polyoxometalate anions. J. Am. Chem. Soc. 1988, 110, 3653–3654. [Google Scholar] [CrossRef]
- Rives, V.; Ulibarri, M.A. Layered double hydroxides (LDH) intercalated with metal coordination compounds and oxometalates. Coord. Chem. Rev. 1999, 181, 61–120. [Google Scholar] [CrossRef]
- Han, K.; Guerlou-Demourgues, L.; Delmas, C. Intercalation process of metavanadate chains into a nickel–cobalt layered double hydroxide. Solid State Ionics 1997, 98, 85–92. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Hall, D.B.; Barnes, T.J. Novel oligovanadate-pillared hydrotalcites. Appl. Clay Sci. 1995, 10, 57–67. [Google Scholar] [CrossRef]
- Twu, J.; Dutta, P.K. Structure and reactivity of oxovanadate anions in layered lithium aluminate materials. J. Phys. Chem. 1989, 93, 7863–7868. [Google Scholar] [CrossRef]
- Węgrzyn, A.; Rafalska-Łasocha, A.; Majda, D.; Dziembaj, R.; Papp, H. The influence of mixed anionic composition of Mg–Al hydrotalcites on the thermal decomposition mechanism based on in situ study. J. Therm. Anal. Calorim. 2009, 99, 443–457. [Google Scholar] [CrossRef]
- Han, K.; Guerlou-Demourgues, L.; Delmas, C. A new metavanadate inserted layered double hydroxide prepared by ‘chimie douce’. Solid State Ionics 1996, 84, 227–238. [Google Scholar] [CrossRef]
- Jurišová, J.; Danielik, V.; Malečková, S.; Guzikiewiczová, E.; Králik, M.; Vizárová, K.; Ambrová, M.; Fellner, P. Preparation and characterisation of hydrotalcites colloid dispersions suitable for deacidification of paper information carriers. Chem. Pap. 2023, 78, 1719–1730. [Google Scholar] [CrossRef]
- Kooli, F.; Rives, V.; Ulibarri, M.A. Preparation and Study of Decavanadate-Pillared Hydrotalcite-like Anionic Clays Containing Transition Metal Cations in the Layers. 1. Samples Containing Nickel-Aluminum Prepared by Anionic Exchange and Reconstruction. Inorg. Chem. 1995, 34, 5114–5121. [Google Scholar] [CrossRef]
- Salinas, E.L.; Ono, Y. Thermal Transformation of a Layered Double Hydroxide Pillared with Decavanadate Isopolyanions. Bull. Chem. Soc. Jpn. 1992, 65, 2465–2470. [Google Scholar] [CrossRef]
- Ulibarri, M.-A.; Labajos, F.M.; Rives, V.; Trujillano, R.; Kagunya, W.; Jones, W. Comparative Study of the Synthesis and Properties of Vanadate-Exchanged Layered Double Hydroxides. Inorg. Chem. 1994, 33, 2592–2599. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Frost, R.L. Fourier Transform Infrared and Raman Spectroscopic Study of the Local Structure of Mg-, Ni-, and Co-Hydrotalcites. J. Solid State Chem. 1999, 146, 506–515. [Google Scholar] [CrossRef]
- Kooli, F.; Crespo, I.; Barriga, C.; Ulibarri, M.A.; Rives, V. Precursor dependence of the nature and structure of non-stoichiometric magnesium aluminium vanadates. J. Mater. Chem. 1996, 6, 1199–1996. [Google Scholar] [CrossRef]
- Griffith, W.P. Vibrational spectra of metaphosphates, meta-arsenates, and metavanadates. J. Chem. Soc. A Inorg. Phys. Theor. 1967, 905–908. [Google Scholar] [CrossRef]
- Griffith, W.P.; Wickins, T.D. Raman studies on species in aqueous solutions. Part I. The vanadates. J. Chem. Soc. A Inorg. Phys. Theor. 1966, 1087–1090. [Google Scholar] [CrossRef]
- Onodera, S.; Ikegami, Y. Infrared and Raman spectra of ammonium, potassium, rubidium, and cesium metavanadates. Inorg. Chem. 1980, 19, 615–618. [Google Scholar] [CrossRef]
- Węgrzyn, A.; Rafalska-Łasocha, A.; Dudek, B.; Dziembaj, R. Nanostructured V-containing hydrotalcite-like materials obtained by non-stoichiometric anion exchange as precursors of catalysts for oxidative dehydrogenation of n-butane. Catal. Today 2006, 116, 74–81. [Google Scholar] [CrossRef]
- Clark, G.; Morley, R. A study of the MgO V2O5 system. J. Solid State Chem. 1976, 16, 429–435. [Google Scholar] [CrossRef]
- Del Arco, M.; Holgado, M.J.; Martín, C.; Rives, V. New route for the synthesis of V2O5-MgO oxidative dehydrogenation catalysts. J. Mater. Sci. Lett. 1987, 6, 616–619. [Google Scholar] [CrossRef]
- Köbel, S.; Schneider, D.; Schüler, C.; Gauckler, L. Sintering of vanadium-doped magnesium oxide. J. Eur. Ceram. Soc. 2003, 24, 2267–2274. [Google Scholar] [CrossRef]
- Labajos, F.; Sánchez-Montero, M.; Holgado, M.; Rives, V. Thermal evolution of V(III)-containing layered double hydroxides. Thermochim. Acta 2001, 370, 99–104. [Google Scholar] [CrossRef]
- Chang, W.; Chen, Y.; Yang, B. Oxidative dehydrogenation of ethylbenzene over VIV and VV magnesium vanadates. Appl. Catal. A 1995, 124, 221–243. [Google Scholar] [CrossRef]
- Węgrzyn, A.; Witkowski, S.; Majda, D.; Białoruski, M.; Miśkowiec, P.; Skórkiewicz, S.; Piskorz, W. Towards Sorption Degradation Mechanism: Transformation of Hydrotalcite to Periclase and Spinel upon Calcination—Experimental Studies and DFT Modelling. Sep. Purif. Technol. 2025, 377, 133963. [Google Scholar] [CrossRef]
