Titanocene Complexes Applied in Organic Transformations
Abstract
1. Introduction
2. Alkenes

3. Epoxides
3.1. Ring Opening of Epoxides

3.2. Radical Arylation of Epoxides
3.3. Radical Alkylation of Epoxides
4. Aldehydes and Ketones

5. Alcohols

6. Nitriles and Amines

7. Others
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Cp | cyclopentadienyl |
| Cp* | pentamethylcyclopentadienyl |
| NHC | N-heterocyclic carbenes |
| -TFA | -O2CCF3 |
| -OMs | -O3SCH3 |
| -OTs | pCH3C6H4SO3- |
| DIPEA | N,N-diisopropylethylamine |
| SET | single electron transfer |
| PR | photoredox |
| PRCat | photoredox catalyst |
| 4CzIPN | 2,4,5,6-tetrakis(carbazol-9-yl)-1,3-dicyanobenzene |
| HE | Hantzsch ester |
| AQCs | aza-quaternary carbons |
| 3DPAFIPN | 1,3-dicyano-5-fluoro-2,4,6-tris(diphenylamino)benzene |
| MHAT | metal hydride hydrogen atom transfer |
| TMSCl | chlorotrimethylsilane |
| TESCl | chlorotriethylsilane |
| PMHS | polymethylhydrosiloxane |
| Coll·HCl | 2,4,6-collidine hydrochloride |
References
- Odom, A.L.; McDaniel, T.J. Titanium-catalyzed multicomponent couplings: Efficient one-pot syntheses of nitrogen heterocycles. Acc. Chem. Res. 2015, 48, 2822–2833. [Google Scholar] [CrossRef]
- Redshaw, C.; Tang, Y. Tridentate ligands and beyond in group IV metal α-olefin homo-/co-polymerization catalysis. Chem. Soc. Rev. 2012, 41, 4484–4510. [Google Scholar] [CrossRef] [PubMed]
- Klosin, J.; Fontaine, P.P.; Figueroa, R. Development of group IV molecular catalysts for high temperature ethylene-α-olefin copolymerization reactions. Acc. Chem. Res. 2015, 48, 2004–2016. [Google Scholar] [CrossRef]
- Bochmann, M. The chemistry of catalyst activation: The case of group 4 polymerization catalysts. Organometallics 2010, 29, 4711–4740. [Google Scholar] [CrossRef]
- Vermeeren, P.; Hamlin, T.A.; Fernández, I.; Bickelhaupt, F.M. How Lewis acids catalyze Diels–Alder reactions. Angew. Chem. Int. Ed. 2020, 59, 6201–6206. [Google Scholar] [CrossRef]
- Rahmatpour, A.; Eeimen, R.; Goodarzi, N. Titanium tetrachloride incorporated crosslinked polystyrene copolymer as an efficient and recyclable polymeric Lewis acid catalyst for the synthesis of Β-amino carbonyl compounds at room temperature. Synth. Commun. 2019, 49, 2915–2930. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.H.; Zhang, G.; Zhang, W.; Wu, Y.; Gao, Z. Tunable titanocene Lewis acid catalysts for selective Friedel-Crafts reaction of indoles and N-sulfonyl-aldimines. Eur. J. Org. Chem. 2016, 2016, 502–507. [Google Scholar]
- Wang, J.; Chen, X.; Wang, X.; Zhang, W.-Q.; Sun, H.-M.; Zhang, G.-F.; Wu, Y.; Gao, Z.-W. Synthesis of air-stable mixed bis-carboxylate titanocene complexes and their catalytic behaviors in cross-aldol and Mannich reactions. Transit. Metal Chem. 2016, 41, 731–738. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, C.; Jia, G.; Zhu, X.; Sun, H.; Zhang, G.; Zhang, W.; Gao, Z. Salicylato titanocene complexes as cooperative organometallic Lewis acid and Bronsted acid catalysts for three-component mannich reactions. Chem. Eur. J. 2014, 20, 8530–8535. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jian, Y.; Wu, Y.; Sun, H.; Zhang, G.; Zhang, W.; Gao, Z. Organometallic titanocene complex as highly efficient bifunctional catalyst for intramolecular Mannich reaction. Appl. Organomet. Chem. 2019, 33, e4925. [Google Scholar] [CrossRef]
- Gansäuer, A.; Kube, C.; Daasbjerg, K.; Sure, R.; Grimme, S.; Fianu, G.D.; Sadasivam, D.V.; Flowers, R.A. Substituent effects and supramolecular interactions of titanocene(III) chloride: Implications for catalysis in single electron steps. J. Am. Chem. Soc. 2014, 136, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Gansäuer, A.; von Laufenberg, D.; Kube, C.; Dahmen, T.; Michelmann, A.; Behlendorf, M.; Sure, R.; Seddiqzai, M.; Grimme, S.; Sadasivam, D.V.; et al. Mechanistic study of the titanocene(III)-catalyzed radical arylation of epoxides. Chem. Eur. J. 2014, 21, 280–289. [Google Scholar] [CrossRef]
