Acetate-Assisted Preparation of High-Cu-Content Cu-SSZ-13 with a Low Si/Al Ratio: Distinguishing Cu Species and Origins
Abstract
1. Introduction
2. Results and Discussion
2.1. Basic Characterizations
2.2. NH3-SCR Performance
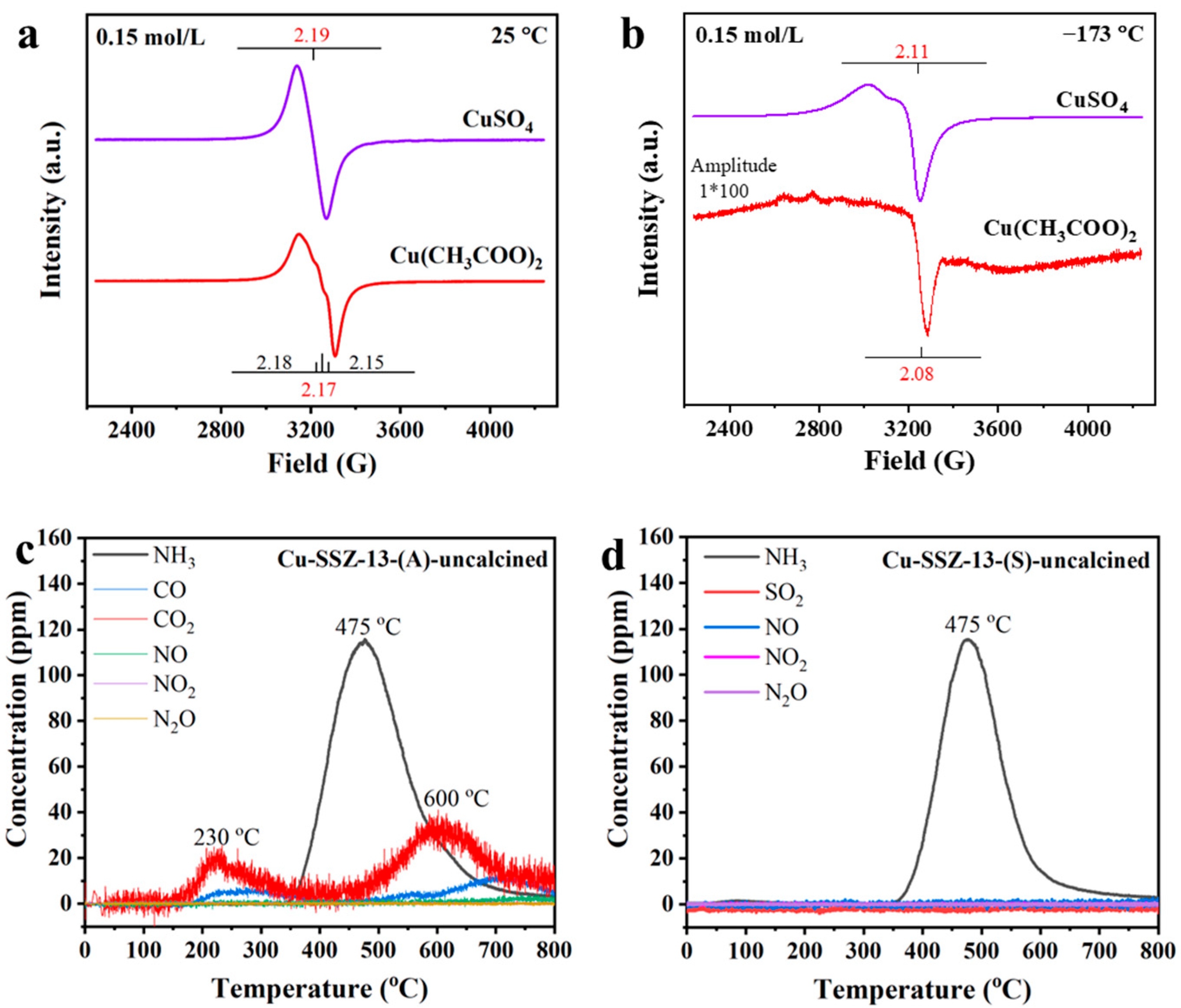
2.3. Characterizations of the Cu Species
2.4. The Redox Properties and Acidity of Cu-SSZ-13
2.5. Framework Si and Al Distributions in Cu-SSZ-13
2.6. The Loading Mechanism of the Cu Species
3. Materials and Methods
3.1. Preparation
3.2. NH3-SCR Activity Tests
3.3. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NOx | nitrogen oxides |
| SCR | selective catalytic reduction |
| ccSCR | close-coupled SCR |
| XRD | X-ray powder diffraction |
| ICP-OES | inductively coupled plasma-optical emission spectrometer |
| SEM | scanning electron microscope |
| XPS | X-ray photoelectron spectroscopy |
| UV-Vis-DRS | ultraviolet-visible diffuse reflectance spectra |
| XAFS | X-ray absorption fine structure |
| EPR | electron paramagnetic resonance |
| MAS NMR | magnetic angle-spinning nuclear magnetic resonance |
| Vis-NIR | visible-near infrared spectrum |
| H2-TPR | H2 temperature-programmed reduction |
| NH3-TPD | NH3 temperature-programmed desorption |
References
- Zhan, J.; Zheng, F.; Xie, R.; Liu, J.; Chu, B.; Ma, J.; Xie, D.; Meng, X.; Huang, Q.; He, H.; et al. The role of NOx in co-occurrence of O3 and PM2.5 pollution driven by wintertime east Asian monsoon in Hainan. J. Environ. Manag. 2023, 345, 118645. [Google Scholar] [CrossRef]
- Chu, B.; Ding, Y.; Gao, X.; Li, J.; Zhu, T.; Yu, Y.; He, H. Coordinated control of fine-particle and ozone pollution by the substantial reduction of nitrogen oxides. Engineering 2022, 15, 13–16. [Google Scholar] [CrossRef]
- Anenberg, S.C.; Miller, J.; Minjares, R.; Du, L.; Henze, D.K.; Lacey, F.; Malley, C.S.; Emberson, L.; Franco, V.; Klimont, Z.; et al. Impacts and mitigation of excess diesel-related NOx emissions in 11 major vehicle markets. Nature 2017, 545, 467–471. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, H.; Li, Y.; Wang, P.; Zhan, S. Selective catalytic reduction of NOx with NH3 over copper-based catalysts: Recent advances and future prospects. EES Catal. 2024, 2, 231–252. [Google Scholar] [CrossRef]
