1. Introduction
The global move toward sustainable energy requires the development of clean, renewable, and efficient energy sources [
1]. Hydrogen, characterized by its high gravimetric energy density, zero carbon emissions upon utilization, and widespread availability, has emerged as a promising alternative to fossil fuels [
2]. Among various hydrogen production technologies, electrochemical water splitting is particularly attractive due to its environmental benignity, scalability, and compatibility with renewable energy sources, such as solar, wind, and hydropower [
3]. However, the practical application of this technology is significantly hindered by the sluggish kinetics of the oxygen evolution reaction (OER) at the anode. As a complex four-electron transfer process, the OER requires high overpotentials to reach desirable current densities, thereby limiting the overall efficiency of water electrolysis [
4].
The current OER catalysts are mainly based on noble metal oxides such as IrO
2 and RuO
2, which offer an excellent catalytic activity and durability [
5,
6]. However, their high cost, limited availability, and poor scalability significantly hinder their commercial application [
7]. Consequently, research has focused on developing abundant and efficient non-precious-metal catalysts [
8,
9]. Transition-metal-based compounds, particularly those involving nickel, cobalt, and iron, have gained attention due to their flexible valence states, diverse coordination environments, and intrinsic catalytic properties [
10].
Among the various transition-metal-based materials, layered double hydroxides (LDHs) have emerged as highly promising candidates for alkaline OER applications [
11]. LDHs feature a characteristic brucite-like layered structure, comprising positively charged metal hydroxide sheets and interlayer anions to maintain charge neutrality [
12]. Their general formula, [M
2+1−xM
x3+ (OH)
2]
x+[A
n−]
x/n·mH
2O, allows for the flexible tuning of the metal cation composition, anion exchange, and structural engineering, enabling the tailoring of their physicochemical properties for an optimized catalytic performance [
13,
14]. The ability to systematically adjust the metal cation ratio and interlayer species imparts LDHs with a remarkable structural versatility, which is advantageous for enhancing catalytic activity and stability in harsh alkaline environments [
15].
Among them, nickel–cobalt LDHs (NiCo LDHs) have received particular attention due to the synergistic interaction between Ni and Co, which enhances the redox activity and electrical conductivity [
16,
17]. Ni sites are known to serve as the primary active centers for oxygen evolution, while Co sites facilitate electron transfer and improve the overall conductivity of the catalyst [
18,
19]. Nevertheless, pristine NiCo LDHs suffer from a moderate conductivity and limited active site exposure, which restrict their OER performance and practical application in large-scale electrolyses. Furthermore, the intrinsic layered structure can lead to aggregation and stacking, reducing the effective surface area and restricting mass transport during the catalytic process.
To address these limitations, various strategies have been developed, including nanostructure designs, hybridization with conductive substrates, defect engineering, and heteroatom doping [
20,
21]. Among them, rare-earth-element doping has emerged as a particularly effective approach to modulate the local electronic environment, increase the density of active sites, and enhance the long-term catalytic stability [
22]. Rare-earth elements, with their unique electronic structures and large ionic radius, can induce lattice distortions and generate oxygen vacancies, which are beneficial for enhancing the intrinsic catalytic activity of LDHs [
23,
24]. Their strong coordination capabilities also help to stabilize the catalyst structure under harsh electrochemical conditions [
25]. Specifically, their partially filled 4f orbitals and multiple valence states introduce additional electronic states that interact with transition-metal centers, tuning the electronic structure and promoting faster electron transfer [
26]. These modifications can lead to enhanced charge transfer kinetics and the increased exposure of catalytically active sites [
27]. Moreover, rare-earth doping can improve the durability of catalysts by suppressing structural degradation during prolonged OER operation, which is critical for practical applications. In particular, Sm doping offers promising advantages due to its 4f electron configuration, which facilitates electron redistribution and catalytic reactivity. However, detailed studies on the structural and electrocatalytic effects of Sm doping in LDH-based systems remain limited. Previous works on Sm doping in other catalytic systems have demonstrated an improved catalytic activity and stability, suggesting a promising role for Sm in enhancing the performance of NiCo LDHs for the OER [
24].
In this work, we employed a one-step hydrothermal method to prepare rare-earth (RE: La, Sm, Pr)-doped NiCo LDHs directly grown on nickel foam (NF), which serves as a conductive and porous substrate. This in situ growth method ensures strong adhesion, maximizes the active surface area, and enhances electrical contact with the conductive substrate. The results demonstrate that Sm doping significantly improves the OER activity of NiCo LDHs by reducing the overpotential, lowering the Tafel slope, increasing the electrochemical active surface area, and decreasing the charge transfer resistance. The optimal Sm-doped catalyst achieved an overpotential as low as 172 mV at 10 mA cm−2 and exhibited an excellent long-term stability. These enhancements are attributed to the synergistic effect of Sm-induced electronic structure modulation and an improved catalyst morphology, which together contribute to faster charge transfer and a greater accessibility of active sites. These findings provide new insights into the rational design of rare-earth-modified LDH catalysts and highlight the potential of Sm as a functional dopant for non-precious-metal-based electrocatalysts in water-splitting applications.
