α-MnO2 Reactive Lattice Oxygen Promotes Peroxymonosulfate-Activated Sulfamethoxazole Degradation
Abstract
1. Introduction
2. Results and Discussion
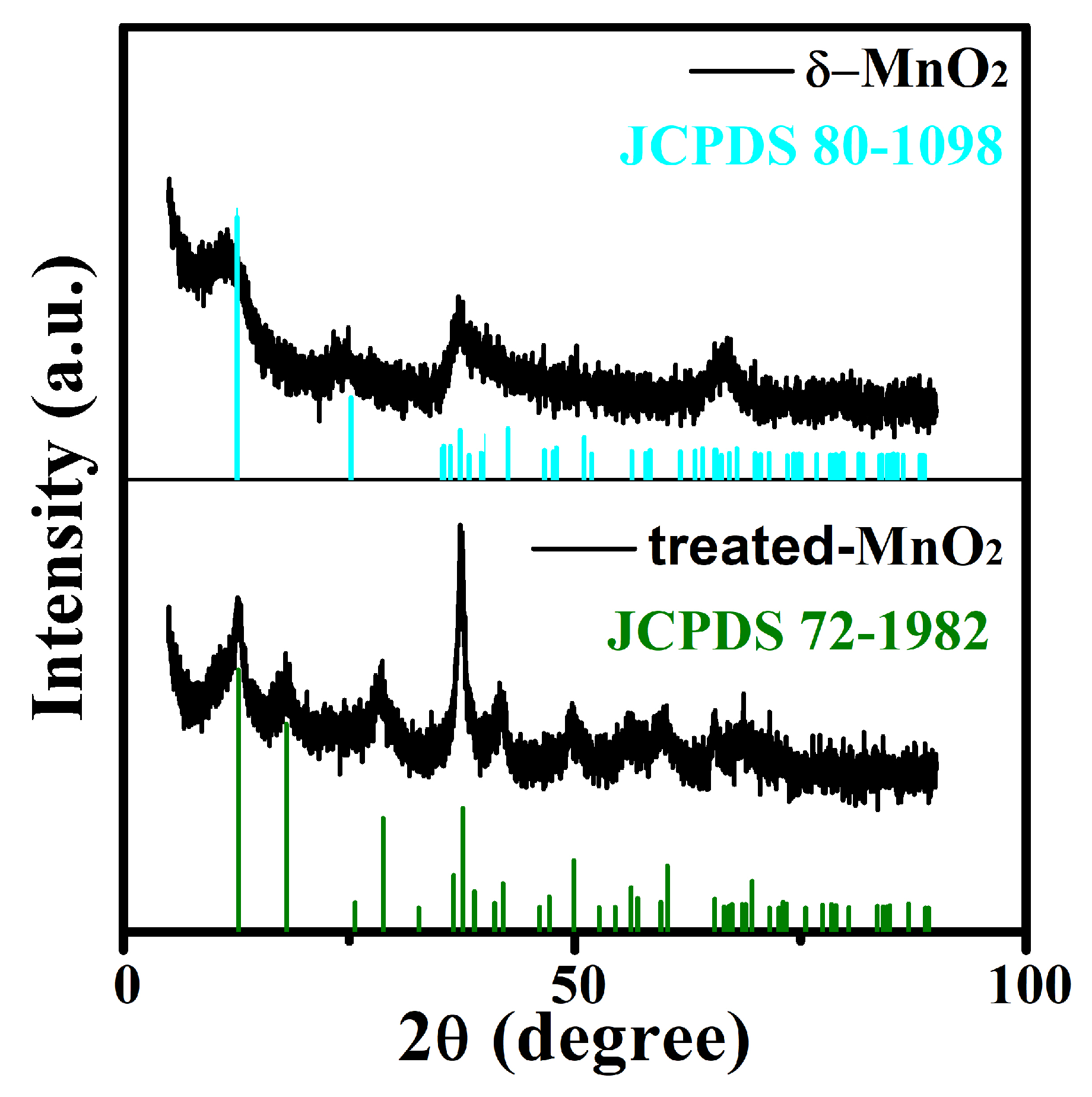
2.1. Characterization of As-Prepared Materials
2.2. Catalytic Activity of Prepared MnO2
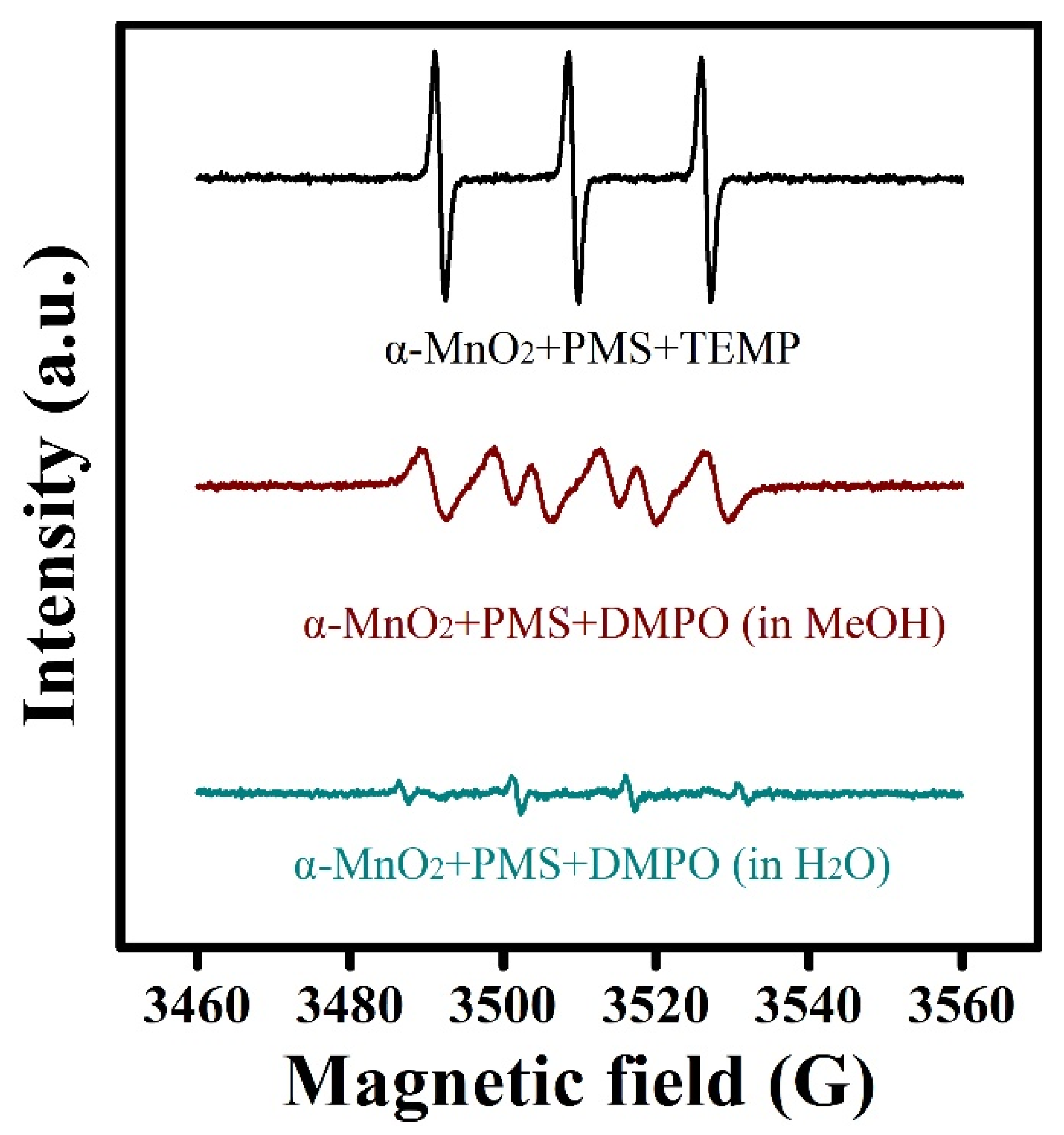
2.3. Identification of Active Species
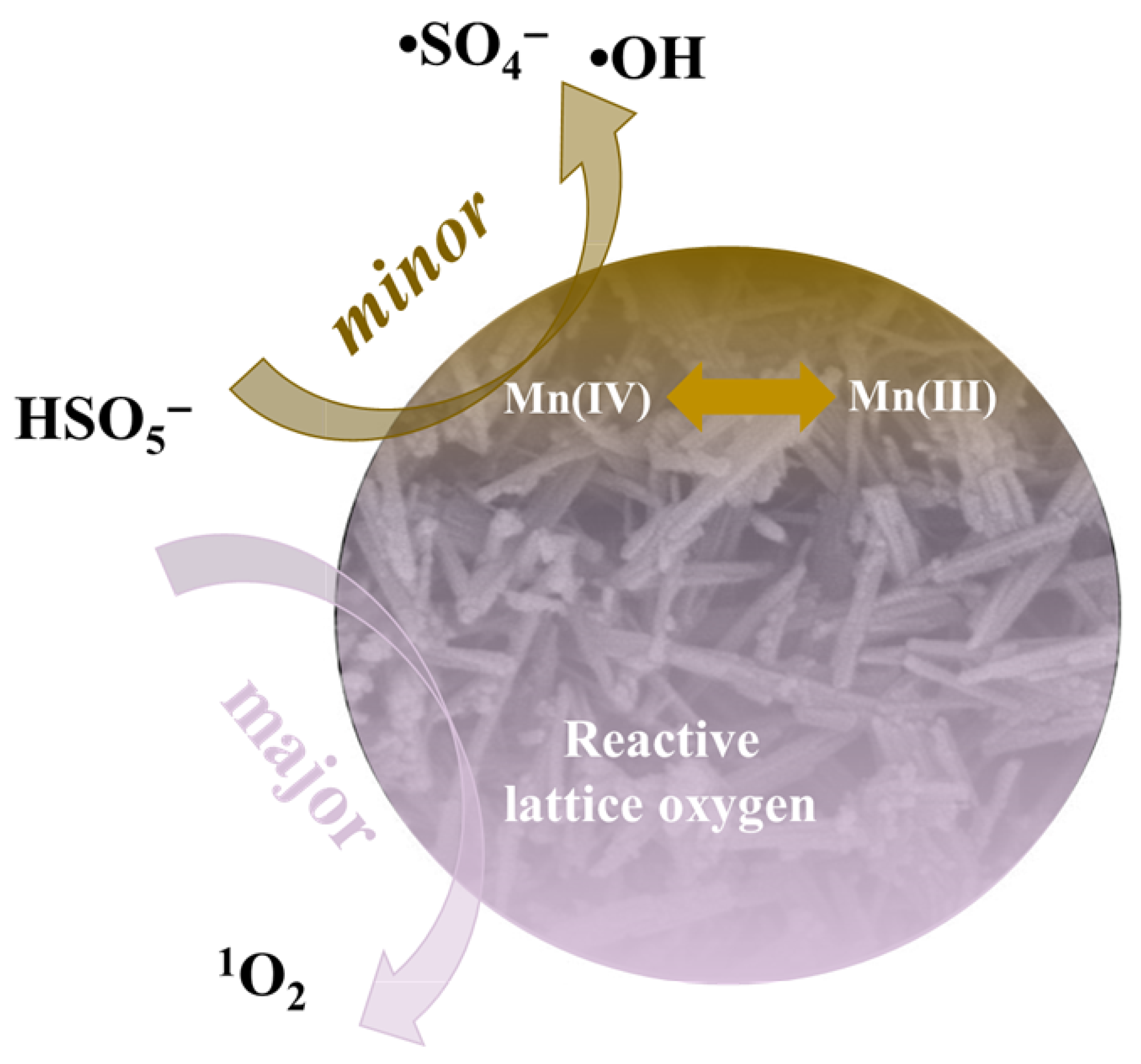
2.4. PMS Activation Mechanism
3. Experimental
3.1. Chemicals
3.2. Catalyst Preparation
3.3. Characterization
3.4. Experimental Setup
3.5. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rahim, K.; Nawaz, M.N.; Almehmadi, M.; Alsuwat, M.A.; Liu, L.; Yu, C.; Khan, S.S. Public health implications of antibiotic resistance in sewage water: An epidemiological perspective. Bioresour. Bioprocess. 2024, 11, 91. [Google Scholar] [CrossRef]
- Gholipour, S.; Shamsizadeh, Z.; Halabowski, D.; Gwenzi, W.; Nikaeen, M. Combating antibiotic resistance using wastewater surveillance: Significance, applications, challenges, and future directions. Sci. Total Environ. 2024, 908, 168056. [Google Scholar] [CrossRef]
- Garcia, J.; Garcia-Galan, M.J.; Day, J.W.; Boopathy, R.; White, J.R.; Wallace, S.; Hunter, R.G. A review of emerging organic contaminants (EOCs), antibiotic resistant bacteria (ARB), and antibiotic resistance genes (ARGs) in the environment: Increasing removal with wetlands and reducing environmental impacts. Bioresour. Technol. 2020, 307, 123228. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Shao, P.; Cui, F. Activation of peroxymonosulfate with magnetic Fe3O4–MnO2 core–shell nanocomposites for 4-chlorophenol degradation. Chem. Eng. J. 2015, 262, 854–861. [Google Scholar] [CrossRef]