- Paolucci, C.; Parekh, A.A.; Khurana, I.; Di Iorio, J.R.; Li, H.; Albarracin Caballero, J.D.; Shih, A.J.; Anggara, T.; Delgass, W.N.; Miller, J.T.; et al. Catalysis in a cage: Condition-dependent speciation and dynamics of exchanged Cu cations in SSZ-13 zeolites. J. Am. Chem. Soc. 2016, 138, 6028–6048. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Tonkyn, R.G.; Kim, D.H.; Szanyi, J.; Peden, C.H.F. Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J. Catal. 2010, 275, 187–190. [Google Scholar] [CrossRef]
- Shan, Y.; Du, J.; Zhang, Y.; Shan, W.; Shi, X.; Yu, Y.; Zhang, R.; Meng, X.; Xiao, F.-S.; He, H. Selective catalytic reduction of NOx with NH3: Opportunities and challenges of Cu-based small-pore zeolites. Natl. Sci. Rev. 2021, 8, nwab010. [Google Scholar] [CrossRef]
- Guo, A.; Liu, H.; Li, Y.; Luo, Y.; Ye, D.; Jiang, J.; Chen, P. Recent progress in novel zeolite catalysts for selective catalytic reduction of nitrogen oxides. Catal. Today 2023, 422, 114212. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Wang, P.; Mansoor, M.; Zhang, J.; Peng, D.; Han, L.; Zhang, D. Challenges and perspectives of environmental catalysis for NOx reduction. JACS Au 2024, 4, 2767–2791. [Google Scholar] [CrossRef]
- Yuan, Y.; Guan, B.; Chen, J.; Zhuang, Z.; Zheng, C.; Zhou, J.; Su, T.; Zhu, C.; Guo, J.; Dang, H.; et al. Research status and outlook of molecular sieve NH3-SCR catalysts. Mol. Catal. 2024, 554, 113846. [Google Scholar] [CrossRef]
- Liu, S.; Wang, B.; Guo, Z.; Wang, B.; Zhang, Z.; Ma, X.; Chang, C.-T.; Wang, P.; He, X.; Sun, X.; et al. Experimental investigation of urea injection strategy for close-coupled SCR aftertreatment system to meet ultra-low NO emission regulation. Appl. Therm. Eng. 2022, 205, 117994. [Google Scholar]
- Li, Y.; Liao, J.; Xu, H.; Wu, Y.; Cai, Z.; Hu, J. Comprehensive experimental analysis of a diesel engine with an electrically heated catalyst and close-coupled SCR during cold/hot WHTC. Appl. Therm. Eng. 2025, 262, 125277. [Google Scholar] [CrossRef]