- Gao, X.; Ruiz, P.; Xin, Q.; Guo, X.; Delmon, B. Preparation and characterization of three pure magnesium vanadate phases as catalysts for selective oxidation of propane to propene. Catal. Lett. 1994, 23, 321–337. [Google Scholar] [CrossRef]
- Di Cosimo, J.; DíeZ, V.; Xu, M.; Iglesia, E.; Apesteguía, C. Structure and Surface and Catalytic Properties of Mg-Al Basic Oxides. J. Catal. 1998, 178, 499–510. [Google Scholar] [CrossRef]
- Kaneko, K. Determination of pore size and pore size distribution. J. Membr. Sci. 1994, 96, 59–89. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Stawiński, W.; Węgrzyn, A.; Dańko, T.; Freitas, O.; Figueiredo, S.; Chmielarz, L. Acid-base treated vermiculite as high performance adsorbent: Insights into the mechanism of cationic dyes adsorption, regeneration, recyclability and stability studies. Chemosphere 2017, 173, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Stawiński, W.; Węgrzyn, A.; Freitas, O.; Chmielarz, L.; Figueiredo, S. Dual-function hydrotalcite-derived adsorbents with sulfur storage properties: Dyes and hydrotalcite fate in adsorption-regeneration cycles. Microporous Mesoporous Mater. 2017, 250, 72–87. [Google Scholar] [CrossRef]
- Stawiński, W.; Węgrzyn, A.; Freitas, O.; Chmielarz, L.; Mordarski, G.; Figueiredo, S. Simultaneous removal of dyes and metal cations using an acid, acid-base and base modified vermiculite as a sustainable and recyclable adsorbent. Sci. Total. Environ. 2017, 576, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Węgrzyn, A.; Stawiński, W.; Freitas, O.; Komędera, K.; Błachowski, A.; Jęczmionek, Ł.; Dańko, T.; Mordarski, G.; Figueiredo, S. Study of adsorptive materials obtained by wet fine milling and acid activation of vermiculite. Appl. Clay Sci. 2018, 155, 37–49. [Google Scholar] [CrossRef]
- Silva, A.; Martinho, S.; Stawiński, W.; Węgrzyn, A.; Figueiredo, S.; Santos, L.H.M.L.M.; Freitas, O. Application of vermiculite-derived sustainable adsorbents for removal of venlafaxine. Environ. Sci. Pollut. Res. 2018, 25, 17066–17076. [Google Scholar] [CrossRef]
- Stawiński, W.; Węgrzyn, A.; Mordarski, G.; Skiba, M.; Freitas, O.; Figueiredo, S. Sustainable adsorbents formed from by-product of acid activation of vermiculite and leached-vermiculite-LDH hybrids for removal of industrial dyes and metal cations. Appl. Clay Sci. 2018, 161, 6–14. [Google Scholar] [CrossRef]
- Węgrzyn, A.; Tsurtsumia, A.; Witkowski, S.; Freitas, O.; Figueiredo, S.; Cybińska, J.; Stawiński, W. Vermiculite as a potential functional additive for water treatment bioreactors inhibiting toxic action of heavy metal cations upsetting the microbial balance. J. Hazard. Mater. 2022, 433, 128812. [Google Scholar] [CrossRef]
- Mendialdua, J.; Casanova, R.; Barbaux, Y. XPS studies of V2O5, V6O13, VO2 and V2O3. J. Electron Spectrosc. Relat. Phenom. 1995, 71, 249–261. [Google Scholar] [CrossRef]
- Demeter, M.; Neumann, M.; Reichelt, W. Mixed-valence vanadium oxides studied by XPS. Surf. Sci. 2000, 454–456, 41–44. [Google Scholar] [CrossRef]
- Taha, M.; Walia, S.; Ahmed, T.; Headland, D.; Withayachumnankul, W.; Sriram, S.; Bhaskaran, M. Insulator–metal transition in substrate-independent VO2 thin film for phase-change devices. Sci. Rep. 2017, 7, 17899. [Google Scholar] [CrossRef]
- Rajeswaran, B.; Pradhan, J.K.; Ramakrishna, S.A.; Umarji, A.M. Thermochromic VO2 thin films on ITO-coated glass substrates for broadband high absorption at infra-red frequencies. J. Appl. Phys. 2017, 122, 163107. [Google Scholar] [CrossRef]
- Idriss, H. On the wrong assignment of the XPS O1s signal at 531–532 eV attributed to oxygen vacancies in photo- and electro-catalysts for water splitting and other materials applications. Surf. Sci. 2021, 712, 121894. [Google Scholar] [CrossRef]
- Harlin, M.; Niemi, V.; Krause, A. Alumina-Supported Vanadium Oxide in the Dehydrogenation of Butanes. J. Catal. 2000, 195, 67–78. [Google Scholar] [CrossRef]
- Owen, O.S.; Kung, M.C.; Kung, H.H. The effect of oxide structure and cation reduction potential of vanadates on the selective oxidative dehydrogenation of butane and propane. Catal. Lett. 1992, 12, 45–50. [Google Scholar] [CrossRef]
- Patel, D. Oxidative dehydrogenation of butane over orthovanadates. J. Catal. 1990, 125, 132–142. [Google Scholar] [CrossRef]
- Albonetti, S.; Cavani, F.; Trifirò, F. Key Aspects of Catalyst Design for the Selective Oxidation of Paraffins. Catal. Rev. 1996, 38, 413–438. [Google Scholar] [CrossRef]
- Grzybowska-Świerkosz, B. Active centres on vanadia-based catalysts for selective oxidation of hydrocarbons. Appl. Catal. A 1997, 157, 409–420. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. Match!—Phase Analysis Using Powder Diffraction, Crystal Impact; GbR: Bonn, Germany, 2025; Available online: https://www.crystalimpact.de/match (accessed on 19 August 2025).
