Electrophoretic Deposition of Gold Nanoparticles on Highly Ordered Titanium Dioxide Nanotubes for Photocatalytic Application
Abstract
1. Introduction
2. Results
2.1. Morphological Analysis
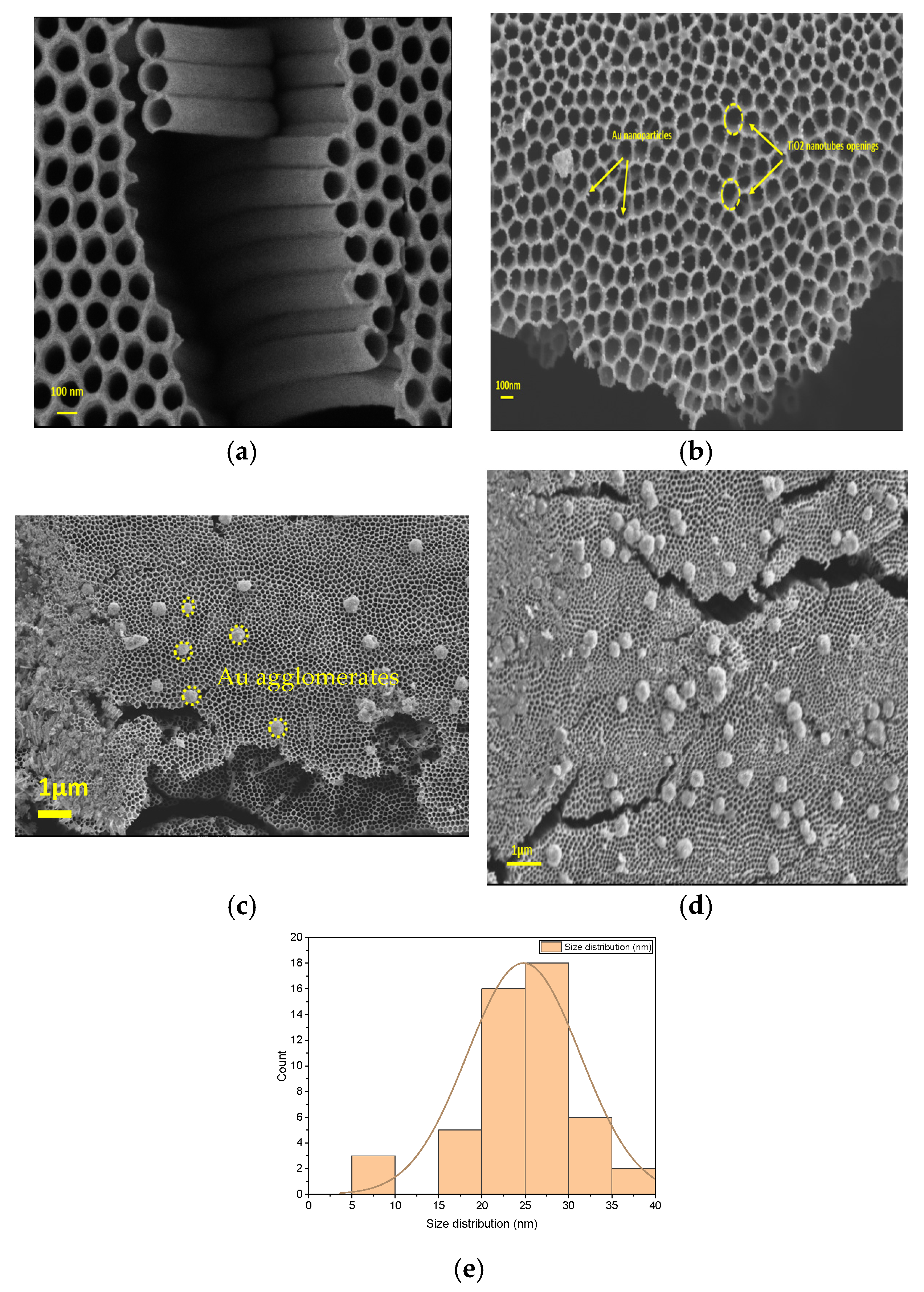
2.1.1. SEM Analysis
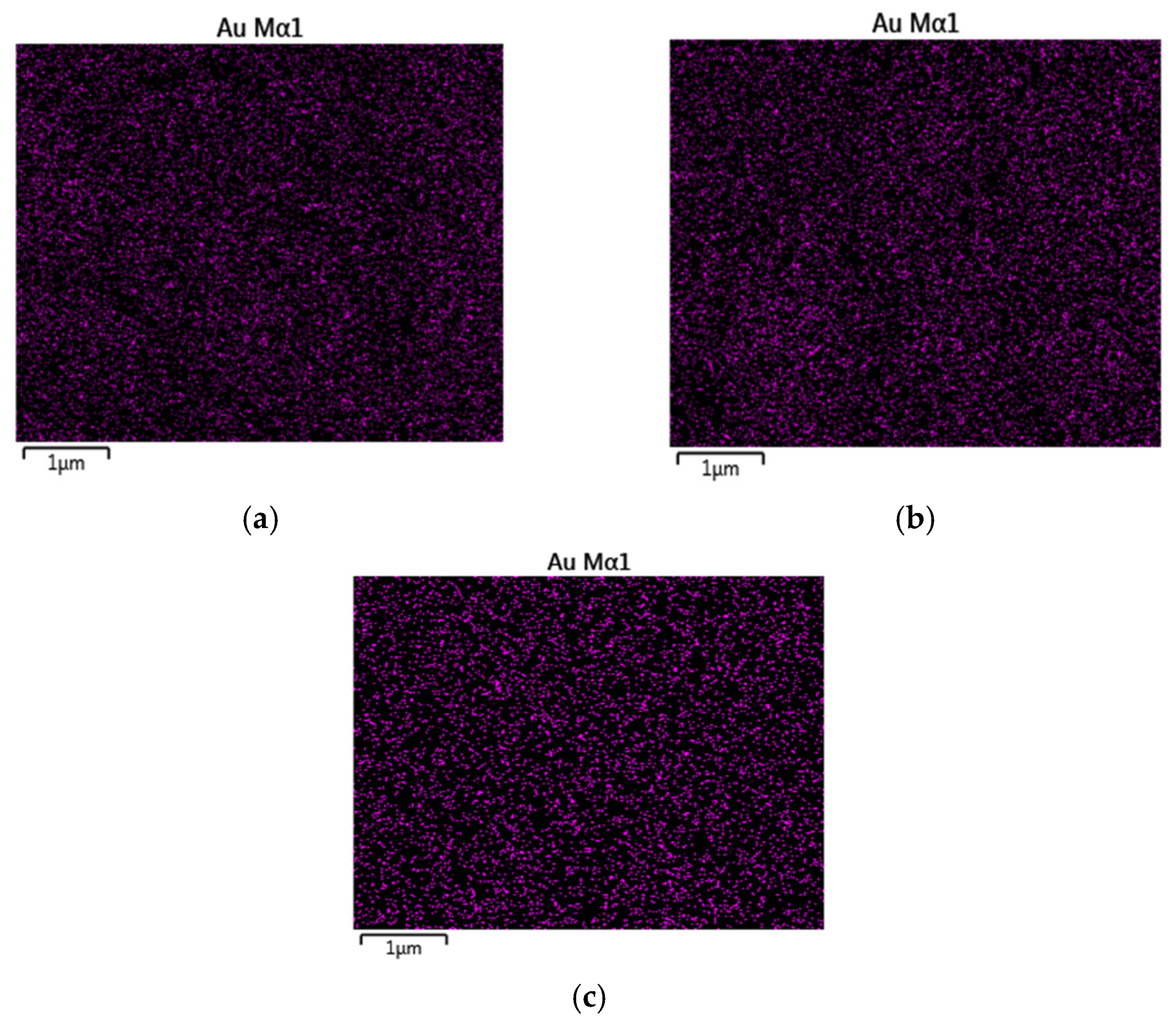
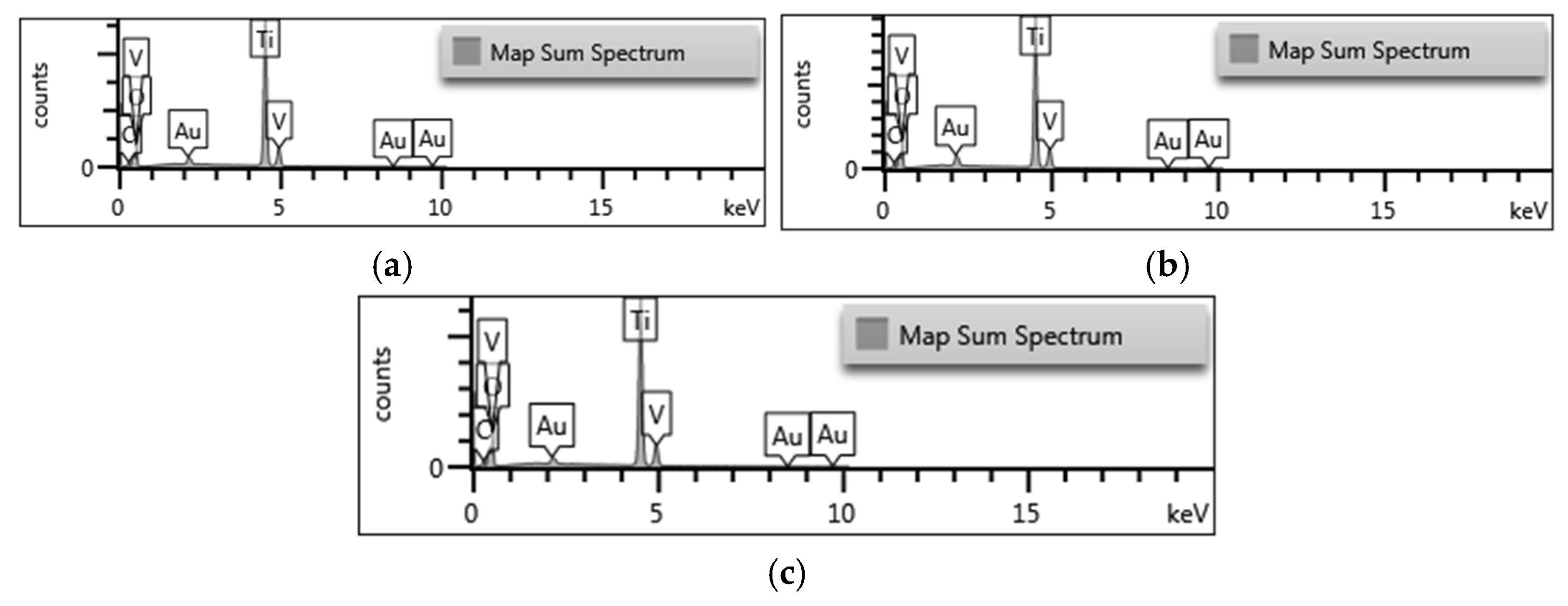
2.1.2. EDX Analysis
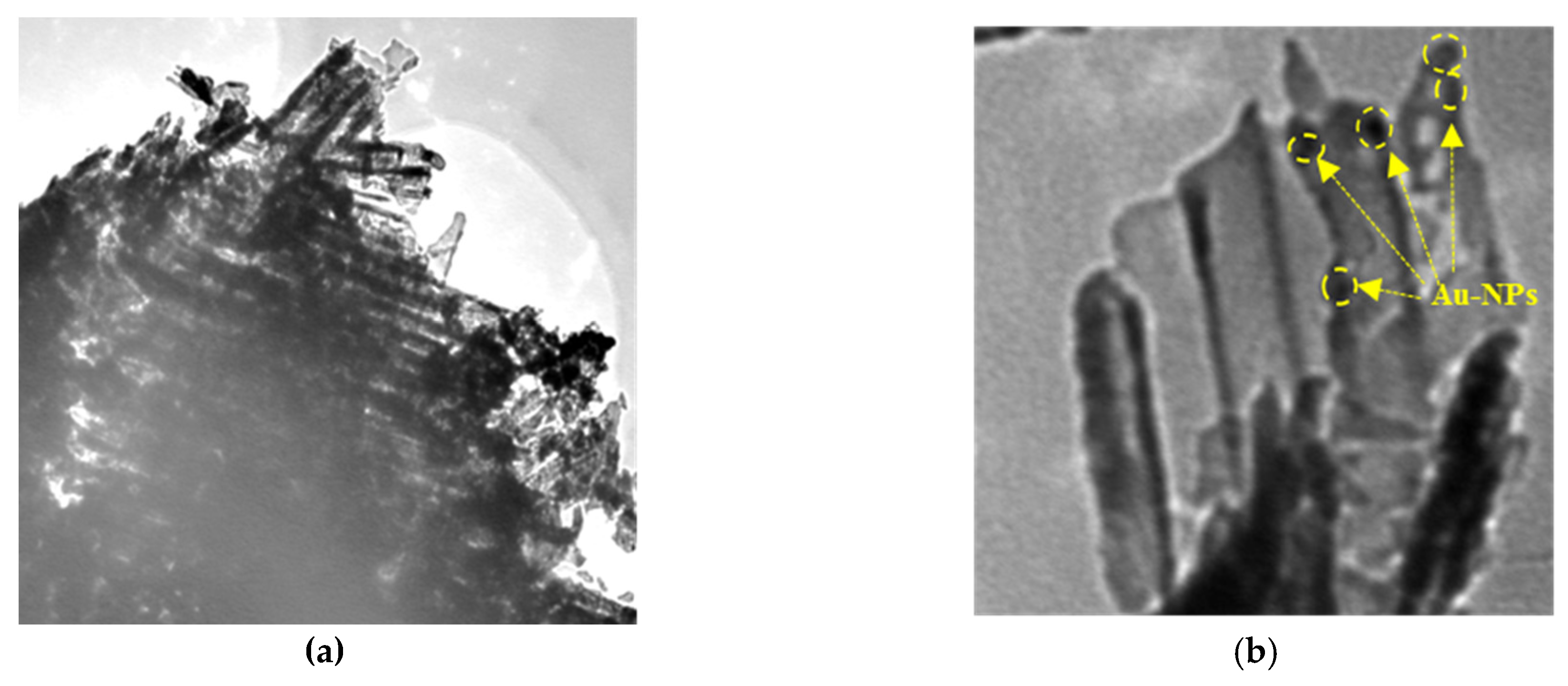
2.1.3. TEM Analysis
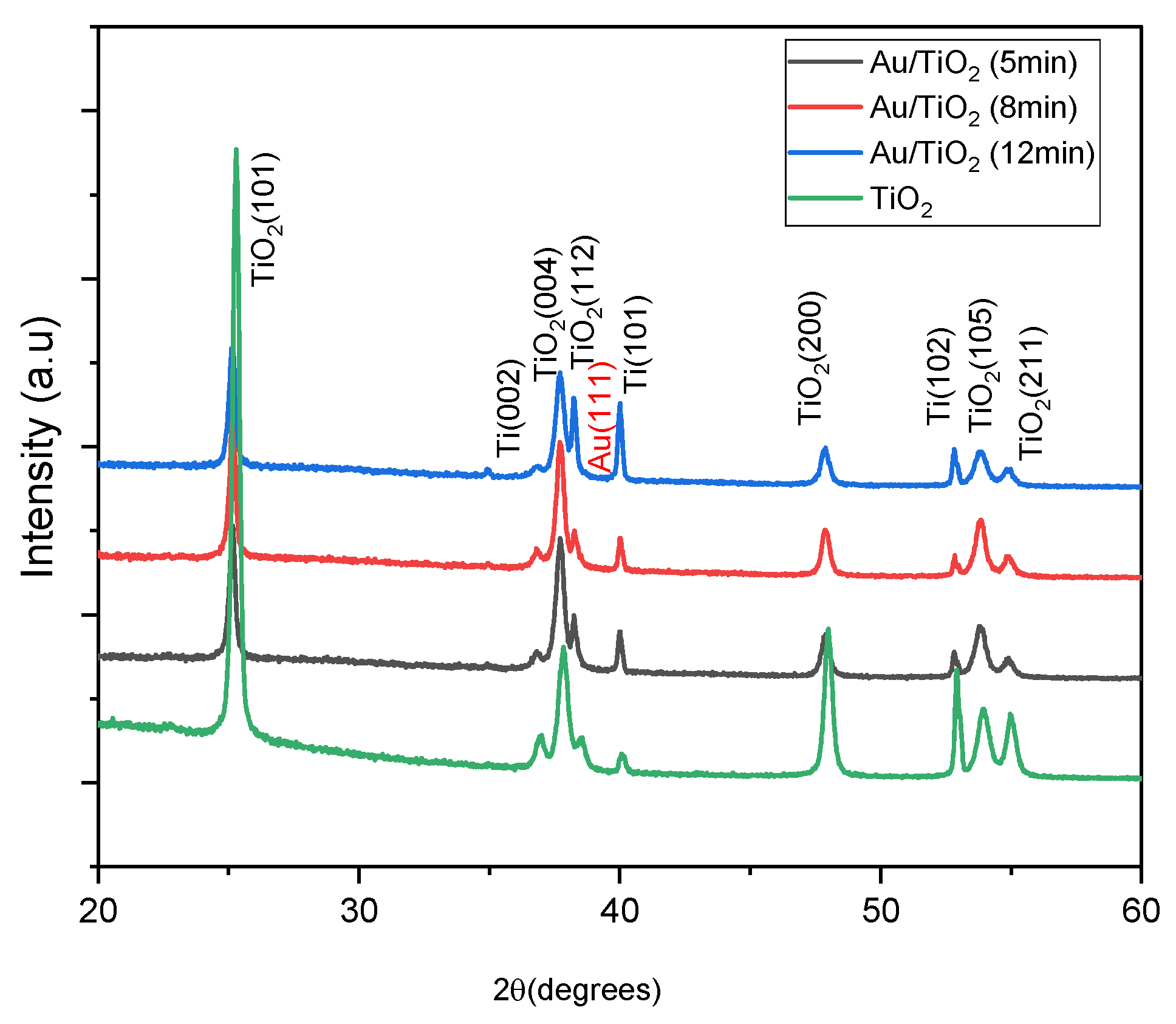
2.2. Structural Analysis
XRD Analysis
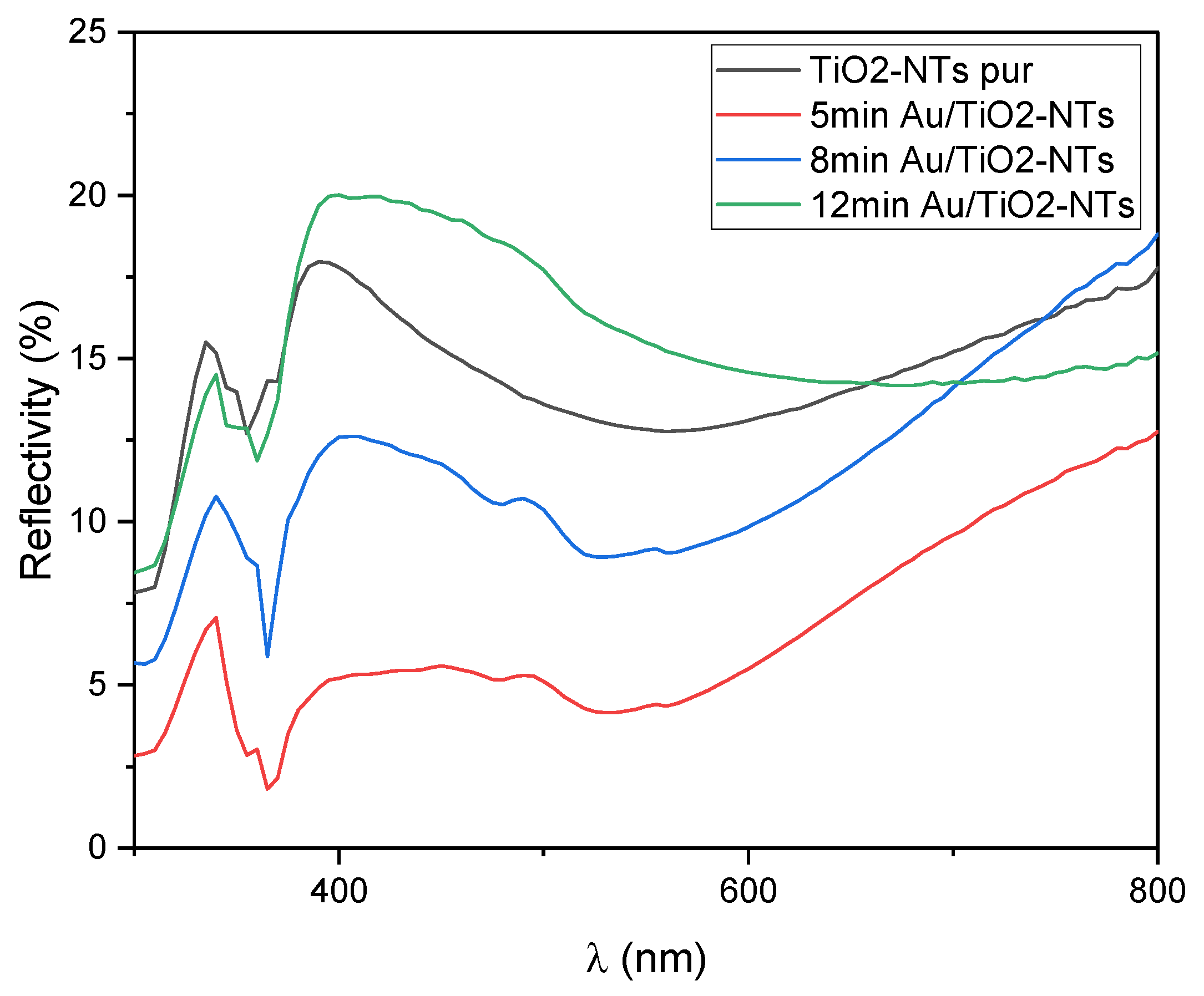
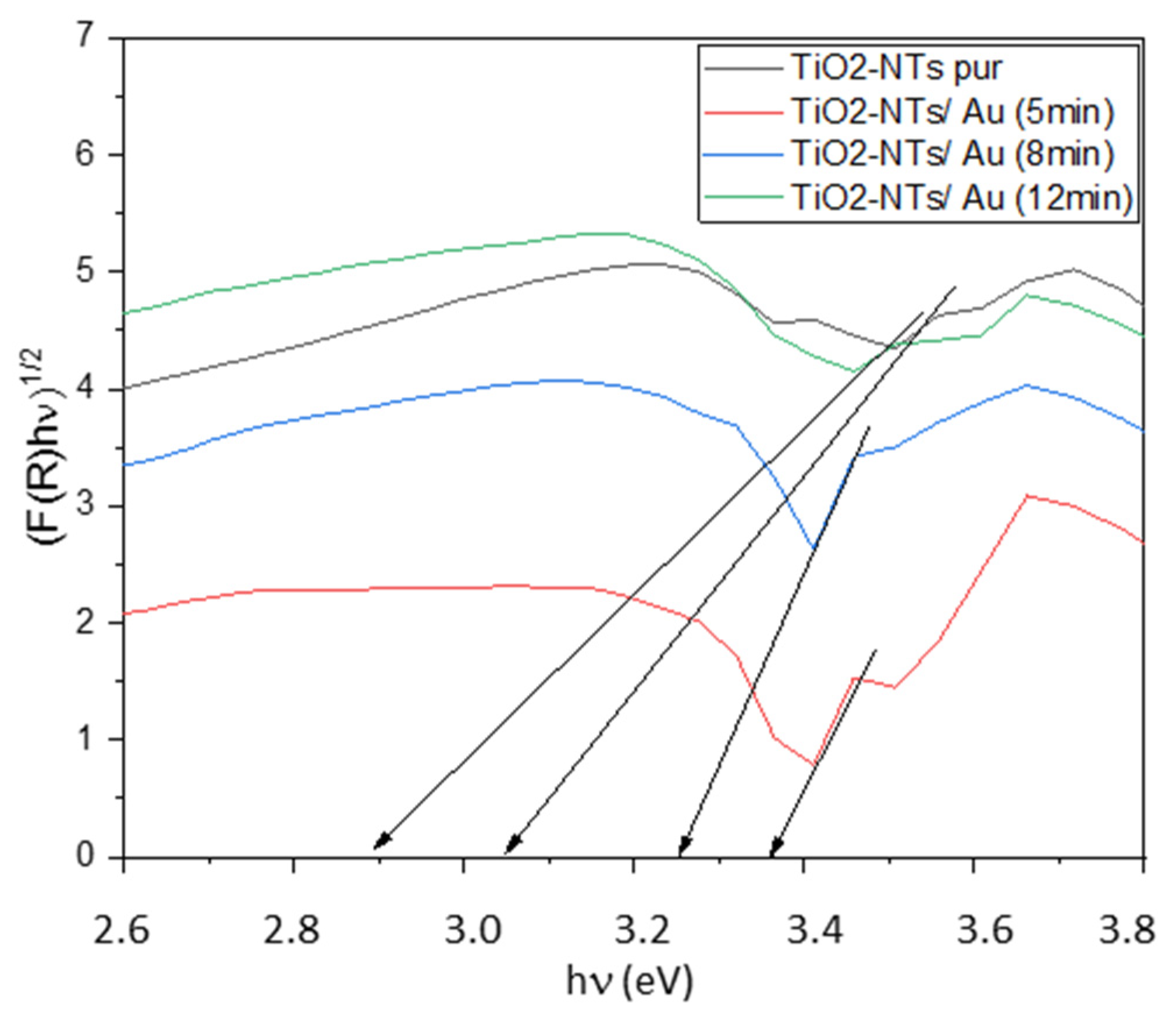
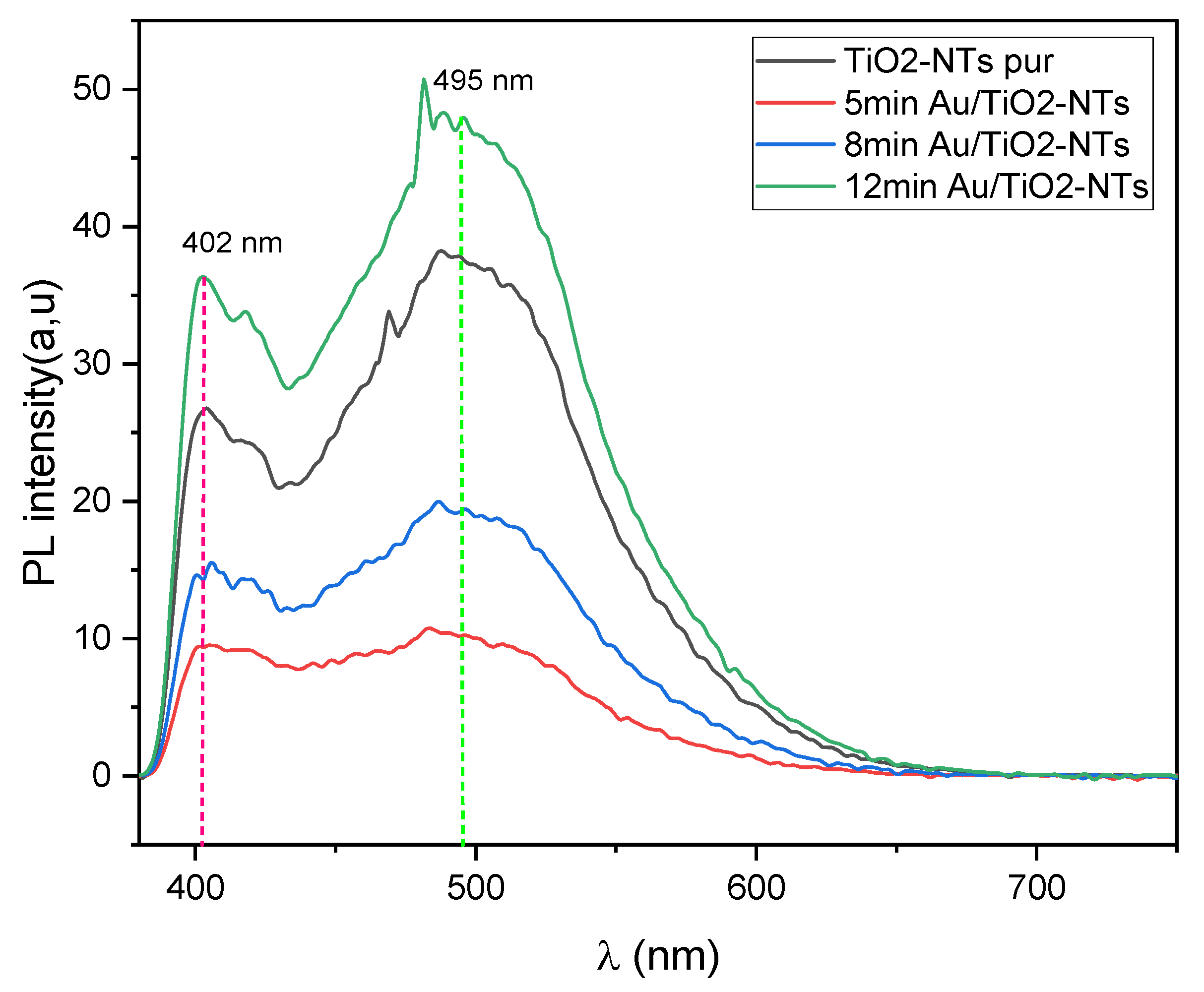
2.3. Optical Analysis
2.3.1. Surface Reflectivity
2.3.2. Photoluminescence Spectroscopy
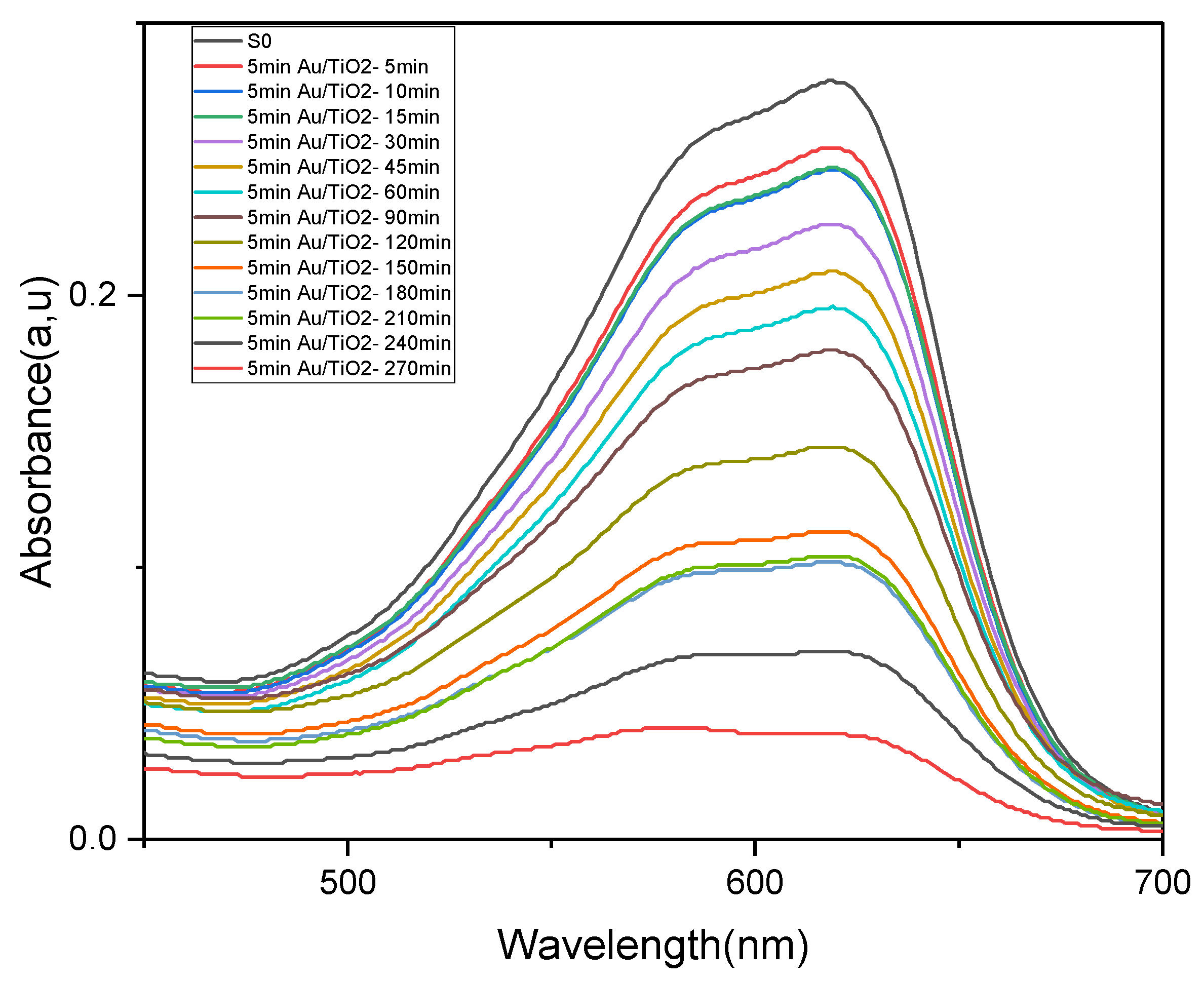
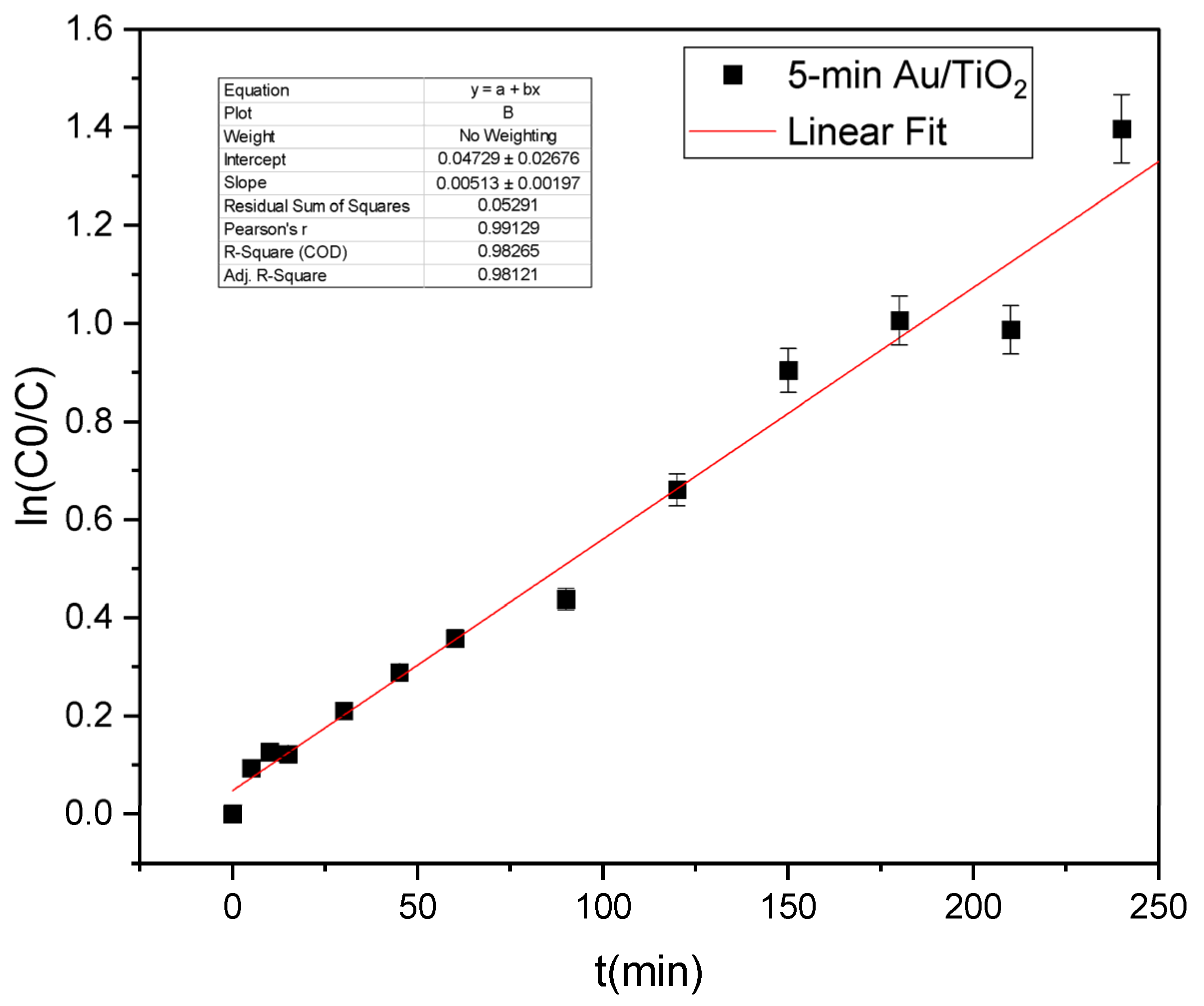
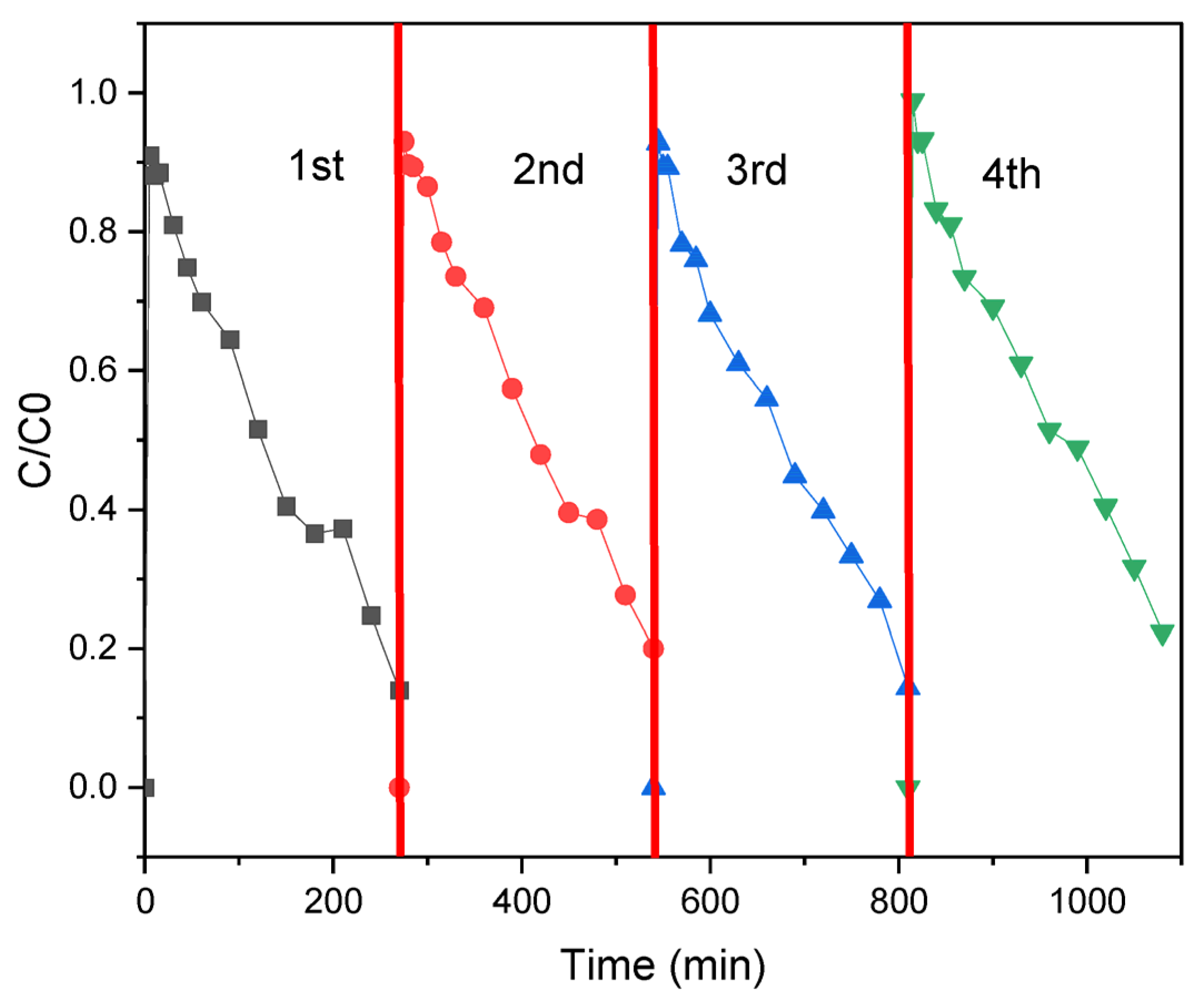
2.4. Photocatalytic Application
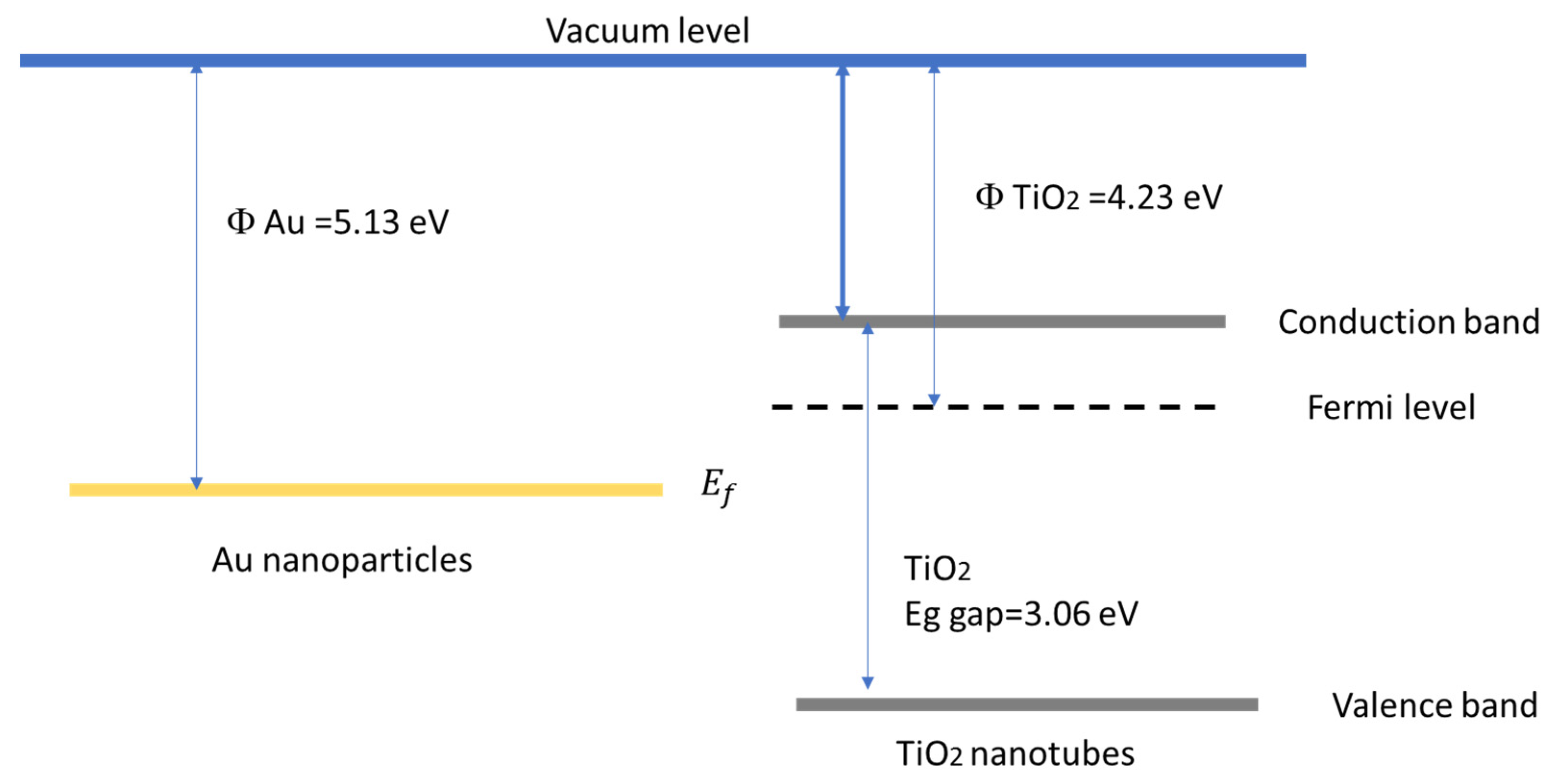
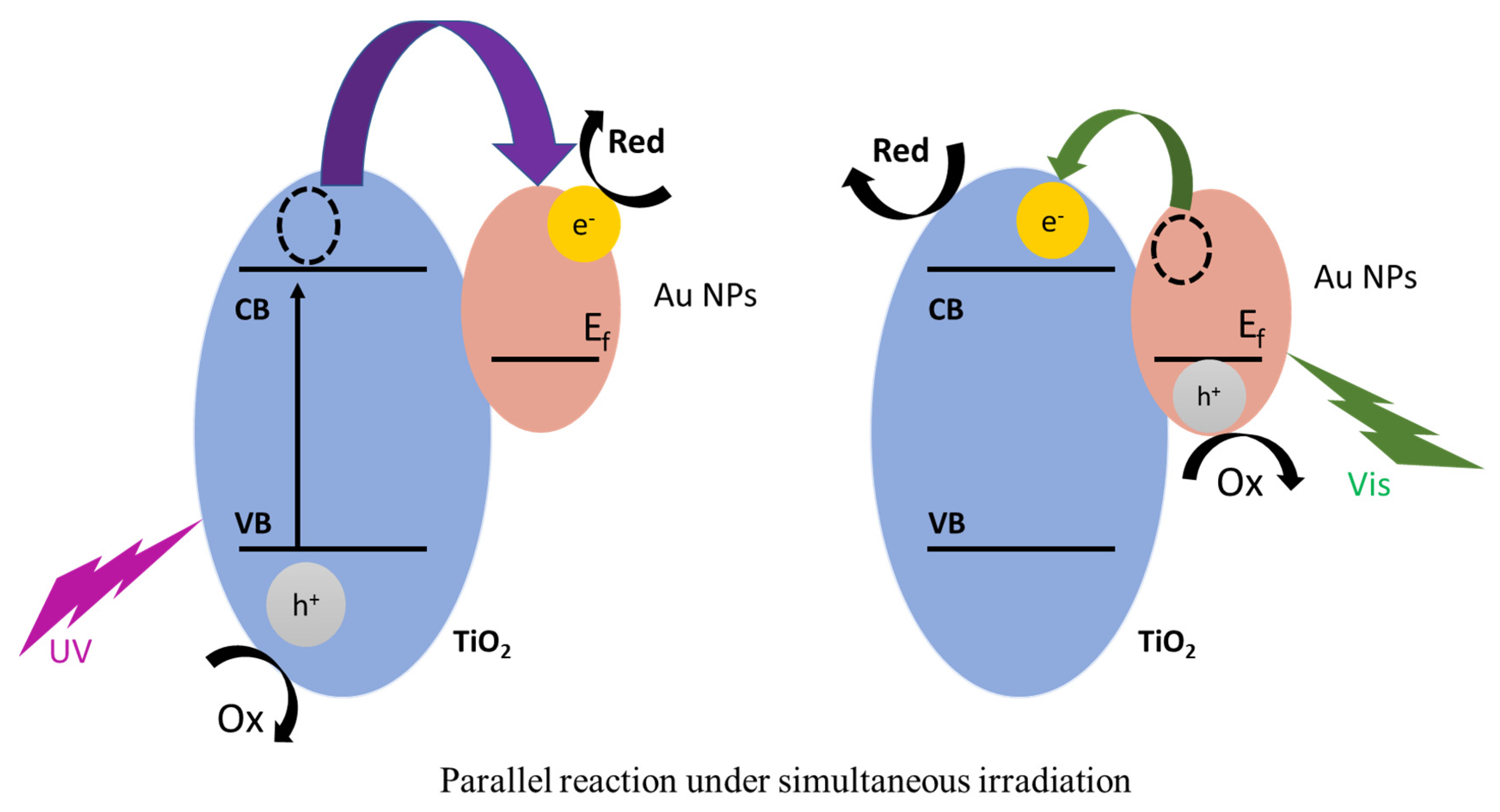
3. Discussion
4. Materials and Methods
4.1. Anodization of Titanium Dioxide Nanotubes
4.2. Electrophoresis of Gold Nanoparticles on Titania Nanotubes
4.3. Characterization of Au-NPs/TiO2-NTs
4.4. Photocatalytic Degradation of Amido Black Dye
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Galstyan, V.; Macak, J.M.; Djenizian, T. Anodic TiO2 nanotubes: A promising material for energy conversion and storage. Appl. Mater. Today 2022, 29, 101613. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review. Appl. Catal. A Gen. 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Chauhan, A.; Verma, R.; Kumari, S.; Sharma, A.; Shandilya, P.; Li, X.; Batoo, K.M.; Imran, A.; Kulshrestha, S.; Kumar, R. Photocatalytic dye degradation and antimicrobial activities of Pure and Ag-doped ZnO using Cannabis sativa leaf extract. Sci. Rep. 2020, 10, 7881. [Google Scholar] [CrossRef]
- Peleyeju, M.G. Electrochemical and Solar Photoelectrocatalytic Oxidation of Selected Organic Compounds at Carbon-Semiconductor Based Electrodes. Ph.D. Thesis, University of Johannesburg, Johannesburg, South Africa, 2017. [Google Scholar]
- Sassi, S.; Trabelsi, K.; El Jery, A.; Abidi, M.; Hajjaji, A.; Khezami, L.; Karrech, A.; Gaidi, M.; Soucase, B.M.; Bessais, B. Synergistic effect of CuxOy-NPs/TiO2-NTs heterostructure on the photodegradation of amido black staining. Optik 2023, 272, 170234. [Google Scholar] [CrossRef]
