1. Introduction
In recent decades, global warming has gained attention, due to the possible consequences of increasing emissions of greenhouse gases. Among these, carbon dioxide (CO
2) is the most abundant, accounting for 79% of global warming, with atmospheric concentrations reaching 410 ppm [
1,
2]. Given the urgency of reducing CO
2 emissions, different strategies are being implemented, including carbon capture and storage (CCS), carbon capture and utilization (CCU) technologies, and conversion into high-value products. Achieving scalable CO
2 conversion into valuable chemicals like methane (CH
4) could be of high interest, aligning with the principles of the circular economy. CO
2 is an apolar, abundant, inexpensive, non-toxic, and thermodynamically stable molecule, with high binding energies primarily associated with the C=O bond. Its standard Gibbs free energy is
, making CO
2 conversion an energy-intensive process [
3]. To overcome this energy barrier, catalytic strategies are widely employed [
4].
Photocatalysis is a promising approach that exploits solar energy, a renewable and lasting energy source, despite its intermittent nature, to drive CO
2 conversion. The photocatalytic process involves the use of semiconducting materials containing conduction and valence bands with the potential of inserting crucial oxidizing/reducing levels/species in the reaction systems to be selectively accelerated. When a semiconductor is irradiated by a radiation of energy equal to or greater than the band gap, excitation occurs, generating a negative excited electron in the conduction band and a corresponding positive hole left on the valence band. The excited electrons can migrate to the semiconductor surface, where they are available to participate in reduction reactions, such as CO
2 activation and conversion, while the holes can catalyze the oxidation reactions, such as water oxidation into oxygen and hydrogen, which are essential reactants in solar fuel production [
3,
4]. Efficient charge carrier separation and mobility are crucial for promoting the desired photocatalytic reactions on the catalyst surface. One of the major limitations in photocatalysis is the electron–hole recombination rate, in which excited electrons return to the valence band and recombine with the holes, dissipating energy as heat or photons [
5]. This phenomenon significantly reduces photocatalytic efficiency and should be maximally prevented.
Titanium dioxide (TiO
2) has been widely explored as a photocatalyst owing to its non-toxicity, availability, cost-effectiveness, and stability [
6]. However, its application is limited to the ultraviolet (UV) region, accounting for only 5% of the solar spectrum, due to its large band gap (3.0–3.2 eV) [
7,
8]. Additionally, TiO
2 suffers from surface degradation and a high recombination rate, which hinder its overall performance [
5]. In this context, perovskites such as titanates are emerging as promising alternatives for photocatalysis. Perovskites represent a class of materials that exhibit ferroelectric, piezoelectric, and photocatalytic properties [
9]. They offer photostability, corrosion resistance in aqueous solution, and a suitable band gap for CO
2 photoreduction. Specifically, barium titanate (BaTiO
3) and calcium titanate (CaTiO
3) possess intrinsic basicity, which improves their interactions with CO
2, which is slightly acidic, facilitating the photoreduction process [
10,
11].
BaTiO
3 and CaTiO
3 can be synthesized via hydrothermal treatment in a simple, cost-effective, and sustainable bottom-up approach [
12,
13]. This method is considered to be environmentally friendly since it uses water as the reaction solvent and allows operation under relatively mild reaction conditions, without requiring calcination. Although perovskites such as BaTiO
3 and CaTiO
3 exhibit promising photocatalytic properties, further strategies are needed to enhance their photoactivity and enable their efficient utilization across the full solar spectrum. One approach to overcoming these limitations is doping impurities, which introduces new intra-gap energy levels, effectively reducing the band gap and extending light absorption into the visible region [
14]. Over the past decades, carbon (C) and nitrogen (N) non-metal elements have been identified as appealing doping candidates for photocatalysis due to their availability, low cost, and non-toxic nature. Nitrogen doping is particularly attractive as it induces formation of intra-gap energy levels between the valence and the conduction bands, enhancing material activation under visible light. In addition to its suitable compatibility for being added to the crystal structure, nitrogen has a lower electronegativity and ionization energy than oxygen. Nitrogen’s ionic radius is similar to oxygen, potentially facilitating the mixing between N 2p and O 2p orbitals [
15,
16]. Furthermore, nitrogen doping can promote CO
2 adsorption and act as an active site for photoreduction. Functional groups of nitrogen also enable strong chemisorption of the reaction intermediates, enhancing selectivity towards methane formation [
12]. On the other hand, carbon doping can enhance the photocatalytic activity by stabilizing the material and promoting molecular adsorption onto the catalyst surface. Also, the C 2p—O 2p orbital mixing phenomenon can help to introduce localized intra-gap energy levels, facilitating visible light absorption [
5]. In recent years, carbon and nitrogen co-doping has frequently been reported to further improve photocatalytic performance under both UV and solar irradiation. Olivo et al. reported that doping TiO
2 with C and N using chitosan as a precursor significant enhanced photocatalytic pollutant degradation under visible light [
17]. Similarly, Chen et al. observed that the synergistic effects of C and N co-doping improved methylene blue degradation under solar irradiation, attributed to the formation of intra-gap energy states between the valence and conduction bands [
18]. In the field of perovskite materials, Humayun et al. synthetized N-doped LaFeO
3 and demonstrated a four-fold enhancement in CO
2 conversion efficiency, compared to the undoped perovskite [
15]. Nitrogen was suggested to facilitate CO
2 interaction and act as an active adsorption site. Additionally carbon doping in NaTaO
3 was shown to reduce the band gap energy, thereby enhancing visible-light activity [
19]. However, carbon and nitrogen doping of Ba- and Ca-based titanates has not been explored yet.
