Tailoring Electrocatalytic Pathways: A Comparative Review of the Electrolyte’s Effects on Five Key Energy Conversion Reactions
Abstract
1. Introduction
1.1. Importance of Electrocatalysis in Energy Conversion and Storage
1.2. How to Use This Review
1.3. Role of the Electrolyte in Electrocatalytic Reactions
1.4. Scope of This Article: Five Key Electrocatalytic Reactions
2. Fundamental Aspects of the Electrolyte’s Effects
2.1. Influences of pH, Ionic Strength, and Ion Identity
2.2. Solvent Effects, Double-Layer Structure, and Hydrogen Bonding
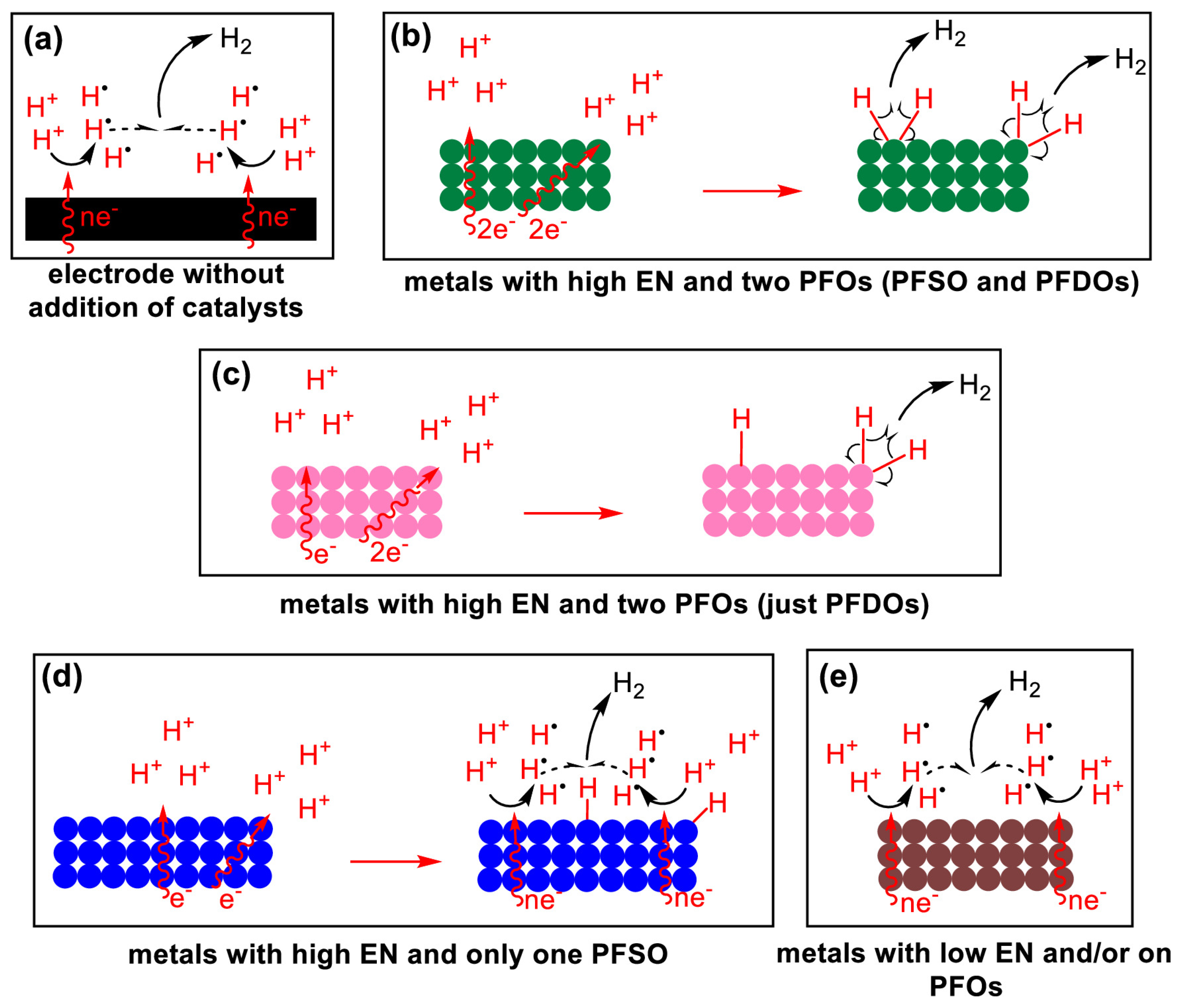
3. Hydrogen Evolution Reaction (HER)
3.1. Mechanism and Rate-Determining Steps
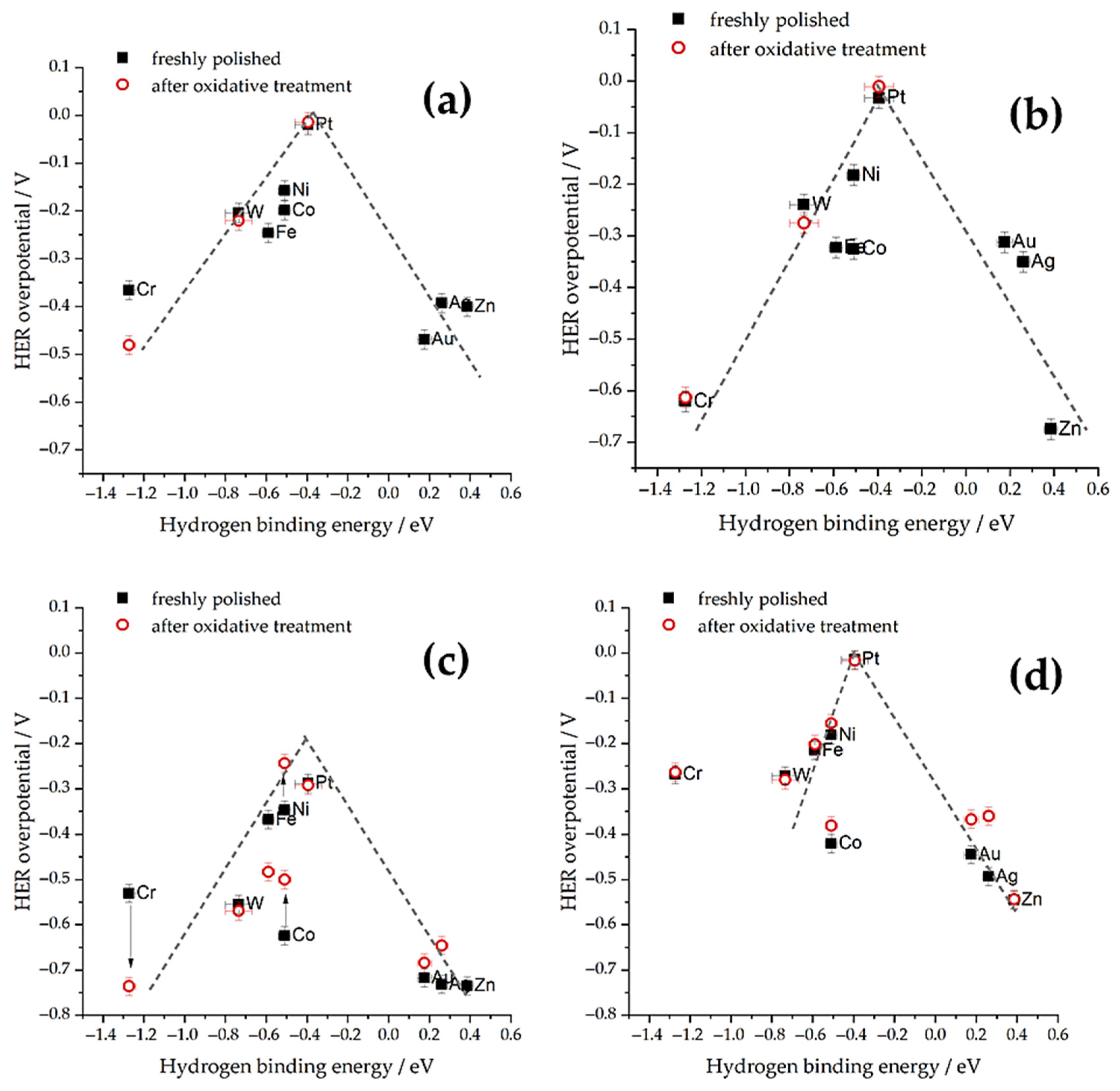
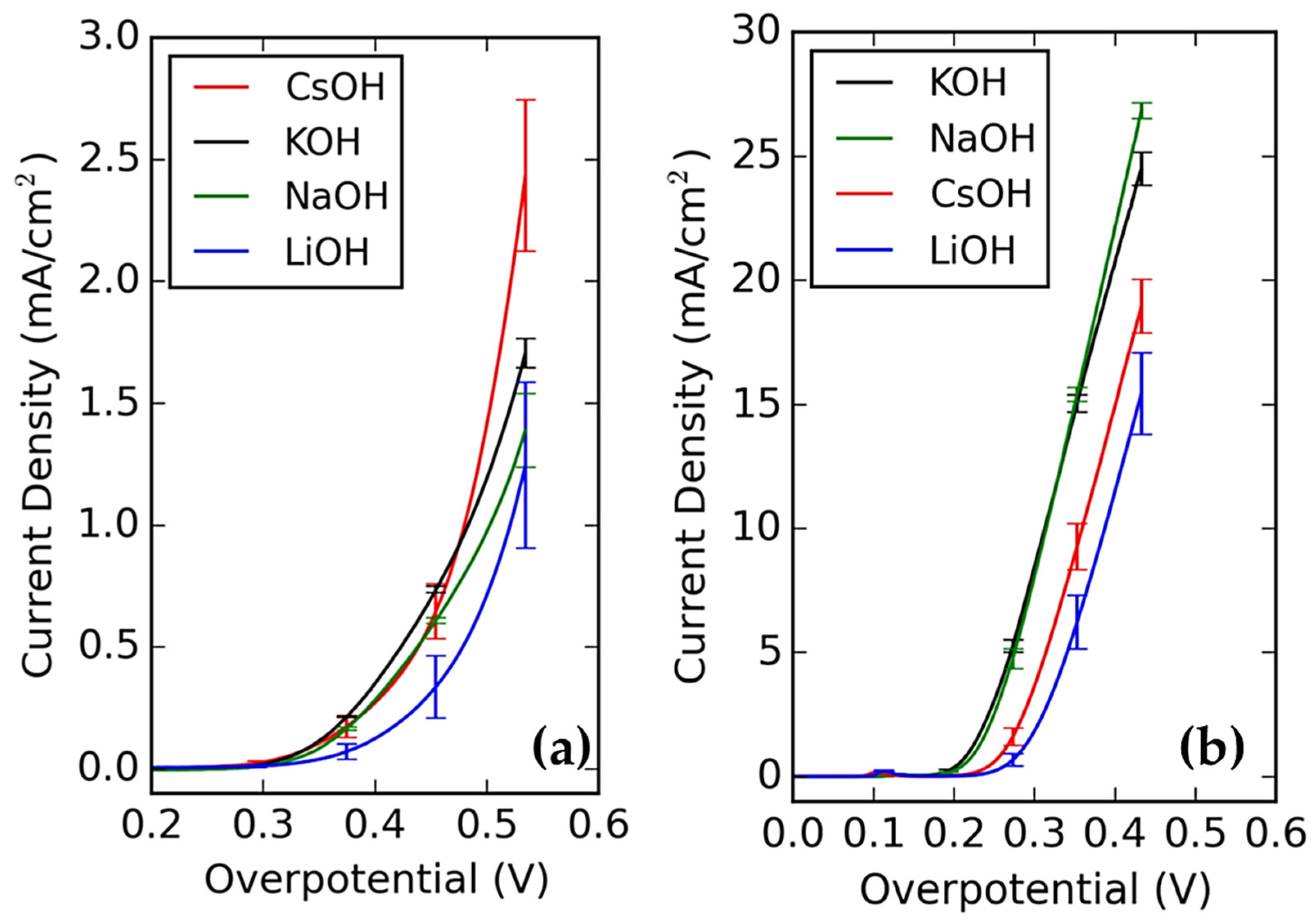
3.2. Impact of the Electrolyte Composition (Acidic vs. Alkaline Media)
3.3. Effects of Specific Ions and the Interfacial Electric Field
3.4. Interim Summary on HER
4. Oxygen Evolution Reaction (OER)
4.1. Mechanistic Pathways in Acidic and Alkaline Media
4.2. Roles of Cations and Anions in Stabilizing Intermediates
4.3. Electrolyte-Dependent Activity and Stability of Catalysts
4.4. Interim Summary of the OER
5. Oxygen Reduction Reaction (ORR)
5.1. Two-Electron vs. Four-Electron Pathways
5.2. pH-Dependent Activity and Selectivity
5.3. Roles of Specific Anions and Cations in Catalyst Performance
5.4. Interim Summary of the ORR
6. Carbon Dioxide Reduction Reaction (CO2RR)
6.1. Influence of the Electrolyte on Product Selectivity
6.2. Buffering Capacity and Local pH Effects
6.3. Effects of Cations on CO2 Activation and Reaction Pathways
6.4. Interim Summary of the CO2RR
7. Nitrogen Reduction Reaction (NRR)
7.1. Challenges in Achieving High Faradaic Efficiency
7.2. Proton Source and Competition with the HER
7.3. Role of Non-Aqueous Electrolytes in Enhancing Selectivity
7.4. Interim Summary of the NRR
8. Comparative Discussion and General Trends
8.1. Common Effects of Electrolytes on Different Electrocatalytic Reactions
8.2. Trade-Offs Between Reaction Rates and Selectivity
8.3. Design Principles for Optimizing Electrocatalytic Performance
9. Conclusions and Future Perspectives
9.1. Summary of the Key Findings
9.2. Open Questions and Potential Research Directions
9.3. Implications for Practical Applications in Energy Conversion and Sustainability
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lai, F.; Shang, H.; Jiao, Y.; Chen, X.; Zhang, T.; Liu, X. Recent progress and perspective on electrocatalysis in neutral media: Mechanisms, materials, and advanced characterizations. Interdiscip. Mater. 2024, 3, 492–529. [Google Scholar] [CrossRef]
- Baglio, V. Electrocatalysts for Energy Conversion and Storage Devices. Catalysts 2021, 11, 1491. [Google Scholar] [CrossRef]
- Wang, H.Y.; Weng, C.C.; Ren, J.T.; Yuan, Z.Y. An overview and recent advances in electrocatalysts for direct seawater splitting. Front. Chem. Sci. Eng. 2021, 15, 1408–1426. [Google Scholar] [CrossRef]
- Chen, M.; Kitiphatpiboon, N.; Feng, C.; Abudula, A.; Ma, Y.; Guan, G. Recent progress in transition-metal-oxide-based electrocatalysts for the oxygen evolution reaction in natural seawater splitting: A critical review. eScience 2023, 3, 100111. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.; Gandionco, K.A.; Bond, A.M.; Zhang, J. Electrocatalytic carbon dioxide reduction from fundamental principles to catalyst design. Materialstoday 2020, 7, 100074. [Google Scholar] [CrossRef]
- Chen, J.G.; Crooks, R.M.; Seefeldt, L.C.; Bren, K.L.; Bullock, R.M.; Darensbourg, M.Y.; Holland, P.L.; Hoffman, B.; Janik, M.J.; Jones, A.K.; et al. Beyond fossil fuel-driven nitrogen transformations. Science 2018, 360, eaar6611. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, G.K.; Jovanović, A.Z.; Pašti, I.A. The Effect of Electrolytes on the Kinetics of the Hydrogen Evolution Reaction. Hydrogen 2023, 4, 776–806. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhang, Z.M.; Zhuang, X.X.; Jia, Z.T.; Wang, T. Electrolyte Effects in Electrocatalytic Kinetics: Critical Review. Chin. J. Chem. 2024, 42, 3533–3552. [Google Scholar] [CrossRef]
- Lu, X.; Tu, W.; Zhou, Y.; Zou, Z. Effects of Electrolyte Ionic Species on Electrocatalytic Reactions: Advances, Challenges, and Perspectives: Review. Adv. Energy Mater. 2023, 13, 2300628. [Google Scholar] [CrossRef]
- Bonnefont, A. Deciphering the effect of pH on electrocatalytic reactions with kinetic modeling. Curr. Opin. Electrochem. 2023, 39, 101294. [Google Scholar] [CrossRef]
- Weber, D.J.; Janssen, M.; Oezaslan, M. Effect of Monovalent Cations on the HOR/HER Activity for Pt in Alkaline Environment. J. Electrochem. Soc. 2019, 166, F66–F73. [Google Scholar] [CrossRef]
- Guha, A.; Kaley, N.M.; Mondal, J.; Narayanan, T.N. Engineering the hydrogen evolution reaction of transition metals: Effect of Li ions. J. Mater. Chem. A Mater. 2020, 8, 15795–15808. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J.K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef]
- Stacy, J.; Regmi, Y.N.; Leonard, B.; Fan, M. The recent progress and future of oxygen reduction reaction catalysis: A review. Renew. Sustain. Energy Rev. 2017, 69, 401–414. [Google Scholar] [CrossRef]
- Attard, G.A.; Bruce, P.G.; Calvo, E.J.; Chen, Y.; Curtiss, L.A.; Dewar, D.; Ellison, J.H.J.; Fernández-Vidal, J.; Freunberger, S.A.; Gao, X.; et al. Mechanism of ORR and OER in non-aqueous electrolytes: General discussion. Faraday Discuss. 2024, 248, 210–249. [Google Scholar] [CrossRef] [PubMed]
- Sarpey, T.K.; Himmelreich, A.V.; Song, K.T.; Gubanova, E.L.; Bandarenka, A.S. The Electrocatalytic Activity of Au Electrodes Changes Significantly in Various Na+/K+ Supporting Electrolyte Mixtures. Small Sci. 2024, 4, 2400042. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, G.K.; Jovanović, A.Z.; Dobrota, A.S.; Skorodumova, N.V.; Pašti, I.A. Hydrogen Evolution Volcano(es)—From Acidic to Neutral and Alkaline Solutions. Catalysts 2022, 12, 1541. [Google Scholar] [CrossRef]
- Shinagawa, T.; Takanabe, K. Identification of intrinsic catalytic activity for electrochemical reduction of water molecules to generate hydrogen. Phys. Chem. Chem. Phys. 2015, 17, 15111–15114. [Google Scholar] [CrossRef]
- Katsounaros, I.; Meier, J.C.; Klemm, S.O.; Topalov, A.A.; Biedermann, P.U.; Auinger, M.; Mayrhofer, K.J.J. The effective surface pH during reactions at the solid–liquid interface. Electrochem. Commun. 2011, 13, 634–637. [Google Scholar] [CrossRef]
- Chung, D.Y.; Yoo, J.M.; Sung, Y.E. Highly Durable and Active Pt-Based Nanoscale Design for Fuel-Cell Oxygen-Reduction Electrocatalysts. Adv. Mater. 2018, 30, 1704123. [Google Scholar] [CrossRef]
- Shinagawa, T.; Takanabe, K. Impact of solute concentration on the electrocatalytic conversion of dissolved gases in buffered solutions. J. Power Sources 2015, 287, 465–471. [Google Scholar] [CrossRef]
- Auinger, M.; Katsounaros, I.; Meier, J.C.; Klemm, S.O.; Biedermann, P.U.; Topalov, A.A.; Rohwerdera, M.; Mayrhofer, K.J.J. Near-surface ion distribution and buffer effects during electrochemical reactions. Phys. Chem. Chem. Phys. 2011, 13, 16384–16394. [Google Scholar] [CrossRef] [PubMed]
- Merrill, M.D.; Logan, B.E. Electrolyte effects on hydrogen evolution and solution resistance in microbial electrolysis cells. J. Power Sources 2009, 191, 203–208. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, C.; Grauer, D.C.; Yano, J.; Long, J.R.; Yang, P.; Chang, C.J. Electrodeposited cobalt-sulfide catalyst for electrochemical and photoelectrochemical hydrogen generation from water. J. Am. Chem. Soc. 2013, 135, 17699–17702. [Google Scholar] [CrossRef]
- Zhou, Z.; Pei, Z.; Wei, L.; Zhao, S.; Jian, X.; Chen, Y. Electrocatalytic hydrogen evolution under neutral pH conditions: Current understandings, recent advances, and future prospects. Energy Envoiorn. Sci. 2020, 13, 3185–3206. [Google Scholar] [CrossRef]
- Huang, B.; Rao, R.R.; You, S.; Myint, K.H.; Song, Y.; Wang, Y.; Ding, W.; Giordano, L.; Zhang, Y.; Wang, T.; et al. Cation- and pH-Dependent Hydrogen Evolution and Oxidation Reaction Kinetics. JACS Au 2021, 1, 1674–1687. [Google Scholar] [CrossRef]
