Piancatelli–Margarita Oxidation and Its Recent Applications in Organic Synthesis
Abstract
1. Introduction
2. The Original Piancatelli–Margarita Oxidation
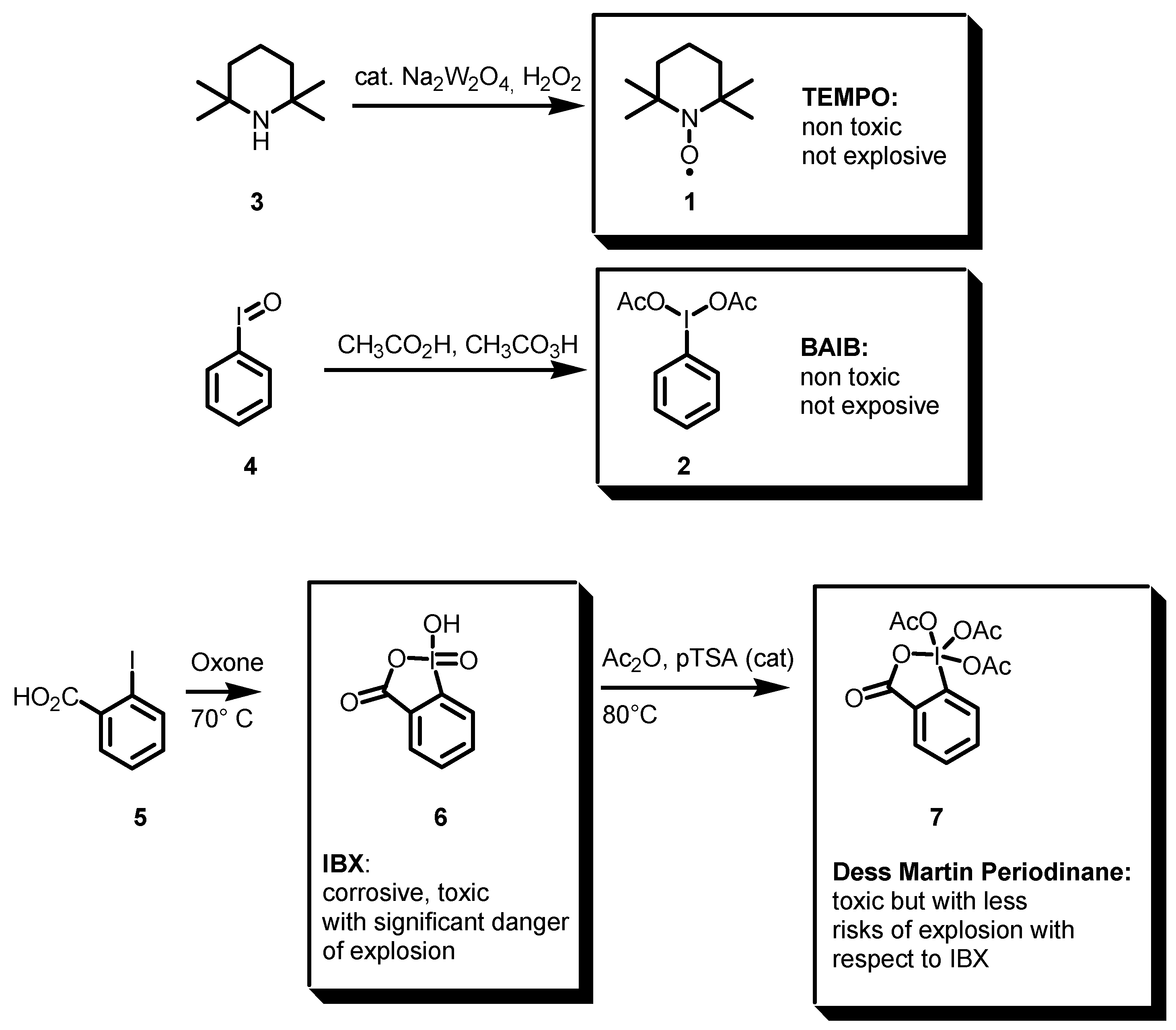
2.1. TEMPO, BAIB, and Comparison with Other Hypervalent Iodine Compounds as Oxidants
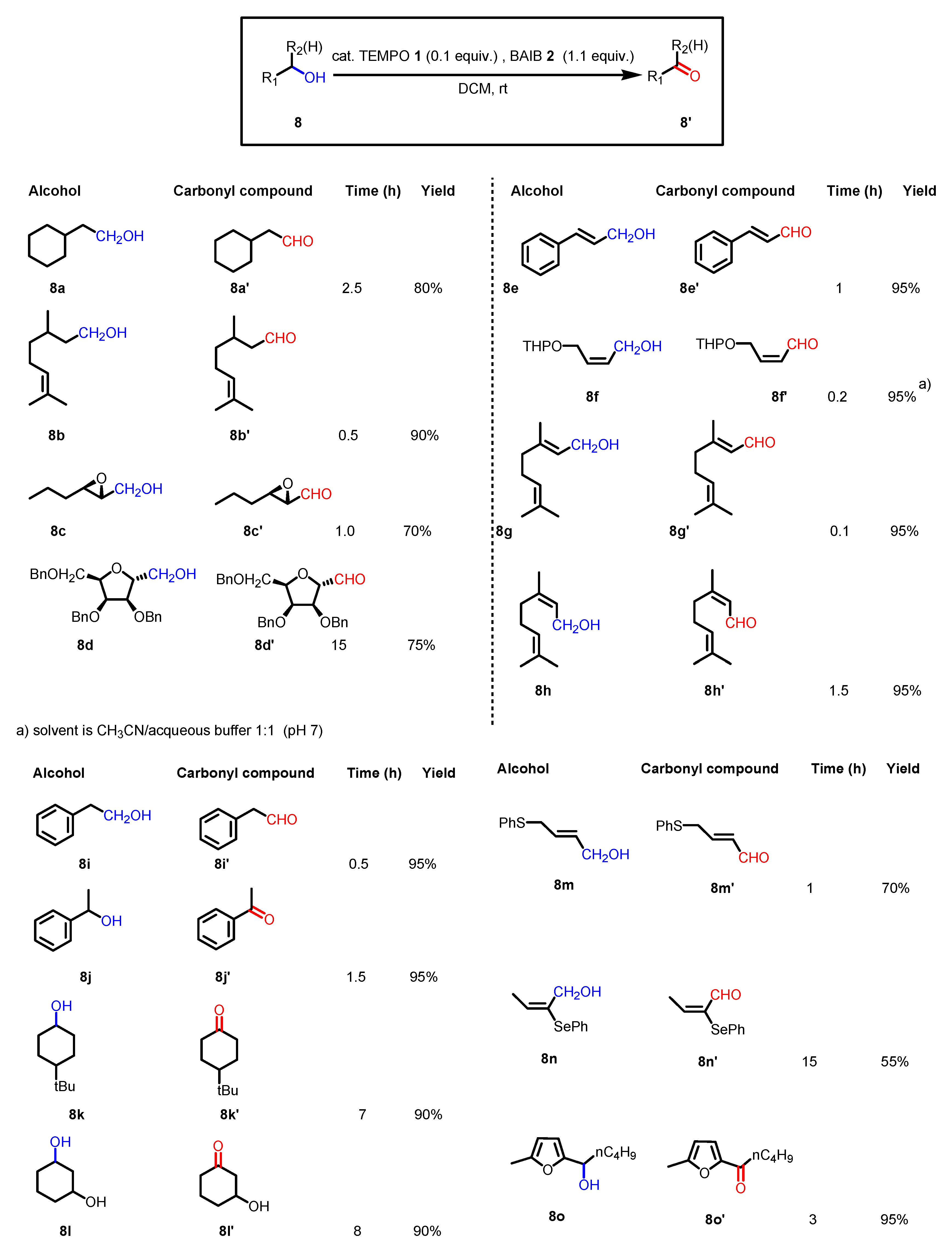
2.2. Reaction Conditions and Substrate Scope in the Original Paper
2.3. Key Features of the Piancatelli–Margarita Oxidation
- -
- The reaction is catalytic with respect to the oxidant; the reagents are non-toxic or explosive and can be considered environmentally benign.
- -
- For most reactions, only slightly more than one equivalent of BAIB 2 is needed. Solid BAIB 2 can be weighed and dosed in a better manner with respect to a solution of NaClO. Therefore, the Piancatelli–Margarita reaction can also be performed on a very small scale, which is the one employed, for example, in the last stages of a total synthesis.
- -
- The reaction is purely organic. No water is necessary.
