Microwave-Assisted Biodiesel Production Using Activated Oat Hull-Derived Biochar as Catalyst
Abstract
1. Introduction
2. Results and Discussion
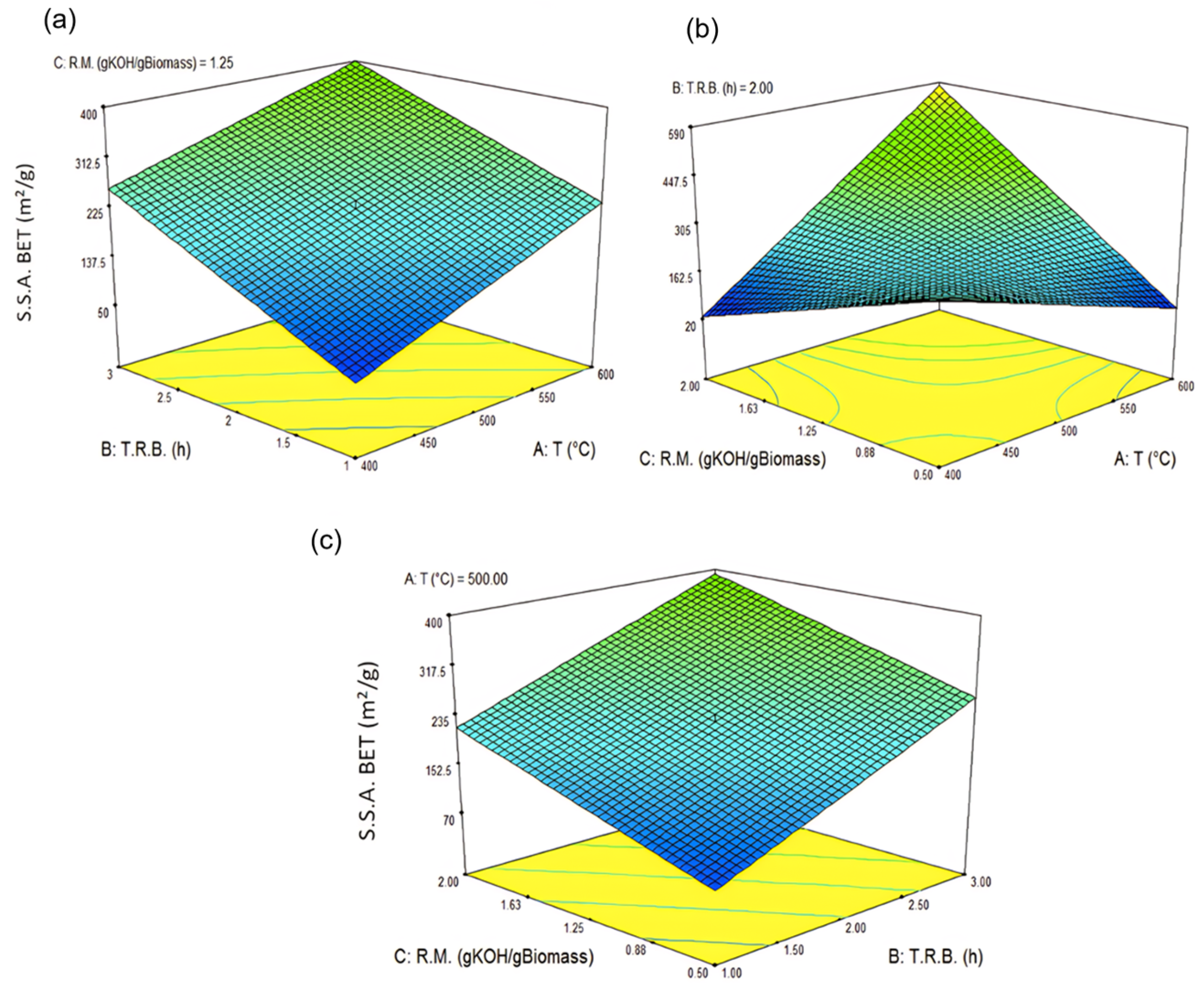
2.1. Effect of Pyrolysis Process Conditions on the Porosity of Biochar
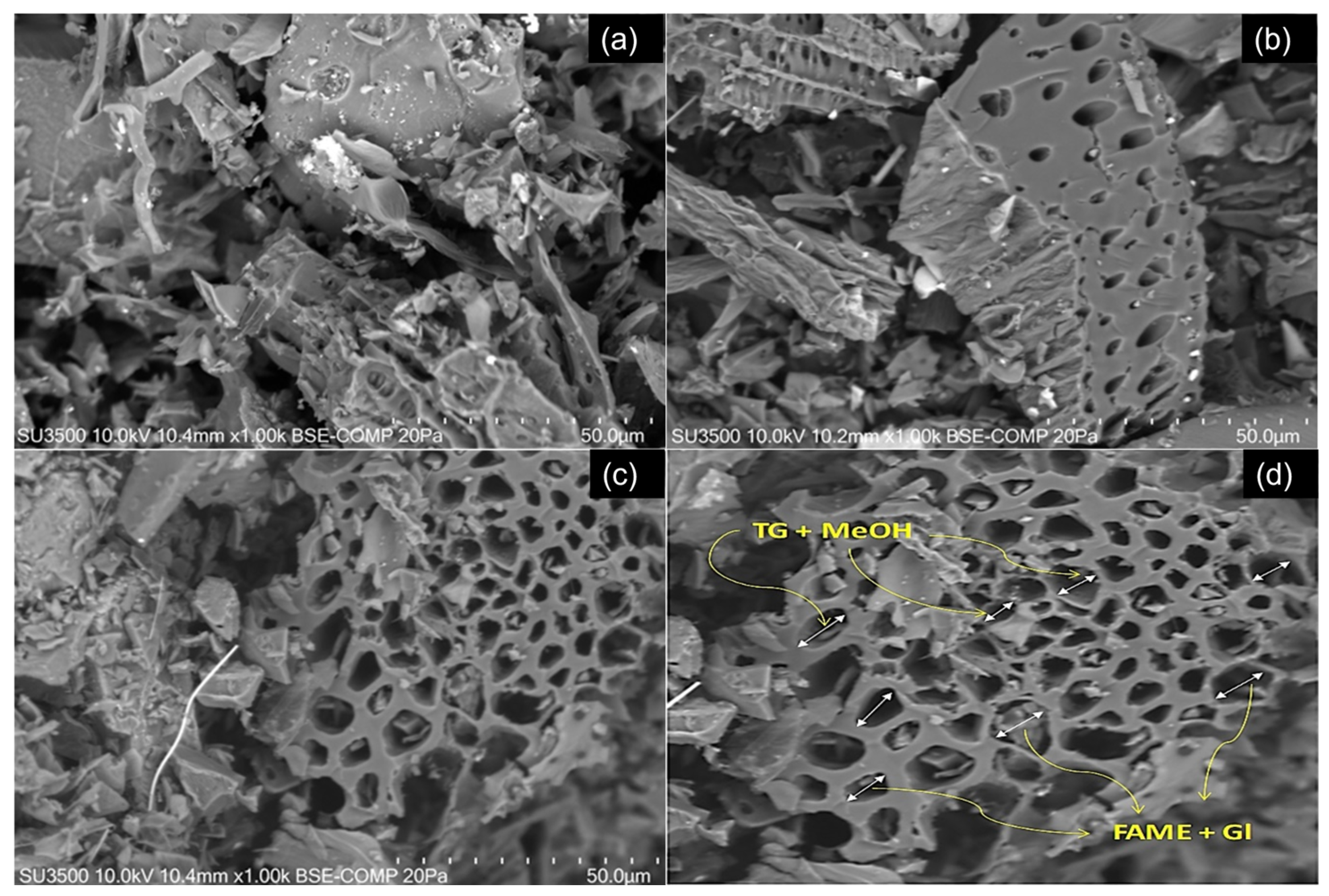
2.2. Effect of Chemical Activation on Catalyst Morphology
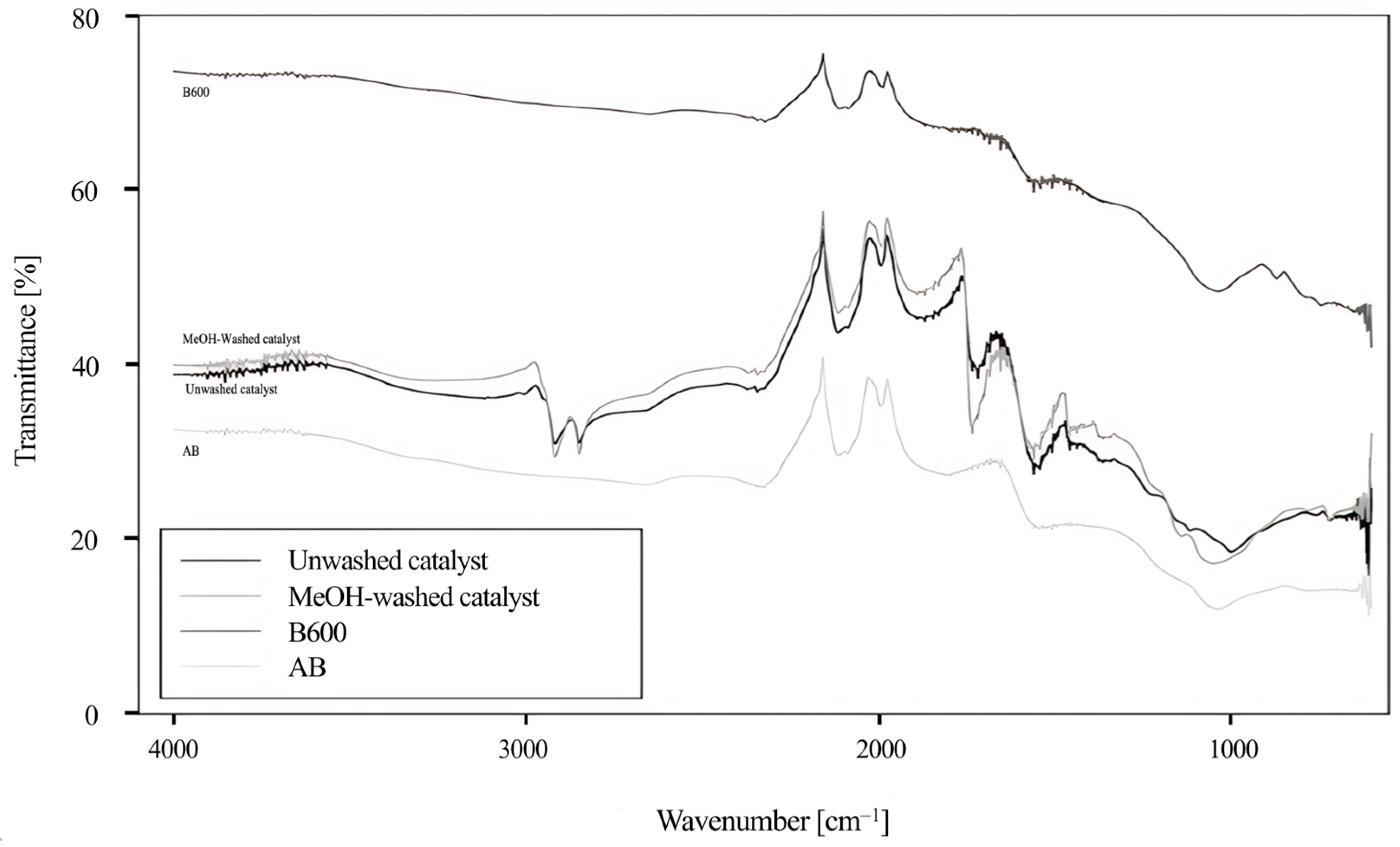
2.3. Effect of Activation Process Conditions in the Composition of Activated Biochar
2.4. Biodiesel Production
2.5. Optimization of Microwave-Assisted Biodiesel Production Using Activated Biochar as a Catalyst
2.6. Effect of Operational Conditions on Biodiesel Yield
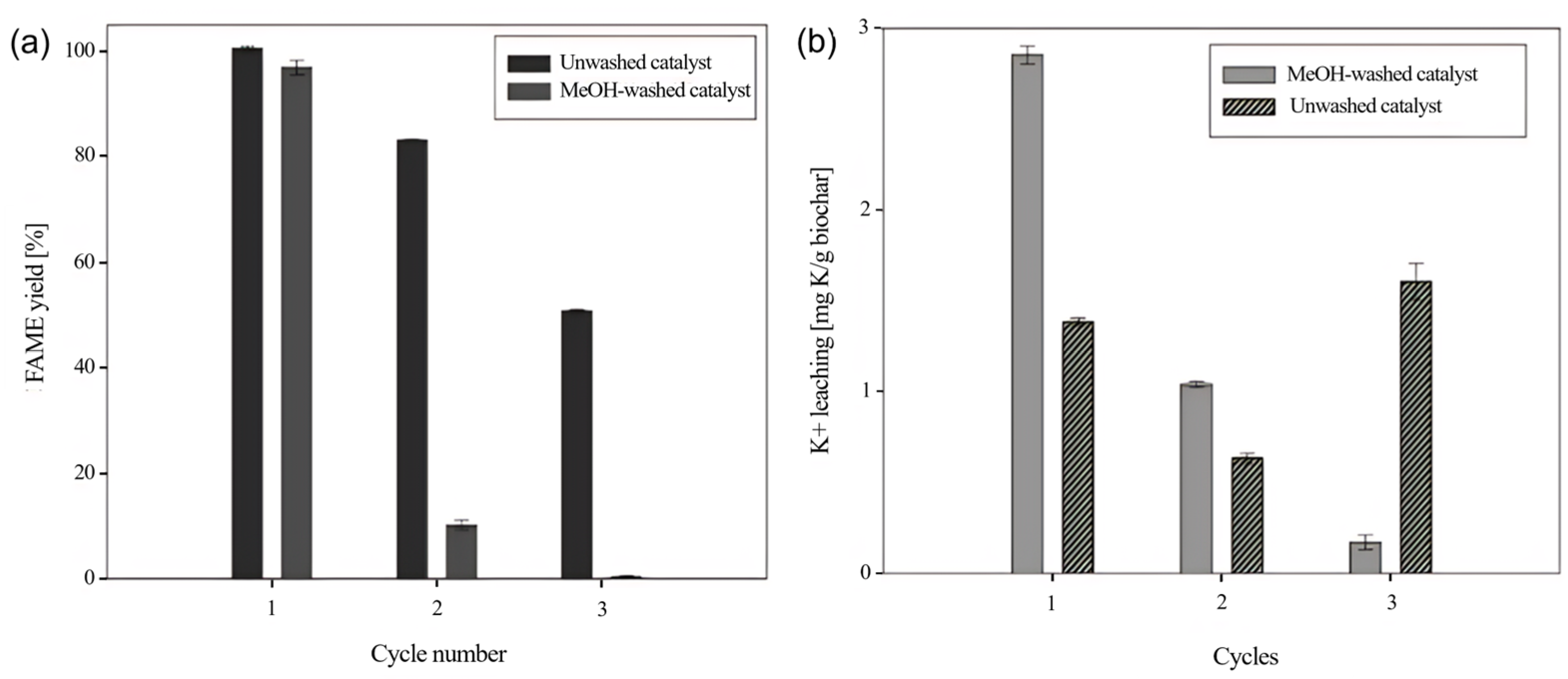
2.7. Catalyst Reuse
2.8. Energetic Demand and Costs Associated with the Catalyst and FAME Production
3. Materials and Methods
3.1. Activated Biochar Production
3.2. Characterization of Activated Biochar
3.3. Biodiesel Production
3.4. FAME Determination
3.5. Energetic Demand and Costs Associated with the Catalyst and FAME Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halkos, G.; Gkampoura, E.C. Assessing Fossil Fuels and Renewables’ Impact on Energy Poverty Conditions in Europe. Energies 2023, 16, 560. [Google Scholar] [CrossRef]
- Child, M.; Kemfert, C.; Bogdanov, D.; Breyer, C. Flexible Electricity Generation, Grid Exchange and Storage for the Transition to a 100% Renewable Energy System in Europe. Renew. Energy 2019, 139, 80–101. [Google Scholar] [CrossRef]