- Wang, J.; Sun, S.; Ding, H.; Chen, W.; Liang, Y. Preparation of a composite photocatalyst with enhanced photocatalytic activity: Smaller TiO2 carried on SiO2 microsphere. Appl. Surf. Sci. 2019, 493, 146–156. [Google Scholar] [CrossRef]
- Baaloudj, O.; Nasrallah, N.; Kebir, M.; Khezami, L.; Amrane, A.; Assadi, A.A. A comparative study of ceramic nanoparticles synthesized for antibiotic removal: Catalysis characterization and photocatalytic performance modeling. Environ. Sci. Pollut. Res. 2021, 28, 13900–13912. [Google Scholar] [CrossRef]
- Hou, W.; Cronin, S.B. A review of surface plasmon resonance-enhanced photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Kunwar, S.; Sui, M.; Pandey, P.; Gu, Z.; Pandit, S.; Lee, J. Improved configuration and LSPR response of platinum nanoparticles via enhanced solid state dewetting of In-Pt bilayers. Sci. Rep. 2019, 9, 1329. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Choi, S.-I.; Xia, X.; Xia, Y. Catalysis on faceted noble-metal nanocrystals: Both shape and size matter. Curr. Opin. Chem. Eng. 2013, 2, 142–150. [Google Scholar] [CrossRef]
- Trabelsi, K.; Hajjaji, A.; Gaidi, M.; Bessais, B.; El Khakani, M.A. Enhancing the photoelectrochemical response of TiO2 nanotubes through their nanodecoration by pulsed-laser-deposited Ag nanoparticles. J. Appl. Phys. 2017, 122, 064503. [Google Scholar] [CrossRef]
- Wu, L.-P.; Wang, W.-G.; Mo, D.; Duan, J.-L.; Li, X.-J. Effect of Au position on the photoelectrochemical and photocatalytic activity of TiO2 nanotubes under UV irradiation. Inorg. Nano-Met. Chem. 2024, 54, 1154–1162. [Google Scholar] [CrossRef]
- Hajjaji, M.; Missaoui, K.; Trabelsi, K.; Bouzaza, A.; Hajjaji, A.; Bessais, B.; Assadi, A. Platinum nanoparticles decorated TiO2 nanotubes for VOCs and bacteria removal in simulated real condition: Effect of the deposition method on the photocatalytic degradation process efficiency. J. Photochem. Photobiol. A Chem. 2025, 458, 115975. [Google Scholar] [CrossRef]
- Hajjaji, M.A.; Missaoui, K.; Trabelsi, K.; Bouzaza, A.; Bessais, B.; Hajjaji, A.; Assadi, A.A. Electrodeposited Platinum Nanoparticles on Highly Ordered Titanium Dioxide Nanotubes for Photocatalytic Application: Enhancement of Photocatalytic Degradation of Amido Black Dye. Catal. Lett. 2024, 154, 1242–1254. [Google Scholar] [CrossRef]