- Zhang, Q.; He, D.; Li, X.; Feng, W.; Lyu, C.; Zhang, Y. Mechanism and performance of singlet oxygen dominated peroxymonosulfate activation on CoOOH nanoparticles for 2,4-dichlorophenol degradation in water. J. Hazard. Mater. 2020, 384, 121350. [Google Scholar] [CrossRef]
- Zhu, S.; Li, X.; Kang, J.; Duan, X.; Wang, S. Persulfate activation on crystallographic manganese oxides: Mechanism of singlet oxygen evolution for nonradical selective degradation of aqueous contaminants. Environ. Sci. Technol. 2019, 53, 307–315. [Google Scholar] [CrossRef]
- Liu, B.; Song, W.; Wu, H.; Liu, Z.; Teng, Y.; Sun, Y.; Xu, Y.; Zheng, H. Degradation of norfloxacin with peroxymonosulfate activated by nanoconfinement Co3O4@CNT nanocomposite. Chem. Eng. J. 2020, 398, 125498. [Google Scholar] [CrossRef]
- Ren, W.; Nie, G.; Zhou, P.; Zhang, H.; Duan, X.; Wang, S. The intrinsic nature of persulfate activation and N-doping in carbocatalysis. Environ. Sci. Technol. 2020, 54, 6438–6447. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, J.; Zhang, Z.; Wei, T.; Wang, J.; Ma, J.; Ren, Y.; Zhang, H. Co3O4 crystal plane regulation to efficiently activate peroxymonosulfate in water: The role of oxygen vacancies. J. Colloid Interface Sci. 2022, 623, 520–531. [Google Scholar] [CrossRef]
- Gao, Q.; Li, H.; Wang, X.; Han, B.; Xia, K.; Wu, J.; Zhou, C.; Dong, J. Doping phosphorus into Co3O4: A new promising pathway to boost the catalytic activity for peroxymonosulfate activation. Appl. Surf. Sci. 2022, 574, 151632. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Gong, M.; Wang, W.; Mu, Y.; Hu, Z.H. α-MnO2/Palygorskite composite as an effective catalyst for heterogeneous activation of peroxymonosulfate (PMS) for the degradation of Rhodamine B. Sep. Purif. Technol. 2020, 230, 115887. [Google Scholar] [CrossRef]