- Gansäuer, A.; Hildebrandt, S.; Michelmann, A.; Dahmen, T.; von Laufenberg, D.; Kube, C.; Fianu, G.D.; Flowers, R.A., II. Cationic titanocene(III) complexes for catalysis in single-electron steps. Angew. Chem. Int. Ed. 2015, 54, 7003–7006. [Google Scholar] [CrossRef]
- Gansäuer, A.; Hildebrandt, S.; Vogelsang, E.; Flowers, R.A., II. Tuning the redox properties of the titanocene(III)/(IV)-couple for atom-economical catalysis in single electron steps. Dalton Trans. 2016, 45, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Rosales, A.; Muñoz-Bascón, J.; Roldan-Molina, E.; Castañeda, M.A.; Padial, N.M.; Gansäuer, A.; Rodríguez-García, I.; Oltra, J.E. Selective reduction of aromatic ketones in aqueous medium mediated by Ti(III)/Mn: A revised mechanism. J. Org. Chem. 2014, 79, 7672–7676. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.-K.; Qi, G.; Stryker, J.M. Synthesis of sterically hindered ortho-substituted tetraphenylethenes. Electronic effects in the McMurry olefination reaction. Org. Lett. 2006, 8, 1491–1494. [Google Scholar] [CrossRef]
- Chen, H.-B.; Yin, J.; Wang, Y.; Pei, J. Unsaturated strained cyclophanes based on Dibenz[a,j]anthracene by an intramolecular McMurry olefination. Org. Lett. 2008, 10, 3113–3116. [Google Scholar] [CrossRef]
- Rosales, A.; Oller-López, J.L.; Justicia, J.; Gansäuer, A.; Oltra, J.E.; Cuerva, J.M. Unprecedented Barbier-type reactions catalysed by titanocene(III). Chem. Commun. 2004, 2628–2629. [Google Scholar] [CrossRef]
- Estévez, R.E.; Justicia, J.; Bazdi, B.; Fuentes, N.; Paradas, M.; Choquesillo-Lazarte, D.; García-Ruiz, J.M.; Robles, R.; Gansäuer, A.; Cuerva, J.M.; et al. Ti-catalyzed Barbier-type allylations and related reactions. Chem. Eur. J. 2009, 15, 2774–2791. [Google Scholar] [CrossRef]
- López-Martínez, J.L.; Torres-García, I.; Rodríguez-García, I.; Muñoz-Dorado, M.; Álvarez-Corral, M. Stereoselective Barbier-type allylations and propargylations mediated by CpTiCl3. J. Org. Chem. 2018, 84, 806–816. [Google Scholar] [CrossRef]
- Frey, G.; Hausmann, J.N.; Streuff, J. Titanium-catalyzed reductive umpolung reactions with a metal-free terminal reducing agent. Chem. Eur. J. 2015, 21, 5693–5696. [Google Scholar] [CrossRef]
- Luu, H.-T.; Wiesler, S.; Frey, G.; Streuff, J. A Titanium(III)-catalyzed reductive umpolung reaction for the synthesis of 1,1-disubstituted tetrahydroisoquinolines. Org. Lett. 2015, 17, 2478–2481. [Google Scholar] [CrossRef]
- Streuff, J.; Feurer, M.; Frey, G.; Steffani, A.; Kacprzak, S.; Weweler, J.; Leijendekker, L.H.; Kratzert, D.; Plattner, D.A. Mechanism of the TiIII-catalyzed acyloin-type umpolung: A catalyst-controlled radical reaction. J. Am. Chem. Soc. 2015, 137, 14396–14405. [Google Scholar] [CrossRef] [PubMed]
- Bichovski, P.; Haas, T.M.; Keller, M.; Streuff, J. Direct conjugate alkylation of α,β-unsaturated carbonyls by TiIII-catalysed reductive umpolung of simple activated alkenes. Org. Biomol. Chem. 2016, 14, 5673–5682. [Google Scholar] [CrossRef]
- Davis-Gilbert, Z.W.; Tonks, I.A. Titanium redox catalysis: Insights and applications of an earth-abundant base metal. Dalton Trans. 2017, 46, 11522–11528. [Google Scholar] [CrossRef]
- Márquez, I.R.; Millán, A.; Campaña, A.G.; Cuerva, J.M. Cp2TiCl-catalyzed highly stereoselective intramolecular epoxide allylation using allyl carbonates. Org. Chem. Front. 2014, 1, 373–381. [Google Scholar] [CrossRef]
- Fernández-Mateos, A.; Madrazo, S.E.; Teijón, P.H.; González, R.R. Radical cyclization of epoxy vinyl- and allylsulfones promoted by titanocene chloride. J. Org. Chem. 2015, 80, 4378–4391. [Google Scholar] [CrossRef]
- Gansäuer, A.; Klatte, M.; Brändle, G.M.; Friedrich, J. Catalytic hydrogen atom transfer (HAT) for sustainable and diastereoselective radical reduction. Angew. Chem. Int. Ed. 2012, 51, 8891–8894. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Jakoby, V.; Stainer, K.; Schmer, A.; Klare, S.; Bauer, M.; Grimme, S.; Cuerva, J.M.; Gansäuer, A. Amide-substituted titanocenes in hydrogen-atom transfer catalysis. Angew. Chem. Int. Ed. 2015, 55, 1523–1526. [Google Scholar] [CrossRef]