- Bera, S.; Lee, J.E.; Rawal, S.B.; Lee, W.I. Size-dependent plasmonic effects of Au and Au@ SiO2 nanoparticles in photocatalytic CO2 conversion reaction of Pt/TiO2. Appl. Catal. B Environ. 2016, 199, 55–63. [Google Scholar] [CrossRef]
- LEI, B.; XUE, J.; JIN, D.; NI, S.; SUN, H. Fabrication, annealing, and electrocatalytic properties of platinum nanoparticles supported on self-organized TiO2 nanotubes. Rare Met. 2008, 27, 445–450. [Google Scholar] [CrossRef]
- Moon, K.-S.; Choi, E.-J.; Bae, J.-M.; Park, Y.-B.; Oh, S. Visible light-enhanced antibacterial and osteogenic functionality of Au and Pt nanoparticles deposited on TiO2 nanotubes. Materials 2020, 13, 3721. [Google Scholar] [CrossRef]
- Tian, M.; Wu, G.; Chen, A. Unique electrochemical catalytic behavior of Pt nanoparticles deposited on TiO2 nanotubes. Acs Catal. 2012, 2, 425–432. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Abe, H.; Ma, T.; Tadaki, D.; Hirano-Iwata, A.; Kanetaka, H.; Watanabe, Y.; Niwano, M. Bactericidal activity of TiO2 nanotube thin films on Si by photocatalytic generation of active oxygen species. Langmuir 2020, 36, 12668–12677. [Google Scholar] [CrossRef] [PubMed]
- Fornari, A.M.D.; de Araujo, M.B.; Duarte, C.B.; Machado, G.; Teixeira, S.R.; Weibel, D.E. Photocatalytic reforming of aqueous formaldehyde with hydrogen generation over TiO2 nanotubes loaded with Pt or Au nanoparticles. Int. J. Hydrog. Energy 2016, 41, 11599–11607. [Google Scholar] [CrossRef]
- Kowalska, E.; Mahaney, O.O.P.; Abe, R.; Ohtani, B. Visible-light-induced photocatalysis through surface plasmon excitation of gold on titania surfaces. Phys. Chem. Chem. Phys. 2010, 12, 2344–2355. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A. Progress, Challenge, and Perspective of Bimetallic TiO2-Based Photocatalysts. J. Nanomater. 2014, 2014, 208920. [Google Scholar] [CrossRef]
- Lincho, J.; Mazierski, P.; Klimczuk, T.; Martins, R.C.; Gomes, J.; Zaleska-Medynska, A. TiO2 nanotubes modification by photodeposition with noble metals: Characterization, optimization, photocatalytic activity, and by-products analysis. J. Environ. Chem. Eng. 2024, 12, 112990. [Google Scholar] [CrossRef]