- -
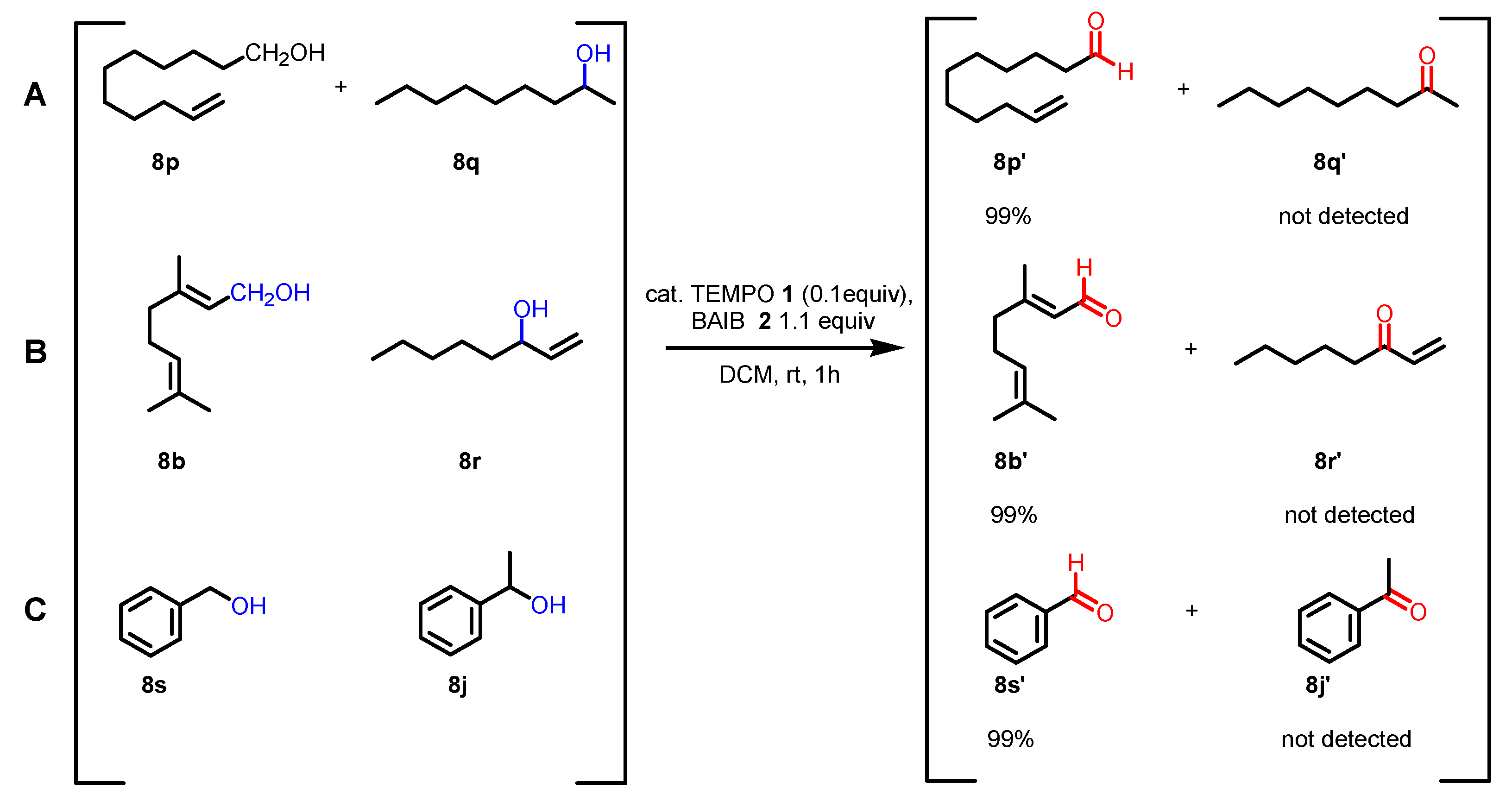
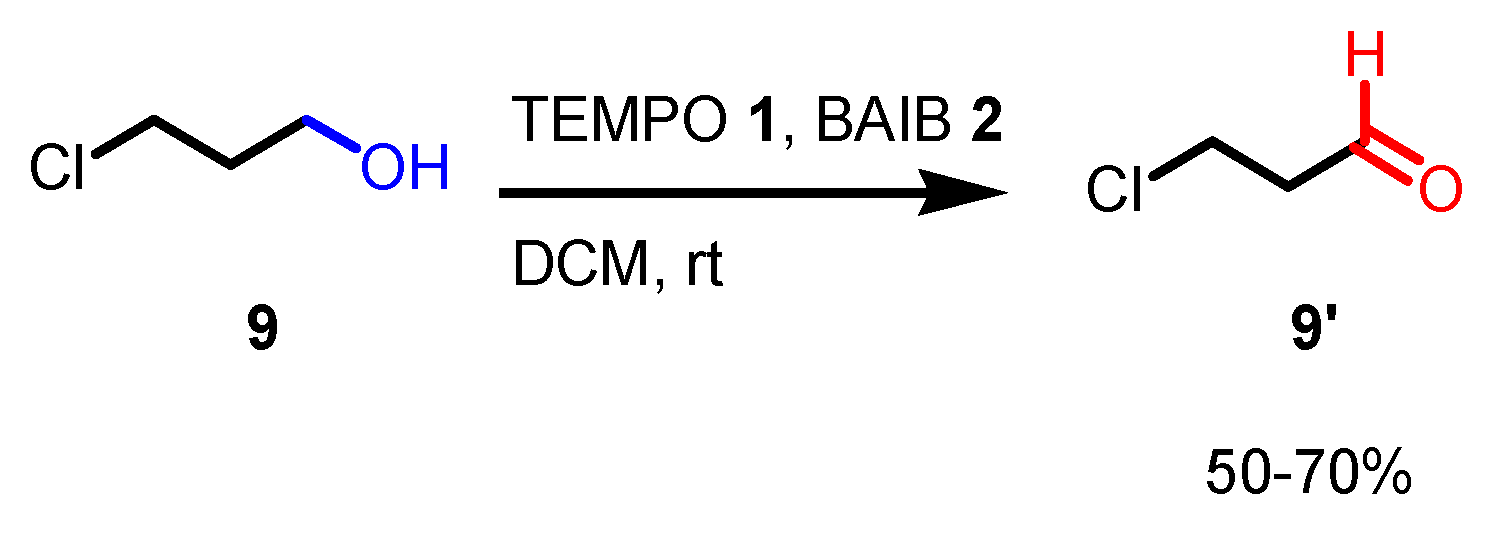
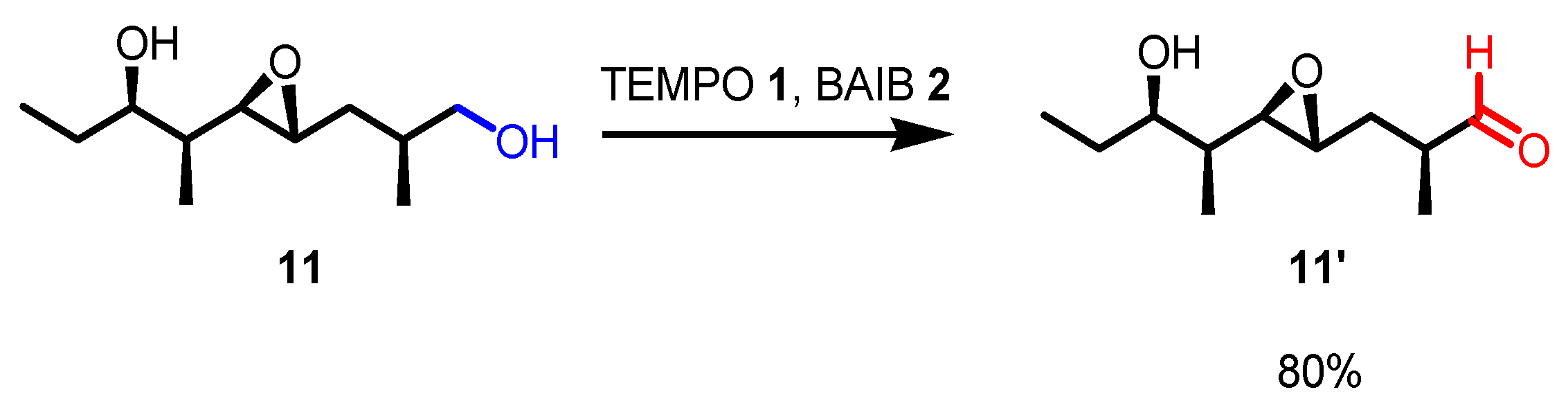
- With primary alcohols, the reaction can lead to the selective formation of aldehydes. The conditions for selective oxidation are short reaction times (one or two hours), room temperature, and the usage of a low-polar solvent such as DCM. If the reaction conditions are forced (longer reaction times, mixtures of acetonitrile and water as the solvent, higher reaction temperatures such as 70 °C), this transformation leads to the formation of carboxylic acids (see following examples for details).
- -