- Bender, J.T.; Petersen, A.S.; Østergaard, F.C.; Wood, M.A.; Heffernan, S.M.J.; Milliron, D.J.; Rossmeisl, J.; Resasco, J. Understanding Cation Effects on the Hydrogen Evolution Reaction. ACS Energy Lett. 2023, 8, 657–665. [Google Scholar] [CrossRef]
- del Rosario, J.A.D.; Li, G.; Labata, M.F.M.; Ocon, J.D.; Chuang, P.Y.A. Unravelling the roles of alkali-metal cations for the enhanced oxygen evolution reaction in alkaline media. Appl. Catal. B 2021, 288, 119981. [Google Scholar] [CrossRef]
- Kamat, G.A.; Zamora Zeledón, J.A.; Kalhara Gunasooriya, G.T.K.; Dull, S.M.; Perryman, J.T.; Nørskov, J.K.; Stevens, M.B.; Jaramillo, T.F. Acid anion electrolyte effects on platinum for oxygen and hydrogen electrocatalysis. Commun. Chem. 2022, 5, 20. [Google Scholar] [CrossRef]
- Xu, Z.; Tan, X.; Chen, C.; Wang, X.; Sui, R.; Zhuang, Z.; Zhang, C.; Chen, C. Recent advances in microenvironment regulation for electrocatalysis. Natl. Sci. Rev. 2024, 11, nwae315. [Google Scholar] [CrossRef]
- Schott, C.M.; Schneider, P.M.; Song, K.; Yu, H.; Götz, R.; Haimerl, F.; Gubanova, E.; Zhou, J.; Schmidt, T.O.; Zhang, Q.; et al. How to Assess and Predict Electrical Double Layer Properties. Implications for Electrocatalysis. Chem. Rev. 2024, 124, 12391–12462. [Google Scholar] [CrossRef]
- Lux, S.F.; Terborg, L.; Hachmöller, O.; Placke, T.; Meyer, H.W.; Passerini, S.; Winter, M.; Nowak, S. LiTFSI Stability in Water and Its Possible Use in Aqueous Lithium-Ion Batteries: pH Dependency, Electrochemical Window and Temperature Stability. J. Electrochem. Soc. 2013, 160, A1694–A1700. [Google Scholar] [CrossRef]
- Du, Y.; Li, B.; Xu, G.; Wang, L. Recent advances in interface engineering strategy for highly-efficient electrocatalytic water splitting. InfoMat 2023, 5, e12377. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, J.; Zhu, Y.; Qiao, Y.; Kang, Z.; Wang, Z.; Tian, X.; Huang, H.; Lai, W. Water in Electrocatalysis. Angew. Chem. Int. Ed. 2025, 64, e202425590. [Google Scholar] [CrossRef]
- Ohno, P.E.; Chang, H.; Spencer, A.P.; Liu, Y.; Boamah, M.D.; Wang, H.; Geiger, F.M. Beyond the Gouy–Chapman Model with Heterodyne-Detected Second Harmonic Generation. J. Phys. Chem. Lett. 2019, 10, 2328–2334. [Google Scholar] [CrossRef]
- Wu, J. Understanding the Electric Double-Layer Structure, Capacitance, and Charging Dynamics. Chem. Rev. 2022, 122, 10821–10859. [Google Scholar] [CrossRef]
- Shi, G.; Lu, T.; Zhang, L. Understanding the interfacial water structure in electrocatalysis. Natl. Sci. Rev. 2024, 11, nwae241. [Google Scholar] [CrossRef]
- Le, J.B.; Fan, Q.Y.; Li, J.Q.; Cheng, J. Molecular origin of negative component of Helmholtz capacitance at electrified Pt(111)/water interface. Sci. Adv. 2020, 6, eabb1219. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Velez, J.J.; Wu, C.H.; Pascal, T.A.; Wan, L.F.; Guo, J.; Prendergast, D.; Salmeron, M. The structure of interfacial water on gold electrodes studied by x-ray absorption spectroscopy. Science 2014, 346, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Loh, Z.H.; Doumy, G.; Arnold, C.; Kjellsson, L.; Southworth, S.H.; Al Haddad, A.; Kumagai, Y.; Tu, M.-F.; Ho, P.J.; March, A.M.; et al. Observation of the fastest chemical processes in the radiolysis of water. Science 2020, 367, 179–182. [Google Scholar] [CrossRef]
- Goyal, A.; Koper, M.T.M. The Interrelated Effect of Cations and Electrolyte pH on the Hydrogen Evolution Reaction on Gold Electrodes in Alkaline Media. Angew. Chem. Int. Ed. Engl. 2021, 60, 13452–13462. [Google Scholar] [CrossRef]
- Li, P.; Jiang, Y.L.; Men, Y.; Jiao, Y.Z.; Chen, S. Kinetic cation effect in alkaline hydrogen electrocatalysis and double layer proton transfer. Nat. Commun. 2025, 16, 1844. [Google Scholar] [CrossRef]
- Alfarano, S.R.; Pezzotti, S.; Stein, C.J.; Lin, Z.; Sebastiani, F.; Funke, S.; Hoberg, C.; Kolling, I.; Ma, C.Y.; Mauelshagen, K.; et al. Stripping away ion hydration shells in electrical double-layer formation: Water networks matter. Proc. Natl. Acad. Sci. USA 2021, 118, e2108568118. [Google Scholar] [CrossRef]
- Sheng, W.; Gasteiger, H.A.; Shao-Horn, Y. Hydrogen Oxidation and Evolution Reaction Kinetics on Platinum: Acid vs Alkaline Electrolytes. J. Electrochem. Soc. 2010, 157, B1529. [Google Scholar] [CrossRef]
- Ryu, J.; Surendranath, Y. Tracking Electrical Fields at the Pt/H2O Interface during Hydrogen Catalysis. J. Am. Chem. Soc. 2019, 141, 15524–15531. [Google Scholar] [CrossRef]
- Li, P.; Jiao, Y.; Huang, J.; Chen, S. Electric Double Layer Effects in Electrocatalysis: Insights from Ab Initio Simulation and Hierarchical Continuum Modeling. JACS Au 2023, 3, 2640. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Gunathunge, C.M.; Waegele, M.M. Hydrogen bonding steers the product selectivity of electrocatalytic CO reduction. Proc. Natl. Acad. Sci. USA 2019, 116, 9220–9229. [Google Scholar] [CrossRef]
- Chai, J.; Zheng, Z.; Pan, H.; Zhang, S.; Lakshmi, K.V.; Sun, Y.Y. Significance of hydrogen bonding networks in the proton-coupled electron transfer reactions of photosystem II from a quantum-mechanics perspective. Phys. Chem. Chem. Phys. 2019, 21, 8721–8728. [Google Scholar] [CrossRef]
- Potts, D.S.; Bregante, D.T.; Adams, J.S.; Torres, C.; Flaherty, D.W. Influence of solvent structure and hydrogen bonding on catalysis at solid–liquid interfaces. Chem. Soc. Rev. 2021, 50, 12308–12337. [Google Scholar] [CrossRef]
- Angeles-Olvera, Z.; Crespo-Yapur, A.; Rodríguez, O.; Cholula-Díaz, J.L.; Martínez, L.M.; Videa, M. Nickel-Based Electrocatalysts for Water Electrolysis. Energies 2022, 15, 1609. [Google Scholar] [CrossRef]
- Cossar, E.; Murphy, F.; Baranova, E.A. Nickel-based anodes in anion exchange membrane water electrolysis: A review. J. Chem. Technol. Biotechnol. 2022, 97, 1611–1624. [Google Scholar] [CrossRef]
- Gutić, S.J.; Dobrota, A.S.; Fako, E.; Skorodumova, N.V.; López, N.; Pašti, I.A. Hydrogen evolution reaction-from single crystal to single atom catalysts. Catalysts 2020, 10, 290. [Google Scholar] [CrossRef]
- Murthy, A.P.; Theerthagiri, J.; Madhavan, J. Insights on Tafel Constant in the Analysis of Hydrogen Evolution Reaction. J. Phys. Chem. C 2018, 122, 23943–23949. [Google Scholar] [CrossRef]
- Štrbac, S.; Smiljanić, M.; Wakelin, T.; Potočnik, J.; Rakočević, Z. Hydrogen evolution reaction on bimetallic Ir/Pt(poly) electrodes in alkaline solution. Electrochim. Acta 2019, 306, 18–27. [Google Scholar] [CrossRef]
- Durst, J.; Simon, C.; Siebel, A.; Rheinländer, P.J.; Schuler, T.; Hanzlik, M.; Herranz, J.; Hasché, F.; Gasteiger, H.A. (Invited) Hydrogen Oxidation and Evolution Reaction (HOR/HER) on Pt Electrodes in Acid vs. Alkaline Electrolytes: Mechanism, Activity and Particle Size Effects. ECS Trans. 2014, 64, 1069–1080. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Liu, C. Enhancing the Understanding of Hydrogen Evolution and Oxidation Reactions on Pt(111) through Ab Initio Simulation of Electrode/Electrolyte Kinetics. J. Am. Chem. Soc. 2020, 142, 4985–4989. [Google Scholar] [CrossRef]
- Intikhab, S.; Snyder, J.D.; Tang, M.H. Adsorbed Hydroxide Does Not Participate in the Volmer Step of Alkaline Hydrogen Electrocatalysis. ACS Catal. 2017, 7, 8314–8319. [Google Scholar] [CrossRef]
- McCrum, I.T.; Koper, M.T.M. The role of adsorbed hydroxide in hydrogen evolution reaction kinetics on modified platinum. Nat. Energy 2020, 5, 891–899. [Google Scholar] [CrossRef]
- Rheinländer, P.J.; Herranz, J.; Durst, J.; Gasteiger, H.A. Kinetics of the Hydrogen Oxidation/Evolution Reaction on Polycrystalline Platinum in Alkaline Electrolyte Reaction Order with Respect to Hydrogen Pressure. J. Electrochem. Soc. 2014, 161, F1448–F1457. [Google Scholar] [CrossRef]
- Lamoureux, P.S.; Singh, A.R.; Chan, K. PH Effects on Hydrogen Evolution and Oxidation over Pt(111): Insights from First-Principles. ACS Catal. 2019, 9, 6194–6201. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Prats, H.; Chan, K. The determination of the HOR/HER reaction mechanism from experimental kinetic data. Phys. Chem. Chem. Phys. 2021, 23, 27150–27158. [Google Scholar] [CrossRef]
