Abstract
In this study, a novel strategy to enhance the performance of palladium (Pd)-based catalysts by doping with metal oxides (Mn3O4, MoO3, and SnO) has been developed in order to overcome the limitations of its low activity and high cost in the catalytic oxidation of formaldehyde (HCHO). The novelty of this strategy lies in the fact that by precisely controlling the types and doping ratios of the metal oxides, a significant enhancement of the electrochemical performance and catalytic activity of the Pd-based catalysts was achieved, while the dependence on precious metals was reduced and the cost-effectiveness of the catalysts was improved. The effects of different metal oxide doping on the catalytic performance were systematically investigated by electrochemical characterization and catalytic activity tests. Among the prepared catalysts, Pd-Mn3O4 showed the most excellent performance, with an electrochemically active surface area of 20.6 m2/g and a formaldehyde oxidation reaction (FOR) current density of 3.5 mA/cm2, which were 31.6% and 169.2% higher than pure Pd, respectively. In a 1000 s timed current method stability test, the limiting current density of Pd-Mn3O4 reached 0.48 mA/cm2, which is 4.4 times higher than that of pure Pd. The excellent catalytic performance is attributed to the abundant surface hydroxyl (-OH) groups provided by Mn3O4, which contribute to the oxidation of formaldehyde intermediates, as well as the electronic synergistic effect between Pd and Mn3O4, which is manifested as a 0.4 eV downshift of the Pd 3d binding energy. In addition, the sensor evaluation showed that the Pd-Mn3O4-based formaldehyde sensor exhibited a high sensitivity (1.5 μA/ppm), excellent linearity (R2 = 0.995), minimal long-term degradation (<7% in 30 days), and ~20-fold selectivity for formaldehyde over interfering gases (e.g., ethanol). This study provides a theoretical basis and practical material reference for the development of efficient and low-cost catalysts for formaldehyde oxidation.
1. Introduction
Formaldehyde (HCHO), widely used in interior decoration materials [1], has become one of the major indoor air pollutants due to its volatility and toxicity [2]. Gas sensors are extensively employed for HCHO detection owing to their rapid response, ease of operation, and high stability [3,4]. Among them, semiconductor-based sensors are favored for their low fabrication cost; however, their performance is often compromised by a poor selectivity and susceptibility to humidity interference, leading to false-positive results during formaldehyde detection [5]. In contrast, electrochemical sensors offer high sensitivity and excellent anti-interference capabilities, making them more suitable for formaldehyde monitoring [6]. Nevertheless, the widespread adoption of electrochemical sensors is hindered by their high production cost, primarily due to the use of platinum black as the sensing material.
Palladium (Pd), known for its high catalytic activity, strong resistance to poisoning, and relatively lower cost compared to platinum, has emerged as a promising alternative [7]. However, the catalytic activity of Pd toward formaldehyde oxidation (FOR) remains inferior to that of Pt, which in turn reduces the sensitivity of Pd-based sensors when used directly as electrode materials. To enhance the catalytic performance of Pd, extensive research has focused on modifying Pd-based catalysts [8,9].
According to the known mechanisms of formaldehyde oxidation [10], doping Pd with metal oxides (such as Mn3O4, MoO3, or SnO) can significantly enhance its catalytic activity. In aqueous environments, hydroxyl groups (–OH) readily accumulate on the surface of metal oxides [11], facilitating the oxidation of intermediate species generated during HCHO oxidation. Additionally, high-valence metal ions produced via disproportionation reactions (e.g., W, Mn, Mo) have a strong oxidation to organic molecules and are an important intermediate to promote the oxidation reaction [12]. Dong et al. reported that in situ-generated SnOx could promote the catalytic formate oxidation activity [13]. Similarly, Han et al. demonstrated that doping Pt catalysts with a small amount of WO2.9 significantly enhanced their formaldehyde oxidation activity, achieving a sensitivity of 53 μA/ppm in sensors based on Pt–WO2.9 (15%) [14].
Although numerous studies have confirmed that doping Pd-based catalysts with transition metal oxides (MxOy, M = Mn, Mo, Sn, etc.) can enhance their catalytic activity [15,16,17], optimizing the catalyst composition to balance the performance and cost remains a significant challenge.
In this work, Pd-based catalysts doped with 10 wt% of metal oxides (Mn3O4, MoO3, SnO) were synthesized and systematically characterized to investigate their physicochemical properties. Their catalytic performance toward HCHO oxidation was evaluated using cyclic voltammetry. Furthermore, the influence of different doped catalysts on the sensor performance was assessed through device fabrication and sensing tests, providing insights into the design of efficient and cost-effective formaldehyde electrochemical sensors.
2. Results and Discussion
2.1. Electrochemical Characterization of Sensitive Materials
Figure 1 presents the cyclic voltammetry (CV) curves of the synthesized catalysts in the 0.5 mol/L H2SO4 solution, recorded within a potential window of –0.3 V to 1.1 V (vs. SCE) at a scan rate of 0.05 V/s. As shown, all catalysts exhibit characteristic hydrogen adsorption/desorption behavior associated with Pd. Notably, two distinct hydrogen adsorption peaks appear between –0.2 V and 0.2 V, corresponding to the strong and weak hydrogen adsorption on the Pd surface, respectively.
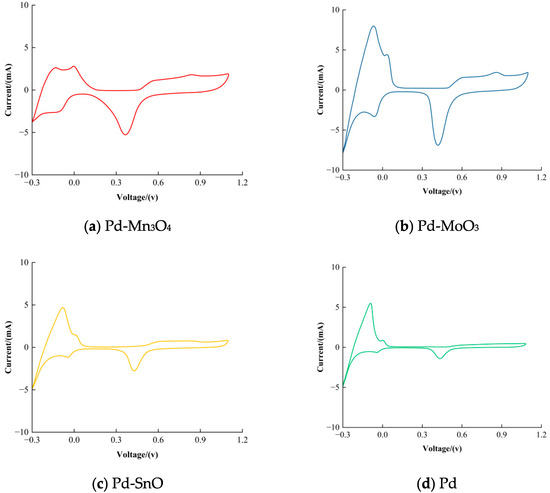
Figure 1.