- Vohra, K.; Vodonos, A.; Schwartz, J.; Marais, E.A.; Sulprizio, M.P.; Mickley, L.J. Global Mortality from Outdoor Fine Particle Pollution Generated by Fossil Fuel Combustion: Results from GEOS-Chem. Environ. Res. 2021, 195, 110754. [Google Scholar] [CrossRef] [PubMed]
- UN. Paris Agreement; United Nations: New York, NY, USA, 2015; pp. 1–27. [Google Scholar]
- Smith, C.J.; Forster, P.M.; Allen, M.; Fuglestvedt, J.; Millar, R.J.; Rogelj, J.; Zickfeld, K. Current Fossil Fuel Infrastructure Does Not yet Commit Us to 1.5 °C Warming. Nat. Commun. 2019, 10, 101. [Google Scholar] [CrossRef]
- Corro, G.; Flores, A.; Pacheco-Aguirre, F.; Pal, U.; Bañuelos, F.; Ramirez, A.; Zehe, A. Biodiesel and Fossil-Fuel Diesel Soot Oxidation Activities of Ag/CeO2 Catalyst. Fuel 2019, 250, 17–26. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel Fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Zulqarnain; Yusoff, M.H.M.; Ayoub, M.; Hamza Nazir, M.; Zahid, I.; Ameen, M.; Abbas, W.; Shoparwe, N.F.; Abbas, N. Comprehensive Review on Biodiesel Production from Palm Oil Mill Effluent. ChemBioEng Rev. 2021, 8, 439–462. [Google Scholar] [CrossRef]
- Muanruksa, P.; Wongsirichot, P.; Winterburn, J.; Kaewkannetra, P. Integrated Cleaner Biocatalytic Process for Biodiesel Production from Crude Palm Oil Comparing to Refined Palm Oil. Catalysts 2021, 11, 734. [Google Scholar] [CrossRef]
- Barreiros, T.; Young, A.; Cavalcante, R.; Queiroz, E. Impact of Biodiesel Production on a Soybean Biorefinery. Renew. Energy 2020, 159, 1066–1083. [Google Scholar] [CrossRef]