- Oshchepkov, A. Investigation of the Hydrogen Electrode Reactions on Ni Electrocatalysts in Alkaline Medium. 2017. Available online: https://tel.archives-ouvertes.fr/tel-02003369 (accessed on 5 April 2025).
- Watzele, S.; Fichtner, J.; Garlyyev, B.; Schwämmlein, J.N.; Bandarenka, A.S. On the Dominating Mechanism of the Hydrogen Evolution Reaction at Polycrystalline Pt Electrodes in Acidic Media. ACS Catal. 2018, 8, 9456–9462. [Google Scholar] [CrossRef]
- Sun, Y. Perspective—A New Viewpoint on the Mechanism of the Hydrogen Evolution Reaction on Various Transition Metal Electrodes. Inst. Phys. 2024, 3, 010503. [Google Scholar] [CrossRef]
- Chen, K.; Xu, B.; Shen, L.; Shen, D.; Li, M.; Guo, L.H. Functions and performance of ionic liquids in enhancing electrocatalytic hydrogen evolution reactions: A comprehensive review. RSC Adv. 2022, 12, 19452–19469. [Google Scholar] [CrossRef] [PubMed]
- Arminio-Ravelo, J.A.; Jensen, A.W.; Jensen, K.D.; Quinson, J.; Escudero-Escribano, M. Electrolyte Effects on the Electrocatalytic Performance of Iridium-Based Nanoparticles for Oxygen Evolution in Rotating Disc Electrodes. ChemPhysChem 2019, 20, 2956–2963. [Google Scholar] [CrossRef]
- Gebremariam, G.K.; Jovanović, A.Z.; Pašti, I.A. Kinetics of Hydrogen Evolution Reaction on Monometallic Bulk Electrodes in Various Electrolytic Solutions. Catalysts 2023, 13, 1373. [Google Scholar] [CrossRef]
- Durst, J.; Siebel, A.; Simon, C.; Hasché, F.; Herranz, J.; Gasteiger, H.A. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 2014, 7, 2255–2260. [Google Scholar] [CrossRef]
- Shinagawa, T.; Takanabe, K. Electrocatalytic Hydrogen Evolution under Densely Buffered Neutral pH Conditions. J. Phys. Chem. C 2015, 119, 20453–20458. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Mechanistic Switching by Hydronium Ion Activity for Hydrogen Evolution and Oxidation over Polycrystalline Platinum Disk and Platinum/Carbon Electrodes. ChemElectroChem 2014, 1, 1497–1507. [Google Scholar] [CrossRef]
- De Silva Muñoz, L.; Bergel, A.; Féron, D.; Basséguy, R. Hydrogen production by electrolysis of a phosphate solution on a stainless steel cathode. Int. J. Hydrogen Energy 2010, 35, 8561–8568. [Google Scholar] [CrossRef]
- Zheng, J.; Sheng, W.; Zhuang, Z.; Xu, B.; Yan, Y. Universal dependence of hydrogen oxidation and evolution reaction activity of platinum-group metals on pH and hydrogen binding energy. Sci. Adv. 2016, 2, e1501602. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Zhuang, Z.; Gao, M.; Zheng, J.; Chen, J.G.; Yan, Y. Correlating hydrogen oxidation and evolution activity on platinum at different pH with measured hydrogen binding energy. Nat. Commun. 2015, 6, 5848. [Google Scholar] [CrossRef]
- Zhu, S.; Qin, X.; Yao, Y.; Shao, M. PH-Dependent Hydrogen and Water Binding Energies on Platinum Surfaces as Directly Probed through Surface-Enhanced Infrared Absorption Spectroscopy. J. Am. Chem. Soc. 2020, 142, 8748–8754. [Google Scholar] [CrossRef]
- Ledezma-Yanez, I.; Wallace, W.D.Z.; Sebastián-Pascual, P.; Climent, V.; Feliu, J.M.; Koper, M.T.M. Interfacial water reorganization as a pH-dependent descriptor of the hydrogen evolution rate on platinum electrodes. Nat. Energy 2017, 2, 17031. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Chang, K.-C.; Strmcnik, D.; Paulikas, A.P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Markovic, N.M. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nat. Mater. 2012, 11, 550–557. [Google Scholar] [CrossRef]
- Strmcnik, D.; Uchimura, M.; Wang, C.; Subbaraman, R.; Danilovic, N.; van der Vliet, D.; Paulikas, A.P.; Stamenkovic, V.R.; Markovic, N.M. Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Nat. Chem. 2013, 5, 300–306. [Google Scholar] [CrossRef]
- Strmcnik, D.; Lopes, P.P.; Genorio, B.; Stamenkovic, V.R.; Markovic, N.M. Design principles for hydrogen evolution reaction catalyst materials. J. Nano Energy 2016, 29, 29–36. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Ross, P.N.; Markovic, N.M. Temperature dependent surface electrochemistry on Pt single crystals in alkaline electrolytes: Part 2. The hydrogen evolution/oxidation reaction. J. Electroanal. Chem. 2002, 524–525, 252–260. [Google Scholar] [CrossRef]
- Liu, E.; Li, J.; Jiao, L.; Doan, H.T.T.; Liu, Z.; Zhao, Z.; Huang, Y.; Abraham, K.M.; Mukerjee, S.; Jia, Q. Unifying the Hydrogen Evolution and Oxidation Reactions Kinetics in Base by Identifying the Catalytic Roles of Hydroxyl-Water-Cation Adducts. J. Am. Chem. Soc. 2019, 141, 3232–3239. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Huang, J.; Mao, B.; An, T.; Wang, J.; Cao, M. Inside solid-liquid interfaces: Understanding the influence of the electrical double layer on alkaline hydrogen evolution reaction. Appl. Catal. B 2021, 293, 120220. [Google Scholar] [CrossRef]
- Shah, A.H.; Zhang, Z.; Huang, Z.; Wang, S.; Zhong, G.; Wan, C.; Alexandrova, A.N.; Huang, Y.; Duan, X. The role of alkali metal cations and platinum-surface hydroxyl in the alkaline hydrogen evolution reaction. Nat. Catal. 2022, 5, 923–933. [Google Scholar] [CrossRef]
- McCrum, I.T.; Janik, M.J. pH and Alkali Cation Effects on the Pt Cyclic Voltammogram Explained Using Density Functional Theory. J. Phys. Chem. C 2016, 120, 457–471. [Google Scholar] [CrossRef]
- Strmcnik, D.; Kodama, K.; Van Der Vliet, D.; Greeley, J.; Stamenkovic, V.R.; Marković, N.M. The role of non-covalent interactions in electrocatalytic fuel-cell reactions on platinum. Nat. Chem. 2009, 1, 466–472. [Google Scholar] [CrossRef]
- Xue, S.; Garlyyev, B.; Watzele, S.; Liang, Y.; Fichtner, J.; Pohl, M.D.; Bandarenka, A.S. Influence of Alkali Metal Cations on the Hydrogen Evolution Reaction Activity of Pt, Ir, Au, and Ag Electrodes in Alkaline Electrolytes. ChemElectroChem 2018, 5, 2326–2329. [Google Scholar] [CrossRef]
- Monteiro, M.C.O.; Goyal, A.; Moerland, P.; Koper, M.T.M. Understanding Cation Trends for Hydrogen Evolution on Platinum and Gold Electrodes in Alkaline Media. ACS Catal. 2021, 11, 14328–14335. [Google Scholar] [CrossRef] [PubMed]
- Frumkin, A.N. Influence of cation adsorption on the kinetics of electrode processes. Trans. Faraday Soc. 1959, 55, 156–167. [Google Scholar] [CrossRef]
- Huang, B.; Myint, K.H.; Wang, Y.; Zhang, Y.; Rao, R.R.; Sun, J.; Muy, S.; Katayama, Y.; Garcia, J.C.; Fraggedakis, D. Cation-Dependent Interfacial Structures and Kinetics for Outer-Sphere Electron-Transfer Reactions. J. Phys. Chem. C 2021, 125, 4397–4411. [Google Scholar] [CrossRef]
- Marković, N.M.; Schmidt, T.J.; Stamenković, V.; Ross, P.N. Oxygen Reduction Reaction on Pt and Pt Bimetallic Surfaces: A Selective Review. Fuel Cells 2018, 1, 105–116. [Google Scholar] [CrossRef]
- Athanasiou, M.; Hasa, B.; Vakros, J.; Sygellou, L.; Katsaounis, A. Electrochemical promotion of carbon supported Pt, Rh and Pd catalysts for H2 oxidation in aqueous alkaline media. J. Chem. Technol. Biotechnol. 2018, 93, 1542–1548. [Google Scholar] [CrossRef]
- Garlyyev, B.; Xue, S.; Watzele, S.; Scieszka, D.; Bandarenka, A.S. Influence of the Nature of the Alkali Metal Cations on the Electrical Double-Layer Capacitance of Model Pt(111) and Au(111) Electrodes. J. Phys. Chem. Lett. 2018, 9, 1927–1930. [Google Scholar] [CrossRef]
- Tang, B.; Fang, Y.; Zhu, S.; Bai, Q.; Li, X.; Wei, L.; Li, Z.; Zhu, C. Tuning hydrogen bond network connectivity in the electric double layer with cations. Chem. Sci. 2024, 15, 7111–7120. [Google Scholar] [CrossRef]
- Li, P.; Jiang, Y.; Hu, Y.; Men, Y.; Liu, Y.; Cai, W.; Chen, S. Hydrogen bond network connectivity in the electric double layer dominates the kinetic pH effect in hydrogen electrocatalysis on Pt. Nat. Catal. 2022, 5, 900–911. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, T.; Cai, Y.-C.; Meng, Z.-D.; Nan, J.-W.; Ye, J.-Y.; Yi, J.; Zhan, D.-P.; Tian, N.; Zhou, Z.-Y. Mechanism of Cations Suppressing Proton Diffusion Kinetics for Electrocatalysis. Angew. Chem. Int. Ed. Engl. 2023, 62, e202218669. [Google Scholar] [CrossRef]
