Recent Advances in Borylation and Suzuki-Type Cross-Coupling—One-Pot Miyaura-Type C–X and C–H Borylation–Suzuki Coupling Sequence
Abstract
1. Introduction
2. Borylation
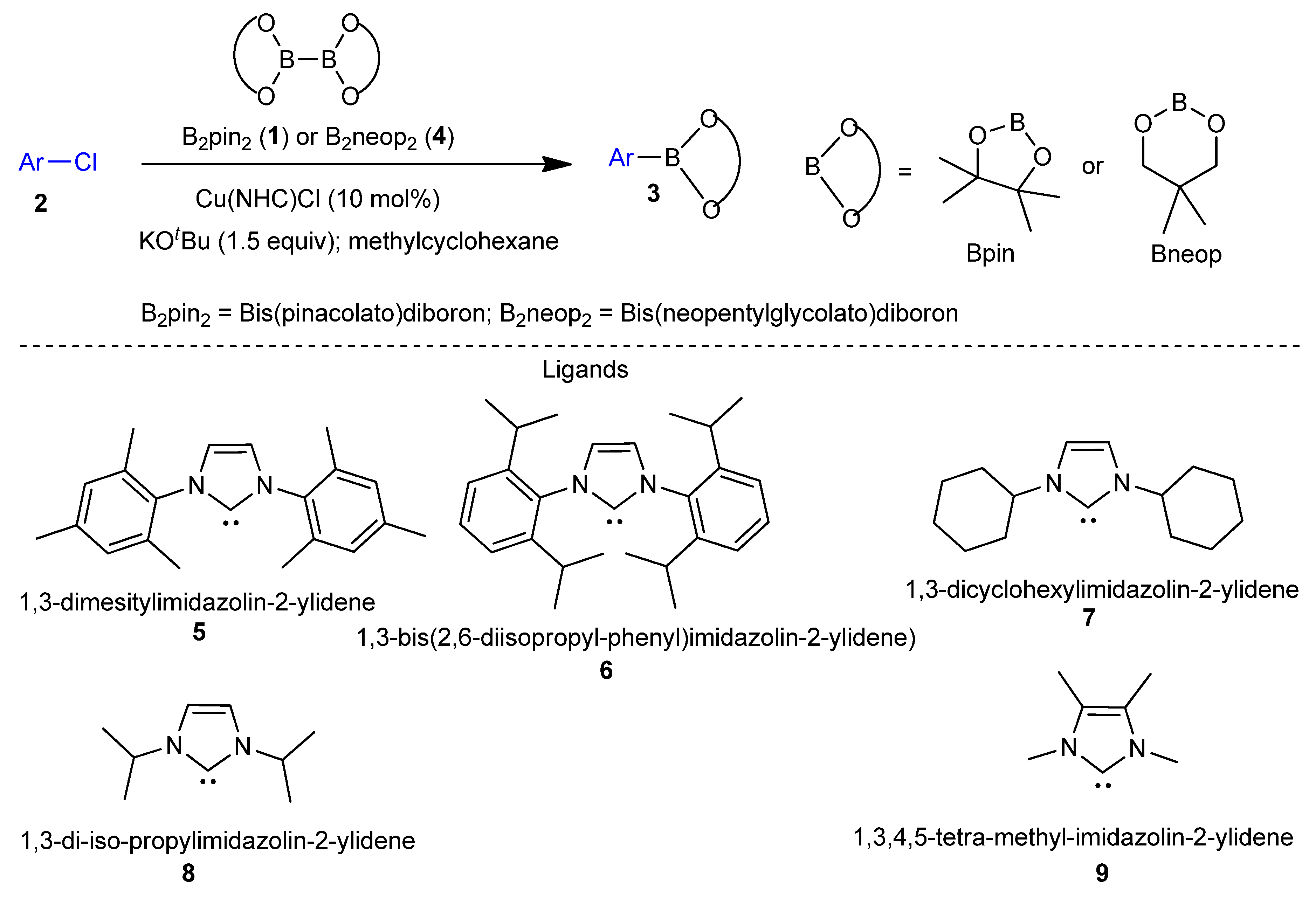
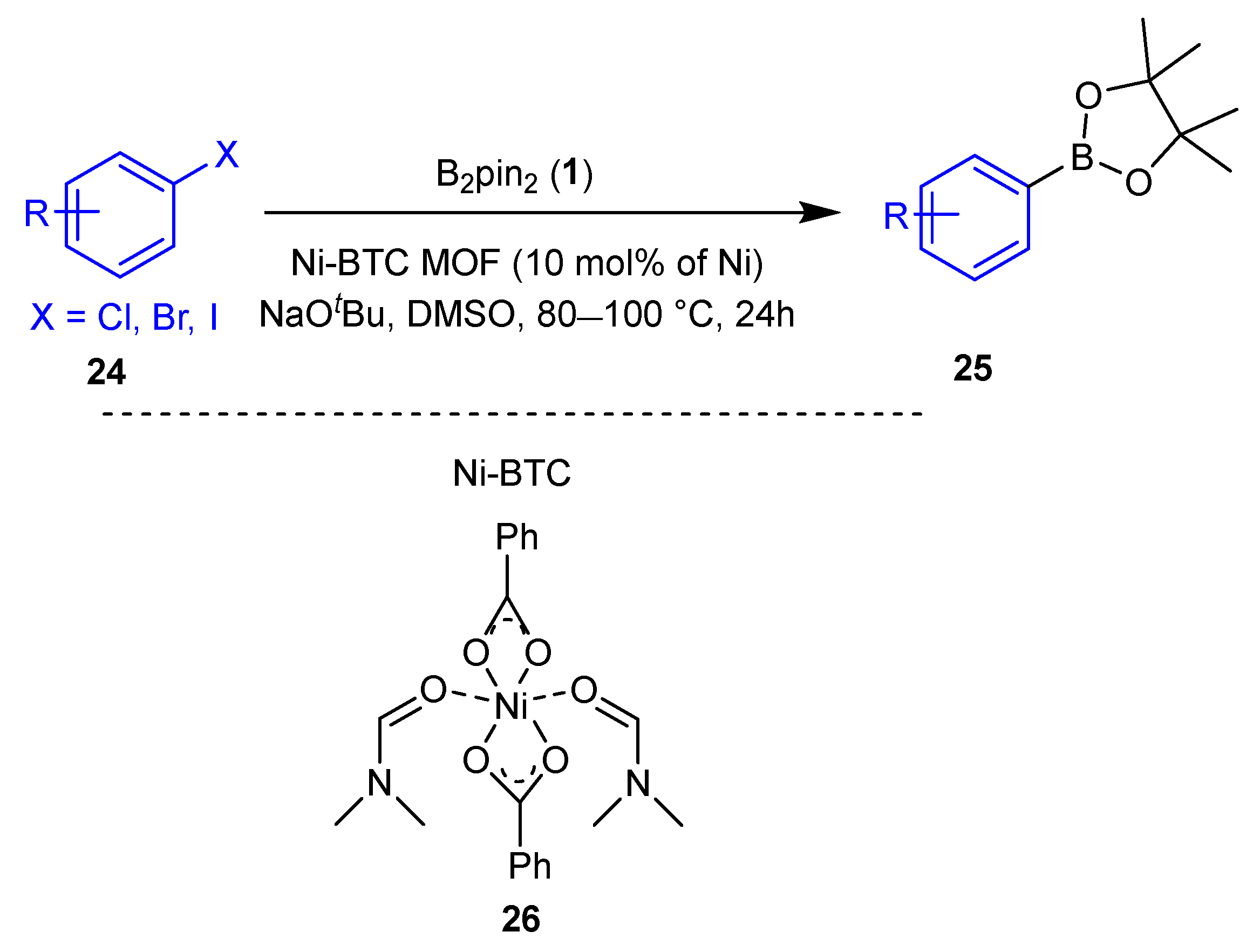
2.1. Transition Metal-Catalyzed C–X Borylation
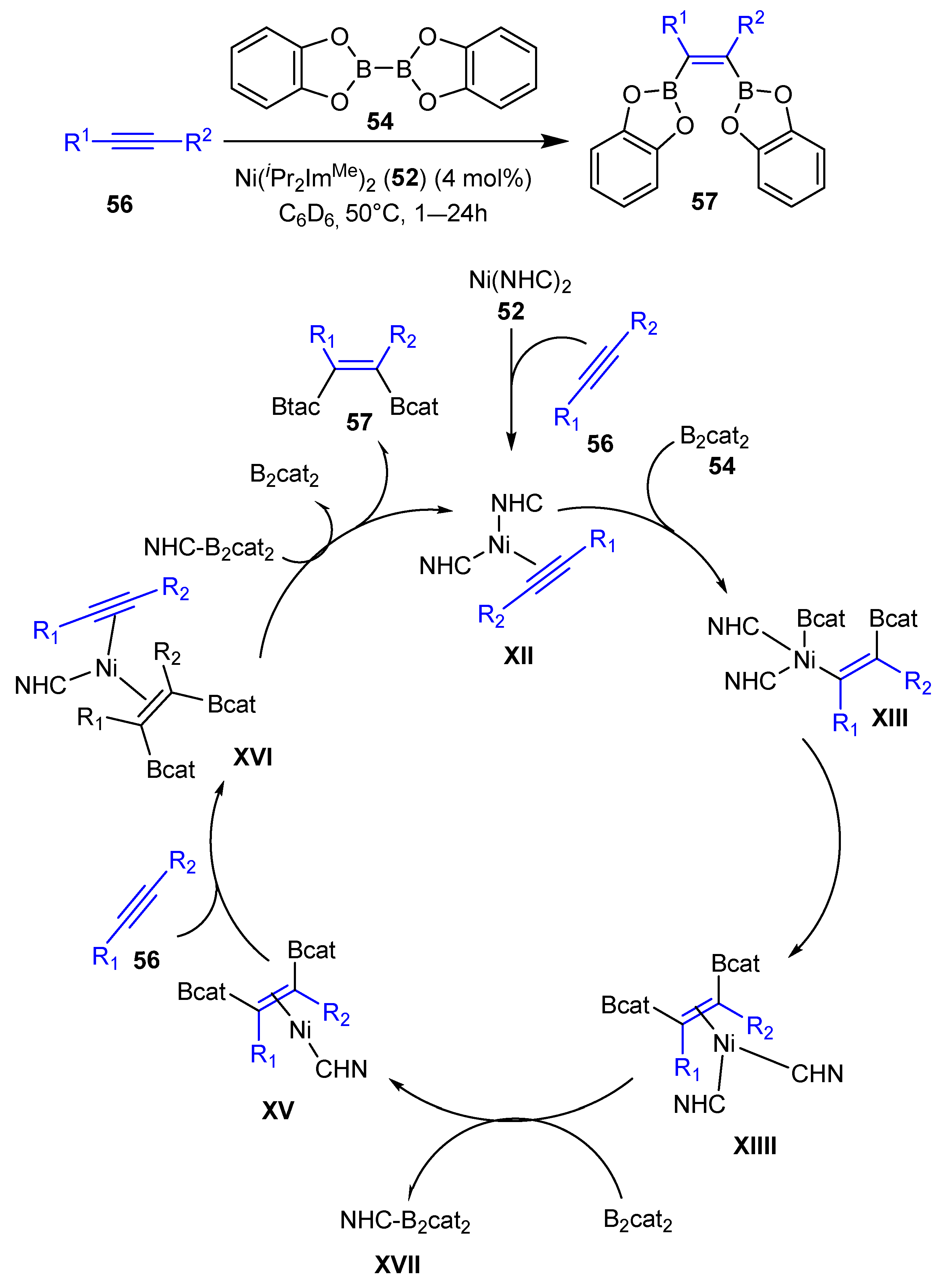
2.2. Transition Metal-Catalyzed C–H Bond Activation and Borylation
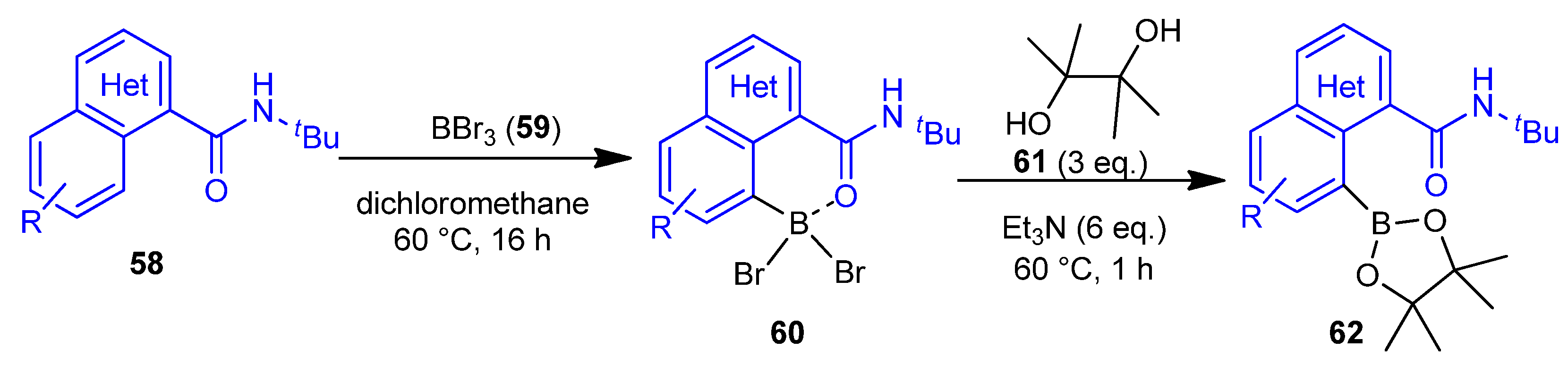
2.3. Metal-Free C–H Borylation
3. Suzuki-Type Cross-Coupling
4. One-Pot C–X and C–H Miyaura-Type Borylation–Suzuki Coupling Sequence
5. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| acac | Acetylacetonate |
| AmPhos | di-tert-Butyl(4-dimethylaminophenyl)phosphine |
| An | Aniline |
| AtaPhos | di-tert-Butyl(4-dimethylaminophenyl)phosphine |
| B2cat2 | Bis(catecholato)diboron |
| BCP | Bicycle[1.1.1]pentane |
| B(EPIN) | Ethyl pinacol boronic ester |
| (R)-BINAP | (R)-(+)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthalene |
| B2(OH)4 | Tetrahydroxydiboron |
| B2eg2 | Bis(ethylene glycolato)diboron |
| B2neop2 | Bis(neopentylglycolato)diboron |
| B2pin2 | Bis(pinacolato)diboron |
| BIDIME | (S)-3-(tert-Butyl)-4-(2,6-dimethoxyphenyl)-2,3-dihydrobenzo[d]oxaphosphole |
| Bpin | Pinacolborane |
| BQ | 1,4-Benzoquinone |
| Bu4NBr | Tetrabutylammonium bromide |
| Bu4NI | Tetrabutylammonium iodide |
| ChCl | choline chloride |
| cin | Cinnamyl |
| COD | 1,5-Cyclooctadiene |
| CPME | Cyclopentyl methyl ether |
| Cy | Cyclohexyl |
| CyJohnPhos | 2-(Dicyclohexylphosphino)biphenyl |
| dba | Dibenzylidene-acetone |
| DCE | Dichloroethane |
| DES | Deep Eutectic Solvent |
| DFT | Density Functional Theory |
| DIPEA | N, N-Diisopropylethylamine |
| dipp | Diisopropyl phthalate |
| DMA | Dimethylacetamide |
| DMSO | Dimethyl sulfoxide |
| DPEPhos | Bis[2-(diphenylphosphino)phenyl] ether |
| dppb | 1,4-Bis(diphenylphosphino)butane |
| dppp | 1,3-Bis(diphenylphosphino)propane |
| dtbbpy | 4,4′-Di-tert-butyl-2,2′-bipyridine |
| dtbpy | 4,4′-Di-tert-butyl-2,2′-bipyridine |
| Gly | Glycerol |
| HBcat | Catecholborane |
| HBMeoCb2 | Bis(1-methyl-ortho-carboranyl)borane (HBMeoCb2) |
| HBpin | Pinacolborane |
| IMes | 1,3-Dimesitylimidazolin-2-ylidene |
| IPAC | Isopropyl acetate |
| IPr | 1,3-Bis(2,6-diisopropylphenyl)imidazolin-2-ylidene |
| iPr2ImMe | 1,3-Di-iso-propyl-4,5-dimethylimidazolin-2-ylidene |
| KEH | Potassium 2-ethylhexanoate |
| KHF2 | Acidic potassium fluoride |
| KOPiv | Potassium pivalate |
| KOtBu | Potassium t-butoxide |
| MBSC | Masuda borylation–Suzuki coupling |
| MIBSC | Miyaura borylation–Suzuki coupling |
| MOF | metal−organic framework |
| MTBE | Methyl t-butyl ether |
| neop | Neopentylglycolato |
| NHC | N-Heterocyclic carbene |
| NHC·BH3 | N-Heterocyclic carbene boranes |
| NMP | N-Methyl-2-pyrrolidone |
| OTf | Triflate |
| Pd(dba)2 | Bis(dibenzylideneacetone) palladium |
| phen | Phenanthroline |
| pin | Pinacolato |
| SIPr | 1,3-Bis(2,6-di-isopropylphenyl)-4,5-dihydroimidazol-2-ylidine |
| SPhos | 2-Dicyclohexylphosphino-2′,6′-dimethoxybiphenyl |
| TBAB | Tetrabutyl ammonium bromide |
| tBuXPhos | 2-Di-tert-butylphosphino-2′,4′,6′-triisopropylbiphenyl |
| TCM | Trichloromethane |
| TDG | Transient directing group |
| Tf2O | Triflic anhydride |
| TFA | Trifluoroacetic acid |