2. Results and Discussion
Figure 1a presents a schematic of the hydrothermal synthesis strategy used to fabricate the catalysts on NF substrates. This one-step method facilitates the uniform nucleation and in situ growth of layered nanosheet arrays, resulting in strong interfacial adhesion and enhanced electrical contact with the conductive substrate.
The surface morphology of the pristine and RE-doped NiCo LDH samples (La, Sm, and Pr) is shown in
Figure 1b–e. The SEM image of the undoped NiCo LDH (
Figure 1b) shows vertically aligned nanosheets uniformly covering the surface of the nickel foam. These nanosheets exhibited a relatively dense microstructure. Such hierarchical features are typical of LDH catalysts and provide a moderate number of active sites [
28]. Upon doping with La
3+ (
Figure 1c), the nanosheet morphology became coarser and thicker, leading to partial stacking and aggregation. This morphological change may reduce the electrochemically active surface area and impede ion diffusion, thus limiting the catalytic efficiency [
5]. In contrast, the Sm-NiCo LDH (
Figure 1d) displayed thinner, more loosely packed nanosheets with a higher degree of structural dispersion. The reduced nanosheet thickness and increased interlayer spacing may be attributed to lattice distortion induced by the large ionic radius of Sm
3+, which disrupts the regular stacking of LDH layers and promotes the formation of defect-rich regions. These features are advantageous for electrocatalysis, as they enhance the exposure of active sites and facilitate electrolyte penetration [
29].
Figure 1e shows the morphology of the Pr-NiCo LDH, which exhibited an intermediate nanosheet density compared to the La- and Sm-doped samples. While the sheets were relatively well-dispersed, partial aggregation was observed at the edges. The Pr
3+ ion, which possesses an ionic radius slightly smaller than La
3+, but larger than Sm
3+, may induce moderate lattice distortion and surface reconstruction, leading to a balanced morphology [
30]. These morphological variations among the RE-doped samples clearly demonstrate that the choice of dopant significantly influences the nanostructure of the catalyst [
31]. In particular, Sm doping leads to the most favorable nanosheet architecture for the catalytic performance, likely contributing to the superior OER activity observed in subsequent electrochemical tests [
32].
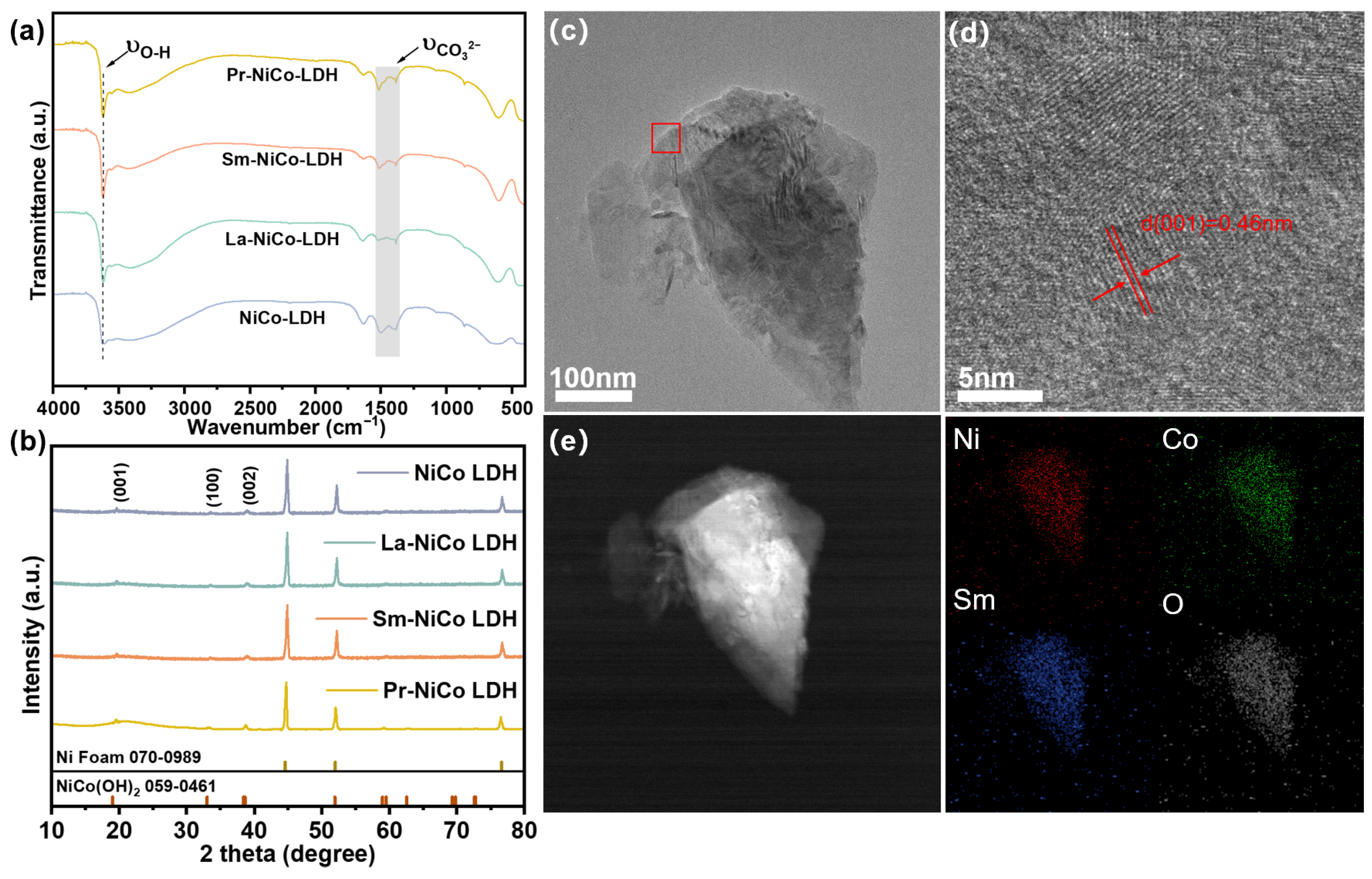
The structural and electronic properties of the pristine and RE-doped NiCo LDH catalysts were systematically investigated using FT-IR, XRD, TEM, and XPS, as shown in
Figure 2.