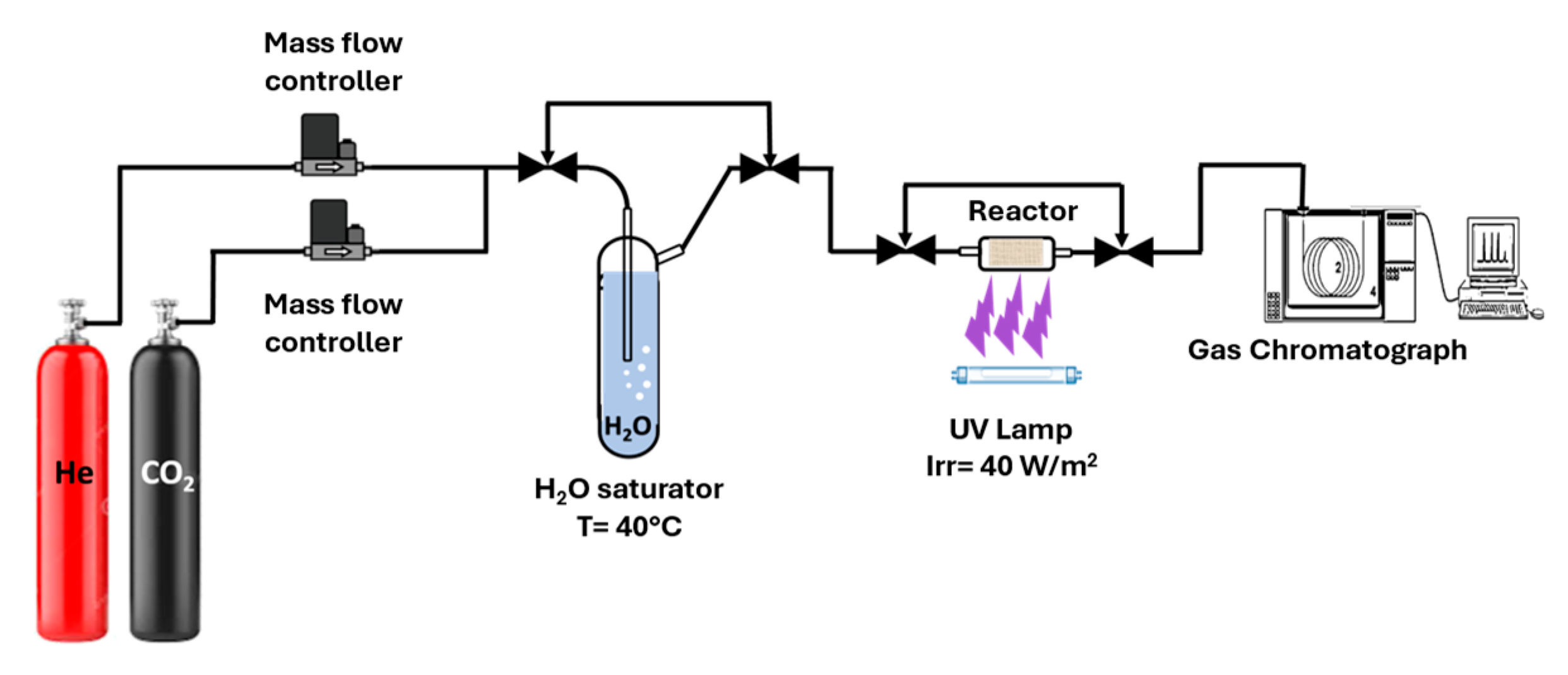
In the present study, bare and non-metal-doped Ba and Ca-based titanates were synthesized via a hydrothermal treatment method. Carbon and nitrogen doping were carried out using two sustainable precursors, glucose (as C source) and chitosan (as a source of C and N). The photocatalytic performance was evaluated for the gas-phase CO2 photoreduction under both UV and solar irradiation.
3. Discussion
The photocatalytic performance of the prepared structures was examined for CO
2 photoreduction in the gaseous phase for methane production. Three different pathways have been proposed in the literature for CO
2 photoreduction reactions [
36,
37]: formaldehyde, carbene, and glyoxal pathways. Of these, the first two are the most broadly accepted. For the BaTiO
3 structure, the main pathway is the carbene one, which is characterized by the following steps: CO
2(g) → CO
2−• → CO+OH
− → CO
−• → C+OH
− → CH
−• → CH
2−• → CH
3−• → CH
4(g) [
38]. In the carbene pathway, CO
2−•, carbon coordinates the semiconductor and reacts with a proton that breaks the C-O bond, forming CO