| THF | Tetrahydrofuran |
| THP | Tetrahydropyran |
| TMAOAc | Tetramethylammonium acetate |
| TMAX | Trisodium Citrate |
| TTSO | Thianthrene S-oxide |
| XPhos | 2-Dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl |
References
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd Metal Catalysts for cross-couplings and related reactions in the 21st century: A critical review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Alonso, F.; Tyurin, V. The Suzuki-Miyaura reaction after the Nobel prize. Coord. Chem. Rev. 2019, 385, 137–173. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Averin, A.D. New trends in the cross-coupling and other catalytic reactions. Pure Appl. Chem. 2017, 89, 1413–1428. [Google Scholar] [CrossRef]
- Kanwal, I.; Mujahid, A.; Rasool, N.; Rizwan, K.; Malik, A.; Ahmad, G.; Shah, S.A.A.; Rashid, U.; Nasir, N.M. Palladium and copper catalyzed Sonogashira cross coupling an excellent methodology for C-C bond formation over 17 years: A review. Catalysts 2020, 10, 443. [Google Scholar] [CrossRef]
- Choi, J.; Fu, G.C. Transition metal–catalyzed alkyl-alkyl bond formation: Another dimension in cross-coupling chemistry. Science 2017, 356, eaaf7230. [Google Scholar] [CrossRef]
- Kumar, S. Recent advances in the Schiff bases and N-heterocyclic carbenes as ligands in the cross-coupling reactions: A comprehensive review. J. Heterocycl. Chem. 2019, 56, 1168–1230. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V.; Hajiabbasi, P.; Hamidi, H. Advances in Kumada–Tamao–Corriu cross-coupling reaction: An update. Monatsh. Chem. 2019, 150, 535–591. [Google Scholar] [CrossRef]
- Sain, S.; Jain, S.; Srivastava, M.; Vishwakarma, R.; Dwivedi, J. Application of palladium-catalyzed cross-coupling reactions in organic synthesis. Curr. Org. Synth. 2020, 16, 1105–1142. [Google Scholar] [CrossRef]
- Kostas, I.D. (Ed.) Transition Metal Catalyzed Cross-Coupling Reactions, 1st ed.; Printed Edition of the Special Issue Published in Catalysts; MDPI: Basel, Switzerland, 2021. [Google Scholar] [CrossRef]
- Kostas, I.D. (Ed.) Suzuki–Miyaura Cross-Coupling Reaction and Potential Applications, 1st ed.; Printed Edition of the Special Issue Published in Catalysts; MDPI: Basel, Switzerland, 2017. [Google Scholar] [CrossRef]
- Suzuki, A. Organoboron Compounds in New Synthetic Reactions. Pure Appl. Chem. 1985, 57, 1749–1758. [Google Scholar] [CrossRef]
- Matteson, D.S. Boronic Esters in Stereodirected Synthesis. Tetrahedron 1989, 45, 1859–1885. [Google Scholar] [CrossRef]
- Bose, S.K.; Deißenberger, A.; Eichhorn, A.; Steel, P.G.; Lin, Z.; Marder, T.B. Zinc-Catalyzed Dual C-X and C-H Borylation of Aryl Halides. Angew. Chem. 2015, 54, 11843–11847. [Google Scholar] [CrossRef]
- London, R.E.; Gabel, S.A. NMR Studies of Fluorobenzeneboronic Acids. 1. Interaction Kinetics with Biologically Significant Ligands. J. Am. Chem. Soc. 1994, 116, 2562–2569. [Google Scholar] [CrossRef]
- Hamachi, I.; Tajiri, Y.; Shinkai, S. Sugar-Responsive Semisynthetic Myoglobin Bearing Phenylboronic Acid Groups as Recognition Sites. J. Am. Chem. Soc. 1994, 116, 7437–7438. [Google Scholar] [CrossRef]
- Ming, Y.; Feng, T.; Chen, B.; Zhou, D. Photoinduced Mechanisms of C-S Borylation of Methyl(p-Tolyl)Sulfane with Bis(Pinacolato)Diboron: A DFT Investigation. Catalysts 2024, 14, 550. [Google Scholar] [CrossRef]