- Gansäuer, A.; Piestert, F.; Huth, I.; Lauterbach, T. Nitriles and carbonyl groups as acceptors in titanocene-catalyzed radical cyclizations. Synthesis 2008, 2008, 3509–3515. [Google Scholar] [CrossRef]
- Gianino, J.B.; Ashfeld, B.L. Titanocene-catalyzed multicomponent coupling approach to diarylethynyl methanes. J. Am. Chem. Soc. 2012, 134, 18217–18220. [Google Scholar] [CrossRef]
- Lepore, A.J.; Pinkerton, D.M.; Ashfeld, B.L. Relay redox and Lewis acid catalysis in the titanocene-catalyzed multicomponent assembly of 1,5-enynes. Adv. Synth. Catal. 2013, 355, 1500–1504. [Google Scholar] [CrossRef]
- Gianino, J.B.; Campos, C.A.; Lepore, A.J.; Pinkerton, D.M.; Ashfeld, B.L. Redox and Lewis acid relay catalysis: A titanocene/zinc catalytic platform in the development of multicomponent coupling reactions. J. Org. Chem. 2014, 79, 12083–12095. [Google Scholar] [CrossRef]
- Nazari, S.H.; Tiempos-Flores, N.; Forson, K.G.; Bourdeau, J.E.; Michaelis, D.J. C–N bond formation from allylic alcohols via cooperative nickel and titanium catalysis. J. Org. Chem. 2018, 83, 10646–10654. [Google Scholar] [CrossRef]
- Rosenthal, U. Reactions of group 4 metallocene bis(trimethylsilyl)acetylene complexes with nitriles and isonitriles. Angew. Chem. Int. Ed. 2018, 57, 14718–14735. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Mateos, A.; Madrazo, S.E.; Teijón, P.H.; Clemente, R.R.; González, R.R.; González, F.S. Synthesis of the BCDE molecular fragment of azadiradione mediated by titanocene(III). J. Org. Chem. 2013, 78, 9571–9578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Vogelsang, E.; Qu, Z.W.; Grimme, S.; Gansäuer, A. Titanocene-catalyzed radical opening of N-acylated aziridines. Angew. Chem. Int. Ed. 2017, 56, 12654–12657. [Google Scholar] [CrossRef]
- Fleury, L.M.; Ashfeld, B.L. Generation of allyl Grignard reagents via titanocene-catalyzed activation of allyl halides. Tetrahedron Lett. 2010, 51, 2427–2430. [Google Scholar] [CrossRef]
- Fleury, L.M.; Kosal, A.D.; Masters, J.T.; Ashfeld, B.L. Cooperative titanocene and phosphine catalysis: Accelerated C–X activation for the generation of reactive organometallics. J. Org. Chem. 2012, 78, 253–269. [Google Scholar] [CrossRef]
- Ryken, S.A.; Schafer, L.L. N,O-Chelating four-membered metallacyclic titanium(IV) complexes for atom-economic catalytic reactions. Acc. Chem. Res. 2015, 48, 2576–2586. [Google Scholar] [CrossRef]
- McCallum, T.; Wu, X.; Lin, S. Recent advances in titanium radical redox catalysis. J. Org. Chem. 2019, 84, 14369–14380. [Google Scholar] [CrossRef]
- Manßen, M.; Schafer, L.L. Titanium catalysis for the synthesis of fine chemicals—Development and trends. Chem. Soc. Rev. 2020, 49, 6947–6994. [Google Scholar] [CrossRef]
- Fortier, S.; Gomez-Torres, A. Redox chemistry of discrete low-valent titanium complexes and low-valent titanium synthons. Chem. Commun. 2021, 57, 10292–10316. [Google Scholar] [CrossRef]
- Wu, X.; Chang, Y.; Lin, S. Titanium radical redox catalysis: Recent innovations in catalysts, reactions, and modes of activation. Chem 2022, 8, 1805–1821. [Google Scholar] [CrossRef]
- Tu, J.-L.; Huang, B. Titanium in photocatalytic organic transformations: Current applications and future developments. Org. Biomol. Chem. 2024, 22, 6650–6664. [Google Scholar] [CrossRef]
- Wang, X.; He, J.; Wang, Y.-N.; Zhao, Z.; Jiang, K.; Yang, W.; Zhang, T.; Jia, S.; Zhong, K.; Niu, L.; et al. Strategies and mechanisms of first-row transition metal-regulated radical C–H functionalization. Chem. Rev. 2024, 124, 10192–10280. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Shu, X.Z. Reductive deoxygenative functionalization of alcohols by first-row transition metal catalysis. Chin. J. Chem. 2023, 41, 1637–1652. [Google Scholar] [CrossRef]
- Rosenthal, U. Equilibria and mesomerism/valence tautomerism of group 4 metallocene complexes. Chem. Soc. Rev. 2020, 49, 2119–2139. [Google Scholar] [CrossRef]
- Rippke, M.; Tian, X.; Reiß, F.; Wu, L.; Beweries, T. Dehydrocoupling of ammonia/amine boranes and related transformations catalysed by group 4 metal complexes. Chem. Eur. J. 2024, 31, e202403982. [Google Scholar] [CrossRef]