- Ding, X.; Garlyyev, B.; Watzele, S.A.; Sarpey, T.K.; Bandarenka, A.S. Spotlight on the Effect of Electrolyte Composition on the Potential of Maximum Entropy: Supporting Electrolytes Are Not Always Inert. Chem.—A Eur. J. 2021, 27, 10016–10020. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. ‘Water-in-salt’ electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 2015, 350, 938–943. [Google Scholar] [CrossRef]
- Guha, A.; Narayanaru, S.; Narayanan, T.N. Tuning the Hydrogen Evolution Reaction on Metals by Lithium Salt. ACS Appl. Energy Mater. 2018, 1, 7116–7122. [Google Scholar] [CrossRef]
- Lamy-Pitara, E.; El Mouahid, S.; Barbier, J. Effect of anions on catalytic and electrocatalytic hydrogenations and on the electrocatalytic oxidation and evolution of hydrogen on platinum. Electrochim. Acta 2000, 45, 4299–4308. [Google Scholar] [CrossRef]
- Guha, A.; Narayanan, T.N. Effect of ‘water-in-salt’ electrolytes in the electrochemical hydrogen evolution reaction of carbon nanotubes. J. Phys. Energy 2020, 2, 034001. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef] [PubMed]
- Zeradjanin, A.R.; Topalov, A.A.; Cherevko, S.; Keeley, G.P. Sustainable generation of hydrogen using chemicals with regional oversupply—Feasibility of the electrolysis in acido-alkaline reactor. Int. J. Hydrogen Energy 2014, 39, 16275–16281. [Google Scholar] [CrossRef]
- Xu, X.; Sun, H.; Jiang, S.P.; Shao, Z. Modulating metal–organic frameworks for catalyzing acidic oxygen evolution for proton exchange membrane water electrolysis. SusMat 2021, 1, 460–481. [Google Scholar] [CrossRef]
- Li, Z.; Wu, X.; Jiang, X.; Shen, B.; Teng, Z.; Sun, D.; Fu, G.; Tang, Y. Surface carbon layer controllable Ni3Fe particles confined in hierarchical N-doped carbon framework boosting oxygen evolution reaction. Adv. Powder Mater. 2022, 1, 100020. [Google Scholar] [CrossRef]
- Marini, S.; Nelli, P.; Pesenti, R.; Villa, M.; Berrettoni, M.; Zangari, G.; Kiros, Y. Advanced alkaline water electrolysis. Electrochim. Acta 2012, 82, 384–391. [Google Scholar] [CrossRef]
- Hong, W.T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef]
- Liao, Y. Research Progress on Electrocatalysts for Electrocatalytic Carbon Dioxide Reduction. E3S Web Conf. 2024, 553, 01005. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef]
- Wang, Y.J.; Fang, B.; Zhang, D.; Li, A.; Wilkinson, D.P.; Ignaszak, A.; Zhang, L.; Zhang, J. A Review of Carbon-Composited Materials as Air-Electrode Bifunctional Electrocatalysts for Metal–Air Batteries. Electrochem. Energy Rev. 2018, 1, 1–34. [Google Scholar] [CrossRef]
- Banti, A.; Zafeiridou, C.; Charalampakis, M.; Spyridou, O.-N.; Georgieva, J.; Binas, V.; Mitrousi, E.; Sotiropoulos, S. IrO2 Oxygen Evolution Catalysts Prepared by an Optimized Photodeposition Process on TiO2 Substrates. Molecules 2024, 29, 2392. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Y.; Zhang, K.; Xie, Z.; Sun, K.; An, W.; Liang, X.; Zou, X. Advances and status of anode catalysts for proton exchange membrane water electrolysis technology. Mater. Chem. Front. 2023, 7, 1025–1045. [Google Scholar] [CrossRef]
- Zhang, L.; Jang, H.; Liu, H.; Kim, M.G.; Yang, D.; Liu, S.; Liu, X.; Cho, J. Sodium-Decorated Amorphous/Crystalline RuO2 with Rich Oxygen Vacancies: A Robust pH-Universal Oxygen Evolution Electrocatalyst. Angew. Chem. Int. Ed. 2021, 60, 18821–18829. [Google Scholar] [CrossRef]
- Reier, T.; Nong, H.N.; Teschner, D.; Schlögl, R.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction in Acidic Environments—Reaction Mechanisms and Catalysts. Adv. Energy Mater. 2017, 7, 1601275. [Google Scholar] [CrossRef]
- Chen, Y.; Shang, C.; Xiao, X.; Guo, W.; Xu, Q. Recent progress of electrocatalysts for acidic oxygen evolution reaction. Coord. Chem. Rev. 2024, 508, 215758. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, Q.; Zhou, J.; Wang, X.; Huang, M.; Jiang, H.; Cölfen, H. Toward Understanding the Formation Mechanism and OER Catalytic Mechanism of Hydroxides by In Situ and Operando Techniques. Angew. Chem. Int. Ed. 2023, 62, e202309293. [Google Scholar] [CrossRef]
- Bajdich, M.; García-Mota, M.; Vojvodic, A.; Nørskov, J.K.; Bell, A.T. Theoretical investigation of the activity of cobalt oxides for the electrochemical oxidation of water. J. Am. Chem. Soc. 2013, 135, 13521–13530. [Google Scholar] [CrossRef] [PubMed]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
- Lee, S.; Shin, Y.; Yeom, K.; Shim, J.; Sung, Y.-E. An overview of design strategies and recent advancements in complex 3d transition metal-based electrocatalysts for alkaline oxygen evolution reaction. Adv. Ind. Eng. Chem. 2025, 1, 2. [Google Scholar] [CrossRef]
- Dau, H.; Limberg, C.; Reier, T.; Risch, M.; Roggan, S.; Strasser, P. The Mechanism of Water Oxidation: From Electrolysis via Homogeneous to Biological Catalysis. ChemCatChem 2010, 2, 724–761. [Google Scholar] [CrossRef]
- Man, I.C.; Su, H.-Y.; Calle-Vallejo, F.; Hansen, H.A.; Martínez, J.I.; Inoglu, N.G.; Kitchin, J.; Jaramillo, T.F.; Nørskov, J.K.; Rossmeisl, J. Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces. ChemCatChem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]
- Trześniewski, B.J.; Diaz-Morales, O.; Vermaas, D.A.; Longo, A.; Bras, W.; Koper, M.T.M.; Smith, W.A. In Situ Observation of Active Oxygen Species in Fe-Containing Ni-Based Oxygen Evolution Catalysts: The Effect of pH on Electrochemical Activity. J. Am. Chem. Soc. 2015, 137, 15112–15121. [Google Scholar] [CrossRef]
- Koper, M.T.M. Theory of multiple proton–electron transfer reactions and its implications for electrocatalysis. Chem. Sci. 2013, 4, 2710–2723. [Google Scholar] [CrossRef]
- Giordano, L.; Han, B.; Risch, M.; Hong, W.T.; Rao, R.R.; Stoerzinger, K.A.; Shao-Horn, Y. pH dependence of OER activity of oxides: Current and future perspectives. Catal. Today 2016, 262, 2–10. [Google Scholar] [CrossRef]
- Yoo, J.S.; Rong, X.; Liu, Y.; Kolpak, A.M. Role of Lattice Oxygen Participation in Understanding Trends in the Oxygen Evolution Reaction on Perovskites. ACS Catal. 2018, 8, 4628–4636. [Google Scholar] [CrossRef]
- Huang, Z.F.; Song, J.; Du, Y.; Xi, S.; Dou, S.; Nsanzimana, J.M.V.; Wang, C.; Xu, Z.J.; Wang, X. Chemical and structural origin of lattice oxygen oxidation in Co–Zn oxyhydroxide oxygen evolution electrocatalysts. Nat. Energy 2019, 4, 329–338. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, X.; Zhong, Y.; Ge, L.; Chen, Y.; Veder, J.-P.M.; Guan, D.; O’Hayre, R.; Li, M.; Wang, G.; et al. Direct evidence of boosted oxygen evolution over perovskite by enhanced lattice oxygen participation. Nat. Commun. 2020, 11, 2002. [Google Scholar] [CrossRef]
- Grimaud, A.; Diaz-Morales, O.; Han, B.; Hong, W.T.; Lee, Y.-L.; Giordano, L.; Stoerzinger, K.A.; Koper, M.T.M.; Shao-Horn, Y. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 2017, 9, 457–465. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, J.; Gong, Z.; Lei, H.; Zhou, D.; Zhang, N.; Mai, W.; Zhao, S.; Chen, Y. Activating lattice oxygen in NiFe-based (oxy)hydroxide for water electrolysis. Nat. Commun. 2022, 13, 2191. [Google Scholar] [CrossRef]
- Li, L.; Wang, P.; Shao, Q.; Huang, X. Recent Progress in Advanced Electrocatalyst Design for Acidic Oxygen Evolution Reaction. Adv. Mater. 2021, 33, 2004243. [Google Scholar] [CrossRef]
- Xie, Y.; Luo, F.; Yang, Z. Acidic oxygen evolution reaction via lattice oxygen oxidation mechanism: Progress and challenges. Energy Mater. 2025, 5, 500026. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, W.; Fu, H.C.; Li, X.L.; Lei, J.L.; Luo, H.Q.; Li, N.B. Unraveling the Mechanism of Self-Repair of NiFe-Based Electrocatalysts by Dynamic Exchange of Iron during the Oxygen Evolution Reaction. ACS Catal. 2023, 13, 14975–14986. [Google Scholar] [CrossRef]
- Feng, Z.; Dai, C.; Shi, P.; Lei, X.; Guo, R.; Wang, B.; Liu, X.; You, J. Seven mechanisms of oxygen evolution reaction proposed recently: A mini review. Chem. Eng. J. 2024, 485, 149992. [Google Scholar] [CrossRef]
- Erdey-Grúz, T.; Shafarik, A. Soviet Electrochemistry: Kinetics and Polarography; Proceedings of the Fourth Conference on Electrochemistry; Consultant Bureau: New York, NY, USA, 1961; Volume 1, Available online: https://www.abebooks.com/Soviet-Electrochemistry-Proceedings-Fourth-Conference-Volume/31149258139/bd (accessed on 15 April 2025).
