High-Performance Pd-Pt/α-MnO2 Catalysts for the Oxidation of Toluene
Abstract
1. Introduction
2. Results and Discussion
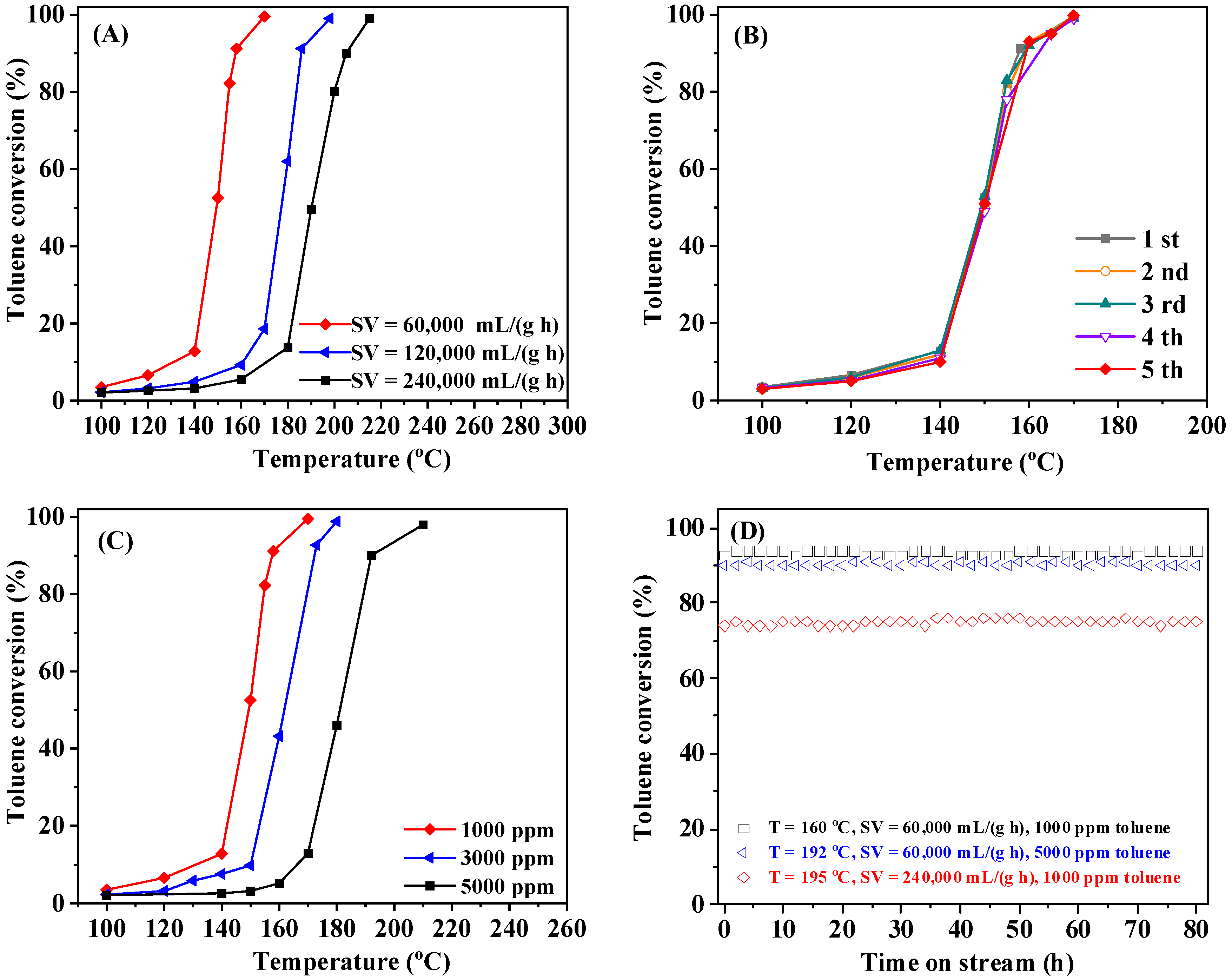
2.1. Catalytic Performance
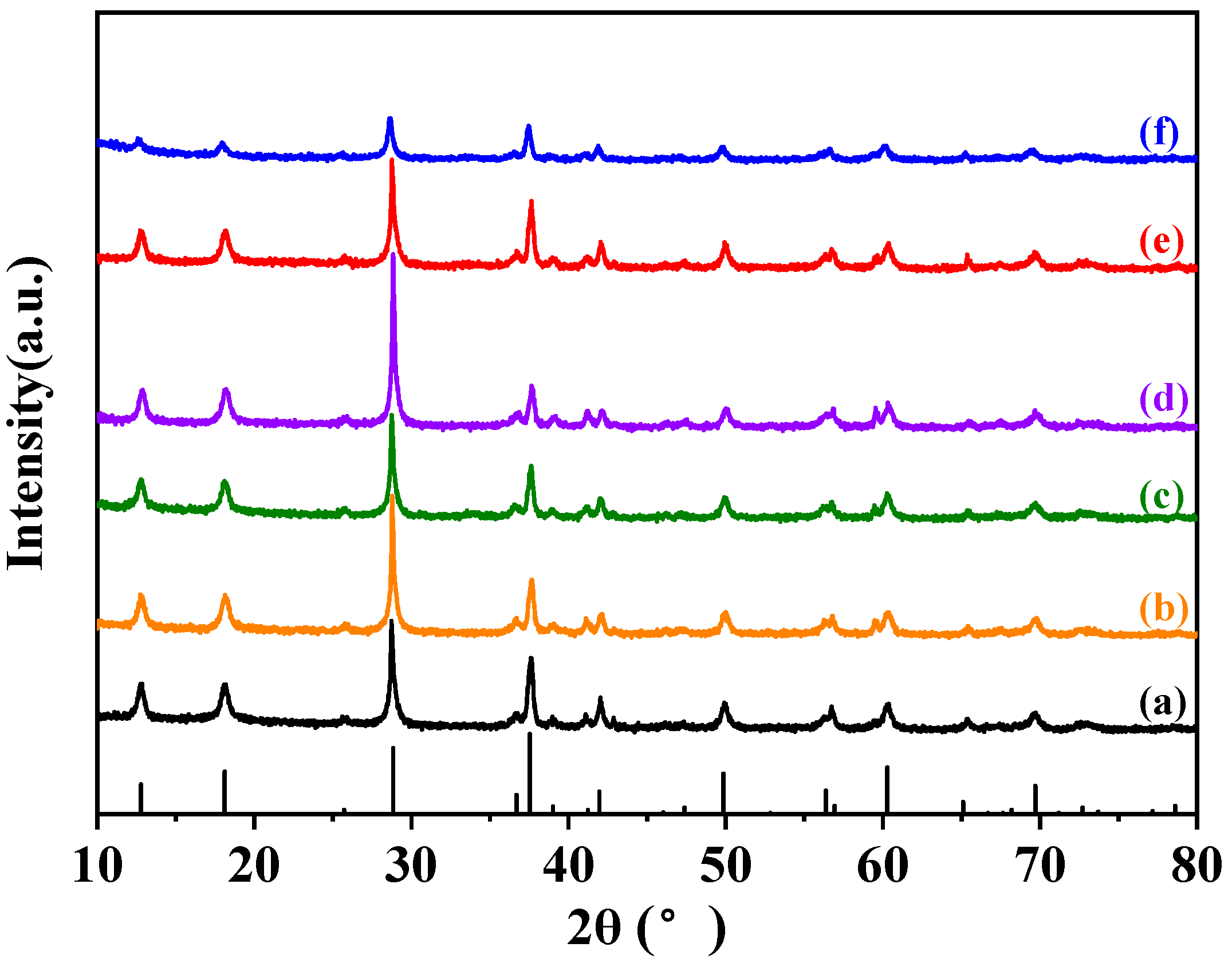
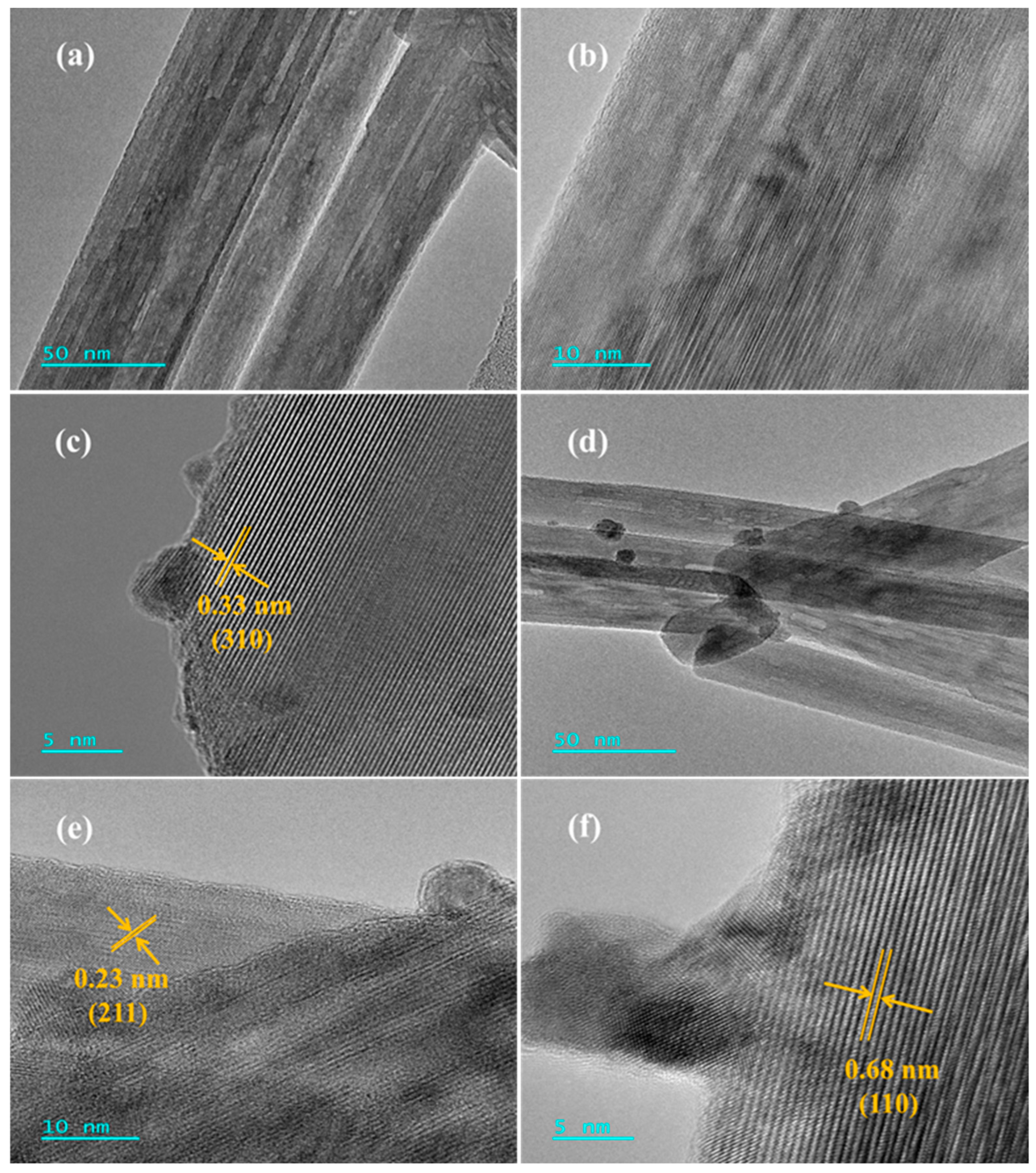
2.2. Crystal Phase Composition, Textural Parameter, and Morphology
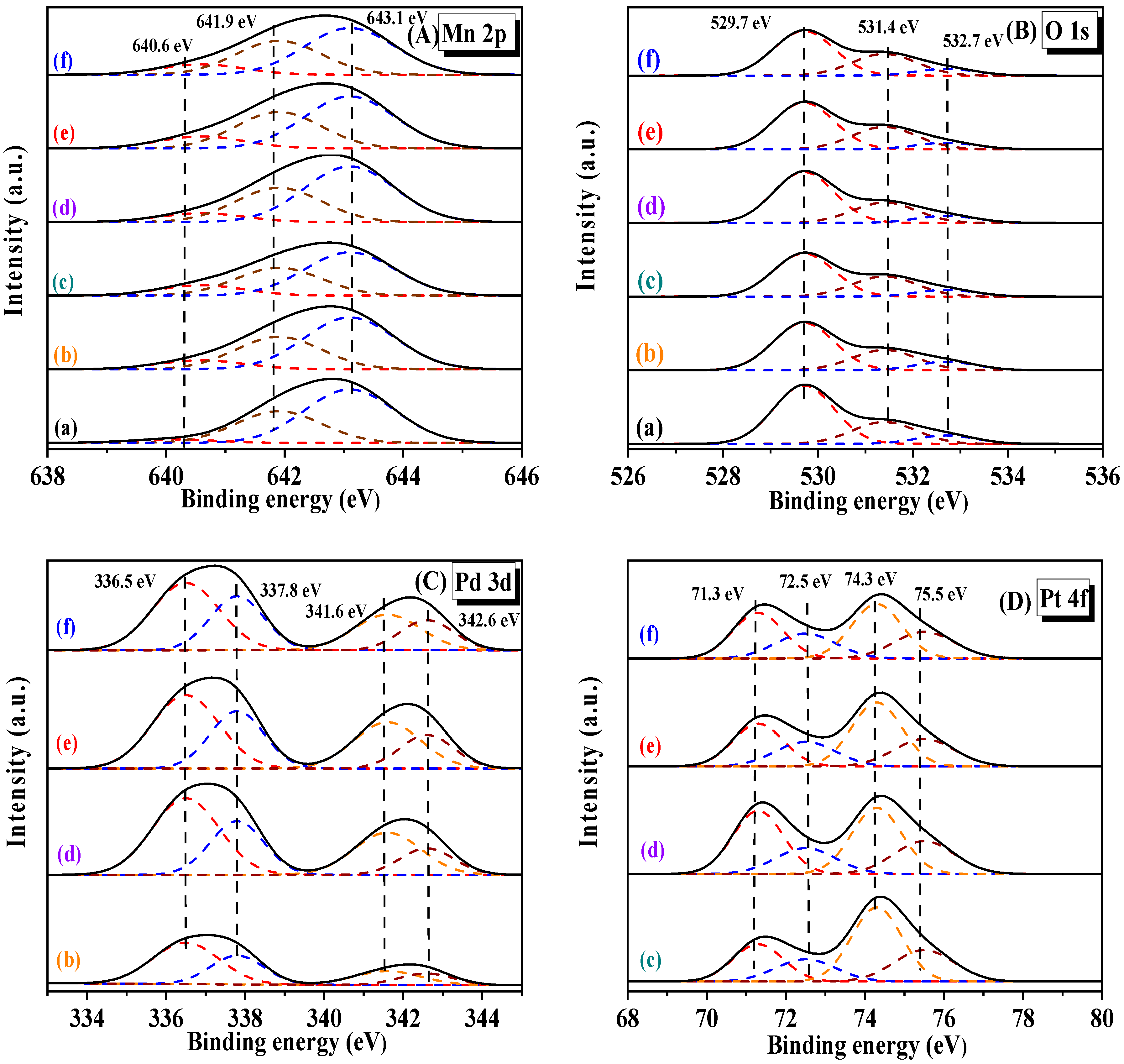
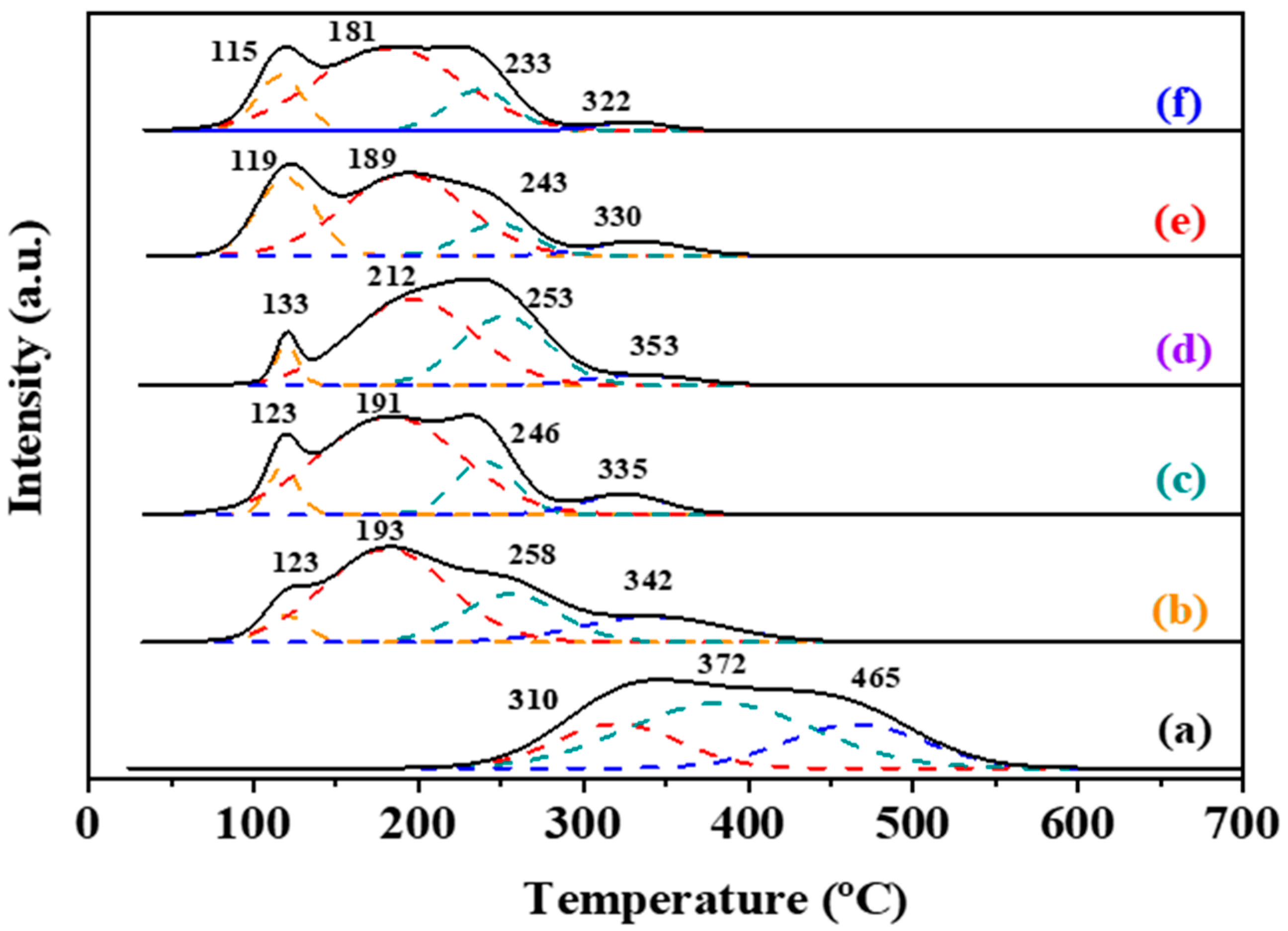
2.3. Surface Properties and Oxygen Desorption Behavior
2.4. Low-Temperature Reducibility
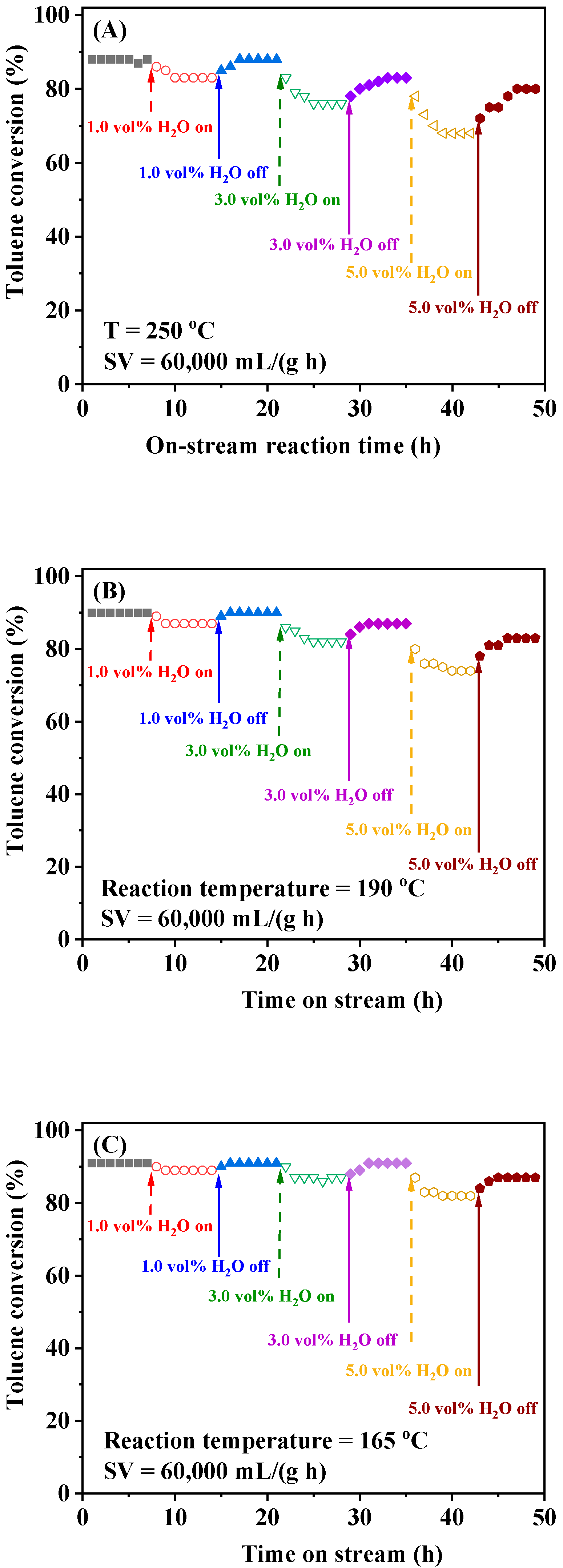
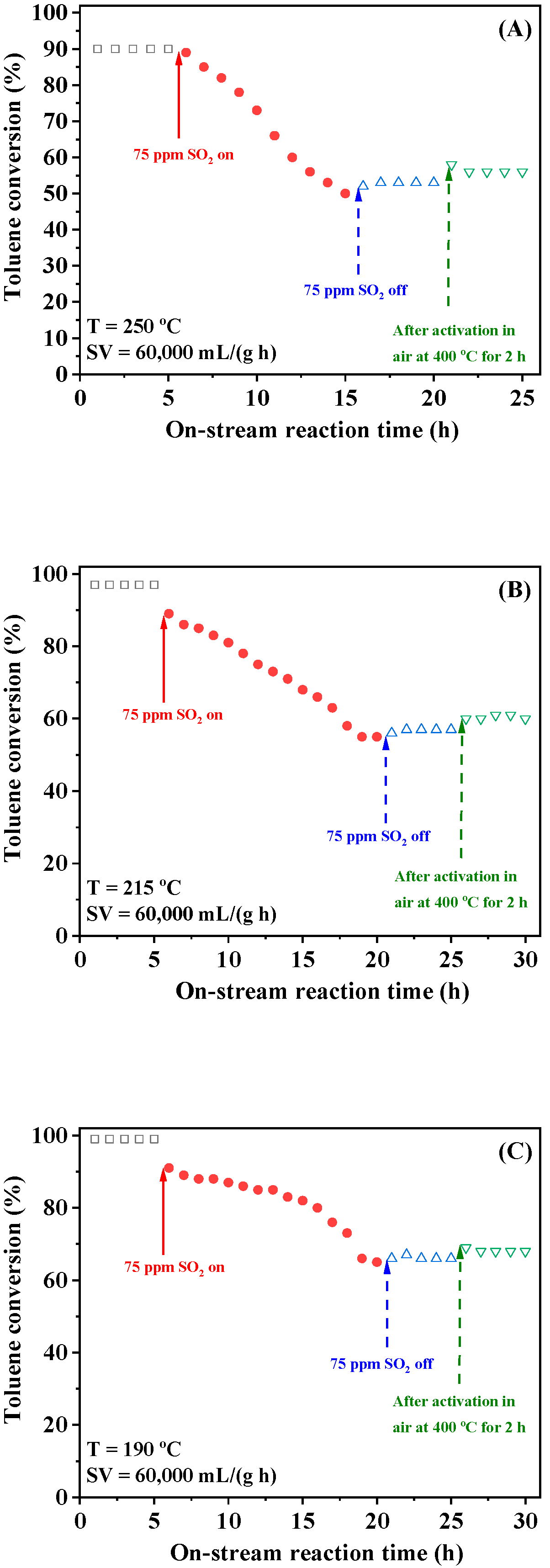
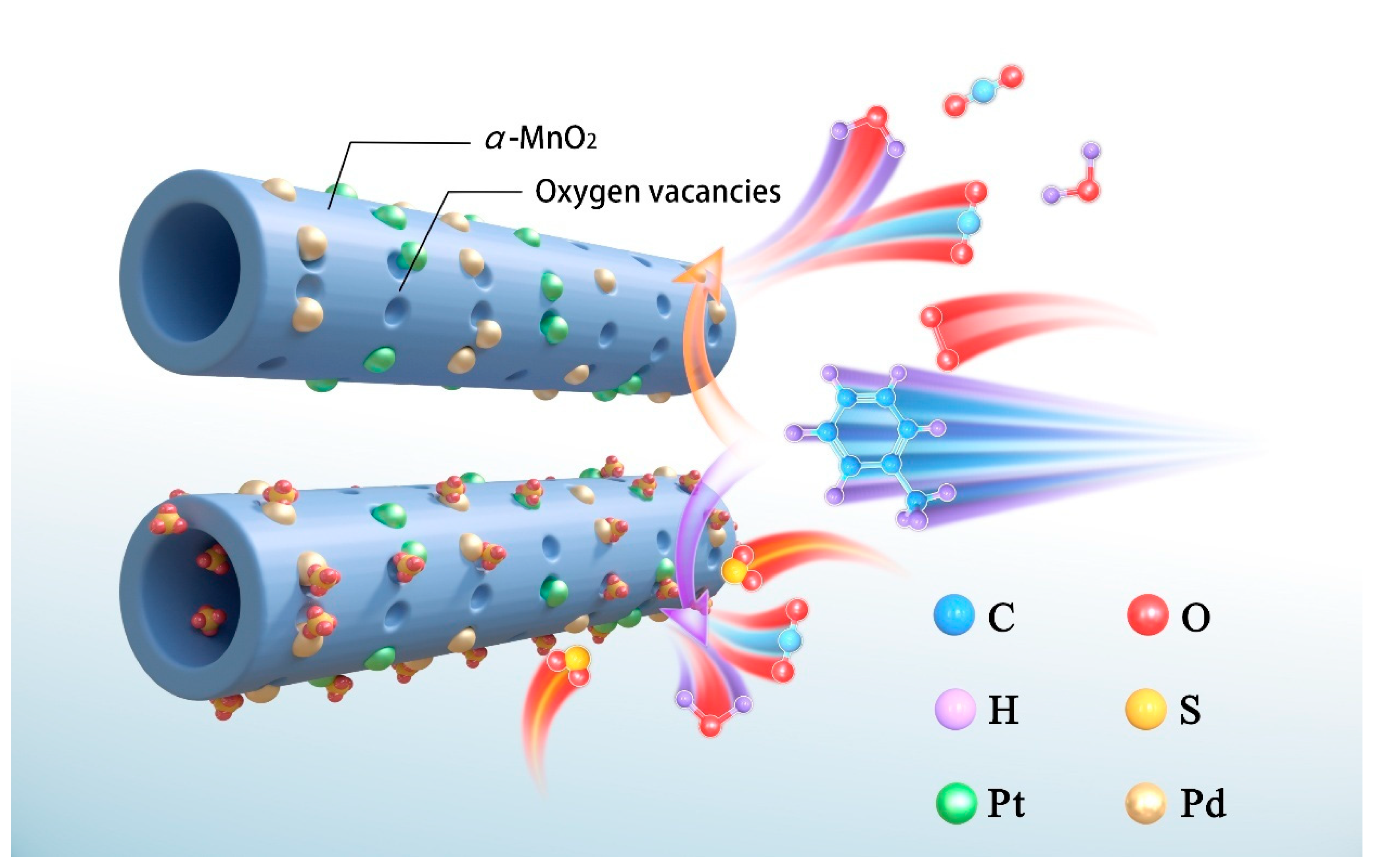
2.5. Catalytic Stability and Effects of SV, Water Vapor, and Sulfur Dioxide
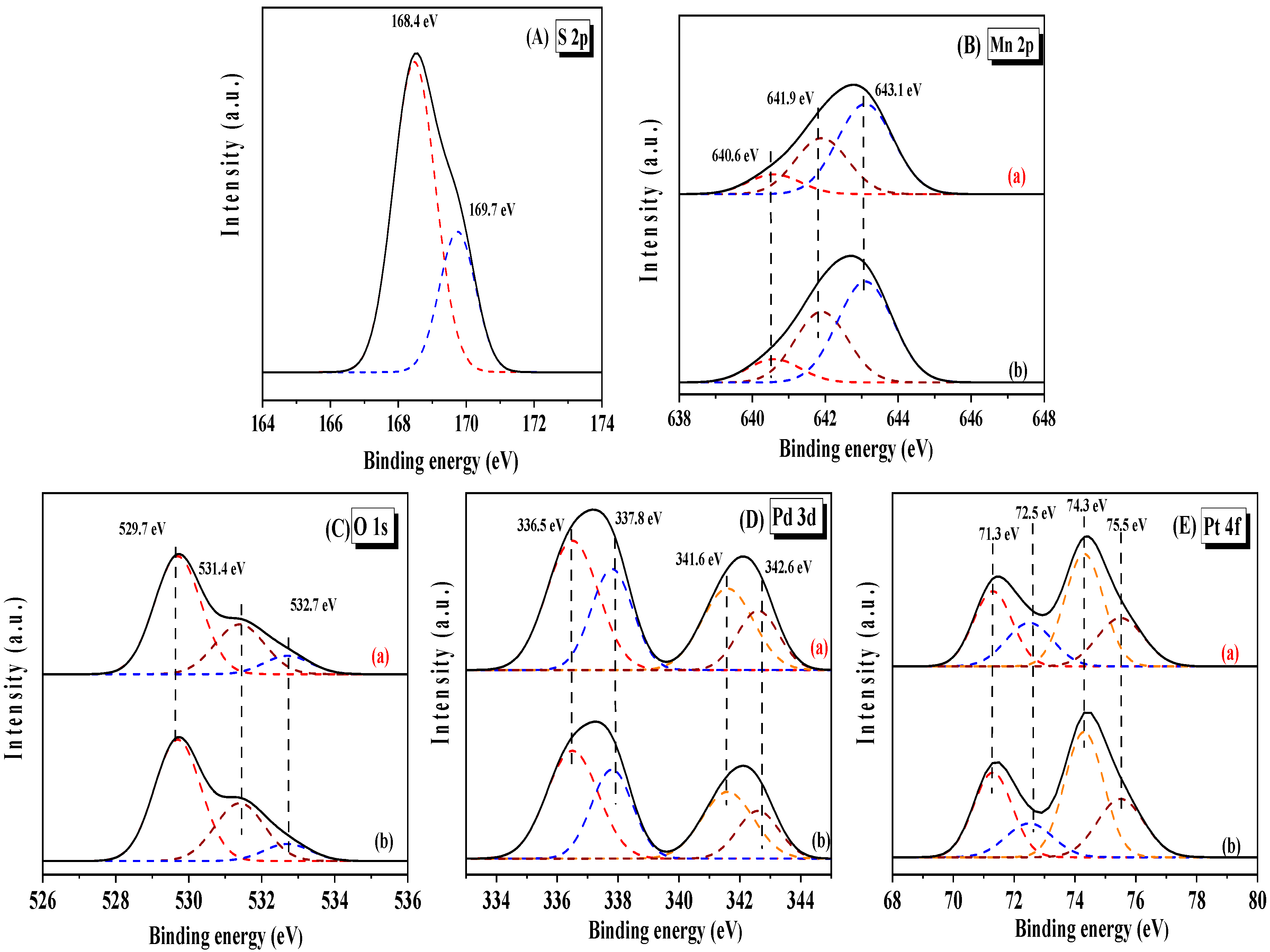
2.6. Physicochemical Properties After Sulfur Dioxide Treatment
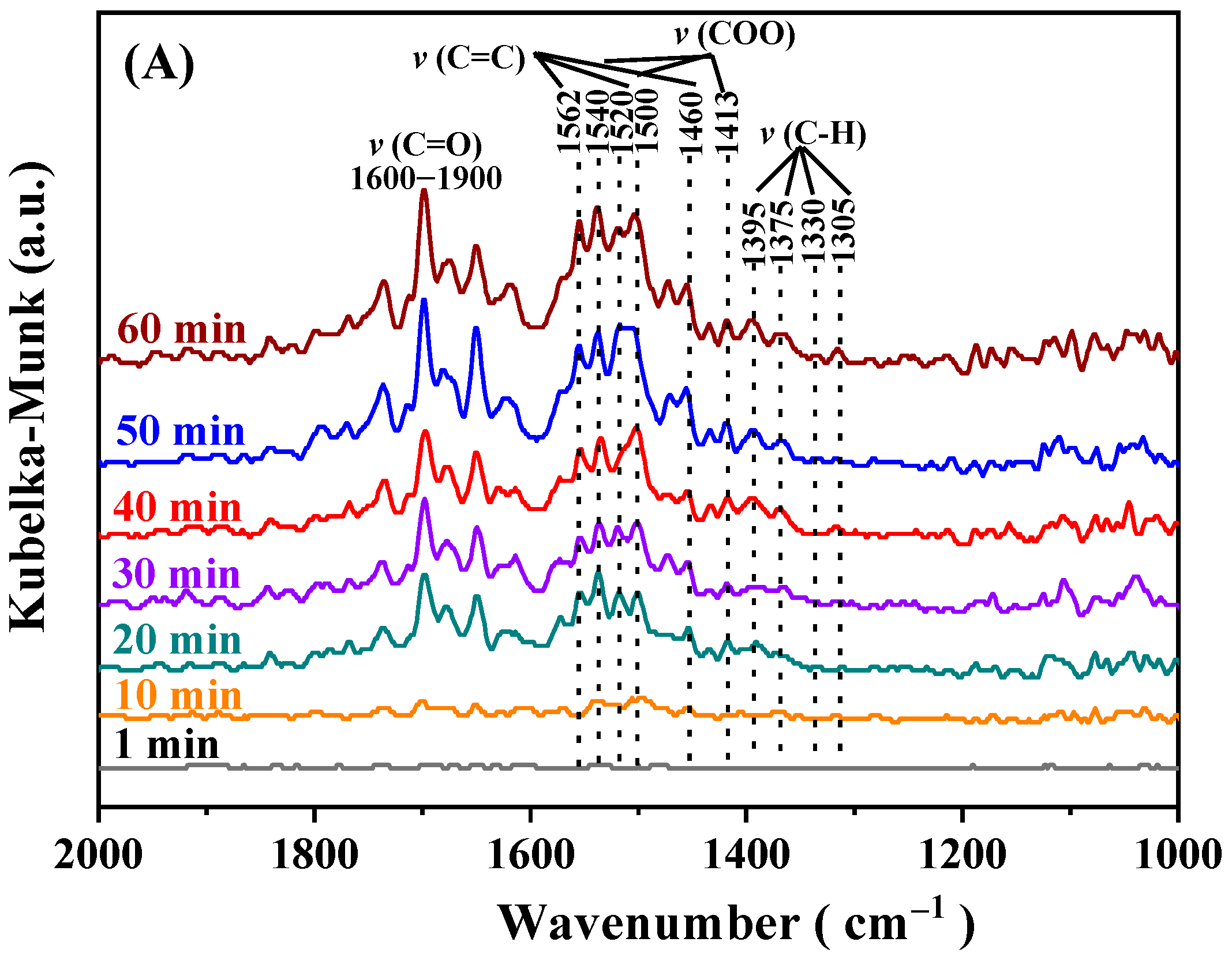
2.7. Reaction Intermediate and Reaction Mechanism
3. Experimental Section
3.1. Catalyst Preparation
3.1.1. Synthesis of α-MnO2
3.1.2. Preparation of xPd-yPt/α-MnO2
3.2. Catalyst Characterization
3.3. Catalytic Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yi, J.; Liu, J.; Gao, B.; Bo, L.; Cao, L.; Sillanpää, M. The comprehensive review of catalysts for catalytic oxidation of volatile organic compounds. J. Environ. Chem. Eng. 2025, 13, 115691. [Google Scholar] [CrossRef]
- He, C.Q.; Zou, Y.; Lv, S.J.; Flores, R.M.; Yan, X.L.; Deng, T.; Deng, X.J. The importance of photochemical loss to source analysis and ozone formation potential: Implications from in-situ observations of volatile organic compounds (VOCs) in Guangzhou, China. Atmos. Environ. 2024, 320, 120320. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, C.; Cao, X. Intermediate volatile organic compounds in Canadian residential air in winter: Implication to indoor air quality. Chemosphere 2023, 328, 138567. [Google Scholar] [CrossRef]
- Zheng, H.; Chang, X.; Wang, S.; Li, S.; Yin, D.; Zhao, B.; Huang, G.; Huang, L.; Jiang, Y.; Dong, Z.; et al. Trends of full-volatility organic emissions in China from 2005 to 2019 and their organic aerosol formation potentials. Environ. Sci. Technol. Lett. 2023, 10, 137–144. [Google Scholar] [CrossRef]