Figure 2a displays the FT-IR spectra of undoped and RE-doped NiCo LDH samples. All the samples exhibited a broad absorption band around 3450 cm
−1, which was attributed to the O-H stretching vibrations of hydroxyl groups and interlayer water molecules coordinated within the metal octahedral structure [
24]. Peaks observed in the region of 400 ~ 800 cm
−1 were attributed to metal–oxygen (M-O) lattice vibrations involving Ni and Co atoms in the LDH layers [
33]. The XRD patterns (
Figure 2b) further confirmed the successful synthesis of the LDH phase for all samples. The diffraction peaks around 19° were indexed to the (001) planes of a typical hydrotalcite-like LDH structure (JCPDS No. 00-059-0461). Upon doping with La
3+, Sm
3+, or Pr
3+, the (001) peak exhibited a small shift toward lower angles, indicating an expansion of the interlayer spacing. This was attributed to the incorporation of larger RE
3+ ions into the LDH lattice, which introduces lattice distortion and alters the stacking periodicity. In the case of the Pr-NiCo LDH, while the (001) peak position remained essentially unchanged, the peak intensity increased and the peak shape became sharper. This indicates that Pr
3+ doping may have a limited effect on the interlayer distance, but promotes an improved crystallinity and a more ordered layer stacking. In contrast, the diffraction features of the La- and Sm-doped samples showed minimal changes in their peak shape or intensity, suggesting that their degree of crystallinity remained similar to that of the undoped LDH. Moreover, no additional peaks corresponding to rare-earth oxides or other impurity phases were observed, indicating that the La
3+, Sm
3+, and Pr
3+ ions were successfully incorporated into the LDH lattice rather than forming separate secondary phases. The TEM images of the Sm-NiCo LDH sample (
Figure 2c) revealed a thin, sheet-like morphology with clearly defined lateral dimensions, consistent with the typical structure of layered double hydroxides. The HRTEM image (
Figure 2d) shows clear lattice fringes with an interplanar spacing of approximately 0.46 nm, consistent with the (001) crystal plane of LDHs. These observations confirm that the introduction of Sm
3+ does not destroy the basic layered structure, but modifies the crystallinity and thickness of the nanosheets. Further structural information was obtained from elemental mapping (
Figure 2e). The spatial distribution maps demonstrate that the Ni, Co, O, and Sm elements were uniformly dispersed across the nanosheet matrix. This homogeneous distribution is indicative of the successful substitutional doping of Sm
3+ within the LDH lattice, rather than the formation of separate Sm-containing phases.