−•; then, CH
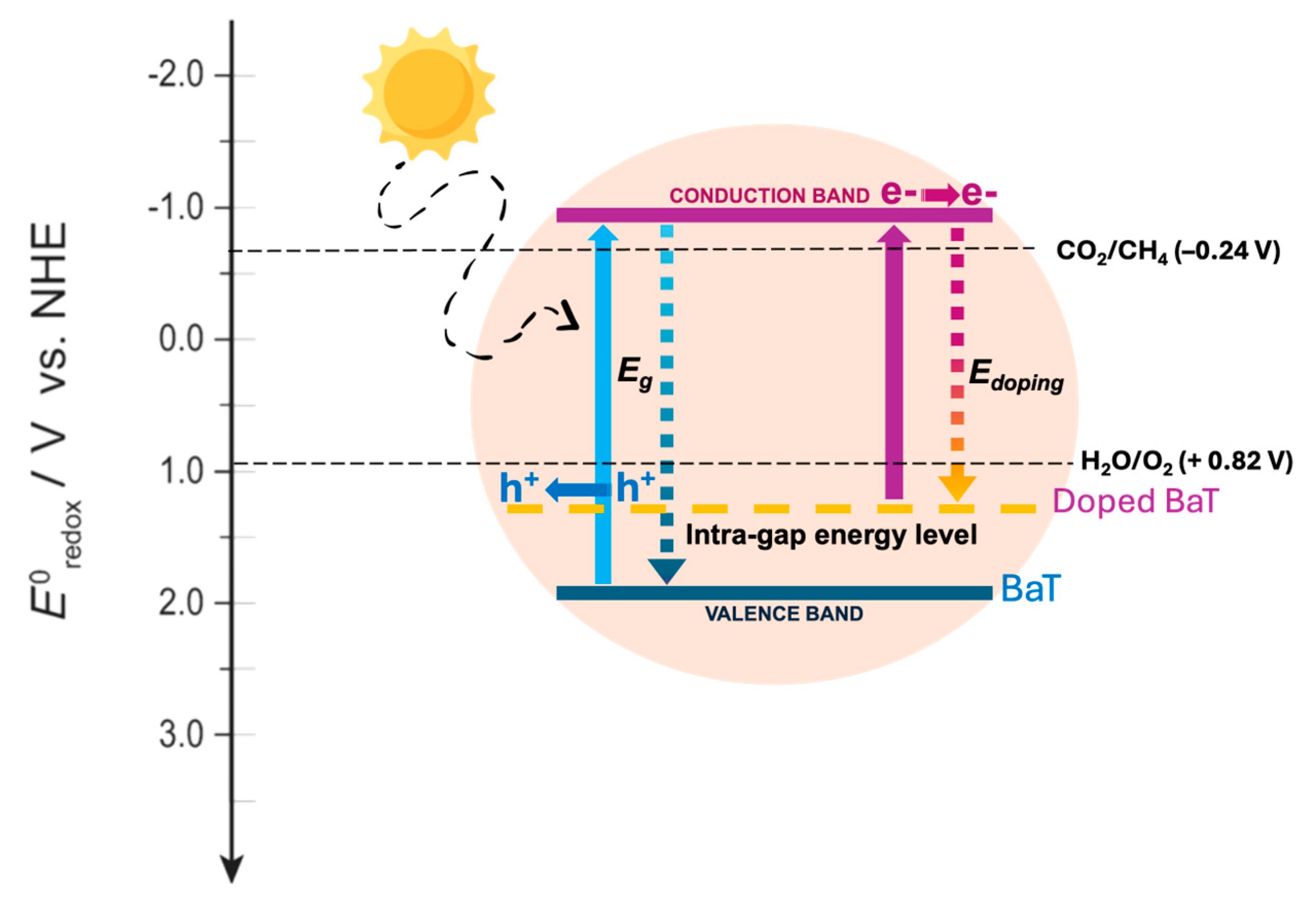
4 is formed through multiple reduction steps. A schematic representation of the electronic behavior in doped titanates is shown in
Figure 8. As an example, pure BaT exhibits band edge positions suitable for both CO
2 reduction and H
2O oxidation: the conduction band is more negative than the CO
2/CH
4 reduction potential, while the valence band is more positive than the H
2O/O
2 oxidation potential. Upon doping with carbon and/or nitrogen, visible-light absorption is improved possibly due to the introduction of intra-gap states, which also helped to reduce charge carrier recombination. Under solar irradiation, the doped material generates electron–hole pairs. Excited electrons in the conduction band participate in CO
2 reduction, while holes in the valence band catalyze water oxidation into protons and hydroxyl radicals. The generated hydrogen ions are crucial for the subsequent CO
2-to-CH
4 conversion.
From the CO
2 photocatalytic results, all materials demonstrated to be active under UV light [