- Leordean, C.; Marta, B.; Gabudean, A.-M.; Focsan, M.; Botiz, I.; Astilean, S. Fabrication of highly active and cost effective SERS plasmonic substrates by electrophoretic deposition of gold nanoparticles on a DVD template. Appl. Surf. Sci. 2015, 349, 190–195. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Hosseingholilou, S.; Dorranian, D.; Ghoranneviss, M. Characterization of gold nanoparticle thin film prepared by electrophoretic deposition method. Gold Bull. 2020, 53, 1–10. [Google Scholar] [CrossRef]
- Lee, M.-S.; Hong, S.-C.; Kim, D. Fabrication of patterned gold electrodes with spin-coated-and-fired Au (1 1 1) film by the soft lithography. Appl. Surf. Sci. 2006, 252, 5019–5025. [Google Scholar] [CrossRef]
- Tian, F.H.; Wang, X.; Zhao, W.; Zhao, L.; Chu, T.; Yu, S. Adsorption of 2-propanol on anatase TiO2 (101) and (001) surfaces: A density functional theory study. Surf. Sci. 2013, 616, 76–84. [Google Scholar] [CrossRef]
- Tanner, A.J.; Robin, K.; Helen, H.F.; Geoff, T. Chemical Modification of Polaronic States in Anatase TiO2 (101). J. Phys. Chem. C 2021, 125, 14348–14355. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H. Facet-dependent catalytic activities of Au nanoparticles enclosed by high-index facets. ACS Catal. 2014, 4, 4027–4033. [Google Scholar] [CrossRef]
- Xiao, C.; Lu, B.-A.; Xue, P.; Tian, N.; Zhou, Z.-Y.; Lin, X.; Lin, W.-F.; Sun, S.-G. High-index-facet-and high-surface-energy nanocrystals of metals and metal oxides as highly efficient catalysts. Joule 2020, 4, 2562–2598. [Google Scholar] [CrossRef]
- Ye, L.; Liang, Y. First principles study on band gap modulation of TiO2 (112) surface for enhancing optical properties. Phys. B Condens. Matter 2024, 674, 415579. [Google Scholar] [CrossRef]
- Chowdhury, R.I.; Hossen, M.A.; Mustafa, G.; Hussain, S.; Rahman, S.N.; Farhad, S.F.U.; Murata, K.; Tambo, T.; Islam, A.B.M.O. Characterization of chemically deposited cadmium sulfide thin films. Int. J. Mod. Phys. B 2010, 24, 5901–5911. [Google Scholar] [CrossRef]
- A, J.K.; Kekuda, D. Performance analysis of a DC magnetron sputtered Cu2O/TiO2 heterojunction photodetector for short-wavelength detection. Sens. Actuators A. Phys. 2025, 388, 116517. [Google Scholar]
- Shabalina, A.; Fakhrutdinova, E.; Chen, Y.-W.; Lapin, I. Preparation of gold-modified F, N-TiO2 visible light photocatalysts and their structural features comparative analysis. J. Sol-Gel Sci. Technol. 2015, 75, 617–624. [Google Scholar] [CrossRef]