- Ishiyama, T.; Murata, M.; Miyaura, N. Palladium (0)-Catalyzed Cross-Coupling Reaction of Alkoxydiboron with Haloarenes: A Direct Procedure for Arylboronic Esters. J. Org. Chem. 1995, 60, 7508–7510. [Google Scholar] [CrossRef]
- Murata, M.; Watanabe, S.; Masuda, Y. Novel Palladium (0)-Catalyzed Coupling Reaction of Dialkoxyborane with Aryl Halides: Convenient Synthetic Route to Arylboronates. J. Org. Chem. 1997, 62, 6458–6459. [Google Scholar] [CrossRef]
- El-Maiss, J.; El Dine, T.M.; Lu, C.-S.; Karamé, I.; Kanj, A.; Polychronopoulou, K.; Shaya, J. Recent advances in metal-catalyzed alkyl–boron (C(sp3))–C(sp2)) Suzuki-Miyaura cross-couplings. Catalysts 2020, 10, 296. [Google Scholar] [CrossRef]
- Ajvazi, N.; Stavber, S. Direct halogenation of alcohols with halosilanes under catalyst- and organic solvent-free reaction conditions. Tetrahedron Lett. 2016, 57, 2430–2433. [Google Scholar] [CrossRef]
- Kruppa, M.; Müller, T.J.J. Masuda Borylation–Suzuki Coupling (MBSC) Sequence: A One-Pot Process to Access Complex (Hetero)Biaryls. Catalysts 2023, 13, 350. [Google Scholar] [CrossRef]
- Baudoin, O.; Guénard, D.; Guéritte, F. Palladium-Catalyzed Borylation of Ortho-Substituted Phenyl Halides and Application to the One-Pot Synthesis of 2,2′-Disubstituted Biphenyls. J. Org. Chem. 2000, 65, 9268–9271. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, H.H.; Zhang, J. Cesium Carbonate Mediated Borylation of Aryl Iodides with Diboron in Methanol. Eur. J. Org. Chem. 2013, 2013, 6263–6266. [Google Scholar] [CrossRef]
- Murata, M.; Oyama, T.; Watanabe, S.; Masuda, Y. Palladium-Catalyzed Borylation of Aryl Halides or Triflates with Dialkoxyborane: A Novel and Facile Synthetic Route to Arylboronates. J. Org. Chem. 2000, 65, 164–168. [Google Scholar] [CrossRef]
- Bello, C.S.; Schmidt-Leithoff, J. Borylation of Organo Halides and Triflates Using Tetrakis(Dimethylamino) Diboron. Tetrahedron Lett. 2012, 53, 6230–6235. [Google Scholar] [CrossRef]
- Iwai, T.; Harada, T.; Tanaka, R.; Sawamura, M. Silica-Supported Tripod Triarylphosphines: Application to Palladium-Catalyzed Borylation of Chloroarenes. Chem. Lett. 2014, 43, 584–586. [Google Scholar] [CrossRef]
- Niwa, T.; Ochiai, H.; Hosoya, T. Copper-Catalyzed Ipso-Borylation of Fluoroarenes. ACS Catal. 2017, 7, 4535–4541. [Google Scholar] [CrossRef]
- Chow, W.K.; Yuen, O.Y.; Choy, P.Y.; So, C.M.; Lau, C.P.; Wong, W.T.; Kwong, F.Y. A decade advancement of transition metal-catalyzed borylation of aryl halides and sulfonates. RSC Adv. 2013, 3, 12518–12539. [Google Scholar] [CrossRef]
- Kuehn, L.; Huang, M.; Radius, U.; Marder, T.B. Copper-Catalysed Borylation of Aryl Chlorides. Org. Biomol. Chem. 2019, 17, 6601–6606. [Google Scholar] [CrossRef]
- Kuehn, L.; Eichhorn, A.F.; Marder, T.B.; Radius, U. Copper(I) Complexes of N-Alkyl-Substituted N-Heterocyclic Carbenes. J. Organomet. Chem. 2019, 881, 25–33. [Google Scholar] [CrossRef]
- Bose, S.K.; Brand, S.; Omoregie, H.O.; Haehnel, M.; Maier, J.; Bringmann, G.; Marder, T.B. Highly Efficient Synthesis of Alkylboronate Esters via Cu (II)-Catalyzed Borylation of Unactivated Alkyl Bromides and Chlorides in Air. ACS Catal. 2016, 6, 8332–8335. [Google Scholar] [CrossRef]
- Yao, W.; Fang, H.; Peng, S.; Wen, H.; Zhang, L.; Hu, A.; Huang, Z. Cobalt-Catalyzed Borylation of Aryl Halides and Pseudohalides. Organometallics 2016, 35, 1559–1564. [Google Scholar] [CrossRef]
