Loading Eu2O3 Enhances the CO Oxidation Activity and SO2 Resistance of the Pt/TiO2 Catalyst
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Activity and SO2 Resistance
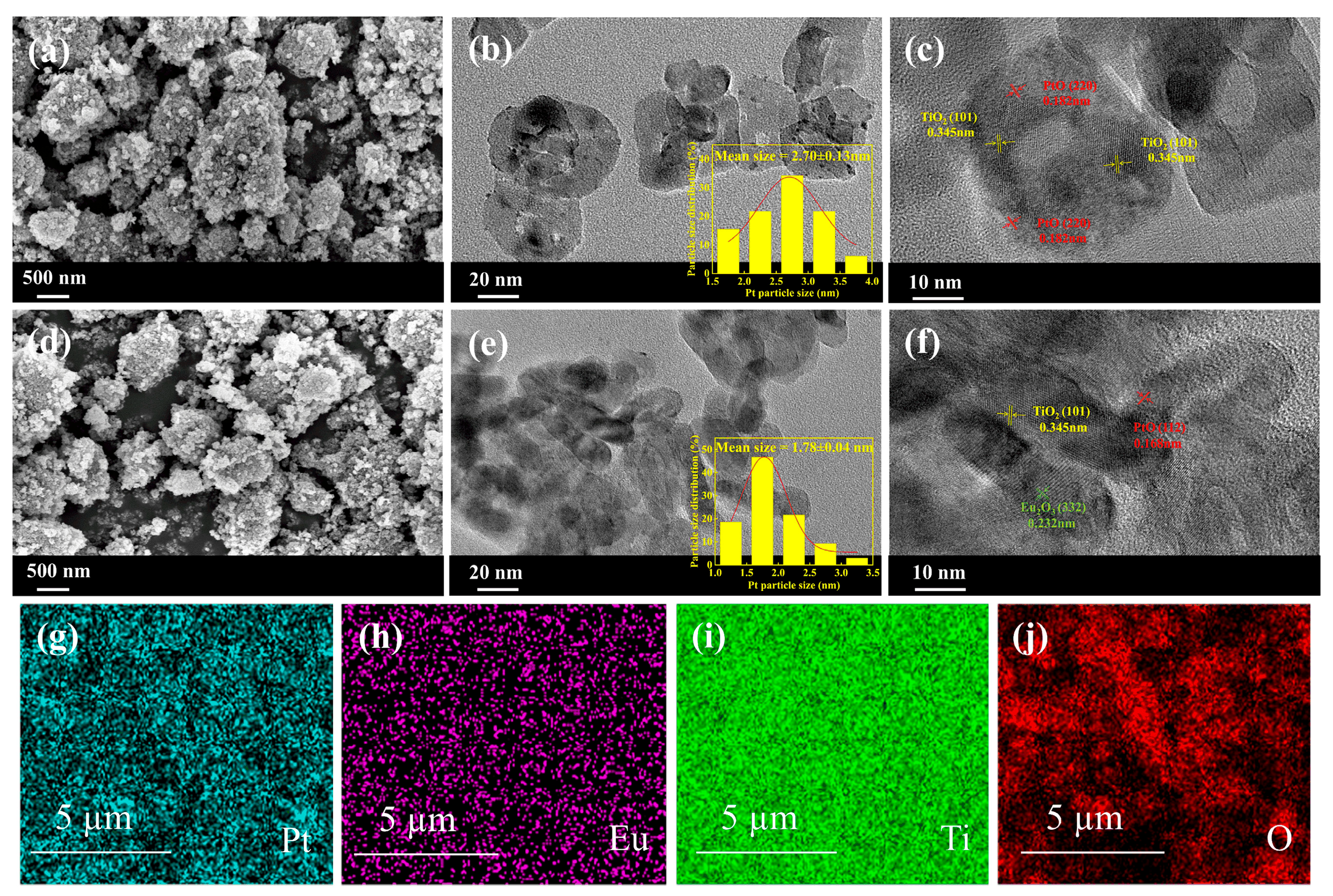
2.2. SEM and TEM Results
2.3. XRD Results
2.4. Specific Surface Area and Pore Structure Characteristics
2.5. CO Chemisorption Results
2.6. H2-TPR Results
2.7. Surface Element Valences
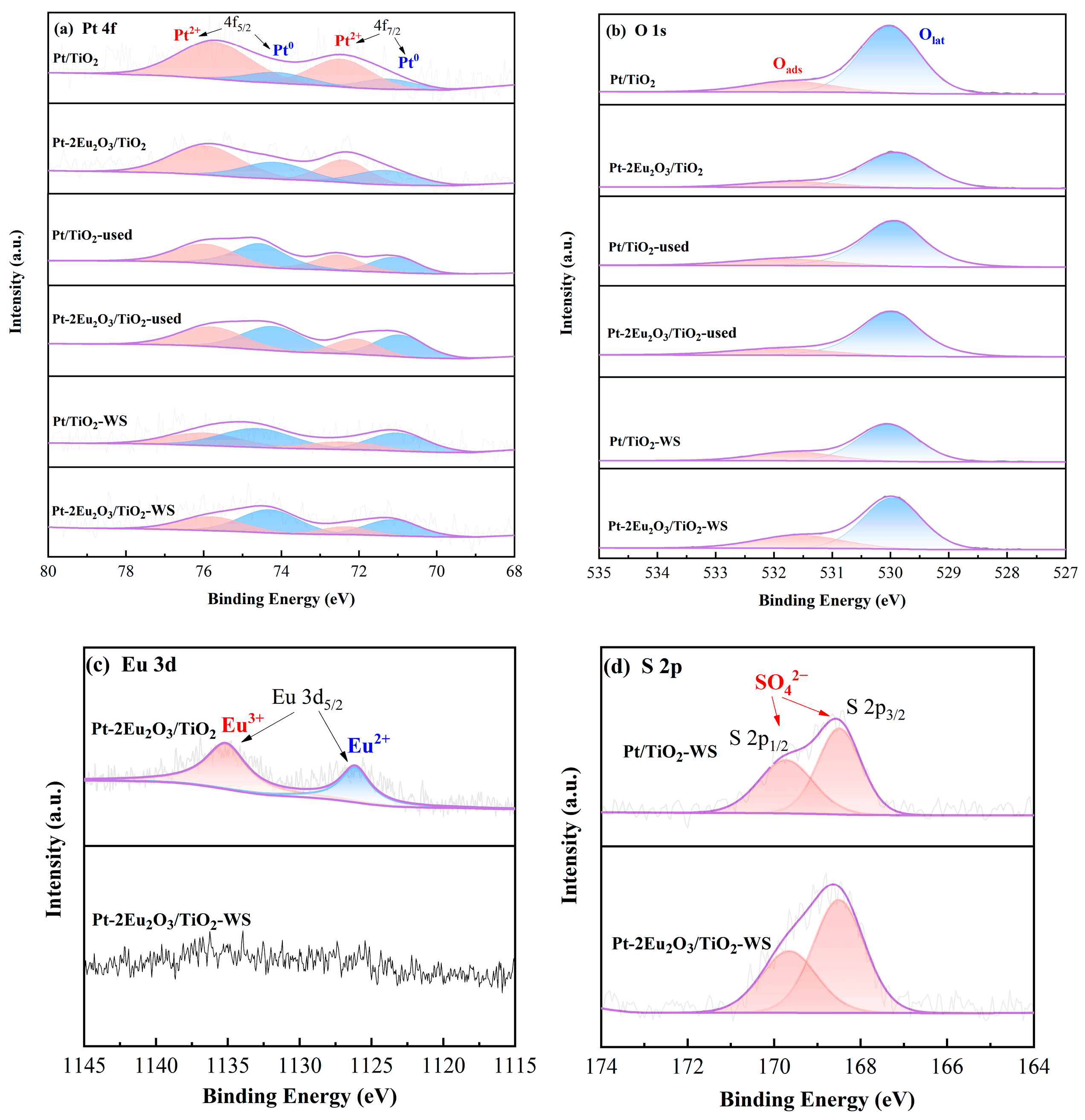
2.7.1. XPS Results
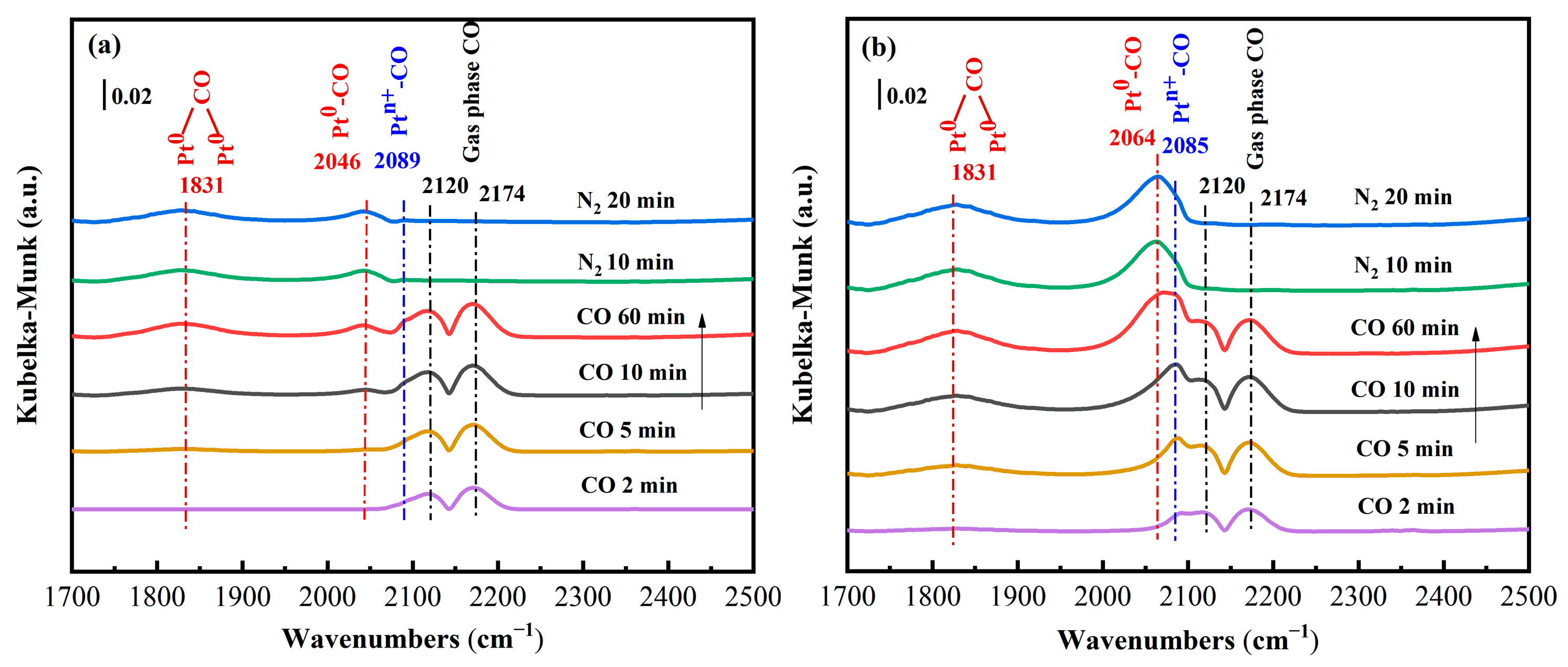
2.7.2. In Situ DRIFTS Results of CO Adsorption
2.8. Catalyst Acidity and Basicity
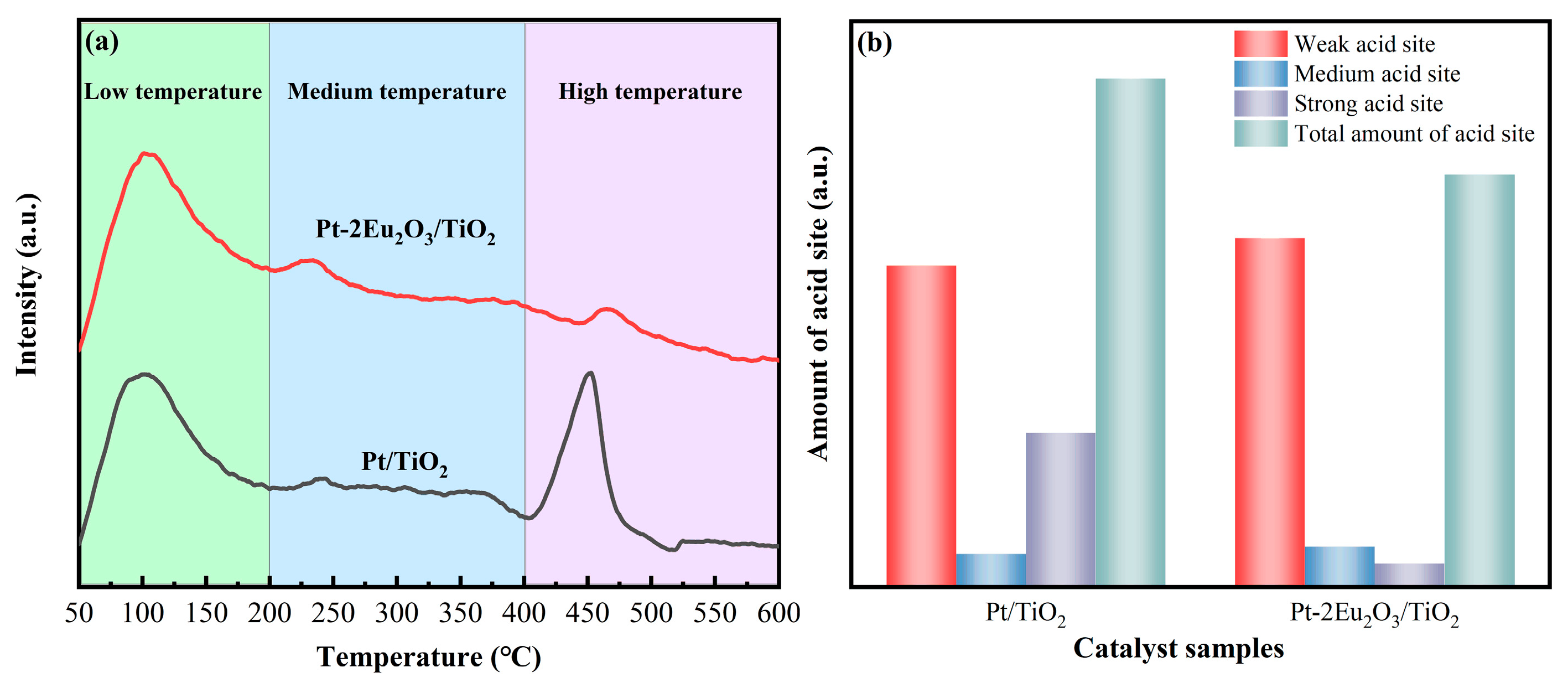
2.8.1. NH3-TPD Results
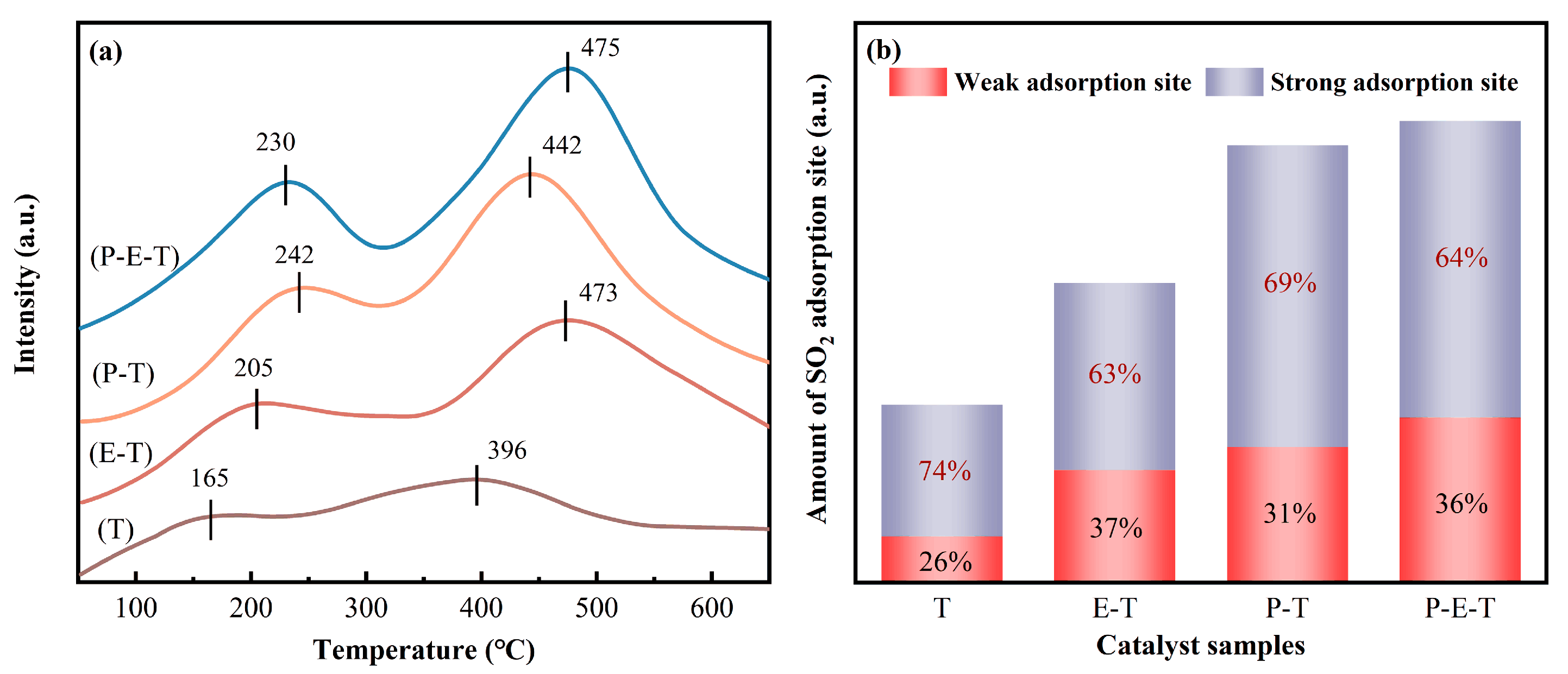
2.8.2. SO2-TPD Results
2.9. H2O-TPD Results
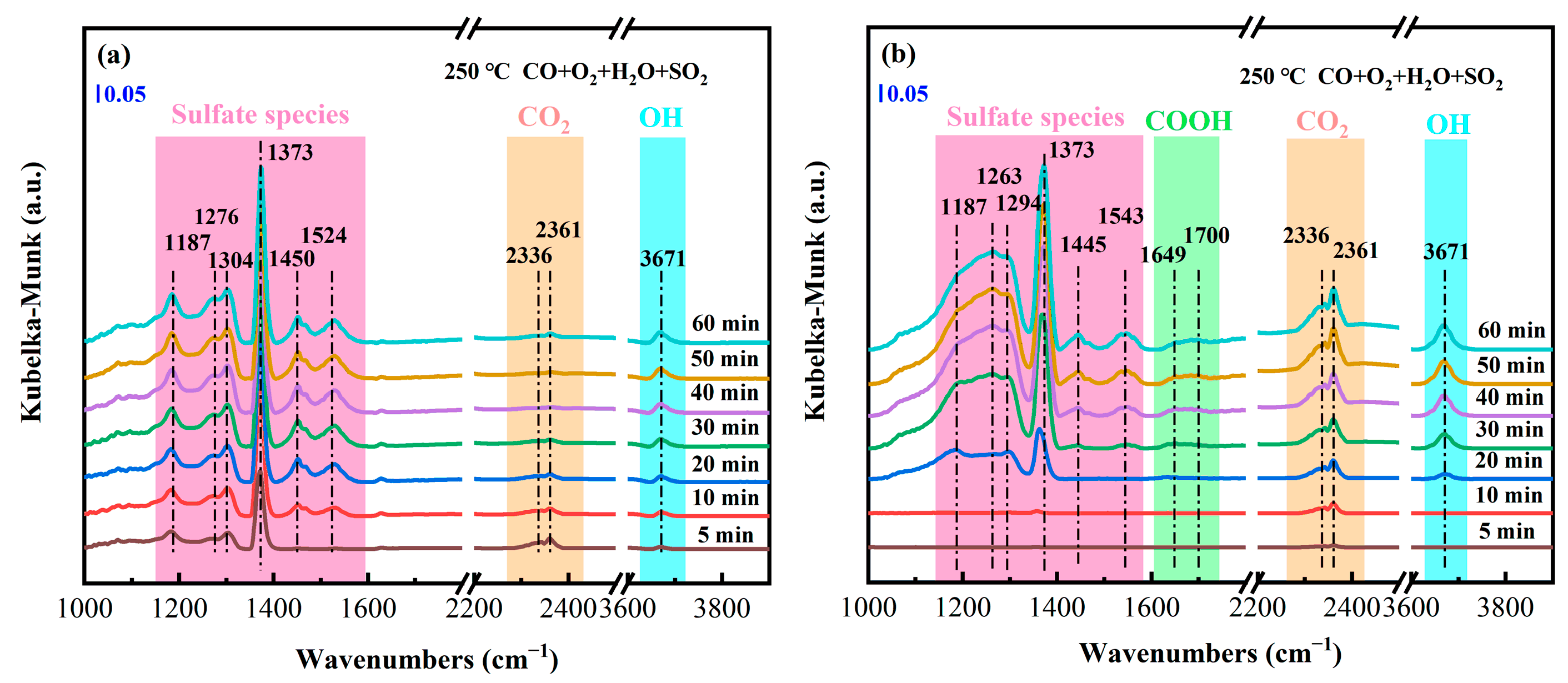
2.10. In Situ DRIFTS Results of CO Oxidation
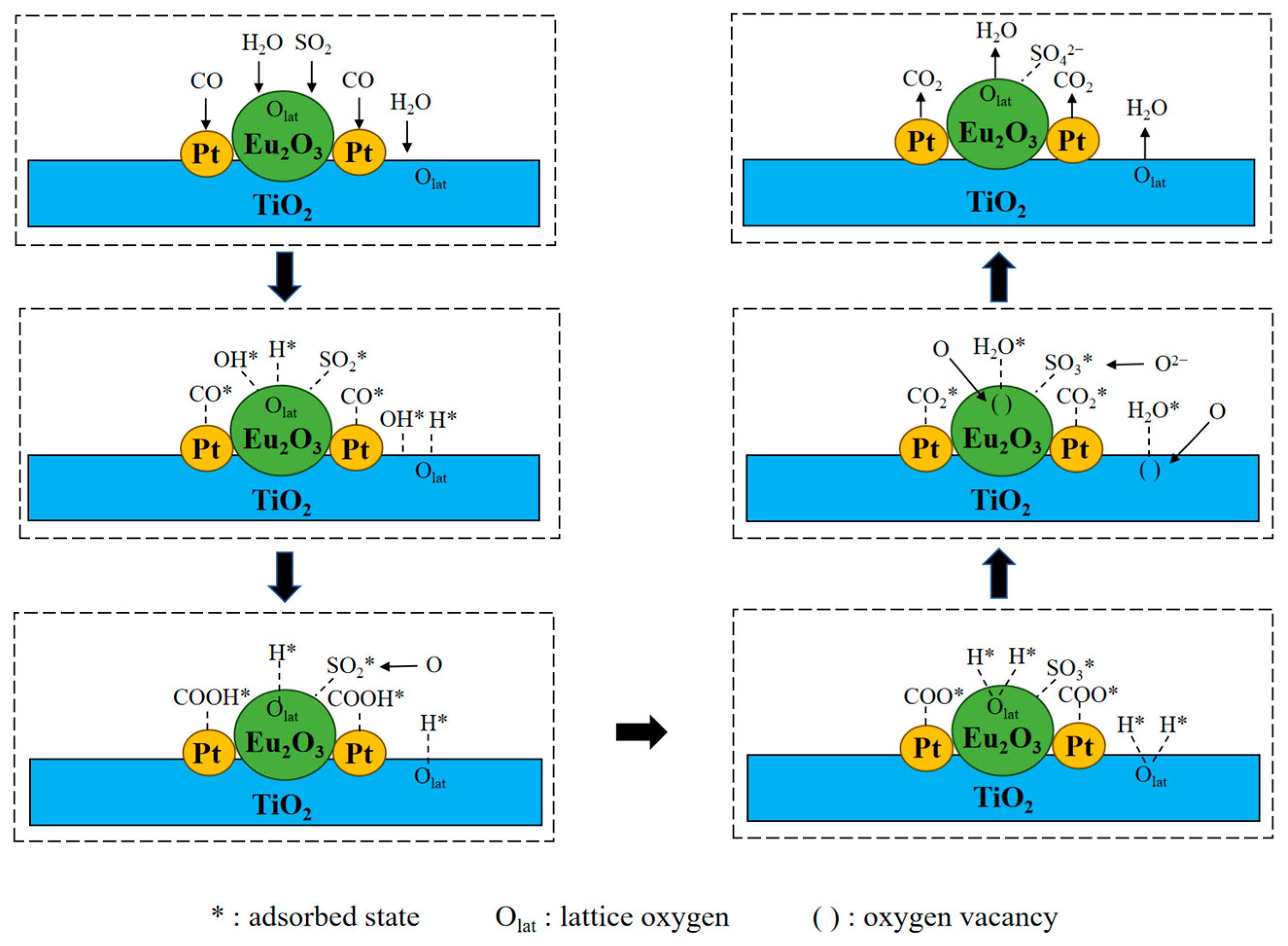
2.11. CO Oxidation Pathway
3. Experimental Section
3.1. Catalyst Preparation
3.2. Catalytic Activity Test
3.3. Kinetic Measurements
3.4. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Y.; Ruan, S.; Xu, K.; He, C.; Qin, C.; Zhang, L. Theoretical investigation of chemical reaction kinetics of CO catalytic combustion over NiNx-Gr. Colloid. Surf. A Physicochem. Eng. Asp. 2022, 653, 129962. [Google Scholar] [CrossRef]
- Taira, K.; Nakao, K.; Suzuki, K.; Einaga, H. SOx tolerant Pt/TiO2 catalysts for CO oxidation and the effect of TiO2 supports on catalytic activity. Environ. Sci. Technol. 2016, 50, 9773–9780. [Google Scholar] [CrossRef]
- Soliman, N.K. Factors affecting CO oxidation reaction over nanosized materials: A review. J. Mater. Res. Technol. JMRT 2019, 8, 2395–2407. [Google Scholar] [CrossRef]
- He, Y.; Liu, H.; Wang, Y.; Liu, X.; Chai, Y.; Kang, J.; Liu, L. Theoretical study on the effects of flue gas impurities on the oxidation/dissociation of CO over Pt catalysts. Surf. Interfaces 2025, 72, 107093. [Google Scholar] [CrossRef]
