Analysis and Application of UV-LED Photoreactors for Phenol Removal
Abstract
1. Introduction
2. Results and Discussion
2.1. Photoreactors
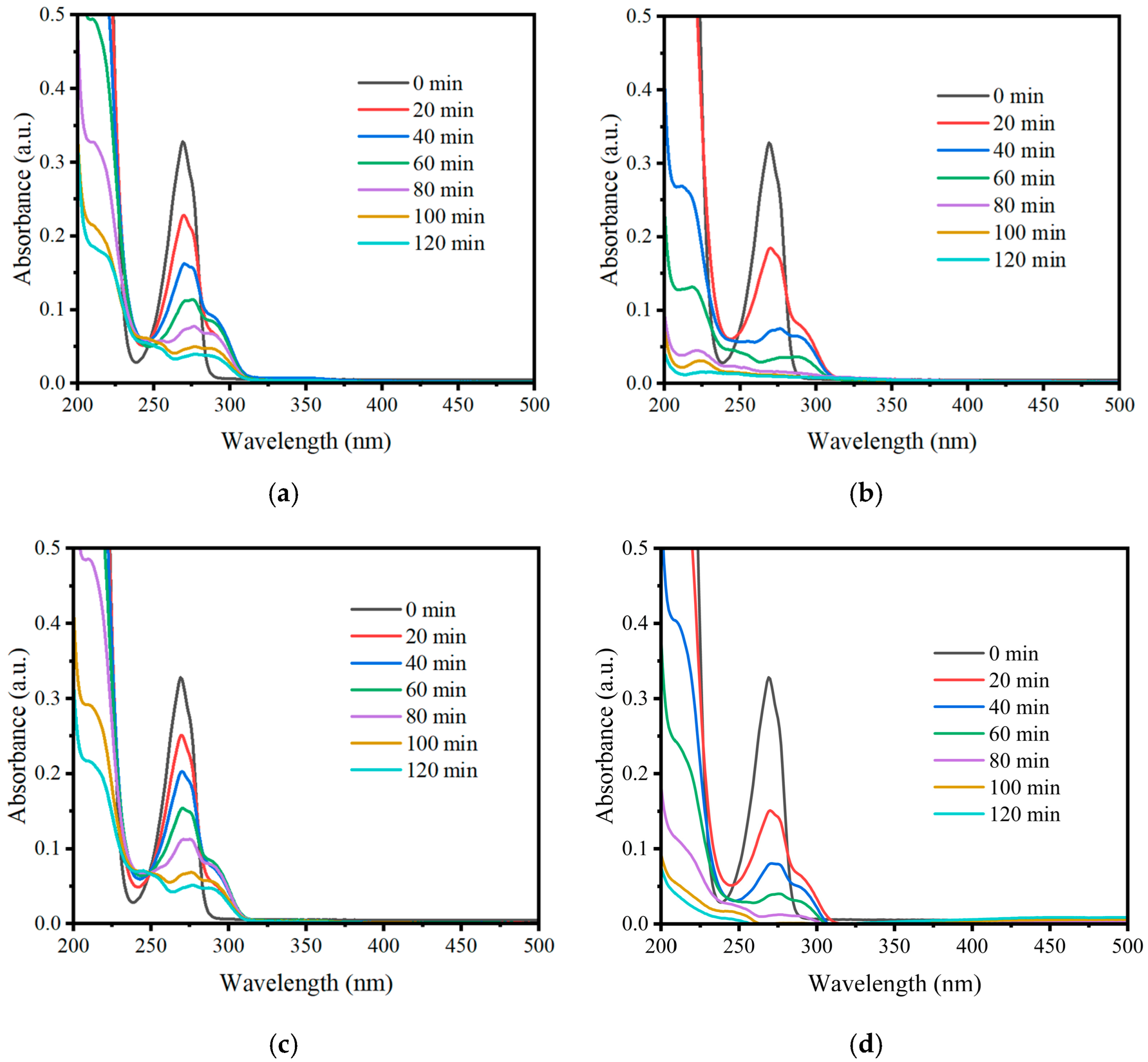