- Deng, Y.; Gao, P.; Wang, L.; Zhang, Y.; Fu, J.; Huang, R.; Zhao, S.; Wang, G.; Wei, Y.; Zhou, S. Activation of peroxymonosulfate by MnO2 with oxygen vacancies: Degradation of organic compounds by electron transfer nonradical mechanism. J. Environ. Chem. Eng. 2022, 10, 107481. [Google Scholar] [CrossRef]
- Wang, Z.; Han, Y.; Fan, W.; Wang, Y.; Huang, L. Shell-core MnO2/Carbon@Carbon nanotubes synthesized by a facile one-pot method for peroxymonosulfate oxidation of tetracycline. Sep. Purif. Technol. 2021, 278, 119558. [Google Scholar] [CrossRef]
- Qin, Q.; Liu, T.; Zhang, J.; Wei, R.; You, S.; Xu, Y. Facile synthesis of oxygen vacancies enriched α-Fe2O3 for peroxymonosulfate activation: A non-radical process for sulfamethoxazole degradation. J. Hazard. Mater. 2021, 419, 126447. [Google Scholar] [CrossRef]
- Zheng, H.; Bao, J.; Huang, Y.; Xiang, L.; Faheem; Ren, B.; Du, J.; Nadagouda, M.N.; Dionysiou, D.D. Efficient degradation of atrazine with porous sulfurized Fe2O3 as catalyst for peroxymonosulfate activation. Appl. Catal. B Environ. 2019, 259, 118056. [Google Scholar] [CrossRef]
- Wu, X.; Guo, H.; Jia, L.; Xiao, Y.; Hou, B.; Li, D. Effect of MnO2 crystal type on the oxidation of furfural to furoic acid. Catalysts 2023, 13, 663. [Google Scholar] [CrossRef]
- Ma, J.; Wang, C.; Xi, W.; Zhao, Q.; Wang, S.; Qiu, M.; Wang, J.; Wang, X. Removal of radionuclides from aqueous solution by manganese dioxide-based nanomaterials and mechanism research: A review. ACS EST Eng. 2021, 1, 685–705. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, S.; Duan, X.; Wang, Y.; Wu, D.; Pang, J.; Wang, X.; Wang, S. Catalytic oxidation of sulfachloropyridazine by MnO2: Effects of crystalline phase and peroxide oxidants. Chemosphere 2021, 267, 129287. [Google Scholar] [CrossRef]
- Huang, J.; Dai, Y.; Singewald, K.; Liu, C.C.; Saxena, S.; Zhang, H. Effects of MnO2 of different structures on activation of peroxymonosulfate for bisphenol A degradation under acidic conditions. Chem. Eng. J. 2019, 370, 906–915. [Google Scholar] [CrossRef]