- Paolucci, C.; Khurana, I.; Parekh, A.; Li, S.; Shih, A.; Li, H.; Iorio, J.; Albarracin-Caballero, J.; Yezerets, A.; Miller, J.; et al. Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 2017, 357, 898–903. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, Y.; Wang, Y.; Rappé, K.G.; Washton, N.M.; Wang, Y.; Walter, E.D.; Gao, F. Rate controlling in low-temperature standard NH3-SCR: Implications from operando EPR spectroscopy and reaction kinetics. J. Am. Chem. Soc. 2022, 144, 9734–9746. [Google Scholar] [CrossRef]
- Wei, L.; Chen, Y.-R.; Marino, S.; Gu, Y.; Epling, W.S. Kinetic modeling of NH3-selective catalytic reduction (SCR) over a mildly hydrothermally aged commercial Cu-SSZ-13 catalyst. Chem. Eng. J. 2023, 467, 143318. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Walter, E.D.; Washton, N.M.; Mei, D.; Kovarik, L.; Engelhard, M.H.; Prodinger, S.; Wang, Y.; Peden, C.H.F.; et al. Toward rational design of Cu/SSZ-13 selective catalytic reduction catalysts: Implications from atomic-level understanding of hydrothermal stability. ACS Catal. 2017, 7, 8214–8227. [Google Scholar] [CrossRef]
- Wang, Y.; Han, J.; Chen, M.; Lv, W.; Meng, P.; Gao, W.; Meng, X.; Fan, W.; Xu, J.; Yan, W.; et al. Low-silica Cu-CHA zeolite enriched with Al pairs transcribed from silicoaluminophosphate seed: Synthesis and ammonia selective catalytic reduction performance. Angew. Chem. Int. Ed. 2023, 62, e202306174. [Google Scholar] [CrossRef]
- Li, P.; Xin, Y.; Zhang, H.; Yang, F.; Tang, A.; Han, D.; Jia, J.; Wang, J.; Li, Z.; Zhang, Z. Recent progress in performance optimization of Cu-SSZ-13 catalyst for selective catalytic reduction of NOx. Front. Chem. 2022, 10, 1033255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, W.; Wang, Q.; Ning, F.; He, Q.; Li, G.; Liu, C.; Li, Z.; Peng, H. Effects of Cu species in Cu-SSZ-13 zeolites on the performance of NOx reduction reactions. Sep. Purif. Technol. 2024, 346, 127544. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Qin, M.; Zhao, Z.; Fan, X.; Li, Q. Insight into the effects of Cu2+ ions and CuO species in Cu-SSZ-13 catalysts for selective catalytic reduction of NO by NH3. J. Colloid. Interface Sci. 2022, 622, 1–10. [Google Scholar] [CrossRef]
- Liu, B.; Lv, N.; Wang, C.; Zhang, H.; Yue, Y.; Xu, J.; Bi, X.; Bao, X. Redistributing Cu species in Cu-SSZ-13 zeolite as NH3-SCR catalyst via a simple ion-exchange. Chin. J. Chem. Eng. 2022, 41, 329–341. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, Y.; Zeng, S.; Zhu, L.; Sun, Q.; Zhang, H.; Yang, C.; Meng, X.; Yang, X.; Xiao, F.-S. Design and synthesis of a catalytically active Cu-SSZ-13 zeolite from a copper-amine complex template. Chin. J. Catal. 2012, 33, 92–105. [Google Scholar] [CrossRef]