Cyclic voltammetric curves of the catalysts in the 0.5 mol/L H2SO4 solution, scanned from −0.3 V to 1.1 V and back at a rate of 0.05 V/s. (a) Pd-Mn3O4, (b) Pd-MoO3, (c) Pd-SnO, and (d) Pd.
The electrochemically active surface area (ECSA) of each catalyst was estimated from the integrated charge under the hydrogen adsorption peaks. The calculated ECSA values for Pd-Mn3O4, Pd-MoO3, Pd-SnO, and pure Pd catalysts were 20.6, 41.6, 19.6, and 27.7 m2/g, respectively. These results indicate that the metal oxide doping significantly influences the surface electroactivity of Pd, with MoO3 doping exhibiting the highest ECSA, followed by pure Pd, Pd-Mn3O4, and Pd-SnO.
Figure 2 displays the cyclic voltammetry (CV) curves of the formaldehyde oxidation catalyzed by the prepared catalysts in a mixed solution of 0.5 mol/L H2SO4 and 0.5 mol/L HCHO. All samples exhibited characteristic CV responses for formaldehyde electrooxidation, indicating their catalytic activity. The onset potential for formaldehyde oxidation on all catalysts was approximately 0.55 V (vs. SCE), while the oxidation peak current appeared in the range of 0.68–0.84 V.
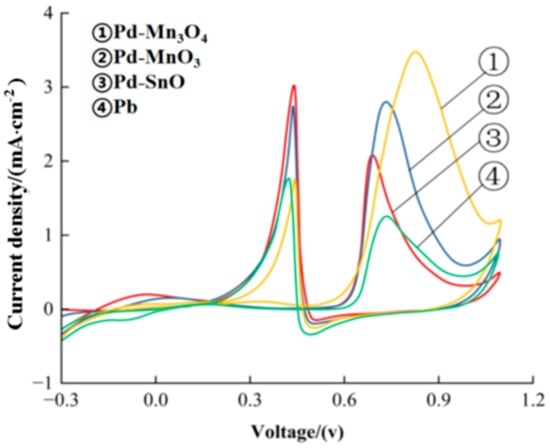
Figure 2.
Cyclic voltammetric curves for formaldehyde oxidation catalyzed by the catalysts in a mixed solution of 0.5 mol/L H2SO4 and 0.5 mol/L HCHO, scanned from −0.3 V to 1.1 V at a rate of 0.05 V/s. The curves show the current density normalized by the electrochemical active area (FOR activity).
To quantitatively evaluate the catalytic performance, the oxidation peak currents were normalized by the electrochemically active surface area (ECSA), representing the formaldehyde oxidation reaction (FOR) activity. Among the catalysts, Pd-Mn3O4 exhibited the highest normalized FOR activity of 3.5 mA/cm2, outperforming Pd-MoO3 (2.8 mA/cm2), Pd-SnO (2.1 mA/cm2), and pure Pd (1.3 mA/cm2).
These results confirm that doping Pd with metal oxides significantly enhances its catalytic activity for formaldehyde oxidation. In particular, the superior performance of the Pd-Mn3O4 catalyst can be attributed to the surface-enriched hydroxyl groups (–OH) on Mn3O4, which facilitate the oxidation of intermediate species, as well as the intrinsic oxidation capacity of Mn3O4. Additionally, Mn3O4 can chemisorb molecular oxygen to form active oxygen species, which further promote the oxidation of formaldehyde [18,19].
Figure 3 presents the chronoamperometric (i–t) curves of the catalysts during the formaldehyde oxidation in a solution containing 0.5 mol/L of H2SO4 and 0.5 mol/L of HCHO at a constant potential of 0.65 V (vs. SCE). As shown, the current densities of all catalysts gradually decreased over time, reaching a relatively stable plateau after approximately 300 s, indicating the establishment of a steady-state electrocatalytic process.
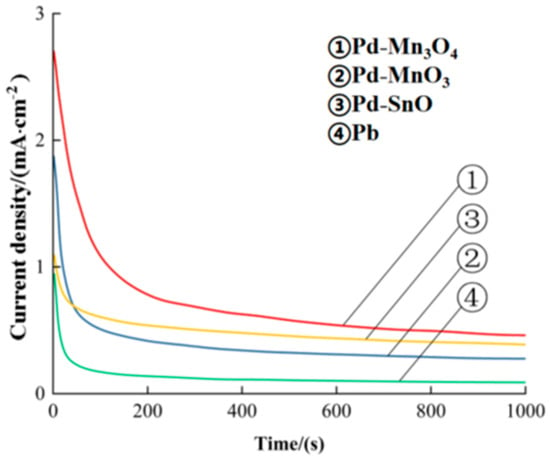
Figure 3.
The timing current curve of the catalysts.
At 1000 s, the limiting current densities of Pd-Mn3O4, Pd-MoO3, Pd-SnO, and pure Pd were 0.48, 0.41, 0.29, and 0.11 mA/cm2, respectively. Based on these results, the electrocatalytic stability of the catalysts followed the order of Pd-Mn3O4 > Pd-MoO3 > Pd-SnO > Pd.
These findings clearly demonstrate that Pd-Mn3O4 not only exhibits the highest catalytic activity for formaldehyde oxidation but also maintains a superior long-term electrochemical stability under operating conditions. This can be attributed to the synergistic interaction between Pd and Mn3O4, which enhances the resistance to catalyst deactivation and improves the durability of the active sites during sustained formaldehyde oxidation.
2.2. Sensor Performance Theory
To comprehensively evaluate the performance of the Pd-Mn3O4 catalyst in formaldehyde sensing, this study introduces the relevant empirical equations describing key sensor characteristics. These include the relationship between the sensor response signal and the formaldehyde concentration, the coefficient of variation (CV) for assessing repeatability, the response and recovery times for evaluating the dynamic behavior, and the long-term stability for monitoring the signal drift over time. The introduction of these equations provides a quantitative foundation for analyzing the sensing performance of the Pd-Mn3O4-based electrochemical sensor.