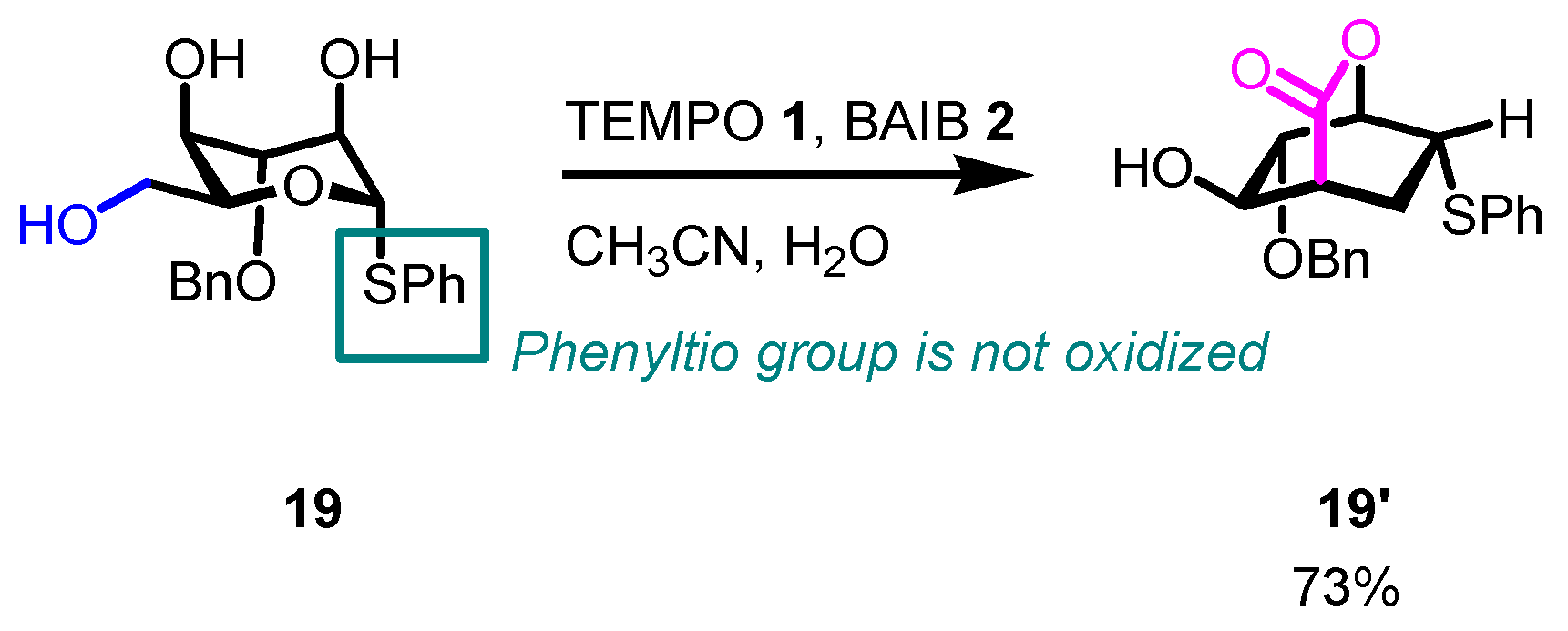
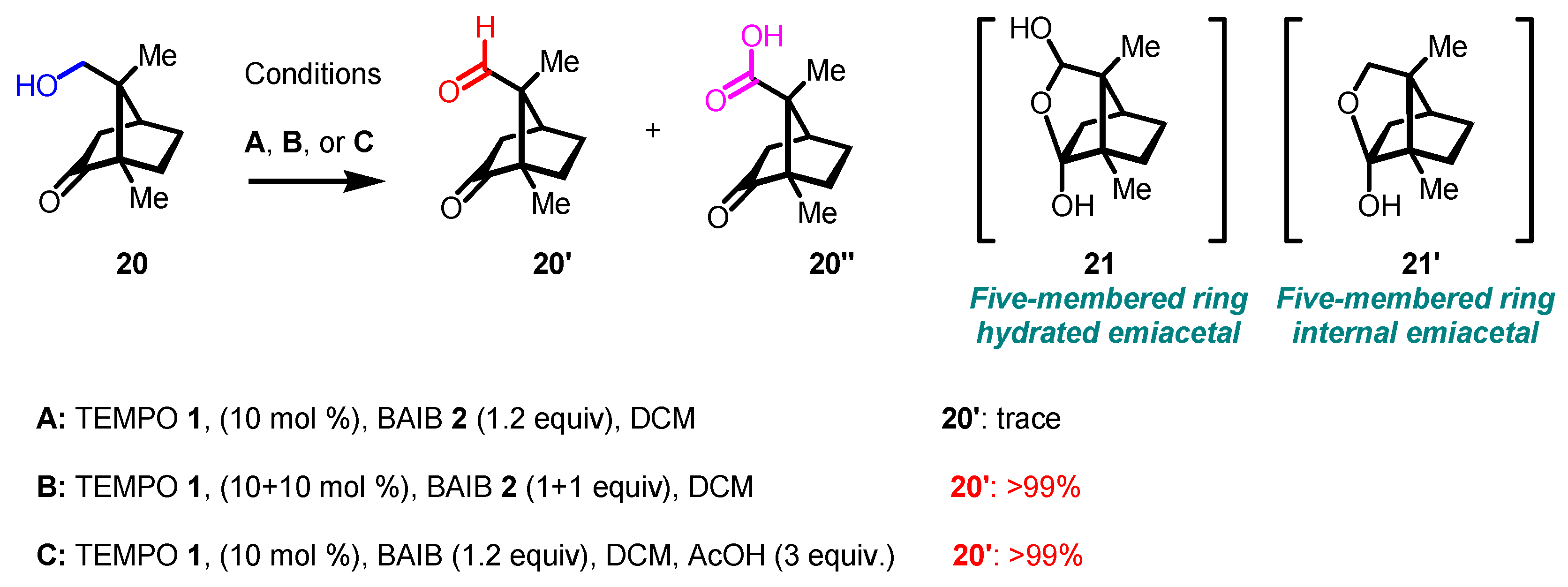
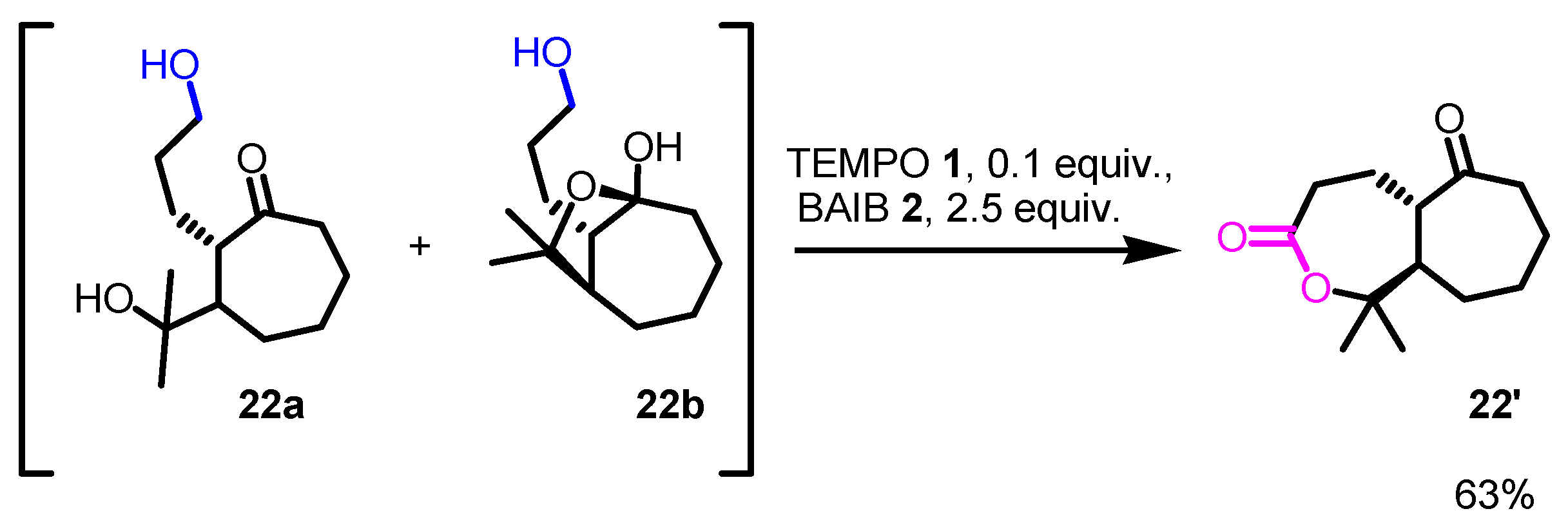
- In some instances, overoxidation cannot be avoided, especially when five-member or larger cyclic hemiacetals are formed. In these cases, only lactones are formed (see following Schemes, with related references).
- -
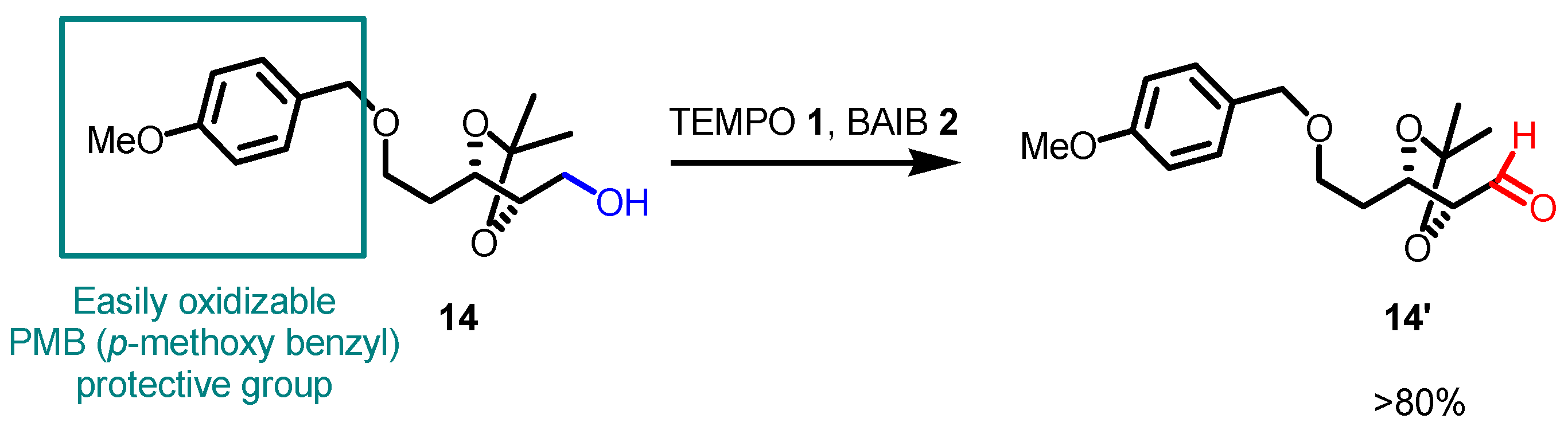
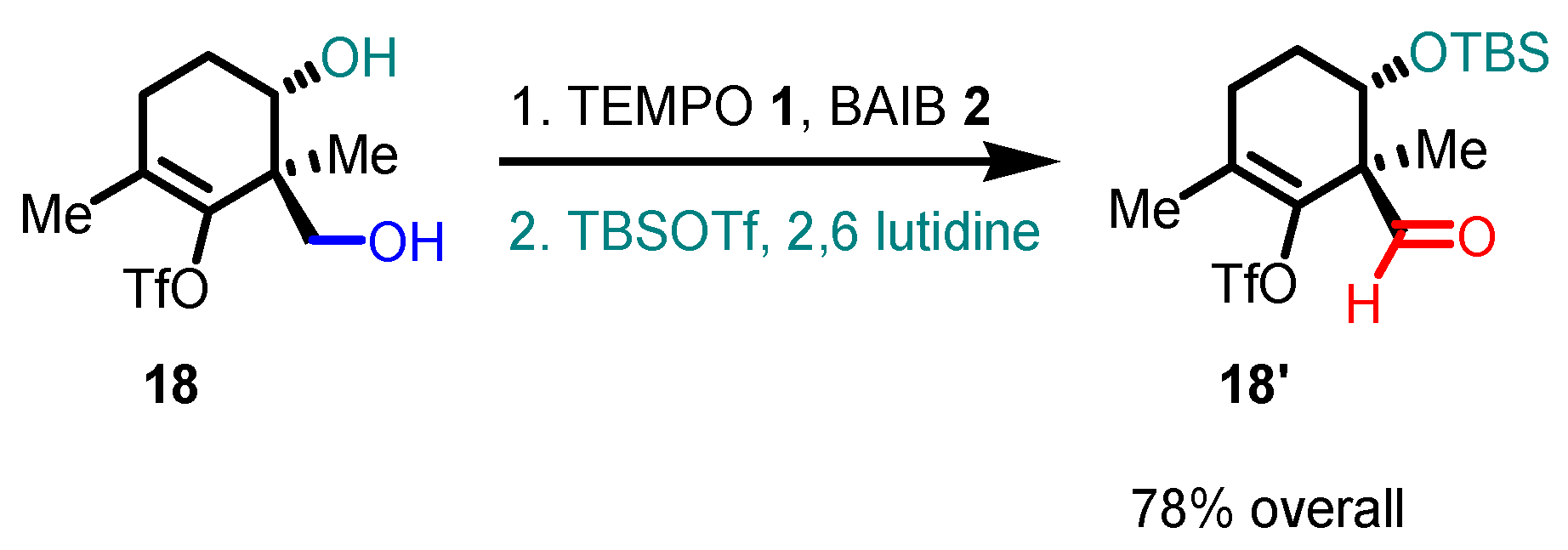
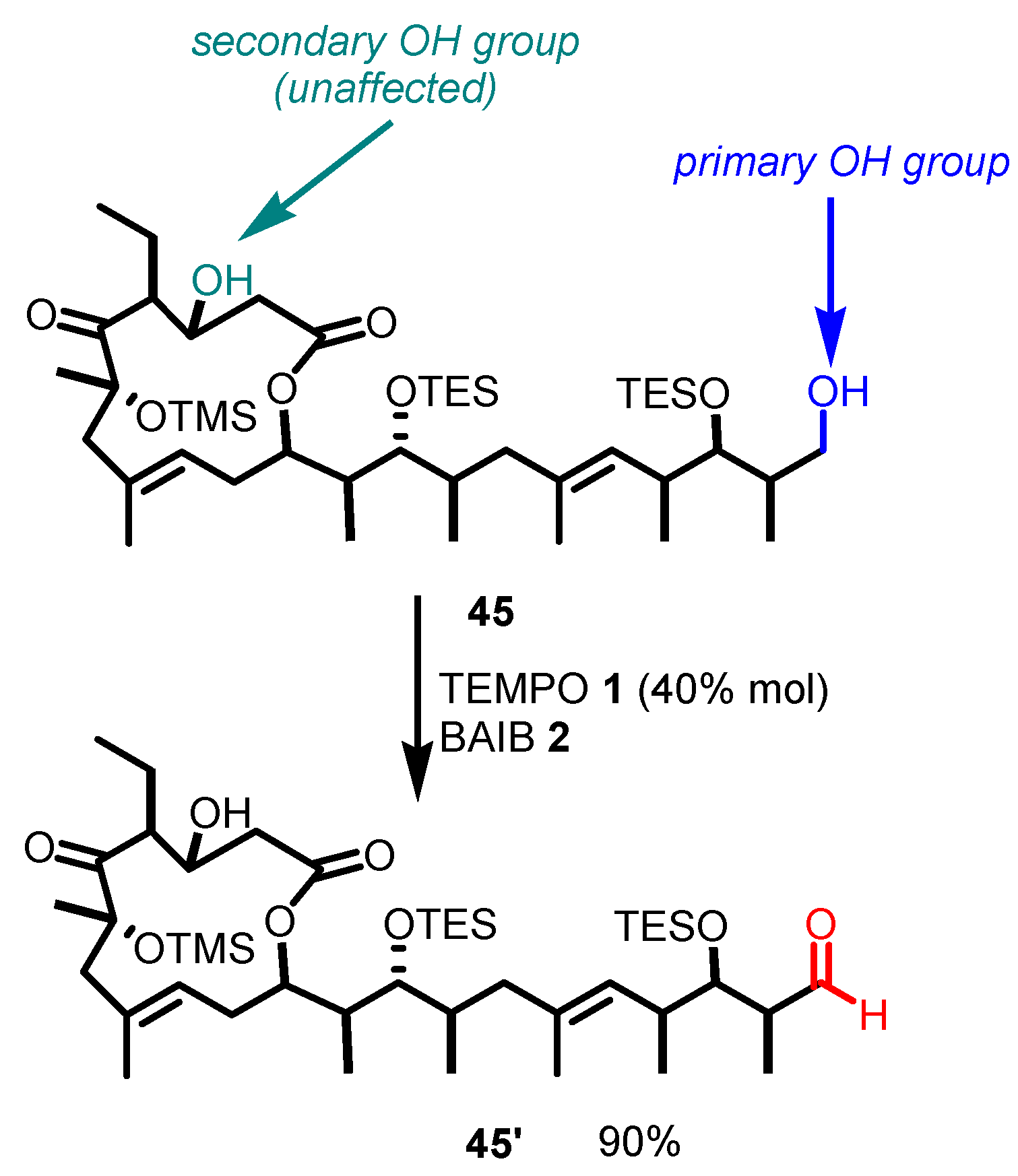
- The reaction is chemoselective: primary alcohols can be selectively oxidized in the presence of secondary alcohols, and easily oxidizable sulfur/selenium functionalities are not affected.