- Shi, G.; Wei, Y.; Liang, F.; Zhang, L. High-activity evolution of adjustable Pd in Pd/P-CeO2-Al2O3 monolithic catalyst for CO oxidation properties: A crucial function of the Brønsted/Lewis acidic site. Appl. Surf. Sci. 2025, 707, 163648. [Google Scholar] [CrossRef]
- Valechha, D.; Megarajan, S.K.; Al-Fatesh, A.; Jiang, H.; Labhasetwar, N. Low Temperature CO Oxidation Over a Novel Nano-Structured, Mesoporous CeO2 Supported Au Catalyst. Catal. Lett. 2019, 149, 127–140. [Google Scholar] [CrossRef]
- Zhao, G.; Guo, Y.; Huang, P.; Zhang, Z.; Zhao, C. Enhanced low-temperature CO oxidation over CuO-Co3O4 catalysts promoted with transition metal oxides. Fuel 2026, 403, 136113. [Google Scholar] [CrossRef]
- Chen, W.; Xu, J.; Huang, F.; Zhao, C.; Guan, Y.; Fang, Y.; Hu, J.; Yang, W.; Luo, Z.; Guo, Y. CO oxidation over CuOx/TiO2 catalyst: The importance of oxygen vacancies and Cu plus species. Appl. Surf. Sci. 2023, 618, 156539. [Google Scholar] [CrossRef]
- Afonasenko, T.N.; Glyzdova, D.V.; Yurpalov, V.L.; Konovalova, V.P.; Rogov, V.A.; Gerasimov, E.Y.; Bulavchenko, O.A. The Study of Thermal Stability of Mn-Zr-Ce, Mn-Ce and Mn-Zr Oxide Catalysts for CO Oxidation. Materials 2022, 15, 7553. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, X.; Zou, Y.; Xie, H.; Zhu, T. Nature of support plays vital roles in H2O promoted CO oxidation over Pt catalysts. J. Catal. 2022, 416, 364–374. [Google Scholar] [CrossRef]
- Wang, T.; Xing, J.; Zhu, L.; Jia, A.; Wang, Y.; Lu, J.; Luo, M. CO oxidation over supported Pt/CrxFe2-xO3 catalysts and their good tolerance to CO2 and H2O. Appl. Catal. B Environ. Energy 2019, 245, 314–324. [Google Scholar] [CrossRef]
- Taira, K.; Einaga, H. The Effect of SO2 and H2O on the Interaction Between Pt and TiO2(P-25) during catalytic CO oxidation. Catal. Lett. 2019, 149, 965–973. [Google Scholar] [CrossRef]