- Kozawa, A. Effects of anions and cations on oxygen reduction and oxygen evolution reactions on platinum electrodes. J. Electroanal. Chem. 1964, 8, 20–39. [Google Scholar] [CrossRef]
- Cha, S.; Cao, X.; Du, W.; Jin, H.; Liu, Y.; Wang, R.; Yang, Y.; Sun, B.; Yang, X.; Gong, M. The ion effect on electrocatalytic oxidation reactions. J. Mater. Chem. A Mater. 2024, 12, 32548–32565. [Google Scholar] [CrossRef]
- Michael, J.D.; Demeter, E.L.; Illes, S.M.; Fan, Q.; Boes, J.R.; Kitchin, J.R. Alkaline Electrolyte and Fe Impurity Effects on the Performance and Active-Phase Structure of NiOOH Thin Films for OER Catalysis Applications. J. Phys. Chem. C 2015, 119, 11475–11481. [Google Scholar] [CrossRef]
- van der Heijden, O.; Eggebeen, J.J.J.; Trzesniowski, H.; Deka, N.; Golnak, R.; Xiao, J.; van Rijn, M.; Mom, R.V.; Koper, M.T.M. Li+ Cations Activate NiFeOOH for Oxygen Evolution in Sodium and Potassium Hydroxide. Angew. Chem. Int. Ed. 2024, 63, e202318692. [Google Scholar] [CrossRef] [PubMed]
- Zaffran, J.; Stevens, M.B.; Trang, C.D.M.; Nagli, M.; Shehadeh, M.; Boettcher, S.W.; Toroker, M.C. Influence of Electrolyte Cations on Ni(Fe)OOH Catalyzed Oxygen Evolution Reaction. Chem. Mater. 2017, 29, 4761–4767. [Google Scholar] [CrossRef]
- Rao, R.R.; Huang, B.; Katayama, Y.; Hwang, J.; Kawaguchi, T.; Lunger, J.R.; Peng, J.; Zhang, Y.; Morinaga, A.; Zhou, H.; et al. PH-and Cation-Dependent Water Oxidation on Rutile RuO2(110). J. Phys. Chem. C 2021, 125, 8195–8207. [Google Scholar] [CrossRef]
- Bockris, J.O.; Devanathan, M.A.V.; Muller, K. On the structure of charged interfaces. Proc. R. Soc. Lond. A Math. Phys. Sci. 1963, 274, 55–79. [Google Scholar] [CrossRef]
- Conway, B.E.; Bockris, J.O.M.; Ammar, I.A. The dielectric constant of the solution in the diffuse and Helmholtz double layers at a charged interface in aqueous solution. Trans. Faraday Soc. 1951, 47, 756–766. [Google Scholar] [CrossRef]
- Huang, J.; Li, M.; Eslamibidgoli, M.J.; Eikerling, M.; Groß, A. Cation Overcrowding Effect on the Oxygen Evolution Reaction. JACS Au 2021, 1, 1752–1765. [Google Scholar] [CrossRef]
- Marcus, Y. Effect of ions on the structure of water: Structure making and breaking. Chem. Rev. 2009, 109, 1346–1370. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.C.; Touzalin, T.; Nieuwland, C.; Perini, N.; Koper, M.T.M. Enhancement of Oxygen Evolution Activity of Nickel Oxyhydroxide by Electrolyte Alkali Cations. Angew. Chem. Int. Ed. 2019, 58, 12999–13003. [Google Scholar] [CrossRef]
- Levell, Z.; Le, J.; Yu, S.; Wang, R.; Ethirajan, S.; Rana, R.; Kulkarni, A.; Resasco, J.; Lu, D.; Cheng, J.; et al. Emerging Atomistic Modeling Methods for Heterogeneous Electrocatalysis. Chem. Rev. 2024, 124, 8620–8656. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Fukushima, T.; Minamimoto, H.; Lyalin, A.; Murakoshi, K.; Taketsugu, T. Enhancing the oxygen evolution reaction by tuning the electrode–electrolyte interface in nickel-based electrocatalysts. Commun. Chem. 2025, 8, 109. [Google Scholar] [CrossRef]
- Li, G.F.; Divinagracia, M.; Labata, M.F.; Ocon, J.D.; Chuang, P.Y.A. Electrolyte-Dependent Oxygen Evolution Reactions in Alkaline Media: Electrical Double Layer and Interfacial Interactions. ACS Appl. Mater. Interfaces 2019, 11, 33748–33758. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Lu, S.; Wang, F.; Zhang, Y.; Hojamberdiev, M.; Chai, Y.; Dong, B.; Zhang, B. Enhancing catalytic durability in alkaline oxygen evolution reaction through squaric acid anion intercalation. Nat. Commun. 2025, 16, 3407. [Google Scholar] [CrossRef]
- Rong, C.; Dastafkan, K.; Wang, Y.; Zhao, C. Breaking the Activity and Stability Bottlenecks of Electrocatalysts for Oxygen Evolution Reactions in Acids. Adv. Mater. 2023, 35, 2211884. [Google Scholar] [CrossRef]
- Cherevko, S.; Geiger, S.; Kasian, O.; Kulyk, N.; Grote, J.-P.; Savan, A.; Shrestha, B.R.; Merzlikin, S.; Breitbach, B.; Ludwig, A.; et al. Oxygen and hydrogen evolution reactions on Ru, RuO2, Ir, and IrO2 thin film electrodes in acidic and alkaline electrolytes: A comparative study on activity and stability. Catal. Today 2016, 262, 170–180. [Google Scholar] [CrossRef]
- Rao, R.R.; Kolb, M.J.; Halck, N.B.; Pedersen, A.F.; Mehta, A.; You, H.; Stoerzinger, K.A.; Feng, Z.; Hansen, H.A.; Zhou, H.; et al. Towards identifying the active sites on RuO2(110) in catalyzing oxygen evolution. Energy Environ. Sci. 2017, 10, 2626–2637. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Sato, E. Electrocatalytic properties of transition metal oxides for oxygen evolution reaction. Mater. Chem. Phys. 1986, 14, 397–426. [Google Scholar] [CrossRef]
- Stoerzinger, K.A.; Qiao, L.; Biegalski, M.D.; Shao-Horn, Y. Orientation-dependent oxygen evolution activities of rutile IrO2 and RuO2. J. Phys. Chem. Lett. 2014, 5, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.; Rao, R.R.; Stoerzinger, K.A.; Hwang, J.; Rossmeisl, J.; Chorkendorff, I.; Shao-Horn, Y.; Stephens, I.E.L. Trends in Activity and Dissolution on RuO2 under Oxygen Evolution Conditions: Particles versus Well-Defined Extended Surfaces. ACS Energy Lett. 2018, 3, 2045–2051. [Google Scholar] [CrossRef]
- Cherevko, S.; Zeradjanin, A.R.; Topalov, A.A.; Kulyk, N.; Katsounaros, I.; Mayrhofer, K.J.J. Dissolution of Noble Metals during Oxygen Evolution in Acidic Media. ChemCatChem 2014, 6, 2219–2223. [Google Scholar] [CrossRef]
- Reier, T.; Pawolek, Z.; Cherevko, S.; Bruns, M.; Jones, T.; Teschner, D.; Selve, S.; Bergmann, A.; Nong, H.N.; Schlögl, R.; et al. Molecular insight in structure and activity of highly efficient, low-Ir Ir-Ni oxide catalysts for electrochemical water splitting (OER). J. Am. Chem. Soc. 2015, 137, 13031–13040. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wang, H.; Zhang, L.; Wilkinson, D.P.; Zhang, J. Recent Progresses in Oxygen Reduction Reaction Electrocatalysts for Electrochemical Energy Applications. Electrochem. Energy Rev. 2019, 2, 518–538. [Google Scholar] [CrossRef]
- Liu, Y.; Ruan, J.; Sang, S.; Zhou, Z.; Wu, Q. Iron and nitrogen co-doped carbon derived from soybeans as efficient electro-catalysts for the oxygen reduction reaction. Electrochim. Acta 2016, 215, 388–397. [Google Scholar] [CrossRef]
- Strbac, S. The effect of pH on oxygen and hydrogen peroxide reduction on polycrystalline Pt electrode. Electrochim. Acta 2011, 56, 1597–1604. [Google Scholar] [CrossRef]
- Golubović, J.; Varničić, M.; Štrbac, S. Study of Oxygen Reduction Reaction on Polycrystalline Rhodium in Acidic and Alkaline Media. Catalysts 2024, 14, 327. [Google Scholar] [CrossRef]
- Zhao, Y.; Saseendran, D.P.A.; Huang, C.; Triana, C.A.; Marks, W.R.; Chen, H.; Zhao, H.; Patzke, G.R. Oxygen Evolution/Reduction Reaction Catalysts: From in Situ Monitoring and Reaction Mechanisms to Rational Design. Chem. Rev. 2023, 123, 6257–6358. [Google Scholar] [CrossRef]
- Li, S.; Shi, L.; Guo, Y.; Wang, J.; Liu, D.; Zhao, S. Selective oxygen reduction reaction: Mechanism understanding, catalyst design and practical application. Chem. Sci. 2024, 15, 11188–11228. [Google Scholar] [CrossRef] [PubMed]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Li, H.; Kelly, S.; Guevarra, D.; Wang, Z.; Wang, Y.; Haber, J.A.; Anand, M.; Gunasooriya, G.T.K.K.; Abraham, C.S.; Vijay, S.; et al. Analysis of the limitations in the oxygen reduction activity of transition metal oxide surfaces. Nat. Catal. 2021, 4, 463–468. [Google Scholar] [CrossRef]
- Huang, H.; Sun, M.; Li, S.; Zhang, S.; Lee, Y.; Li, Z.; Fang, J.; Chen, C.; Zhang, Y.-X.; Wu, Y.; et al. Enhancing H2O2 Electrosynthesis at Industrial-Relevant Current in Acidic Media on Diatomic Cobalt Sites. J. Am. Chem. Soc. 2024, 146, 9434–9443. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, W.; Shang, H.; Chen, W.; Sun, T.; Li, H.; Dong, J.; Zhou, J.; Li, Z.; Wang, Y.; et al. Atomic interface effect of a single atom copper catalyst for enhanced oxygen reduction reactions. Energy Environ. Sci. 2019, 12, 3508–3514. [Google Scholar] [CrossRef]
- Diesen, E.; Dudzinski, A.M.; Reuter, K.; Bukas, V.J. Origin of Electrocatalytic Selectivity during the Oxygen Reduction Reaction on Au(111). ACS Catal. 2025, 15, 5403–5411. [Google Scholar] [CrossRef]
- Chen, J.W.; Zhang, Z.; Yan, H.M.; Xia, G.J.; Cao, H.; Wang, Y.G. Pseudo-adsorption and long-range redox coupling during oxygen reduction reaction on single atom electrocatalyst. Nat. Commun. 2022, 13, 1734. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, X.; Zhu, C.; Liu, Y.; Tan, H.; Ku, R.; Zhang, Y.; Zhou, L.; Liu, Z.; Hwang, S.-J.; et al. Atomically Dispersed Co2-N6 and Fe-N4 Costructures Boost Oxygen Reduction Reaction in Both Alkaline and Acidic Media. Adv. Mater. 2021, 33, 2104718. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhao, X.; Xia, C.; Wu, Z.-Y.; Zhu, P.; Kim, J.Y.; Bai, X.; Gao, G.; Hu, Y.; Zhong, J.; et al. Highly active and selective oxygen reduction to H2O2 on boron-doped carbon for high production rates. Nat. Commun. 2021, 12, 4225. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Ge, B.; Zheng, F.; Zhang, N.; Zuo, M.; Yang, Y.; Chen, Q. Constructing Graphitic-Nitrogen-Bonded Pentagons in Interlayer-Expanded Graphene Matrix toward Carbon-Based Electrocatalysts for Acidic Oxygen Reduction Reaction. Adv. Mater. 2021, 33, 2103133. [Google Scholar] [CrossRef]
- Yang, S.; Verdaguer-Casadevall, A.; Arnarson, L.; Silvioli, L.; Čolić, V.; Frydendal, R.; Rossmeisl, J.; Chorkendorff, I.; Stephens, I.E.L. Toward the Decentralized Electrochemical Production of H2O2: A Focus on the Catalysis. ACS Catal. 2018, 8, 4064–4081. [Google Scholar] [CrossRef]
- Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E.A.; Frydendal, R.; Hansen, T.W.; et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 2013, 12, 1137–1143. [Google Scholar] [CrossRef]