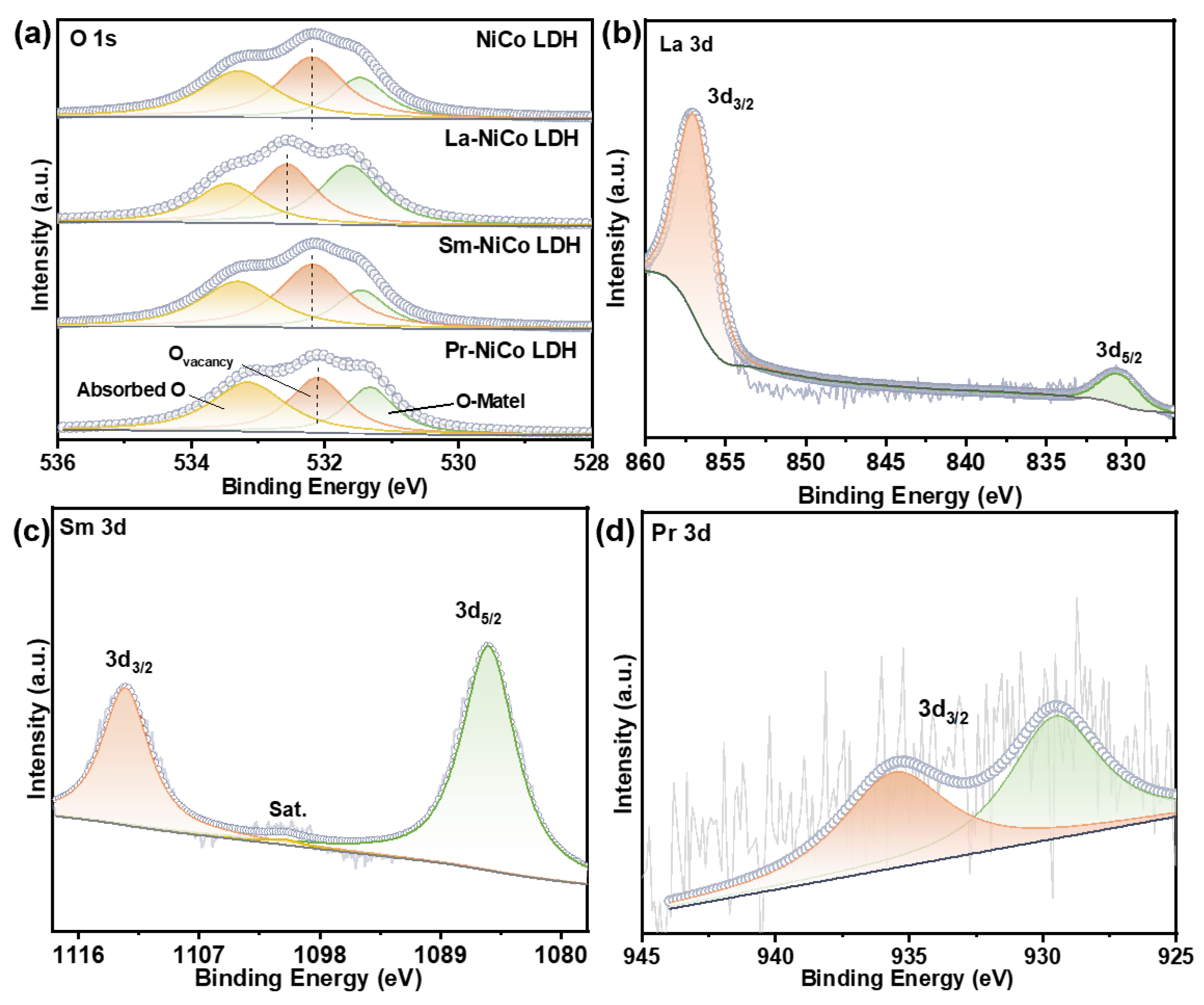
To further investigate the surface chemistry and electronic states, an XPS analysis was conducted.