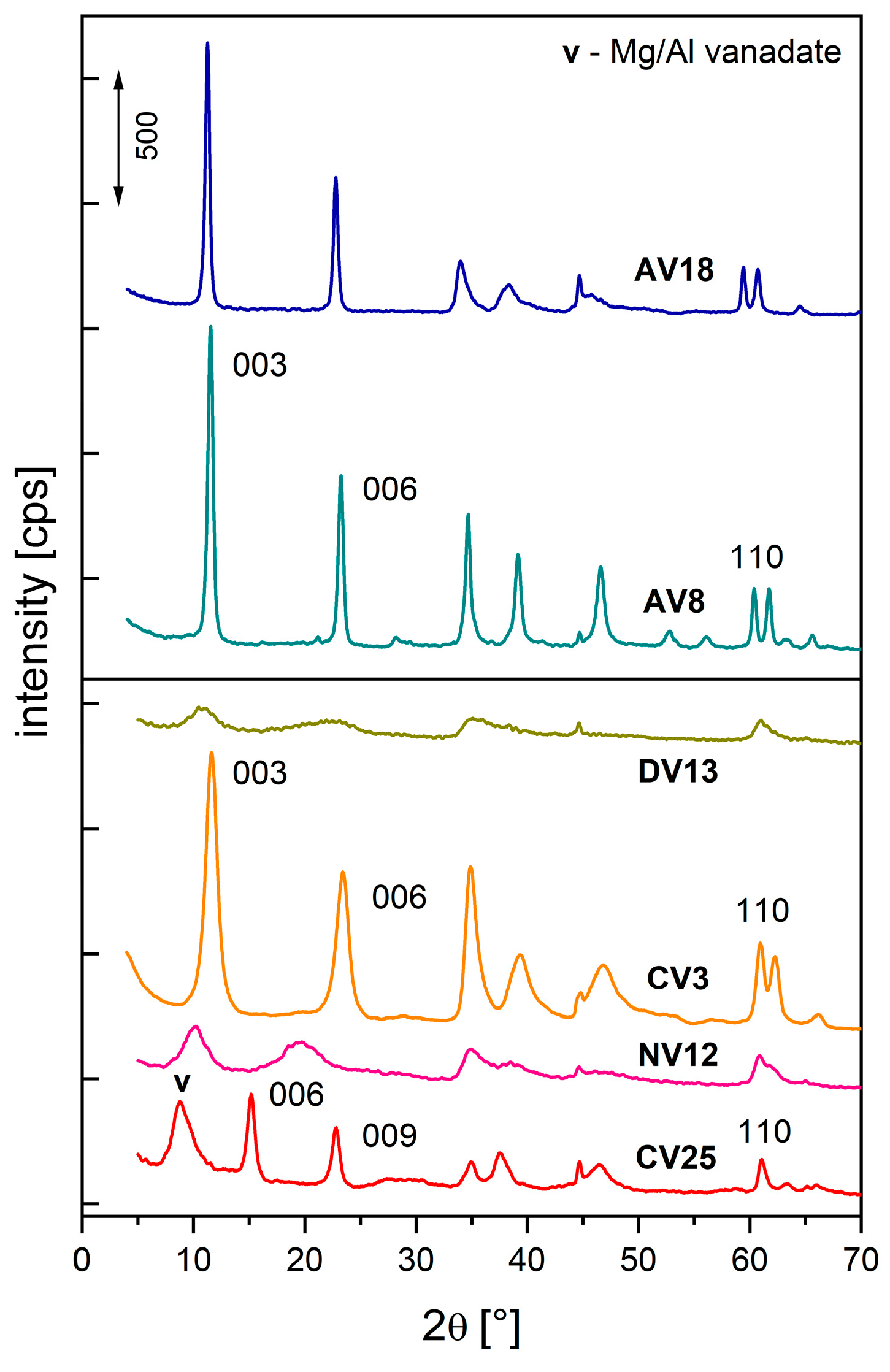
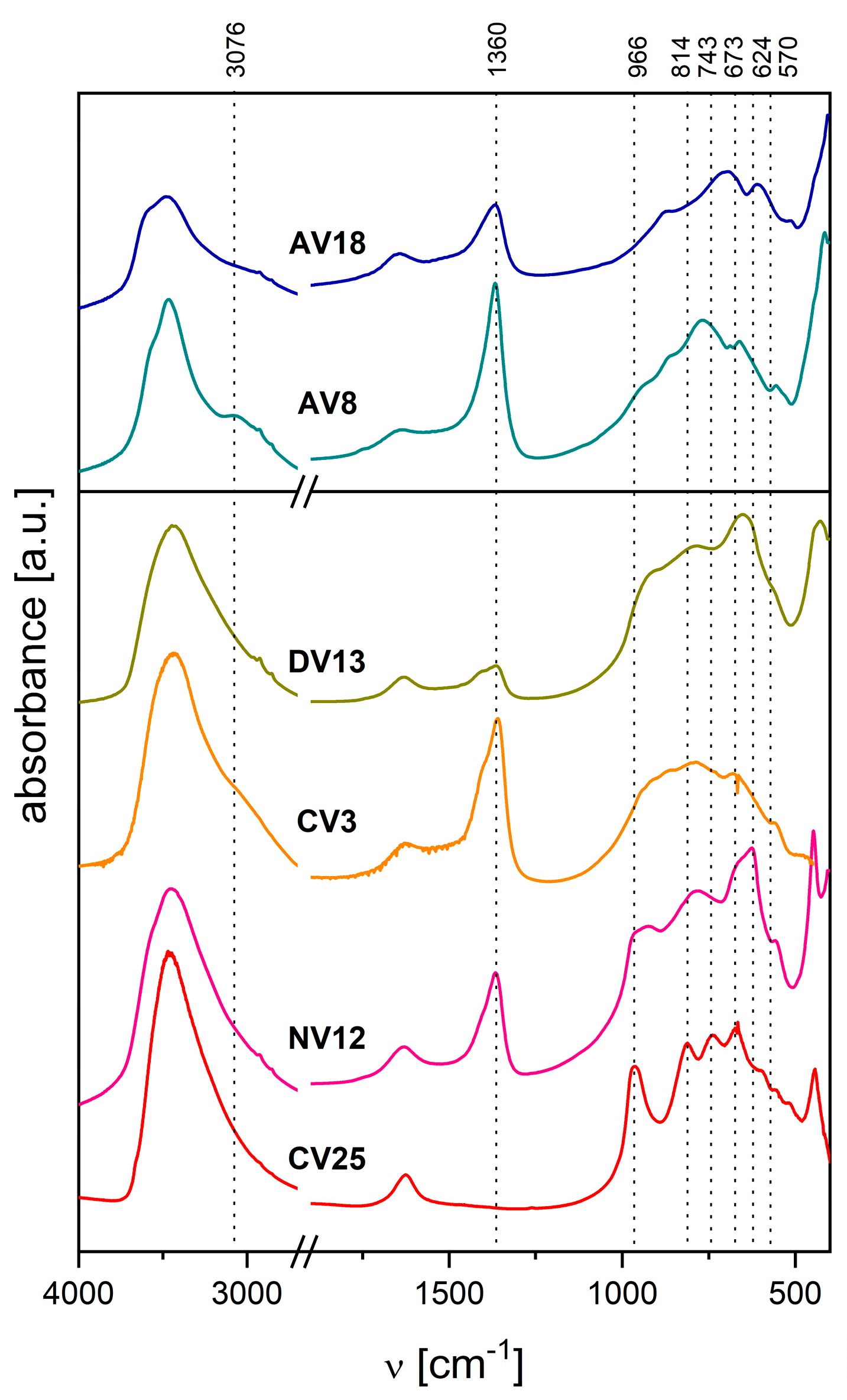
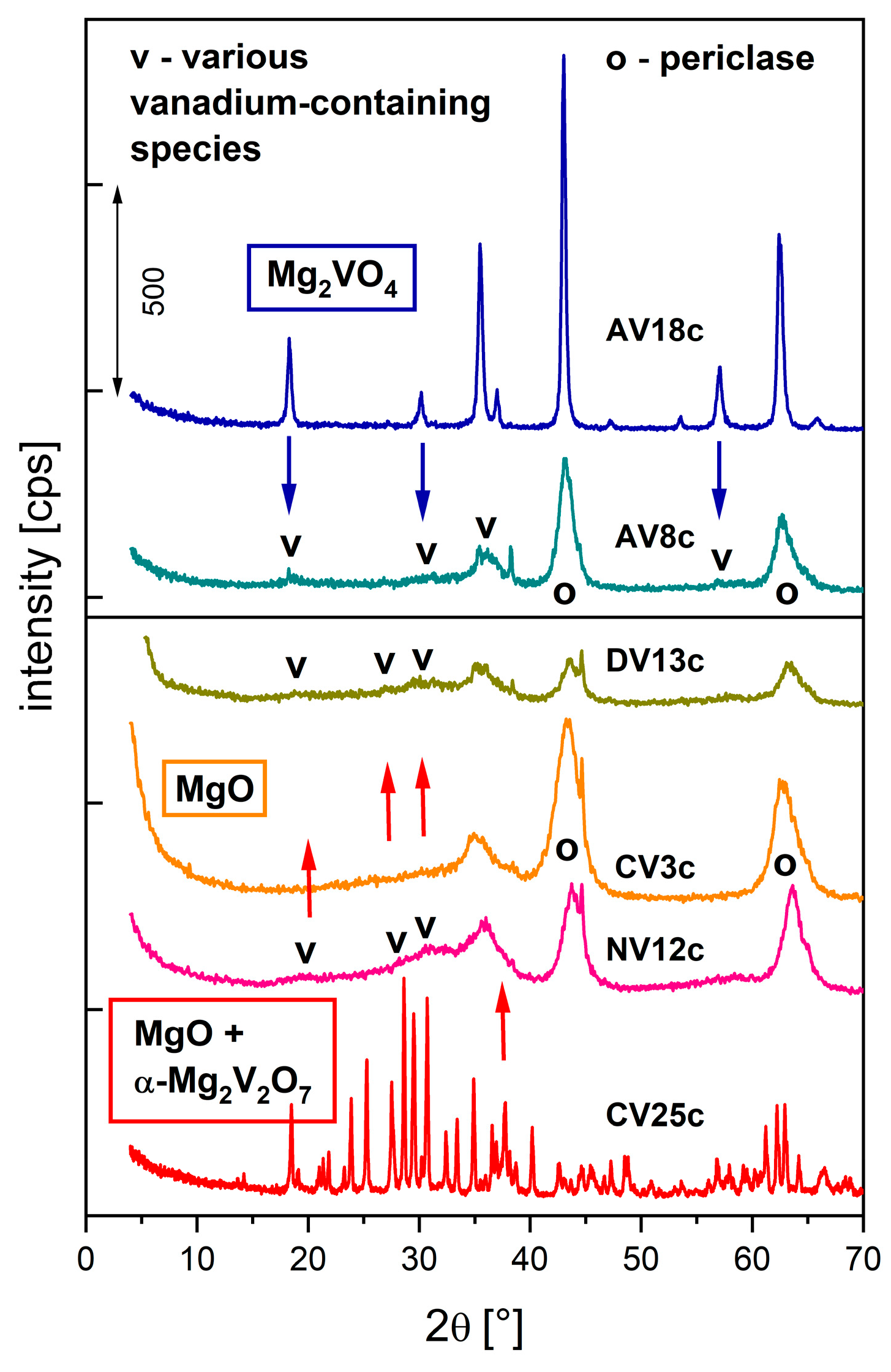
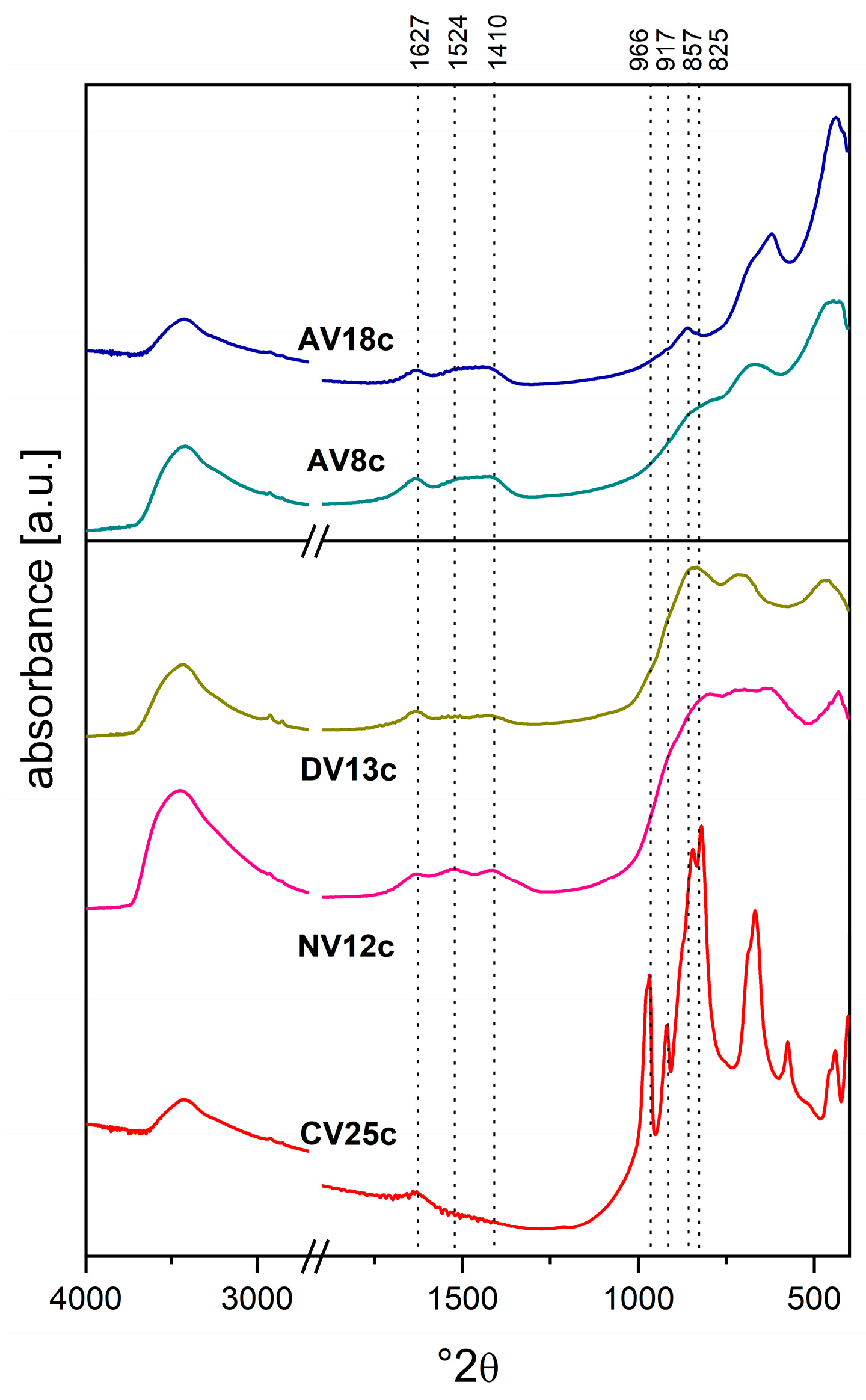
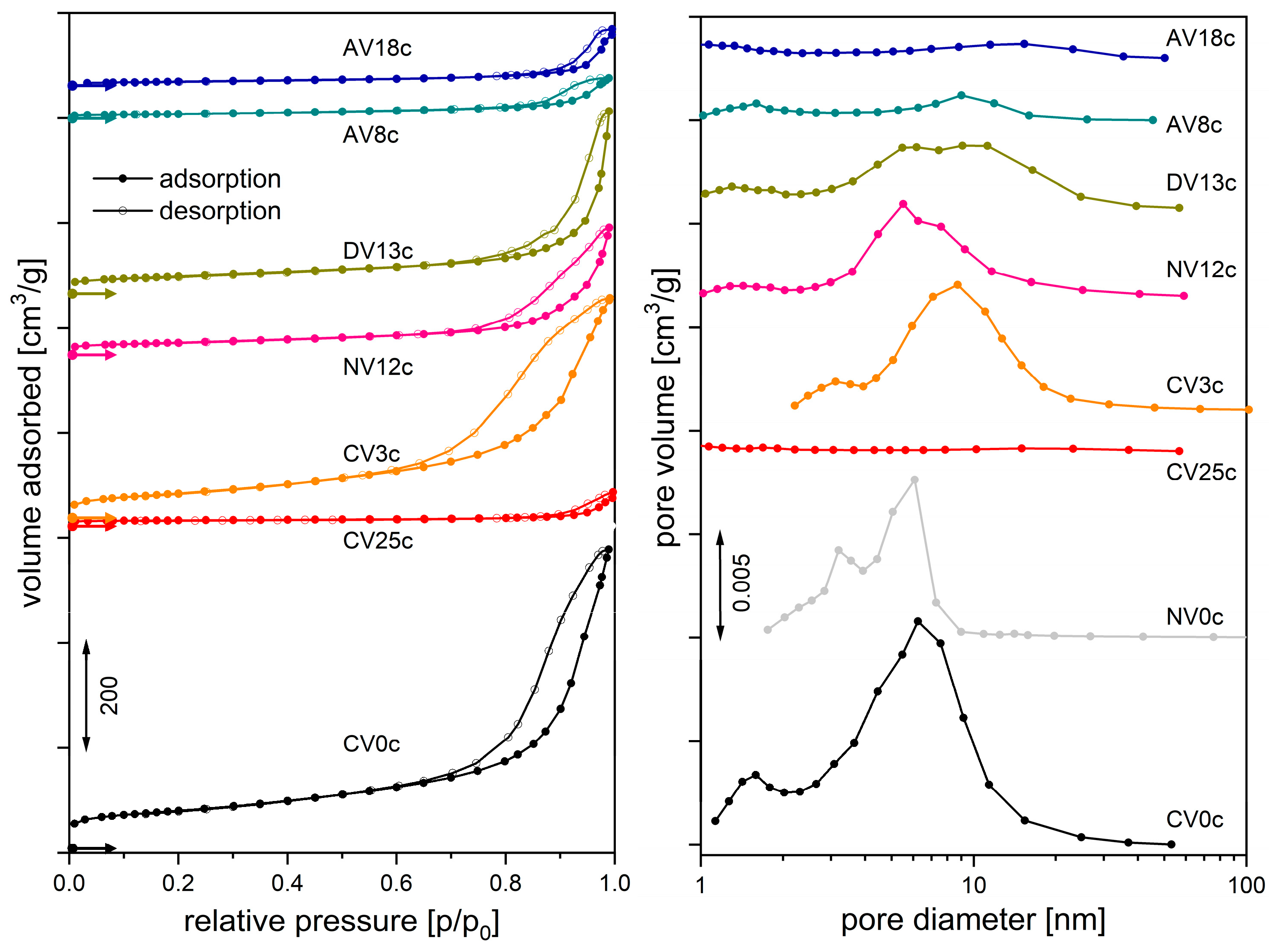
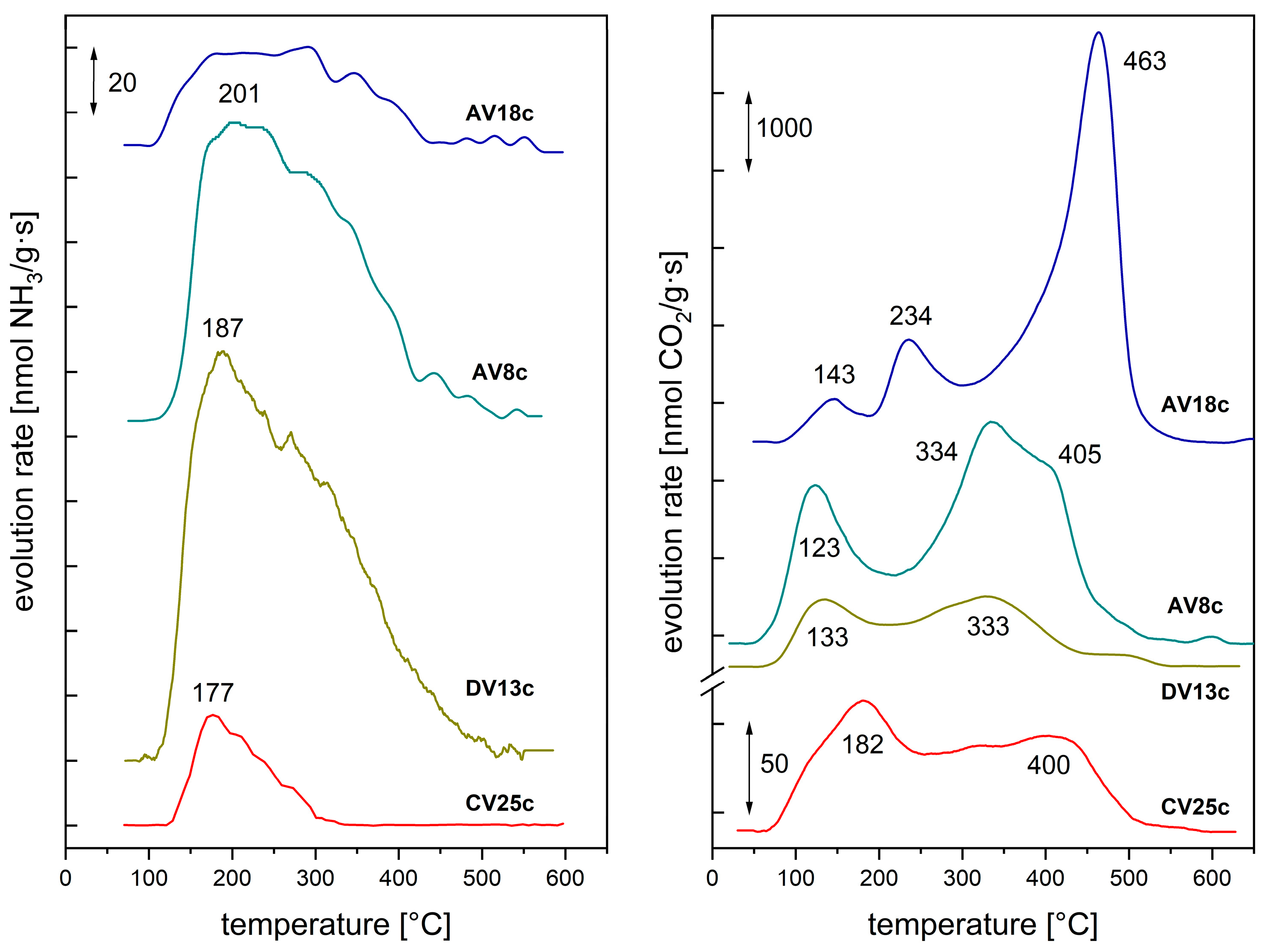
| Synthesis or Route of Vanadium Incorporation | Sample Code * | Chemical Composition of Hydrotalcite (Formula) |
|---|---|---|
| starting material for ion exchange | CV0 | Mg0.654 Al0.346 (OH)2 (NO3−)0.002 (CO32−)0.172·0.645 H2O |
| starting material for ion exchange | NV0 | Mg0.657 Al0.343 (OH)2 (NO3−)0.302 (CO32−)0.020·0.889 H2O |
| ion exchange, pH = 9.5 | CV3 | Mg0.654 Al0.346 (OH)2 (CO32−)0.133 (V2O74−)0.002·0.392 H2O EIE ** = 14.1% |
| ion exchange, pH = 9.5 | CNV7 | Mg0.600 Al0.400 (OH)2 (CO32−)0.100 (V2O74−)0.050·0.474 H2O EIE ** = 51.4% |
| ion exchange, pH = 9.5 | NV12 | Mg0.704 Al0.296 (OH)2 (CO32−)0.019 (V2O74−)0.064·0.354 H2O EIE ** = 86.9% |
| ion exchange, pH = 4.5 | CV25 | Mg0.627 Al0.376 (OH)2 (CO32−)0.009 (V10O286−)0.040·0.450 H2O EIE ** = 52.2% |