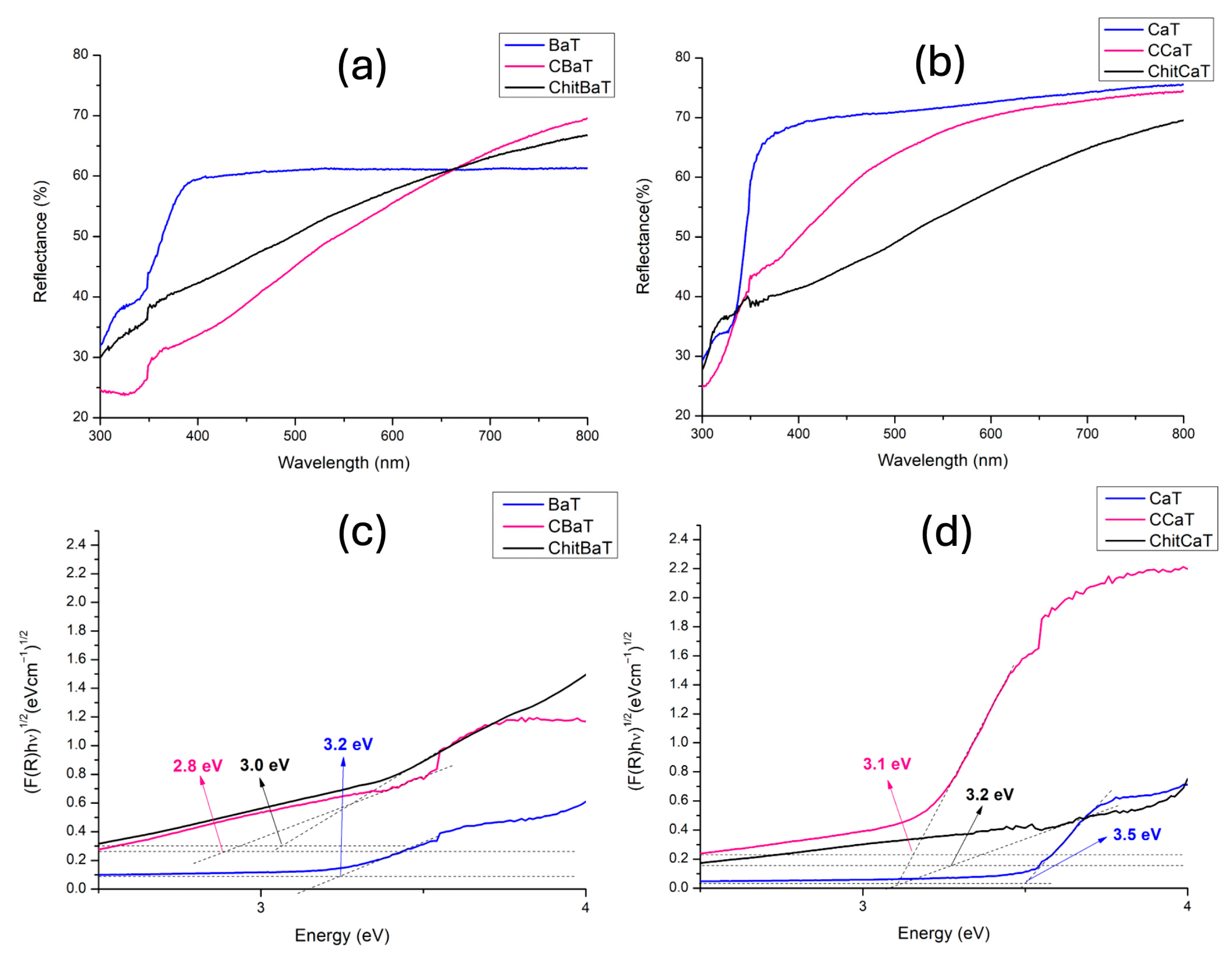
39]. Concerning solar light activity, undoped titanates’ performance was hindered by their large band gaps, as demonstrated by Tauc plot analyses reported in
Figure 4c,d.
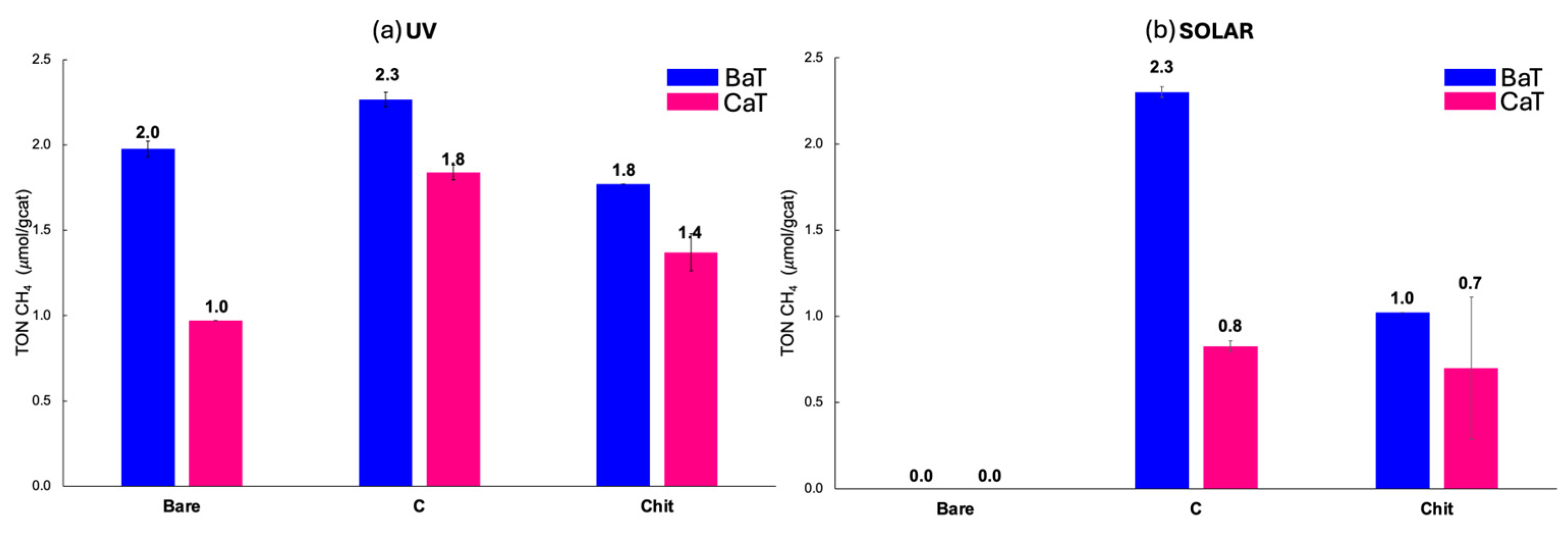
CBaT emerged as the best catalyst under both solar and UV light conditions, reaching TON of 2.3 μmol/g
cat. Moreover, recycling tests on CBaT showed that the photocatalytic efficiency remained constant with the same CH
4 selectivity after five cycles under solar light, suggesting a good stability of CBaT photocatalyst (
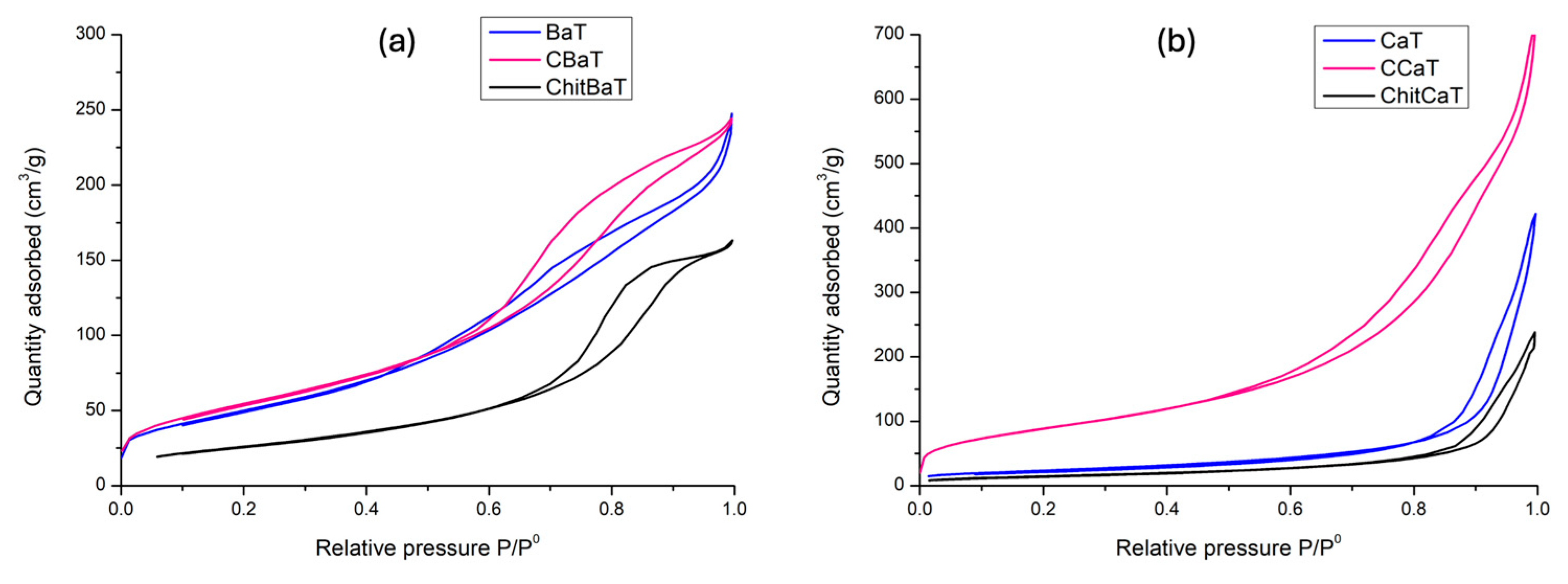
Figure S5). It is possible that the high surface area (196 m
2/g) and pore volume (0.34 cm
3/g) of the sample facilitated the reactants’ interaction and consequent adsorption on the titanate surface as a means to promote the reaction mechanism. Dolat et al. reported that the surface charge decreases as the carbon content on the surface increases, preventing particle agglomeration and enhancing the specific surface area [
40]. In addition, Tauc plot analysis (