Figure 3a presents the O 1s spectra of all the samples, which were deconvoluted into three components. The peak at 533.30 eV was attributed to surface-adsorbed oxygen species (O
ads), while the peak at 532.20 eV was assigned to oxygen-vacancy-associated oxygen (O
v). The lower binding energy peak at 531.45 eV originated from lattice oxygen involved in metal–oxygen bonding (M-O) [
34]. Notably, compared with the undoped NiCo LDH, all the RE-doped samples exhibited a distinct chemical shift in the oxygen vacancy peak position within the O 1s spectra. This shift strongly indicates significant electronic interactions between the dopant ions and the NiCo LDH host lattice, which were likely caused by local electronic redistribution and changes in the crystal field environment induced by dopant incorporation. Among them, the La-doped NiCo LDH sample showed a positive shift toward a higher binding energy, suggesting a reduction in the local electron cloud density surrounding oxygen species, which in turn implies a more stabilized oxygen state within the lattice. The XPS spectra of the RE-NiCo LDH 3d regions are shown in
Figure 3b–d. The La 3d spectrum exhibited two main peaks at 857.05 eV and 830.58 eV, corresponding to the La 3d
3/2 and La 3d
5/2 spin-orbit components, confirming the trivalent oxidation state of La
3+ in the LDH framework [
35]. Due to the possible overlap with Ni 2p satellite peaks and a limited spectral resolution, the La 3d spectrum showed an abnormal 3d
3⁄2-to-3d
5⁄2 intensity ratio. Therefore, the La signal is used here only as qualitative evidence of La incorporation, and no quantitative or mechanistic conclusions were drawn from this region. Similarly, the Sm 3d spectrum showed peaks at 1108.25 eV and 1091.52 eV, assigned to Sm 3d
5/2 and 3d
3/2, respectively [
24], while the Pr 3d spectrum displayed features consistent with Pr
3+ species [
36]. The Pr 3d XPS spectrum showed a characteristic doublet structure, which may indicate the coexistence of Pr
3+ and Pr
4+ oxidation states. This mixed-valence feature is consistent with previous reports on Pr-doped transition-metal oxides and layered hydroxides [
36]. The presence of Pr
4+ may contribute to local charge redistribution and enhanced electron transfer between metal centers, which could potentially influence the adsorption of oxygen intermediates and thus improve the overall OER kinetics. While further confirmation via advanced spectroscopy is needed, this observation suggests that Pr doping may tune the electronic structure of the LDH host lattice in a beneficial way. The detection of RE
3+ states in all doped samples confirms the successful incorporation of La
3+, Sm
3+, and Pr
3+ ions into the LDH lattice, likely via the partial substitution of Ni or Co cations. This substitution is expected to induce electronic structure modulation and alter the local bonding environment, thereby influencing the electrocatalytic behavior of the materials.
Overall, these results provide strong evidence that rare-earth doping, particularly with Sm3+, induces significant structural modulation and enhances the surface chemistry of NiCo LDH catalysts. The resulting improvements in the crystallinity, oxygen vacancy content, and active site exposure are expected to contribute directly to the superior OER performance observed in subsequent electrochemical analyses.
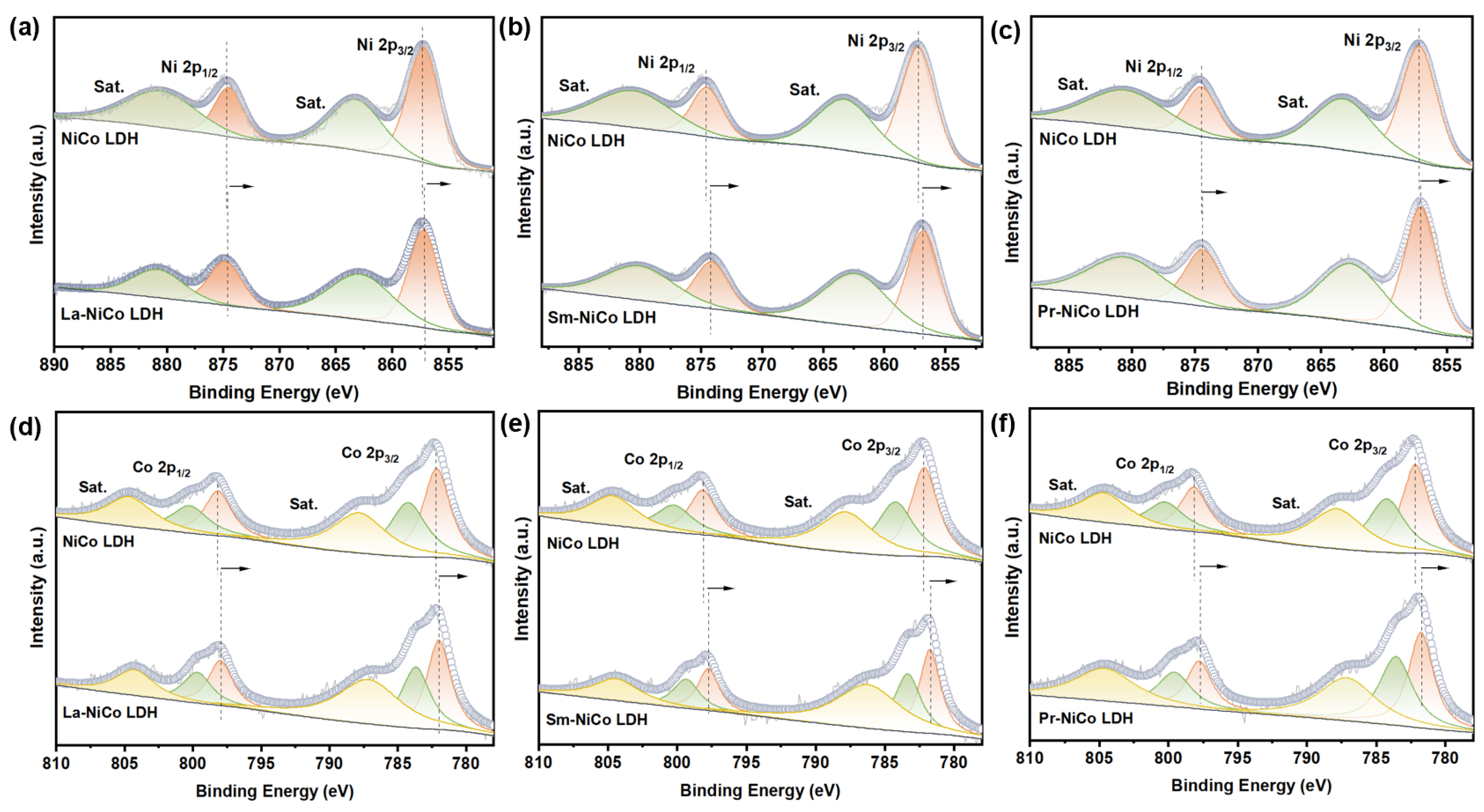
As shown in