- Olding, A.; Lucas, N.T.; Ho, C.C.; Bissember, A.C. Acridine-based copper(I) PNP pincer complexes: Catalysts for alkyne hydroboration and borylation of aryl halides. Dalton Trans. 2024, 53, 4471–4478. [Google Scholar] [CrossRef]
- Bedford, R.B. How Low Does Iron Go? Chasing the Active Species in Fe-Catalyzed Cross-Coupling Reactions. Acc. Chem. Res. 2015, 48, 1485–1493. [Google Scholar] [CrossRef]
- Yoshida, T.; Ilies, L.; Nakamura, E. Iron-Catalyzed Borylation of Aryl Chlorides in the Presence of Potassium t-Butoxide. ACS Catal. 2017, 7, 3199–3203. [Google Scholar] [CrossRef]
- Liu, X.W.; Echavarren, J.; Zarate, C.; Martin, R. Ni-Catalyzed Borylation of Aryl Fluorides via C–F Cleavage. J. Am. Chem. Soc. 2015, 137, 12470–12473. [Google Scholar] [CrossRef]
- Zhou, J.; Kuntze-Fechner, M.W.; Bertermann, R.; Paul, U.S.D.; Berthel, J.H.J.; Friedrich, A.; Du, Z.; Marder, T.B.; Radius, U. Preparing (Multi)Fluoroarenes as Building Blocks for Synthesis: Nickel-Catalyzed Borylation of Polyfluoroarenes via C-F Bond Cleavage. J. Am. Chem. Soc. 2016, 138, 5250–5253. [Google Scholar] [CrossRef]
- Prakash, A.; Basappa, S.; Jeebula, B.; Nagaraju, D.H.; Dhayal, R.S.; Bose, S.K. A Simple Nickel Metal−Organic Framework-Catalyzed Borylation of Aryl Chlorides and Bromides. Org. Lett. 2024, 26, 2569–2573. [Google Scholar] [CrossRef]
- Barroso, S.; Joksch, M.; Puylaert, P.; Tin, S.; Bell, S.J.; Donnellan, L.; Duguid, S.; Muir, C.; Zhao, P.; Farina, V.; et al. Improvement in the Palladium-Catalyzed Miyaura Borylation Reaction by Optimization of the Base: Scope and Mechanistic Study. J. Org. Chem. 2021, 86, 103–109. [Google Scholar] [CrossRef]
- Molander, G.A.; Trice, S.L.J.; Dreher, S.D. Palladium-Catalyzed, Direct Boronic Acid Synthesis from Aryl Chlorides: A Simplified Route to Diverse Boronate Ester Derivatives. J. Am. Chem. Soc. 2010, 132, 17701–17703. [Google Scholar] [CrossRef]
- Munteanu, C.; Spiller, T.E.; Qiu, J.; Delmonte, A.J.; Wisniewski, S.R.; Simmons, E.M.; Frantz, D.E. Pd- and Ni-Based Systems for the Catalytic Borylation of Aryl (Pseudo)Halides with B2(OH)4. J. Org. Chem. 2020, 85, 10334–10349. [Google Scholar] [CrossRef]
- Budiman, Y.P.; Lorenzen, S.; Liu, Z.; Radius, U.; Marder, T.B. Base-Free Pd-Catalyzed C−Cl Borylation of Fluorinated Aryl Chlorides. Chem. Eur. J. 2021, 27, 3869–3874. [Google Scholar] [CrossRef]
- Niwa, T.; Takimoto, T.; Sakata, Y.; Hosoya, T. Palladium-Catalyzed ipso-Borylation of Aryl Halides Promoted by Lewis Acid-Mediated Electrophilic Activation of Aryl(halo)palladium (II) Complex. Org. Lett. 2023, 25, 8173–8177. [Google Scholar] [CrossRef]
- Gay, B.L.; Prendeville, L.A.; Wang, Y.-N.; Hull, K.L. Base-Free Borylation of Aryl Halides Enabled by Zn-Promoted Halide Abstraction. Org. Lett. 2024, 26, 10481–10486. [Google Scholar] [CrossRef]
- Li, X.; Tian, X.; Jiao, H.; Wu, L. Titanium-Catalyzed C—X and C—H Borylation of Aryl Halides for the Synthesis of Bis(boronate)benzenes. Angew. Chem. Int. Ed. 2025, 64, e202509778. [Google Scholar] [CrossRef]
- Dhawa, U.; Kaplaneris, N.; Ackermann, L. Green Strategies for Transition Metal-Catalyzed C-H Activation in Molecular Syntheses. Org. Chem. Front. 2021, 8, 4886–4913. [Google Scholar] [CrossRef]