The response signal equation of the sensor is shown in Equation (1)
where I is the response signal of the sensor, S is the sensitivity of the sensor, C is the concentration of formaldehyde, and b is a constant.
The coefficient of variation is a common metric for evaluating the stability of the sensor response. As shown in Equation (2).
where CV is the coefficient of variation, is the standard deviation of the response signal, and is the mean value of the response model. The smaller the coefficient of variation, the more stable the response of the sensor under different concentrations of formaldehyde, with better repeatability.
The response time and recovery time are important parameters for evaluating the performance of a sensor. The response time t1 is the time required for the sensor to reach a certain response value from the initial state, while the recovery time t2 is the time required for the sensor to return to the initial state after reaching the response value. The response time can usually be described by an exponential model, which is given as shown in Equation (3).
where I(t) is the sensor response signal at time t; Imax is the steady-state response signal finally reached by the sensor; and τ is a time constant indicating the speed at which the sensor reaches the steady state.
The long-term stability of a sensor reflects the degradation of its performance after a long period of use. Long-term stability is usually evaluated by the decay rate of the response signal, as shown in Equation (4).
where Iinitial is the response signal at the initial time of the sensor, and Ifianl is the response signal after a period of time. The smaller the degradation rate, the less the performance degradation of the sensor during long-term use and a better long-term stability.
2.3. Sensor Performance Test
Based on the previous experimental results, Pd-Mn3O4 was selected for a further detailed investigation of its formaldehyde sensing performance. For comparison, a commercial material containing platinum black (denoted as Pt reference) was also tested under identical conditions. Figure 4a,b show the response signals of Pd-Mn3O4 and the Pt reference sensors toward formaldehyde exposure. Although the Pt reference sensor exhibited a higher sensitivity of 22 μA/ppm, this advantage is counterbalanced by several limitations: (1) the high cost of Pt impedes its widespread practical application; (2) the response linearity of the Pt reference (R2 = 0.961) is inferior to that of Pd-Mn3O4 (R2 = 0.995), indicating the better quantitative reliability of Pd-Mn3O4; and (3) long-term stability tests revealed a 20% decrease in the Pt reference’s response over 30 days, whereas Pd-Mn3O4 demonstrated a significantly lower signal loss of less than 7%. Collectively, Pd-Mn3O4 exhibits a well-balanced performance profile, combining a satisfactory sensitivity with a low cost, high precision, and superior durability, making it a more practical candidate for formaldehyde detection.
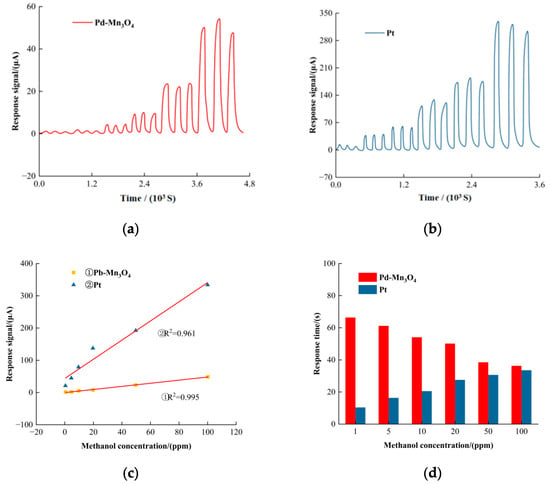
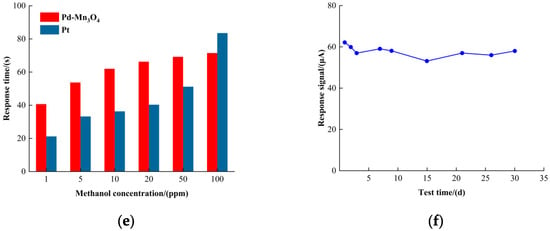
Figure 4.
The circular voltammetric curve of the catalyst. (a) The Pd-Mn3O4 reference response to formaldehyde; (b) the Pt reference response to formaldehyde; (c) the linearity of the response signal; (d) the response time; (e) the recovery time; and (f) the long-term stability of Pd-Mn3O4 in formaldehyde detection.
Response and recovery times are critical parameters for evaluating the sensor performance. As illustrated in Figure 4d, the Pd-Mn3O4 sensor showed a response time of 11 s at 1 ppm of formaldehyde, increasing to 36 s at 100 ppm. In contrast, the Pt reference sensor exhibited a response time of 34 s at 100 ppm of formaldehyde. These results indicate that Pd-Mn3O4 performs particularly well at low formaldehyde concentrations, with its response time approaching that of the Pt reference as the concentration increases. Figure 4e presents the recovery times: the recovery time of Pd-Mn3O4 remained relatively stable across different formaldehyde concentrations, whereas the recovery time of the Pt reference increased significantly at higher concentrations. This disparity can be attributed to the strong resistance of Pd-Mn3O4 to poisoning by formaldehyde oxidation intermediates, whereas platinum black is more susceptible to such deactivation effects.
Finally, Figure 4f demonstrates the long-term stability of the Pd-Mn3O4 sensor. After 30 days of continuous testing, the response signal loss was less than 7%, confirming the excellent durability of Pd-Mn3O4 for sustained formaldehyde detection applications.
The selectivity of the Pd-Mn3O4 sensor was evaluated against common interfering gases, including ethanol, methanol, carbon monoxide (CO), and hydrogen sulfide (H2S), as presented in Figure 5. The sensor demonstrated a formaldehyde sensitivity of 1.5 μA/ppm, which is approximately 20 times higher than its response to the interfering gases—for instance, only 0.07 μA/ppm toward ethanol. This pronounced selectivity is attributed to the unique surface chemistry of Mn3O4, which preferentially adsorbs formaldehyde molecules due to specific interactions, while effectively suppressing the adsorption and oxidation of polar or reducing gases, thereby minimizing the cross-sensitivity.