Figure 4, high-resolution XPS spectra of the Ni 2p and Co 2p regions were collected for all the samples, including the NiCo LDH, La-NiCo LDH, Sm-NiCo LDH, and Pr-NiCo LDH. The Ni 2p spectra for the NiCo LDH exhibited two principal peaks centered around 857.1 eV and 874.7 eV, corresponding to Ni 2p
3/2 and Ni 2p
1/2, respectively, along with accompanying satellite peaks [
37]. Among the RE-doped samples, the Sm-NiCo LDH displayed a slight shift of the Ni 2p peaks to lower binding energies, suggesting an increased electron density around the Ni centers due to electronic interaction with the dopant ion. This shift implies that Sm
3+ doping modulates the local electronic structure, potentially facilitating redox transitions involved in the oxygen OER process. The Co 2p spectra exhibited characteristic peaks for Co
2+ at 784.28 eV and 800.37 eV, while the peaks at 782.18 eV and 798.14 eV were attributed to Co
3+ species [
38]. These peaks indicate the presence of both the Co
2+ and Co
3+ oxidation states, which are known to play critical roles in OER catalysis. As with the Ni 2p spectra, the Co 2p peaks in the Sm-doped sample exhibited a small, but consistent, negative shift in the binding energy, implying enhanced electron delocalization. This shift reflects a change in the local coordination environment and electron distribution due to Sm incorporation. Although the XPS spectra indicate the presence of La, Sm, and Pr elements, it should be noted that XPS is a surface-sensitive technique and provides only semi-quantitative information regarding elemental composition. Therefore, the precise dopant concentrations and their distribution in the bulk phase could not be confirmed in this work. This limitation has been clearly acknowledged, and we have revised the mechanistic interpretation accordingly. Further verification using bulk elemental analysis techniques such as ICP-OES and operando spectroscopy will be conducted in future studies.
In both the La-NiCo LDH and Pr-NiCo LDH, the Ni 2p
3/2 and Co 2p
3/2 peaks also shifted toward lower binding energies, although the extent of these shifts was smaller than that observed for the Sm-NiCo LDH. This suggests that electron transfer between the dopants and Ni/Co is less pronounced in the La- and Pr-doped samples. Such binding energy variations indicate that rare-earth doping alters the chemical states of Ni and Co, thereby modulating the electronic structure and potentially affecting the catalytic performance of the materials. The XPS results suggest that Sm doping may induce changes in the electronic environment of Ni and Co species. While this modulation could contribute to the improved OER performance, further verification through operando spectroscopy or theoretical studies is warranted. An enhanced electron density at active centers may promote the adsorption and conversion of oxygen intermediates, thus accelerating the overall reaction kinetics [
39]. The observed improvement in the OER performance may be partially associated with changes in the electronic environment induced by Sm doping, as suggested by the XPS analysis. However, a definitive mechanistic understanding requires further validation through operando spectroscopy or theoretical calculations.
The OER performance of the pristine NiCo LDH, RE-doped NiCo LDH (La, Sm, and Pr), and reference materials (RuO
2 and bareNF) was systematically evaluated in 1.0 M KOH electrolytes. The electrochemical data are summarized in
Figure 5.