| direct precipitation (D) | DV13 | Mg0.696 Al0.304 (OH)2 (CO32−)0.031 (V2O74−)0.075·0.337 H2O EIE ** = 98.5% |
| precipitation followed by hydrothermal aging in autoclave (A) | AV8 | Mg0.690 Al0.239 V0.071 (OH)2 (CO32−)0.295 (NO3−)0.002·0.378 H2O |
| precipitation followed by hydrothermal aging in autoclave (A) | AV18 | Mg0.831 V0.169 (OH)2 (CO32−)0.269 (NO3−)0.004·0.624 H2O |
| Sample | Intercalated Anions | Cell Parameter [nm] | Crystallite Size [nm] * | ||
|---|---|---|---|---|---|
| c | a | kc | ka | ||
| CV0 NV0 | CO32− NO3− | 2.293 2.689 | 0.3045 0.3045 | 16 17 | 31 21 |
| CV25 | V10O286− | 3.508 | 0.3035 | 22 | 22 |
| CV3 NV12 | CO32− ** CO32−/V2O74− | 2.283 2.554–2.854 | 0.3041 0.3046 | 15 (~6) | 31 23 |
| DV13 | CO32−/V2O74− | 2.358–2.738 | 0.3034 | - | 12 |
| AV8 AV18 | CO32− CO32− | 2.300 2.349 | 0.3066 0.3111 | 36 43 | 49 59 |
| Sample | Phase Composition | x * [MIII/(MIII + MII)] | Mg [wt%] | Al [wt%] | V * [wt%] |
|---|---|---|---|---|---|