- He, L.; Duan, Y.; Zhang, Y.; Yu, Q.; Huo, J.; Chen, J.; Cui, H.; Li, Y.; Ma, W. Effects of VOC emissions from chemical industrial parks on regional O3-PM2.5 compound pollution in the Yangtze River Delta. Sci. Total. Environ. 2024, 906, 167503. [Google Scholar] [CrossRef]
- Li, L.; Zhang, D.; Hu, W.; Yang, Y.; Zhang, S.; Yuan, R.; Lv, P.; Zhang, W.; Zhang, Y.; Zhang, Y. Improving VOC control strategies in industrial parks based on emission behavior, environmental effects, and health risks: A case study through atmospheric measurement and emission inventory. Sci. Total. Environ. 2023, 865, 161235. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Dong, C.; Song, C.; Qu, Z. Spinel-based catalysts that enable catalytic oxidation of volatile organic compounds. Environ. Sci. Technol. 2024, 58, 20785–20811. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Yang, J.; Pan, C. The surface/interface modulation of platinum group metal (PGM)-free catalysts for VOCs and CO catalytic oxidation. ACS Appl. Mater. Interfaces 2024, 16, 37379–37389. [Google Scholar] [CrossRef]
- Sun, K.; Kong, F.; Gong, X.; Shi, Y.; Jin, D.; Jin, H.; Guo, X.; Zhou, R. Hierarchical porous S-1 zeolite supported Pt catalyst for catalytic oxidation of various VOCs at low temperature with strong water resistance and excellent stability. Appl. Catal. A 2025, 699, 120284. [Google Scholar] [CrossRef]
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review. Appl. Catal. B 2021, 281, 119447. [Google Scholar] [CrossRef]
- Gong, P.; He, F.; Xie, J.; Fang, D. Catalytic removal of toluene using MnO2-based catalysts: A review. Chemosphere 2023, 318, 137938. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, C.; Du, F.; Zhang, C.; Zhang, R.; Liu, P.; Li, J. Mutual inhibition effects on the synchronous conversion of benzene, toluene, and xylene over mnox catalysts. J. Colloid Interface Sci. 2023, 641, 791–802. [Google Scholar] [CrossRef]
- Xiang, W.; Liu, W.; Liu, G.; He, Y.; Yang, S.; Chen, X.; Song, Z.; Zhang, X.; Tsubaki, N. Decoding the role of oxygen defects in MnO2-based catalysts for enhanced VOCs oxidation. Mater. Today Chem. 2025, 44, 102604. [Google Scholar] [CrossRef]
- Nguyen, D.M.T.; Nguyen, C.C.; Thi, P.N.H.; Nguyen, P.H.D.; Van, L.Q.; Kim, S.Y.; Nguyen-Dinh, L. Tailoring oxygen vacancies and active surface oxygen species in copper-doped MnO2 catalysts for total catalytic oxidation of VOCs. Ind. Eng. Chem. Res. 2023, 62, 6908–6919. [Google Scholar] [CrossRef]