- Meng, G.; Lam, N.Y.S.; Lucas, E.L.; Saint-Denis, T.G.; Verma, P.; Chekshin, N.; Yu, J.Q. Achieving Site-Selectivity for C-H Activation Processes Based on Distance and Geometry: A Carpenter’s Approach. J. Am. Chem. Soc. 2020, 142, 10571–10591. [Google Scholar] [CrossRef]
- Mkhalid, I.A.I.; Barnard, J.H.; Marder, T.B.; Murphy, J.M.; Hartwig, J.F. C–H Activation for the Construction of C–B Bonds. Chem. Rev. 2010, 110, 890–931. [Google Scholar] [CrossRef]
- Hassan, M.M.M.; Guria, S.; Dey, S.; Das, J.; Chattopadhyay, B. Transition Metal-Catalyzed Remote C—H Borylation: An Emerging Synthetic Tool. Sci. Adv. 2023, 9, eadg3311. [Google Scholar] [CrossRef]
- Zhang, H.; Hagihara, S.; Itami, K. Aromatic C–H Borylation by Nickel Catalysis. Chem. Lett. 2015, 44, 779–781. [Google Scholar] [CrossRef]
- Furukawa, T.; Tobisu, M.; Chatani, N. Nickel-Catalyzed Borylation of Arenes and Indoles via C-H Bond Cleavage. Chem. Commun. 2015, 51, 6508–6511. [Google Scholar] [CrossRef]
- Das, A.; Hota, P.K.; Mandal, S.K. Nickel-Catalyzed C(sp2)−H Borylation of Arenes. Organometallics 2019, 38, 3286–3293. [Google Scholar] [CrossRef]
- Tian, Y.M.; Guo, X.N.; Wu, Z.; Friedrich, A.; Westcott, S.A.; Braunschweig, H.; Radius, U.; Marder, T.B. Ni-Catalyzed Traceless, Directed C3-Selective C-H Borylation of Indoles. J. Am. Chem. Soc. 2020, 142, 13136–13144. [Google Scholar] [CrossRef]
- Tendera, L.; Fantuzzi, F.; Marder, T.B.; Radius, U. Nickel boryl complexes and nickel-catalyzed alkyne borylation. Chem. Sci. 2023, 14, 2215–2228. [Google Scholar] [CrossRef] [PubMed]
- Rej, S.; Chatani, N. Regioselective Transition-Metal-Free C(sp2)−H Borylation: A Subject of Practical and Ongoing Interest in Synthetic Organic Chemistry. Angew. Chem. Int. Ed. 2022, 61, e202209539. [Google Scholar] [CrossRef] [PubMed]
- Nad, P.; Mukherjee, A. Metal-free C-H Borylation and Hydroboration of Indoles. ACS Omega 2023, 8, 37623–37640. [Google Scholar] [CrossRef]
- Bhanja, R.; Bera, S.K.; Mal, P. Photocatalyst- and Transition Metal-Free Light-Induced Borylation Reactions. Chem. Asian J. 2023, 18, e202300691. [Google Scholar] [CrossRef]
- Shang, Z.-H.; Pan, J.; Wang, Z.; Zhang, Z.-X.; Wu, J. Transition-Metal-Free Radical Borylation Reactions. Eur. J. Org. Chem. 2023, 26, e202201379. [Google Scholar] [CrossRef]
- Banerjee, M.; Chatterjee, A.; Aneja, S.; Chatterjee, A. Mechanochemical Functionalization of Heterocycles by C–H Activation: An Update. J. Org. Chem. 2025, 90, 5323–5335. [Google Scholar] [CrossRef]
- Cheung, T.L.; Lyu, H. Electrochemical Borylation of C−C and C−Het Bonds. ChemElectroChem 2025, 12, e202400560. [Google Scholar] [CrossRef]
- Maji, S.; Rawal, P.; Ghosh, A.; Pidiyar, K.; Al-Thabaiti, S.A.; Gupta, P.; Maiti, D. Metal-Free Borylation of α-Naphthamides and Phenylacetic Acid Drug. JACS Au 2024, 4, 3679–3689. [Google Scholar] [CrossRef]
- Liang, Y.; Du, C.; Dong, C.; Cao, J.; Xu, Y.; Zhang, H. BBr3-Mediated ortho C−H Borylation of Benzamides. Org. Lett. 2025, 27, 4650–4655. [Google Scholar] [CrossRef]
- Lv, J.; Liang, Y.; Ouyang, Y.; Zhang, H. Metal-Free ortho C−H Borylation of Thiobenzamides. Org. Lett. 2024, 26, 3709–3714. [Google Scholar] [CrossRef]
- Chen, W.; Xia, J.; Huang, J.; Zhou, L.; Wu, G. Chemoselective C−H Hydroxylation and Borylation of N-Phenylbenzamides Using BBr3. Org. Lett. 2024, 26, 4631–4636. [Google Scholar] [CrossRef]
