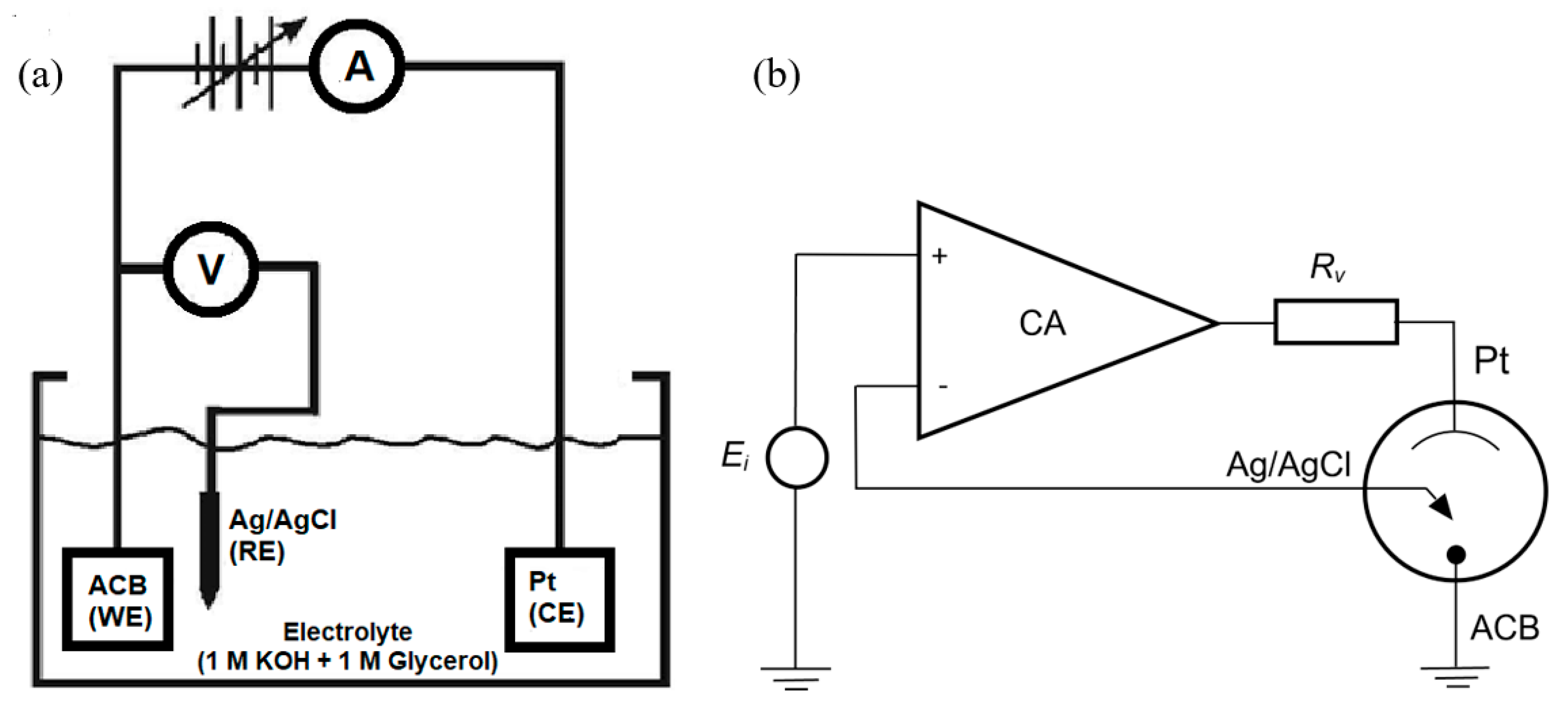
2.1. Impedance Spectra
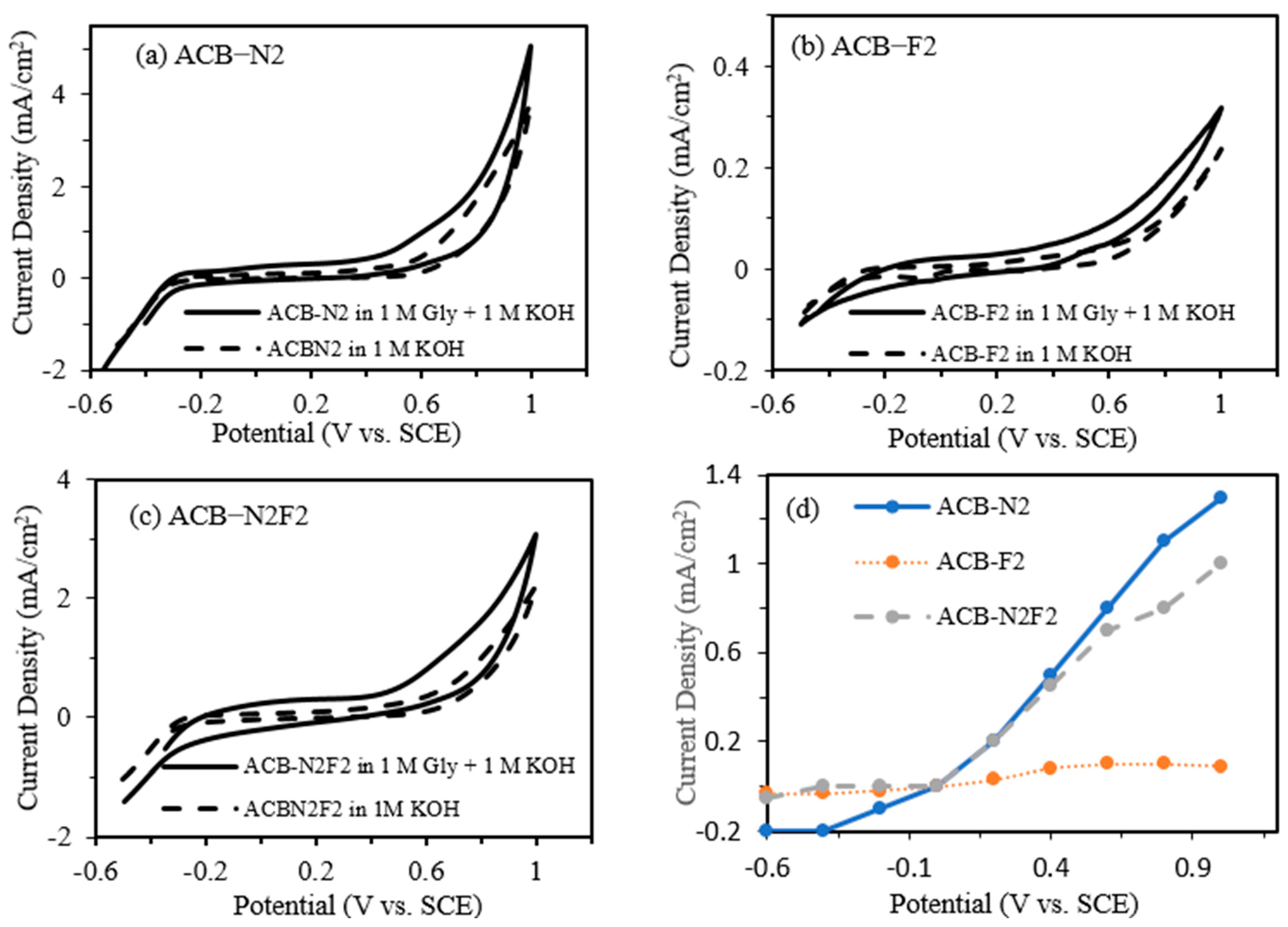
Figure 1 gives the CV results comparing the performance of the electrodes with and without 1 M glycerol. Adding 1 M glycerol considerably improves the electrochemical response of the composite electrodes. The electrocatalytic activity toward glycerol oxidation is more accurately assessed by the difference in current densities between the two conditions. For example, at 1.0 V vs. SCE, the ACB-N2F2 electrode exhibits a current density of 3.08 mA/cm
2 in the presence of 1 M glycerol, compared to 1.99 mA/cm
2 in its absence, resulting in a net glycerol oxidation current of approximately 1.09 mA/cm
2 (
Figure 1d). This net enhancement, rather than the absolute current, serves as a more reliable indicator of catalytic activity. Notably, this difference reflects the contribution of glycerol electrooxidation over the background capacitive and surface oxidation processes. Similar behavior is observed for the ACB-N2 and ACB-F2 electrodes. These observations align with the literature reports, which emphasize the importance of subtracting baseline currents to isolate true electrocatalytic effects. Accordingly, we have revised our analysis to emphasize these differences as a more accurate reflection of the electrode’s activity.
The corrected values (
Figure 1d) indicate that the ACB-N2 electrode consistently exhibits the highest net current density across the potential window, with a maximum of 1.3 mA/cm
2 at 1.0 V, followed by ACB-N2F2 (1.0 mA/cm
2) and ACB-F2 (0.09 mA/cm
2). This trend highlights ACB-N2 as the most active catalyst for glycerol oxidation based on instantaneous reaction current, contrary to earlier interpretations.
However, the glycerol electrooxidation activity of these electrodes, as measured using cyclic amperometry (CA), was previously reported [
31]. The outstanding activity of ACB-N2F2 is attributed to the high electronegativity difference between fluorine and nitrogen. Therefore, when long-term performance and reaction kinetics are considered, ACB-N2F2 displays notable advantages. Despite its lower ECSA (1057.10 cm
2/g) compared to ACB-N2 (1309.84 cm
2/g) and ACB-F2 (1751.39 cm
2/g), ACB-N2F2 exhibits the lowest Tafel slope (177.97 mV dec
−1), indicating faster kinetics and more efficient charge transfer during glycerol oxidation. This kinetic advantage suggests that the synergistic effect of dual N-F doping enhances active site reactivity and electronic conductivity, compensating for the reduction in surface area.
Additionally, chronoamperometry results confirm the superior stability of ACB-N2F2, which maintains a stable current over extended periods, unlike ACB-N2 and ACB-F2, which show significant current decay. This behavior reflects the enhanced structural integrity and electrochemical robustness conferred by dual doping, supporting its suitability for long-term catalytic applications.