Figure 5a presents the linear sweep voltammetry (LSV) curves of all samples. The undoped NiCo LDH required an overpotential of 318 mV to reach 10 mA cm
−2, while the Sm-doped NiCo LDH showed a substantially reduced overpotential of 172 mV. This value is even lower than that of the benchmark RuO
2 catalyst, indicating excellent intrinsic activity. In contrast, the La-NiCo LDH and Pr-NiCo LDH showed moderate improvements over the pristine NiCo LDH, but did not surpass the Sm-doped counterpart. The superior performance of the Sm-NiCo LDH is further visualized in
Figure 5b, which compares the overpotentials at 10 and 50 mA cm
−2. At both current densities, the Sm-doped sample consistently delivered the lowest overpotential among all the tested materials. The catalytic kinetics were analyzed by Tafel slope fitting, as shown in
Figure 5c. The Tafel slope of the pristine NiCo LDH was measured at 187 mV dec
−1, which decreased to 84 mV dec
1 for the Sm-NiCo LDH. This reduction indicates a more favorable reaction mechanism and faster electron transfer dynamics. The La- and Pr-doped samples showed intermediate values, again confirming the superior kinetic advantages conferred by Sm doping.
To optimize the Sm-doping concentration, a series of Sm-NiCo LDH samples with different Sm contents were synthesized and tested. The LSV curves in
Figure 5d demonstrate that increasing the Sm content enhanced the OER performance up to an optimal point. As seen in
Figure 5e, the overpotential was minimized at a doping level of 1.0 mmol. The Tafel slope analysis (
Figure 5f) further supports this conclusion, showing the lowest slope for the 1 mmol sample and confirming that it offers the best balance between structural distortion and electronic enhancement.
The ECSA was assessed by estimating the C
dl from cyclic voltammetry. As shown in
Figure 5g, the Sm-NiCo LDH sample exhibited the highest C
dl value, indicating a greater density of accessible active sites. The trend followed Sm, La, Pr, and the pristine NiCo LDH, correlating well with the observed catalytic performance. EIS was performed to study the charge transfer resistance (Rct) at the electrode/electrolyte interface.
Figure 5h shows the Nyquist plots of all samples. The Sm-NiCo LDH sample had the smallest semicircle, lower than those of other doped or pristine LDHs. This result confirms the improved conductivity and faster charge transport kinetics imparted by Sm incorporation. The long-term stability of the optimal Sm-NiCo LDH catalyst was evaluated by a chronoamperometric stability test at a constant potential for 48 h (
Figure 5i). The current density remained at its initial value throughout the test, demonstrating an excellent durability and structural stability under harsh alkaline conditions. No obvious decay or fluctuations were observed, indicating that Sm doping not only enhances the activity, but also significantly improves the stability of the LDH catalyst.
Taken together, the structural, electronic, and electrochemical characterizations collectively demonstrate that Sm incorporation into NiCo LDHs induces significant lattice distortion, enhances the oxygen vacancy concentration, and modulates the electronic structure of active metal centers. These modifications result in thinner, well-dispersed nanosheet morphologies; increased surface hydroxylation; and more favorable charge redistribution, as evidenced by the XRD, TEM, and XPS analyses. Electrochemical evaluations further confirmed that Sm doping reduces the overpotential, lowers the Tafel slope, increases the Cdl, and decreases the charge transfer resistance, while also imparting an excellent long-term stability under alkaline conditions. The Sm-NiCo LDH catalyst delivered a lower overpotential than benchmark RuO2 and maintained its initial current over 48 h of continuous operation. Compared to other non-precious catalysts such as NiFe-LDHs, which suffer from Fe leaching and stability issues, an Sm-doped NiCo LDH offers a more robust framework without introducing redox-inactive blocking species. These synergistic enhancements firmly establish the Sm-NiCo LDH as a highly promising, earth-abundant electrocatalyst for efficient and durable water oxidation.
3. Experimental Section
3.1. Materials and Reagents
All chemical reagents were of analytical grade and used without further purification; they included nickel nitrate hexahydrate (Ni(NO3)2·6H2O, ≥99.0%,Aladdin Reagent Co., Ltd. Shanghai, China), cobalt nitrate hexahydrate (Co(NO3)2·6H2O, ≥99.0%, Aladdin Reagent Co., Ltd. Shanghai, China), samarium nitrate hexahydrate (Sm(NO3)3·6H2O, ≥99.0%, Macklin Biochemical Co., Ltd. Shanghai, China), lanthanum nitrate hexahydrate (La(NO3)3·6H2O, ≥99.9%, Macklin Biochemical Co., Ltd. Shanghai, China), praseodymium(III) nitrate hexahydrate (Pr(NO3)3·6H2O, ≥99.9%, Macklin Biochemical Co., Ltd. Shanghai, China), urea (CO(NH2)2, ≥99.0%, Aladdin Reagent Co., Ltd. Shanghai, China), and ammonium fluoride (NH4F, ≥99.0%, Aladdin Reagent Co., Ltd. Shanghai, China). Deionized (DI) water was used throughout all experimental procedures. Commercial nickel foam (NF, 1.5 mm thick) was cut into 2 cm × 3 cm pieces. Before the experiment, the NF was cleaned with ethanol, 2 mol/L hydrochloric acid, and water ultrasonically for 15 min each and then dried in vacuum at 60 °C.