- Sahar; Sadaf, S.; Iqbal, J.; Ullah, I.; Bhatti, H.N.; Nouren, S.; Habib-ur-Rehman; Nisar, J.; Iqbal, M. Biodiesel Production from Waste Cooking Oil: An Efficient Technique to Convert Waste into Biodiesel. Sustain. Cities Soc. 2018, 41, 220–226. [Google Scholar] [CrossRef]
- Wang, B.; Wang, B.; Shukla, S.K.; Wang, R. Enabling Catalysts for Biodiesel Production via Transesterification. Catalysts 2023, 13, 740. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Mohammadi, R.; Peighambardoust, S.H.; Ramavandi, B. Application of Waste Chalk/CoFe2O4/K2CO3 Composite as a Reclaimable Catalyst for Biodiesel Generation from Sunflower Oil. Chemosphere 2022, 289, 133226. [Google Scholar] [CrossRef]
- Tobío-Pérez, I.; Domínguez, Y.D.; Machín, L.R.; Pohl, S.; Lapuerta, M.; Piloto-Rodríguez, R. Biomass-Based Heterogeneous Catalysts for Biodiesel Production: A Comprehensive Review. Int. J. Energy Res. 2022, 46, 3782–3809. [Google Scholar] [CrossRef]
- Balajii, M.; Niju, S. Biochar-Derived Heterogeneous Catalysts for Biodiesel Production. Environ. Chem. Lett. 2019, 17, 1447–1469. [Google Scholar] [CrossRef]
- He, Y.; Chen, X.; Tang, X.; Chen, S.; Evrendilek, F.; Chen, T.; Dai, W.; Liu, J. Co-Combustion Dynamics and Products of Textile Dyeing Sludge with Waste Rubber versus Polyurethane Tires of Shared Bikes. J. Environ. Chem. Eng. 2023, 11, 109196. [Google Scholar] [CrossRef]
- Mo, Z.; He, Y.; Liu, J.; Tu, J.; Li, D.; Hu, C.; Zhang, Q.; Wang, K.; Wang, T. Biomass Steam Gasification for Hydrogen-Rich Syngas Production over Fly Ash-Based Catalyst Pretreated by Coupling of Washing and Calcination. Int. J. Hydrogen Energy 2024, 49, 164–176. [Google Scholar] [CrossRef]