In summary, while ACB-N2 demonstrates the highest net glycerol oxidation current, ACB-N2F2 excels in kinetic efficiency and electrochemical stability, making it a promising candidate for durable electrocatalysis in glycerol oxidation systems. The performance differences underscore the importance of evaluating both instantaneous activity and stability when selecting electrocatalysts for real-world applications.
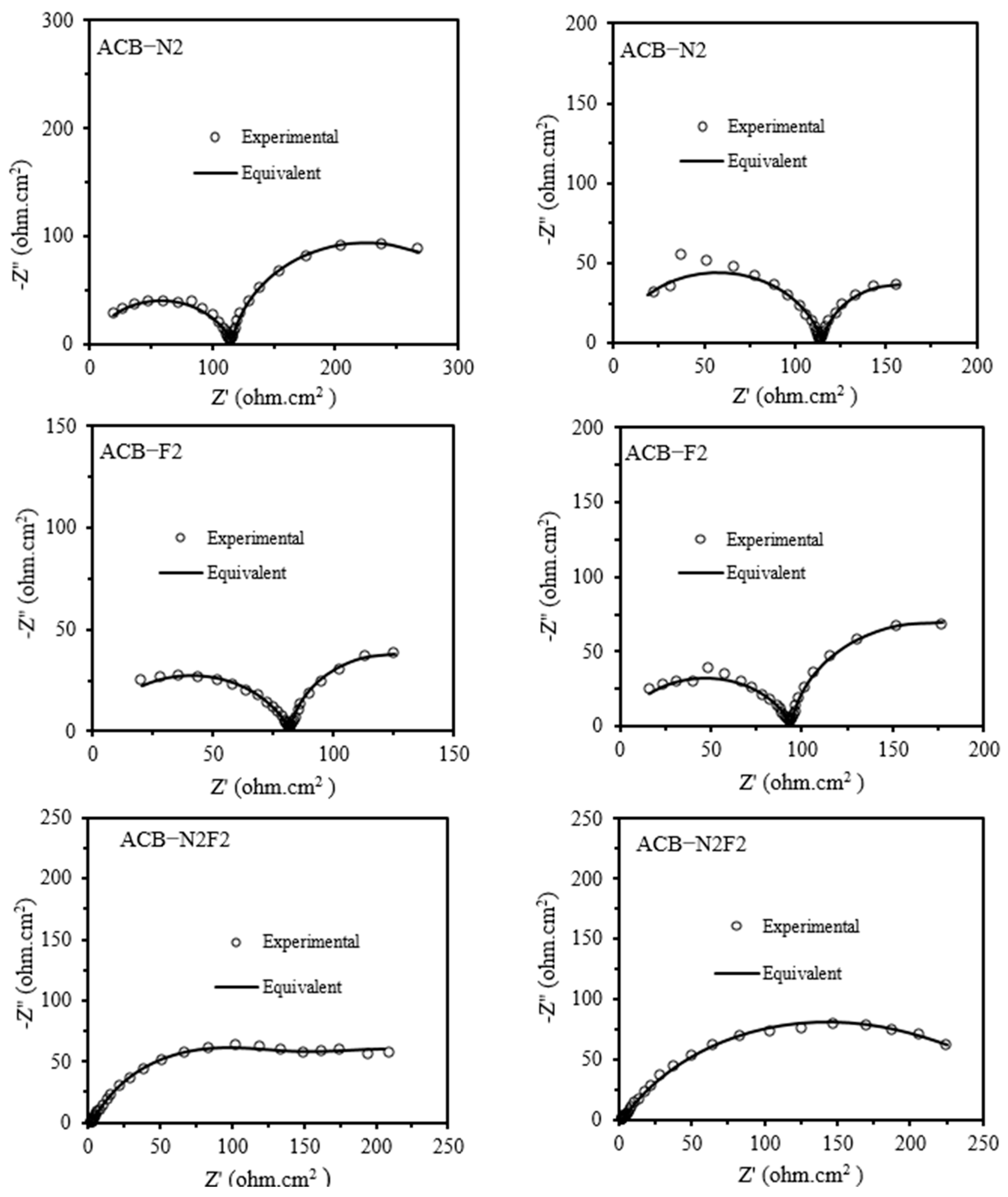
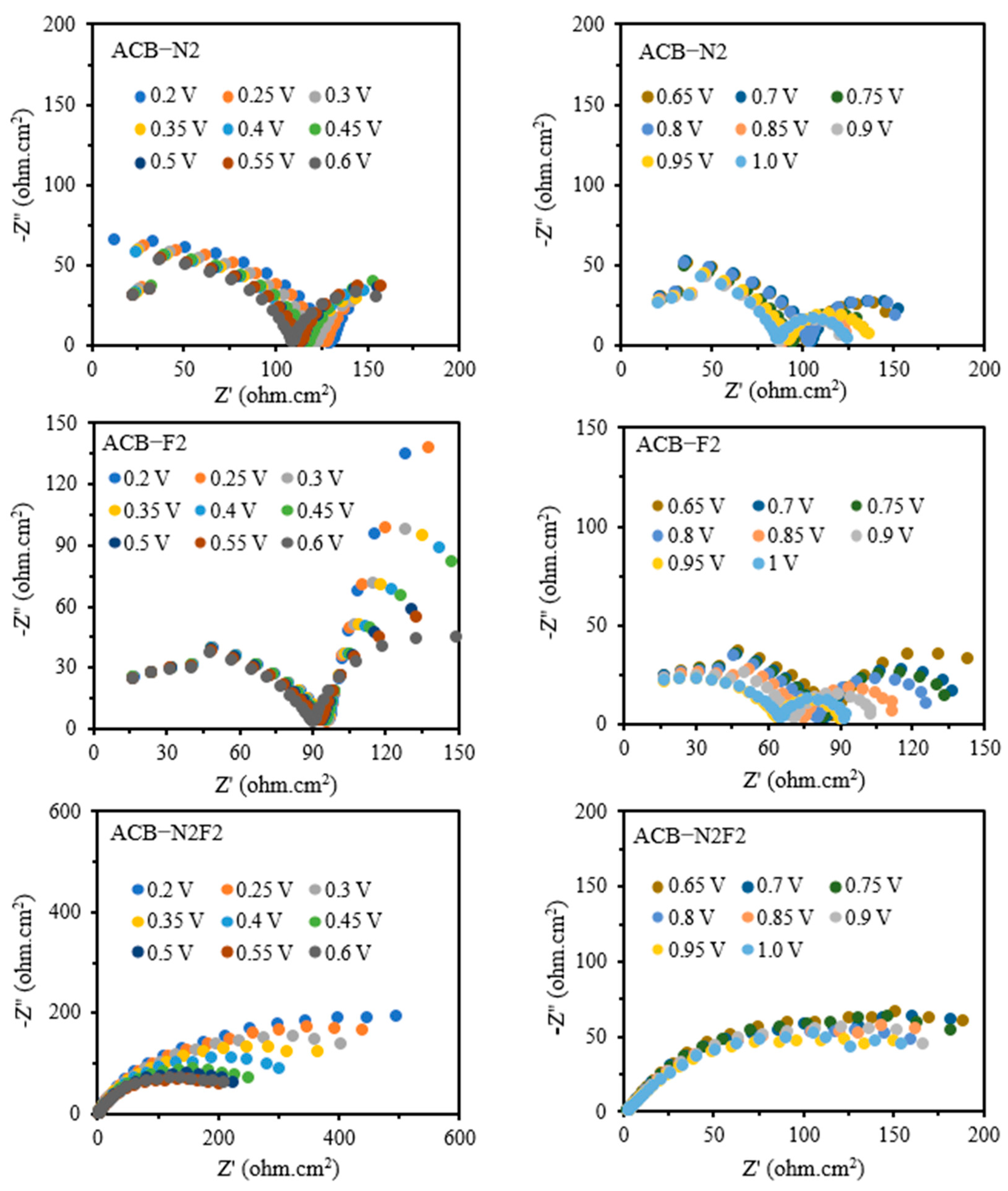
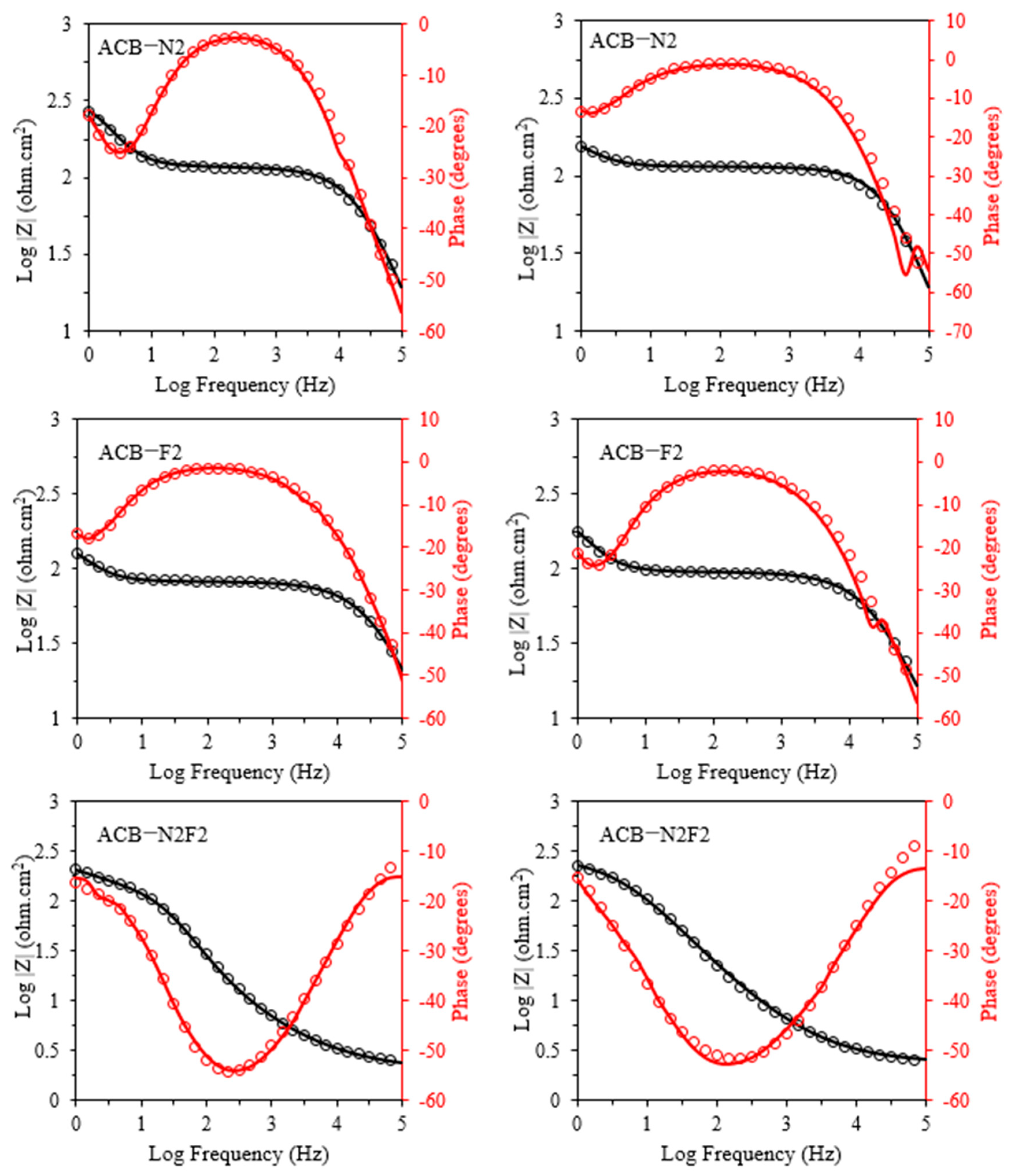
Figure 2 gives the EIS complex plane plot determined for the anode under potentiostatic conditions. The number of elementary frequencies is N = 31. The experimental spectra of the ACB-N2 and the fluorine-doped activated carbon black composite electrode (ACB-F2) show two suppressed semicircles, whereas that of ACB-N2F2 exhibits only one. The catalytic layer of the anode and the processes that occur there are detected in a mid-frequency range. A low-frequency loop seems the most interesting as it provides information about diffusion. A low-frequency range is assigned to slow oxygen diffusion or diffusion of generated water through the catalyst layer [
34].