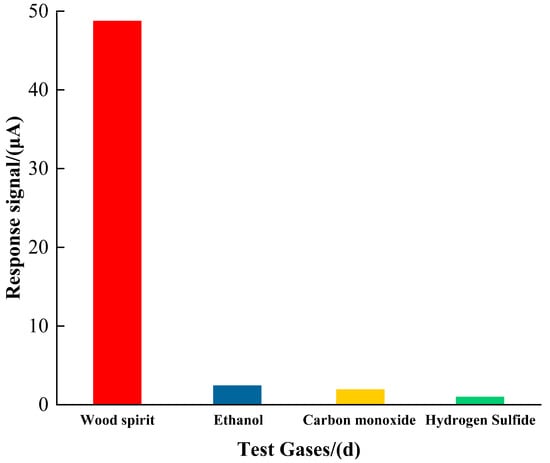
Figure 5.
Selectivity of Pd-Mn3O4 for formaldehyde and interfering gases.
2.4. The In-Depth Analysis of the Catalyst Performance
From the electrochemical active surface area (ECSA) data, the values for Pd-MoO3 and Pd-SnO are relatively close and comparatively larger, which may be attributed to the fact that doping with MoO3 and SnO alters the catalyst microstructure to some extent, thereby increasing the exposure of active sites. However, Pd-Mn3O4 distinctly outperforms these catalysts in terms of the formaldehyde oxidation reaction (FOR) activity, suggesting a unique synergistic interaction between Mn3O4 and Pd.
The surface of Mn3O4 likely provides a special chemical environment that facilitates the adsorption and activation of formaldehyde molecules more efficiently, thereby promoting their oxidation. Furthermore, the limiting current density data at 1000 s demonstrate that Pd-Mn3O4 maintains a high current density over extended reaction times, indicative of a superior stability. This enhanced durability may be attributed to the stable chemical bonding or strong electronic interactions between Mn3O4 and Pd, which inhibit catalyst agglomeration and mitigate the loss of active sites during a prolonged operation. Recycling of different catalysts FOR retention of activity as shown in Table 1.

Table 1.
Recycling of different catalysts FOR retention of activity.
2.5. An Investigation of the Influence Mechanism of Metal Oxide Doping
2.5.1. Changes in Electron Cloud Density of Pd Before and After Doping with Different Metal Oxides
The Pd-Mn3O4 tight interface formed by the stepwise method promotes electron transfer (binding energy ↓0.4 eV) and accelerates the oxidation of HCHO intermediates (FOR current density ↑169.2%). Taking Pd-Mn3O4 as an example, the binding energy of Pd decreased by 0.4 eV upon doping, indicating that the electron transfer occurred from Mn3O4 to Pd. This electron donation increases the electron cloud density around Pd atoms, which facilitates the adsorption and activation of formaldehyde molecules. The enhanced electron density strengthens the interaction between the Pd and formaldehyde, thereby lowering the activation energy of the oxidation reaction and promoting a more efficient catalytic performance. The stepwise impregnation method was employed to precisely control the degree of the modification of metal oxides on the surface of Pd nanoparticles and to optimize the interfacial electronic synergies (see Table 2).

Table 2.
Investigating the variation in binding energy densities in Pd.
2.5.2. Specific Surface Area and Pore Size Distribution Data for Different Catalysts
The BET analysis results reveal that the specific surface areas of all catalysts increased after doping with metal oxides, with Pd-Mn3O4 exhibiting the largest specific surface area among the samples. A higher specific surface area provides more active sites for catalytic reactions, which is beneficial for the adsorption of formaldehyde molecules.
Moreover, the pore size distribution plays a crucial role in influencing the diffusion rates of reactants and products within the catalyst structure. For instance, the average pore size of Pd-Mn3O4 was measured to be approximately 5.2 nm. This moderate pore size effectively facilitates the diffusion and reaction of formaldehyde molecules within the pore channels without inducing significant diffusion limitations. Such favorable textural properties contribute substantially to the superior catalytic performance observed for Pd-Mn3O4.
The weaving parameters of the four palladium-based catalysts are summarized in Table 3, aiming to systematically compare the carrier effect on the pore structure of the materials in terms of the specific surface area, pore size distribution, and pore volume. It can be seen that the BET specific surface areas of the loaded catalysts (Pd-Mn3O4, Pd-MoO3, and Pd-SnO) were all significantly higher than that of the unloaded pure Pd (90 m2 g−1), with Pd-Mn3O4 being the highest at 120 m2 g−1. 1, indicating that the Mn3O4 carrier has the best performance in dispersing the active phase and inhibiting particle agglomeration; the average pore size of the three loading systems ranged from 5.2 to 6.1 nm, which was higher than that of the pure Pd (4.5 nm), suggesting that the introduction of metal oxide carriers can moderately expand the pore size, which is conducive to the reduction in the diffusion resistance. The trend of pore volume changes was consistent with the specific surface area, with Pd-Mn3O4 reaching 0.32 cm3 g−1, which was about 45% higher than that of pure Pd, further confirming that its more developed pore structure could provide abundant accessible active sites for reactants/intermediates. In conclusion, the carrier type has a significant modulating effect on the weaving performance of the palladium-based catalysts, among which the Mn3O4 carrier is particularly prominent in simultaneously increasing the specific surface area and pore volume, which is expected to provide a structural basis for the subsequent optimization of the catalytic activity and stability.

Table 3.
Specific surface area and pore size distribution of catalysts.
2.6. Kinetic Analysis of Catalytic Reactions
2.6.1. Reaction Rate Constants for Each Catalyst at Different Temperatures
The reaction rate constants listed in Table 4 are apparent first-order rate constants denoted as k, describing the kinetics of the formaldehyde oxidation reaction on different Pd-based catalysts under specific temperature conditions. These constants were determined by fitting the experimental data to the first-order rate law:
where k is the rate constant, and HCHO is the formaldehyde concentration.

Table 4.
Effect of reaction temperature on apparent rate constants for formaldehyde oxidation.
Since the reaction follows first-order kinetics, the dimension of these rate constants is s−1 (inverse seconds), representing the rate at which the concentration of formaldehyde decreases over time under the influence of the catalyst. A higher value of k indicates a faster oxidation rate at a given temperature and formaldehyde concentration.