- Collins, R.A.; Russell, A.F.; Mountford, P. Group 4 metal complexes for homogeneous olefin polymerisation: A short tutorial review. Appl. Petrochem. Res. 2015, 5, 153–171. [Google Scholar] [CrossRef]
- Martínez, A.R.; Rodríguez, M.C.; Rodríguez-García, I.; Morales, L.P.; Maecker, R.N.R. Titanocene dichloride: A new green reagent in organic chemistry. Chin. J. Catal. 2017, 38, 1659–1663. [Google Scholar] [CrossRef]
- Nugent, W.A.; RajanBabu, T.V. Four mechanistic mysteries: The benefits of writing a critical review. Angew. Chem. Int. Ed. 2020, 60, 2194–2201. [Google Scholar] [CrossRef]
- Hilche, T.; Younas, S.L.; Gansäuer, A.; Streuff, J. A guide to low-valent titanocene complexes as tunable single-electron transfer catalysts for applications in organic chemistry. ChemCatChem 2022, 14, e202200530. [Google Scholar] [CrossRef]
- Höthker, S.; Gansäuer, A. Formal anti-Markovnikov addition of water to olefins by titanocene-catalyzed epoxide hydrosilylation: From stoichiometric to sustainable catalytic reactions. Glob. Chall. 2023, 7, 2200240. [Google Scholar] [CrossRef]
- Nomura, K.; Pengoubol, S.; Apisuk, W. Synthesis of ultrahigh molecular weight polymers with low PDIs by polymerizations of 1-decene, 1-dodecene, and 1-tetradecene by Cp*TiMe2(O-2,6-iPr2C6H3)–Borate Catalyst. Molecules 2019, 24, 1634. [Google Scholar] [CrossRef] [PubMed]
- Unruean, P.; Apisuk, W.; Kawabata, Y.; Murayama, T.; Kitiyanan, B.; Nomura, K. Effect of supported MAO cocatalysts in ethylene polymerization and ethylene/1-hexene copolymerization using Cp*TiCl2(O-2,6-iPr2C6H3) catalyst. Mol. Catal. 2019, 475, 110490. [Google Scholar]
- Nomura, K.; Nagai, G.; Nasr, A.; Tsutsumi, K.; Kawamoto, Y.; Koide, K.; Tamm, M. Synthesis of half-titanocenes containing anionic N-heterocyclic carbenes that contain a weakly coordinating borate moiety, Cp′TiX2(WCA-NHC), and their use as catalysts for ethylene(co)polymerization. Organometallics 2019, 38, 3233–3244. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, C.; Guo, W.; Zhang, Y.; Sun, M.; Yang, W.; He, L.; Huang, Q. Prominent spatial structure and synergistic linkage effects in bimetallic titanium olefin polymerization aatalysts. Ind. Eng. Chem. Res. 2022, 61, 17017–17026. [Google Scholar] [CrossRef]
- Khusainova, L.I.; Khafizova, L.O.; Ryazanov, K.S.; Tyumkina, T.V.; Dzhemilev, U.M. Cp2TiCl2-catalyzed borylation and hydroboration of α-olefins with dichloro(diisopropylamino)borane. J. Organomet. Chem. 2019, 898, 120858. [Google Scholar] [CrossRef]
- Hang, W.; Zou, S.; Xi, C. Titanocene-catalyzed sequential carbocarboxylation of dienes and alkenes with organic halides and carbon dioxide in the presence of nBuMgCl. ChemCatChem 2019, 11, 3814–3817. [Google Scholar] [CrossRef]
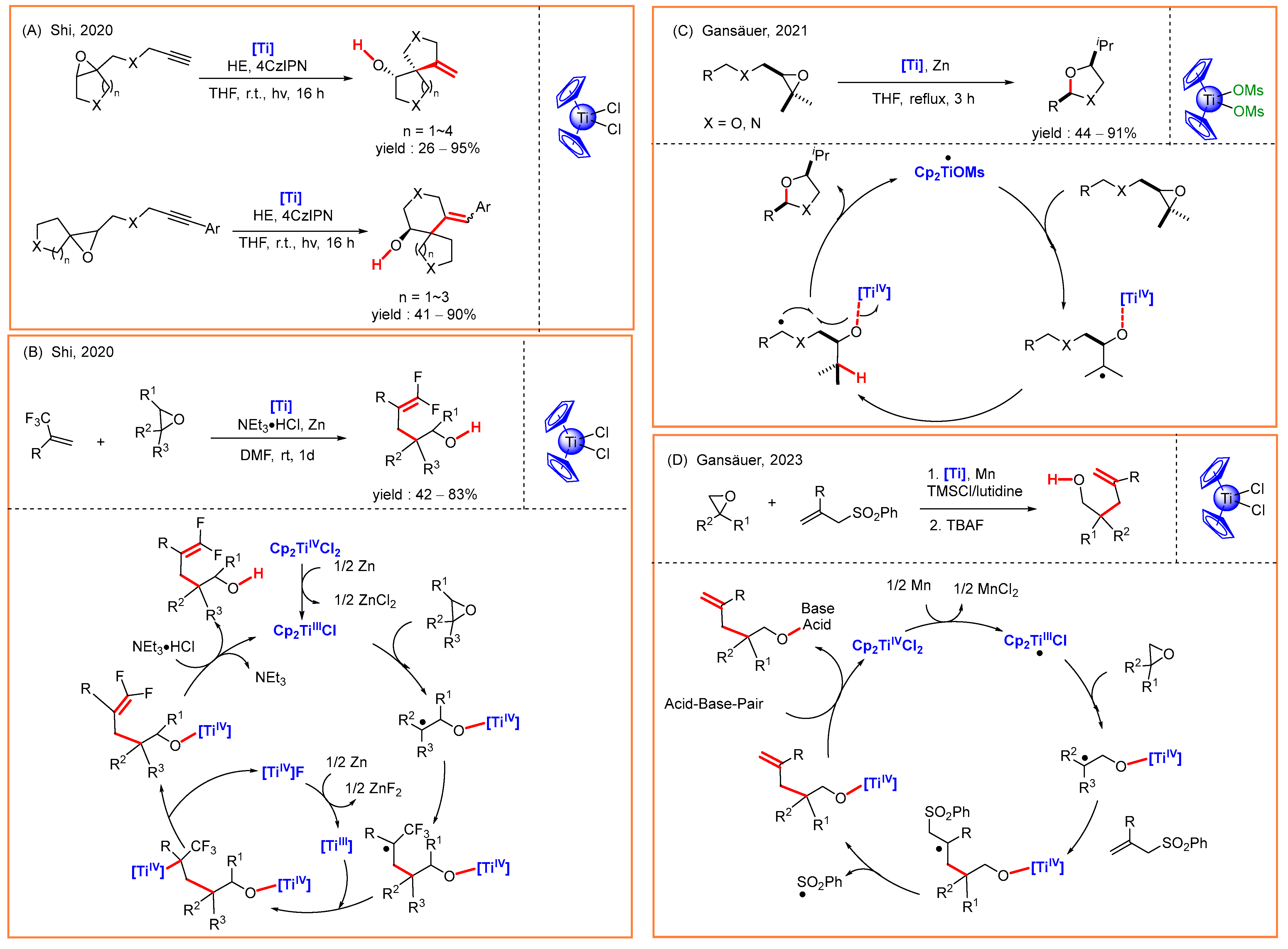
- Zhang, Z.; Stückrath, J.B.; Grimme, S.; Gansäuer, A. Titanocene-catalyzed [2+2] cycloaddition of bisenones and comparison with photoredox catalysis and established methods. Angew. Chem. Int. Ed. 2021, 60, 14339–14344. [Google Scholar] [CrossRef]
- Ni, J.; Xia, X.; Zheng, W.-F.; Wang, Z. Ti-catalyzed diastereoselective cyclopropanation of carboxylic derivatives with terminal olefins. J. Am. Chem. Soc. 2022, 144, 7889–7900. [Google Scholar] [CrossRef]
- Inoue, M.; Stropp, J.; Ashuiev, A.; Kakiuchi, Y.; Payard, P.-A.; Teraishi, T.; Mizukami, M.; Tsurugi, H.; Klose, D.; Copéret, C.; et al. Branch-selective olefin hydroaminoalkylation from Ti(III)–Al bimetallic intermediates evidenced by EPR hyperfine spectroscopy and DFT calculations. J. Am. Chem. Soc. 2025, 147, 16438–16449. [Google Scholar] [CrossRef]
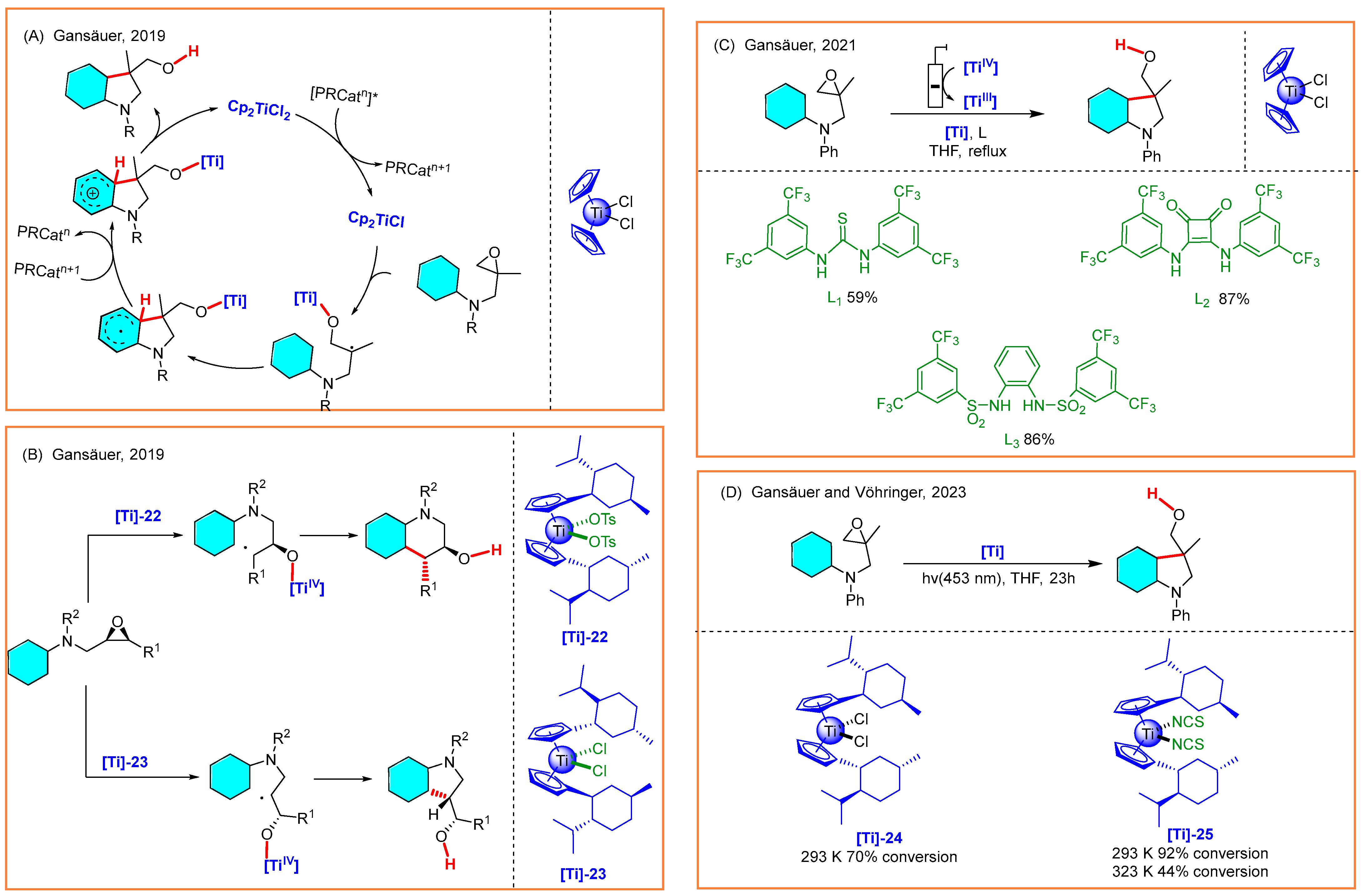
- Yao, C.; Dahmen, T.; Gansäuer, A.; Norton, J. Anti-Markovnikov alcohols via epoxide hydrogenation through cooperative catalysis. Science 2019, 364, 764–767. [Google Scholar] [CrossRef]
- Zhang, Z.; Hilche, T.; Slak, D.; Rietdijk, N.R.; Oloyede, U.N.; Flowers II, R.A.; Gansäuer, A. Titanocenes as photoredox catalysts using green-light irradiation. Angew. Chem. Int. Ed. 2020, 59, 9355–9359. [Google Scholar] [CrossRef]