However, various equivalent circuits have revealed that the choice of a suitable equivalent circuit for a complete and universal description of a redox reaction remains a herculean task. A Randles equivalent electrical circuit is the most commonly used basic circuit to assess electrode mechanisms. In an equivalent circuit, double-layer capacitance (CDL) or constant phase element (CPE) represents the double-layer capacity [
25,
35]. In the Randles equivalent circuit, we also have
Rs and
Rct. CPE is used for electrodes with a porous structure and electrical inhomogeneity. Warburg semi-infinite impedance represents the diffusion of the reagent to the surface of the electrode, whereas Warburg impedance accounts for finite diffusion. The spectra of ACB-N2 and ACB-F2 were analyzed using
Rs +
Q1/
Rct +
Q2/
R3, while that of ACB-N2F2 was analyzed using
Rs +
Q1/(
Rct +
W). The presence of both nitrogen and fluorine dopants modifies the electronic structure of the carbon matrix, reducing charge transfer resistance. This results in a more homogenous electrochemical environment, leading to a single dominant time constant in the impedance response. Nitrogen doping enhances electron-donating properties, improving conductivity and facilitating charge transfer. Fluorine doping modifies surface hydrophobicity and electronic density, reducing undesired side reactions and stabilizing intermediate species. The co-doping of nitrogen and fluorine harmonizes the catalyst surface, streamlining reaction pathways and eliminating secondary resistive processes, typically resulting in multiple time constants.
In ACB-N2 and ACB-F2, isolated doping sites may introduce multiple reaction centers with varying charge transfer resistances, leading to a double time constant model. However, in ACB-N2F2, the optimized distribution of active sites mitigates these discrepancies, resulting in a more uniform electrochemical response. The dual-doped system enhances electrode–electrolyte interactions, facilitating faster charge transfer and mitigating diffusion-related impedance effects. This further supports the single time constant model, which features a streamlined and efficient charge transfer pathway.
The capacity CPE accounts for the sum of the capacities of the double layer for glycerol oxidation diffusion and the double layer for the oxidation process via the adsorption of glycerol to form the product. The use of CPEs typically reflects surface heterogeneity and non-ideal capacitive behavior. This indirectly accounts for phenomena like the adsorption of glycerol and its oxidation intermediates. It is used to describe the nonfaradaic capacitances of oxidation of anode pores. The values of the CPE exponent α are more stable across potential regions. Moreover, the presence of Warburg impedance in the equivalent circuit of ACB-N2F2 hints at deeper electrochemical dynamics beyond simple charge transfer. In glycerol oxidation reactions, this element captures not just diffusion phenomena but also potentially adsorption-mediated transport processes, which are critical in systems involving polyols.
2.3. Forward Sweep
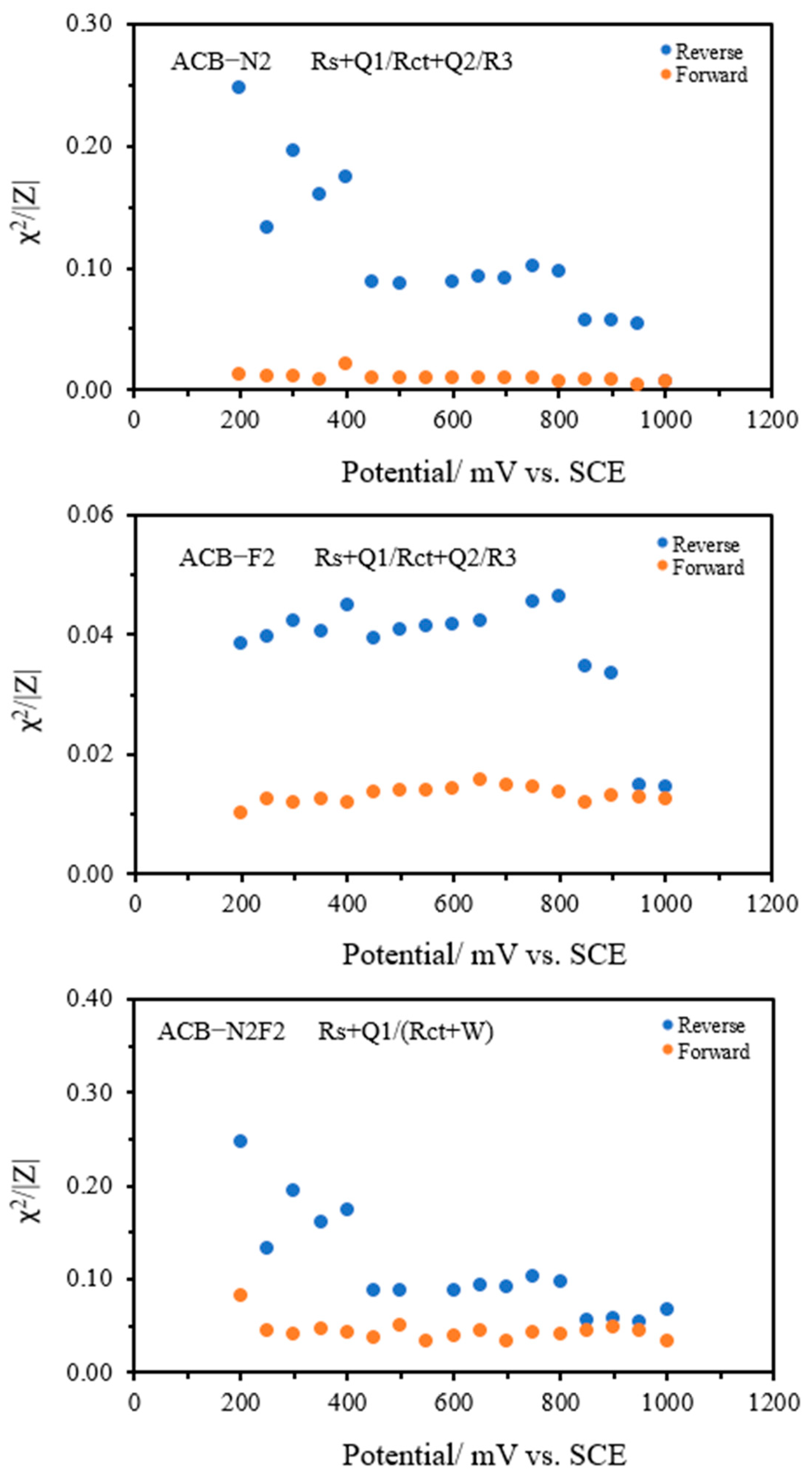
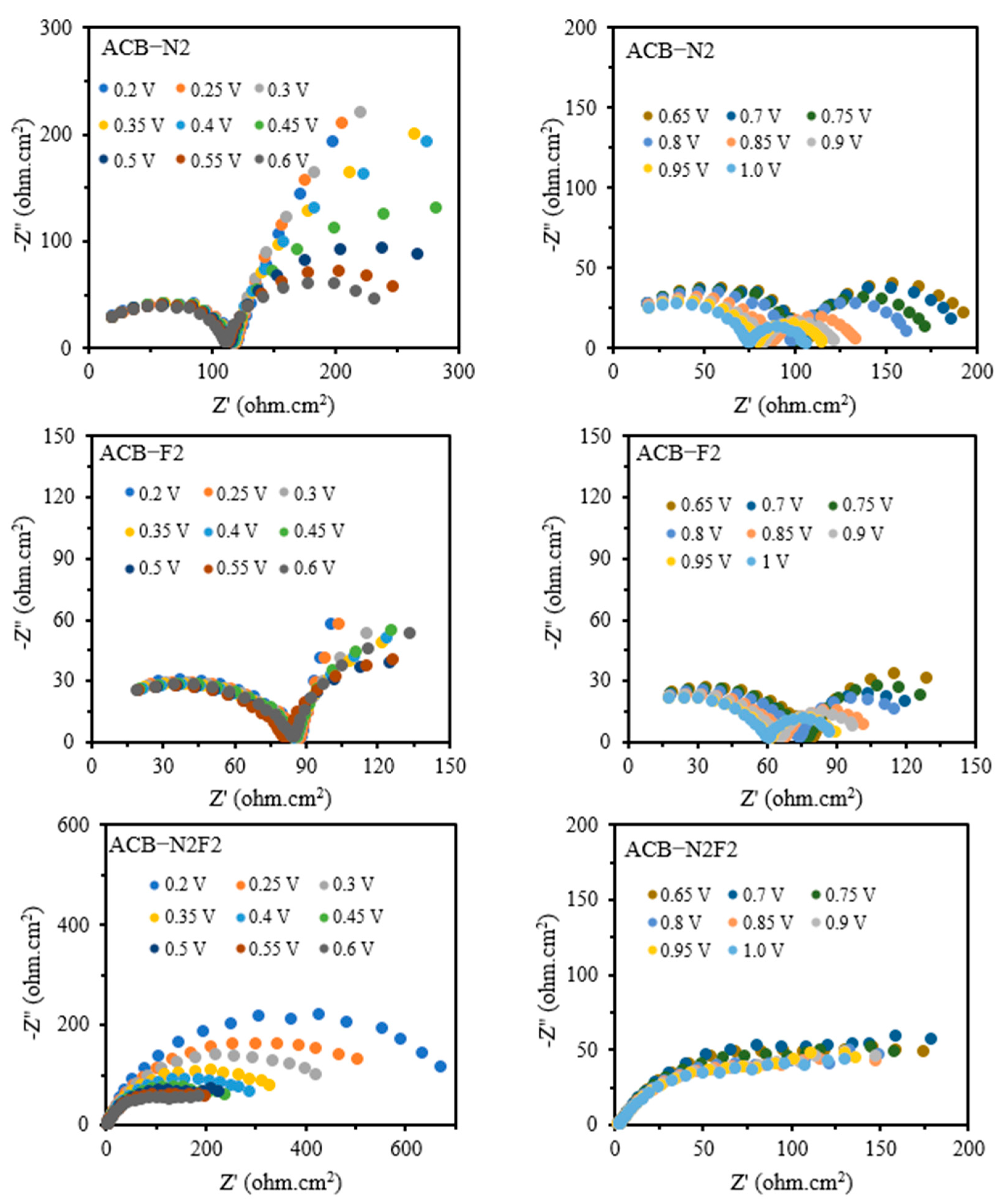
Figure 4 illustrates the Nyquist (complex plane) plots for the forward potential sweeps of the three electrodes, ACB-F2, ACB-N2, and ACB-N2F2, across the investigated potential cycles. The onset of charge transfer, identified by a deviation from the baseline in the corresponding direct current (DC) voltammograms, occurs at approximately 0.36 V for ACB-F2, 0.16 V for ACB-N2, and 0.37 V for ACB-N2F2. This deviation marks the initiation of faradaic processes and reflects the activation of surface redox sites. The impedance data across all potential cycles were fitted using the same equivalent circuit model for each electrode, achieving residual fitting errors below 3%, 2%, and 9% for ACB-F2, ACB-N2, and ACB-N2F2, respectively. This confirms the adequacy and robustness of the selected model in capturing the electrode interfacial phenomena under varied electrochemical conditions.