- Shan, Y.; Du, J.; Yu, Y.; Shan, W.; Shi, X.; He, H. Precise control of post-treatment significantly increases hydrothermal stability of in-situ synthesized Cu-zeolites for NH3-SCR reaction. Appl. Catal. B Environ. 2020, 266, 118655. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, J.; Meng, Y.; Pang, L.; Guo, Y.; Luo, Z.; Fang, Y.; Dong, Y.; Cai, W.; Li, T. Insight into solid-state ion-exchanged Cu-based zeolite (SSZ-13, SAPO-18, and SAPO-34) catalysts for the NH3-SCR reaction: The promoting role of NH4-form zeolite substrates. Appl. Surf. Sci. 2022, 571, 151328. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, H.; Zhang, T.; Li, J.; Chen, J.; Peng, Y.; Li, J. Revealing the synergistic deactivation mechanism of hydrothermal aging and SO2 poisoning on Cu/SSZ-13 under SCR condition. Environ. Sci. Technol. 2021, 56, 1917–1926. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, W.; Ahn, S.H.; Wang, Y.; Walter, E.D.; Chen, Y.; Derewinski, M.A.; Washton, N.M.; Rappé, K.G.; Wang, Y.; et al. Interplay between copper redox and transfer and support acidity and topology in low temperature NH3-SCR. Nat. Commun. 2023, 14, 2633. [Google Scholar] [CrossRef]
- Groen, J.; Peffer, L.; Perez-Ramırez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Fu, G.; Yang, R.; Liang, Y.; Yi, X.; Li, R.; Yan, N.; Zheng, A.; Yu, L.; Yang, X.; Jiang, J. Enhanced hydrothermal stability of Cu/SSZ-39 with increasing Cu contents, and the mechanism of selective catalytic reduction of NO. Microporous Mesoporous Mater. 2021, 320, 111060. [Google Scholar] [CrossRef]
- Singh, S.; Janssens, T.V.W.; Grönbeck, H. Mechanism for Cu-enhanced hydrothermal stability of Cu–CHA for NH3-SCR. Catal. Sci. Technol. 2024, 14, 3407–3415. [Google Scholar] [CrossRef]
- Chen, M.; Li, J.; Xue, W.; Wang, S.; Han, J.; Wei, Y.; Mei, D.; Li, Y.; Yu, J. Unveiling secondary-ion-promoted catalytic properties of Cu-SSZ-13 zeolites for selective catalytic reduction of NOx. J. Am. Chem. Soc. 2022, 144, 12816–12824. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhang, R.; Jiao, W.; Jia, J.; Wang, H.; Hou, X.; Lv, W.; Lv, B. Synthesis of Cu-SSZ-13 with different Si/Al ratios by zeolite Y conversion and its NH3-SCR activity and hydrothermal stability. Appl. Catal. A 2024, 683, 119842. [Google Scholar] [CrossRef]