- Shen, S.; Zhou, X.; Zhao, Q.; Jiang, W.; Wang, J.; He, L.; Ma, Y.; Yang, L.; Chen, Z. Understanding the nonradical activation of peroxymonosulfate by different crystallographic MnO2: The pivotal role of Mn(III) content on the surface. J. Hazard. Mater. 2022, 439, 129613. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Y.; Gao, M.; Xin, Y.; Zhang, G.; Xu, P.; Ma, D. Degradation of dimethyl phthalate by morphology controlled β-MnO2 activated peroxymonosulfate: The overlooked roles of high-valent manganese species. J. Hazard. Mater. 2023, 459, 132199. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, P.; Hu, K.; Duan, X.; Ren, Y.; Sun, H.; Wang, S. Sustainable redox processes induced by peroxymonosulfate and metal doping on amorphous manganese dioxide for nonradical degradation of water contaminants. Appl. Catal. B Environ. 2021, 286, 119903. [Google Scholar] [CrossRef]
- Huang, Y.; Tian, X.; Nie, Y.; Yang, C.; Wang, Y. Enhanced peroxymonosulfate activation for phenol degradation over MnO2 at pH 3.5-9.0 via Cu(II) substitution. J. Hazard. Mater. 2018, 360, 303–310. [Google Scholar] [CrossRef]
- Ndayiragije, S.; Zhang, Y.; Zhou, Y.; Song, Z.; Wang, N.; Majima, T.; Zhu, L. Mechanochemically tailoring oxygen vacancies of MnO2 for efficient degradation of tetrabromobisphenol A with peroxymonosulfate. Appl. Catal. B Environ. 2022, 307, 121168. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chen, D.Z.; Zhang, Z.X.; Zhou, T.L.; Zou, J.P. Singlet oxygen-dominated activation of peroxymonosulfate by 3D hierarchical MnO2 nanostructures for degradation of organic pollutants in water: Surface defect and catalytic mechanism. Sep. Purif. Technol. 2022, 303, 122177. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Zhang, P.; Hu, T.; Wang, X. Highly active copper-intercalated weakly crystallized δ-MnO2 for low-temperature oxidation of CO in dry and humid air. Front. Environ. Sci. Eng. 2024, 18, 62. [Google Scholar] [CrossRef]