- Bai, B.; Huang, Y.; Chen, J.; Lei, J.; Wang, S.; Wang, J. Ultrathin MnO2 with strong lattice disorder for catalytic oxidation of volatile organic compounds. J. Colloid Interface Sci. 2024, 653, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Lin, F.; Guo, X.; Li, Y. Catalytic acetone oxidation over mnox catalysts: Regulating their crystal structures and surface properties. Processes 2024, 12, 326. [Google Scholar] [CrossRef]
- Li, J.; Zhu, D.; Di, S.; Xu, L.; Wu, Z.; Yao, S. Construction of Pt-MnO2 interface with strong electron coupling effect for plasma catalytic oxidation of aromatic VOCs. Colloids Surf. A 2023, 665, 131248. [Google Scholar] [CrossRef]
- Ru, X.; Li, W.; Wang, X.; Shi, Z.; Wen, X.; Mo, S.; Zhang, Q.; Mo, D. Regulating the surface local environment of MnO2 materials via metal-support interaction in Pt/MnO2 hetero-catalysts for boosting methanol oxidation. Chem. Eng. Sci. 2023, 281, 119079. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, L.; Liu, Y.; Yang, T.; Deng, J.; Dai, H. Concurrent catalytic removal of typical volatile organic compound mixtures over Au-Pd/α-MnO2 nanotubes. J. Environ. Sci. 2018, 64, 276–288. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, M.; Xia, Y. Enhancing the catalytic and electrocatalytic properties of Pt-based catalysts by forming bimetallic nanocrystals with Pd. Chem. Soc. Rev. 2012, 41, 8035–8049. [Google Scholar] [CrossRef]
- Liao, F.; Lo, T.W.B.; Tsang, S.C.E. Recent developments in palladium-based bimetallic catalysts. ChemCatChem 2015, 7, 1998–2014. [Google Scholar] [CrossRef]
- Rosseler, O.; Ulhaq-Bouillet, C.; Bonnefont, A.; Pronkin, S.; Savinova, E.; Louvet, A.; Keller, V.; Keller, N. Structural and electronic effects in bimetallic PdPt nanoparticles on TiO2 for improved photocatalytic oxidation of CO in the presence of humidity. Appl. Catal. B 2015, 166, 381–392. [Google Scholar] [CrossRef]
- Kim, S.; Im, M.; Cho, E.; Jang, H.; Jang, S.Y.; Kim, D.W.; Kim, K.W.; Heo, I.; Kim, Y.J.; Lee, J.H. Effects of thermal aging on the electronic and structural properties of Pt-Pd and toluene oxidation activity. Sci. Total. Environ. 2022, 847, 157482. [Google Scholar] [CrossRef] [PubMed]