- Shan, Y.; He, G.; Du, J.; Sun, Y.; Liu, Z.; Fu, Y.; Liu, F.; Shi, X.; Yu, Y.; He, H. Strikingly distinctive NH3-SCR behavior over Cu-SSZ-13 in the presence of NO2. Nat. Commun. 2022, 13, 4606. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Karp, E.M.; Luo, J.; Tonkyn, R.G.; Kwak, J.H.; Szanyi, J.; Peden, C.H.F. Structure–activity relationships in NH3-SCR over Cu-SSZ-13 as probed by reaction kinetics and EPR studies. J. Catal. 2013, 300, 20–29. [Google Scholar] [CrossRef]
- Shan, Y.; Shan, W.; Shi, X.; Du, J.; Yu, Y.; He, H. A comparative study of the activity and hydrothermal stability of Al-rich Cu-SSZ-39 and Cu-SSZ-13. App. Catal. B Environ. 2020, 264, 118511. [Google Scholar] [CrossRef]
- Wu, Y.; Andana, T.; Wang, Y.; Chen, Y.; Walter, E.D.; Engelhard, M.H.; Rappé, K.G.; Wang, Y.; Gao, F.; Menon, U.; et al. A comparative study between real-world and laboratory accelerated aging of Cu/SSZ-13 SCR catalysts. App. Catal. B Environ. 2022, 318, 121807. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Li, J.; Groden, K.; McEwen, J.-S.; Walter, E.D.; Chen, Y.; Wang, Y.; Gao, F. Probing active-site relocation in Cu/SSZ-13 SCR catalysts during hydrothermal aging by in situ EPR spectroscopy, kinetics studies, and DFT calculations. ACS Catal. 2020, 10, 9410–9419. [Google Scholar] [CrossRef]
- Cha, Y.H.; Lee, K.B. Examining the impact of different anions in Cu precursors on sulfur adsorption through zeolites with Cu ion-exchange. Chem. Eng. J. 2023, 468, 143461. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Wen, Z.; Zhao, J.; Wei, X.; Liao, J.; Chang, L.; Xie, K. Insight into the relationship between effective active sites and ultra-deep adsorption desulfurization performance of CuCeY with different Cu precursors. Fuel Process. Technol. 2023, 250, 107930. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Dong, M.; Ding, Z.; Wang, Q.; Ning, F.; He, Q.; Chen, Y.; Zhao, Z.; Li, Z.; et al. Low-silica chabazite zeolites for NOx reduction: Tailoring the active center of Cu2+-2Z and [Cu(OH)]+-Z through the regulation of CHA-cages opening. Sep. Purif. Technol. 2025, 354, 129201. [Google Scholar] [CrossRef]