- Zhang, H.; Sui, S.; Zheng, X.; Cao, R.; Zhang, P. One-pot synthesis of atomically dispersed Pt on MnO2 for efficient catalytic decomposition of toluene at low temperatures. Appl. Catal. B Environ. 2019, 257, 117878. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Lin, Y.L.; Zhang, T.Y.; Hu, C.Y.; Pan, Y.; Pan, R.; Tang, Y.L.; Xu, B.; Gao, N.Y. Enhanced coagulation and oxidation by the Mn(VII)-Fe(III)/peroxymonosulfate process: Performance and mechanisms. Water Res. 2022, 226, 119200. [Google Scholar] [CrossRef]
- Xie, J.; Wei, Y.; Song, X.; Chen, Y.; Zou, Q.; Wang, M.; Xu, A.; Li, X. Controlled growth of γ-MnO2 nanoflakes on OMS-2 for efficient decomposition of organic dyes in aqueous solution via peroxymonosulfate activation. J. Colloid Interface Sci. 2018, 529, 476–485. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Gao, M.; Zhou, C.; Xin, Y.; Zhang, G.; Xu, P.; Ma, D. Indium-doped β-MnO2 catalyst for activation of peroxymonosulfate to generate singlet oxygen with complete selectivity. J. Cleaner Prod. 2022, 380, 134953. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Wang, R.; Gao, M.; Xin, Y.; Zhang, G.; Xu, P.; Ma, D. Activation of peroxymonosulfate with cobalt embedded in layered δ-MnO2 for degradation of dimethyl phthalate: Mechanisms, degradation pathway, and DFT calculation. J. Hazard. Mater. 2023, 451, 130901. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Miao, J.; Guan, D.; Zhong, Y.; Ran, R.; Wang, S.; Zhou, W.; Shao, Z. Efficient wastewater remediation enabled by self-assembled perovskite oxide heterostructures with multiple reaction pathways. ACS Sustain. Chem. Eng. 2020, 8, 6033–6042. [Google Scholar] [CrossRef]