- Hamzehlouyan, T.; Sampara, C.S.; Li, J.; Kumar, A.; Epling, W.S. Kinetic study of adsorption and desorption of SO2 over γ-Al2O3 and Pt/γ-Al2O3. Appl. Catal. B Environ. Energy 2016, 181, 587–598. [Google Scholar] [CrossRef]
- Happel, M.; Luckas, N.; Viñes, F.; Sobota, M.; Laurin, M.; Görling, A.; Libuda, J. SO2 adsorption on Pt(111) and oxygen precovered Pt(111): A combined infrared reflection absorption spectroscopy and density functional study. J. Phys. Chem. C 2011, 115, 479–491. [Google Scholar] [CrossRef]
- Zhu, H.; Qiu, W.; Wu, R.; Li, K.; He, H. Spatial confinement: An effective strategy to improve H2O and SO2 resistance of the expandable graphite-modified TiO2-supported Pt nanocatalysts for CO oxidation. J. Environ. Sci. 2025, 148, 57–68. [Google Scholar] [CrossRef]
- Park, K.Y.; Lee, M.J.; Kim, W.G.; Kim, S.J.; Jeong, B.; Ye, B.; Kim, H.D. Oxidation of CO and slipped NH3 over a WS2-Loaded Pt/TiO2 catalyst with an extended reaction temperature range. Mater. Chem. Phys. 2023, 309, 128341. [Google Scholar] [CrossRef]
- Xu, T.; Liu, X.; Zhu, T.; Feng, C.; Hu, Y.; Tian, M. New insights into the influence mechanism of H2O and SO2 on Pt-W/Ti catalysts for CO oxidation. Catal. Sci. Technol. 2022, 12, 1574–1585. [Google Scholar] [CrossRef]
- Yu, C.; Yang, C.; Wang, R.; Dai, G.; Chen, H.; Huang, Z.; Zhao, H.; Zhou, Z.; Wu, X.; Jing, G. Mechanistic insight into the catalytic CO oxidation and SO2 resistance over Mo-decorated Pt/TiO2 catalyst: The essential role of Mo. Chem. Eng. J. 2024, 486, 150319. [Google Scholar] [CrossRef]
- Li, H.; Tang, Y.; Kang, J.; Wang, Y.; Liu, S.; Ding, L.; Long, H. Study on CO oxidation in sintering flue gas based on Pt-Ce-Ti catalyst. J. Cent. South Univ. 2022, 53, 4886–4895. [Google Scholar]
- Wang, F.; Lu, G. High performance rare earth oxides LnOx (Ln = La, Ce, Nd, Sm and Dy)-modified Pt/SiO2 catalysts for CO oxidation in the presence of H2. J. Power Sources 2008, 181, 120–126. [Google Scholar] [CrossRef]