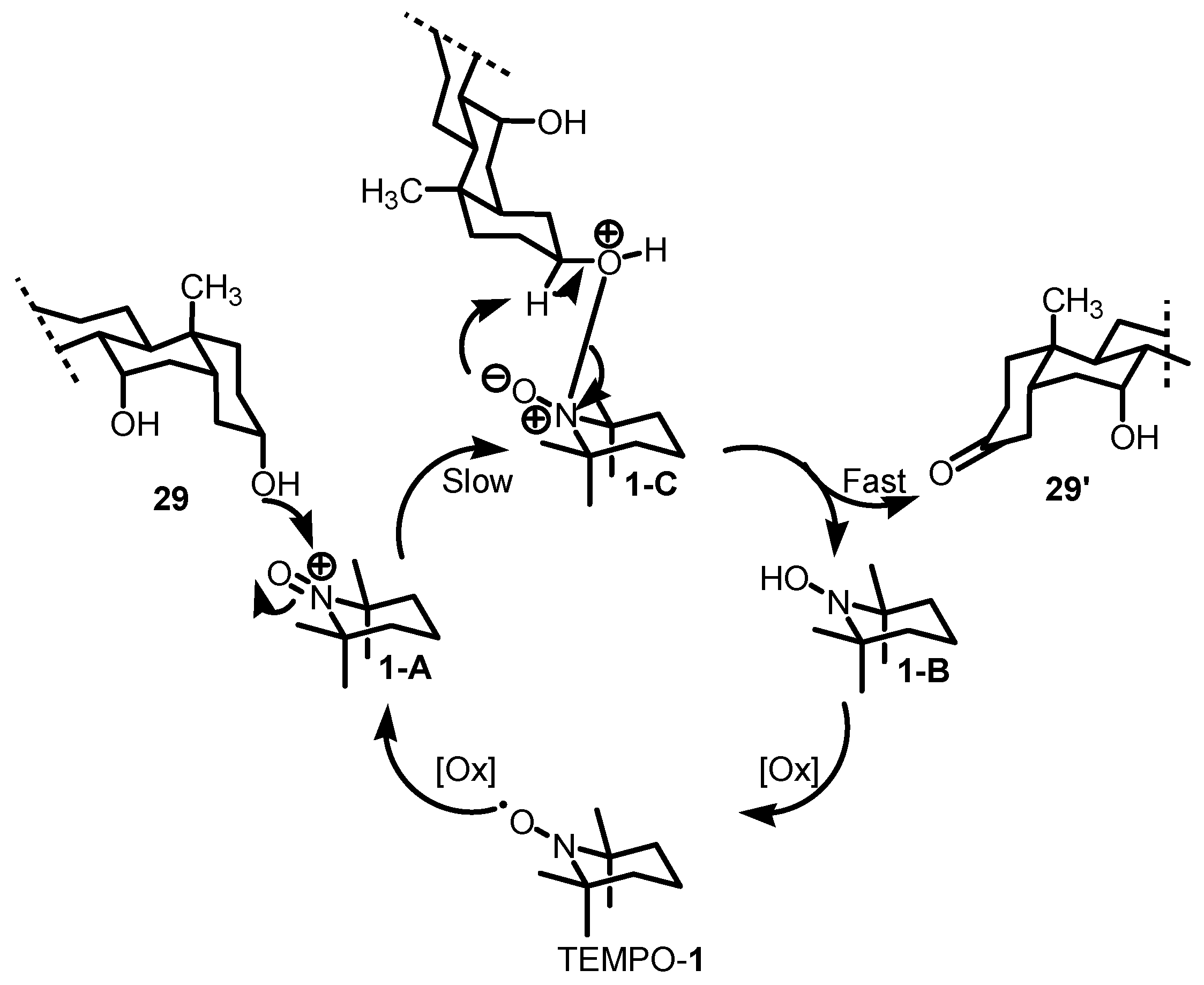
2.4. Proposed Reaction Mechanism
3. Selected Examples of Piancatelli–Margarita Oxidation Applications in Synthesis: Large-Scale Reactions and Small Molecules
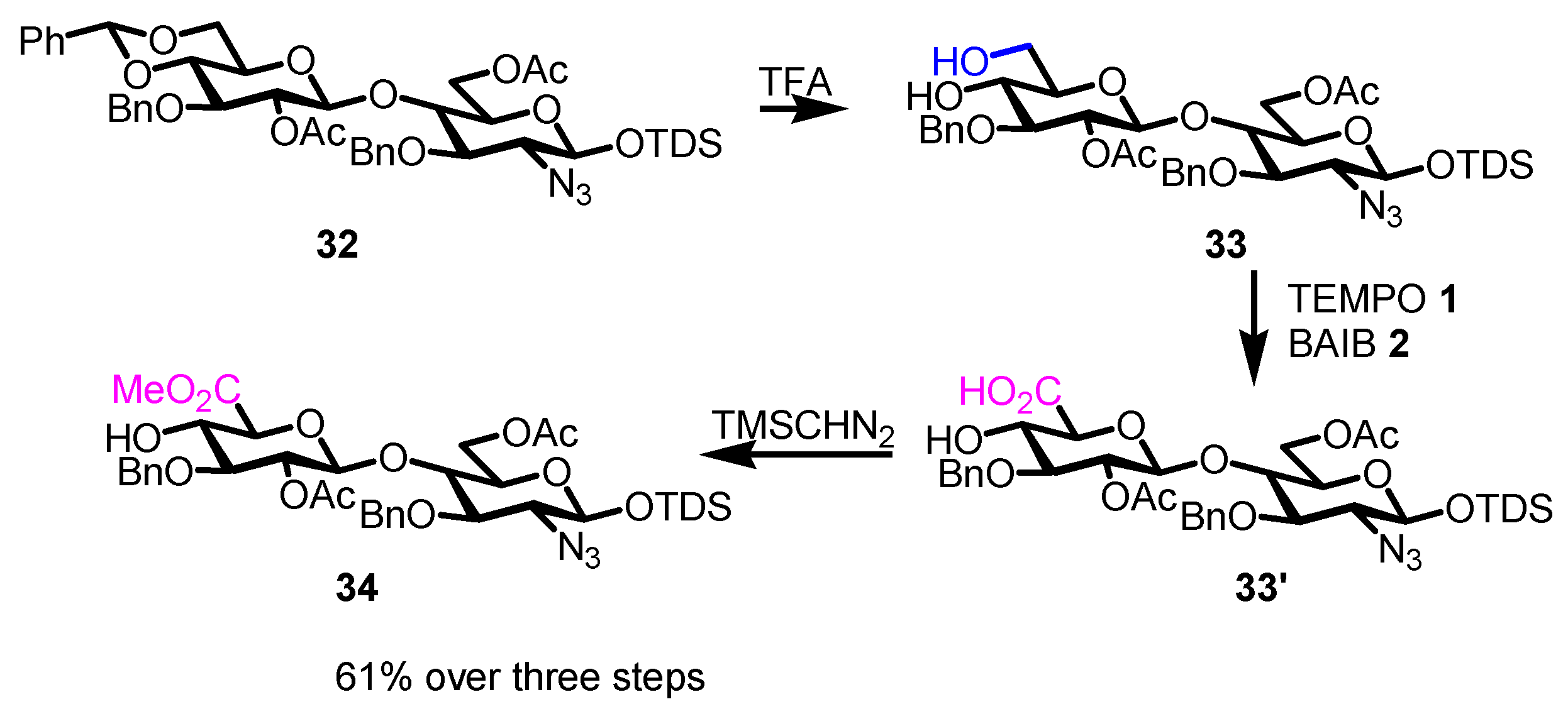
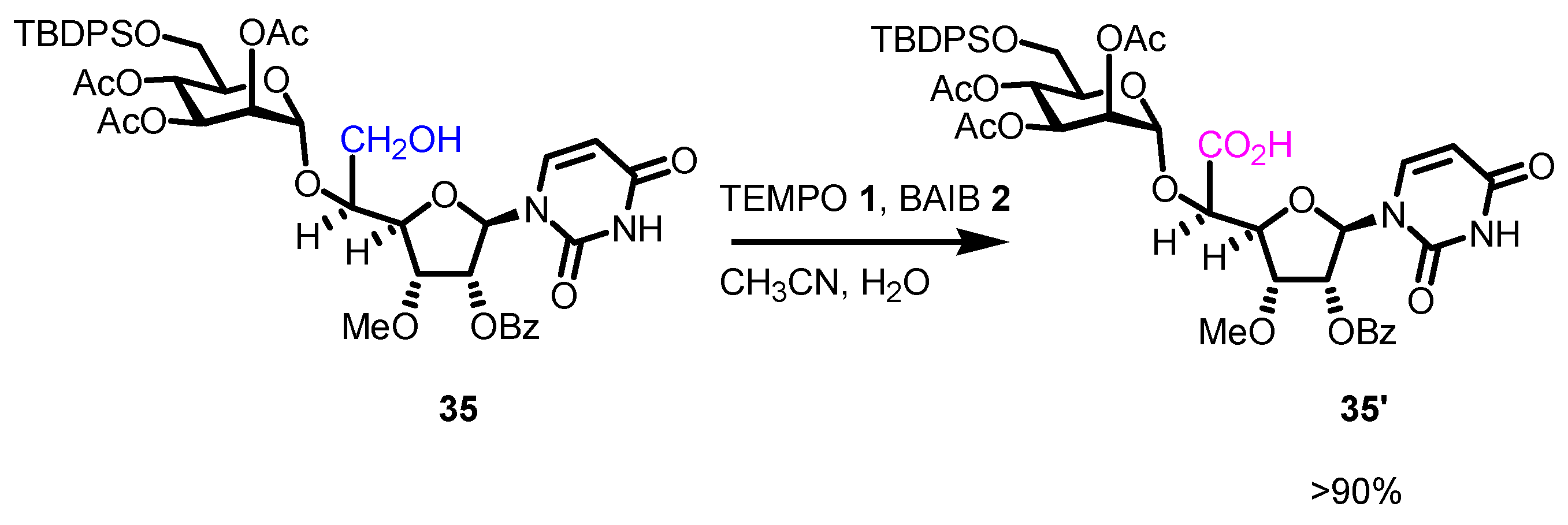
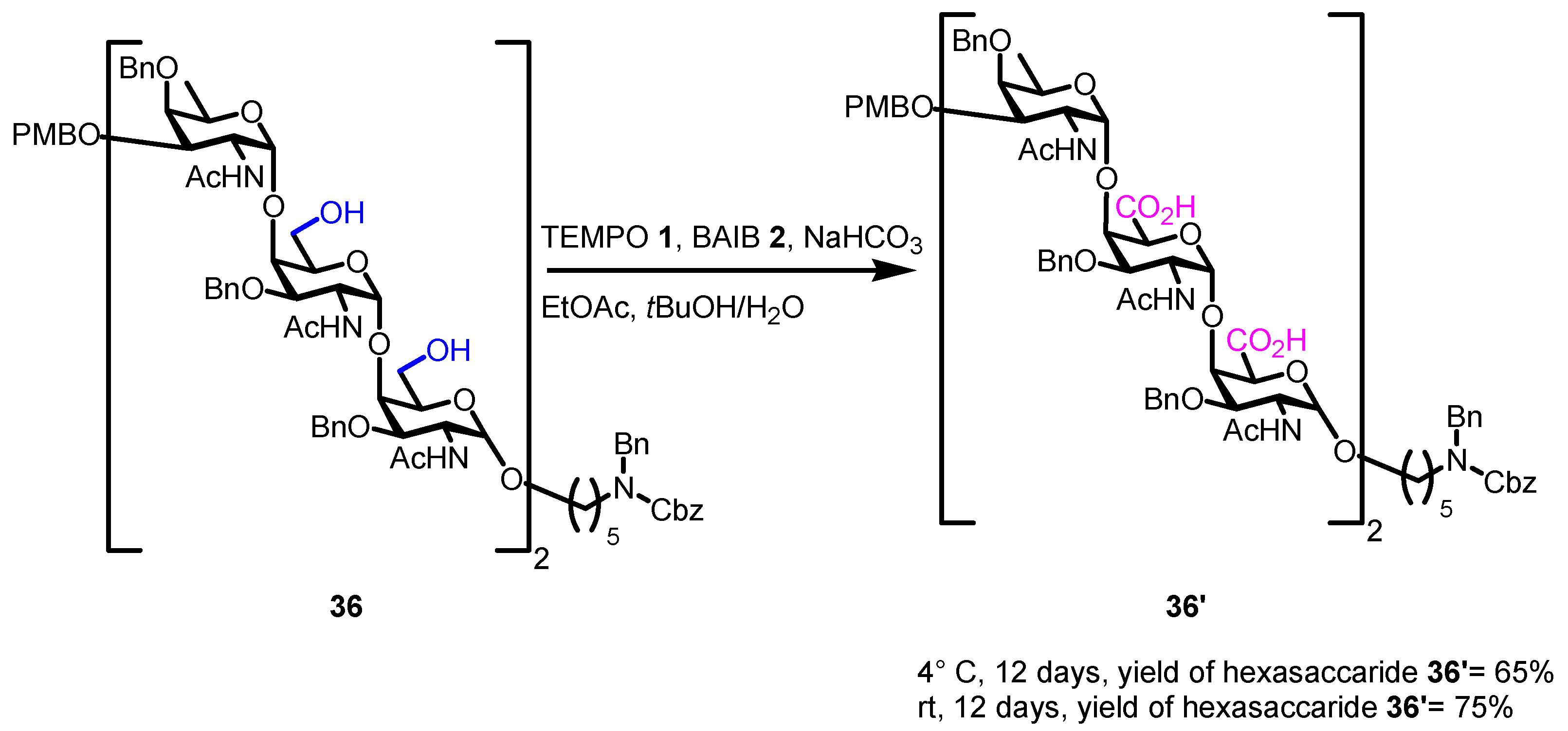
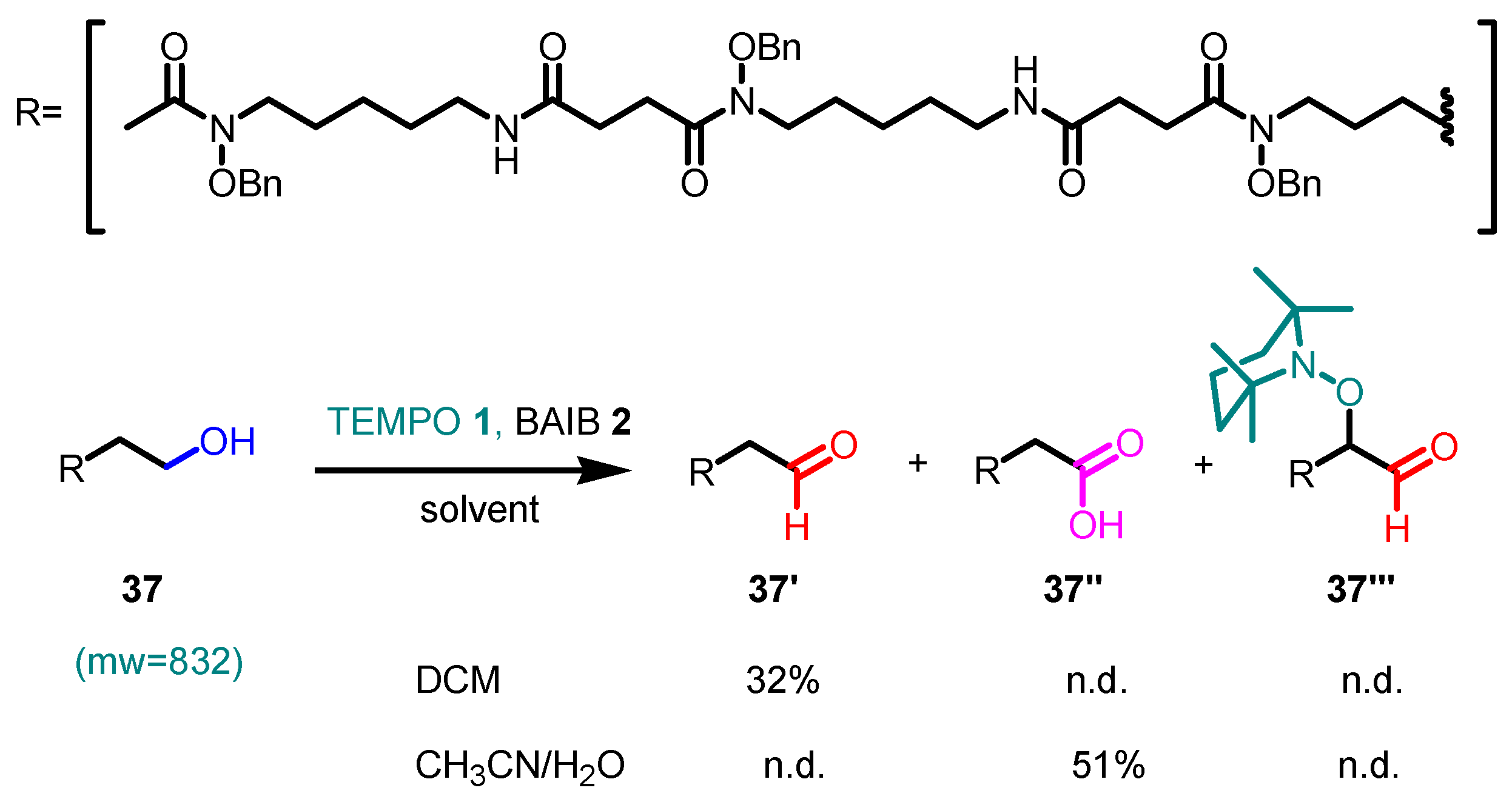
4. Noteworthy Applications of Piancatelli–Margarita Oxidation in Carbohydrate Chemistry: Examples from the Recent Literature
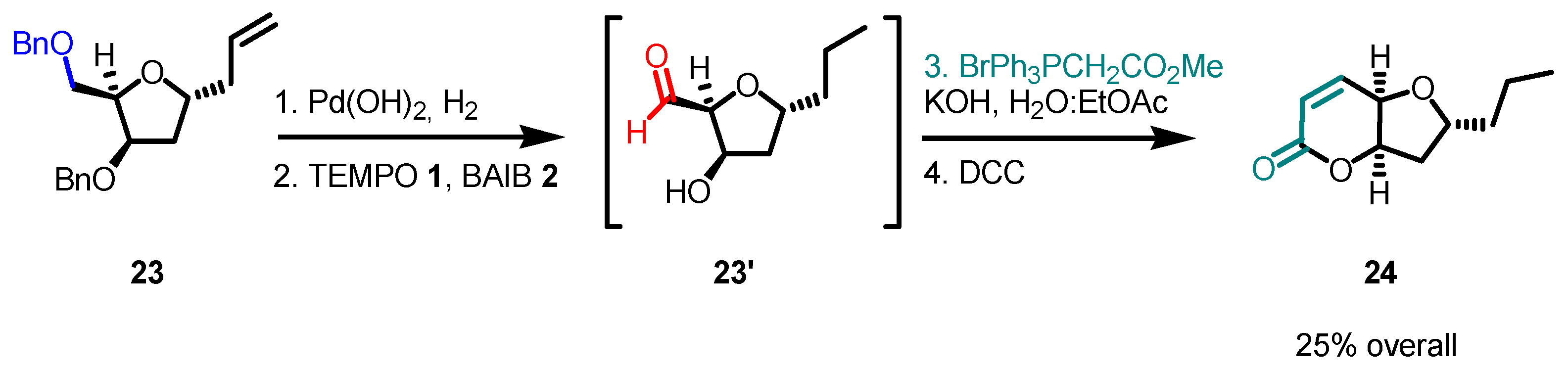
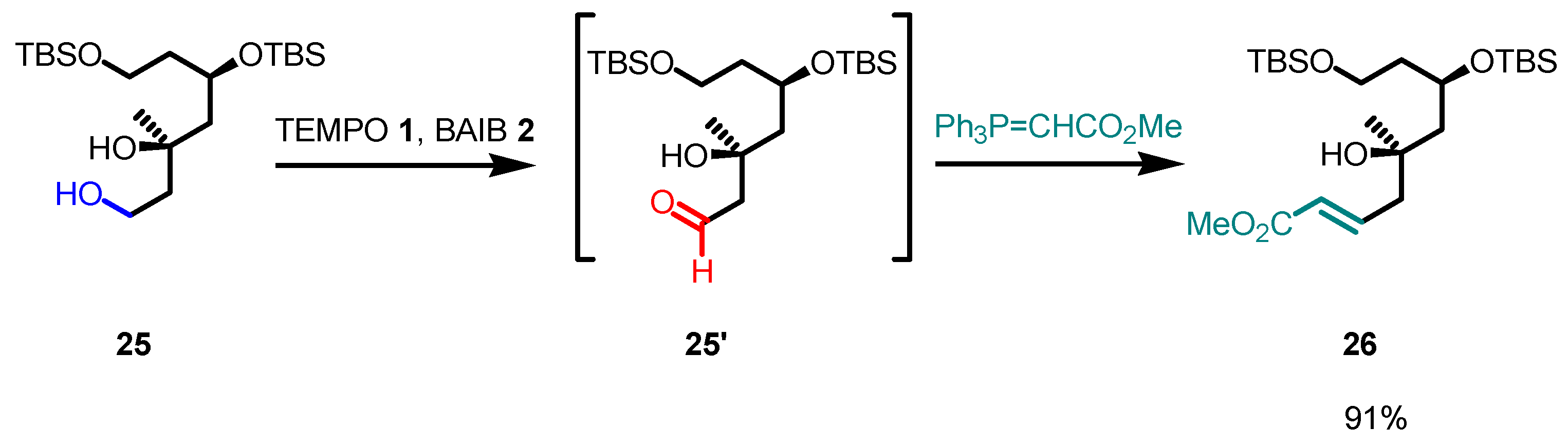
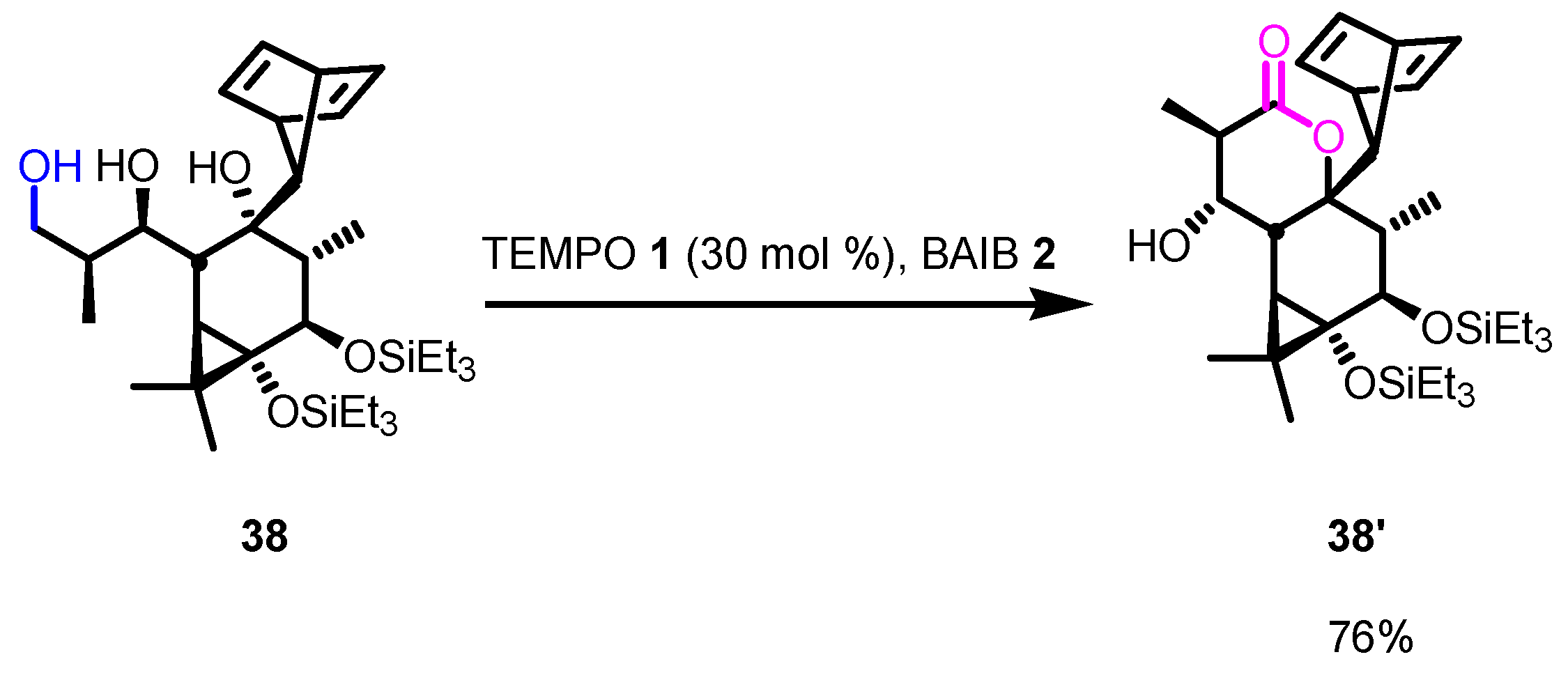
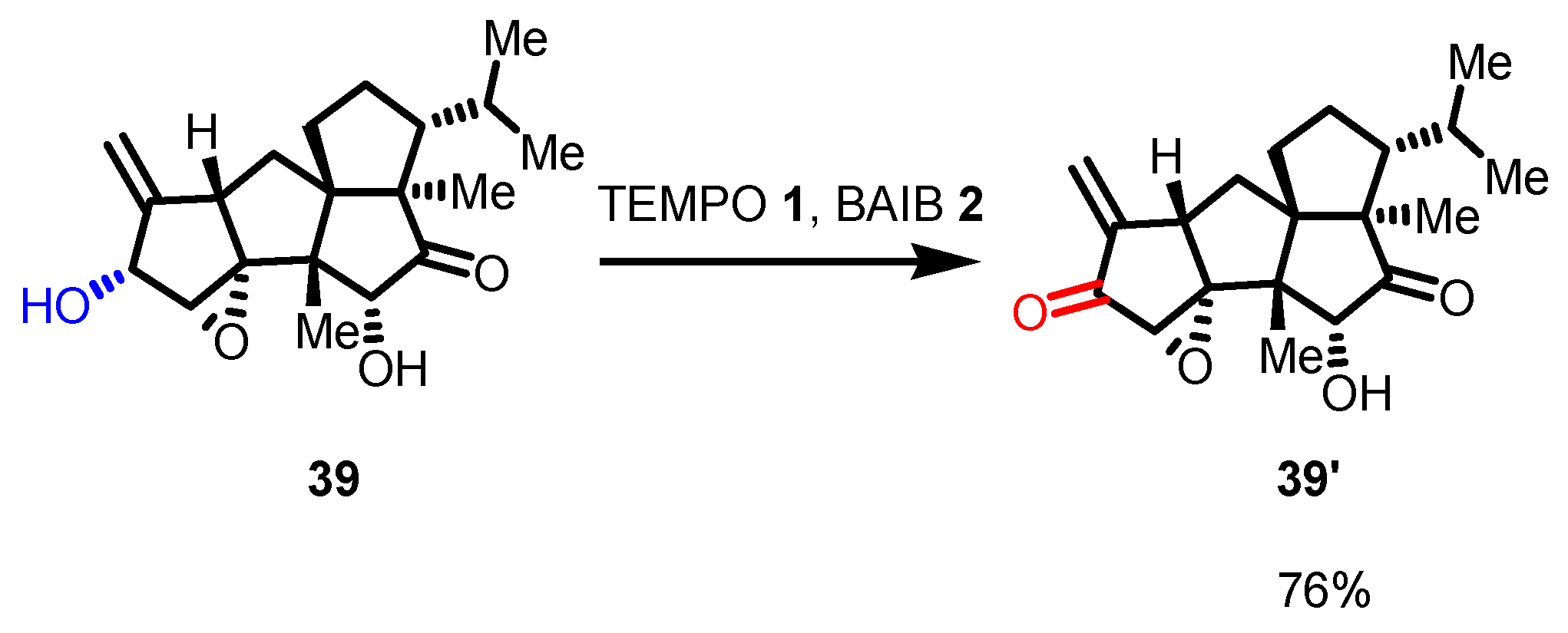
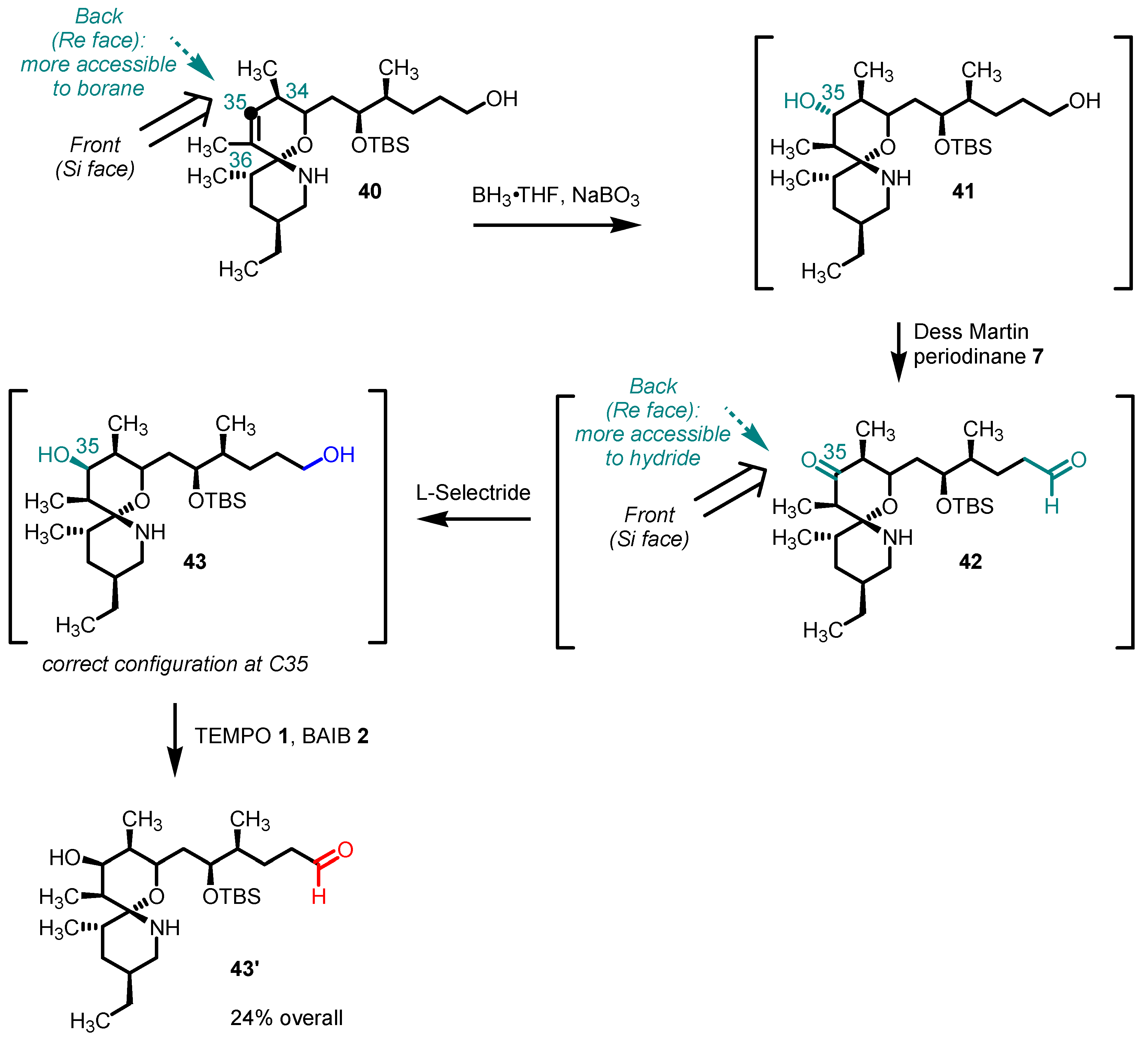
5. Selected Examples of Piancatelli–Margarita Oxidation Applications in Total Synthesis: Late-Stage Intermediates and Endgame
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TEMPO | (2,2,6,6-tetramethylpiperidin-1-yl)oxyl |
| BAIB | bis(acetoxy)iodobenzene |
| IBX | 1-hydroxy-1λ5,2-benziodoxole-1,3-dione |
| DCM | dichloromethane |