- Zhan, B.; Lv, J.; Wu, J.; Zhang, H. Synthesis of C5-boryl indoles via a borane-catalyzed borylation/hydride transfer cascade. Chem Catal. 2024, 4, 100975. [Google Scholar] [CrossRef]
- Begum, A.; Akram, M.O.; Martin, C.D. Dearomative C2-borylation of indoles. Dalton Trans. 2025, 54, 5664–5667. [Google Scholar] [CrossRef] [PubMed]
- Keerthika, K.; Muhammed, S.B.; Geetharani, K. A Metal-Free and Operationally Simple Radical Trifluoromethylative Borylation of Unactivated Alkenes. Chem. Eur. J. 2024, 30, e202303468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, J.; Xiao, Y.; Wang, Z.; Wu, W.; Xue, D. Metal and photocatalyst-free alkylboration of [1.1.1]propellane enabled by red-light-induced electron transfer. Org. Chem. Front. 2025, 12, 4050–4057. [Google Scholar] [CrossRef]
- Patil, K.S.; Reddappa, S.; Kumar, R.; Mane, M.V.; Bose, S.K. Synthesis of 1,2-Bis- and 1,1,2-Tris-Borylalkanes under Transition Metal-Free and Solvent-Free Conditions. J. Org. Chem. 2025, 90, 4140–4148. [Google Scholar] [CrossRef]
- Kader, D.A.; Sidiq, M.K.; Taher, S.G.; Aziz, D.M. Recent advances in palladium-catalyzed Suzuki-Miyaura cross-coupling reactions: Exploration of catalytic systems, reaction parameters, and ligand influences: A review. J. Organomet. Chem. 2025, 1030, 123569. [Google Scholar] [CrossRef]
- Pasricha, S.; Sachidanand; Srivastava, A.; Yadav, S.; Sunny, A.; Tuwani, N.; Rangarajan, T.M.; Mittal, K. Greener media for nano catalysts in Suzuki Miyaura reaction. Coord. Chem. Rev. 2025, 528, 216431. [Google Scholar] [CrossRef]
- Kostas, I.D.; Coutsolelos, A.G.; Charalambidis, G.; Skondra, A. The first use of porphyrins as catalysts in cross-coupling reac-tions: A water-soluble palladium complex with a porphyrin ligand as an efficient catalyst precursor or the Suzuki–Miyaura reaction in aqueous media under aerobic conditions. Tetrahedron Lett. 2007, 48, 6688–6691. [Google Scholar] [CrossRef]
- Kostas, I.D.; Andreadaki, F.J.; Kovala-Demertzi, D.; Prentjas, C.; Demertzis, M.A. Suzuki-Miyaura cross-coupling reaction of aryl bromides and chlorides with phenylboronic acid under aerobic conditions catalyzed by palladium complexes with thiosemicarbazone ligand. Tetrahedron Lett. 2005, 46, 1967–1970. [Google Scholar] [CrossRef]
- Hasan, H.; Mhaibes, R.M.; Al-Bahrani, H.A.; Kadhum, A.A.H.; Imeer, A.T.A.; Al-Rashedi, N.A.M.; Shu, G. Recent advances on Pd schiff base catalysts in Suzuki-Miyaura cross-coupling reaction: A review. J. Organomet. Chem. 2025, 1024, 123444. [Google Scholar] [CrossRef]
- Liu, B.; Qin, X.; Li, K.; Li, X.; Guo, Q.; Lan, J.; You, J. A Palladium/Copper Bimetallic Catalytic System: Dramatic Improvement for Suzuki-Miyaura-Type Direct C-H Arylation of Azoles with Arylboronic Acids. Chem. Eur. J. 2010, 16, 11836–11839. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Ikeda, Y.; Kuroda, A.; Tanaka, S.; Minakata, S. Pd/NHC-Catalyzed Enantiospecific and Regioselective Suzuki-Miyaura Arylation of 2-Arylaziridines: Synthesis of Enantioenriched 2-Arylphenethylamine Derivatives. J. Am. Chem. Soc. 2014, 136, 8544–8547. [Google Scholar] [CrossRef]
- Chen, X.Y.; Nie, X.X.; Wu, Y.; Wang, P. Para-Selective Arylation and Alkenylation of Monosubstituted Arenes Using Thianthrene S-Oxide as a Transient Mediator. Chem. Commun. 2020, 56, 5058–5061. [Google Scholar] [CrossRef]