- Zhao, B.; Jian, Y.; Jiang, Z.; Albilali, R.; He, C. Revealing the unexpected promotion effect of EuO on Pt/CeO2 catalysts for catalytic combustion of toluene. Chin. J. Catal. 2019, 40, 543–552. [Google Scholar] [CrossRef]
- Jung, C.H.; Yun, J.; Qadir, K.; Naik, B.; Yun, J.Y.; Park, J.Y. Catalytic activity of Pt/SiO2 nanocatalysts synthesized via ultrasonic spray pyrolysis process under CO oxidation. Appl. Catal. B Environ. Energy 2014, 154, 171–176. [Google Scholar] [CrossRef]
- Cai, J.; Yu, Z.; Fan, X.; Li, J. Effect of TiO2 calcination pretreatment on the performance of Pt/TiO2 catalyst for CO oxidation. Molecules 2022, 27, 3875. [Google Scholar] [CrossRef]
- Jain, N.; Ravishankar, N.; Madras, G. Spectroscopic and kinetic insights of Pt-dispersion over microwave-synthesized GO-supported Pt-TiO2 for CO oxidation. Mol. Catal. 2017, 432, 88–98. [Google Scholar]
- He, J.; Li, J.; Liang, W.; Song, L.; Yuan, J.; Cai, J.; Yu, Z. Unveiling the promotional performance of CO oxidation over Na-doped Pt-0.5P&W/TiO2 catalyst: “SMSI” effect of Na in catalysis. Fuel 2024, 372, 132199. [Google Scholar]
- He, J.; Li, J.; Yu, Z.; Li, S.; Yuan, J.; Cai, J. Strong metal support interaction (SMSI) and MoO3 synergistic effect of Pt-based catalysts on the promotion of CO activity and sulfur resistance. Environ. Sci. Pollut. Res. 2024, 31, 1530–1542. [Google Scholar] [CrossRef] [PubMed]
- Einaga, H.; Urahama, N.; Tou, A.; Teraoka, Y. CO Oxidation Over TiO2-Supported Pt-Fe Catalysts Prepared by Coimpregnation Methods. Catal. Lett. 2014, 144, 1653–1660. [Google Scholar] [CrossRef]
- Rathore, R.R.S.; Paul, D.; Chaure, N.B.; Rathore, D. Investigation of novel BaFe2O4/SiC/TiO2 nanocomposites as gas sensor towards NH3 (g): Structural, morphological, optical and dielectric properties. Appl. Phys. A Mater. Sci. Process. 2024, 130, 36. [Google Scholar] [CrossRef]
- Buttersack, C. Modeling of type IV and V sigmoidal adsorption isotherms. Phys. Chem. Chem. Phys. 2019, 21, 5614–5626. [Google Scholar] [CrossRef]