- Han, D.; Xin, Y.; Jia, J.; Wang, J.; Zhang, Z. Mild thermal treatment-driven transformation of CuOx assisted with the rearrangement of Al sites in Cu-SSZ-13. Appl. Catal. O Open 2025, 205, 207054. [Google Scholar] [CrossRef]
- Wang, M.; Peng, Z.; Zhang, C.; Liu, M.; Han, L.; Hou, Y.; Huang, Z.; Wang, J.; Bao, W.; Chang, L. Effect of copper precursors on the activity and hydrothermal stability of CuII−SSZ−13 NH3−SCR catalysts. Catalysts 2019, 9, 781. [Google Scholar] [CrossRef]
- Shan, Y.; Sun, Y.; Du, J.; Zhang, Y.; Shi, X.; Yu, Y.; Shan, W.; He, H. Hydrothermal aging alleviates the inhibition effects of NO2 on Cu-SSZ-13 for NH3-SCR. Appl. Catal. B Environ. 2020, 275, 119105. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Gao, L.; Tian, X.; Hao, J.; Ning, P.; Chen, J.; Zhang, Q. Pr-functionalized Cu/SAPO-34 with superior hydrothermal stability for NH3-SCR: The copper species and framework stabilization effect. Fuel 2022, 327, 125229. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Z.; Liu, N.; Dai, C.; Zhang, J.; Chen, B. Understanding Zn functions on hydrothermal stability in a one-pot-synthesized Cu&Zn-SSZ-13 catalyst for NH3 selective catalytic reduction. ACS Catal. 2020, 10, 6197–6212. [Google Scholar]
- Zhou, C.; Zhang, H.; Yang, L.; Wu, D.; He, S.; Yang, H.; Xiong, H. Methane-selective oxidation to methanol and ammonia selective catalytic reduction of NOx over monolithic Cu/SSZ-13 catalysts: Are hydrothermal stability and active sites same? Fuel 2022, 309, 122178. [Google Scholar] [CrossRef]
- Deka, D.J.; Daya, R.; Ladshaw, A.; Trandal, D.; Joshi, S.Y.; Partridge, W.P. Assessing impact of real-world aging on Cu-redox half cycles of a Cu-SSZ-13 SCR catalyst via transient response measurements and kinetic modeling. App. Catal. B Environ. 2022, 309, 121233. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, J.; Yang, L.; Wu, X.; Gao, Y.; Ran, R.; Weng, D. Flexible Al coordination with H2O explaining the deviation of strong acid amount from the framework Al content in Al-rich SSZ-13. J. Phys. Chem. C 2023, 127, 16598–16606. [Google Scholar] [CrossRef]