- Wen, J.; Qin, S.; Ma, L.F.; Dong, L.; Zhang, J.; Liu, S.S.; Duan, Y.S.; Chen, S.Y.; Hu, C.W.; Yu, X.Q. Iron-Mediated Direct Suzuki-Miyaura Reaction: A New Method for the Ortho-Arylation of Pyrrole and Pyridine. Org. Lett. 2010, 12, 2694–2697. [Google Scholar] [CrossRef]
- Chao, B.; Bai, C.; Yan, H.; Zhao, R.; Liu, D.; Muschin, T.; Bao, A.; Eerdun, C.; Bao, Y.-S. Suzuki−Miyaura Type Regioselective C−H Arylation of Aromatic Aldehydes by a Transient Directing Strategy. Org. Lett. 2023, 25, 6823–6829. [Google Scholar] [CrossRef]
- Naik, V.; Khan, F.A. Palladium-catalyzed synthesis of indenoindoles via C-H activation and tandem synthesis of indenoisoquinolines via Suzuki-Miyaura coupling and annulations. Arkivoc 2023, 2023, 12095. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, J.; Dong, G. Suzuki-Miyaura Coupling of Simple Ketones via Activation of Unstrained Carbon-Carbon Bonds. J. Am. Chem. Soc. 2018, 140, 5347–5351. [Google Scholar] [CrossRef]
- Prabhu, R.N.; Ramesh, R. Synthesis and Structural Characterization of Pd(II) Thiosemicarbazonato Complex: Catalytic Evaluation in Synthesis of Diaryl Ketones from Aryl Aldehydes and Arylboronic Acids. Tetrahedron Lett. 2017, 58, 405–409. [Google Scholar] [CrossRef]
- Dai, J.J.; Liu, J.H.; Luo, D.F.; Liu, L. Pd-Catalysed Decarboxylative Suzuki Reactions and Orthogonal Cu-Based O-Arylation of Aromatic Carboxylic Acids. Chem. Commun. 2011, 47, 677–679. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, Y.; Ye, J.; Ma, Z.; Feng, J.; Liu, X.; Lei, P.; Szostak, M. Suzuki-Miyaura Cross-Coupling of 2-Pyridyl Trimethylammonium Salts by N-C Activation Catalyzed by Air- and Moisture-Stable Pd-NHC Precatalysts: Application to the Discovery of Agrochemicals. Org. Lett. 2023, 25, 2975–2980. [Google Scholar] [CrossRef]
- Weires, N.A.; Baker, E.L.; Garg, N.K. Nickel-Catalysed Suzuki-Miyaura Coupling of Amides. Nat. Chem. 2016, 8, 75–79. [Google Scholar] [CrossRef]
- Gao, P.; Zhu, Y.; Zhou, T.; Utecht-Jarzyńska, G.; Szostak, R.; Szostak, M. Pd-Catalyzed Decarbonylative Suzuki−Miyaura Cross-Coupling of Pyramidalized N-Mesyl Amides by a Tandem N−C(O)/C−C Bond Activation. J. Org. Chem. 2024, 89, 17463–17474. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-D.; Xie, L.-J.; Kong, D.-L.; Liu, L.; Cheng, L. Metal-free allylation of electron-rich heteroaryl boronic acids with allylic alcohols. Tetrahedron 2016, 72, 1873–1880. [Google Scholar] [CrossRef]
- Ji, H.; Cai, J.; Gan, N.; Wang, Z.; Wu, L.; Li, G.; Yi, T. Palladium-Catalyzed Borylation of Aryl (Pseudo)Halides and Its Applications in Biaryl Synthesis. Chem. Cent. J. 2018, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Boontiem, P.; Kiatisevi, S. Facile and Economical Miyaura Borylation and One-Pot Suzuki–Miyaura Cross-Coupling Reaction. Inorg. Chim. Acta 2020, 506, 119538. [Google Scholar] [CrossRef]
- Nelson, C.B.; L’Heureux, S.J.; Wong, M.J.; Kuhn, S.L.; Ghiglietti, E.; Lipshutz, B.H. Environmentally friendly Miyaura Borylations allowing for green, 1-pot borylation/Suzuki–Miyaura couplings. Green Chem. 2024, 26, 10115–10122. [Google Scholar] [CrossRef]
- Luo, B.; Dong, W.; Ma, Q.; Yang, H.; Tang, W. Synthesis of Biheteroaryls by Pd-Catalyzed Homocoupling of Heteroaryl Bromides. Org. Lett. 2024, 26, 8736–8740. [Google Scholar] [CrossRef]