- Wang, H.; Yao, R.; Zhang, R.; Ma, H.; Gao, J.; Liang, M.; Zhao, Y.; Miao, Z. CeO2-supported TiO2-Pt nanorod composites as efficient catalysts for CO oxidation. Molecules 2023, 28, 1867. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Hirabayashi, T.; Dohmae, K.; Takagi, N.; Minami, T.; Shinjoh, H.; Matsumoto, S. Sintering inhibition mechanism of platinum supported on ceria-based oxide and Pt-oxide-support interaction. J. Catal. 2006, 242, 103–109. [Google Scholar] [CrossRef]
- Cai, J.; Yu, Z.; Li, J. Effect of preparation methods on the performance of Pt/TiO2 catalysts for the catalytic oxidation of carbon monoxide in simulated sintering flue gas. Catalysts 2021, 11, 804. [Google Scholar] [CrossRef]
- Ye, J.; Zhu, B.; Cheng, B.; Jiang, C.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. Synergy between platinum and gold nanoparticles in oxygen activation for enhanced room-temperature formaldehyde oxidation. Adv. Funct. Mater. 2022, 32, 2110423. [Google Scholar] [CrossRef]
- Rahm, M.; Zeng, T.; Hoffmann, R. Electronegativity seen as the ground-state average valence electron binding energy. J. Am. Chem. Soc. 2019, 141, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Wang, Y.; Dong, W.; Liu, C.; Xu, C. Pt nanoclusters controllably prepared by impregnation method and its catalytic property for glycerol oxidation. Catal. Lett. 2021, 151, 593–599. [Google Scholar] [CrossRef]
- Kim, G.J.; Kwon, D.W.; Hong, S.C. Effect of Pt particle size and valence state on the performance of Pt/TiO2 catalysts for CO oxidation at room temperature. J. Phys. Chem. C 2016, 120, 17996–18004. [Google Scholar] [CrossRef]