- Taheri, A.; Hamzehlouyan, T. Effect of hydrothermal aging on CO and C3H6 oxidation over a Pt-Pd-based wiremesh catalyst as a motorcycle aftertreatment device. Appl. Catal. A 2023, 656, 119128. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Ning, P.; Tang, T.; Hu, J.; Su, W. One-pot synthesis of mesoporous Al2O3-supported Pt-Pd catalysts for toluene combustion. Catal. Commun. 2018, 115, 26–30. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Deng, J.; Xie, S.; Zhao, X.; Zhang, Y.; Zhang, K.; Arandiyan, H.; Guo, G.; Dai, H. Enhanced catalytic performance for methane combustion of 3Dom CoFe2O4 by co-loading MnO and Pd-Pt alloy nanoparticles. Appl. Surf. Sci. 2017, 403, 590–600. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Y.; Deng, J.; Jing, L.; Zhang, X.; Zhang, K.; Han, Z.; Jiang, X.; Dai, H. Intermetallic compound PtMny-derived Pt-MnOx supported on mesoporous CeO2: Highly efficient catalysts for the combustion of toluene. Appl. Catal. A 2020, 595, 117509. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Zheng, P.; Dang, F.; Han, J. Carbon deposits during catalytic combustion of toluene on Pd-Pt-based catalysts. Catal. Sci. Technol. 2020, 1, 2452–2461. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, L.; Liu, F.; Dou, Y.; Liu, S.; Fan, Y.; Cheng, G.; Song, W.; Zhou, J. Core-shell structure Ag@Pd nanoparticles supported on layered MnO2 substrate as toluene oxidation catalyst. J. Nanoparticle Res. 2019, 21, 28. [Google Scholar] [CrossRef]
- Pei, W.; Liu, Y.; Deng, J.; Zhang, K.; Hou, Z.; Zhao, X.; Dai, H. Partially embedding Pt nanoparticles in the skeleton of 3DOM Mn2O3: An effective strategy for enhancing catalytic stability in toluene combustion. Appl. Catal. B 2019, 256, 117814. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, W.; Ning, P.; Liu, X.; Wang, H.; Hu, J. Catalytic performance and mechanistic study of toluene combustion over the Pt-Pd-HMS catalyst. Chem. Eng. Sci. 2019, 205, 230–237. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, S.; Wang, H.; Chen, R.; Sun, P.; Chen, T. Platinum nanoparticles supported on hierarchically porous aluminosilicate nanospheres for low-temperature catalytic combustion of volatile organic compounds. ACS Appl. Nano Mater. 2020, 3, 8472–8482. [Google Scholar] [CrossRef]