Figure 4c) suggested the formation of intra-gap energy levels, narrowing the band gap to 2.8 eV, which can contribute to suppress the recombination events and improve the photocatalytic activity [
41]. Carbon atoms could be replaced with some oxygen atoms or gained interstitial positions in the bulk structure, generating intra-gap energy levels and lattice distortions, thereby shifting the perovskite-type absorption into the visible region [
5]. Teng et al. studied the density of states of the orbitals for CBaT and suggested that the C 2p states of the dopant are distributed on both sides of the Fermi level, resulting in a narrower band gap [
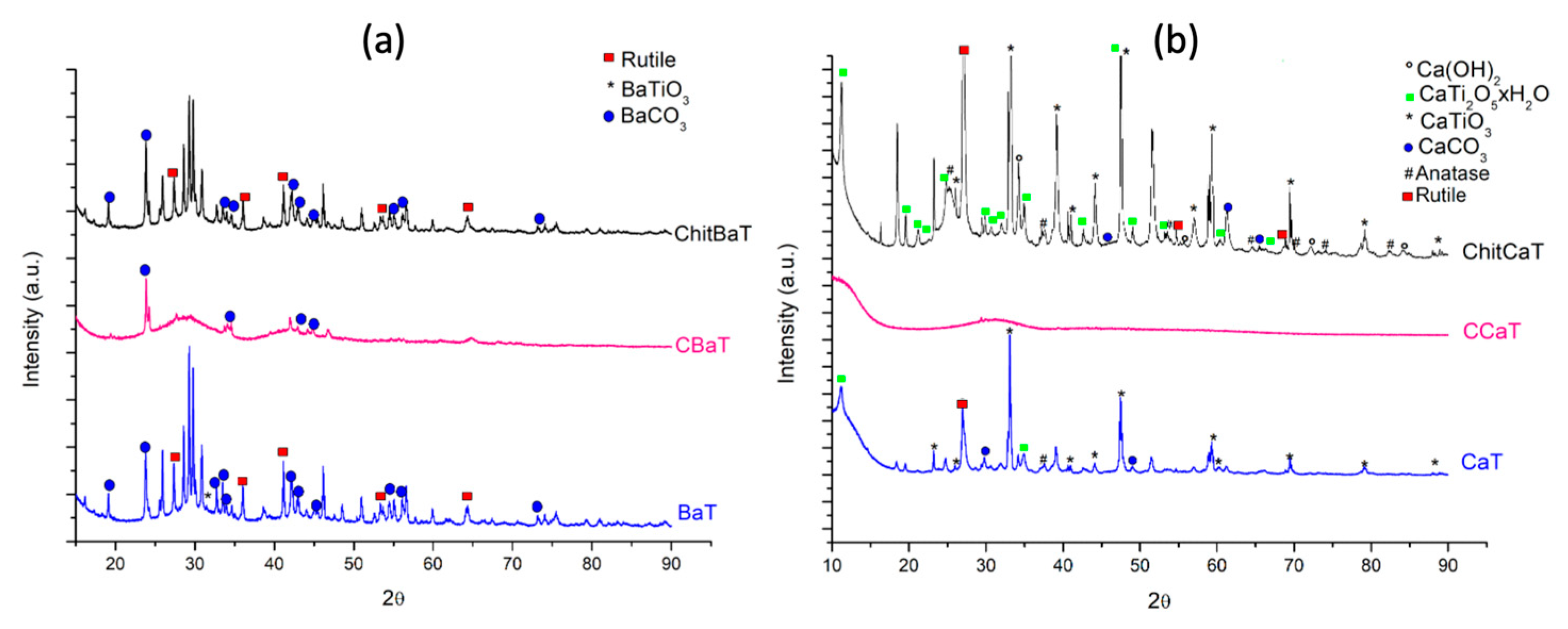
42]. As supported by elemental analyses, the successful doping of carbon was demonstrated, in accordance with the XRD pattern (
Figure 1a), in which CBaT showed the occurence of an amorphous phase, which can promote photoactivity. CBaT (
Figure 2b) exhibited aggregates on the BaT surface, which could be attributed to carbon deposition, as supported by EDX analysis (
Figure S1a,b).