- Liu, J.; Ding, T.; Zhang, H.; Li, G.; Cai, J.; Zhao, D.; Tian, Y.; Xian, H.; Bai, X.; Li, X. Engineering surface defects and metal-support interactions on Pt/TiO2(B) nanobelts to boost the catalytic oxidation of CO. Catal. Sci. Technol. 2018, 8, 4934–4944. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, Y.; Jiang, H.; Yu, J.; Jiang, R.; Li, C. Engineering TiO2 supported Pt sub-nanoclusters via introducing variable valence Co ion in high-temperature flame for CO oxidation. Nanoscale 2018, 10, 13384–13392. [Google Scholar] [CrossRef]
- Feng, C.; Liu, X.; Zhu, T.; Hu, Y.; Tian, M. Catalytic oxidation of CO over Pt/TiO2 with low Pt loading: The effect of H2O and SO2. Appl. Catal. A Gen. 2021, 622, 118218. [Google Scholar] [CrossRef]
- Kumar, S.; Prakash, R.; Choudhary, R.J.; Phase, D.M. Structural, XPS and magnetic studies of pulsed laser deposited Fe doped Eu2O3 thin film. Mater. Res. Bull. 2015, 70, 392–396. [Google Scholar] [CrossRef]
- Unal, F.; Alp, E.; Kazmanli, K. Production, characterization, and photocatalytic properties of Eu:Y2O3 nanopowders. J. Chin. Chem. Soc. 2022, 69, 1744–1753. [Google Scholar] [CrossRef]
- Mashkovtsev, M.A.; Kosykh, A.S.; Ishchenko, A.V.; Chukin, A.V.; Kukharenko, A.I.; Troshin, P.A.; Zhidkov, I.S. Unraveling oxygen vacancies effect on chemical composition, electronic structure and optical properties of Eu doped SnO2. Nanomaterials 2024, 14, 1675. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, M.; Xu, W.; Chen, J.; Hong, Z.; Jia, H. Incorporating Mn cation as anchor to atomically disperse Pt on TiO2 for low-temperature removal of formaldehyde. Appl. Catal. B Environ. Energy 2019, 259, 118013. [Google Scholar] [CrossRef]
- Meunier, F.C.; Cardenas, L.; Kaper, H.; Smíd, B.; Vorokhta, M.; Grosjean, R.; Aubert, D.; Dembélé, K.; Lunkenbein, T. Synergy between metallic and oxidized Pt sites unravelled during room temperature CO oxidation on Pt/Ceria. Angew. Chem. Int. Ed. Energy 2021, 60, 7472. [Google Scholar] [CrossRef]