- Khezami, L.; Lounissi, I.; Hajjaji, A.; Guesmi, A.; Assadi, A.A.; Bessais, B. Synthesis and characterization of TiO2 nanotubes (TiO2-NTs) decorated with platine nanoparticles (Pt-NPs): Photocatalytic performance for simultaneous removal of microorganisms and volatile organic compounds. Materials 2021, 14, 7341. [Google Scholar] [CrossRef] [PubMed]
- Sassi, S.; Bouich, A.; Hajjaji, A.; Khezami, L.; Bessais, B.; Soucase, B.M. Cu-Doped TiO2 Thin Films by Spin Coating: Investigation of Structural and Optical Properties. Inorganics 2024, 12, 188. [Google Scholar] [CrossRef]
- Sassi, S.; Bouich, A.; Bessais, B.; Khezami, L.; Soucase, B.M.; Hajjaji, A. Comparative Analysis of Anodized TiO2 Nanotubes and Hydrothermally Synthesized TiO2 Nanotubes: Morphological, Structural, and Photoelectrochemical Properties. Materials 2024, 17, 5182. [Google Scholar] [CrossRef] [PubMed]
- Yuwono, A.H.; Sofyan, N.; Kartini, I.; Ferdiansyah, A.; Pujianto, T.H. Nanocrystallinity enhancement of TiO2 nanotubes by post-hydrothermal treatment. Adv. Mater. Res. 2011, 277, 90–99. [Google Scholar] [CrossRef]
- Zare, M.; Mortezaali, A.; Shafiekhani, A. Photoelectrochemical determination of shallow and deep trap states of platinum-decorated TiO2 nanotube arrays for photocatalytic applications. J. Phys. Chem. C 2016, 120, 9017–9027. [Google Scholar] [CrossRef]
- Perillo, P.M.; Rodríguez, D.F. Anodization growth of self-organized ZrO2 nanotubes on zircaloy-4. Evaluation of the photocatalytic activity. Matéria 2015, 20, 627–635. [Google Scholar] [CrossRef]
- Saquib, M. TiO2-mediated photocatalytic degradation of a triphenylmethane dye (gentian violet), in aqueous suspensions. Dye. Pigment. 2003, 56, 37–49. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, X.; Liu, J.; Tian, Z.; Dai, L.; He, B.; Han, C.; Wu, Y.; Zeng, Z.; Hu, Z. On the role of localized surface plasmon resonance in UV-Vis light irradiated Au/TiO2 photocatalysis systems: Pros and cons. Nanoscale 2015, 7, 4114–4123. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, K.; Jemai, S.; El Jery, A.; Sassi, S.; Guesmi, A.; Khezami, L.; Hajjaji, A.; Gaidi, M.; Bessais, B. Ag-NPs coating influence on TiO2-NTs photocatalytic performances on Amido Black staining. Res. Sq. 2022, 1–19. [Google Scholar] [CrossRef]