| CV0c NV0c | MgO MgO | 0.346 (0.33) 0.343 (0.33) | 36.1 36.7 | 21.2 20.7 | - - |
| CV25c | MgO + α-Mg2V2O7 | 0.376 (0.33) | 18.9 | 12.5 | 25.5 (30) |
| CV3c NV12c | MgO MgO + vanadate | 0.346 (0.33) 0.296 (0.33) | 34.4 31.0 | 20.2 14.5 | 2.7 (-) 11.9 (13) |
| DV13c | MgO + vanadate | 0.304 (0.33) | 29.6 | 14.4 | 13.4 (13) |
| AV8c AV18c | MgO + spinel Mg2VO4 | 0.310 (0.33) 0.169 (0.25) | 36.1 41.3 | 13.9 - | 7.8 (12) 17.6 (26) |
| Sample | SBET [m2/g] | VTOTAL [cm3/g] | Pore Size [nm] | Amount of Adsorbed Molecules [μmol/g] | |
|---|---|---|---|---|---|
| CO2 | NH3 | ||||
| CV0c NV0c | 216 164 | 0.757 0.270 | 1.6, 6.4 3.2, 5.8 | 678.8 n.d. | 97.5 n.d. |
| CV25c | 14 | 0.044 | - | 117.4 | 20.5 |
| CV3c NV12c | 196 79 | 0.593 0.272 | 3.2, 8.4 1.4, 5.6 | n.d. n.d. | n.d. n.d. |
| DV13c | 104 | 0.305 | 1.8, 6.8, 12 | * | 142.7 |
| AV8c AV18c | 28 34 | 0.099 0.109 | - - | * * | 117.5 41.4 |
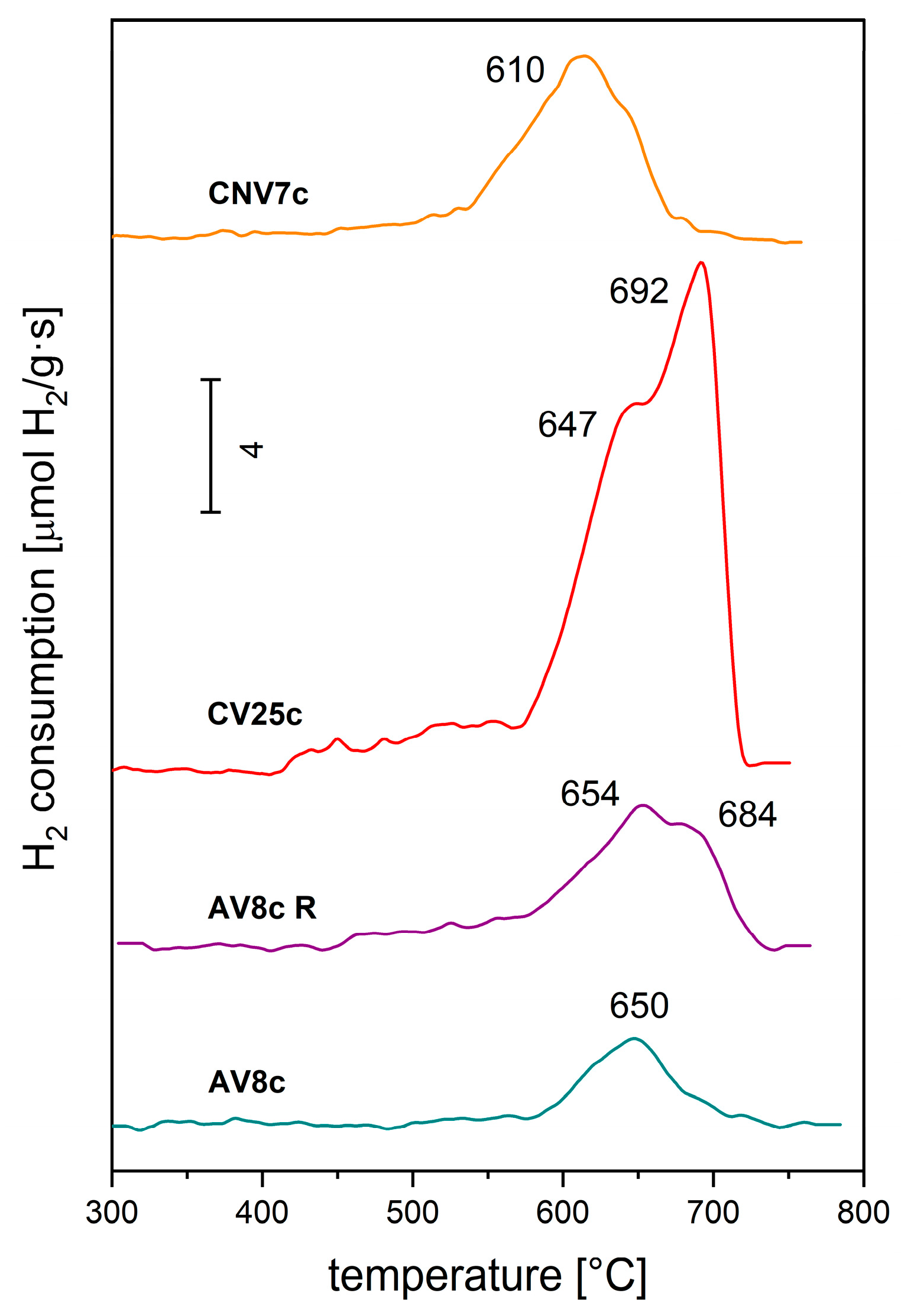
| Sample | Consumption H2 [mmol/g] | Reduction Degree | Temperature [°C] | ||