- Ma, C.; Yang, C.; Wang, B.; Chen, C.; Wang, F.; Yao, X.; Song, M. Effects of H2O on HCHO and CO oxidation at room-temperature catalyzed by MCo2O4 (M = Mn, Ce and Cu) materials. Appl. Catal. B Environ. Energy 2019, 254, 76–85. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, F.; Zhu, Z.; Yang, Y.; Zhao, S.; Chen, J.; Lv, X.; Wang, Y.; Xu, J.; Liu, N. The promoting effect of H2O on rod-like MnCeOx derived from MOFs for toluene oxidation: A combined experimental and theoretical investigation. Appl. Catal. B Environ. Energy 2021, 297, 120393. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, L.; Liu, Y.; Deng, J.; Jing, L.; Yu, X.; Han, Z.; Zhang, K.; Dai, H. 3DOM CeO2-supported RuyM (M = Au, Pd, Pt) alloy nanoparticles with improved catalytic activity and chlorine-tolerance in trichloroethylene oxidation. Catal. Sci. Technol. 2020, 10, 3755–3770. [Google Scholar] [CrossRef]
- Zhou, J.; He, J.; Wang, T.; Chen, X.; Sun, D. Synergistic effect of RE2O3 (RE = Sm, Eu and Gd) on Pt/mesoporous carbon catalyst for methanol electro-oxidation. Electrochim. Acta 2009, 54, 3103–3108. [Google Scholar] [CrossRef]
- He, K. In situ DRIFTS and TPD studies on surface properties affecting SO2-resistance of Pt/TiO2 catalyst in low-temperature CO oxidation. Surf. Sci. 2023, 734, 122315. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, W.; Zhu, H.; Ding, X.; Wu, R.; He, H. Promotion effect of the keggin structure on the sulfur and water resistance of Pt/CeTi catalysts for CO oxidation. Catalysts 2022, 12, 4. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Y.; Wang, Y.; Cen, W.; Wu, Z.; Wang, H.; Weng, X. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature. Appl. Catal. B Environ. Energy 2014, 148, 582–588. [Google Scholar] [CrossRef]
- Ye, D.; Qu, R.; Song, H.; Gao, X.; Luo, Z.; Ni, M.; Cen, K. New insights into the various decomposition and reactivity behaviors of NH4HSO4 with NO on V2O5/TiO2 catalyst surfaces. Chem. Eng. J. 2016, 283, 846–854. [Google Scholar] [CrossRef]