- Yang, F.; Xin, Y.; Zhu, X.; Tang, A.; Yu, L.; Han, D.; Jia, J.; Lu, Y.; Zhang, Z. Hard template-assisted trans-crystallization synthesis of hierarchically porous Cu-SSZ-13 with enhanced NH3-SCR performance. Catalysts 2023, 13, 1217. [Google Scholar] [CrossRef]
- Yoshioka, T.; Iyoki, K.; Hotta, Y.; Kamimura, Y.; Yamada, H.; Han, Q.; Kato, T.; Fisher, C.; Liu, Z.; Ohnishi, R.; et al. Dealumination of small-pore zeolites through pore-opening migration process with the aid of pore-filler stabilization. Sci. Adv. 2022, 8, eabo3093. [Google Scholar] [CrossRef] [PubMed]
- Nishitoba, T.; Yoshida, N.; Kondo, J.N.; Yokoi, T. Control of Al distribution in the CHA-Type aluminosilicate zeolites and its impact on the hydrothermal stability and catalytic properties. Ind. Eng. Chem. Res. 2018, 57, 3914–3922. [Google Scholar] [CrossRef]
- Sun, L.; Yang, M.; Cao, L.; Cao, Y.; Xu, S.; Zhu, D.; Tian, P.; Liu, Z. Fabrication of Cu-CHA composites with enhanced NH3-SCR catalytic performances and hydrothermal stabilities. Microporous Mesoporous Mater. 2020, 309, 110585. [Google Scholar] [CrossRef]
- Chen, K.; Gan, Z.; Horstmeier, S.; White, J.L. Distribution of aluminum species in zeolite catalysts: 27Al NMR of framework, partially-coordinated framework, and non-framework moieties. J. Am. Chem. Soc. 2021, 143, 6669–6680. [Google Scholar] [CrossRef]
- Li, D.; Yang, G.; Chen, M.; Pang, L.; Guo, Y.; Yu, J.; Li, T. Na co-cations promoted stability and activity of Pd/SSZ-13 for low-temperature NO adsorption. Appl. Catal. B Environ. 2022, 309, 121266. [Google Scholar] [CrossRef]
- Ding, X.; Liu, C.; Niu, J.; Chen, N.; Xu, S.; Wei, Y.; Liu, Z. Solid-state NMR study of the stability of MOR framework aluminum. Chin. J. Struct. Chem. 2024, 43, 100247. [Google Scholar] [CrossRef]
- Itadani, A.; Kuroda, Y.; Nagao, M. Elucidation of a preparation method for copper ion-exchanged ZSM-5 samples exhibiting extremely efficient N2-adsorption at room temperature: Effect of counter ions in the exchange solution. Microporous Mesoporous Mater. 2004, 70, 119–126. [Google Scholar] [CrossRef]
- DědeČek, J.; Wichterlová, B. Role of hydrated Cu ion complexes and aluminum distribution in the framework on the Cu ion siting in ZSM-5. J. Phys. Chem. B 1997, 101, 10233–10240. [Google Scholar] [CrossRef]