|---|---|---|---|---|---|
| [H2]/[V] a | Change of Oxidation State | Maximum | Onset | ||
| AV8c AV8c R | 0.675 1.467 | 0.443 0.963 | 0.9 1.9 | (650) (654, 684) | (550) (500) |
| CNV7c CV22c | 1.549 4.222 | 1.074 0.981 | 2.1 2.0 | (610) (647, 692) | (450) (510) |
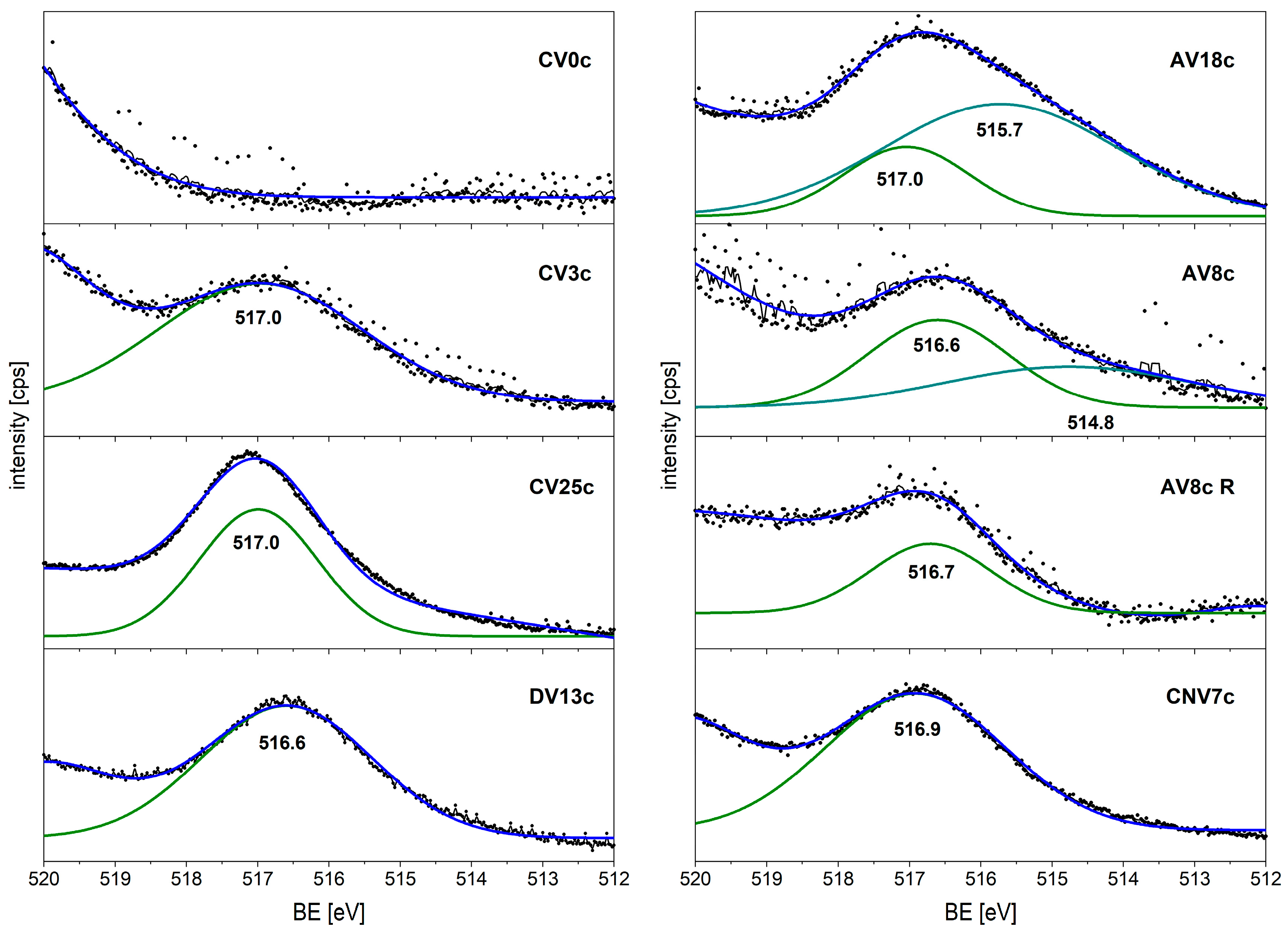
| Surface Atomic % | ||||||
|---|---|---|---|---|---|---|
| Mg | Al | V | C | O | ||
| CV0c | 19.0 | 13.7 | 0.0 | 4.0 | 63.3 | |
| CV3c | 16.1 | 18.8 | 1.1 | 2.1 | 61.9 | |
| CV25c | 10.3 | 19.2 | 7.3 | 1.7 | 61.4 | |
| CNV7c | 14.6 | 19.2 | 2.4 | 1.8 | 62.1 | |
| DV13c | 16.4 | 16.7 | 3.6 | 2.6 | 60.7 | |
| AV8c | 32.8 | 20.9 | 1.4 | 5.1 | 39.7 | |
| AV8c R | 14.8 | 8.3 | 0.5 | 30.9 | 45.5 | |
| AV18c | 26.4 | 0.0 | 6.3 | 6.4 | 61.0 | |
| Surface Molar Ratio | Bulk Molar Ratio | |||||
| Mg | Al | V | Mg | Al | V | |
| CV0c | 0.581 | 0.419 | 0.000 | 0.654 | 0.346 | 0.000 |
| CV3c | 0.447 | 0.523 | 0.030 | 0.638 | 0.338 | 0.024 |
| CV25c | 0.280 | 0.521 | 0.199 | 0.447 | 0.266 | 0.287 |