- Joshi, N.C.; Sinha, S.; Bhatnagar, P.; Nath, Y.; Negi, B.; Kumar, V.; Gururani, P. A Concise Review on Waste Biomass Valorization through Thermochemical Conversion. Curr. Res. Microb. Sci. 2024, 6, 100237. [Google Scholar] [CrossRef]
- Okolie, J.A.; Epelle, E.I.; Tabat, M.E.; Orivri, U.; Amenaghawon, A.N.; Okoye, P.U.; Gunes, B. Waste Biomass Valorization for the Production of Biofuels and Value-Added Products: A Comprehensive Review of Thermochemical, Biological and Integrated Processes. Process Saf. Environ. Prot. 2022, 159, 323–344. [Google Scholar] [CrossRef]
- Chen, W.; Meng, J.; Han, X.; Lan, Y.; Zhang, W. Past, Present, and Future of Biochar. Biochar 2019, 1, 75–87. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and Its Importance on Nutrient Dynamics in Soil and Plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Srivastav, A.L.; Pham, T.D.; Izah, S.C.; Singh, N.; Singh, P.K. Biochar Adsorbents for Arsenic Removal from Water Environment: A Review. Bull. Environ. Contam. Toxicol. 2022, 108, 616–628. [Google Scholar] [CrossRef]
- Ghanbarpour Mamaghani, Z.; Hawboldt, K.A.; MacQuarrie, S. Adsorption of CO2 Using Biochar—Review of the Impact of Gas Mixtures and Water on Adsorption. J. Environ. Chem. Eng. 2023, 11, 109643. [Google Scholar] [CrossRef]
- Jung, J.M.; Oh, J.I.; Baek, K.; Lee, J.; Kwon, E.E. Biodiesel Production from Waste Cooking Oil Using Biochar Derived from Chicken Manure as a Porous Media and Catalyst. Energy Convers. Manag. 2018, 165, 628–633. [Google Scholar] [CrossRef]