| PCC | pyridinium chlorochromate |
References
- De Mico, A.; Margarita, R.; Parlanti, L.; Vescovi, A.; Piancatelli, G. A Versatile and Highly Selective Hypervalent Iodine (III)/2,2,6,6-Tetramethyl-1-Piperidinyloxyl-Mediated Oxidation of Alcohols to Carbonyl Compounds. J. Org. Chem. 1997, 62, 6974–6977. [Google Scholar] [CrossRef]
- Piancatelli, G.; Scettri, A.; Barbadoro, S. A useful preparation of 4-substituted 5-hydroxy-3-oxocyclopentene. Tetrahedron Lett. 1976, 39, 3555–3558. [Google Scholar] [CrossRef]
- Piancatelli, G.; Leonelli, F. Oxidation of nerol to neral with iodobenzene and TEMPO. Org. Synth. 2006, 83, 18–23. [Google Scholar] [CrossRef]
- Leonelli, F.; Margarita, R.; Piancatelli, G. Discussion addendum for: Oxidation of Nerol to Neral with iodosobenzene and TEMPO. Org. Synth. 2012, 89, 311–322. [Google Scholar] [CrossRef]
- Montanari, F.; Quici, S.; Henry-Riyad, H.; Tidwell, T.T. 2,2,6,6-Tetramethylpiperidin-1-oxyl. In Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 0471936235. [Google Scholar] [CrossRef]
- Anelli, P.L.; Biffi, C.; Montanari, F.; Quici, S. Fast and selective oxidation of primary alcohols to aldehydes or to carboxylic acids and of secondary alcohols to ketones mediated by oxoammonium salts under two-phase conditions. J. Org. Chem. 1987, 52, 2559–2562. [Google Scholar] [CrossRef]
- De Luca, L.; Giacomelli, G.; Porcheddu, A. A Very Mild and Chemoselective Oxidation of Alcohols to Carbonyl Compounds. Org. Lett. 2001, 3, 3041–3043. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, J.; Einhorn, C.; Ratajczak, F.; Pierre, J.-L. Efficient and Highly Selective Oxidation of Primary Alcohols to Aldehydes by N-Chlorosuccinimide Mediated by Oxoammonium Salts. J. Org. Chem. 1996, 61, 7452–7454. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Pagliaro, M. Industrial Oxidations with Organocatalyst TEMPO and Its Derivatives. Org. Process Res. Dev. 2010, 14, 245–251. [Google Scholar] [CrossRef]
- Available online: https://www.sigmaaldrich.com/US/en/substance/tempo156252564832 (accessed on 12 July 2025).