- Miao, J.; Li, J.; Dai, J.; Guan, D.; Zhou, C.; Zhou, W.; Duan, X.; Wang, S.; Shao, Z. Postsynthesis oxygen nonstoichiometric regulation: A new strategy for performance enhancement of perovskites in advanced oxidation. Ind. Eng. Chem. Res. 2019, 59, 99–109. [Google Scholar] [CrossRef]
- Liang, C.; Huang, C.F.; Mohanty, N.; Kurakalva, R.M. A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere 2008, 73, 1540–1543. [Google Scholar] [CrossRef] [PubMed]






| Sample | BET (m2/g) | AOS of Mn | Mn 2p3/2 | OII/(Ototal) by XPS | ||
|---|---|---|---|---|---|---|
| Mn2+ | Mn3+ | Mn4+ | ||||
| δ-MnO2 | 135 | 3.33 | 11.2% | 42.7% | 46.1% | 25.2% |
| α-MnO2 | 97 | 3.53 | 9.4% | 37.4% | 53.2% | 37.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; He, J.; Ma, C.; Zhang, Y.; He, Y.; Yu, Y.; Meng, T.; Zhang, M. α-MnO2 Reactive Lattice Oxygen Promotes Peroxymonosulfate-Activated Sulfamethoxazole Degradation. Catalysts 2025, 15, 824. https://doi.org/10.3390/catal15090824
Zhang H, He J, Ma C, Zhang Y, He Y, Yu Y, Meng T, Zhang M. α-MnO2 Reactive Lattice Oxygen Promotes Peroxymonosulfate-Activated Sulfamethoxazole Degradation. Catalysts. 2025; 15(9):824. https://doi.org/10.3390/catal15090824
Chicago/Turabian StyleZhang, Hao, Junhui He, Chao Ma, Yue Zhang, Ying He, Yangyang Yu, Tan Meng, and Min Zhang. 2025. "α-MnO2 Reactive Lattice Oxygen Promotes Peroxymonosulfate-Activated Sulfamethoxazole Degradation" Catalysts 15, no. 9: 824. https://doi.org/10.3390/catal15090824
APA StyleZhang, H., He, J., Ma, C., Zhang, Y., He, Y., Yu, Y., Meng, T., & Zhang, M. (2025). α-MnO2 Reactive Lattice Oxygen Promotes Peroxymonosulfate-Activated Sulfamethoxazole Degradation. Catalysts, 15(9), 824. https://doi.org/10.3390/catal15090824