- Azman, N.S.; Khairuddin, N.; Tengku Azmi, T.S.M.; Seenivasagam, S.; Hassan, M.A. Application of Biochar from Woodchip as Catalyst Support for Biodiesel Production. Catalysts 2023, 13, 489. [Google Scholar] [CrossRef]
- Farouk, S.M.; Tayeb, A.M.; Abdel-Hamid, S.M.S.; Osman, R.M. Recent Advances in Transesterification for Sustainable Biodiesel Production, Challenges, and Prospects: A Comprehensive Review. Environ. Sci. Pollut. Res. 2024, 31, 12722–12747. [Google Scholar] [CrossRef]
- Yadav, N.; Yadav, G.; Ahmaruzzaman, M. Fabrication of Surface-Modified Dual Waste-Derived Biochar for Biodiesel Production by Microwave-Assisted Esterification of Oleic Acid: Optimization, Kinetics, and Mechanistic Studies. Renew. Energy 2023, 218, 119308. [Google Scholar] [CrossRef]
- Sultana, M.; Ahmaruzzaman, M. Transforming Waste Biomass to a Heterogenous Acid Catalyst for Microwave-Assisted Biodiesel Production: Empirical Evaluation and Kinetic Study. Biomass Convers. Biorefin. 2025, 10, 1–19. [Google Scholar] [CrossRef]
- Bizualem, Y.D.; Nurie, A.G. A Review on Recent Biodiesel Intensification Process through Cavitation and Microwave Reactors: Yield, Energy, and Economic Analysis. Heliyon 2024, 10, e24643. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH Activation of Carbon-Based Materials for Energy Storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Durán, A.; Alonso, M.; Stoica, A.I. Investigation of Effectiveness of KOH-Activated Olive Pomace Biochar for Efficient Direct Air Capture of CO2. Sep. Purif. Technol. 