In the high-frequency region of the impedance spectra typically associated with rapid charge transfer and double-layer capacitance dynamics, distinct semicircular arcs are evident. For ACB-F2 and ACB-N2, these arcs initiate around mid-to-high frequencies and extend toward the mid-frequency region. The partial nature of these arcs indicates overlapping electrochemical processes, primarily governed by interfacial kinetics and double-layer effects. A second semicircle emerges as frequency further decreases, attributed to slower phenomena, such as intermediate adsorption or surface-bound species relaxation.
Conversely, the ACB-N2F2 electrode presents a more coherent impedance profile, with a semicircle forming from the onset of the high-frequency region and gradually transitioning to the low-frequency domain within the first quadrant. This continuous progression suggests improved charge carrier mobility and a more unified response across time constants, implying enhanced electrochemical performance and lower recombination losses.
The semicircle diameter, representing the effective charge transfer resistance, decreases with increasing applied potential for all electrodes. This trend highlights improved electrode kinetics and increased conductivity at elevated potentials, particularly for ACB-N2F2, which consistently exhibits lower impedance across the voltage range, affirming its superior electrochemical reactivity.
2.4. Reverse Sweep
In the reverse sweep (
Figure 5), the same trends as the forward sweep persist with notable differences. The semicircle diameters for all electrodes decrease significantly, suggesting enhanced conductivity or surface activation effects following the forward polarization. However, the quality of fitting, expressed by the
χ2/|
Z| parameter, deteriorates—especially for ACB-N2 and ACB-N2F2 at low potentials—indicating more complex interfacial dynamics during cathodic scanning. Additionally, the appearance of noise in the second quadrant for ACB-F2 and ACB-N2 and in the first quadrant for ACB-N2F2 at high potentials suggests external perturbations, likely from acoustic or mechanical vibrations, which become more pronounced at low impedance or under highly capacitive conditions.
Overall, the combination of EIS features, including the number and size of semicircles, noise patterns, and model fitting fidelity, points to distinct electrochemical behaviors among the electrode systems. ACB-N2F2, in particular, exhibits a more stable and unified response, possibly due to synergistic effects between nitrogen and fluorine doping that streamline interfacial charge transport.
2.5. Zeroes of the Interfacial Impedance
The zeroes of the interfacial impedance are determined from the fitted capacitances and resistances. The successful fitting of circuits, as shown in
Figure 3, indicates the validity of the zeroes. The transfer function is the interfacial admittance, given that the current is measured as the interfacial potential is controlled. This is referred to as a “chemical” or “purely potentiostatic” case. System stability is evaluated by analyzing the zeroes and poles of the interfacial impedance function. Stability is indicated when the poles (or inverse of time constants) of the impedance have negative real parts. This ensures that the system’s response decays over time rather than growing unbounded. In practice, the equivalent circuit model for interfacial impedance often comprises elements with physically meaningful positive values, such as resistors and capacitors. However, in modeling, idealized elements (e.g., negative inductances or resistances) may sometimes be mathematically valid when representing specific behaviors.