- Yashnik, S.; Ismagilov, Z. Cu-substituted ZSM-5 catalyst: Controlling of DeNO reactivity via ion-exchange mode with copper–ammonia solution. Appl. Catal. B Environ. 2015, 170–171, 241–254. [Google Scholar] [CrossRef]
- Zhang, Y.; Sarofim, A.; Hu, Z.; Flytzani-Stephanopoulos, M. Preparation effects on the activity of Cu-ZSM-5 catalysts for NO decomposition. Catal. Lett. 1995, 31, 75–89. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, S.; Wu, X.; Shi, Y.; Cao, L.; Liu, L.; Ran, R.; Si, Z.; Liu, J.; Weng, D. Low-temperature solid-state ion-exchange method for preparing Cu-SSZ-13 selective catalytic reduction catalyst. ACS Catal. 2019, 9, 6962–6973. [Google Scholar] [CrossRef]

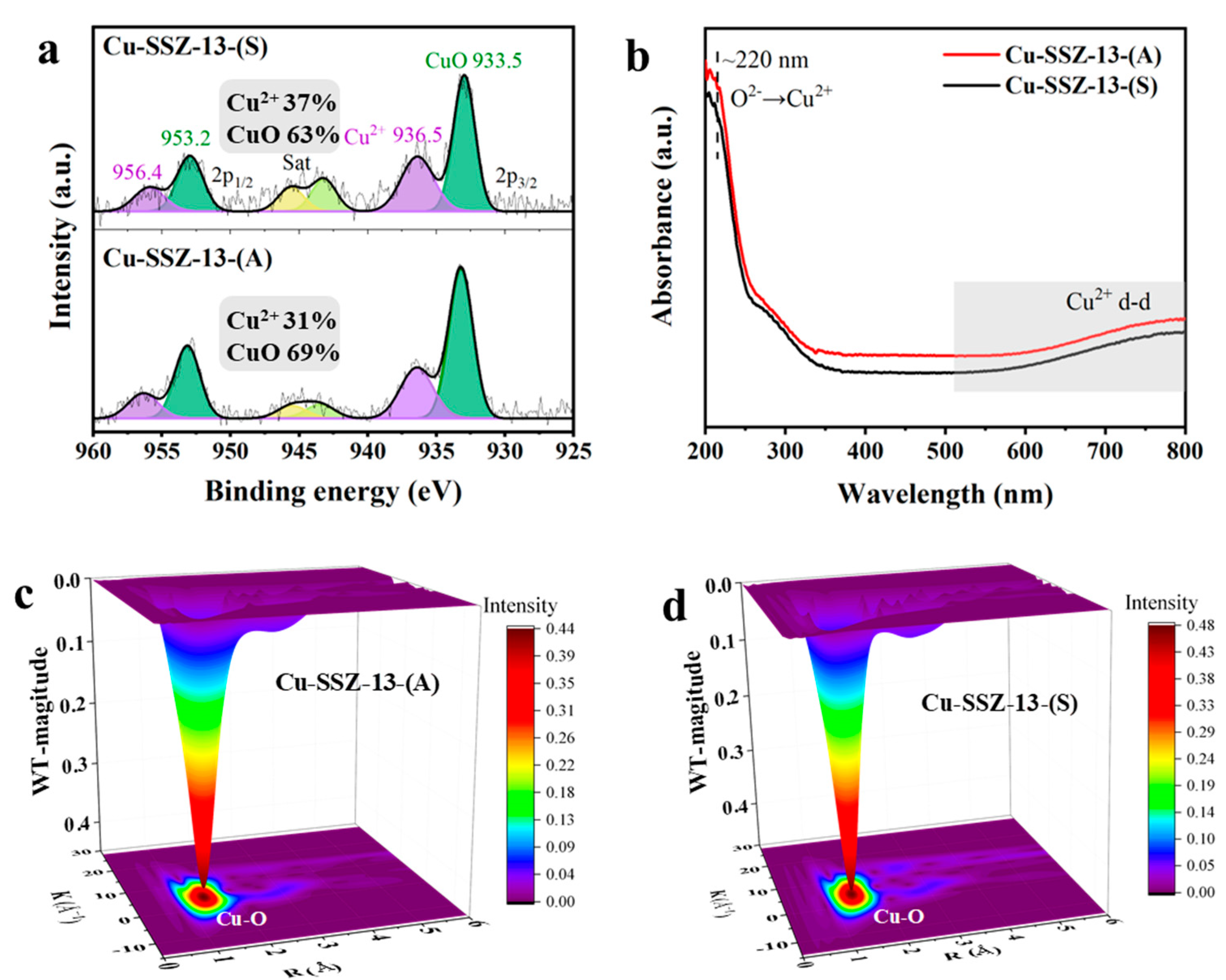
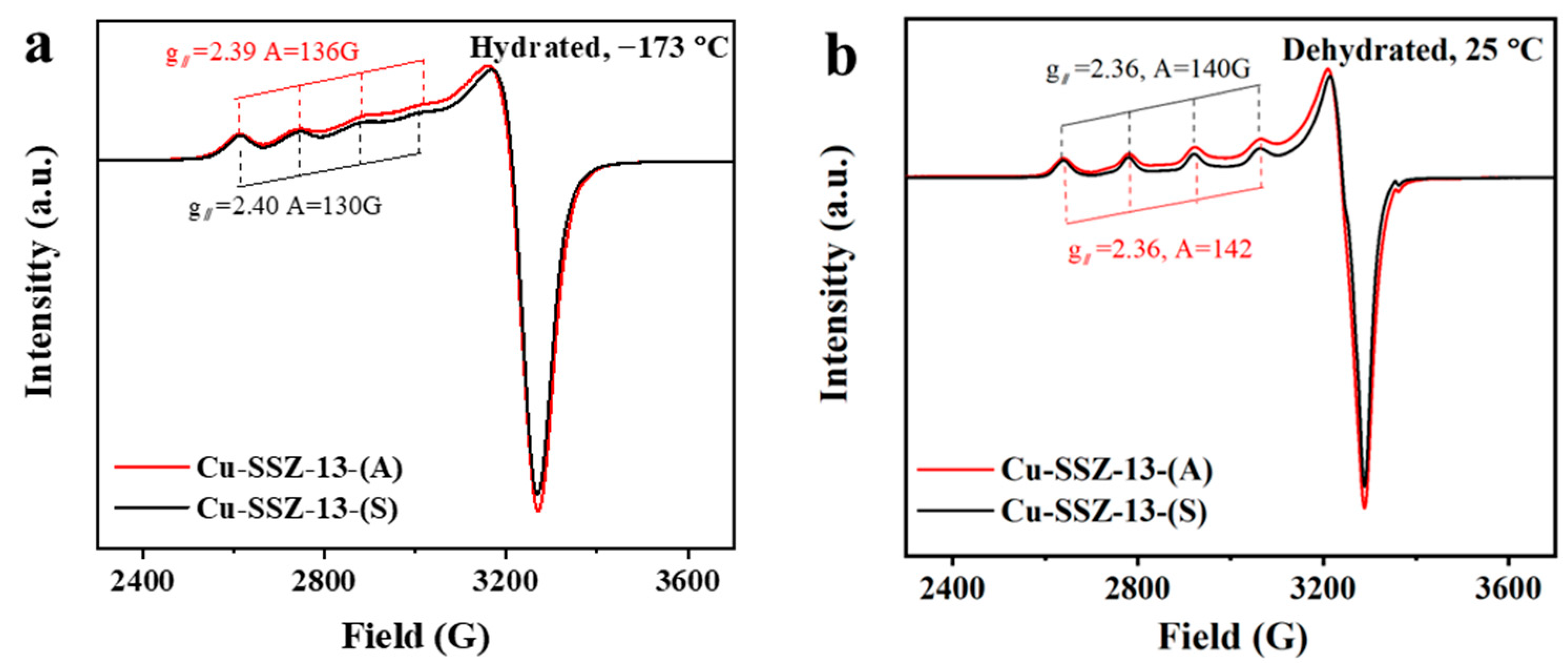
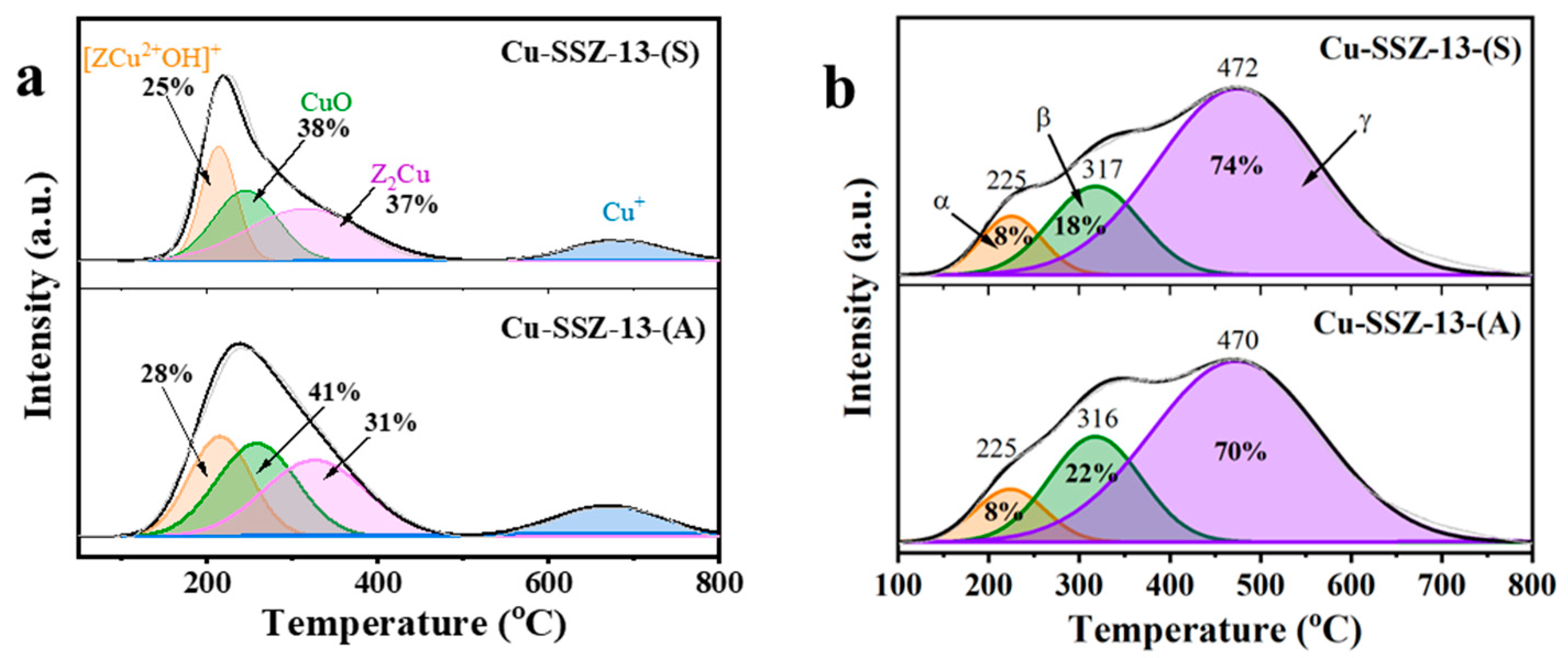
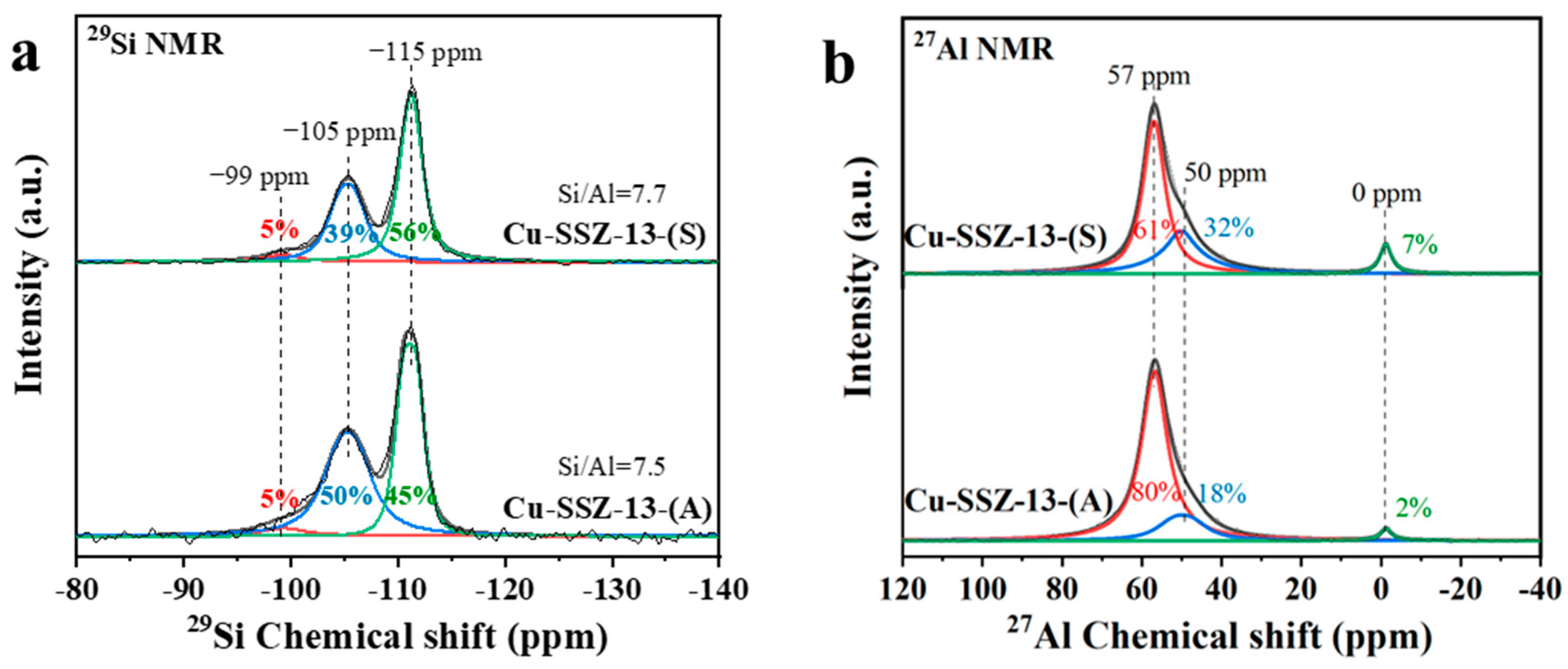
| Samples | Si/Al Ratio | Surface Area c (m2∙g−1) | Total Pore Volume c (cm3∙g−1) | Total Cu a (wt.%) | Total Cu2+ d (wt.%) | Cu (wt.%) | ||
|---|---|---|---|---|---|---|---|---|
| Z2Cu2+ e | Z[Cu2+(OH)]+ | CuOx | ||||||
| Cu-SSZ-13-(A) | 6.3 a/7.5 b | 515.5 | 0.28 | 5.0 | 3.7 | 1.4 | 2.3 | 1.3 |
| Cu-SSZ-13-(S) | 6.4 a/7.7 b | 516.4 | 0.27 | 3.9 | 3.3 | 1.3 | 2.0 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Xin, Y.; Jia, J.; Wang, J.; Zhang, Z. Acetate-Assisted Preparation of High-Cu-Content Cu-SSZ-13 with a Low Si/Al Ratio: Distinguishing Cu Species and Origins. Catalysts 2025, 15, 741. https://doi.org/10.3390/catal15080741
Han D, Xin Y, Jia J, Wang J, Zhang Z. Acetate-Assisted Preparation of High-Cu-Content Cu-SSZ-13 with a Low Si/Al Ratio: Distinguishing Cu Species and Origins. Catalysts. 2025; 15(8):741. https://doi.org/10.3390/catal15080741
Chicago/Turabian StyleHan, Dongxu, Ying Xin, Junxiu Jia, Jin Wang, and Zhaoliang Zhang. 2025. "Acetate-Assisted Preparation of High-Cu-Content Cu-SSZ-13 with a Low Si/Al Ratio: Distinguishing Cu Species and Origins" Catalysts 15, no. 8: 741. https://doi.org/10.3390/catal15080741
APA StyleHan, D., Xin, Y., Jia, J., Wang, J., & Zhang, Z. (2025). Acetate-Assisted Preparation of High-Cu-Content Cu-SSZ-13 with a Low Si/Al Ratio: Distinguishing Cu Species and Origins. Catalysts, 15(8), 741. https://doi.org/10.3390/catal15080741