- Henriques, D.S.G.; Rojo-Wiechel, E.; Klare, S.; Mika, R.; Höthker, S.; Schacht, J.H.; Schmickler, N.; Gansäuer, A. Titanocene(III)-catalyzed precision deuteration of epoxides. Angew. Chem. Int. Ed. 2021, 61, e202114198. [Google Scholar] [CrossRef]
- Li, T.-Z.; Yang, X.-T.; Wang, J.-P.; Geng, C.-A.; Ma, Y.-B.; Su, L.-H.; Zhang, X.-M.; Chen, J.-J. Biomimetic synthesis of lavandiolides H, I, and K and artematrolide F via Diels–Alder reaction. Org. Lett. 2021, 23, 8380–8384. [Google Scholar] [CrossRef]
- Höthker, S.; Mika, R.; Goli, H.; Gansäuer, A. Converging stereodivergent reactions: Highly stereoselective formalanti-Markovnikov addition of H2O to mixtures of olefins. Chem. Eur. J. 2023, 29, e202301031. [Google Scholar] [CrossRef]
- Höthker, S.; Goli, H.; Klare, S.; Krebs, T.; Schacht, J.H.; Gansäuer, A. Attenuating nucleophilicity of titanocene hydrides beyond steric effects en route to fatty alcohols. Chem. Eur. J. 2024, 30, e202402694. [Google Scholar] [CrossRef]
- Schacht, J.H.; Wu, S.; Klare, S.; Höthker, S.; Schmickler, N.; Gansäuer, A. Polymethylhydrosiloxane (PMHS) as sustainable reductant in the titanocene catalyzed epoxide hydrosilylation. ChemCatChem 2022, 14, e202200852. [Google Scholar] [CrossRef]
- Heinz, M.; Weiss, G.; Shizgal, G.; Panfilova, A.; Gansäuer, A. Coupling titanium and chromium catalysis in a reaction network for the reprogramming of [BH4]− as electron transfer and hydrogen atom transfer reagent for radical chemistry. Angew. Chem. Int. Ed. 2023, 62, e202308680. [Google Scholar] [CrossRef]
- Zhang, Z.; Slak, D.; Krebs, T.; Leuschner, M.; Schmickler, N.; Kuchuk, E.; Schmidt, J.; Domenianni, L.I.; Kleine Büning, J.B.; Grimme, S.; et al. A chiral titanocene complex as regiodivergent photoredox catalyst: Synthetic scope and mechanism of catalyst generation. J. Am. Chem. Soc. 2023, 145, 26667–26677. [Google Scholar] [CrossRef]
- Li, G.; Norton, J.R. Ti(III)-catalyzed anti-Markovnikov reduction of epoxides with borohydride. Org. Lett. 2024, 26, 1382–1386. [Google Scholar] [CrossRef]
- Zhang, Z.; Richrath, R.B.; Gansäuer, A. Merging catalysis in single electron steps with photoredox catalysis—Efficient and sustainable radical chemistry. ACS Catal. 2019, 9, 3208–3212. [Google Scholar] [CrossRef]
- Mühlhaus, F.; Weißbarth, H.; Dahmen, T.; Schnakenburg, G.; Gansäuer, A. Merging regiodivergent catalysis with atom-economical radical arylation. Angew. Chem. Int. Ed. 2019, 58, 14208–14212. [Google Scholar] [CrossRef]
- Liedtke, T.; Hilche, T.; Klare, S.; Gansäuer, A. Condition screening for sustainable catalysis in single-electron steps by cyclic voltammetry: Additives and solvents. ChemSusChem 2019, 12, 3166–3171. [Google Scholar] [CrossRef]
- Khan, H.P.A.; Chakraborty, T.K. Application of Cp2TiCl-promoted radical-induced cyclization: An expeditious access to [a]-annelated indoles. J. Org. Chem. 2020, 85, 8000–8012. [Google Scholar] [CrossRef]
- Parasram, M.; Shields, B.J.; Ahmad, O.; Knauber, T.; Doyle, A.G. Regioselective cross-electrophile coupling of epoxides and (hetero)aryl iodides via Ni/Ti/photoredox catalysis. ACS Catal. 2020, 10, 5821–5827. [Google Scholar] [CrossRef]
- Hilche, T.; Reinsberg, P.H.; Klare, S.; Liedtke, T.; Schäfer, L.; Gansäuer, A. Design platform for sustainable catalysis with radicals: Electrochemical activation of Cp2TiCl2 for catalysis unveiled. Chem. Eur. J. 2021, 27, 4903–4912. [Google Scholar] [CrossRef]
- Schmidt, J.; Domenianni, L.I.; Leuschner, M.; Gansäuer, A.; Vöhringer, P. Observing the entry events of a titanium-based photoredox catalytic cycle in real time. Angew. Chem. Int. Ed. 2023, 62, e202307178. [Google Scholar] [CrossRef]
- Nallasivam, J.L.; Chakraborty, T.K. Titanocene(III)-mediated 5-exo-trig radical cyclization: En route to spirooxindole-based tetrahydrofuran and bicyclic lactone. J. Org. Chem. 2019, 84, 16124–16138. [Google Scholar] [CrossRef]
- Lin, S.; Chen, Y.; Li, F.; Shi, C.; Shi, L. Visible-light-driven spirocyclization of epoxides via dual titanocene and photoredox catalysis. Chem. Sci. 2020, 11, 839–844. [Google Scholar] [CrossRef]