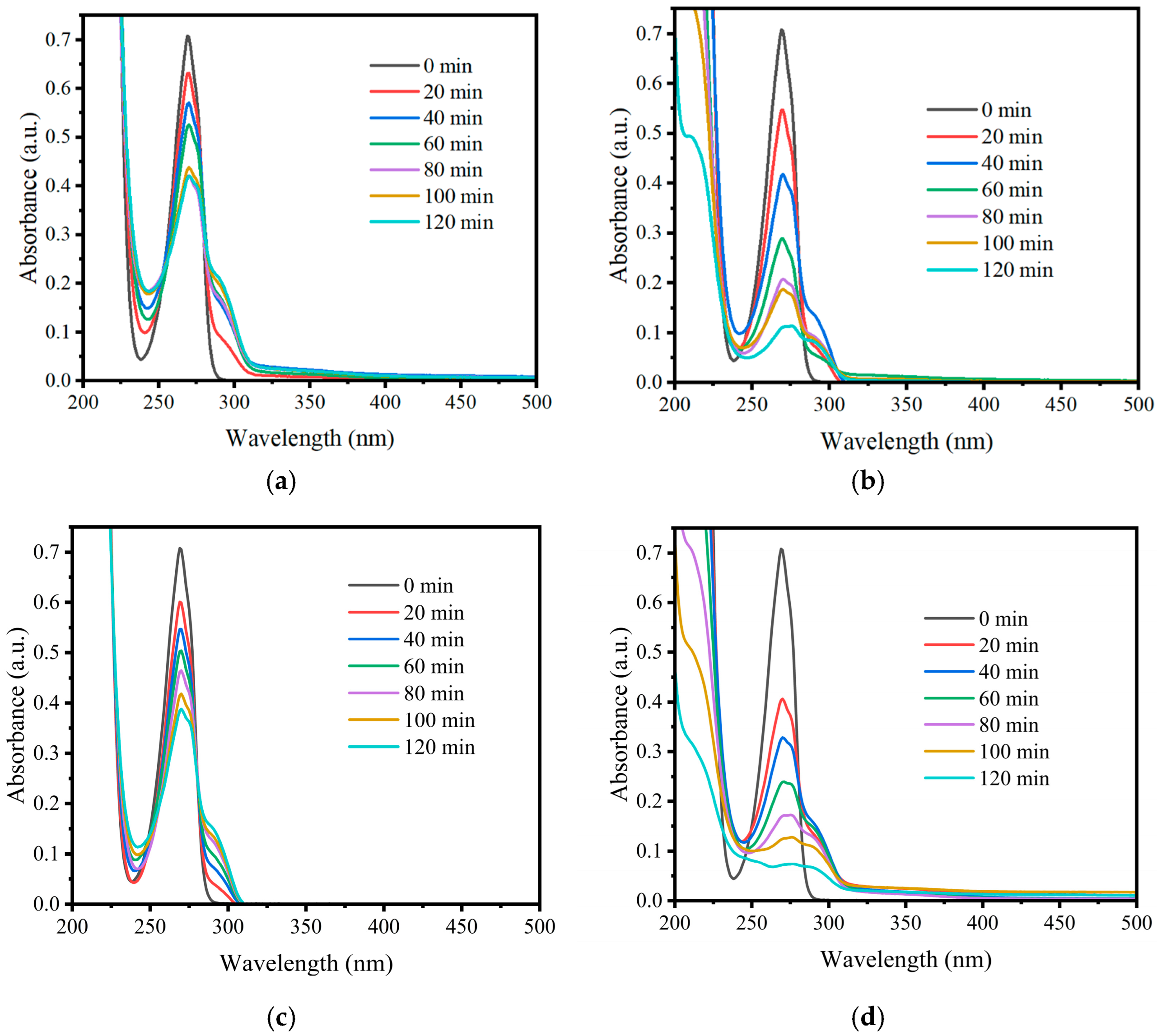
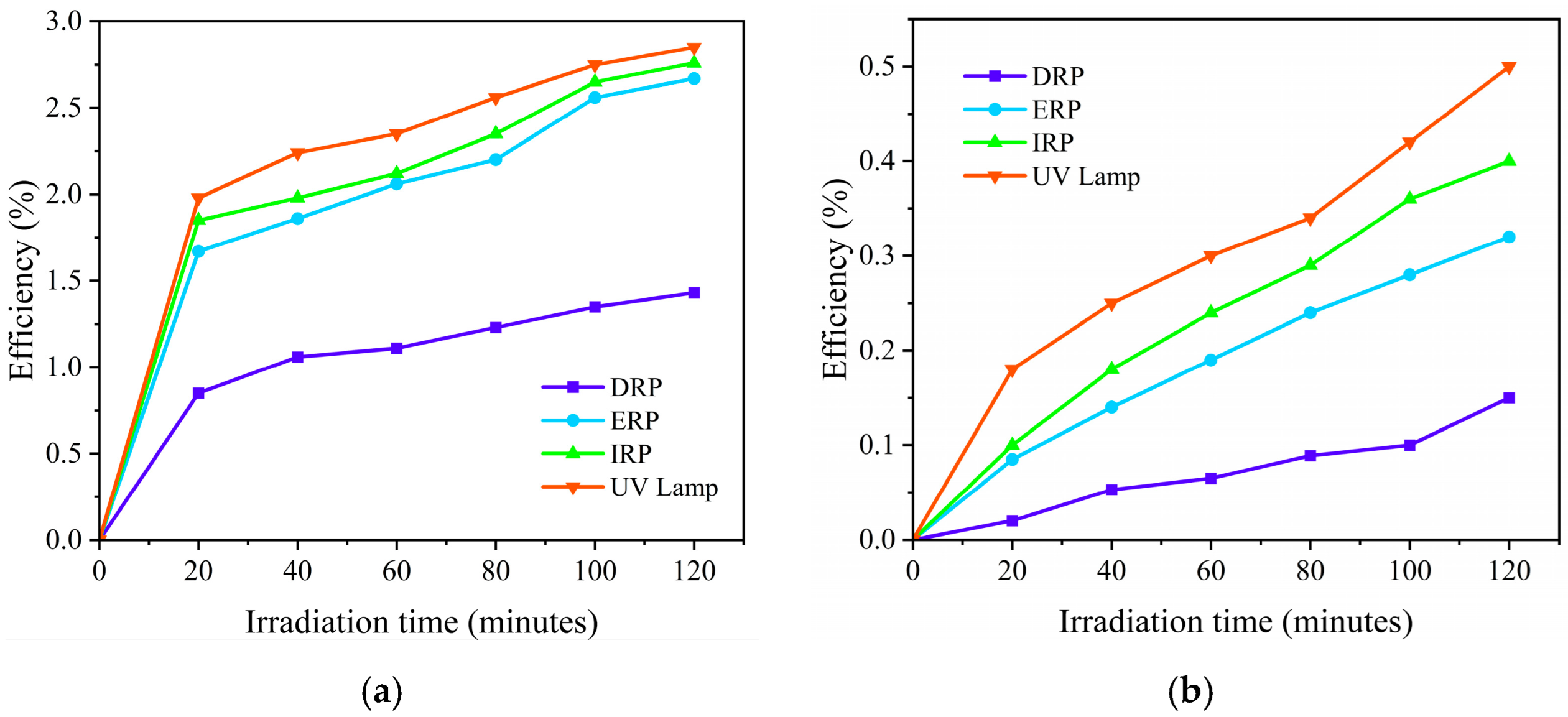
2.2. Photocatalytic Evaluation
3. Methods and Experiments
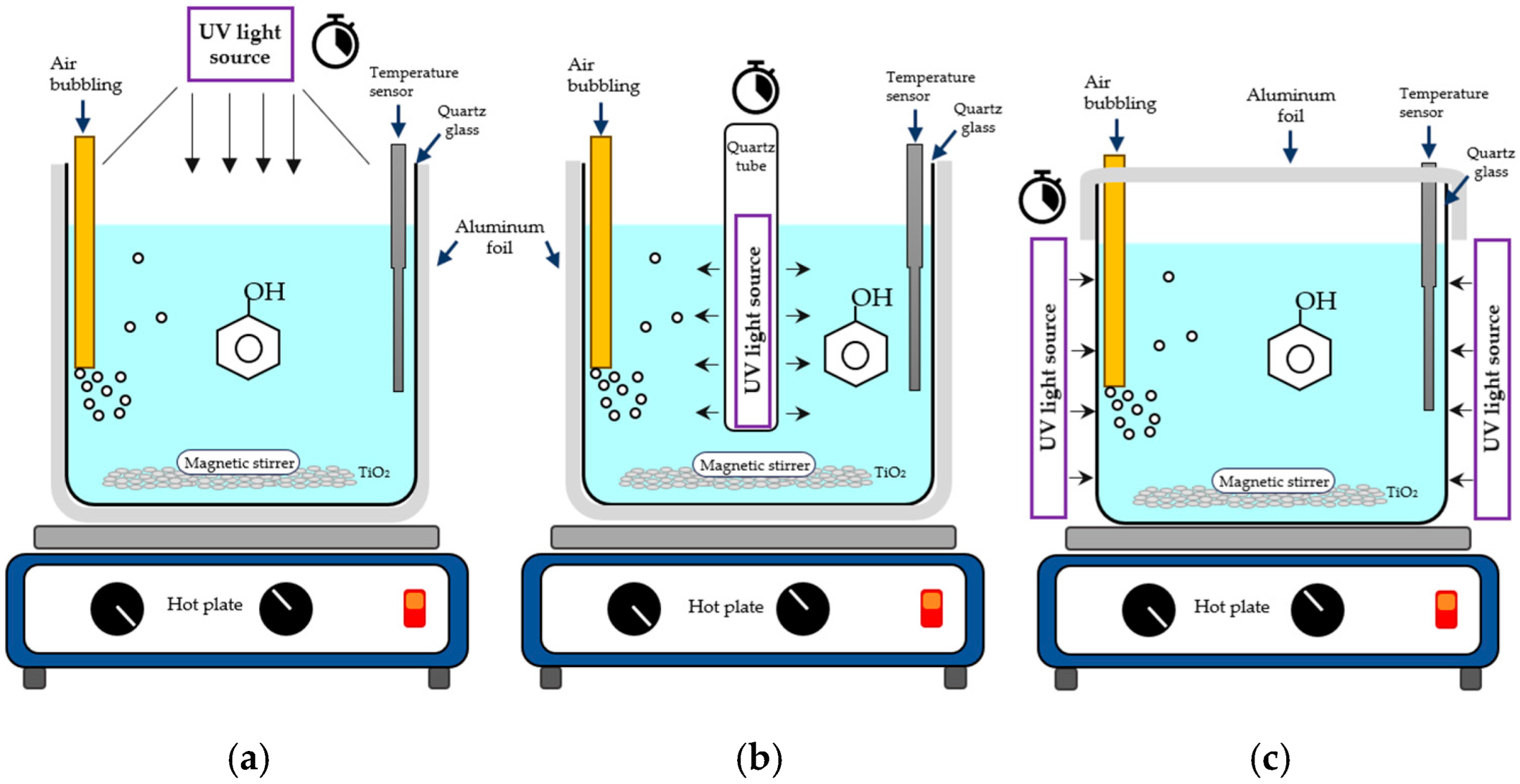
3.1. Photoreactors’ Configuration
- (a)
- DRP. In this configuration, a single LED was positioned 4 cm above the solution, as shown in Figure 8a. The LED was mounted in a dedicated aluminum heatsink.