Based on the experimentally determined reaction rate data at various temperatures, the reaction rate constants for each catalyst were calculated using established kinetic equations. It is evident that the reaction rate constants for all catalysts increase with the rising temperature, which is consistent with typical catalytic behavior. However, the reaction rate constant of Pd-Mn3O4 remains consistently higher than those of the other catalysts across the entire temperature range, exhibiting a more pronounced increase. This observation further confirms that Pd-Mn3O4 possesses superior catalytic activity over a wide temperature range and demonstrates an enhanced adaptability and robustness under varying thermal conditions.
The primary reaction rate constants (k, in s−1) of the four palladium-based catalysts in the interval of 25–40 °C are systematically recorded in Table 4 to quantitatively evaluate the influence of the carrier effect on the low-temperature catalytic activity. The data showed that the k values of all samples increased monotonically for every 5 °C increase in the reaction temperature, with Pd-Mn3O4 showing the most significant increase (k increased from 0.025 to 0.048 s−1 for 25 °C→40 °C, an increase of 92%) and maintaining the highest activity at all temperature points; Pd-MoO3 was the next highest, with a k value interval of 0.018–0.033 sPd−1; Pd-SnO again; and pure Pd had the lowest activity (0.010–0.019 s−1). This sequence was positively correlated with the BET specific surface area and pore volume size (see Table 3), suggesting that the carriers synergistically enhanced the intrinsic reaction rate by improving the dispersion and mass transfer efficiency. In addition, the linear relationship of ln k against 1/T was good (R2 > 0.99), which is consistent with Arrhenius behavior and can be used for subsequent activation energy calculations and kinetic modeling.
2.6.2. Reaction Rates of Each Catalyst at Different Formaldehyde Concentrations (30 °C)
Table 5 systematically examined the variation patterns of the apparent reaction rates of the four palladium-based catalysts in the formaldehyde inlet concentration range of 10–40 ppm at room temperature (25 °C) to elucidate the effect of the substrate concentration on the catalytic oxidation kinetics. The results showed that the apparent reaction rates of all catalysts increased linearly with the increase in the formaldehyde concentration from 10 ppm to 40 ppm, which was consistent with the first-order kinetic characteristics (R2 > 0.99), among which the increase in Pd-Mn3O4 was the most significant (0.005 → 0.020 s−1), and the activity was always ahead of that of the other systems: system Pd-MoO3 followed (0.003 → 0.012 s−1), Pd-SnO3 again (0.0025 → 0.010 s−1), and pure Pd had the lowest activity (0.0015 → 0.006 s−1). The order of the rate magnitude of the four catalysts is fully consistent with the high and low BET specific surface area and pore volume in Table 3, which further verifies that the carrier-induced weaving optimization is decisive for enhancing the density of surface-available active sites and the reactant mass transfer efficiency.

Table 5.
Effect of formaldehyde concentration on apparent rate constants at 30 °C.
The experiments were conducted at 30 °C by varying the formaldehyde concentration to obtain reaction rate data for the different catalysts. The results showed that the reaction rate of each catalyst increased proportionally with the formaldehyde concentration, exhibiting an approximately linear relationship consistent with first-order reaction kinetics. Notably, at identical formaldehyde concentrations, the reaction rate of Pd-Mn3O4 was significantly higher than those of the other catalysts, indicating its superior adsorption and activation capability toward formaldehyde molecules. This enhanced activity effectively promotes the oxidation of formaldehyde.
These findings provide a comprehensive analysis of the catalytic performance and reaction kinetics from multiple perspectives, offering deeper insight and a solid foundation for further study.
2.7. Long-Term Stability and Cycling Performance Analysis
2.7.1. Recycling Performance of Different Catalysts
Table 6 quantitatively evaluated the activity retention ability of the four palladium-based catalysts in the room temperature formaldehyde oxidation reaction by 10 consecutive cycling tests. The results showed that Pd-Mn3O4 exhibited the highest cycling stability with a 92% activity retention, which was significantly better than that of the Pd-MoO3 (85%), Pd-SnO (80%), and pure Pd (70%).

Table 6.
Recycling performance of different catalysts.
After 10 consecutive cycles of formaldehyde oxidation catalysis, the FOR activity of each catalyst was re-evaluated, and the retention rate was calculated. The data indicate that Pd-Mn3O4 maintains a high catalytic activity throughout the cycling tests, with a retention rate of approximately 92%, which can be attributed to its stable structural integrity and excellent resistance to sintering. In contrast, the activity retention rates of Pd-MoO3, Pd-SnO4, and pure Pd catalysts exhibited a gradual decline over the cycles, suggesting that their catalytic performance may have been compromised due to the deactivation of active sites or structural changes occurring during the repeated reaction processes.
2.7.2. Changes in Catalyst Performance After Long-Term Storage
Table 7 systematically compares the decay of the formaldehyde oxidation activity at room temperature (FOR) of the four palladium-based catalysts after 3 months of storage under ambient conditions in order to assess their long-term storage stability. The rate of the change in activity (%) was calculated as the difference between the “initial activity” and the “activity after storage”. The results showed that the initial activity of Pd-Mn3O4 was the highest (3.5 a.u.) and only decreased by 5.7% after 3 months of storage, which maintained the best activity; the initial activities of Pd-MoO3 and Pd-SnO were 2.8 and 2.1 a.u., and the loss in the activity of the two was the same (−14.3%), which indicated that the activity of Pd-Mn3O3 was the highest (3.5 a.u.), and that the activity of Pd-Mn3O4 was the best. MoO3 and SnO carriers had similar effects on the enhancement of the active site stability; the unloaded pure Pd had the lowest initial activity (1.3 a.u.) and the most significant decrease in activity after storage (−23.1%). In comparison, the Mn3O4 carrier showed the optimal “encapsulation–stabilization” effect in inhibiting the agglomeration or surface oxidation of metal particles during storage, which ensured that the catalysts could maintain a high activity after long-term storage.

Table 7.
Performance after long-term storage.