- Wang, W.; Xu, D.; Cheng, B.; Yu, J.; Jiang, C. Hybrid carbon@TiO2 hollow spheres with enhanced photocatalytic CO2 reduction activity. J. Mater. Chem. A 2017, 5, 5020–5029. [Google Scholar] [CrossRef]



| Samples | BET Specific Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Diameter (nm) | Pt Dispersion (%) |
|---|---|---|---|---|
| Pt/TiO2 | 69.4 | 0.39 | 21.7 | 47.5 |
| Pt/TiO2-WS | 67.0 | 0.39 | 22.7 | / |
| Pt-2Eu2O3/TiO2 | 72.1 | 0.41 | 22.2 | 53.5 |
| Pt-2Eu2O3/TiO2-WS | 61.4 | 0.38 | 23.9 | / |
| Samples | H2 Consumption (μmol/gcat) | ||
|---|---|---|---|
| Peak I | Peak II | Total | |
| Pt/TiO2 | 9.7 | 673.2 | 682.9 |
| Pt-1Eu2O3/TiO2 | 13.1 | 491.1 | 504.2 |
| Pt-1.5Eu2O3/TiO2 | 16.6 | 509.8 | 526.4 |
| Pt-2Eu2O3/TiO2 | 19.0 | 498.8 | 517.8 |
| Pt-2.5Eu2O3/TiO2 | 23.0 | 492.0 | 515 |
| Samples | Oads/(Olat + Oads) (%) | Pt0/(Pt0 + Pt2+) (%) |
|---|---|---|
| Pt/TiO2 | 18.7 | 20.0 |
| Pt-2Eu2O3/TiO2 | 18.0 | 38.7 |
| Pt/TiO2-used | 22.1 | 52.0 |
| Pt-2Eu2O3/TiO2-used | 22.2 | 56.5 |
| Pt/TiO2-WS | 24.5 | 62.3 |
| Pt-2Eu2O3/TiO2-WS | 27.6 | 63.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Cai, J.; Meng, Y.; Li, J.; Liang, W.; Fan, X. Loading Eu2O3 Enhances the CO Oxidation Activity and SO2 Resistance of the Pt/TiO2 Catalyst. Catalysts 2025, 15, 783. https://doi.org/10.3390/catal15080783
Yu Z, Cai J, Meng Y, Li J, Liang W, Fan X. Loading Eu2O3 Enhances the CO Oxidation Activity and SO2 Resistance of the Pt/TiO2 Catalyst. Catalysts. 2025; 15(8):783. https://doi.org/10.3390/catal15080783
Chicago/Turabian StyleYu, Zehui, Jianyu Cai, Yudong Meng, Jian Li, Wenjun Liang, and Xing Fan. 2025. "Loading Eu2O3 Enhances the CO Oxidation Activity and SO2 Resistance of the Pt/TiO2 Catalyst" Catalysts 15, no. 8: 783. https://doi.org/10.3390/catal15080783
APA StyleYu, Z., Cai, J., Meng, Y., Li, J., Liang, W., & Fan, X. (2025). Loading Eu2O3 Enhances the CO Oxidation Activity and SO2 Resistance of the Pt/TiO2 Catalyst. Catalysts, 15(8), 783. https://doi.org/10.3390/catal15080783