- Moriarty, R.M.; Chany, C.J.; Kosmeder, J.W.; Du Bois, J. (Diacetoxyiodo)benzene. In Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2001; ISBN 9780470842898. [Google Scholar] [CrossRef]
- Frigerio, M.; Santagostino, M.; Sputore, S. A User-Friendly Entry to 2-Iodoxybenzoic Acid (IBX). J. Org. Chem. 1999, 64, 4537–4538. [Google Scholar] [CrossRef]
- Boeckman, R.J.; George, K.M. 1,1,1-Triacetoxy-1,1-dihydro-1,2-benziodoxol-3(1H)-one. In Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 978-0471936237. [Google Scholar] [CrossRef]
- Available online: https://www.sigmaaldrich.com/US/en/search/ibx?focus=products&page=1&perpage=30&sort=relevance&term=ibx&type=product (accessed on 12 July 2025).
- Available online: https://www.sigmaaldrich.com/US/en/search/dess-martin-periodinane?focus=products&page=1&perpage=30&sort=relevance&term=Dess-Martin%20periodinane&type=product (accessed on 12 July 2025).
- Available online: https://www.sigmaaldrich.com/US/en/sds/aldrich/178721?userType=anonymous (accessed on 12 July 2025).
- Available online: https://www.sigmaaldrich.com/US/en/search/baib?focus=products&page=1&perpage=30&sort=relevance&term=BAIB&type=product (accessed on 12 July 2025).
- Zelch, D.; Russo, C.M.; Ruud, K.J.; O’Reilly, M.C. A General and Scalable Method toward Enantioenriched C2-Substituted Azetidines Using Chiral Tert-Butanesulfinamides. J. Org. Chem. 2024, 89, 15137–15144. [Google Scholar] [CrossRef]
- Lee, M.; Wu, J.-P.; Lee, J.; Wang, J.; White, J.A.H.; Rugg, K.W.; Sienkiewicz, A.; Lorenz, J.C.; Greb, P.; Bunner, M.H.; et al. A Chiral Pool Strategy for the Synthesis of a SMARCA2 Degrading PROTAC. Org. Process Res. Dev. 2024, 28, 1239–1252. [Google Scholar] [CrossRef]
- Rhoades, D.; Rheingold, A.L.; O’Malley, B.W.; Wang, J. Expedient Total Syntheses of Pladienolide-Derived Spliceosome Modulators. J. Am. Chem. Soc. 2021, 143, 4915–4920. [Google Scholar] [CrossRef]
- Chourreu, P.; Guerret, O.; Guillonneau, L.; Gayon, E.; Lefèvre, G. Short and Easily Scalable Synthesis of the Sex Pheromone of the Horse-Chestnut Leaf Miner (Cameraria ohridella) Relying on a Key Ligand- and Additive-Free Iron-Catalyzed Cross-Coupling. Org. Process Res. Dev. 2020, 24, 1335–1340. [Google Scholar] [CrossRef]
- Kaden, F.; Nowotni, S.; Höfner, F.; Lorenz, M.; Barthel, A.; Jäger, A.; Hennersdorf, F.; Weigand, J.J.; Metz, P. Asymmetric Total Synthesis of (−)-Dehydrocostus Lactone by Domino Metathesis. Eur. J. Org. Chem. 2021, 2021, 3579–3586. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Hsu, C.-S. Enantioselective Total Synthesis of (+)-EBC-23, a Potent Anticancer Agent from the Australian Rainforest. J. Org. Chem. 2021, 86, 6351–6360. [Google Scholar] [CrossRef] [PubMed]
- Movahhed, S.; Westphal, J.; Kempa, A.; Schumacher, C.E.; Sperlich, J.; Neudörfl, J.; Teusch, N.; Hochgürtel, M.; Schmalz, H. Total Synthesis of (+)-Erogorgiaene and the Pseudopterosin A−F Aglycone via Enantioselective Cobalt-Catalyzed Hydrovinylation. Chem. A Eur. J. 2021, 27, 11574–11579. [Google Scholar] [CrossRef]
- Vece, V.; Jakkepally, S.; Hanessian, S. Total Synthesis and Absolute Stereochemical Assignment of the Insecticidal Metabolites Yaequinolones J1 and J2. Org. Lett. 2018, 20, 4277–4280. [Google Scholar] [CrossRef]
- Kobayakawa, T.; Azuma, C.; Watanabe, Y.; Sawamura, S.; Taniguchi, A.; Hayashi, Y.; Tsuji, K.; Tamamura, H. Development of Methods for Convergent Synthesis of Chloroalkene Dipeptide Isosteres and Its Application. J. Org. Chem. 2021, 86, 5091–5101. [Google Scholar] [CrossRef] [PubMed]
- Perea, M.A.; Wang, B.; Wyler, B.C.; Ham, J.S.; O’Connor, N.R.; Nagasawa, S.; Kimura, Y.; Manske, C.; Scherübl, M.; Nguyen, J.M.; et al. General Synthetic Approach to Diverse Taxane Cores. J. Am. Chem. Soc. 2022, 144, 21398–21407. [Google Scholar] [CrossRef]
- Jeanneret, R.A.; Dalton, C.E.; Gardiner, J.M. Synthesis of Heparan Sulfate- and Dermatan Sulfate-Related Oligosaccharides via Iterative Chemoselective Glycosylation Exploiting Conformationally Disarmed [2.2.2] l-Iduronic Lactone Thioglycosides. J. Org. Chem. 2019, 84, 15063–15078. [Google Scholar] [CrossRef]
- Lusi, R.F.; Sennari, G.; Sarpong, R. Strategy Evolution in a Skeletal Remodeling and C–H Functionalization-Based Synthesis of the Longiborneol Sesquiterpenoids. J. Am. Chem. Soc. 2022, 144, 17277–17294. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, P.; Li, T.; Zhang, M.; Liu, W.; Li, J.; Wang, L.; Chen, Y. Synthesis of the ABC Ring System of Kadlongilactones. Org. Biomol. Chem. 2023, 21, 1704–1708. [Google Scholar] [CrossRef]