After storing the catalysts under ambient temperature and pressure conditions for three months, their formaldehyde oxidation reaction (FOR) activities were re-evaluated and compared with the initial values. The results showed that the activity of Pd-Mn3O4 exhibited only a minor decline of approximately 5.7%, indicating an excellent stability during long-term storage. In contrast, the activities of the Pd-MoO3, Pd-SnO, and pure Pd catalysts decreased significantly, likely due to the surface oxidation or the adsorption of airborne impurities. These findings further underscore the superior long-term stability of Pd-Mn3O4, highlighting its potential advantage for practical applications.
2.8. Cost–Benefit Assessment
2.8.1. Comparison of Feedstock Costs for Different Catalysts
The market prices (RMB g−1) of the main feedstocks in the four palladium-based catalysts and their actual feedstock costs converted to each gram of the catalyst were calculated in Table 8 using PdCl2 as the palladium precursor. The results showed that the feedstock costs of the three loaded catalysts varied very little between RMB 0.455 and 0.460 g−1, with Pd-Mn3O4 being the lowest (RMB 0.455 g−1), Pd-SnO4 being the highest (RMB 0.460 g−1), indicating that different carrier precursors have a limited influence on the overall cost; the feedstock cost of the unloaded pure Pd catalyst was RMB 0.50 g−1, which was slightly higher than that of the loaded system and was mainly attributed to the higher amount of precious metals used.

Table 8.
Costs of different catalysts.
The feedstock cost per gram of the catalyst was estimated based solely on the price and proportion of the primary raw materials, excluding other preparation-related expenses. Although the cost of Pd-Mn3O4 is relatively low, the difference compared to the pure Pd is not substantial. However, when considering its superior catalytic performance, Pd-Mn3O4 demonstrates a more favorable cost-to-performance ratio, suggesting greater economic advantages for potential large-scale applications.
2.8.2. The Estimation of the Cost-Effectiveness of Different Catalysts in Practical Applications
Table 9 comprehensively evaluates the actual dosages, corresponding treatment costs, and purification efficiencies of the four palladium-based catalysts under the conditions of 1000 ppm of formaldehyde and a 1 m3 treatment volume. The results show that Pd-Mn3O4 only needs 0.5 g to achieve a 95% removal rate, with a converted treatment cost of RMB 0.2275, which is the lowest among the four; Pd-MoO3 needs 0.6 g, with a cost of RMB 0.2748 and an efficiency of 88%; Pd-SnO needs 0.7 g, with a cost of RMB 0.322 and an efficiency of 82%; and the pure Pd needs 0.8 g, with a cost of RMB 0.4 and an efficiency of only 75%. Pd-SnO requires 0.7 g at a cost of RMB 0.322 and an efficiency of 82%; the pure Pd requires 0.8 g at a cost of RMB 0.4 and an efficiency of only 75%. Comprehensively, Pd-Mn3O4 has the best performance in terms of the dosage, economy, and purification efficiency, which highlights its potential application in formaldehyde treatments in the atmosphere.

Table 9.
Practical applications of various catalysts.
Assuming a practical scenario where 1 m3 of gas containing 1000 ppm of formaldehyde requires treatment, the catalyst dosage and associated treatment cost were estimated based on the catalytic activity of each catalyst and empirical data from real-world applications. Concurrently, the formaldehyde removal efficiency of each catalyst under these conditions was experimentally determined.
The results demonstrate that the Pd-Mn3O4 catalyst achieves an optimal balance between the treatment cost and removal efficiency, enabling a higher formaldehyde degradation at a lower cost compared to other catalysts. This favorable cost–performance profile underscores its promising application potential and economic feasibility for large-scale formaldehyde remediation.
By integrating the aforementioned data, tables, and analyses, a comprehensive evaluation of the catalysts’ performance and applicability was conducted. This provides a more detailed and practical reference framework to guide future research and facilitate the transition toward real-world implementation.
3. Experiment
3.1. Instruments and Reagents
The instruments used in this study included an X-ray diffractometer (XRD, D8 Advance, Bruker, Billerica, MA, USA), a transmission electron microscope (TEM, JEM-2010, JEOL, Peabody, MA, USA), and an electrochemical workstation (CHI660D, Chenhua Instruments, Shanghai, China). Electrochemical measurements were performed using a conventional three-electrode system, consisting of a glassy carbon electrode (GCE, 3 mm diameter) as the working electrode, a platinum wire as the counter electrode, and a saturated calomel electrode (SCE) as the reference. All potentials reported in this work are referenced to the SCE. The chemicals used were palladium chloride (PdCl2), formaldehyde aqueous solution (37–40%), sodium borohydride (NaBH4), isopropyl alcohol [(CH3)2CHOH], absolute ethanol (C2H5OH), sulfuric acid (H2SO4), hydrochloric acid (HCl), potassium permanganate (KMnO4), ammonium molybdate [(NH4)6Mo7O24·4H2O], tin(II) chloride dihydrate (SnCl2·2H2O), Nafion solution, polytetrafluoroethylene (PTFE) emulsion, and high-purity nitrogen gas (N2, 99.99%). All reagents were of analytical grade and used as received without further purification.
3.2. Preparation of the Catalyst
All catalysts in this study were synthesized via a sequential dipping method, optimized to ensure uniform doping and high catalytic performance.
In the first step, Pd nanoparticles were synthesized by chemical reduction. Specifically, 25 mL of a 10 g/L PdCl2 aqueous solution (0.14 M, containing 2.5 mmol Pd) was stirred in an ice-water bath (0–4 °C) for 30 min to ensure temperature control and stability during nucleation. A freshly prepared 50 mL NaBH4 solution (0.1 M, 5 mmol; 10:1 molar ratio to Pd) was then added dropwise at a rate of 1 mL/min under continuous stirring at 500 rpm. The reaction was allowed to proceed for 2 h. The resulting black suspension was centrifuged at 8000 rpm for 15 min, and the precipitate was washed three times with 20 mL deionized water. The purified Pd nanoparticles were then dried at 70 °C for 6 h to obtain dry Pd powder.