- Lin, Z.; Lan, Y.; Wang, C. Titanocene-catalyzed reductive domino epoxide ring opening/defluorinative cross-coupling reaction. Org. Lett. 2020, 22, 3509–3514. [Google Scholar] [CrossRef]
- Begum, S.; Chakraborty, T.K. Cp2TiCl-mediated reductive cyclization: Total synthesis of pestalotiolactone A, myrotheciumone A, and scabrol A. J. Org. Chem. 2021, 86, 11812–11821. [Google Scholar] [CrossRef]
- Funk, P.; Richrath, R.B.; Bohle, F.; Grimme, S.; Gansäuer, A. Oxidation under reductive conditions: From benzylic ethers to acetals with perfect atom-economy by titanocene(III) catalysis. Angew. Chem. Int. Ed. 2021, 60, 5482–5488. [Google Scholar] [CrossRef]
- Schrempp, M.; Wagner, R.; Gleich, H.; Gansäuer, A.; Menche, D. Quaternary carbon synthesis by titanocene catalyzed radical allyl transfer on epoxides. Org. Lett. 2023, 25, 8089–8094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, H.; Yi, D.; Tu, S.; Qi, Z.; Wei, S.; Fu, Q.; Fu, H.; Du, X. Ti-catalyzed regioselective ring-opening alkynylation of epoxides with haloalkynes. Tetrahedron Lett. 2021, 85, 153461. [Google Scholar] [CrossRef]
- Li, J.; Cao, C.; Wu, H.; Dong, K. Nickel/Titanocene-catalyzed electrophilic acylation coupling of styrene oxides. Org. Lett. 2023, 25, 6959–6963. [Google Scholar] [CrossRef] [PubMed]
- Höthker, S.; Plato, A.; Grimme, S.; Qu, Z.-W.; Gansäuer, A. Stereoconvergent approach to the enantioselective construction of α-quaternary alcohols by radical epoxide allylation. Angew. Chem. Int. Ed. 2024, 63, e202405911. [Google Scholar] [CrossRef] [PubMed]
- Torres-García, I.; López-Martínez, J.L.; Martínez-Martínez, R.; Oltra, J.E.; Muñoz-Dorado, M.; Rodríguez-García, I.; Álvarez-Corral, M. The half-sandwich titanocene CpTiIIICl2 as efficient system for the preparation of 2,5-dihydrofurans via α-allenols. Appl. Organomet. Chem. 2019, 34, e5244. [Google Scholar] [CrossRef]
- Li, F.; Lin, S.; Chen, Y.; Shi, C.; Yan, H.; Li, C.; Wu, C.; Lin, L.; Duan, C.; Shi, L. Photocatalytic generation of π-allyltitanium complexes via radical intermediates. Angew. Chem. Int. Ed. 2020, 60, 1561–1566. [Google Scholar] [CrossRef]
- Calogero, F.; Gualandi, A.; Matteo, M.D.; Potenti, S.; Fermi, A.; Bergamini, G.; Cozzi, P.G. Photoredox propargylation of aldehydes catalytic in titanium. J. Org. Chem. 2021, 86, 7002–7009. [Google Scholar] [CrossRef]
- Younas, S.L.; Streuff, J. Kinetic analysis uncovers hidden autocatalysis and inhibition pathways in titanium(III)-catalyzed ketone-nitrile couplings. ACS Catal. 2021, 11, 11451–11458. [Google Scholar] [CrossRef]
- Pinosa, E.; Gualandi, A.; Fermi, A.; Ceroni, P.; Cozzi, P.G.; Calogero, F. A dual photoredox- and Cp2TiCl2-catalyzed approach for the direct access to α-vinyl-β-hydroxy esters. Eur. J. Org. Chem. 2023, 26, e202300421. [Google Scholar] [CrossRef]
- Cheng, J.-T.; Zheng, X.; Huang, P.-Q. Construction of multifunctional heterocycles bearing aza-quaternary carbons by titanocene-catalyzed umpolung reactions. Tetrahedron 2019, 75, 1612–1623. [Google Scholar] [CrossRef]
- Gualandi, A.; Calogero, F.; Mazzarini, M.; Guazzi, S.; Fermi, A.; Bergamini, G.; Cozzi, P.G. Cp2TiCl2-catalyzed photoredox allylation of aldehydes with visible light. ACS Catal. 2020, 10, 3857–3863. [Google Scholar] [CrossRef]
- Li, F.-Z.; Chen, Y.-Q.; Lin, S.-J.; Shi, C.-Z.; Li, X.-Y.; Sun, Y.-C.; Guo, Z.-W.; Shi, L. Visible-light-mediated Barbier allylation of aldehydes and ketones via dual titanium and photoredox catalysis. Org. Chem. Front. 2020, 7, 3434–3438. [Google Scholar] [CrossRef]
- Yan, H.; Liao, Q.; Chen, Y.; Gurzadyan, G.G.; Lu, B.; Wu, C.; Shi, L. Photocatalytic metal hydride hydrogen atom transfer mediated allene functionalization by cobalt and titanium dual catalysis. Angew. Chem. Int. Ed. 2023, 62, e202302483. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.; Lu, B.; Liu, Y.; Yang, T.; Yan, H.; Ding, X.; Jin, Y.; Shi, L. Difunctionalization of 1,3-butadiene via sequential radical thiol-ene reaction and allylation by dual photoredox and titanium catalysis. Org. Lett. 2023, 25, 5094–5099. [Google Scholar] [CrossRef]