- Fu, C.; Li, M.; Li, H.; Li, C.; Wu, X.G.; Yang, B. of Au nanoparticle/TiO2 hybrid films for photoelectrocatalytic degradation of methyl orange. J. Alloys Compd. 2017, 692, 727–733. [Google Scholar] [CrossRef]
- Musa, I.; Ghabboun, J. Work function, electrostatic force microscopy, tunable photoluminescence of gold nanoparticles, and plasmonic interaction of gold nanoparticles/rhodamine 6G nanocomposite. Plasmonics 2024, 20, 2531–2540. [Google Scholar] [CrossRef]
- Surah, S.S.; Vishwakarma, M.; Kumar, R.; Nain, R.; Sirohi, S.; Kumar, G. Tuning the electronic band alignment properties of TiO2 nanotubes by boron doping. Results Phys. 2019, 12, 1725–1731. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, W.; Qian, X.-Y.; Liu, J.-B.; Wu, J.-M. UV and visible light photocatalytic activity of Au/TiO2 nanoforests with Anatase/Rutile phase junctions and controlled Au locations. Sci. Rep. 2017, 7, 41253. [Google Scholar] [CrossRef]
- Koushki, E.; Mowlavi, A.A.; Hoseini, S.T. Application of Localized Surface Plasmon Resonance of Conjugated Gold Nanoparticles in Spectral Diagnosis of SARS-CoV-2: A Numerical Study. Plasmonics 2023, 18, 1847–1855. [Google Scholar] [CrossRef]
- Manuel, A.P.; Shankar, K. Hot electrons in TiO2–noble metal nano-heterojunctions: Fundamental science and applications in photocatalysis. Nanomaterials 2021, 11, 1249. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, P.; Kumar, A.; Camargo, P.H.C.; Krishnan, V. Recent advances in plasmonic photocatalysis based on TiO2 and noble metal nanoparticles for energy conversion, environmental remediation, and organic synthesis. Small 2022, 18, 2101638. [Google Scholar] [CrossRef]
- Okuno, T.; Kawamura, G.; Muto, H.; Matsuda, A. Photocatalytic properties of Au-deposited mesoporous SiO2–TiO2 photocatalyst under simultaneous irradiation of UV and visible light. J. Solid State Chem. 2016, 235, 132–138. [Google Scholar] [CrossRef]
- She, P.; Xu, K.; Zeng, S.; He, Q.; Sun, H.; Liu, Z. Investigating the size effect of Au nanospheres on the photocatalytic activity of Au-modified ZnO nanorods. J. Colloid Interface Sci. 2017, 499, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kang, D.; Jeong, S.; Do, H.T.; Kim, J.H. Photocatalytic degradation of rhodamine B dye by TiO2 and gold nanoparticles supported on a floating porous polydimethylsiloxane sponge under ultraviolet and visible light irradiation. ACS Omega 2020, 5, 4233–4241. [Google Scholar] [CrossRef]