In the second step, the doped Pd catalysts were prepared by introducing selected metal oxides (Mn3O4, MoO3, or SnO) onto the Pd surface. A total of 90 mg of dried Pd nanoparticles was dispersed in 30 mL of deionized water using ultrasonic treatment (40 kHz, 300 W) for 30 min to achieve a uniform suspension. The metal precursors were individually dissolved in 5 mL of deionized water as follows: 10.5 mg KMnO4 (0.066 mmol) for Mn doping, 12.4 mg (NH4)6Mo7O24·4H2O (0.01 mmol) for Mo doping, and 8.5 mg SnCl2·2H2O (0.038 mmol) for Sn doping. The selected metal precursor solution was added dropwise (1 mL/min) into the Pd suspension under a nitrogen atmosphere to prevent premature oxidation. The loading of all three metal oxides (Mn3O4, MoO3, and SnO) on palladium was consistently 10 wt%, as controlled by the mass ratio of metal precursors to Pd nanoparticles (1:9) during the stepwise impregnation synthesis. This uniform doping ratio allows for a systematic comparison of the effects of different metal oxides on catalytic performance.
After 30 min of stirring, 10 mL of a 0.05 M NaBH4 solution (0.5 mmol) was introduced to facilitate the in situ reduction in the metal precursor and promote nucleation on the Pd surface. The pH of the solution was then adjusted to 2.5 using 0.1 M HCl to enhance metal oxide formation. The reaction mixture was stirred for an additional hour to complete the doping process. The suspension was then centrifuged at 10,000 rpm for 20 min to collect the solid product, which was thoroughly washed three times with a 1:1 (v/v) ethanol/deionized water mixture to remove residual ions and byproducts.
Finally, the collected powder was dried at 80 °C for 4 h and subjected to thermal treatment at 300 °C for 2 h under a nitrogen atmosphere (50 mL/min), using a temperature ramp rate of 5 °C/min. This sintering step ensured the formation of well-dispersed, stable metal oxide-doped Pd catalysts suitable for electrochemical and sensor applications.
3.3. Catalytic Test
The Pd-Mn3O4 catalyst was employed as the sensitive material for the working electrode, while platinum black was used as a reference electrode material. To prepare the sensing ink, the catalyst powder was mixed with 20 wt% polytetrafluoroethylene (PTFE) emulsion and isopropyl alcohol, followed by ultrasonication for 30 min to ensure homogeneous dispersion. The resulting ink was then screen-printed onto a polyvinyl chloride (PVC) membrane to form a membrane electrode assembly (MEA). After printing, the membrane was thoroughly rinsed with deionized water to remove any residual solvent and subsequently subjected to pressing to enhance adhesion. Finally, the fabricated membrane electrode was immersed in 0.5 mol/L H2SO4 electrolyte solution to activate the electrode surface, and the sensor assembly was completed. In the sensor test, the current standard gas is required to ensure accuracy.
To ensure accuracy in sensor testing, a calibrated standard gas preparation protocol was employed. First, 95 μL of formaldehyde aqueous solution (37 wt%) was injected into a gas generator, producing formaldehyde vapor at a concentration of approximately 2500 ppm (1 ppm = 10−6). According to the desired test concentration, a measured volume of this high-concentration gas was transferred into a 5 L aluminum foil gas sampling bag and subsequently diluted with clean air to prepare the required formaldehyde standard gas.
During sensor testing, the standard gas was introduced into the test chamber at a controlled flow rate of 0.3 L/min. The current response of the sensor was recorded using the CHI660D electrochemical workstation. Following each measurement, fresh air was purged through the chamber for 30 s to fully remove any residual formaldehyde gas, ensuring reliable and repeatable testing conditions. Table 10 shows the Comparison of Pd-Mn3O4 with reported catalysts for formaldehyde oxidation.

Table 10.
Comparison of Pd-Mn3O4 with reported catalysts for formaldehyde oxidation.
While some Pt-based catalysts offer higher absolute sensitivity, they often suffer from longer response times, higher costs, and poorer long-term stability. In contrast, the Pd-Mn3O4 catalyst strikes a balance between performance and cost, showing excellent selectivity and durability, which is crucial for practical applications in indoor air quality monitoring and industrial formaldehyde detection. Therefore, although the Pd-Mn3O4 catalyst does not exhibit the highest sensitivity, it offers a more comprehensive and stable sensing profile, especially considering its economic advantages over Pt-based systems.
4. Conclusions
This study systematically investigated the influence of doping metal oxides (Mn3O4, MoO3, and SnO) on the catalytic activity of Pd-based materials for formaldehyde oxidation. Electrochemical tests demonstrated that metal oxide doping significantly enhanced the electrocatalytic oxidation performance of Pd, with Mn3O4 exhibiting the most pronounced promotional effect.
Sensor evaluations further revealed that formaldehyde sensors utilizing Pd-Mn3O4 as the sensitive material exhibit a superior performance in detecting high formaldehyde concentrations and display strong resistance to catalyst poisoning. Moreover, the sensor demonstrated an excellent selectivity, achieving a formaldehyde sensitivity approximately 20 times greater than that for common interfering gases. These results indicate that Pd-Mn3O4-based sensors possess substantial potential for practical applications in formaldehyde detection.
The ability to detect high concentrations of formaldehyde is particularly important in various practical applications. In industrial settings, such as chemical plants and formaldehyde production facilities, monitoring high levels of formaldehyde is crucial for worker safety and environmental compliance. High-concentration formaldehyde sensors can provide real-time feedback to ensure that exposure levels are within safe limits and to prevent potential health hazards. Additionally, in residential and commercial buildings undergoing extensive renovations, high-concentration sensors can help identify and mitigate formaldehyde hotspots, ensuring that the indoor air quality meets health standards. The Pd-Mn3O4 sensor, with its high sensitivity, selectivity, and stability, is well-suited for these demanding applications, offering reliable and cost-effective formaldehyde detection solutions.
Author Contributions
Formal analysis, writing—original draft preparation, B.C. and Z.R.; investigation X.L.; Conceptualization, software, X.W.; methodology, writing—review and editing, Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
The State Sponsored Postdoctoral Fellowship Programme under Grant (No. GZC20231280). This research is supported by the National Key R&D Program of China (No. 2021YFB4001501), Open Foundation of Laboratory of Low frequency Electromagnetic Communication Technology with the WMCRT CSSC, (No. DPJJ-2022-03), and Open Foundation of State Key Laboratory of High-End Compressor and System Technology, (No SKL-YSJ202201).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
There are no conflicts of interest in this article.