- Yan, H.; Shan, J.-R.; Zhang, F.; Chen, Y.; Zhang, X.; Liao, Q.; Hao, E.; Shi, L. Radical crotylation of aldehydes with 1,3-butadiene by photoredox cobalt and titanium dual catalysis. Org. Lett. 2023, 25, 7694–7699. [Google Scholar] [CrossRef]
- Xie, H.; Guo, J.; Wang, Y.-Q.; Wang, K.; Guo, P.; Su, P.-F.; Wang, X.; Shu, X.-Z. Radical dehydroxylative alkylation of tertiary alcohols by Ti catalysis. J. Am. Chem. Soc. 2020, 142, 16787–16794. [Google Scholar] [CrossRef]
- Lin, Q.; Tong, W.; Shu, X.-Z.; Chen, Y. Ti-catalyzed dehydroxylation of tertiary alcohols. Org. Lett. 2022, 24, 8459–8464. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, R.; Shi, L.; Zhao, C.; Wang, X. Divergent synthesis of organofluorinated molecules from titanium mediated deoxygenation of free alcohols. Chin. J. Chem. 2023, 41, 1783–1790. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Yuan, W.; Qu, A.; Chen, K.; Zhu, Y. Photoredox/Ti dual-catalyzed dehydroxylative ring-opening Giese reaction of cyclobutanone oximes. Green Synth. Catal. 2023, 5, 159–164. [Google Scholar] [CrossRef]
- Han, B.; Ren, C.; Jiang, M.; Wu, L. Titanium-catalyzed exhaustive reduction of oxo-chemicals. Angew. Chem. Int. Ed. 2022, 61, e202209232. [Google Scholar] [CrossRef]
- Xie, H.; Wang, S.; Wang, Y.; Guo, P.; Shu, X.-Z. Ti-catalyzed reductive dehydroxylative vinylation of tertiary alcohols. ACS Catal. 2022, 12, 1018–1023. [Google Scholar] [CrossRef]
- Matsunaga, K.; Endo, R.; Nagasawa, K.; Kishida, A.; Takatori, K. Synthesis of succinonitrile derivatives by homocoupling from cyanohydrin derivatives with a low-valent titanium reagent. J. Org. Chem. 2022, 87, 3707–3711. [Google Scholar] [CrossRef]
- Căciuleanu, A.; Vöhringer, F.; Fleischer, I. Titanium-catalysed deoxygenation of benzylic alcohols and lignin model compounds. Org. Chem. Front. 2023, 10, 2927–2935. [Google Scholar] [CrossRef]
- Ni, J.; Xia, X.; Gu, D.; Wang, Z. Ti-Catalyzed modular ketone synthesis from carboxylic derivatives and gem-dihaloalkanes. J. Am. Chem. Soc. 2023, 145, 14884–14893. [Google Scholar] [CrossRef]
- Xie, H.; Wang, S.; Shu, X.-Z. C–OH bond activation for stereoselective radical C-glycosylation of native saccharides. J. Am. Chem. Soc. 2024, 146, 32269–32275. [Google Scholar] [CrossRef]
- Haupt, A.; Fleischer, I. Dual nickel/titanium catalyzed cross-electrophile coupling of thioesters with benzylic alcohols. ChemCatChem 2025, 17, e202500221. [Google Scholar] [CrossRef]
- Khafizova, L.O.; Shaibakova, M.G.; Rikhter, N.A.; Tyumkina, T.V.; Dzhemilev, U.M. One-pot synthesis of 2,3,5-substituted 1H-pyrroles via the reaction of terminal acetylenes with nitriles and EtAlCl2 catalyzed by Cp2TiCl2. Tetrahedron 2019, 75, 906–911. [Google Scholar] [CrossRef]
- Leijendekker, L.H.; Weweler, J.; Leuther, T.M.; Kratzert, D.; Streuff, J. Development, scope, and applications of titanium(III)-catalyzed cyclizations to aminated N-heterocycles. Chem. Eur. J. 2019, 25, 3382–3390. [Google Scholar] [CrossRef]
- Weweler, J.; Younas, S.L.; Streuff, J. Titanium(III)-catalyzed reductive decyanation of geminal dinitriles by a non-free-radical mechanism. Angew. Chem. Int. Ed. 2019, 58, 17700–17703. [Google Scholar] [CrossRef] [PubMed]
- Wiesler, S.; Younas, S.L.; Kratzert, D.; Streuff, J. Titanocene catalysts with modifiable C-symmetric chiral ligands. J. Organomet. Chem. 2020, 919, 121327. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Zheng, Y.-Q.; Huang, H.-G.; Wang, K.-H.; Gong, J.-L.; Liu, W.-B. From quaternary carbon to tertiary C(sp3)–Si and C(sp3)–Ge bonds: Decyanative coupling of malononitriles with chlorosilanes and chlorogermanes enabled by Ni/Ti dual catalysis. J. Am. Chem. Soc. 2024, 146, 14445–14452. [Google Scholar] [CrossRef]
- Unkrig-Bau, M.A.; Leijendekker, S.L.; Streuff, J. Exploring dinuclear titanium complexes in titanium(III) catalysis. ChemCatChem 2024, 17, e202401337. [Google Scholar] [CrossRef]
- Zeng, F.; Ma, B.; Wang, Y.; Li, H.; Zhang, M.; Li, Z. Titanium catalyzed the cross-coupling of nitriles and alkyl halides for the synthesis of ketones. Adv. Synth. Catal. 2025, 367, e202500222. [Google Scholar] [CrossRef]