- (b)
- IRP. Nine LEDs were arranged in three strips, each 60 mm long and spaced 10 mm apart. These strips were mounted on an aluminum triangular profile placed inside a quartz tube, irradiating the solution from inside out and covering 360°, as illustrated in Figure 8b.
- (c)
- ERP. This configuration involved distributing the light sources around a glass container, irradiating from the outside toward the center of the solution, also 360°. Similarly to the IRP, three LED strips, each containing three LEDs, were used. In this case, a circular 2 ¼′′ aluminum heatsink was used. A representative image of the ERP setup is shown in Figure 8c.
3.2. Photodegradation of Phenol
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOPs | Advanced oxidation processes |
| ROS | Reactive oxygen species |
| LEDs | Light emitting diodes |
| LED | Light emitting diode |
| UV | Ultraviolet |
| DRP | Direct radiation photoreactor |
| IRP | Internal radiation photoreactor |
| ERP | External radiation photoreactor |
| TOC | Total Organic Carbon |
| DOC | Dissolved Organic Carbon |
| LC-MS | Liquid Chromatography-Mass Spectrometry |
References
- Colcha, P.D.G. Degradación del Fenol Presente en las Descargas de Aguas Residuales Industriales de la Refinería de Shushufindi Mediante foto Oxidación Catalítica Homogénea y Heterogénea para Descontaminación de sus Descargas. Bachelor’s Thesis, Escuela Superior Politécnica de Chimborazo, Riobamba, Ecuador, 2016. [Google Scholar]
- Mohd, A. Presence of phenol in wastewater effluent and its removal: An overview. Int. J. Environ. Anal. Chem. 2022, 102, 1362–1384. [Google Scholar] [CrossRef]
- Kulkarni, S.; Kaware, J. Review on research for removal of phenol from wastewater. Int. J. Sci. Res. Publ. 2018, 3, 2250–3153. [Google Scholar]
- Wang, Y.; Song, J.; Zhao, W.; He, X.; Chen, J.; Xiao, M. In situ degradation of phenol and promotion of plant growth in contaminated environments by a single Pseudomonas aeruginosa strain. J. Hazard. Mater. 2011, 192, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Soto, H.M.; Palma, T.M.; García, M.; García-Mateos, R. Phenolic Compounds: Natural Sources, Importance and Applications; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Dang, T.T.T.; Le, S.T.T.; Channei, D.; Khanitchaidecha, W.; Nakaruk, A.D. Photodegradation mechanisms of phenol in the photocatalytic process. Res. Chem. Intermed. 2016, 42, 5961–5974. [Google Scholar] [CrossRef]
- Oller, I.; Gernjak, W.; Maldonado, M.I.; Pérez-Estrada, L.A.; Sánchez-Pérez, J.A.; Malato, S. Solar photocatalytic degradation of some hazardous water-soluble pesticides at pilot-plant scale. J. Hazard. Mater. 2006, 138, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Heterogeneous photocatalytic degradation of phenols in wastewater: A review on current status and developments. Desalination 2010, 261, 3–18. [Google Scholar] [CrossRef]
- Granda, F.; Hicapié, M.G. Sistemas fotocatalíticos para el tratamiento de VOCs en corrientes gaseosas: Aspectos básicos y aplicaciones. Vector 2015, 10, 122. [Google Scholar]