- Wu, L.; Li, F.; Xu, Y.; Zhang, J.W.; Zhang, D.; Li, G.; Li, H. Plasmon-induced photoelectrocatalytic activity of Au nanoparticles enhanced TiO2 nanotube arrays electrodes for environmental remediation. Appl. Catal. B Environ. 2015, 164, 217–224. [Google Scholar] [CrossRef]
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, R.M.; Martins, R.C. N–TiO2 photocatalysts: A review of their characteristics and capacity for emerging contaminants removal. Water 2019, 11, 373. [Google Scholar] [CrossRef]
- Hajjaji, A.; Jemai, S.; Rebhi, A.; Trabelsi, K.; Gaidi, M.; Alhazaa, A.; Al-Gawati, M.; El Khakani, M.; Bessais, B. Enhancement of photocatalytic and photoelectrochemical properties of TiO2 nanotubes sensitized by SILAR-Deposited PbS nanoparticles. J. Mater. 2020, 6, 62–69. [Google Scholar] [CrossRef]
- Abidi, M.; Hajjaji, A.; Bouzaza, A.; Trablesi, K.; Makhlouf, H.; Rtimi, S.; Assadi, A.; Bessais, B. Simultaneous removal of bacteria and volatile organic compounds on Cu2O-NPs decorated TiO2 nanotubes: Competition effect and kinetic studies. J. Photochem. Photobiol. A Chem. 2020, 400, 112722. [Google Scholar] [CrossRef]
- Ali, I.; Alharbi, O.M.L.; Alothman, Z.A.; Badjah, A.Y. Kinetics, thermodynamics, and modeling of amido black dye photodegradation in water using Co/TiO2 nanoparticles. Photochem. Photobiol. 2018, 94, 935–941. [Google Scholar] [CrossRef]

| Atomic % | |||
|---|---|---|---|
| Samples | Titanium (Ti) | Oxygen (O) | Gold (Au) |
| 5 min Au/TiO2-NTs | 62.51 | 32.61 | 0.13 |
| 8 min Au/TiO2-NTs | 59.03 | 34.61 | 0.16 |
| 12 min Au/TiO2-NTs | 58.31 | 38.13 | 0.17 |
| TiO2-NTs | 5 Min Au/TiO2-NTs | 8 Min Au/TiO2-NTs | 12 Min Au/TiO2-NTs | |
|---|---|---|---|---|
| I(004)/I(101) | 0.21 | 0.91 | 0.83 | 0.82 |
| I(112)/I(101) | 0.064 | 0.42 | 0.29 | 0.64 |
| Samples | 2θ (°) | FWHM (rad) | D (nm) | Microstrain (%) × 10−3 |
|---|---|---|---|---|
| Bare TiO2-NTs | 25.156 | 0.002267 | 62 | 2.5 |
| 5 min Au/TiO2-NTs | 25.154 | 0.002189 | 64.2 | 2.4 |
| 8 min Au/TiO2-NTs | 25.156 | 0.002171 | 64.7 | 2.4 |
| 12 min Au/TiO2-NTs | 25.142 | 0.002477 | 56.7 | 2.8 |