- Jafarihaghighi, F.; Payan, A.; Soltan, J. Unraveling the role of support in the catalytic performance of α-MnO2 for enhanced O3-assisted VOC decomposition: Influence of operational parameters, catalytic synergies, and stability. Colloid Surf. A 2025, 718, 136922. [Google Scholar] [CrossRef]
- Wu, S.; Yuan, C.; Huang, Z.; Xu, H.; Shen, W. Engineering Mn-O strength in manganese oxide catalyst to enhance propane catalytic oxidation. Chem. Eng. J. 2024, 479, 147928. [Google Scholar] [CrossRef]
- Xing, X.; Wang, Y.; Hao, M.; Li, Z.; Liu, D.; Yan, K. Simultaneous catalytic oxidation of benzene and toluene over Pd-CeZrOx catalysts. Atmosphere 2024, 15, 1301. [Google Scholar] [CrossRef]
- Lu, Y.; Deng, H.; Zhang, X.; Ding, J.; Bai, S.; Wang, L.; He, H. Tuning Pt-TiO2 interactions to switch inhibition to synergy in toluene–acetone mixture combustion. Environ. Sci. Technol. 2025, 59, 11875–11884. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, C.; Yi, C. Construction of CeO2/δ-MnO2 heterojunction for photothermal catalysis of toluene. Appl. Surf. Sci. 2025, 686, 162009. [Google Scholar] [CrossRef]
- He, J.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Pt-Pd bimetallic nanoparticles anchored on uniform mesoporous MnO2 sphere as an advanced nanocatalyst for highly efficient toluene oxidation. Green Energy Environ. 2022, 7, 1349–1360. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, D.; Liang, C.; Shen, B. Water poisoning and resistance in catalytic oxidation of VOCs from industrial flue gas. Fuel Process. Technol. 2025, 273, 108231. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, K.; Zhang, X.; Shen, B.; Smith, R.L.; Guo, H. Siloxane-modified mnox catalyst for oxidation of coal-related o-xylene in presence of water vapor. J. Hazard. Mater. 2022, 436, 129109. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, X.; Zhu, T.; Zhu, T. Catalytic oxidation of ethyl acetate over silver catalysts supported on CeO2 with different morphologies. Mater. Chem. Phys. 2019, 229, 32–38. [Google Scholar] [CrossRef]