- Jo, W.K.; Tayade, R.J. Recent developments in photocatalytic dye degradation upon irradiation with energy-efficient light emitting diodes. Chin. J. Catal. 2014, 35, 1781–1792. [Google Scholar] [CrossRef]
- Dutta, G.S.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Hölz, K.; Lietard, J.; Somoza, M.M. High-power 365 nm UV LED mercury arc lamp replacement for photochemistry and chemical photolithography. ACS Sustain. Chem. Eng. 2017, 5, 828–834. [Google Scholar] [CrossRef]
- Bertagna, S.D.; Buttiglieri, G.; Babić, T.; Ćurković, L.; Babić, S. Impact of UV-LED photoreactor design on the degradation of contaminants of emerging concern. Process Saf. Environ. Prot. 2021, 153, 94–106. [Google Scholar] [CrossRef]
- Khademalrasool, M.; Farbod, M.; Talebzadeh, M.D. The improvement of photocatalytic processes: Design of a photoreactor using high-power LEDs. J. Sci. Adv. Mater. Devices 2016, 1, 382–387. [Google Scholar] [CrossRef]
- Dai, K.; Lu, L.; Dawson, G. Development of UV-LED/TiO2 device and their application for photocatalytic degradation of methylene blue. J. Mater. Eng. Perform. 2012, 22, 1035–1040. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Fernandes, J.R.; Peres, J.A.; Tavares, P.B.; Lucas, M.S. Wireless UV-A LEDs-driven AOP in the treatment of agro-industrial wastewaters. Environ. Res. 2021, 200, 111430. [Google Scholar] [CrossRef] [PubMed]
- Nyangaresi, P.O.; Qin, Y.; Chen, G.; Zhang, B.; Lu, Y.; Shen, L. Comparison of UV-LED photolytic and UV-LED/TiO2 photocatalytic disinfection for Escherichia coli in water. Catal. Today 2018, 335, 200–207. [Google Scholar] [CrossRef]
- Triquet, T.; Tendero, C.; Latapie, L.; Manero, M.; Richard, R.; Andriantsiferana, C. TiO2 MOCVD coating for photocatalytic degradation of ciprofloxacin using 365 nm UV LEDs—Kinetics and mechanisms. J. Environ. Chem. Eng. 2020, 8, 104544. [Google Scholar] [CrossRef]
- Schwarze, M.; Borchardt, S.; Frisch, M.L.; Collis, J.; Walter, C.; Menezes, P.W.; Strasser, P.; Driess, M.; Tasbihi, M. Degradation of phenol via an advanced oxidation process (AOP) with immobilized commercial titanium dioxide (TiO2) photocatalysts. Nanomaterials 2023, 13, 1249. [Google Scholar] [CrossRef]
- Mazierski, P.; Bajorowicz, B.; Grabowska, E.; Zaleska, M.A. Photoreactor design aspects and modeling of light. In Heterogeneous Photocatalysis; Green Chemistry and Sustainable Technology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 211–248. [Google Scholar] [CrossRef]
- Lefèvre, S.; Boutin, O.; Ferrasse, J.H.; Malleret, L.; Faucherand, R.; Viand, A. Thermodynamic and kinetic study of phenol degradation by a non-catalytic wet air oxidation process. Chemosphere 2011, 84, 1208–1215. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Khyave, A.E.; Mafigholmi, R.; Davood, A.; Mahvi, A.; Salimi, L. Photocatalytic degradation of azithromycin and ceftriaxone using synthesized Ag/g-C3N4/Fe3O4 nanocomposites in aqueous solution. Sci. Rep. 2025, 15, 18726. [Google Scholar] [CrossRef]
- Takhar, V.; Singh, S. Nanomaterials ROS: A Comprehensive Review for Environmental applications. Environ. Sci. Nano 2025, 12, 2516–2550. [Google Scholar] [CrossRef]