Regarding the CCaT photocatalyst, this showed greater photoactivity than CaT (0.8 μmol/g
cat) under solar light, attributed to the narrow band gap, high surface area (314 m
2/g), and small particle size, as revealed by the physisorption analysis (
Figure 3b), which supported more efficient CO
2 adsorption on its surface compared with bare CaT [
43]. The absorption at lower relative pressures could be attributed to its higher BET surface area of 314 m
2/g, probably reflecting the higher C% detected by elemental analysis. The XRD pattern (
Figure 1) highlights the amorphous nature of carbon-doped materials. However, the CCaT catalyst showed lower performance than CBaT for CO
2 degradation, although this sample exhibited a high surface area. As reported in
Table 3, the lower photocatalytic activity of CCaT under solar light could be attributed to its wider band gap energy (3.1 eV) compared with CBaT (2.8 eV), restricting the CCaT’s photoactivity to the UV region. Additionally, the lower pore volume of CCaT (
Table 2) might hinder the interactions between the reactants and the catalyst surface [
44]. Furthermore, the ferroelectric properties of BaTiO
3 enhance the electron mobility and charge separation in CBaT, thereby promoting the target reaction more effectively than in CCaT [
45].
Regarding Chit-based titanates, as reported, ChitBaT produced 1.8 μmol/g
cat of CH
4 compared with 1.4 μmol/g
cat for ChitCaT under UV irradiation. The solar photocatalytic activity of chitosan-treated materials (1.0 and 0.7 μmol/g
cat of CH
4) was found to be lower than that of carbon-doped ones, but still higher than the bare materials. From the SEM images (
Figure 2c,f), chitosan seemed to be deposited on the titanate surface, possibly acting as an active or anchoring site, participating in the reaction. SEM images showed that mainly the Chit-doped materials presented carbon deposited on their surfaces, although without completely excluding the possibility of C incorporation into the crystal structure. Beyond acting as a dopant source, chitosan appears to function as a templating agent that influences crystal growth. Preethi et al. demonstrated the chitosan templating effect during the hydrothermal synthesis of TiO
2, which affected crystallite orientation and limited crystallite size. This behavior was attributed to strong interactions between the functional groups of chitosan and titania, promoting the controlled growth of titania nanoparticles [
46]. Similarly, Hamden et al. reported that chitosan templating enabled TiO