- Halder, S.; Datta, A. Highly efficient chemoselective synthesis of 2-aryl-1-arylmethyl-1H-benzimidazoles by using TiCp2Cl2 catalyst. Orient. J. Chem. 2020, 36, 1173–1178. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.; Jian, Y.; Leng, D.; Zhang, W.; Zhang, G.; Sun, H.; Gao, Z. A sustainable water-tolerant catalyst with enhanced Lewis acidity: Dual activation of Cp2TiCl2 via ligand and solvent. Mol. Catal. 2020, 498, 111247. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, M.; Yao, Y.; Yu, B.; Wang, Y.; Sun, H.; Zhang, W.; Zhang, G.; Gao, Z. One-pot synthesis of benzo[b][1,4]diazepines via the carbonylative Sonogashira reaction and aza-Michael addition cyclocondensation. New J. Chem. 2022, 46, 5927–5931. [Google Scholar] [CrossRef]
- Zhuang, M.; Tu, L.; Wu, Y.; Jian, Y.; Wang, Y.; Zhang, W.; Sun, H.; Gao, Z. Selective synthesis of benzimidazoles and benzodiazepines catalyzed by Brønsted Acid/ base-cooperative Titanocene dichloride. Mol. Catal. 2022, 524, 112181. [Google Scholar] [CrossRef]
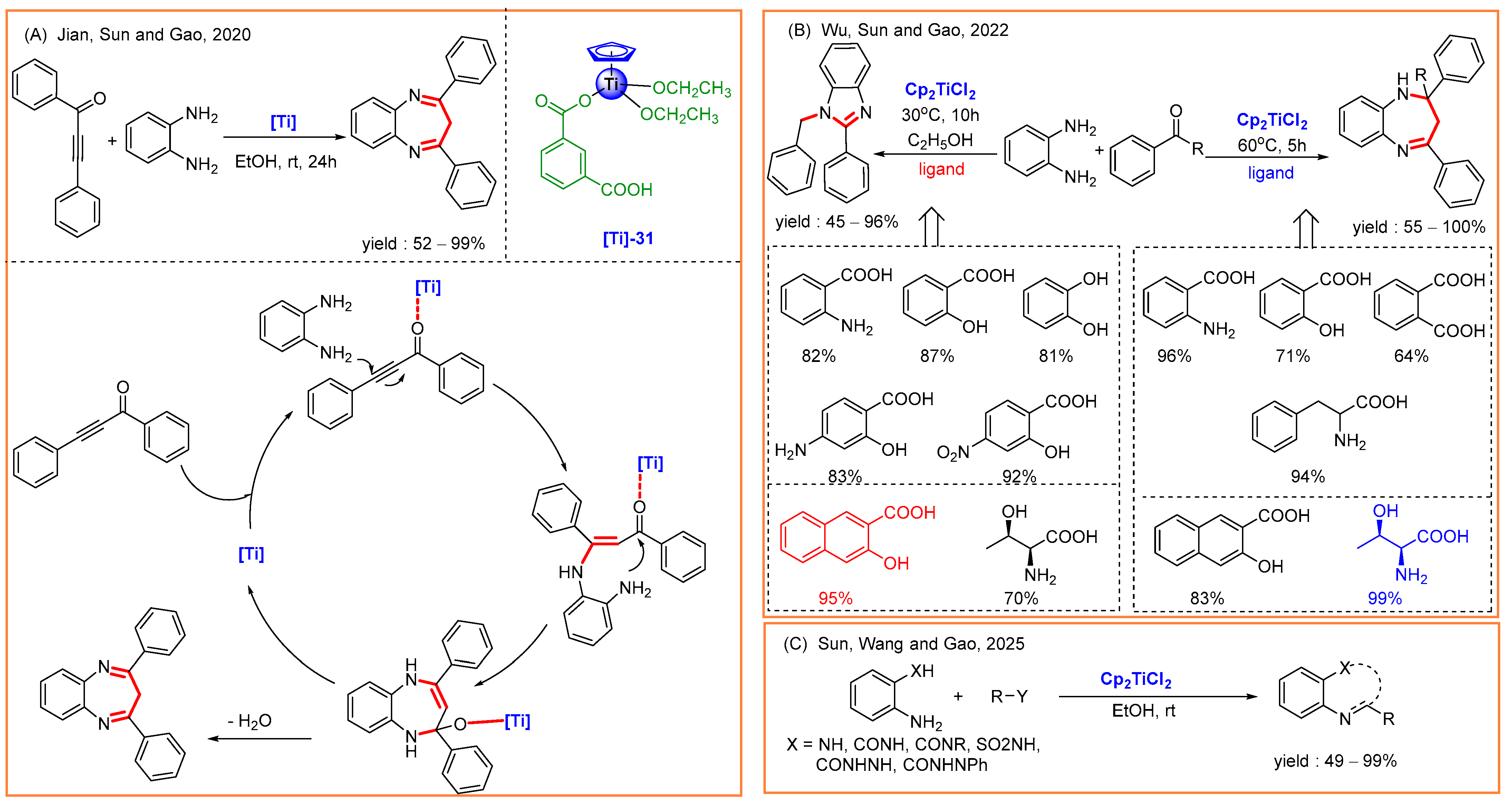
- Yao, Y.-X.; Tu, L.; Wang, X.; Wu, Y.; Jian, Y.-J.; Sun, H.-M.; Wang, Z.-H.; Gao, Z.-W. Titanocene dichloride-catalyzed synthesis of heterocycles accelerated by in-situ formed Lewis and Brønsted acids. Mol. Catal. 2025, 573, 114822. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, Y.; Wang, Y.; Sun, H.; Xie, Z.; Zhang, W.; Gao, Z. Ethanol promoted titanocene Lewis acid catalyzed synthesis of quinazoline derivatives. RSC Adv. 2016, 6, 66074–66077. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Luo, Y.; Wang, J.; Jian, Y.; Sun, H.; Zhang, G.; Zhang, W.; Gao, Z. Solvent strategy for unleashing the Lewis acidity of titanocene dichloride for rapid Mannich reactions. RSC Adv. 2016, 6, 15298–15303. [Google Scholar] [CrossRef]
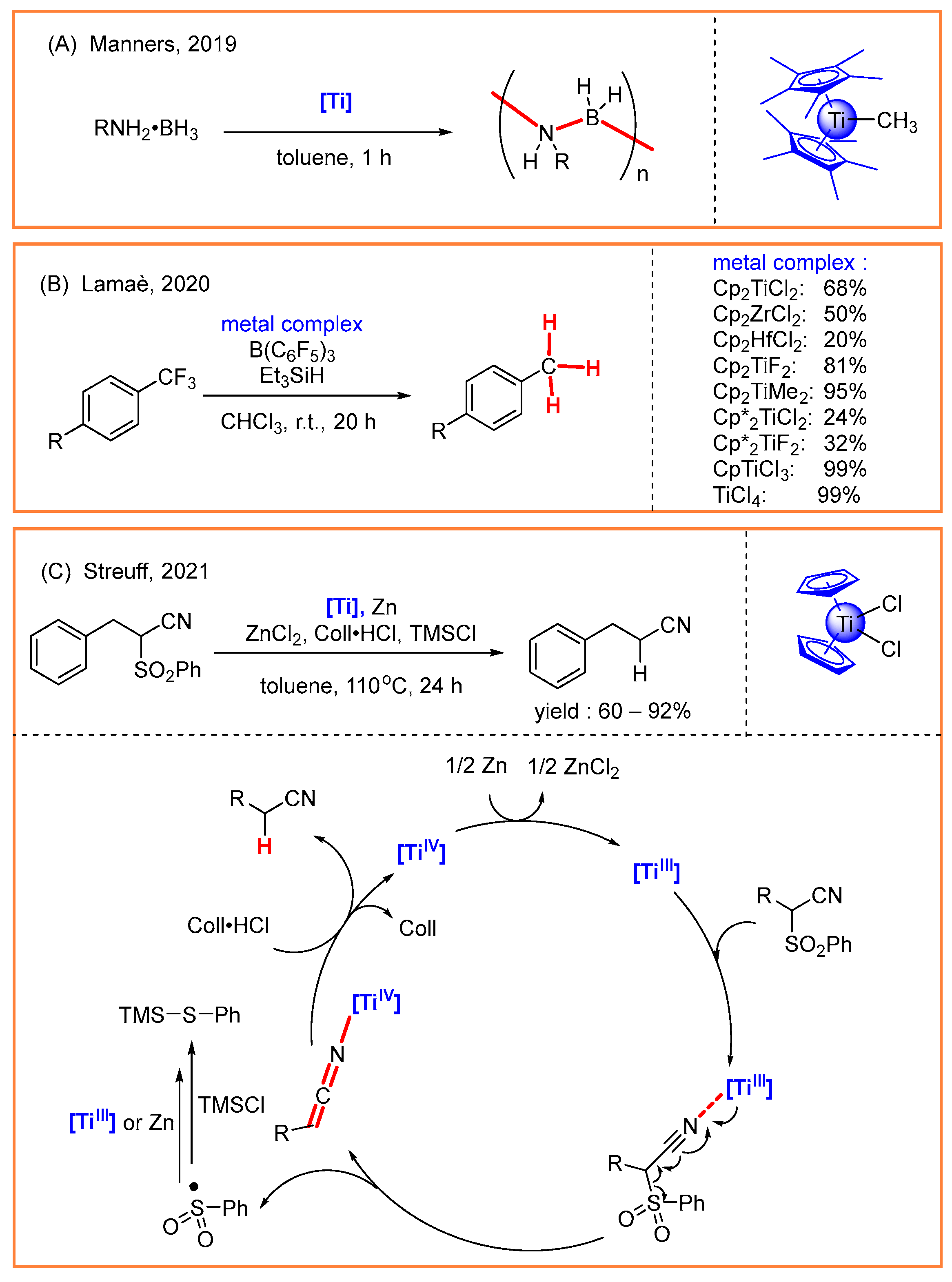
- LaPierre, E.A.; Patrick, B.O.; Manners, I. Trivalent titanocene alkyls and hydrides as well-defined, highly active, and broad scope precatalysts for dehydropolymerization of amine-boranes. J. Am. Chem. Soc. 2019, 141, 20009–20015. [Google Scholar] [CrossRef]
- Dunlop, D.; Pinkas, J.; Horáček, M.; Žilková, N.; Lamač, M. Hydrodehalogenation of organohalides by Et3SiH catalysed by group 4 metal complexes and B(C6F5)3. Dalton Trans. 2020, 49, 2771–2775. [Google Scholar] [CrossRef] [PubMed]
- Kern, C.; Selau, J.; Streuff, J. A titanium-catalyzed reductive α-desulfonylation. Chem. Eur. J. 2021, 27, 6178–6182. [Google Scholar] [CrossRef]
- Wang, X.; Cui, P.; Xia, C.; Wu, L. Catalytic boration of alkyl halides with borane without hydrodehalogenation enabled by titanium catalyst. Angew. Chem. Int. Ed. 2021, 60, 12298–12303. [Google Scholar] [CrossRef]
- Liu, L.; Mendoza-Espinosa, D.; Quiroz-Guzmán, M.; Rheingold, L.A.; Hanna, A.T.; Saha, G.; Tang, L.; Chen, Y.; Gilbert, M.; Dutta, A.; et al. Radical and ring-opening polymerizations with aryl-substituted methylene-bridged titanium bisphenolates. Organometallics 2023, 42, 414–434. [Google Scholar] [CrossRef]
- Oniani, D.; Jia, X.; Mane, E.L.; Charboneau, D.J.; Chow, J.L.; Hazari, N.; Huang, H.; Lee, M.; Mercado, B.Q.; Uehling, M.R.; et al. Ni/Ti dual catalyzed cross-electrophile coupling between unactivated alkyl chlorides and aryl halides. ACS Catal. 2025, 15, 11726–11738. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Leng, D.; Wang, Z.; Wang, X.; Gao, Z. Titanocene Complexes Applied in Organic Transformations. Catalysts 2025, 15, 779. https://doi.org/10.3390/catal15080779
Yang M, Leng D, Wang Z, Wang X, Gao Z. Titanocene Complexes Applied in Organic Transformations. Catalysts. 2025; 15(8):779. https://doi.org/10.3390/catal15080779
Chicago/Turabian StyleYang, Mingming, Deying Leng, Zhenhua Wang, Xiu Wang, and Ziwei Gao. 2025. "Titanocene Complexes Applied in Organic Transformations" Catalysts 15, no. 8: 779. https://doi.org/10.3390/catal15080779
APA StyleYang, M., Leng, D., Wang, Z., Wang, X., & Gao, Z. (2025). Titanocene Complexes Applied in Organic Transformations. Catalysts, 15(8), 779. https://doi.org/10.3390/catal15080779