- D’Amico, F.; Papucci, C.; Franchi, D.; Reginato, G.; Taddei, M.; Mordini, A.; Zani, L.; Dessì, A.; Calamante, M. Pd-Catalyzed Miyaura Borylation and Telescopic Borylation/Suzuki−Miyaura Cross-Coupling Processes in Deep-Eutectic Solvents. J. Org. Chem. 2024, 89, 6991–7003. [Google Scholar] [CrossRef]
- Pei, X.; Zhou, G.; Li, X.; Xu, Y.; Panicker, R.C.; Srinivasan, R. Sterically Controlled C-H/C-H Homocoupling of Arenes: Via C-H Borylation. Org. Biomol. Chem. 2019, 17, 5703–5707. [Google Scholar] [CrossRef]
- Esteves, H.A.; Goldfogel, M.J.; Shemet, A.; Peng, C.; Hritzko, B.; Simmons, E.M.; Wisniewski, S.R. Advancing Base-Metal Catalysis: Developing Nickel Catalysis for the Direct Telescope of Miyaura Borylation and Suzuki−Miyaura Cross Coupling Reactions. Org. Process Res. Dev. 2024, 28, 4039–4045. [Google Scholar] [CrossRef]
- Ng, S.S.; Chen, Z.; Yuen, O.Y.; So, C.M. Palladium-Catalyzed Chemoselective Borylation of (Poly)Halogenated Aryl Triflates and Their Application in Consecutive Reactions. Adv. Synth. Catal. 2022, 364, 1596–1601. [Google Scholar] [CrossRef]
- Yadav, P.; Das, S.; Saito, M.; Evans, T.; Das, B.C. One-Pot Borylation/Arylation of 4-Chloroquinolines and Its Application for the Synthesis of Various Quinoline-Based Pharmacophores. Tetrahedron Lett. 2023, 131, 154780. [Google Scholar] [CrossRef]
- Pang, Y.; Ishiyama, T.; Kubota, K.; Ito, H. Iridium(I)-Catalyzed C−H Borylation in Air by Using Mechanochemistry. Chem. Eur. J. 2019, 25, 4654–4659. [Google Scholar] [CrossRef]
- Nagano, T.; Nakamura, K.; Tokimaru, Y.; Ito, S.; Miyajima, D.; Aida, T.; Nozaki, K. Functionalization of Azapentabenzocorannulenes by Fivefold C−H Borylation and Cross-Coupling Arylation: Application to Columnar Liquid-Crystalline Materials. Chem. Eur. J. 2018, 24, 14075–14078. [Google Scholar] [CrossRef]
- Moore, M.J.; Qu, S.; Tan, C.; Cai, Y.; Mogi, Y.; Jamin Keith, D.; Boger, D.L. Next-Generation Total Synthesis of Vancomycin. J. Am. Chem. Soc. 2020, 142, 16039–16050. [Google Scholar] [CrossRef]












































Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahyoune, N.; Eddahmi, M.; Diamantopoulou, P.; Kostas, I.D.; Bouissane, L. Recent Advances in Borylation and Suzuki-Type Cross-Coupling—One-Pot Miyaura-Type C–X and C–H Borylation–Suzuki Coupling Sequence. Catalysts 2025, 15, 738. https://doi.org/10.3390/catal15080738
Bahyoune N, Eddahmi M, Diamantopoulou P, Kostas ID, Bouissane L. Recent Advances in Borylation and Suzuki-Type Cross-Coupling—One-Pot Miyaura-Type C–X and C–H Borylation–Suzuki Coupling Sequence. Catalysts. 2025; 15(8):738. https://doi.org/10.3390/catal15080738
Chicago/Turabian StyleBahyoune, Nouhaila, Mohammed Eddahmi, Perikleia Diamantopoulou, Ioannis D. Kostas, and Latifa Bouissane. 2025. "Recent Advances in Borylation and Suzuki-Type Cross-Coupling—One-Pot Miyaura-Type C–X and C–H Borylation–Suzuki Coupling Sequence" Catalysts 15, no. 8: 738. https://doi.org/10.3390/catal15080738
APA StyleBahyoune, N., Eddahmi, M., Diamantopoulou, P., Kostas, I. D., & Bouissane, L. (2025). Recent Advances in Borylation and Suzuki-Type Cross-Coupling—One-Pot Miyaura-Type C–X and C–H Borylation–Suzuki Coupling Sequence. Catalysts, 15(8), 738. https://doi.org/10.3390/catal15080738