2 crystallization at low temperatures of about 200 °C, generating TiO
2 nanoparticles. Therefore, the presence of chitosan may have contributed to the observed structural and morphological features of the doped samples [
47]. The lower surface area observed for ChitBaT (
Table 2) was attributed to the accumulation of high molecular weight chitosan (and/or its derivatives) inside the pores of BaT during the synthesis, reducing the gas interaction at the surface of the perovskite. Additionally, as reported by Hussein et al. [
48], chitosan modifies the electronic structure of TiO
2 by forming surface complexes between their functional groups. This creates intermediate energy levels within the band gap, facilitating electronic transitions at lower photon energies. These states can effectively reduce the band gap and alter charge distribution, enhancing light absorption. A similar mechanism was hypothesized for Ba- and Ca-based titanates doped with chitosan, resulting in enhanced Ba- and Ca-based titanates, as also suggested by the calculated band gap values,
Table 3 [
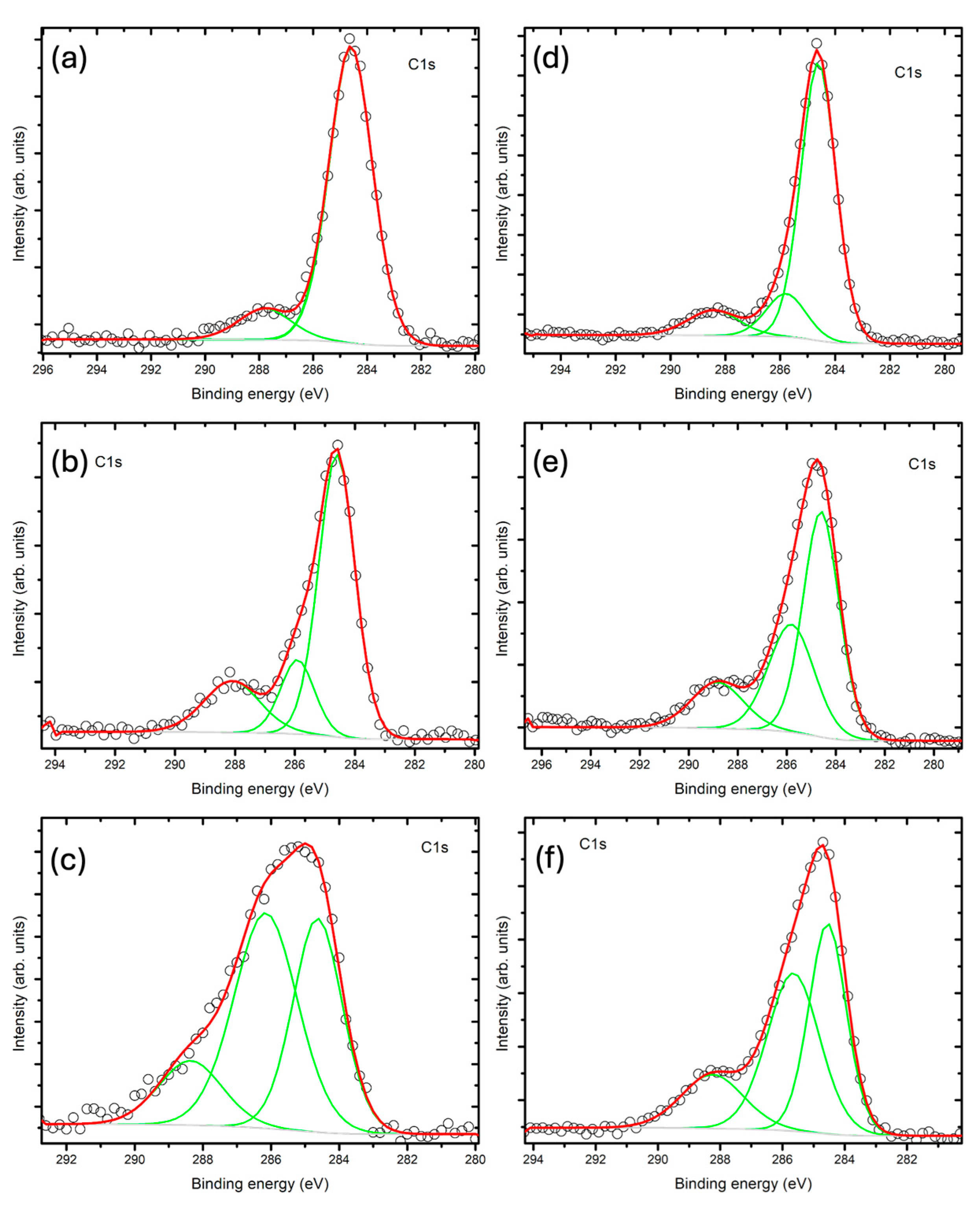
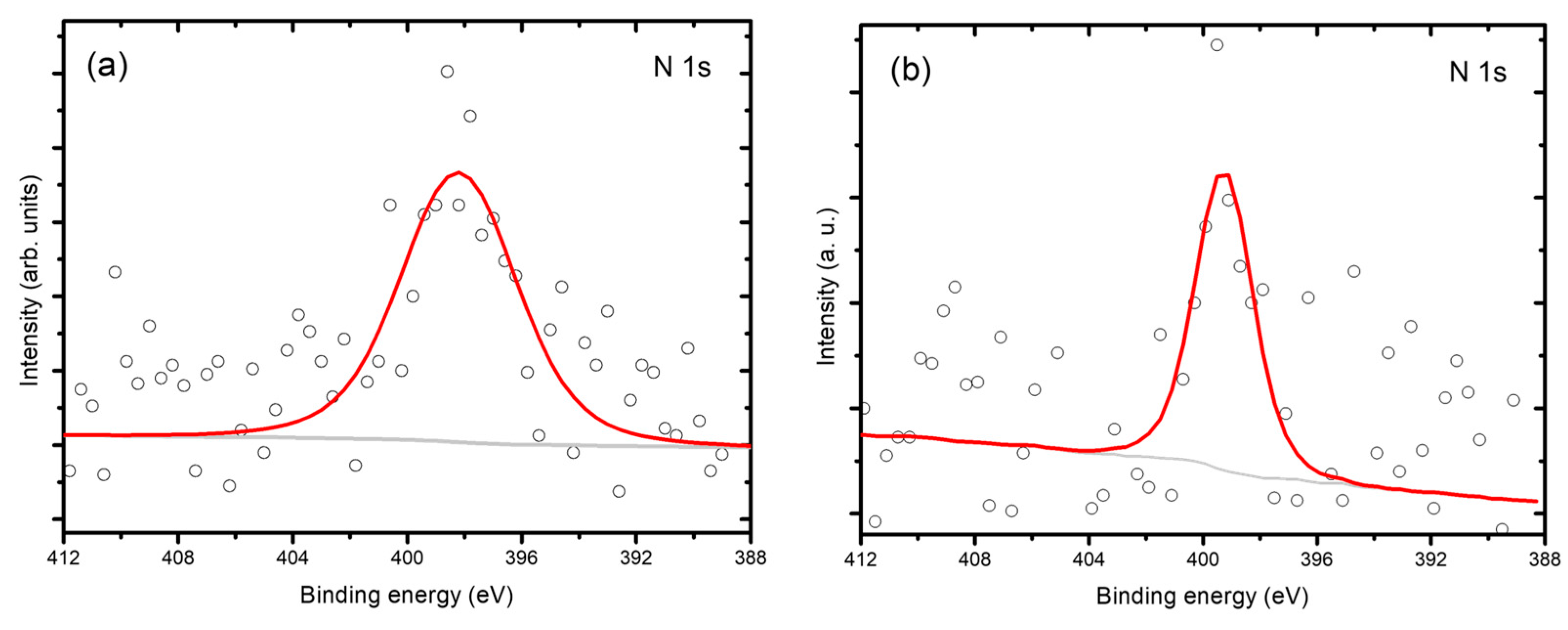
48]. In the XPS spectra (
Figure 5), ChitBaT and ChitCaT exhibited peaks which can be attributed to the aromatic carbon C-OH, C-N, C-O, and C=O bonds typical of chitosan, suggesting the possible deposition of carbon on the surface rather than its incorporation within the bulk material, as also highlighted by SEM analyses,
Figure 2 [
20]. Lastly, as reported by Zhang et al., it was not possible to distinguish carbon potentially incorporated into interstitial sites of the perovskite-related structure forming Ti-O-C bonds [
31]. From these results, although the reflectance spectra (
Figure 4a,b) suggested that CaT is more strongly affected by the presence of chitosan than BaT, the ChitBaT sample actually shows a higher amount of chitosan deposited on its surface compared to ChitCaT. Beside acting as doping and templating agent, chitosan tended to deposit on the titanate surface, possibly due to its high molecular weight. In contrast, glucose doping is more likely to have favored the incorporation of C into the titanate lattice while also contributing to particle size reduction. Concerning ChitCaT, the heterogeneous composition was related to a polycrystalline phase of the material, as supported by the XRD profile in
Figure 1b. Badovinac et al. reported that these nanoparticles or small grains can play an important role in prompting the catalytic route due to the increase of the active surface area [
49].