The zeroes of the interfacial impedance, denoted as B, are governed not directly by potential but by the partial derivatives of reaction rates with respect to surface coverage and potential. Specifically, Equations (1) and (2) [
30] express these relationships as follows:
F is the Faraday constant (
C mol
−1),
E is the electric field, and
θ is the depression angle of a semicircular arc below the real axis.
C is pseudo-capacitance (
F cm
−2) and
L is a pseudo-inductance (
H cm
2), provided that the signs of the elements are not constrained to always be positive, but for real data, one or the other circuit gives better fits. For the galvanostatic and potentiostatic cases, the relationship between the mechanistic parameters and the zeroes and poles has been given in general [
29]. Therefore, the zeroes of the interfacial impedance are directly associated with the chemical mechanism (chemical reaction step), disregarding the potential dependency of the rate constants.
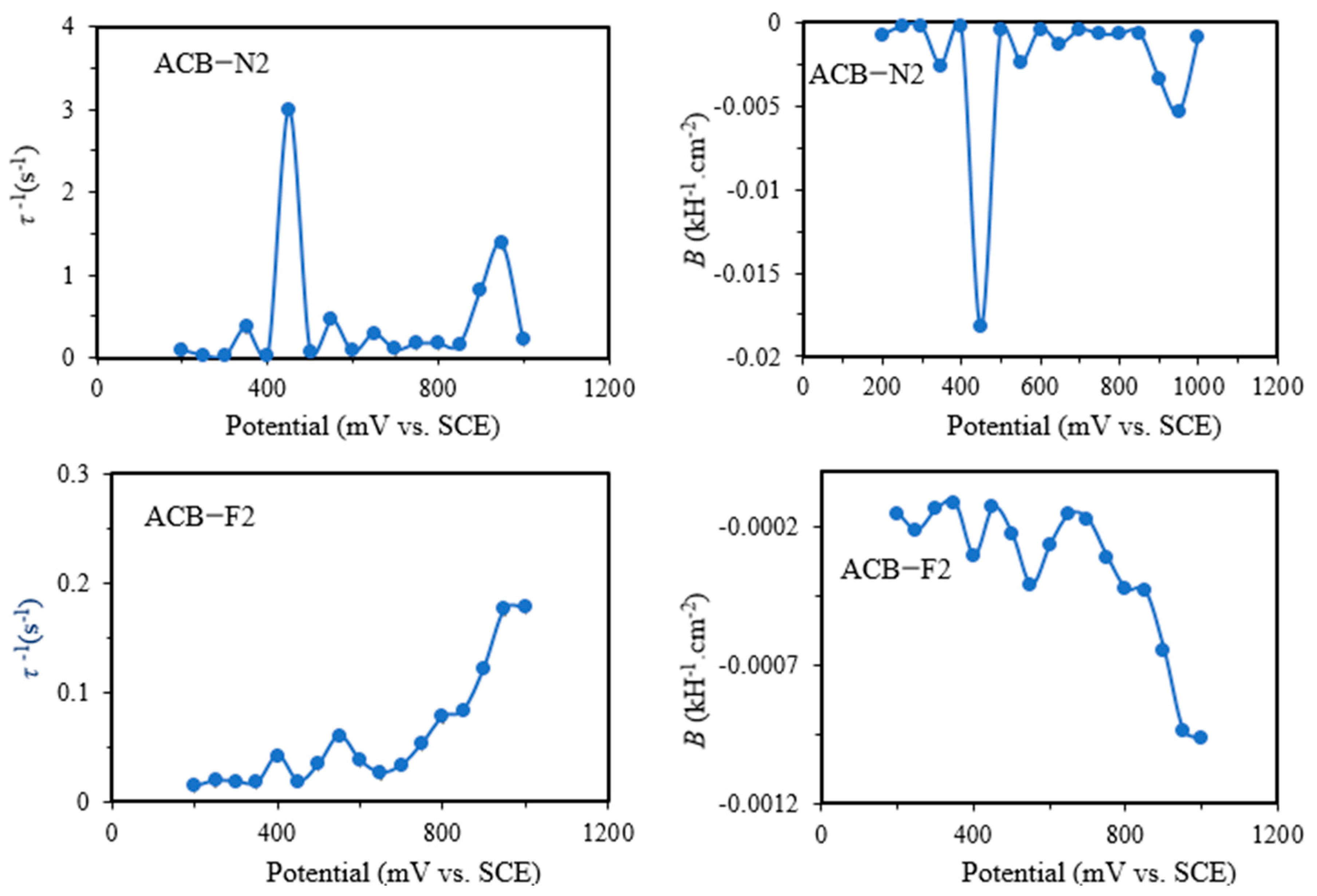
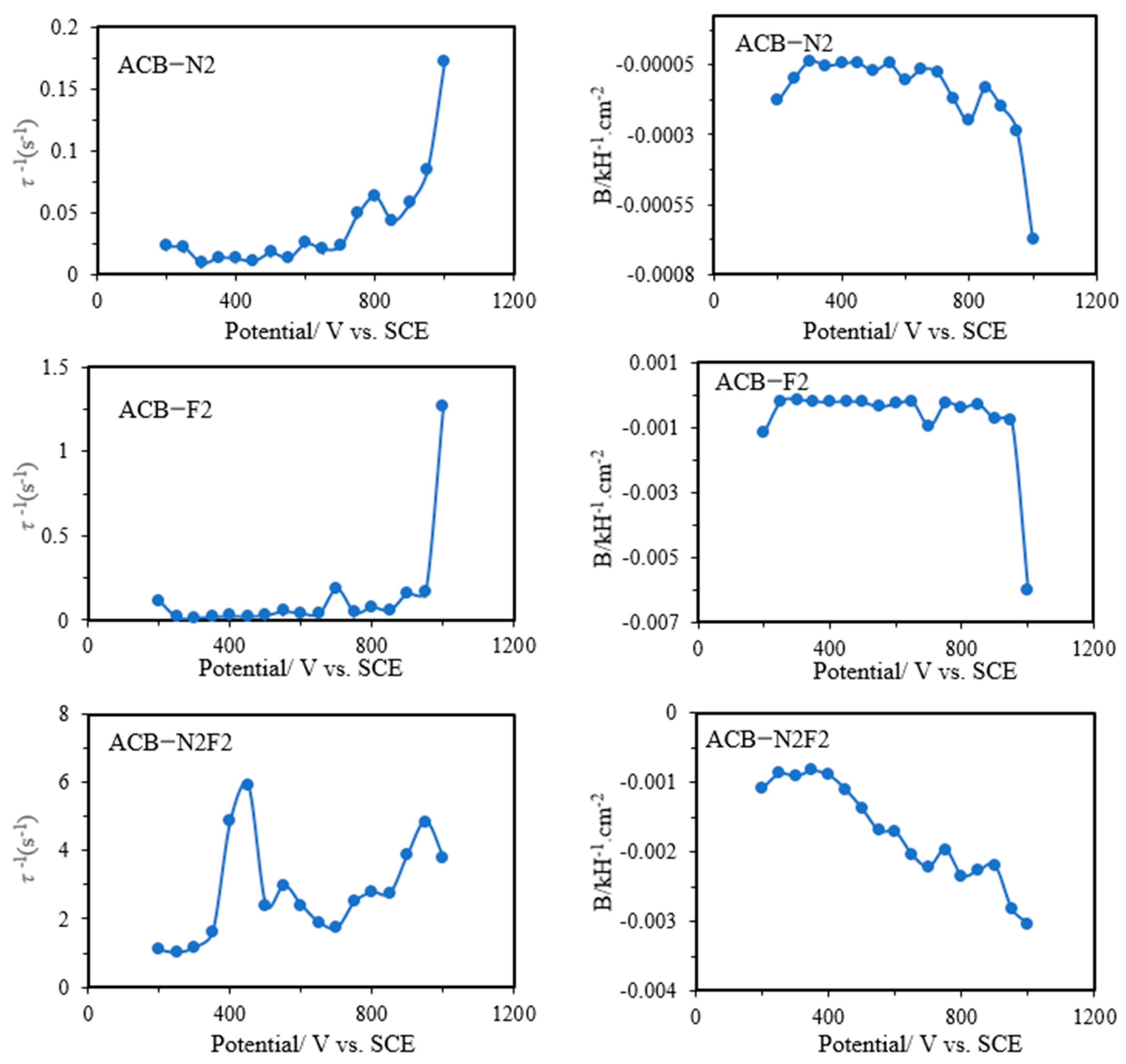
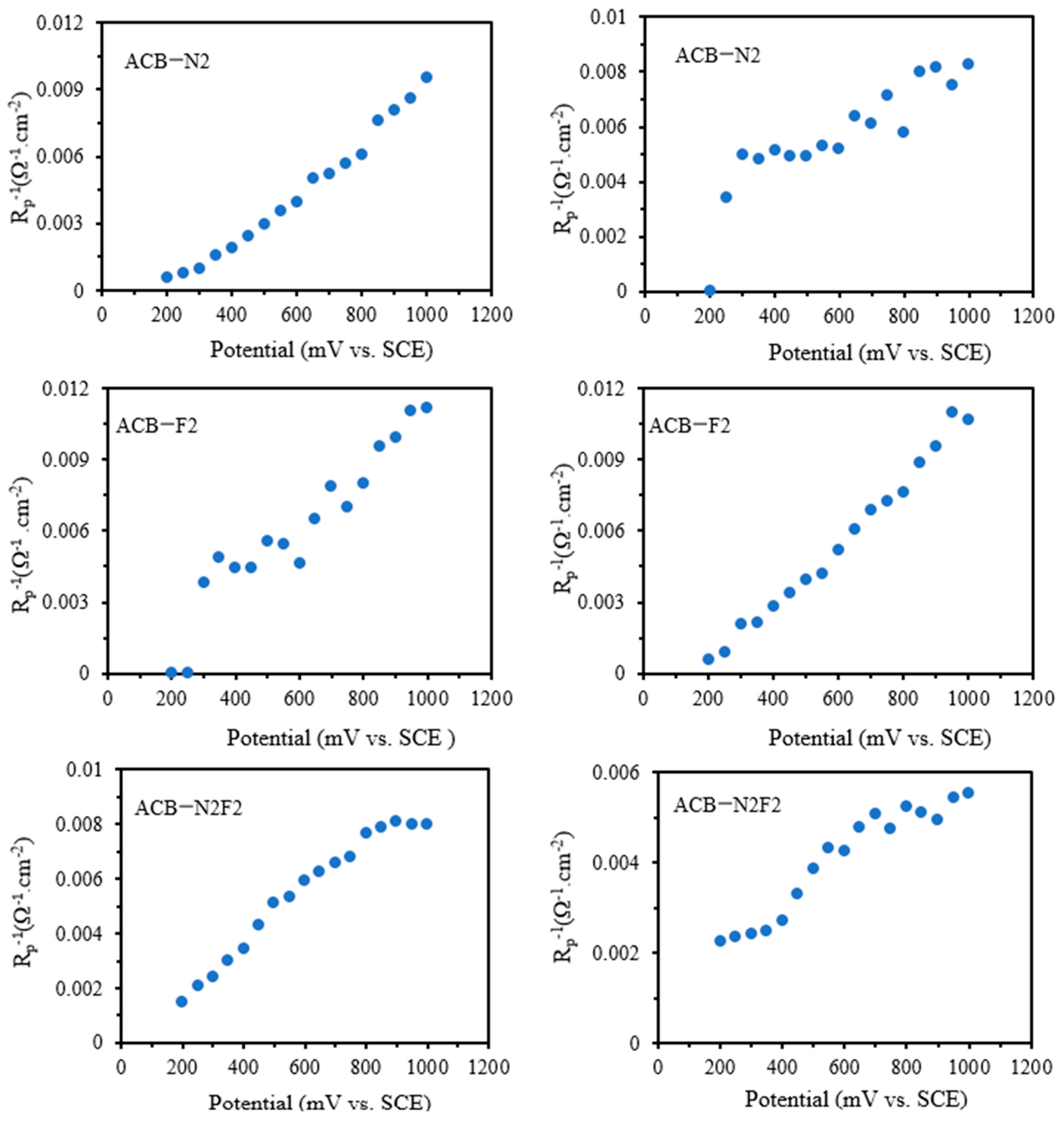
Figure 6 shows the values of the derived parameters
B = 1/
C2Rct2 and the time constant
τ,
τ−1 = (
Rct + R2)/(
C2RctR2) as a function of applied potential. The parameters have a simpler meaning than the elements of the equivalent circuit, which have a higher degree of complexity, especially for reaction mechanisms involving only one absorbed intermediate species that is not controlled by diffusion. The parameter
τ quantifies the rate at which the fractional surface coverage of the adsorbed carbon monoxide (
θCO) relaxes to a new value after a change in potential. In addition to the faradaic contribution, the adsorption of intermediates could influence the double-layer charge, making CDL a function of
θCO. This claim could be justified by the two-time constant exhibited by the electrodes, complemented by the two semicircles exhibited by ACB-N2 and ACB-F2, and the presence of the Warburg element exhibited by ACB-N2F2. The parameter exhibits a somewhat constant slope as a function of potential and a change at the shoulder peak potential at higher overpotential for ACB-N2 and ACB-F2 and lower overpotential for ACB-N2F2 (
Figure 7). This trend could be due to the partial derivative of the net generation of
rCO concerning the
θCO at a constant potential. Since the
rCO parameter depends on both the reaction intermediate step and the oxidation step of the intermediate species in the glycerol oxidation pathway, the changes attributed to
CO are expected to be larger for the reaction intermediate step, particularly if it involves more than one available site [
36].
Parameter B also reveals the shoulder peaks, which can be attributed to charge transfer resistance, resulting in a change in the coverage of intermediate species. Experimentally, the two-time constant was merged into a single semicircle. For all the electrodes, the values of parameter B are negative across the cycled potential, and all the capacitive circuit (τ) elements are positive, indicating that the electrodes exhibit a stable chemical mechanism consistent with their natural characteristics.
The noise generated by initial instability in the chemistry of the electrodes during the reverse sweeps causes the values and variability of the parameters B and
τ−1 in
Figure 7 to differ significantly from those of the forward reaction. The estimated zero-frequency resistance shown in
Figure 8 reveals that the impedance crosses into the second quadrant, and all the data points fall within this quadrant.