- Yang, K.; Liu, Y.; Deng, J.; Zhao, X.; Yang, J.; Han, Z.; Hou, Z.; Dai, H. Three-dimensionally ordered mesoporous iron oxide-supported single-atom platinum: Highly active catalysts for benzene combustion. Appl. Catal. B 2019, 244, 650–659. [Google Scholar] [CrossRef]
- Xie, S.; Dai, H.; Deng, J.; Yang, H.; Han, W.; Arandiyan, H.; Guo, G. Preparation and high catalytic performance of Au/3DOM Mn2O3 for the oxidation of carbon monoxide and toluene. J. Hazard. Mater. 2014, 279, 392–401. [Google Scholar] [CrossRef]
- Guo, M.; Zhao, P.; Liu, Q.; Liu, C.; Han, J.; Ji, N.; Song, C.; Ma, D.; Lu, X.; Liang, X.; et al. Improved low-temperature activity and H2O resistance of Fe-doped Mn-Eu catalysts for no removal by NH3-SCR. ChemCatChem 2019, 11, 4954–4965. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Wang, X.; Lu, J.; Feng, J.; Zhao, S.; Qiu, W.; Huang, Z.; Lin, H. Directional induction of hydrogen spillover enhancing H2O resistance of Ca-Ni-based dual-function materials for integrated CO2 capture and in-situ methanation. Chem. Eng. J. 2025, 505, 159691. [Google Scholar] [CrossRef]
- Feng, C.; Liu, X.; Zhu, T.; Hu, Y.; Tian, M. Catalytic oxidation of Co over Pt/TiO2 with low Pt loading: The effect of H2O and SO2. Appl. Catal. A 2021, 622, 118218. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Cao, Y.; Yao, X.; Ge, C.; Gao, F.; Deng, Y.; Tang, C.; Dong, L. Getting insight into the influence of SO2 on TiO2/CeO2 for the selective catalytic reduction of NO by NH3. Appl. Catal. B 2015, 165, 589–598. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, Y.; Zheng, J.; Zhang, D.; Chen, W.; Yuan, W.; Chen, X.; Liu, R.; Chen, B.; Li, L.; et al. Diverse effects of SO2-induced Pt-O-SO3 on the catalytic oxidation of C3H6 and C3H8. Environ. Sci. Technol. 2024, 58, 18020–18032. [Google Scholar] [CrossRef] [PubMed]
- Uz Zaman, M.W.; Phule, A.D.; Senthamaraikannan, T.G.; Kim, S.Y.; Elkaee, S.; Yang, J.H.; Lim, D. Design of bifunctional nitrogen-doped biochar with adsorption and catalytic oxidation capabilities for enhanced toluene emission control. Environ. Res. 2025, 269, 120867. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, S.; Mo, S.; Zhong, J.; Chen, D.; Ren, Q.; Fu, M.; Chen, P.; Ye, D. Enhancement of catalytic toluene combustion over Pt–Co3O4 catalyst through in-situ metal-organic template conversion. Chemosphere 2021, 262, 127738. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, L.; Dai, Z.; Jiang, W.; Ma, S.; Yao, L.; Chen, Y.; Zhou, Q.; Zheng, J. Zeolite-induced defect engineering for synthesis of cubtc-derived novel carbon-zeolite bifunctional support catalyst for multicomponent VOCs catalytic oxidation removal. Chem. Eng. J. 2024, 500, 156830. [Google Scholar] [CrossRef]
- Yang, X.; Yu, X.; Jing, M.; Song, W.; Liu, J.; Ge, M. Defective mnxzr1-XO2 solid solution for the catalytic oxidation of toluene: Insights into the oxygen vacancy contribution. ACS Appl. Mater. Interfaces 2019, 11, 730–739. [Google Scholar] [CrossRef]
- Lai, Y.T.; Chen, T.C.; Lan, Y.K.; Chen, B.S.; You, J.H.; Yang, C.M.; Lai, N.C.; Wu, J.H.; Chen, C.S. Pt/SBA-15 as a highly efficient catalyst for catalytic toluene oxidation. ACS Catal. 2014, 4, 3824–3836. [Google Scholar] [CrossRef]
- Huo, Y.; Liu, K.; Liu, J.; He, H. Effects of SO2 on standard and fast SCR over CeWo: A quantitative study of the reaction pathway and active sites. Appl. Catal. B 2022, 301, 120784. [Google Scholar] [CrossRef]
- Kwon, D.W.; Kim, D.H.; Lee, S.; Kim, J.; Ha, H.P. A dual catalytic strategy by the nature of the functionalization effect as well as active species on vanadium-based catalyst for enhanced low temperature SCR. Appl. Catal. B 2021, 289, 120032. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Li, J.; Zhao, S.; Wang, J.; Chu, C.; Li, C. Promotional mechanisms of activity and SO2 tolerance of Co- or Ni-doped MnOx-CeO2 catalysts for SCR of NOx with NH3 at low temperature. Chem. Eng. J. 2017, 317, 20–31. [Google Scholar] [CrossRef]
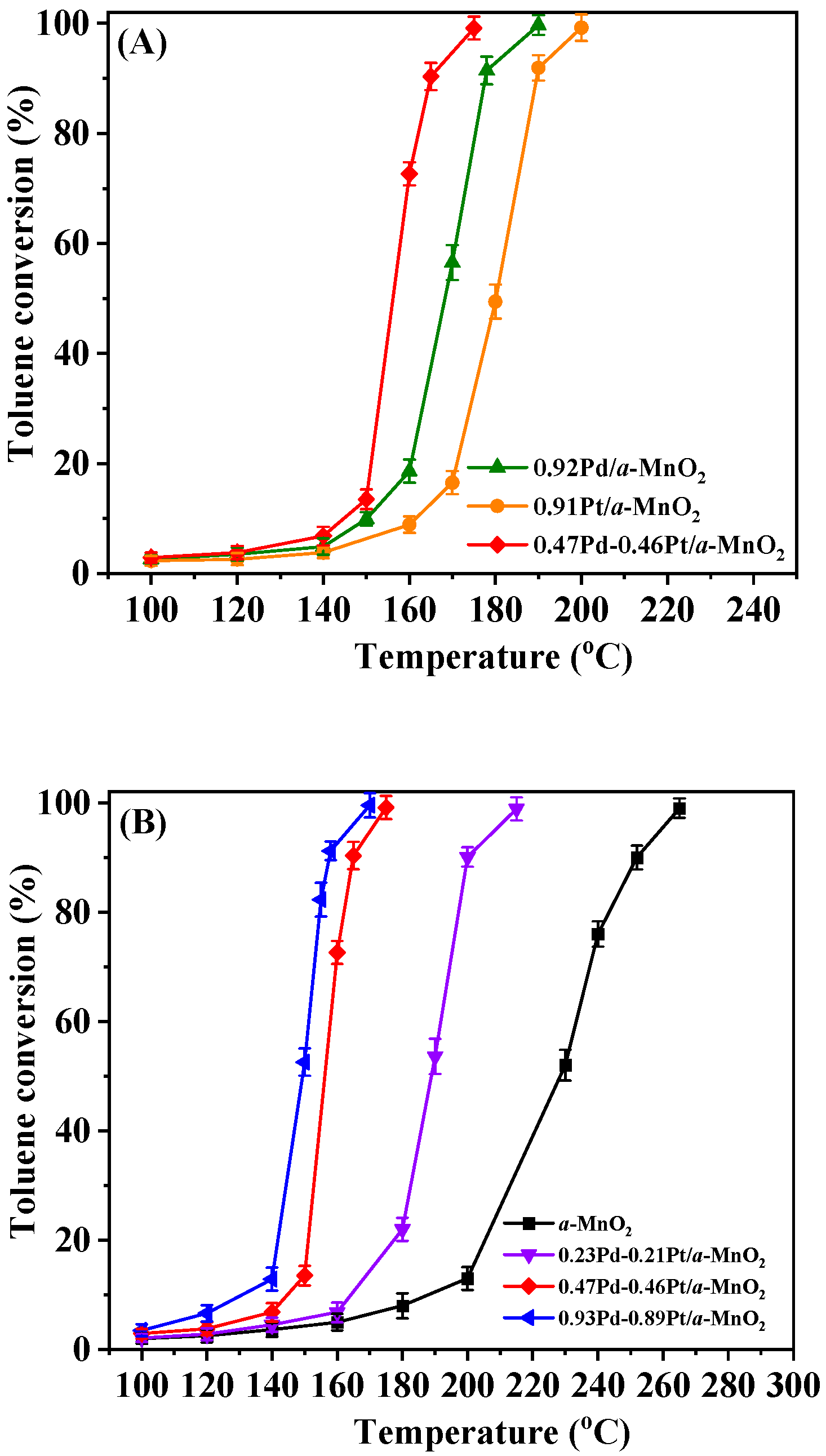


| Sample | Toluene Oxidation Activity | Ea (kJ/mol) | rcat (mol/(g s)) | |
|---|---|---|---|---|
| T50% (°C) | T90% (°C) | |||
| α-MnO2 | 222 | 252 | 54.3 | 3.54 × 10−5 |
| 0.92Pd/α-MnO2 | 177 | 192 | 41.5 | 6.13 × 10−5 |
| 0.91Pt/α-MnO2 | 161 | 178 | 37.3 | 1.31 × 10−4 |
| 0.23Pd-0.21Pt/α-MnO2 | 186 | 201 | 46.9 | 4.77 × 10−5 |
| 0.47Pd-0.46Pt/α-MnO2 | 155 | 165 | 33.6 | 4.98 × 10−4 |
| 0.93Pd-0.89Pt/α-MnO2 | 148 | 156 | 31.9 | 6.34 × 10−4 |
| Sample | Surface Area a (m2/g) | Pore Volume a (cm3/g) | Average Pore Diameter b (nm) | D c (nm) | Noble Metal Particle Size d (nm) | Actual Pd Content e (wt%) | Actual Pt Content e (wt%) |