As expected and demonstrated by the large band gaps in the Tauc plot analyses reported in
Figure 4c,d [
43,
50], undoped titanates (both BaT and CaT) had lower activity under solar irradiation. However, they exhibited higher photoactivity if irradiated by UV light, reaching TON values of 2.0 and 1.0 μmol/g
cat of CH
4, respectively. Although the band gap values were similar, the higher activity of BaT compared with CaT could be attributed to the ferroelectricity of BaTiO
3, which promotes the electron mobility and improves charge separation, enhancing chemical reactions on its surface [
43,
51]. However, CaTiO
3 does not possess ferroelectricity, possibly leading to a less efficient charge separation process. In addition, Ba
2+ polarizability is higher than Ca
2+; so, Ba
2+ can more efficiently interact with CO
2, stabilizing it on the surface. The XRD results (
Figure 1b) indicate that the high crystallinity of Ca-based materials could negatively affect the photocatalytic activity, due to the lower presence of defects [
52]. Furthermore, Kwak et al. reported that BaTiO
3 shows a higher photocatalytic activity for CO
2 photoreduction compared to SrTiO
3 and CaTiO
3, because of the lower recombination rate, the higher surface area, and the better interaction with carbon dioxide [
53]. In the BaT’s XRD pattern, a small amount of barium carbonate (BaCO
3) (PDF 45-1471) was identified, which was attributed to the superficial adsorption of CO
2 from air during the synthesis steps [
54]. Although an increased affinity for CO
2 is desirable, a too high affinity could be an issue, resulting in the fast saturation of active superficial sites [
21]. Therefore, the BaT’s XRD pattern in
Figure 1a highlights the polycrystalline nature of Ba-derived titanate, which is also indicated by the SEM image (
Figure 2a) [
32].
In general, in the SEM images, carbon deposition on the surface is visible only on Chit-based materials. The observed increase in surface areas in both Ba and Ca samples was partially linked to the carbon deposition on the surface, as discussed above and as already reported [
40]. The number of active sites, specific surface area, and the type of porosity played a crucial role in the photocatalytic reaction by supplying stronger interactions with the substrates and enhanced charge migration at the interfaces.
Among the C-doped materials, CBaT was the best photocatalyst under both solar and UV lights, reaching the same TON values of 2.3 μmol/gcat. Concerning the calcium-based titanates, they were just active under UV light, with CH4 productivity ranging from 1.4, for ChitCaT, to 1.8 μmol/gcat, for CCaT. The improved performance of the doped samples compared with bare CaT could be attributed to the presence of residual chitosan precursors in ChitCaT, which improves the catalyst–reactant interaction. On the other hand, CCaT exhibits a higher surface area, increasing the number of accessible active sites for CO2 reduction.
Concerning the Chit-based photocatalysts, chitosan was employed as a dual carbon and nitrogen source. As reported by Chen et al., who used urea as a C/N dopant, the synergistic effect of carbon and nitrogen doping can be explained by the fact that carbon acts as a photosensitizer, facilitating the accumulation of electrons in the conduction band, while nitrogen introduces intra-bandgap energy states. These N-induced states can contribute to band gap narrowing, thereby enhancing light absorption and potentially accelerating electron transfer.
Overall, doped catalysts achieved higher TON values under both UV and solar light compared to the bare ones, which were inactive under solar irradiation, supporting that doping is a valuable strategy to both enhance the photoactivity and broaden the absorption spectrum of photocatalysts. Furthermore, the use of glucose and chitosan as carbon and C/N dopants, respectively, highlights the potential of employing bio-sourced materials for CO2 photoreduction under solar light, aligning with principles of sustainability and green chemistry, such as the use of renewably sourced dopants, without compromising photocatalytic performance.
Table 4 shows a comparison with similar perovskite-containing structures applied in the same reaction in some recent works. The reported doped titanates demonstrate such similar or lower activity than our samples. In addition, carbon-doped Ba or Ca-based titanates have not been investigated under solar irradiating conditions, highlighting the novelty of this study within the literature.