References
- Dorieh, A.; Selakjani, P.P.; Shahavi, M.H.; Pizzi, A.; Movahed, S.G.; Pour, M.F.; Aghaei, R. Recent developments in the performance of micro/nanoparticle-modified urea-formaldehyde resins used as wood-based composite binders: A review. Int. J. Adhes. Adhes. 2022, 114, 103106. [Google Scholar] [CrossRef]
- Luo, B.; Cao, L.; Luo, F.; Mu, R.; Wang, C.; Chen, L.; Sun, K.; Xu, Y.; Liu, S.; Zhou, H.; et al. Breakthrough progress of thin-film thermocouple development on special-shaped superalloy surface. Aeronaut. Mater. J. 2023, 43, 117–120. [Google Scholar]
- Zhang, Y.; Yu, Y.; Zhang, C.; Song, N.; Guo, Z.; Liang, M. Highly sensitive and selective detection of formaldehyde via bio-electrocatalysis over aldehyde dehydrogenase. Anal. Chem. 2022, 94, 15827–15831. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wang, R.; Wang, L.; Qu, H.; Zheng, L. Breath alcohol sensor based on hydrogel-gated graphene field-effect transistor. Biosens. Bioelectron. 2022, 210, 114319. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yu, Z.; Huang, L.; Zeng, Y.; Liu, X.; Tang, D. Photoinduced electron transfer modulated photoelectric signal: Toward an organic small molecule-based photoelectrochemical platform for formaldehyde detection. Anal. Chem. 2023, 95, 9130–9137. [Google Scholar] [CrossRef] [PubMed]
- Inobeme, A.; Natarajan, A.; Pradhan, S.; Adetunji, C.O.; Ajai, A.I.; Inobeme, J.; Tsado, M.J.; Jacob, J.O.; Pandey, S.S.; Singh, K.R.; et al. Chemical Sensor Technologies for Sustainable Development: Recent Advances, Classification, and Environmental Monitoring. Adv. Sens. Res. 2024, 3, 2400066. [Google Scholar] [CrossRef]
- Ruiz-López, E.; Ribota Peláez, M.; Blasco Ruz, M.; Domínguez Leal, M.I.; Martinez Tejada, M.; Ivanova, S.; Centeno, M.Á. Formic acid dehydrogenation over Ru-and Pd-based catalysts: Gas-vs. liquid-phase reactions. Materials 2023, 16, 472. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, X.; Tay, S.W. Nanostructured PdRu/C catalysts for formic acid oxidation. J. Solid State Electrochem. 2012, 16, 545–550. [Google Scholar] [CrossRef]
- Lou, Z.; Zhou, J.; Sun, M.; Xu, J.; Yang, K.; Lv, D.; Zhao, Y.; Xu, X. MnO2 enhances electrocatalytic hydrodechlorination by Pd/Ni foam electrodes and reduces Pd needs. Chem. Eng. J. 2018, 352, 549–557. [Google Scholar] [CrossRef]
- Batista, E.A.; Iwasita, T. Adsorbed intermediates of formaldehyde oxidation and their role in the reaction mechanism. Langmuir 2006, 22, 7912–7916. [Google Scholar] [CrossRef] [PubMed]
- Kulesza, P.J.; Pieta, I.S.; Rutkowska, I.A.; Wadas, A.; Marks, D.; Klak, K.; Stobinski, L.; Cox, J.A. Electrocatalytic oxidation of small organic molecules in acid medium: Enhancement of activity of noble metal nanoparticles and their alloys by supporting or modifying them with metal oxides. Electrochim. Acta 2013, 110, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Li, W.; Ma, J.; Lei, Y.; Zhu, Y.; Huang, Q.; Dou, X. A review of the preparation and applications of MnO2 composites in formaldehyde oxidation. J. Ind. Eng. Chem. 2018, 66, 126–140. [Google Scholar] [CrossRef]
- Dong, Q.; Wu, M.; Mei, D.; Shao, Y.; Wang, Y.; Liu, J.; Li, H.; Hong, L. Multifunctional Pd–Sn electrocatalysts enabled by in situ formed SnOx and TiC triple junctions. Nano Energy 2018, 53, 940–948. [Google Scholar] [CrossRef]
- Han, F.; Li, F.; Liu, S.; Niu, L. Sub-stoichiometric WO2.9 as co-catalyst with platinum for formaldehyde gas sensor with high sensitivity. Sens. Actuators B Chem. 2018, 263, 369–376. [Google Scholar] [CrossRef]
- Zhou, J.; Qin, L.; Xiao, W.; Zeng, C.; Li, N.; Lv, T.; Zhu, H. Oriented growth of layered-MnO2 nanosheets over α-MnO2 nanotubes for enhanced room-temperature HCHO oxidation. Appl. Catal. B Environ. 2017, 207, 233–243. [Google Scholar] [CrossRef]
- Song, C.; Khanfar, M.; Pickup, P.G. Mo oxide modified catalysts for direct methanol, formaldehyde and formic acid fuel cells. J. Appl. Electrochem. 2006, 36, 339–345. [Google Scholar] [CrossRef]
- Güntner, A.T.; Abegg, S.; Wegner, K.; Pratsinis, S.E. Zeolite membranes for highly selective formaldehyde sensors. Sens. Actuators B Chem. 2018, 257, 916–923. [Google Scholar] [CrossRef]
- Li, Y.; Qu, J.; Gao, F.; Lv, S.; Shi, L.; He, C.; Sun, J. In situ fabrication of Mn3O4-decorated graphene oxide as a synergistic catalyst for degradation of methylene blue. Appl. Catal. B Environ. 2015, 162, 268–274. [Google Scholar] [CrossRef]
- Bigiani, L.; Maccato, C.; Carraro, G.; Gasparotto, A.; Sada, C.; Comini, E.; Barreca, D. Tailoring vapor-phase fabrication of Mn3O4 nanosystems: From synthesis to gas-sensing applications. ACS Appl. Nano Mater. 2018, 1, 2962–2970. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).