| Samples | TiO2-NTs | TiO2 NTs/Au (5 min) | TiO2 NTs/Au (8 min) | TiO2 NTs/Au (12 min) |
| Band gap (eV) | 3.06 | 3.36 | 3.25 | 2.89 |
| Time(s) | 5 | 15 | 30 | 45 | 60 | 90 | 120 | 150 | 180 | 210 | 240 | 270 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D% | 8.86 | 11.47 | 18.99 | 25.09 | 30.10 | 35.48 | 48.38 | 59.49 | 63.44 | 62.72 | 75.27 | 86.02 |
| Catalyst | Conditions | Photodegradation Efficiency (%) | Photodegradation Time (min) | Ref |
|---|---|---|---|---|
| Pt-NPs/TiO2-NTs | [Black Amido] = 5 mg L−1 [catalyst] = thin film (2.5 × 2.5 cm) UV lamp = 15 W (256 nm) | 97 | 90 | [16] |
| Ag-NPs/TiO2-NTs | [Black Amido] = 5 mg L−1 [catalyst] = thin film (2.5 × 2.5 cm2) UV lamp = 15 W (256 nm) | 96.4 | 270 | [46] |
| Au-NPs/TiO2-NTs | [Methyl Orange] = 5.2 mg L−1 [catalyst] = thin film (Ti wires Ø = 1.5 mm and L = 6 cm) UV lamp = 350 W (365 nm) | 72.5 | 200 | [47] |
| Au-NPs/TiO2-NTs | [Black Amido] = 5 mg L−1 [catalyst] = thin film (2.5 × 1 cm2) UV lamp = 15 W (256 nm) | 86 | 270 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benghanoum, H.; Khezami, L.; Zaghouani, R.B.; Sassi, S.; Guesmi, A.; Bouich, A.; Soucase, B.M.; Hajjaji, A. Electrophoretic Deposition of Gold Nanoparticles on Highly Ordered Titanium Dioxide Nanotubes for Photocatalytic Application. Catalysts 2025, 15, 781. https://doi.org/10.3390/catal15080781
Benghanoum H, Khezami L, Zaghouani RB, Sassi S, Guesmi A, Bouich A, Soucase BM, Hajjaji A. Electrophoretic Deposition of Gold Nanoparticles on Highly Ordered Titanium Dioxide Nanotubes for Photocatalytic Application. Catalysts. 2025; 15(8):781. https://doi.org/10.3390/catal15080781
Chicago/Turabian StyleBenghanoum, Halima, Lotfi Khezami, Rabia Benabderrahmane Zaghouani, Syrine Sassi, Ahlem Guesmi, Amal Bouich, Bernabé Mari Soucase, and Anouar Hajjaji. 2025. "Electrophoretic Deposition of Gold Nanoparticles on Highly Ordered Titanium Dioxide Nanotubes for Photocatalytic Application" Catalysts 15, no. 8: 781. https://doi.org/10.3390/catal15080781
APA StyleBenghanoum, H., Khezami, L., Zaghouani, R. B., Sassi, S., Guesmi, A., Bouich, A., Soucase, B. M., & Hajjaji, A. (2025). Electrophoretic Deposition of Gold Nanoparticles on Highly Ordered Titanium Dioxide Nanotubes for Photocatalytic Application. Catalysts, 15(8), 781. https://doi.org/10.3390/catal15080781