- Michalec, K.; Mozgawa, B.; Kusior, A.; Pietrzyk, P.; Sojka, Z.; Radecka, M. Tunable Generation of Reactive Oxygen Species in SnO2/SnS2 Nanostructures: Mechanistic Insights into Indigo Carmine Photodegradation. J. Phys. Chem. C 2024, 128, 5011–5029. [Google Scholar] [CrossRef]
- Schneider, J.T.; Firak, D.S.; Ribeiro, R.R.; Peralta-Zamora, P. Use of scavenger agents in heterogeneous photocatalysis: Truths, half-truths, and misinterpretations. Phys. Chem. Chem. Phys. 2020, 22, 15723–15733. [Google Scholar] [CrossRef]
- Bai, X.; Nie, M.; Diwu, Z.; Nie, H.; Wang, Y. Enhanced degradation and mineralization of phenol by combining two highly efficient strains with divergent ring-cleavage pathways. J. Water Process Eng. 2021, 39, 101743. [Google Scholar] [CrossRef]
- Hammouda, S.B.; Zhao, F.; Safaei, Z.; Srivastava, V.; Lakshmi, R.D.; Iftekhar, S.; Kalliola, S.; Sillanpää, M. Degradation and mineralization of phenol in aqueous medium by heterogeneous monopersulfate activation on nanostructured cobalt based-perovskite catalysts ACoO3 (A=La, Ba, Sr and Ce): Characterization, kinetics and mechanism study. Appl. Catal. B Environ. 2017, 215, 60–73. [Google Scholar] [CrossRef]
- Rangel, V.I.; Del Angel, G.; Ramos, R.E.; González, F.; Acevedo, P.P.; Gómez, C.M.; Tzompantzi, F.; Gutiérrez, O.N.; Torres, J.G. Improvement of photocatalytic activity in the degradation of 4-chlorophenol and phenol in aqueous medium using tin-modified TiO2 photocatalysts. RSC Adv. 2023, 13, 13862–13879. [Google Scholar] [CrossRef]
- Górska, P.; Zaleska, A.; Hupka, J. Photodegradation of phenol by UV/TiO2 and Vis/N,C-TiO2 processes: Comparative mechanistic and kinetic studies. Sep. Purif. Technol. 2009, 68, 90–96. [Google Scholar] [CrossRef]
- Zhang, T.; Sa, G.; Xu, A. Photocatalytic degradation of phenol with one-step hydrothermal synthesized Bi-TiO2. Vacuum 2024, 233, 113982. [Google Scholar] [CrossRef]
- Marcì, G.; Sclafani, A.; Augugliaro, V.; Palmisano, L.; Schiavello, M. Influence of some aromatic and aliphatic compounds on the rate of photodegradation of phenol in aqueous suspensions of TiO2. J. Photochem. Photobiol. A Chem. 1995, 89, 69–74. [Google Scholar] [CrossRef]
- Pera-Titus, M.; García-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B Environ. Energy 2003, 47, 219–256. [Google Scholar] [CrossRef]
- Herrmann, J. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Turki, A.; Guillard, C.; Dappozze, F.; Ksibi, Z.; Berhault, G.; Kochkar, H. Phenol photocatalytic degradation over anisotropic TiO2 nanomaterials: Kinetic study, adsorption isotherms and formal mechanisms. Appl. Catal. B Environ. 2015, 163, 404–414. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Xu, Y.-J. (Eds.) Heterogeneous Photocatalysis: From Fundamentals to Green Applications; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Udom, I.; Myers, P.D.; Ram, M.K.; Hepp, A.F.; Archibong, E.; Stefanakos, E.K.; Goswami, D.Y. Optimization of photocatalytic degradation of phenol using simple photocatalytic reactor. Am. J. Anal. Chem. 2014, 05, 743–750. [Google Scholar] [CrossRef]