The impedance at zero frequency was determined from the parameters of the fitted equivalent circuit to assess the adequacy of the EIS spectra, as the features cannot be measured at a lower frequency. At 0 frequency, the impedance is real and estimated to be the inverse of the slope of the quasi-steady-state polarization curve for equivalent circuits with a direct current path [
30] as follows:
For ACB-N2F2, the circuits exhibit blocking characteristics at direct current. The impedance tends to be significantly higher at zero frequency and low potential. This condition also fulfils the above equation. Furthermore, the relationship holds for all the potential cycle regions of the three electrodes since
τ > 0. The polarization curves in
Figure 9 resemble the voltammetry curves of all the electrodes due to little or no perturbation.
The obtained values of
Rp from the equivalent circuit agree in sign with
djss/
dE on the forward sweep for all the potential cycles. In the Tafel region,
Rct =
Rp, meaning that the Ts for
Rct agree with the Ts of the current at a steady state [
30]. Considering ACB-N2F2 with an equivalent containing a Warburg element, the last part of the forward sweep may exhibit unquantifiable features at lower frequencies, which are not accessible in the experiment. Similar to the voltammetry slope, the values of
Rp remain positive throughout the potential circuit during the reverse sweep.
2.6. Charge-Transfer Parameters
The EIS spectra in
Figure 3 revealed the qualitative features of the electrodes in the electrooxidation reaction. The spectra were analyzed using the equivalent circuits inserted in
Figure 3. The values of the circuit’s elements were transformed to parameters applicable in the kinetic theory for a single adsorbed species mechanism [
36] as follows:
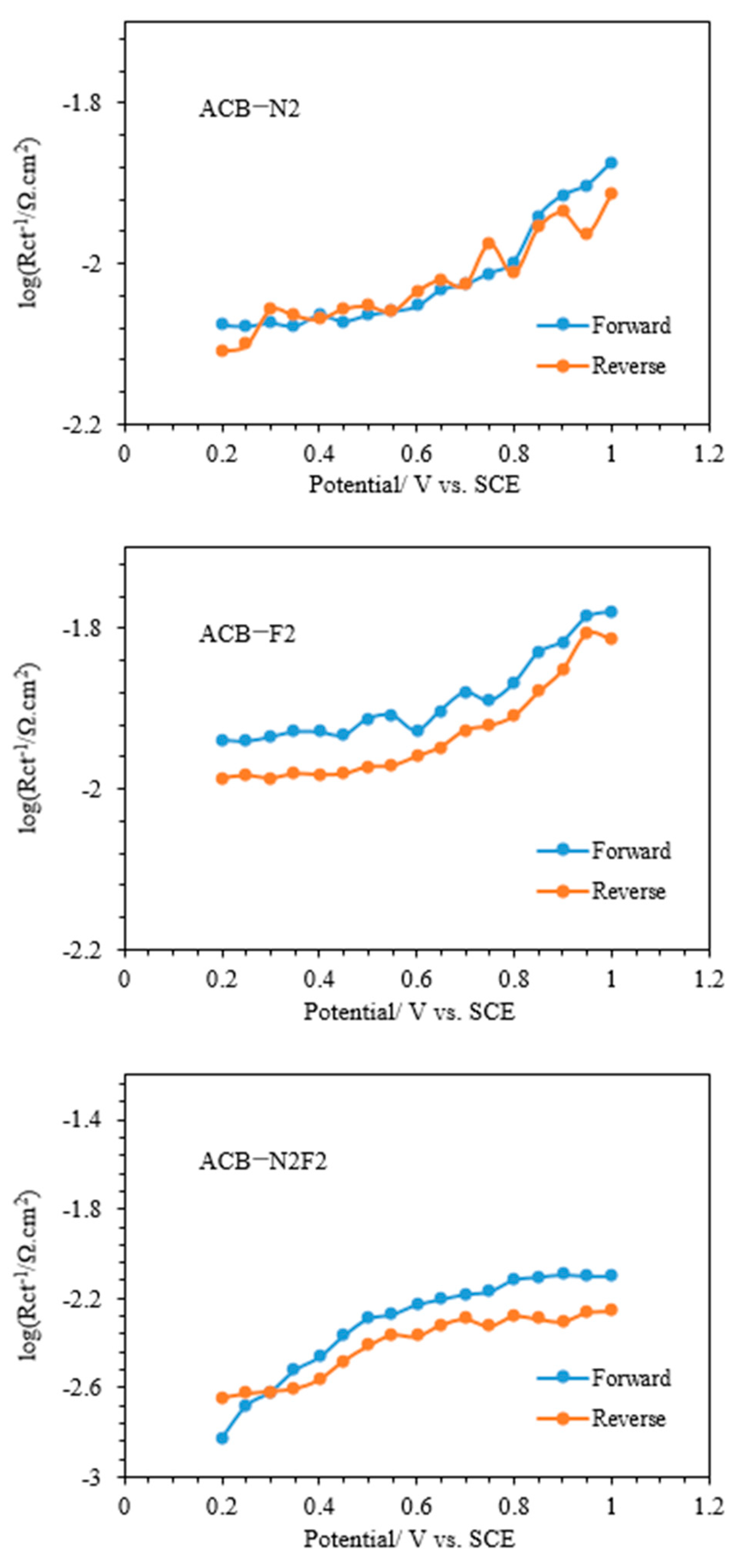
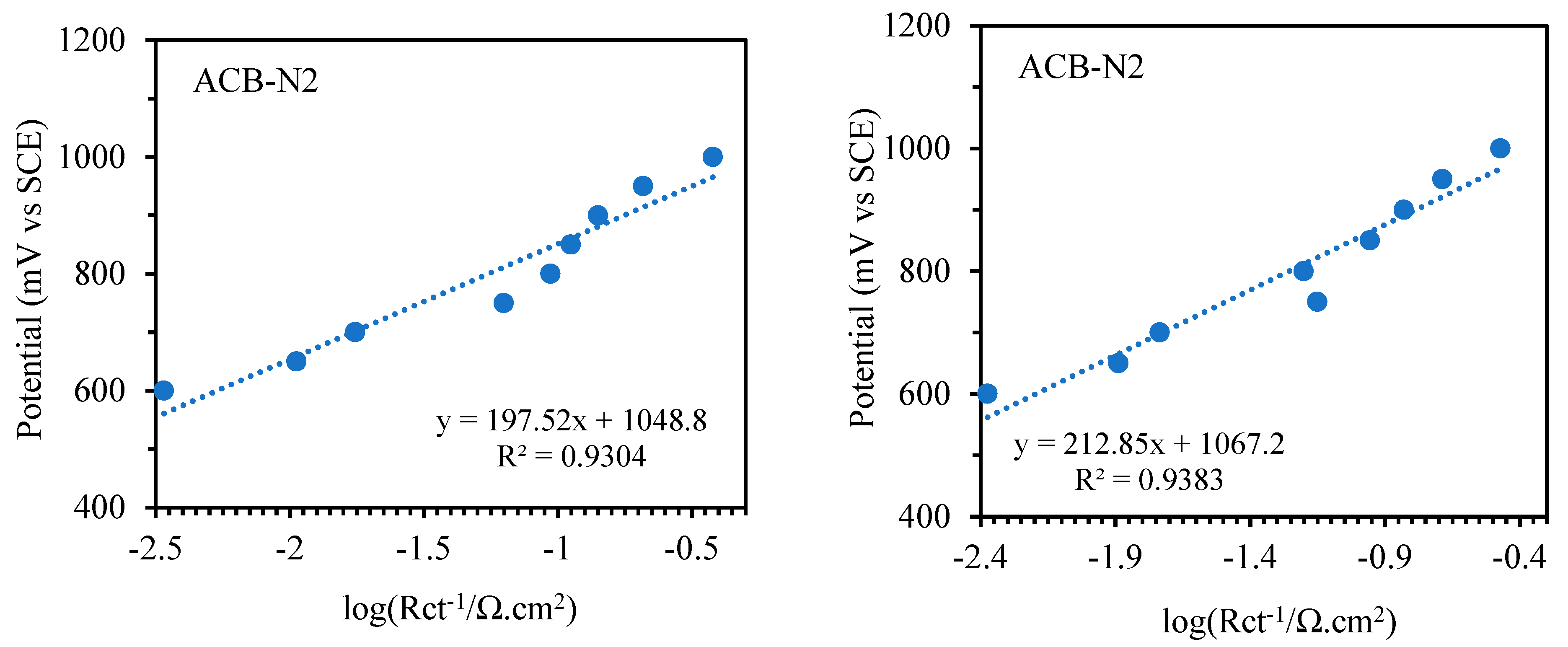
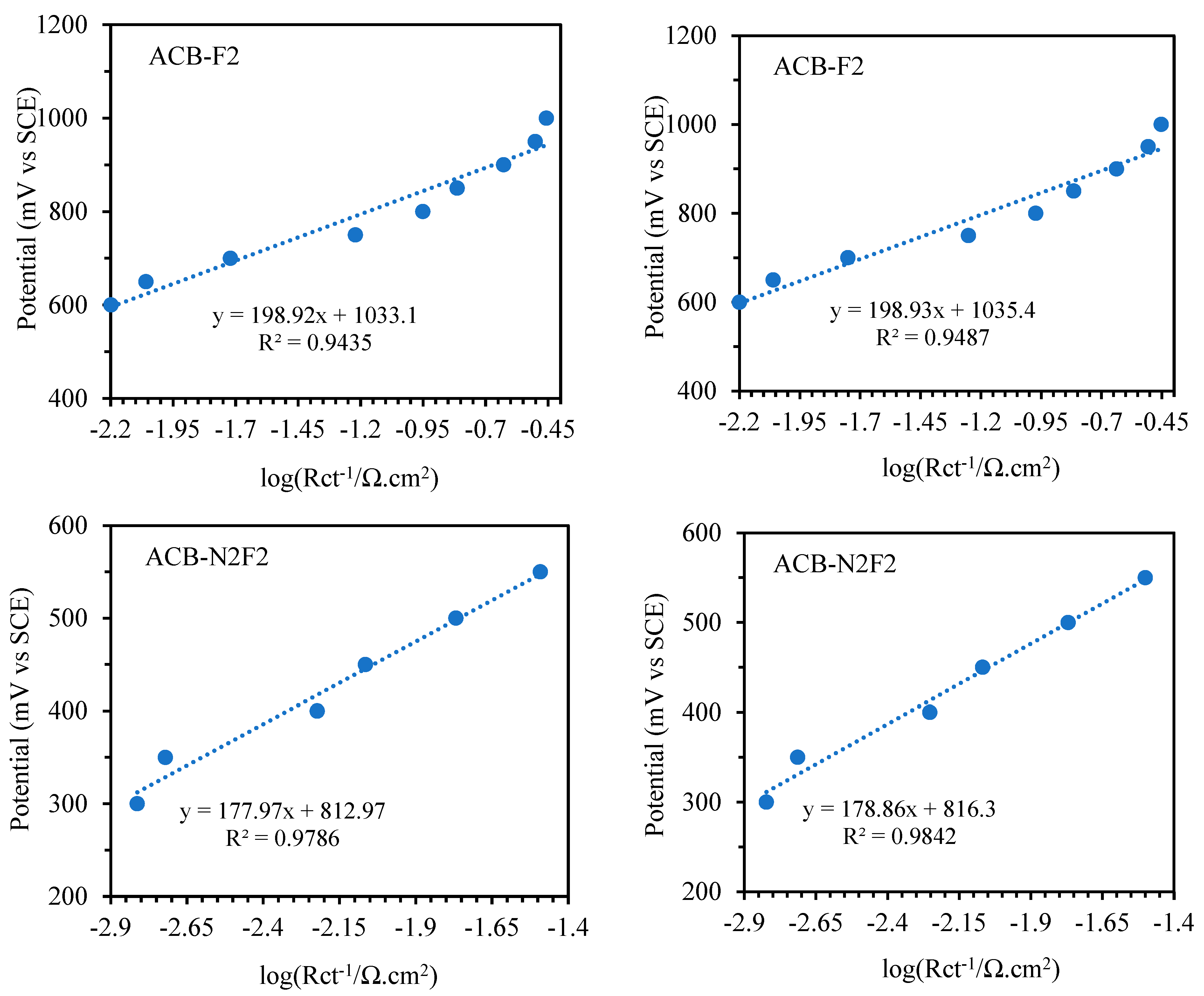
To explore the kinetic parameters of the functionalized carbon composite catalysts for glycerol electrooxidation, the Tafel plots in
Figure 10, which are plotted according to
E (vs. SCE) vs. log (
Rct−1) [
2], follow the Tafel equation below:
Figure 11 presents the curves of the charge-transfer resistances obtained from the equivalent circuits. A Ts (
b) of 197.52 and 198.92 mV dec
−1 were achieved for ACB-F2 and ACB-N2, respectively, in the potential range of 0.6 to 1 V vs. SCE. In contrast, 172.61 mV dec
−1 was obtained for ACB-N2F2 in the potential range of 0.25 to 0.5 V vs. SCE for the forward sweep. The reverse sweep showed a higher Ts than the forward sweep of the oxidation peak for all the electrodes within the same potential region.