- Luo, M.; Koper, M.T.M. A kinetic descriptor for the electrolyte effect on the oxygen reduction kinetics on Pt(111). Nature Catalysis 2022, 5, 615–623. [Google Scholar] [CrossRef]
- Srejic, I.; Rakocevic, Z.; Nenadovic, M.; Strbac, S. Oxygen reduction on polycrystalline palladium in acid and alkaline solutions: Topographical and chemical Pd surface changes. Electrochim. Acta 2015, 169, 22–31. [Google Scholar] [CrossRef]
- Štrbac, S.; Adžić, R.R. The influence of OH− chemisorption on the catalytic properties of gold single crystal surfaces for oxygen reduction in alkaline solutions. J. Electroanal. Chem. 1996, 403, 169–181. [Google Scholar] [CrossRef]
- Prieto, A.; Hernández, J.; Herrero, E.; Feliu, J.M. The role of anions in oxygen reduction in neutral and basic media on gold single-crystal electrodes. J. Solid. State Electrochem. 2003, 7, 599–606. [Google Scholar] [CrossRef]
- Zhu, S.; Hu, X.; Shao, M. Impacts of anions on the oxygen reduction reaction kinetics on platinum and palladium surfaces in alkaline solutions. Phys. Chem. Chem. Phys. 2017, 19, 7631–7641. [Google Scholar] [CrossRef] [PubMed]
- Tripkovic, D.V.; Strmcnik, D.; Van Der Vliet, D.; Stamenkovic, V.; Markovic, N.M. The role of anions in surface electrochemistry. Faraday Discuss. 2008, 140, 25–40. [Google Scholar] [CrossRef]
- Rademaker, D.; Tanase, S.; Hetterscheid, D.G.H. Effect of the Electrolyte on the Oxygen Reduction Reaction with a MOF Embedded Co-Porphyrin. ChemSusChem 2013, 18, e202402295. [Google Scholar] [CrossRef]
- Suntivich, J.; Perry, E.E.; Gasteiger, H.A.; Shao-Horn, Y. The Influence of the Cation on the Oxygen Reduction and Evolution Activities of Oxide Surfaces in Alkaline Electrolyte. Electrocatalysis 2012, 4, 49–55. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Huang, B.; Cai, B.; Rao, R.R.; Giordano, L.; Sun, S.-G.; Shao-Horn, Y. Enhancing oxygen reduction electrocatalysis by tuning interfacial hydrogen bonds. Nat. Catal. 2021, 4, 753–762. [Google Scholar] [CrossRef]
- Wang, Y.R.; Liu, M.; Gao, G.-K.; Yang, Y.-L.; Yang, R.-X.; Ding, H.-M.; Chen, Y.; Li, S.-L.; Lan, Y.-Q. Implanting Numerous Hydrogen-Bonding Networks in a Cu-Porphyrin-Based Nanosheet to Boost CH4 Selectivity in Neutral-Media CO2 Electroreduction. Angew. Chem. Int. Ed. Engl. 2021, 60, 21952–21958. [Google Scholar] [CrossRef]
- Khani, H.; Santiago, A.R.P.; He, T. An Interfacial View of Cation Effects on Electrocatalysis Systems. Angew. Chem. Int. Ed. Engl. 2023, 62, e202306103. [Google Scholar] [CrossRef]
- Ren, Q.; Xu, L.; Lv, M.; Zhang, Z.; Li, Z.; Shao, M.; Duan, X. Cation effects in electrocatalytic reduction reactions: Recent advances. Chin. J. Catal. 2024, 63, 16–32. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Zhu, P.; Adler, Z.; Wu, Z.-Y.; Liu, Y.; Wang, H. Electrochemical oxygen reduction to hydrogen peroxide at practical rates in strong acidic media. Nat. Commun. 2022, 13, 2880. [Google Scholar] [CrossRef]
- Hübner, J.L.; Lucchetti, L.E.B.; Nong, H.N.; Sharapa, D.I.; Paul, B.; Kroschel, M.; Kang, J.; Teschner, D.; Behrens, S.; Studt, F.; et al. Cation Effects on the Acidic Oxygen Reduction Reaction at Carbon Surfaces. ACS Energy Lett. 2024, 9, 1331–1338. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Strmcnik, D.; van der Vliet, D.F.; Chang, K.-C.; Komanicky, V.; Kodama, K.; You, H.; Stamenkovic, V.R.; Marković, N.M. Effects of Li+, K+, and Ba2+ Cations on the ORR at Model and High Surface Area Pt and Au Surfaces in Alkaline Solutions. J. Phys. Chem. Lett. 2011, 2, 2733–2736. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Mukerjee, S. Influence of inner- and outer-sphere electron transfer mechanisms during electrocatalysis of oxygen reduction in alkaline media. J. Phys. Chem. C 2011, 115, 18015–18026. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Stamenkovic, V.; Ross, P.N.; Markovic, N.M. Temperature dependent surface electrochemistry on Pt single crystals in alkaline electrolyte. Phys. Chem. Chem. Phys. 2003, 5, 400–406. [Google Scholar] [CrossRef]
- Marković, N.M.; Gasteiger, H.A.; Ross, P.N. Oxygen Reduction on Platinum Low-Index Single-Crystal Surfaces in Alkaline Solution: Rotating Ring DiskPt(hkl) Studies. J. Phys. Chem. 1996, 100, 6715–6721. [Google Scholar] [CrossRef]
- Kristoffersen, H.H.; Chan, K.; Vegge, T.; Hansen, H.A. Energy–entropy competition in cation–hydroxyl interactions at the liquid water–Pt(111) interface. Chem. Commun. 2020, 56, 427–430. [Google Scholar] [CrossRef]
- Kumeda, T.; Tajiri, H.; Sakata, O.; Hoshi, N.; Nakamura, M. Effect of hydrophobic cations on the oxygen reduction reaction on single–crystal platinum electrodes. Nat. Commun. 2018, 9, 4378. [Google Scholar] [CrossRef]
- Rizo, R.; Herrero, E.; Feliu, J.M. Oxygen reduction reaction on stepped platinum surfaces in alkaline media. Phys. Chem. Chem. Phys. 2013, 15, 15416–15425. [Google Scholar] [CrossRef]
- Garlyyev, B.; Xue, S.; Pohl, M.D.; Reinisch, D.; Bandarenka, A.S. Oxygen Electroreduction at High-Index Pt Electrodes in Alkaline Electrolytes: A Decisive Role of the Alkali Metal Cations. ACS Omega 2018, 3, 15325–15331. [Google Scholar] [CrossRef] [PubMed]
- Kumeda, T.; Laverdure, L.; Honkala, K.; Melander, M.M.; Sakaushi, K. Cations Determine the Mechanism and Selectivity of Alkaline Oxygen Reduction Reaction on Pt(111). Angew. Chem. Int. Ed. Engl. 2023, 62, e202312841. [Google Scholar] [CrossRef]
- Lee, C.H.; Kanan, M.W. Controlling H+ vs CO2 Reduction Selectivity on Pb Electrodes. ACS Catal. 2015, 5, 465–469. [Google Scholar] [CrossRef]
- Gupta, N.; Gattrell, M.; MacDougall, B. Calculation for the cathode surface concentrations in the electrochemical reduction of CO2 in KHCO3 solutions. J. Appl. Electrochem. 2006, 36, 161–172. [Google Scholar] [CrossRef]
- Peterson, A.A.; Abild-Pedersen, F.; Studt, F.; Rossmeisl, J.; Nørskov, J.K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 2010, 3, 1311–1315. [Google Scholar] [CrossRef]
- Tu, C.; Tripp, B.C.; Ferry, J.G.; Silverman, D.N. Bicarbonate as a Proton Donor in Catalysis by Zn(II)- and Co(II)-Containing Carbonic Anhydrases. J. Am. Chem. Soc. 2001, 123, 5861–5866. [Google Scholar] [CrossRef] [PubMed]
- Ooka, H.; Figueiredo, M.C.; Koper, M.T.M. Competition between Hydrogen Evolution and Carbon Dioxide Reduction on Copper Electrodes in Mildly Acidic Media. Langmuir 2017, 33, 9307–9313. [Google Scholar] [CrossRef]
- Cave, E.R.; Montoy, J.H.; Kuhl, K.P.; Abram, D.N.; Hatsukade, T.; Shi, C.; Hahn, C.; Nørskov, J.K.; Jaramillo, T.F. Electrochemical CO2 reduction on Au surfaces: Mechanistic aspects regarding the formation of major and minor products. Phys. Chem. Chem. Phys. 2017, 19, 15856–15863. [Google Scholar] [CrossRef]
- Clark, E.L.; Ringe, S.; Tang, M.; Walton, A.; Hahn, C.; Jaramillo, T.F.; Chan, K.; Bell, A.T. Influence of Atomic Surface Structure on the Activity of Ag for the Electrochemical Reduction of CO2 to CO. ACS Catal. 2019, 9, 4006–4014. [Google Scholar] [CrossRef]
- Moreno-Garciá, P.; Kovács, N.; Grozovski, V.; Gálvez-Vázquez, M.D.J.; Vesztergom, S.; Broekmann, P. Toward CO2 Electroreduction under Controlled Mass Flow Conditions: A Combined Inverted RDE and Gas Chromatography Approach. Anal. Chem. 2020, 92, 4301–4308. [Google Scholar] [CrossRef]
- Newman, J.S.; Delacourt, C.; Ridgway, P.; Kerr, J.; Newman, J.S. Design of an electrochemical cell making syngas (CO + H2) from CO2 and H2O reduction at room temperature. J. Electrochem. Soc. 2008, 155, B42. [Google Scholar] [CrossRef]
- Bondue, C.J.; Graf, M.; Goyal, A.; Koper, M.T.M. Suppression of Hydrogen Evolution in Acidic Electrolytes by Electrochemical CO2 Reduction. J. Am. Chem. Soc. 2021, 143, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Claus, S. The Electrolyte Effects on Carbon Dioxide Electro-catalysis. Org. Chem. Curr. Res. 2022, 11, 264. [Google Scholar]
- Jouny, M.; Luc, W.; Jiao, F. General Techno-Economic Analysis of CO2 Electrolysis Systems. Ind. Eng. Chem. Res. 2018, 57, 2165–2177. [Google Scholar] [CrossRef]
- Kortlever, R.; Shen, J.; Schouten, K.J.P.; Calle-Vallejo, F.; Koper, M.T.M. Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, X.; Stucky, G.D.; Boettcher, S.W. Thermodynamic, Kinetic, and Transport Contributions to Hydrogen Evolution Activity and Electrolyte-Stability Windows for Water-in-Salt Electrolytes. J. Am. Chem. Soc. 2024, 146, 3438–3448. [Google Scholar] [CrossRef]
- Lin, S.; Fishler, Y.; Kwon, S.; Böhme, A.E.; Nie, W.; Richter, M.H.; Yang, M.Y.; Matthews, J.E.; Iton, Z.W.B.; Lee, B.C.; et al. Cooperative effects associated with high electrolyte concentrations in driving the conversion of CO2 to C2H4 on copper. Chem. Catal. 2025, 5, 101338. [Google Scholar] [CrossRef]
- Khalil, M.; Kadja, G.T.M.; Nugroho, F.A.A.; Sutanto, L.G.; Jiwanti, P.K.; Abdi, F.F.; Hussin, F.; Aroua, M.K. Suppressing the competing hydrogen evolution reaction in CO2 electroreduction: A review. Renew. Sustain. Energy Rev. 2024, 206, 114869. [Google Scholar] [CrossRef]
- Marcandalli, G.; Monteiro, M.C.O.; Goyal, A.; Koper, M.T.M. Electrolyte Effects on CO2 Electrochemical Reduction to CO. Acc. Chem. Res. 2022, 55, 1900–1911. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Murata, A.; Takahashi, R. Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1989, 85, 2309–2326. [Google Scholar] [CrossRef]
- Varela, A.S.; Kroschel, M.; Reier, T.; Strasser, P. Controlling the selectivity of CO2 electroreduction on copper: The effect of the electrolyte concentration and the importance of the local pH. Catal. Today 2016, 260, 8–13. [Google Scholar] [CrossRef]