- Liu, Z.; Qian, M.; Cheng, X.; Liu, Z. Recent advances in the photocatalytic degradation of phenol over Bi-based oxide catalysts. Processes 2024, 12, 1799. [Google Scholar] [CrossRef]
- Suhadolnik, L.; Pohar, A.; Likozar, B.; Čeh, M. Mechanism and kinetics of phenol photocatalytic, electrocatalytic and photoelectrocatalytic degradation in a TiO2-nanotube fixed-bed microreactor. Chem. Eng. J. 2016, 303, 292–301. [Google Scholar] [CrossRef]
- Mills, A.; Hunte, S.L. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Jamil, Q.; Rana, K.B.; Matoh, L.A. CFD Study on Optimization of Mass Transfer and Light Distribution in a Photocatalytic Reactor with Immobilized Photocatalyst on Spheres. Water 2024, 16, 1828. [Google Scholar] [CrossRef]
- Turchi, C. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack. J. Catal. 1990, 122, 178–192. [Google Scholar] [CrossRef]
- Khan, Z.; Kamal, M.; Jaafar, J.; Mir, F.; Rehman, G.U.; Khan, A.A.; Ismail, A.; Matsuura, T.; Othman, M.; Rahman, M.A. Recent advances in photoreactor designs for the degradation of persistent organic contaminants with influential effects of configuration and parameters: A review. J. Water Process Eng. 2024, 69, 106825. [Google Scholar] [CrossRef]
- Tran, H.D.; Nguyen, D.Q.; Do, P.T.; Tran, U.N.P. Kinetics of photocatalytic degradation of organic compounds: A mini-review and new approach. RSC Adv. 2023, 13, 16915–16925. [Google Scholar] [CrossRef]
- Martin, M.V.; Ipiña, A.; Villabrille, P.I.; Rosso, J.A. Combination of sunlight, oxidants, and Ce-doped TiO2 for phenol degradation. Environ. Sci. Pollut. Res. Int. 2017, 24, 6013–6021. [Google Scholar] [CrossRef]
- Subagio, D.P.; Srinivasan, M.; Lim, M.; Lim, T.T. Photocatalytic degradation of bisphenol-A by nitrogen-doped TiO2 hollow sphere in a vis-LED photoreactor. Appl. Catal. B Environ. 2010, 95, 414–422. [Google Scholar] [CrossRef]
- Belekbir, S.; El Azzouzi, M.; El Hamidi, A.; Rodríguez, L.L.; Santaballa, J.A.; Canle, M. Improved photocatalyzed degradation of phenol as a model pollutant over metal-impregnated nanosized TiO2. Nanomaterials 2020, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- Jamali, A.; Vanraes, R.; Hanselaer, P.; Gerven, T.V. A batch LED reactor for the photocatalytic degradation of phenol. Chem. Eng. Process.—Process. Intensif. 2013, 71, 43–50. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Miller, J.S. Rose bengal-sensitized photooxidation of 2-chlorophenol in water using solar simulated light. Water Res. 2004, 39, 412–422. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A Chem. 2003, 162, 317–322. [Google Scholar] [CrossRef]
- Hübner, U.; Spahr, S.; Lutze, H.; Wieland, A.; Rüting, S.; Gernjak, W.; Wenk, J. Advanced oxidation processes for water and wastewater treatment—Guidance for systematic future research. Heliyon 2024, 10, e30402. [Google Scholar] [CrossRef] [PubMed]
- Bekkouche, S.; Bouhelassa, M.; Salah, N.H.; Meghlaoui, F. Study of adsorption of phenol on titanium oxide (TiO2). Desalination 2004, 166, 355–362. [Google Scholar] [CrossRef]