Table 1 presents the deduced Ts and
β (charge transfer coefficient) at room temperature for the reaction, considering both reverse and forward sweeps, assuming the process is based on the adsorption of one-electron species. ACB-F2 and ACB-N2 have relatively comparable Ts and exhibit a similar reaction mechanism for glycerol electrooxidation. ACB-N2F2 exhibits the fastest kinetics, having the highest charge transfer coefficient.
In comparison with metal-based electrocatalysts, ACB-N2F2 is similar to Au-based (Ts = 0.177 V dec
−1) but significantly slower than Pt (Ts = 160 to 169 mV dec
−1) [
32,
37] and Pd-based (Ts = 136 mV dec
−1) electrodes (
Table 2). The elevated Tafel slopes observed in our study highlight the fundamental differences in charge transfer kinetics between metal-free and metal-supported systems. The absence of metal sites likely contributes to a less efficient electron transfer pathway, increasing reaction resistance [
29,
30,
31,
32,
33]. However, despite slower kinetics, functionalized composite carbon materials offer a cost-effective and sustainable alternative for glycerol oxidation, reducing dependency on noble metals.
2.7. Rate-Determining Step (rds)
Tafel plot analysis is the primary approach for investigating the kinetic parameters of glycerol electrooxidation. Refs. [
38,
39] employed a mathematical method to derive kinetic parameters for anodic reactions, such as the oxygen evolution reaction (OER) on lead electrodes. Their study presents the relationship between parameters like Tafel slope, rate-determining step resistance (
R), apparent charge transfer coefficient (α
apa), and exchange current density as described below:
R* is a function of exchange current density. The overpotential (E − E0) is an independent variable; αapa can be determined from the slope obtained from the plot of log (R−1) against (E − E0). The catalyst exchange current density can be obtained using the intercept value of the plot.
A microkinetic modeling technique can also be used to determine kinetic parameters such as exchange current density and Tafel slope of the electrocatalysts based on the above Costal model.
For the anodic process, Costa and Da Silva [
38] proposed microkinetic modeling kinetic parameters for the anode catalyst, expressing the total resistance (
R) as Equation (7) as follows:
Meanwhile, the Butler–Volmer equation describes the relationship between electrode potential (
E) and current density (
j), and the equation can be transformed into Equation (7), considering that the rds of the electrooxidation process is the adsorption of hydroxyl species on the electrode.
where
v is the rate of electrooxidation.
A is the ECSA of the composite electrocatalyst and
kapa is the apparent rate constant.
θ is the rate of coverage of the adsorbed OH species. [H
2O]
gly is the glycerol concentration, and
βapa is the rds-based apparent charge transfer coefficient. Hence, the current density (
j) can be expressed as Equation (9) as follows:
According to Costa et al., the total resistance (
R) of the electrooxidation process can be demonstrated by introducing a term denoted by
Y (reaction admittance), which is the reciprocal of
R. From the foregoing, Equation (10) was obtained as follows:
Take R as the rds, the overpotential (E − E0) as the independent variable, and R as the dependent variable.
The curves follow a logarithmic expression proposed by Costa et al. [
40] as follows:
where
b represents the Tafel slope.
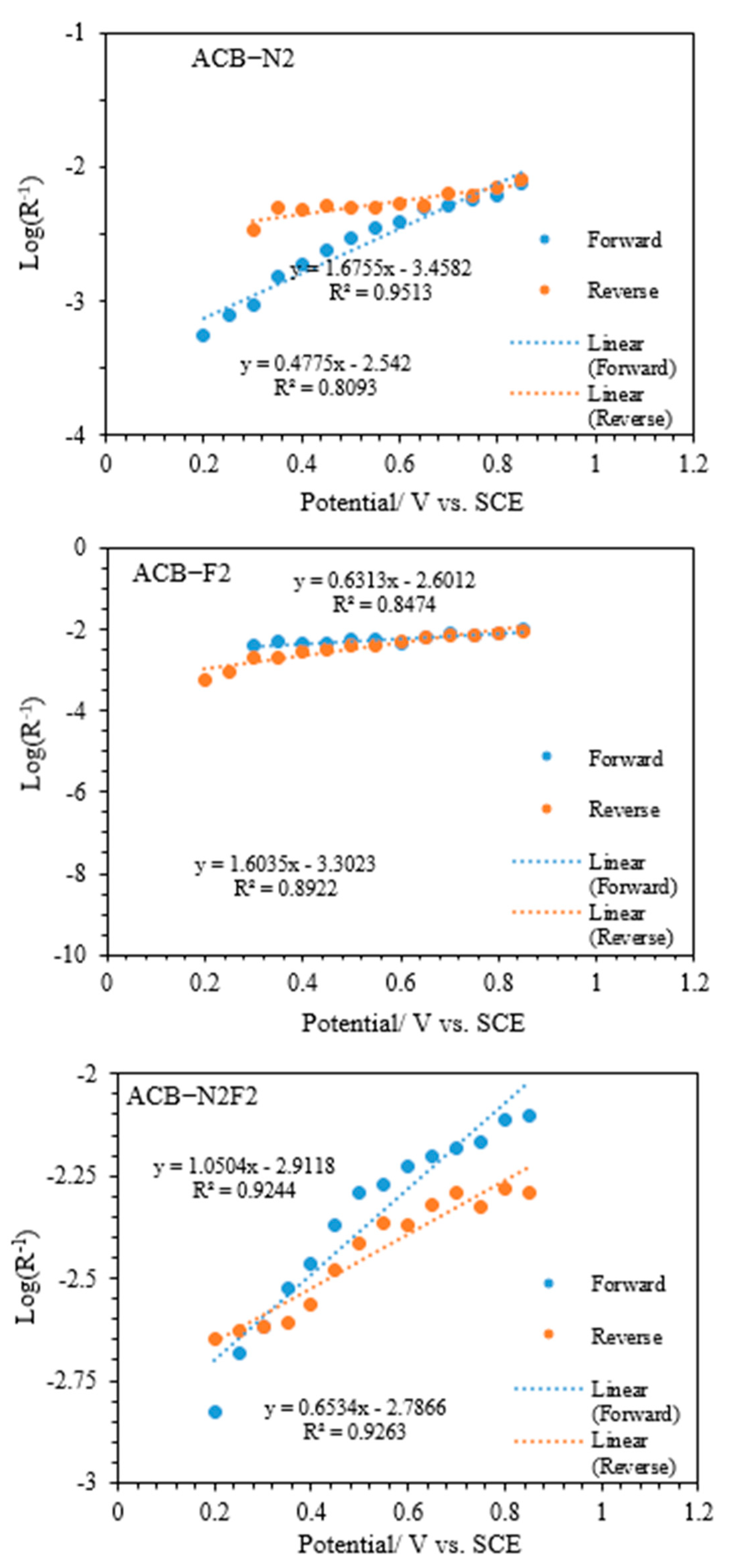
Take the overpotential (V) as the independent variable and log (
R−1) as the dependent variable to obtain a straight-line plot, as presented in
Figure 8. Then, the value of the slope is
And, the intercept is log (
R*
−1), according to Equation (5). According to this microkinetic model,
Table 3 presents the calculated exchange current density and Tafel slope for all the electrodes. ACB-N2F2 exhibits the lowest Tafel slope and the highest exchange current density, indicating the fastest electron transfer, while ACB-F2 has the highest Tafel slope. The relativity of the Tafel slope and exchange current density for ACB-N2 and ACB-F2 observed in both forward and reverse sweeps is irregular due to a complex mechanism. The complexity of the ACB-N2 and ACB-F2 mechanisms is a function of how closely the curves follow the logarithmic expression proposed by Costa et al. [
39]. The simpler mechanism of ACB-N2F2 relative to ACB-N2 and ACB-F2 is due to the synergistic effect of both nitrogen and fluorine on the composite carbon. The higher exchange current density exhibited by ACB-N2F2 indicates a faster electrode oxidation reaction, a vital factor for efficient electrocatalysts and remarkable glycerol oxidation performance [
32].