- Monteiro, M.C.O.; Mirabal, A.; Jacobse, L.; Doblhoff-Dier, K.; Barton, S.C.; Koper, M.T.M. Time-Resolved Local pH Measurements during CO2 Reduction Using Scanning Electrochemical Microscopy: Buffering and Tip Effects. JACS Au 2021, 1, 1915–1924. [Google Scholar] [CrossRef]
- Ruiz-López, E.; Gandara-Loe, J.; Baena-Moreno, F.; Reina, T.R.; Odriozola, J.A. Electrocatalytic CO2 conversion to C2 products: Catalysts design, market perspectives and techno-economic aspects. Renew. Sustain. Energy Rev. 2022, 161, 112329. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Liu, Y.; Li, W.; Wen, C.F.; Fu, H.Q.; Yuan, H.Y.; Liu, P.F.; Yang, H.G. A focus on the electrolyte: Realizing CO2 electroreduction from aqueous solution to pure water. Chem. Catal. 2023, 3, 100471. [Google Scholar] [CrossRef]
- Raciti, D.; Mao, M.; Park, J.H.; Wang, C. Local pH Effect in the CO2 Reduction Reaction on High-Surface-Area Copper Electrocatalysts. J. Electrochem. Soc. 2018, 165, F799–F804. [Google Scholar] [CrossRef]
- Hori, Y.; Kikuchi, K.; Suzuki, S. Production of CO and CH4 in electrochemical reduction of CO2 at metal electrodes in aqueous hydrogencarbonate solution. Chem. Lett. 1985, 14, 1695–1698. [Google Scholar] [CrossRef]
- Hashiba, H.; Weng, L.-C.; Chen, Y.; Sato, H.K.; Yotsuhashi, S.; Xiang, C.; Weber, A.Z. Effects of electrolyte buffer capacity on surface reactant species and the reaction rate of CO2 in Electrochemical CO2 reduction. J. Phys. Chem. C 2018, 122, 3719–3726. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, K.; Zheng, S.; Cheng, X.; He, Y.; Qin, W.; Zhang, X.; Chang, H.; Zhong, N.; He, X. Advancements in electrochemical CO2 reduction reaction: A review on CO2 mass transport enhancement strategies. Chem. Eng. J. 2024, 486, 150169. [Google Scholar] [CrossRef]
- Patniboon, T.; Hansen, H.A. Effects of electrolyte anion adsorption on the activity and stability of single atom electrocatalysts. Chem. Phys. Rev. 2023, 4, 011401. [Google Scholar] [CrossRef]
- Qin, X.; Sechi, R.; Hansen, H.A. Recent progress in mechanistic insights into cation effects on electrochemical CO2 reduction reactions. Curr. Opin. Electrochem. 2025, 49, 101614. [Google Scholar] [CrossRef]
- Gu, J.; Liu, S.; Ni, W.; Ren, W.; Haussener, S.; Hu, X. Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium. Nat. Catal. 2022, 5, 268–276. [Google Scholar] [CrossRef]
- Resasco, J.; Chen, L.D.; Clark, E.; Tsai, C.; Hahn, C.; Jaramillo, T.F.; Chan, K.; Bell, A.T. Promoter Effects of Alkali Metal Cations on the Electrochemical Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2017, 139, 11277–11287. [Google Scholar] [CrossRef]
- Chen, L.D.; Urushihara, M.; Chan, K.; Nørskov, J.K. Electric Field Effects in Electrochemical CO2 Reduction. ACS Catal. 2016, 6, 7133–7139. [Google Scholar] [CrossRef]
- Ringe, S.; Clark, E.L.; Resasco, J.; Walton, A.; Seger, B.; Bell, A.T.; Chan, K. Understanding cation effects in electrochemical CO2 reduction. Energy Environ. Sci. 2019, 12, 3001–3014. [Google Scholar] [CrossRef]
- Gao, D.; McCrum, I.T.; Deo, S.; Choi, Y.-W.; Scholten, F.; Wan, W.; Chen, J.G.; Janik, M.J.; Cuenya, B.R. Activity and Selectivity Control in CO2 Electroreduction to Multicarbon Products over CuOx Catalysts via Electrolyte Design. ACS Catal. 2018, 8, 10012–10020. [Google Scholar] [CrossRef]
- Monteiro, M.C.O.; Dattila, F.; Hagedoorn, B.; García-Muelas, R.; López, N.; Koper, M.T.M. Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution. Nat. Catal. 2021, 4, 654–662. [Google Scholar] [CrossRef]
- Monteiro, M.C.O.; Dattila, F.; López, N.; Koper, M.T.M. The Role of Cation Acidity on the Competition between Hydrogen Evolution and CO2Reduction on Gold Electrodes. J. Am. Chem. Soc. 2022, 144, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.-G.; Li, F.-Z.; Du, Y.-F.; Yang, L.-F.; Wang, H.; Bai, Y.-Y.; Lin, M.; Gu, J. Quantitative Understanding of Cation Effects on the Electrochemical Reduction of CO2 and H+ in Acidic Solution. ACS Catal. 2025, 13, 916–926. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, X.; Liu, X.; Tang, Z.; Wang, L.; Tang, Q. Understanding the Role of Potential and Cation Effect on Electrocatalytic CO2 Reduction in All-Alkynyl-Protected Ag15 Nanoclusters. J. Am. Chem. Soc. 2025, 147, 2699–2713. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Wang, T.; Cai, Y.-C.; Li, X.-Y.; Ye, J.-Y.; Zhou, Y.; Tian, N.; Zhou, Z.-Y.; Sun, S.-G. Probing electrolyte effects on cation-enhanced CO2 reduction on copper in acidic media. Nat. Catal. 2024, 7, 807–817. [Google Scholar] [CrossRef]
- Yu, S.; Yamauchi, H.; Wang, S.; Aggarwal, A.; Kim, J.; Gordiz, K.; Huang, B.; Xu, H.; Zheng, D.J.; Wang, X. CO2-to-methanol electroconversion on a molecular cobalt catalyst facilitated by acidic cations. Nat. Catal. 2024, 7, 1000–1009. [Google Scholar] [CrossRef]
- Singh, M.R.; Kwon, Y.; Lum, Y.; Ager, J.W.; Bell, A.T. Hydrolysis of Electrolyte Cations Enhances the Electrochemical Reduction of CO2 over Ag and Cu. J. Am. Chem. Soc. 2016, 138, 13006–13012. [Google Scholar] [CrossRef] [PubMed]
- Kas, R.; Kortlever, R.; Yılmaz, H.; Koper, M.T.M.; Mul, G. Manipulating the Hydrocarbon Selectivity of Copper Nanoparticles in CO2 Electroreduction by Process Conditions. ChemElectroChem 2015, 2, 354–358. [Google Scholar] [CrossRef]
- Shah, A.H.; Gong, Y.; Wang, Y.; Woldu, A.R.; He, T. Understanding the Role of Electrolyte Cations on Activity and Product Selectivity of CO2 Reduction over Cu Electrode. Catalysts 2023, 13, 1092. [Google Scholar] [CrossRef]
- Hoffman, B.M.; Lukoyanov, D.; Yang, Z.Y.; Dean, D.R.; Seefeldt, L.C. Mechanism of nitrogen fixation by nitrogenase: The next stage. Chem. Rev. 2014, 114, 4041–4062. [Google Scholar] [CrossRef]
- Légaré, M.A.; Bélanger-Chabot, G.; Dewhurst, R.D.; Welz, E.; Krummenacher, I.; Engels, B.; Braunschweig, H. Nitrogen fixation and reduction at boron. Science 2018, 359, 896–900. [Google Scholar] [CrossRef]
- Wang, L.; Xia, M.; Wang, H.; Huang, K.; Qian, C.; Maravelias, C.T.; Ozin, G.A. Greening Ammonia toward the Solar Ammonia Refinery. Joule 2018, 2, 1055–1074. [Google Scholar] [CrossRef]
- Jesudass, S.C.; Surendran, S.; Kim, J.Y.; An, T.-Y.; Janani, G.; Kim, T.-H.; Kim, J.K.; Sim, U. Pathways of the Electrochemical Nitrogen Reduction Reaction: From Ammonia Synthesis to Metal-N2 Batteries. Electrochem. Energy Rev. 2023, 6, 1–43. [Google Scholar] [CrossRef]
- Schwalbe, J.A.; Statt, M.J.; Chosy, C.; Singh, A.R.; Rohr, B.A.; Nielander, A.C.; Andersen, S.Z.; McEnaney, J.M.; Baker, J.G.; Jaramillo, T.F.; et al. A Combined Theory-Experiment Analysis of the Surface Species in Lithium-Mediated NH3 Electrosynthesis. ChemElectroChem 2020, 7, 1542–1549. [Google Scholar] [CrossRef]
- Cai, X.; Fu, C.; Iriawan, H.; Yang, F.; Wu, A.; Luo, L.; Shen, S.; Wei, G.; Shao-Horn, Y.; Zhang, J. Lithium-mediated electrochemical nitrogen reduction: Mechanistic insights to enhance performance. iScience 2021, 24, 103105. [Google Scholar] [CrossRef]
- Lazouski, N.; Schiffer, Z.J.; Williams, K.; Manthiram, K. Understanding Continuous Lithium-Mediated Electrochemical Nitrogen Reduction. Joule 2019, 3, 1127–1139. [Google Scholar] [CrossRef]
- Hu, C.; Chen, X.; Jin, J.; Han, Y.; Chen, S.; Ju, H.; Cai, J.; Qiu, Y.; Gao, C.; Wang, C.; et al. Surface Plasmon Enabling Nitrogen Fixation in Pure Water through a Dissociative Mechanism under Mild Conditions. J. Am. Chem. Soc. 2019, 141, 7807–7814. [Google Scholar] [CrossRef] [PubMed]
- Skúlason, E.; Bligaard, T.; Gudmundsdóttir, S.; Studt, F.; Rossmeisl, J.; Abild-Pedersen, F.; Vegge, T.; Jónssonac, H.; Nørskov, J.K. A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction. Phys. Chem. Chem. Phys. 2012, 14, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Abghoui, Y.; Garden, A.L.; Hlynsson, V.F.; Björgvinsdóttir, S.; Ólafsdóttir, H.; Skúlason, E. Enabling electrochemical reduction of nitrogen to ammonia at ambient conditions through rational catalyst design. Phys. Chem. Chem. Phys. 2015, 17, 4909–4918. [Google Scholar] [CrossRef]
- Wan, Y.; Xu, J.; Lv, R. Heterogeneous electrocatalysts design for nitrogen reduction reaction under ambient conditions. Mater. Today 2019, 27, 69–90. [Google Scholar] [CrossRef]
- Suryanto, B.H.R.; Du, H.-L.; Wang, D.; Chen, J.; Simonov, A.N.; MacFarlane, D.R. Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia. Nat. Catal. 2019, 2, 290–296. [Google Scholar] [CrossRef]
- Montoya, J.H.; Tsai, C.; Vojvodic, A.; Nørskov, J.K. The Challenge of Electrochemical Ammonia Synthesis: A New Perspective on the Role of Nitrogen Scaling Relations. ChemSusChem 2015, 8, 2180–2186. [Google Scholar] [CrossRef]
- Singh, A.R.; Rohr, B.A.; Schwalbe, J.A.; Cargnello, M.; Chan, K.; Jaramillo, T.F.; Chorkendorff, I.; Nørskov, J.K. Electrochemical Ammonia Synthesis—The Selectivity Challenge. ACS Catal. 2017, 7, 706–709. [Google Scholar] [CrossRef]
- Guo, Y.; Gu, J.; Zhang, R.; Zhang, S.; Li, Z.; Zhao, Y.; Huang, Z.; Fan, J.; Chen, Z.; Zhi, C. Molecular Crowding Effect in Aqueous Electrolytes to Suppress Hydrogen Reduction Reaction and Enhance Electrochemical Nitrogen Reduction. Adv. Energy Mater. 2021, 11, 2101699. [Google Scholar] [CrossRef]
- Shen, P.; Li, X.; Luo, Y.; Guo, Y.; Zhao, X.; Chu, K. High-Efficiency N2 Electroreduction Enabled by Se-Vacancy-Rich WSe2-x in Water-in-Salt Electrolytes. ACS Nano 2022, 16, 7915–7925. [Google Scholar] [CrossRef]
- Jiang, L.; Bai, X.; Zhi, X.; Davey, K.; Jiao, Y. Advancing electrochemical N2 reduction: Interfacial electrolyte effects and operando computational approaches. EES Catal. 2025, 3, 57–79. [Google Scholar] [CrossRef]
- Waegele, M.M.; Gunathunge, C.M.; Li, J.; Li, X. How cations affect the electric double layer and the rates and selectivity of electrocatalytic processes. J. Chem. Phys. 2019, 151, 160902. [Google Scholar] [CrossRef]