- Bharali, D.; Saikia, S.; Devi, R.; Choudary, B.M.; Gour, N.K.; Deka, R.C. Photocatalytic degradation of phenol and its derivatives over ZnFe layered double hydroxide. J. Photochem. Photobiol. A Chem. 2023, 438, 114509. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, S.A.; Sarathi, M.P. Study of UV assisted photocatalytic degradation of organic dye. Mater. Today Proc. 2022, 66, 3244–3249. [Google Scholar] [CrossRef]


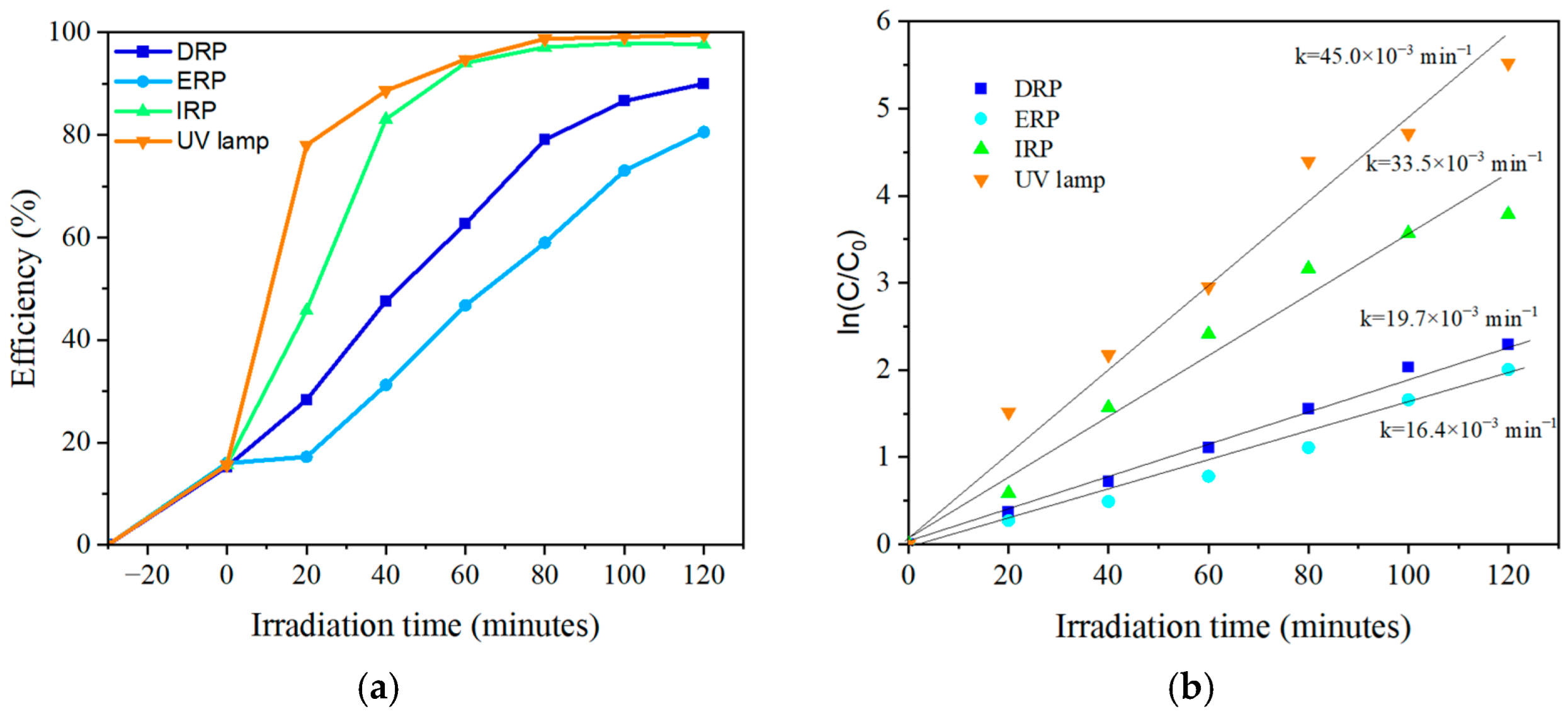
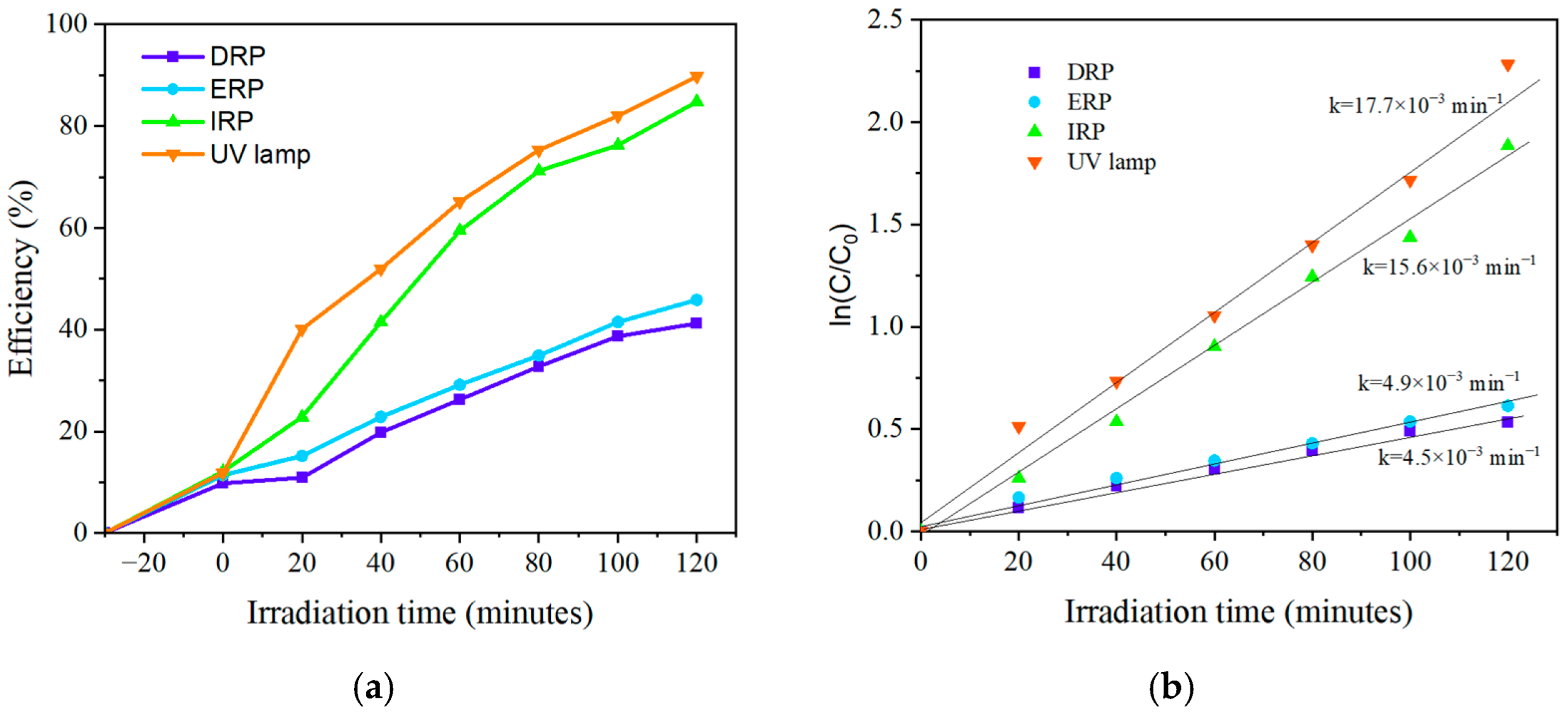
| System | 25 ppm | 50 ppm | ||
|---|---|---|---|---|
| k (min−1) | R2 | k (min−1) | R2 | |
| DRP | 0.0197 | 0.99 | 0.0045 | 0.98 |
| ERP | 0.0164 | 0.97 | 0.0049 | 0.98 |
| IRP | 0.0335 | 0.96 | 0.0156 | 0.98 |
| UV lamp | 0.0450 | 0.97 | 0.0177 | 0.98 |
| System | t1/2 (min) | |
|---|---|---|
| 25 ppm | 50 ppm | |
| DRP | 35.18 | 154 |
| ERP | 42.26 | 141.43 |
| IRP | 20.69 | 44.42 |
| UV lamp | 15.4 | 39.15 |
| Reference | Study Conditions | Photodegradation Efficiency/Rate Constant | Time |
|---|---|---|---|
| Turki et al. [35] | 30 mL of phenol (10 mg/L), 30 mg TiO2, mercury lamp (365 nm, 125 W) | 95% k = 6.56 × 10−3 min−1 | 60 min |
| Jamali et al. [49] | 10 mg/L de phenol, 20 mm × 50 mm cell, LED (375 nm) | 42% k = 2.37 × 10−5 min−1 | 480 min |
| Belekbir et al. [48] | 200 mL of phenol (50 ppm), mercury lamp (366 nm), immersion photoreactor with quartz tube with water circulation | 70% k = 76.5 × 10−3 min−1 | 60 min |
| This study (FRD; FRE; FRI; UV lamp) | 100 mL of phenol (25 ppm), 100 mg TiO2 P25, LEDs (365 nm) and UV lamp (254 nm), quartz glass 150 mL | 90.17%, 85.24%, 97.79%, 99.6%. k = 19.7 × 10−3 min−1, 16.4 × 10−3 min−1, 33.5 × 10−3 min−1, 45.0 × 10−3 min−1. | 120 min |
| 100 mL of phenol (50 ppm), 100 mg TiO2 P25, LEDs (365 nm) and UV lamp (254 nm), quartz glass 150 mL | 41.21%, 45.83%, 84.81%, 89.80%. k = 4.5 × 10−3 min−1, 4.9 × 10−3 min−1, 15.9 × 10−3 min−1, 17.7 × 10−3 min−1 | 120 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ildefonso-Ojeda, B.; Hernández-Chávez, M.; Álvarez-Lemus, M.A.; López-González, R.; Contreras-Bárbara, J.R.; Roa-Tort, K.; Rivera-Fernández, J.D.; Fabila-Bustos, D.A. Analysis and Application of UV-LED Photoreactors for Phenol Removal. Catalysts 2025, 15, 748. https://doi.org/10.3390/catal15080748
Ildefonso-Ojeda B, Hernández-Chávez M, Álvarez-Lemus MA, López-González R, Contreras-Bárbara JR, Roa-Tort K, Rivera-Fernández JD, Fabila-Bustos DA. Analysis and Application of UV-LED Photoreactors for Phenol Removal. Catalysts. 2025; 15(8):748. https://doi.org/10.3390/catal15080748
Chicago/Turabian StyleIldefonso-Ojeda, Betsabé, Macaria Hernández-Chávez, Mayra A. Álvarez-Lemus, Rosendo López-González, José R. Contreras-Bárbara, Karen Roa-Tort, Josué D. Rivera-Fernández, and Diego A. Fabila-Bustos. 2025. "Analysis and Application of UV-LED Photoreactors for Phenol Removal" Catalysts 15, no. 8: 748. https://doi.org/10.3390/catal15080748
APA StyleIldefonso-Ojeda, B., Hernández-Chávez, M., Álvarez-Lemus, M. A., López-González, R., Contreras-Bárbara, J. R., Roa-Tort, K., Rivera-Fernández, J. D., & Fabila-Bustos, D. A. (2025). Analysis and Application of UV-LED Photoreactors for Phenol Removal. Catalysts, 15(8), 748. https://doi.org/10.3390/catal15080748