|---|---|---|---|---|---|---|---|
| α-MnO2 | 39 ± 1.5 | 0.087 ± 0.01 | 9.0 ± 1.4 | 28 ± 1.6 | − | − | − |
| 0.92Pd/α-MnO2 | 42 ± 1.5 | 0.071 ± 0.01 | 7.0 ± 1.4 | 30 ± 1.6 | 3.4 ± 1.0 | 0.92 ± 0.01 | − |
| 0.91Pt/α-MnO2 | 42 ± 1.5 | 0.078 ± 0.01 | 7.3 ± 1.4 | 28 ± 1.6 | 3.2 ± 1.0 | − | 0.91 ± 0.01 |
| 0.23Pd-0.21Pt/ α-MnO2 | 41 ± 1.5 | 0.071 ± 0.01 | 7.2 ± 1.4 | 29 ± 1.6 | 2.9 ± 0.9 | 0.23 ± 0.01 | 0.21 ± 0.01 |
| 0.47Pd-0.46Pt/ α-MnO2 | 42 ± 1.5 | 0.069 ± 0.01 | 7.1 ± 1.4 | 30 ± 1.6 | 2.6 ± 1.1 | 0.47 ± 0.01 | 0.46 ± 0.01 |
| 0.93Pd-0.89Pt/ α-MnO2 | 43 ± 1.5 | 0.079 ± 0.01 | 6.8 ± 1.4 | 32 ± 1.6 | 2.5 ± 0.6 | 0.93 ± 0.01 | 0.89 ± 0.01 |
| Sample | Surface Element Composition (Molar Ratio) | H2 Consumption (mmol/g) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mn3+/Mn4+ | Pt2+/Pt0 | Pd2+/Pd0 | Oads/Olatt | Peak α | Peak β | Peak γ | Total | |
| α-MnO2 | 0.56 | − | − | 0.40 | 12.3 | 13.5 | 12.8 | 38.6 |
| 0.92Pd/α-MnO2 | 0.59 | − | 0.59 | 0.46 | 19.6 | 11.5 | 9.3 | 40.4 |
| 0.91Pt/α-MnO2 | 0.61 | 0.58 | − | 0.50 | 22.3 | 11.4 | 9.4 | 43.1 |
| 0.23Pd-0.21Pt/α-MnO2 | 0.58 | 0.54 | 0.54 | 0.43 | 19.1 | 13.2 | 8.2 | 40.5 |
| 0.47Pd-0.46Pt/α-MnO2 | 0.66 | 0.59 | 0.61 | 0.51 | 22.6 | 13.1 | 8.8 | 44.5 |
| 0.93Pd-0.89Pt/α-MnO2 | 0.69 | 0.62 | 0.65 | 0.57 | 23.3 | 13.6 | 10.3 | 47.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, N.; Wang, W.; Zheng, X.; Liu, H.; Zhang, J.; Ye, Q.; Dai, H. High-Performance Pd-Pt/α-MnO2 Catalysts for the Oxidation of Toluene. Catalysts 2025, 15, 746. https://doi.org/10.3390/catal15080746
Dong N, Wang W, Zheng X, Liu H, Zhang J, Ye Q, Dai H. High-Performance Pd-Pt/α-MnO2 Catalysts for the Oxidation of Toluene. Catalysts. 2025; 15(8):746. https://doi.org/10.3390/catal15080746
Chicago/Turabian StyleDong, Ning, Wenjin Wang, Xuelong Zheng, Huan Liu, Jingjing Zhang, Qing Ye, and Hongxing Dai. 2025. "High-Performance Pd-Pt/α-MnO2 Catalysts for the Oxidation of Toluene" Catalysts 15, no. 8: 746. https://doi.org/10.3390/catal15080746
APA StyleDong, N., Wang, W., Zheng, X., Liu, H., Zhang, J., Ye, Q., & Dai, H. (2025). High-Performance Pd-Pt/α-MnO2 Catalysts for the Oxidation of Toluene. Catalysts, 15(8), 746. https://doi.org/10.3390/catal15080746