3.2. Synthesis of Doped NiCo LDH Catalysts
The Sm-doped NiCo layered double hydroxide (Sm-NiCo LDH) was synthesized via a one-step hydrothermal method. In a typical procedure, 1 mmol of Ni(NO3)2·6H2O, 1 mmol of Co(NO3)2·6H2O, and 0.5 mmol of Sm(NO3)3·6H2O were dissolved in 35 mL of DI water. Subsequently, 6 mmol of urea and 6 mmol of NH4F were added. The mixed solution was stirred vigorously for 30 min to ensure complete dissolution and homogenization. The resulting solution was transferred into a 50 mL Teflon-lined stainless-steel autoclave, where a piece of pre-cleaned NF was added.
The autoclave was maintained at 120 °C for 6 h in an electric oven. After cooling to room temperature naturally, the NF substrate was removed, cleaned thoroughly with DI water and ethanol, and then dried at 60 °C in air. The resulting product was denoted as the Sm-NiCo LDH. For comparison, an undoped NiCo LDH was prepared using the same procedure, but without the addition of Sm(NO3)3·6H2O. To prepare the La-NiCo LDH and Pr-NiCo LDH, Sm(NO3)3·6H2O was replaced by a certain amount of La(NO3)3·6H2O or Pr(NO3)3·6H2O, respectively. The Sm-doped NiCo LDH samples with different Sm-doping levels were also synthesized using the same hydrothermal procedure, with different amounts of Sm(NO3)3·6H2O (0.1 mmol, 0.5 mmol, 0.75 mmol, or 1.0 mmol).
3.3. Catalyst Characterization
The crystalline structures of the synthesized products were investigated by X-ray diffraction (XRD, Empyrean, Malvern Panalytical, Almelo, The Netherlands, Cu Kα). The surface chemical states and electronic structures of the prepared products were probed by X-ray photoelectron spectroscopy (XPS, AXIS SUPRA, Kratos Analytical Ltd., Manchester, UK). The morphologies and microstructures of the products were examined by scanning electron microscopy (SEM, ZEISS Sigma 500, Carl Zeiss Microscopy GmbH, Jena, Germany). Transmission electron microscopy (TEM, FEI Talos F200S, Thermo Fisher Scientific, Hillsboro) was employed to directly observe the surface morphology and lattice structure. Fourier transform infrared (FT-IR, Thermo Nexus 470 spectrometer, Perkin-Elmer, Thermo Nicolet, Madison, Wisconsin) spectroscopy was employed to analyze the surface functional groups of the catalysts, with the samples being prepared by the KBr pellet method.
3.4. Electrochemical Measurements
All electrochemical measurements were performed using a CHI660E electrochemical workstation (Shanghai Chenhua Instruments Co., Ltd., Shanghai, China) in a standard three-electrode configuration. The catalyst-loaded NF was used directly as the working electrode (0.5 cm2), a carbon electrode served as the counter electrode, and a Hg/HgO (1 M KOH) electrode was used as the reference. The electrolyte was a 1.0 M KOH aqueous solution, purged with nitrogen for 30 min before each test to remove dissolved oxygen.
All potentials were converted to the reversible hydrogen electrode (RHE) scale using the equation: ERHE = EHg/HgO + 0.098 + 0.059 × pH. The linear sweep voltammetry (LSV) polarization curves were recorded in 1.0 M KOH at a scan rate of 5 mV s−1 using a three-electrode system with the catalyst-loaded Ni foam as the working electrode, Hg/HgO (1.0 M KOH) as the reference electrode, and a carbon rod as the counter electrode. The calculation formula of the Tafel slope was as follows: η = b log j + a, where η was the overpotential, b was the Tafel slope, a was the Tafel intercept, and j was the current density (mA cm−2). The electrochemical double-layer capacitance (Cdl) was determined from the CV curves and measured in a potential range without the Faradaic current by Cdl = ∆j/∆v. The Δv was the scan rate (20 ~ 120 mV s −1) and ∆j was the half-value of the difference between the anode and cathode current density (∆j = (ja − jc)/2).