- López-Mendoza, P.; Porras-Santos, L.F.; Pérez-Bautista, J.A.; Quintero, L.; Bautista-Nava, J.; León-Rayo, D.F.; Cordero-Vargas, A.; Sartillo-Piscil, F. En Route to Furan-Fused Naphthopyrones: Formal Synthesis of the (+)-Lasionectrin and Its C12-Epimer. J. Org. Chem. 2023, 88, 17409–17419. [Google Scholar] [CrossRef]
- Takamura, H.; Sugitani, Y.; Morishita, R.; Yorisue, T.; Kadota, I. Total Synthesis and Structure–Antifouling Activity Relationship of Scabrolide F. Org. Biomol. Chem. 2024, 22, 5739–5747. [Google Scholar] [CrossRef]
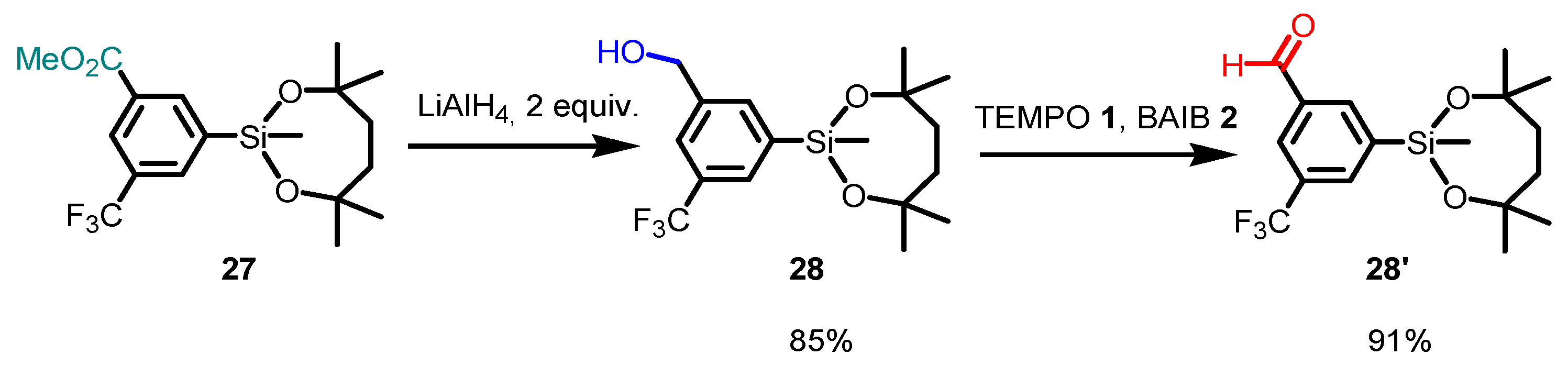
- Saito, H.; Shimokawa, J.; Yorimitsu, H. The Dioxasilepanyl Group as a Versatile Organometallic Unit: Studies on Stability, Reactivity, and Utility. Chem. Sci. 2021, 12, 9546–9555. [Google Scholar] [CrossRef]
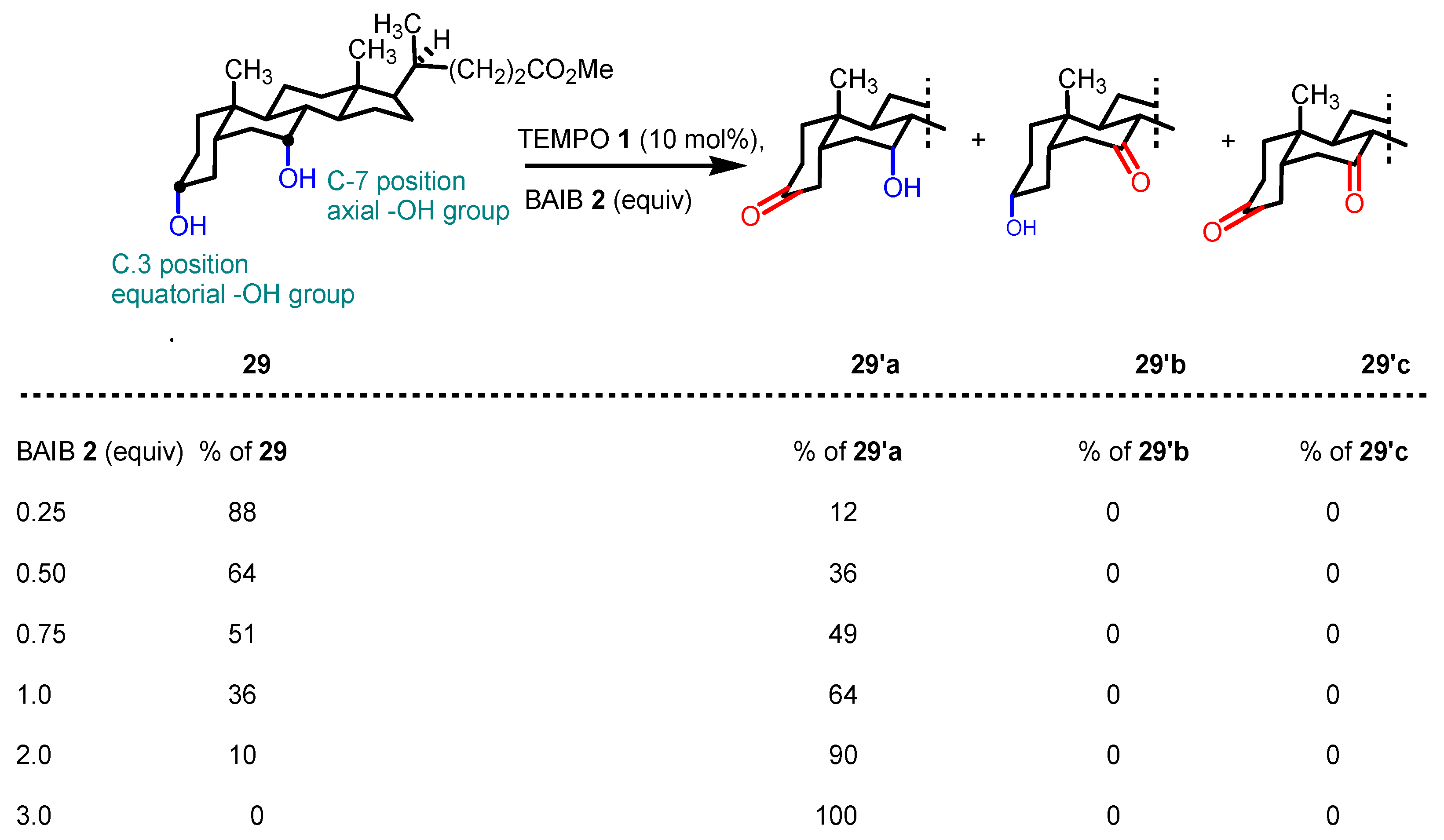
- Kaspar, M.; Kudova, E. Selectivity of Oxidizing Agents toward Axial and Equatorial Hydroxyl Groups. J. Org. Chem. 2022, 87, 9157–9170. [Google Scholar] [CrossRef]
- Li, T.; Wang, J.; Zhu, X.; Zhou, X.; Sun, S.; Wang, P.; Cao, H.; Yu, G.; Li, M. Synthesis of Rare 6-Deoxy-d-/l-Heptopyranosyl Fluorides: Assembly of a Hexasaccharide Corresponding to Campylobacter jejuni Strain CG8486 Capsular Polysaccharide. J. Am. Chem. Soc. 2021, 143, 11171–11179. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chopra, P.; Boons, G.-J. Modular Synthesis of Heparan Sulfate Oligosaccharides Having N-Acetyl and N-Sulfate Moieties. J. Org. Chem. 2020, 85, 16082–16098. [Google Scholar] [CrossRef]
- He, H.; Xu, L.; Sun, R.; Zhang, Y.; Huang, Y.; Chen, Z.; Li, P.; Yang, R.; Xiao, G. An Orthogonal and Reactivity-Based One-Pot Glycosylation Strategy for Both Glycan and Nucleoside Synthesis: Access to TMG-Chitotriomycin, Lipochitooligosaccharides and Capuramycin. Chem. Sci. 2021, 12, 5143–5151. [Google Scholar] [CrossRef] [PubMed]
- Østerlid, K.E.; Cergano, R.; Overkleeft, H.S.; Van Der Marel, G.A.; Codée, J.D.C. Synthesis of a Set of Staphylococcus aureus Capsular Polysaccharide Type 1 Oligosaccharides Carrying Taurine Esters. Chem. A Eur. J. 2025, 31, e202500132. [Google Scholar] [CrossRef]
- Zujew, L.; Raibaut, L.; Chambron, J. From Desferrioxamine B Umpolung to an Enantiopure Bifunctional Chelator for 89Zr-immunoPET. Chem. A Eur. J. 2025, 31, e202501114. [Google Scholar] [CrossRef] [PubMed]
- Fadel, M.; Carreira, E.M. Enantioselective Total Synthesis of (+)-Pedrolide. J. Am. Chem. Soc. 2023, 145, 8332–8337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, J.; Chen, R.; Xiong, F.; Xie, H.; Ding, H. Divergent Total Syntheses of (−)-Crinipellins Facilitated by a HAT-Initiated Dowd–Beckwith Rearrangement. J. Am. Chem. Soc. 2022, 144, 2495–2500. [Google Scholar] [CrossRef]
- Chang, C.; Flaxman, H.A.; Woo, C.M. Enantioselective Synthesis and Biological Evaluation of Sanglifehrin A and B and Analogs. Angew. Chem. Int. Ed. 2021, 60, 17045–17052. [Google Scholar] [CrossRef]
- Xin, Z.; Wang, H.; He, H.; Zhao, X.; Gao, S. Asymmetric Total Synthesis of Norzoanthamine. Angew. Chem. Int. Ed. 2021, 60, 12807–12812. [Google Scholar] [CrossRef]
- Anketell, M.J.; Sharrock, T.M.; Paterson, I. Total Synthesis of the Actinoallolides and a Designed Photoaffinity Probe for Target Identification. Org. Biomol. Chem. 2020, 18, 8109–8118. [Google Scholar] [CrossRef]
- Anketell, M.J.; Sharrock, T.M.; Paterson, I. A Unified Total Synthesis of the Actinoallolides, a Family of Potent Anti-Trypanosomal Macrolides. Angew. Chem. Int. Ed. 2020, 59, 1572–1576. [Google Scholar] [CrossRef]
- Moore, M.J.; Qu, S.; Tan, C.; Cai, Y.; Mogi, Y.; Jamin Keith, D.; Boger, D.L. Next-Generation Total Synthesis of Vancomycin. J. Am. Chem. Soc. 2020, 142, 16039–16050. [Google Scholar] [CrossRef]
- Moore, M.J.; Qin, P.; Yamasaki, N.; Zeng, X.; Keith, D.J.; Jung, S.; Fukazawa, T.; Graham-O’Regan, K.; Wu, Z.-C.; Chatterjee, S.; et al. Tetrachlorovancomycin: Total Synthesis of a Designed Glycopeptide Antibiotic of Reduced Synthetic Complexity. J. Am. Chem. Soc. 2023, 145, 21132–21141. [Google Scholar] [CrossRef]









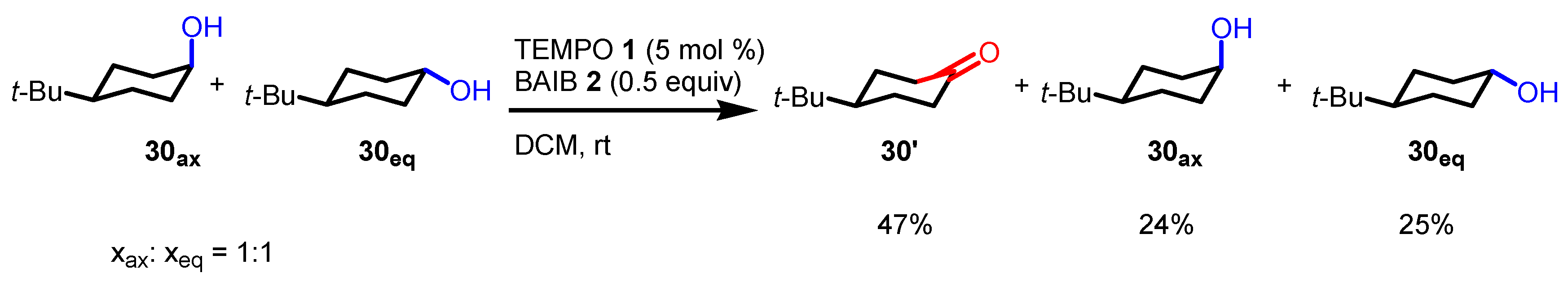



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bella, M. Piancatelli–Margarita Oxidation and Its Recent Applications in Organic Synthesis. Catalysts 2025, 15, 780. https://doi.org/10.3390/catal15080780
Bella M. Piancatelli–Margarita Oxidation and Its Recent Applications in Organic Synthesis. Catalysts. 2025; 15(8):780. https://doi.org/10.3390/catal15080780
Chicago/Turabian StyleBella, Marco. 2025. "Piancatelli–Margarita Oxidation and Its Recent Applications in Organic Synthesis" Catalysts 15, no. 8: 780. https://doi.org/10.3390/catal15080780
APA StyleBella, M. (2025). Piancatelli–Margarita Oxidation and Its Recent Applications in Organic Synthesis. Catalysts, 15(8), 780. https://doi.org/10.3390/catal15080780