Using Equation (9), important electrooxidation kinetic parameters, like the apparent charge transfer coefficient (
βapa) and the apparent rate constant (
), are also computed, and their values are presented in
Table 3. ACB-N2F2 has the highest rate constant, while ACB-N2 has the lowest for both the forward and reverse sweeps. This result agrees with the Tafel slope, which shows that ACB-N2F2 is the best catalyst. The apparent charge transfer coefficient value mainly depends on the catalyst [
40].
The results of the electrooxidation kinetic process study and the electrocatalyst microkinetic modeling investigated in this work are important for future studies of novel electrooxidation catalysts. The catalytic glycerol electrooxidation activity evaluation techniques in this study provide a suitable protocol for electrooxidation kinetic studies.
2.8. Double-Layer Capacitance
The double-layer capacitance was estimated from the value of the CPE parameters using the equation proposed by Brug et al. [
41] for complex plane plots in the form of a depressed semicircle with the assumption that the simple distribution of time constants
τ =
Rs·Cdl.
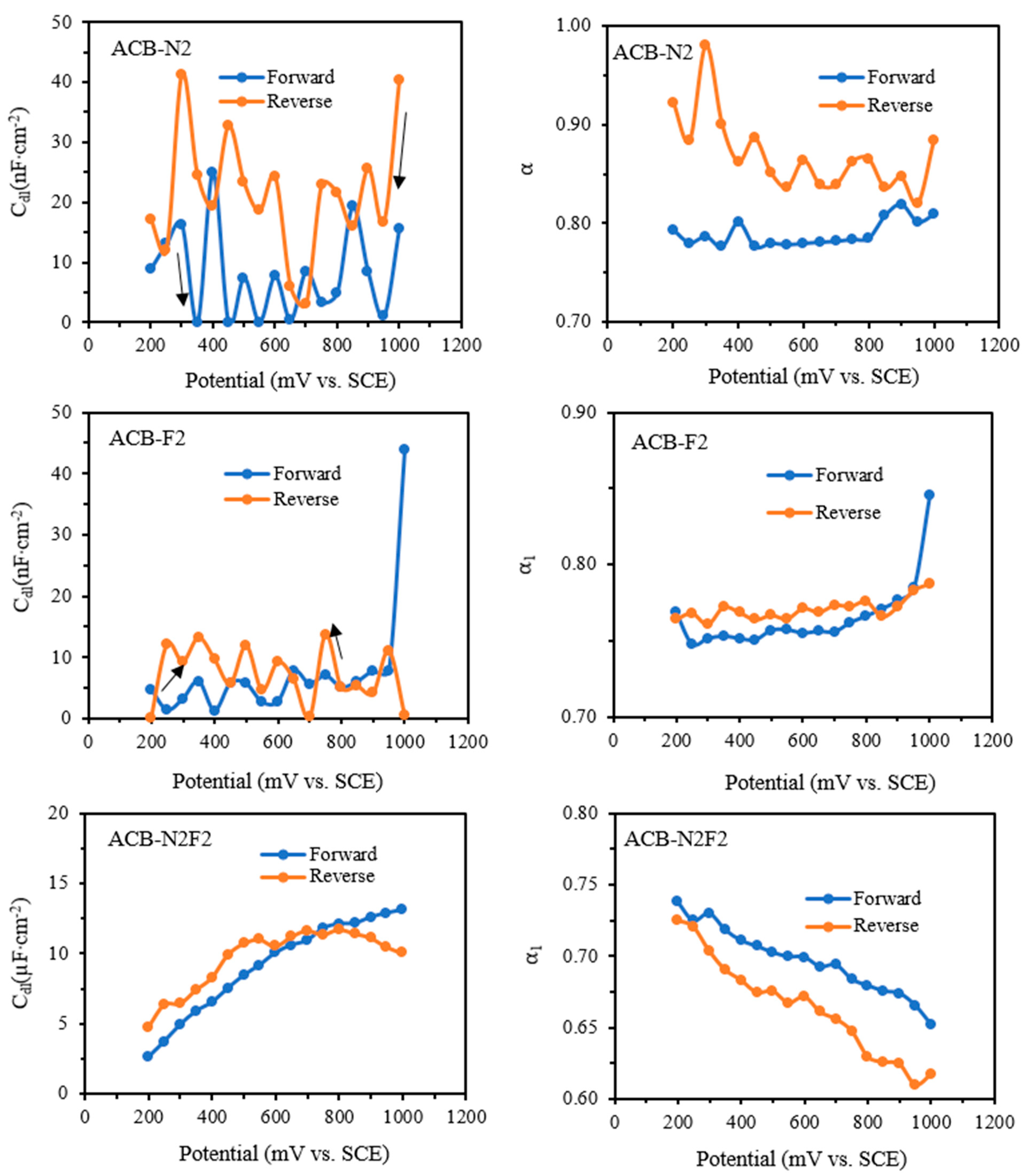
Figure 12 illustrates the impact of overpotential on double-layer capacitance and the CPE exponent α from fits to all circuits in both forward and reverse sweeps. For ACB-N2 and ACB-F2, the capacitance exhibits a disproportionate relationship with the overpotential, while the capacitance increases with an increase in the overpotential for the ACB-N2F2 electrode. This trend indicates that glycerol oxidation increases significantly with an increase in overpotential using ACB-N2F2, and it exhibits better kinetics at lower potentials. The trend holds for both forward and backward sweeps, but the increase is more linear for the forward sweep. This is because the heteroatoms act as oxidizing agents, and the oxidizing effect becomes more stable when synergistically and rationally combined.
All the electrodes exhibit a dip in the phase in the region where the direct current departs from the baseline of the forward and reverse sweep. The dip in phase could be ascribed to a double-layer effect.
Figure 13 reveals a dip in phase at 0.5 V on both forward and reverse sweeps, indicating a phase change that occurs around 200 Hz. For ACB-N2 and ACB-F2, the dip in phase occurred at a high frequency, whereas for ACB-N2F2, it occurred at a mid-frequency. Moreover, the dip in phase is more severe for ACB-N2F2, showing a higher value of CDL. The dip in phase justifies why both ACB-N2 and ACB-F2 exhibit two depressed semicircle equivalent circuits (
Rs +
Q1/
Rct +
Q2/
R3) rather than a single depressed semicircle equivalent circuit, which is also evident in the complex plane plot. Although the complex plane plot of ACB-N2F2 exhibits a vivid depressed semicircle, an element of Warburg for infinite diffusion can be slightly noticed at a lower frequency (especially at a lower potential), which is justified by the phase angles in
Figure 13 and the equivalent circuit (
Rs +
Q1/(
Rct +
W)).
The values of capacitance in the forward sweep circuit of ACB-N2 and ACB-F2 are in the nano range (7.45 and 5.7 nF cm−2 at 0.5 V), while that of ACB-N2F2 is in the micro range. This means that the process is based on a double-layer rather than an adsorption relaxation, and the capacitance can be combined with CPE to obtain a more accurate fit of the double-layer phase in this region. This trend is also observed in the reverse sweep of the electrodes, confirming that the feature is fundamental. The electrodes exhibited high-frequency relaxation with a resistance of several tens of ohms/cm2, which declined with increasing potential.
2.9. Kinetic Process of Glycerol Oxidation and Integration with Impedance Data
Glycerol oxidation in an alkaline medium follows a complex reaction pathway that involves multiple electron transfer steps, adsorption/desorption equilibria, and the formation of various intermediate species. The overall reaction mechanism can be understood through the following key steps.
- 1.
Glycerol Adsorption and Initial Deprotonation
Glycerol (C
3H
8O
3) is adsorbed onto the functionalized carbon electrode surface, where the hydroxyl (-OH) groups interact with active sites. The first deprotonation step occurs via hydroxide ions (OH
−) from the electrolyte, leading to the formation of glyceroxide species as follows:
The impedance response at this stage typically shows high charge transfer resistance (Rct), as the initial adsorption and electron transfer processes are not yet fully established.
- 2.
Formation of Glyceraldehyde and Intermediates
The adsorbed glyceroxide undergoes successive oxidation steps, forming intermediates, such as glyceraldehyde (C
3H
6O
2) and dihydroxyacetone. These intermediates are crucial in determining the overall oxidation efficiency as follows:
The emergence of these intermediates leads to a decrease in impedance (lower Rct), indicating an increased electron transfer rate.
- 3.
Further Oxidation to Carboxylates. As the potential increases, glyceraldehyde is further oxidized to glyceric acid, which can subsequently be cleaved into smaller carboxylate species, such as formate and oxalate, as follows:
The impedance spectrum at this stage reflects a more stable charge transfer process, with the Warburg impedance (W) indicating diffusion-controlled kinetics at higher potentials.
- 4.
Rate-Determining Step and Water Adsorption Influence
The observed Tafel slopes suggest that the rate-determining step for ACB-N2F2 occurs at lower overpotentials than ACB-N2 and ACB-F2 (0.4 V vs. SCE for ACB-N2F2). This indicates that water adsorption plays a crucial role in controlling the reaction rate, as water molecules are required for the oxidative cleavage of C–C bonds in glycerol as follows:
Here, the impedance model for ACB-N2F2 (single time constant) suggests that the reaction proceeds via a direct, streamlined pathway with minimal secondary resistive components.
- 5.
Electrochemical Impedance Spectroscopy (EIS) Interpretation
For ACB-N2 and ACB-F2 (double time constant model), two distinct charge transfer resistances suggest sequential reaction steps with intermediate charge accumulation, likely due to multiple active sites with varying electron transfer efficiencies. For ACB-N2F2 (single time constant model), the single dominant charge transfer resistance (Rct) in the impedance model indicates a uniform and highly conductive electron transport pathway facilitated by the co-doping of nitrogen and fluorine. This suggests that the synergistic effects of both dopants create a more homogenous catalyst surface, reducing the occurrence of intermediate resistance phases.