| CNV7c | 0.404 | 0.531 | 0.066 | 0.559 | 0.372 | 0.069 |
| DV13c | 0.448 | 0.455 | 0.097 | 0.605 | 0.265 | 0.131 |
| AV8c | 0.595 | 0.379 | 0.026 | 0.690 | 0.239 | 0.071 |
| AV8c R | 0.628 | 0.352 | 0.019 | n.d. | n.d. | n.d. |
| AV18c | 0.808 | 0.000 | 0.192 | 0.831 | 0.000 | 0.169 |
| TPR | NH3-TPD | CO2-TPD |
|---|---|---|
| outgassing: RT-600 °C, β~30 °C/min He = 20 mL/min | outgassing: RT-600 °C, β~30 °C/min He = 20 mL/min | outgassing: RT-600 °C, β~30 °C/min N2 = 85 mL/min |
| - | adsorption NH3: 70 °C, 45 min 1% NH3/He = 20 mL/min | adsorption CO2: 70 °C, 4 h CO2 = 85 mL/min |
| TPR: 200–800 °C, β = 15 °C/min 6% H2/Ar = 16 mL/min mass = 30 mg particle size: 355–500 μm detector: QMS | TPD: 70–600 °C β = 10 °C/min He = 20 mL/min mass = 50 mg particle size: 355–500 μm detector: QMS | TPD: RT-600 °C β = 10 °C/min He = 20 mL/min mass = 50 mg particle size: 355–500 μm detector: QMS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Węgrzyn, A.; Katarzyńska, A.; Miśkowiec, P.; Makowski, W. Beyond Fossil Fuels: The Role of V-Doped Hydrotalcites in n-Butane Oxidative Dehydrogenation for a Circular Economy. Catalysts 2025, 15, 841. https://doi.org/10.3390/catal15090841
Węgrzyn A, Katarzyńska A, Miśkowiec P, Makowski W. Beyond Fossil Fuels: The Role of V-Doped Hydrotalcites in n-Butane Oxidative Dehydrogenation for a Circular Economy. Catalysts. 2025; 15(9):841. https://doi.org/10.3390/catal15090841
Chicago/Turabian StyleWęgrzyn, Agnieszka, Alicja Katarzyńska, Paweł Miśkowiec, and Wacław Makowski. 2025. "Beyond Fossil Fuels: The Role of V-Doped Hydrotalcites in n-Butane Oxidative Dehydrogenation for a Circular Economy" Catalysts 15, no. 9: 841. https://doi.org/10.3390/catal15090841
APA StyleWęgrzyn, A., Katarzyńska, A., Miśkowiec, P., & Makowski, W. (2025). Beyond Fossil Fuels: The Role of V-Doped Hydrotalcites in n-Butane Oxidative Dehydrogenation for a Circular Economy. Catalysts, 15(9), 841. https://doi.org/10.3390/catal15090841