- Shen, P.; Li, X.; Luo, Y.; Zhang, N.; Zhao, X.; Chu, K. Ultra-efficient N2 electroreduction achieved over a rhodium single-atom catalyst (Rh1/MnO2) in water-in-salt electrolyte. Appl. Catal. B 2022, 316, 121651. [Google Scholar] [CrossRef]
- Hu, L.; Xing, Z.; Feng, X. Understanding the Electrocatalytic Interface for Ambient Ammonia Synthesis. ACS Energy Lett. 2020, 5, 430–436. [Google Scholar] [CrossRef]
- Wang, D.; Azofra, L.M.; Harb, M.; Cavallo, L.; Zhang, X.; Suryanto, B.H.R.; MacFarlane, D.R. Energy-Efficient Nitrogen Reduction to Ammonia at Low Overpotential in Aqueous Electrolyte under Ambient Conditions. ChemSusChem 2018, 11, 3416–3422. [Google Scholar] [CrossRef]
- Yang, D.; Chen, T.; Wang, Z. Electrochemical reduction of aqueous nitrogen (N2) at a low overpotential on (110)-oriented Mo nanofilm. J. Mater. Chem. A Mater. 2017, 5, 18967–18971. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Li, Z.; Li, G.; Liu, X. Recent Advances in Electrochemical Synthesis of Ammonia through Nitrogen Reduction under Ambient Conditions. ChemElectroChem 2020, 7, 1067–1079. [Google Scholar] [CrossRef]
- Geng, Z.; Liu, Y.; Kong, X.; Li, P.; Li, K.; Liu, Z.; Du, J.; Shu, M.; Si, R.; Zeng, J. Achieving a Record-High Yield Rate of 120.9 for N2 Electrochemical Reduction over Ru Single-Atom Catalysts. Adv. Mater. 2018, 30, 1803498. [Google Scholar] [CrossRef]
- Huang, L.; Wu, J.; Han, P.; Al-Enizi, A.M.; Almutairi, T.M.; Zhang, L.; Zheng, G. NbO2 Electrocatalyst Toward 32% Faradaic Efficiency for N2 Fixation. Small Methods 2019, 3, 1800386. [Google Scholar] [CrossRef]
- Du, H.L.; Matuszek, K.; Hodgetts, R.Y.; Dinh, K.N.; Cherepanov, P.V.; Bakker, J.M.; MacFarlane, D.R.; Simonov, A.N. The chemistry of proton carriers in high-performance lithium-mediated ammonia electrosynthesis. Energy Environ. Sci. 2023, 16, 1082–1090. [Google Scholar] [CrossRef]
- Tsuneto, A.; Kudo, A.; Sakata, T. Efficient Electrochemical Reduction of N2 to NH3 Catalyzed by Lithium. Chem. Lett. 1993, 22, 851–854. [Google Scholar] [CrossRef]
- Fu, X.; Xu, A.; Pedersen, J.B.; Li, S.; Sažinas, R.; Zhou, Y.; Andersen, S.Z.; Saccoccio, M.; Deissler, N.H.; Mygind, J.B.V. Phenol as proton shuttle and buffer for lithium-mediated ammonia electrosynthesis. Nat. Commun. 2024, 15, 2417. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, D.; Lazouski, N.; Gala, M.L.; Manthiram, K.; Viswanathan, V. Closed-Loop Electrolyte Design for Lithium-Mediated Ammonia Synthesis. ACS Cent. Sci. 2021, 7, 2073–2082. [Google Scholar] [CrossRef]
- Lazouski, N.; Steinberg, K.J.; Gala, M.L.; Krishnamurthy, D.; Viswanathan, V.; Manthiram, K. Proton Donors Induce a Differential Transport Effect for Selectivity toward Ammonia in Lithium-Mediated Nitrogen Reduction. ACS Catal. 2022, 12, 5197–5208. [Google Scholar] [CrossRef]
- Kim, K.; Yoo, C.-Y.; Kim, J.-N.; Yoon, H.C.; Han, J.-I. Electrochemical Synthesis of Ammonia from Water and Nitrogen in Ethylenediamine under Ambient Temperature and Pressure. J. Electrochem. Soc. 2016, 163, F1523–F1526. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Wang, H.; Liu, L.; Hu, W. Electrocatalytic Reduction of Nitrogen to Ammonia in Ionic Liquids. ACS Sustain. Chem. Eng. 2022, 10, 4345–4358. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, L.; Chen, C.; Zheng, Y.; Qiao, S.Z. Catalyst–electrolyte interface engineering propels progress in acidic CO2 electroreduction. Energy Environ. Sci. 2025, 18, 2025–2049. [Google Scholar] [CrossRef]
- Ringe, S. Cation effects on electrocatalytic reduction processes at the example of the hydrogen evolution reaction. Curr. Opin. Electrochem. 2023, 39, 101268. [Google Scholar] [CrossRef]
- Goyal, A.; Louisia, S.; Moerland, P.; Koper, M.T.M. Cooperative Effect of Cations and Catalyst Structure in Tuning Alkaline Hydrogen Evolution on Pt Electrodes. J. Am. Chem. Soc. 2024, 146, 7305–7312. [Google Scholar] [CrossRef] [PubMed]
- Golru, S.S.; May, A.S.; Biddinger, E.J. Modifying Copper Local Environment with Electrolyte Additives to Alter CO2 Electroreduction vs Hydrogen Evolution. ACS Catal. 2023, 13, 7831–7843. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, J.; Raciti, D.; Hall, A.S. Promoting Cu-catalysed CO2 electroreduction to multicarbon products by tuning the activity of H2O. Nat. Catal. 2023, 6, 807–817. [Google Scholar] [CrossRef]
- Schreier, M.; Kenis, P.; Che, F.; Hall, A.S. Trends in Electrocatalysis: The Microenvironment Moves to Center Stage. ACS Energy Lett. 2023, 8, 3935–3940. [Google Scholar] [CrossRef]
- Yoo, J.M.; Ingenmey, J.; Salanne, M.; Lukatskaya, M.R. Anion Effect in Electrochemical CO2 Reduction: From Spectators to Orchestrators. J. Am. Chem. Soc. 2024, 146, 31768–31777. [Google Scholar] [CrossRef]
- Li, P.; Jiao, Y.; Ruan, Y.; Fei, H.; Men, Y.; Guo, C.; Wu, Y.; Chen, S. Revealing the role of double-layer microenvironments in pH-dependent oxygen reduction activity over metal-nitrogen-carbon catalysts. Nat. Commun. 2023, 14, 6936. [Google Scholar] [CrossRef] [PubMed]





| Under Acidic Conditions (pH = 0) | Under Alkaline Conditions (pH = 14) | |
|---|---|---|
| Cathode reaction | 2H+ + 2e− → H2 E° = 0 V | 2H2O + 2e− → H2 + 2OH− E° = 0.83 V |
| Anode reaction | 2H2O → O2 + 4H+ + 4e− E° = 1.23 V | 4OH− → O2 + 2H2O + 4e− E° = 0.40 V |
| Overall reaction | 2H2O → 2H2 + O2 E°cell = 1.23 V | |
| Mechanism | Primary Reaction Stages |
|---|---|
| Dissociative pathway | N2 + 2* → 2*N |
| 2*N + 2e− + 2H+ → 2*NH | |
| 2*NH + 2e− + 2H+ → 2*NH2 | |
| 2*NH2 + 2e− + 2H+ → 2NH3 + 2* | |
| Associative distal pathway | N2 + * → *N2 |
| *N2 + e− + H+ → *NNH | |
| *NNH + e− + H+ → *NNH2 | |
| *NNH2 + e− + H+ → *N + NH3 | |
| *N + e− + H+ → *NH | |
| *NH + e− + H+ → *NH2 | |
| *NH2 + e− + H+ → NH3 + * | |
| Associative alternating pathway | N2 + * → *N2 |
| *N2 + e− + H+ → *NNH | |
| *NNH + e− + H+ → *NHNH | |
| *NHNH + e− + H+ → *NHNH2 | |
| *NHNH2 + e− + H+ → *NH2NH2 | |
| *NH2NH2 + e− + H+ → *NH2 + NH3 | |
| *NH2 + e− + H+ → NH3 + * |
| Reaction | Key Electrolyte Factors | Most Sensitive to | HER Competition | Cation Role |
|---|---|---|---|---|
| HER | pH, cation hydration | Interfacial water structure | — | Strong (Li+ slows, Cs+ speeds) |
| OER | Anion adsorption, pH | Surface oxidation | No | Moderate (Fe/Ni shifts) |
| ORR | pH, anion competition | Adsorption site blocking | Yes (esp. acidic) | Strong (Pt activity cation-dependent) |
| CO2RR | pH, cation size, concentration | C–C coupling barriers | Yes (dominant) | Critical (Cu/C2+ tuning) |
| NRR | Proton donors, solvation | Competing HER | Major limitation | Emerging topic |
| Reaction | pH Effect | Cation’s Effect | Anion’s Effect | Solvent/Buffering | Notable Outcomes |
|---|---|---|---|---|---|
| HER | Strong pH dependence; faster in acid but influenced by the EDL in base 1 | Hydrated cations (e.g., Li+) may inhibit activity in base; larger cations (e.g., K+) often enhance activity 2 | Adsorbing anions may block active sites (e.g., SO42−) 3 | Interfacial water structure is critical; buffering stabilizes the local pH 4 | Activity modulated by both outer-sphere (double layer) and inner-sphere interactions |
| OER | Faster in base due to easier OH− activation; pH affects the pathway (AEM vs. LOM) 5 | Larger cations (K+ and Cs+) can promote activity via EDL effects 6 | Some anions (e.g., PO43−) adsorb and poison the surface 7 | Buffering is important at a high current density; it affects stability | Electrolytes impact lattice oxygen participation and catalyst durability |
| ORR | pH governs selectivity (2e− vs. 4e−); faster in acid but more stable in base | Cation size and hydration impact O2 binding and the interfacial field | Cl− and other adsorbing anions suppress activity on Pt | Weakly coordinated solvents enhance O2 solubility and transport | The electrolyte composition controls activity and product selectivity |
| CO2RR | Local pH affects selectivity (CO vs. hydrocarbons); lower pH promotes the HER | Alkali cations stabilize *CO2− and intermediates; Cs+ > K+ > Na+ > Li+ 8 | Weakly coordinating anions (e.g., ClO4−) are beneficial; strong adsorption blocks sites | Buffering is essential to maintain the pH near the interface; water-in-salt systems explored | Cation effects dominate; the interfacial environment controls the product distribution |
| NRR | Mildly acidic to neutral favored; high pH may suppress N2 adsorption | Weakly hydrated cations (e.g., Cs+) may facilitate N2 activation 9 | Strongly adsorbing anions hinder the reaction | The proton source and water activity must be finely tuned | Electrolyte selection is key to suppress the HER and enable NRR selectivity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebremariam, G.K.; Siraj, K.; Pašti, I.A. Tailoring Electrocatalytic Pathways: A Comparative Review of the Electrolyte’s Effects on Five Key Energy Conversion Reactions. Catalysts 2025, 15, 835. https://doi.org/10.3390/catal15090835
Gebremariam GK, Siraj K, Pašti IA. Tailoring Electrocatalytic Pathways: A Comparative Review of the Electrolyte’s Effects on Five Key Energy Conversion Reactions. Catalysts. 2025; 15(9):835. https://doi.org/10.3390/catal15090835
Chicago/Turabian StyleGebremariam, Goitom K., Khalid Siraj, and Igor A. Pašti. 2025. "Tailoring Electrocatalytic Pathways: A Comparative Review of the Electrolyte’s Effects on Five Key Energy Conversion Reactions" Catalysts 15, no. 9: 835. https://doi.org/10.3390/catal15090835
APA StyleGebremariam, G. K., Siraj, K., & Pašti, I. A. (2025). Tailoring Electrocatalytic Pathways: A Comparative Review of the Electrolyte’s Effects on Five Key Energy Conversion Reactions. Catalysts, 15(9), 835. https://doi.org/10.3390/catal15090835