2025, 352, 127997. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, P.; Li, L.; Wang, S.; Pan, Z.; Song, C.; Wang, T. Fabrication of Biomass-Derived Activated Carbon with Interconnected Hierarchical Architecture via H3PO4-Assisted KOH Activation for High-Performance Symmetrical Supercapacitors. J. Electroanal. Chem. 2021, 903, 115828. [Google Scholar] [CrossRef]
- Mitome, T.; Uchida, Y.; Egashira, Y.; Hayashi, K.; Nishiura, A.; Nishiyama, N. Adsorption of Indole on KOH-Activated Mesoporous Carbon. Colloids Surf. A Physicochem. Eng. Asp. 2013, 424, 89–95. [Google Scholar] [CrossRef]
- Khamkeaw, A.; Asavamongkolkul, T.; Perngyai, T.; Jongsomjit, B.; Phisalaphong, M. Interconnected Micro, Meso, and Macro Porous Activated Carbon from Bacterial Nanocellulose for Superior Adsorption Properties and Effective Catalytic Performance. Molecules 2020, 25, 4063. [Google Scholar] [CrossRef]
- Sevilla, M.; Díez, N.; Fuertes, A.B. More Sustainable Chemical Activation Strategies for the Production of Porous Carbons. ChemSusChem 2021, 14, 94–117. [Google Scholar] [CrossRef]
- Chen, W.; Gong, M.; Li, K.; Xia, M.; Chen, Z.; Xiao, H.; Fang, Y.; Chen, Y.; Yang, H.; Chen, H. Insight into KOH Activation Mechanism during Biomass Pyrolysis: Chemical Reactions between O-Containing Groups and KOH. Appl. Energy 2020, 278, 115730. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.P.; Qiu, L.L.; Xiao, J.; Wu, F.P.; Cao, J.P.; Bai, Y.H.; Liu, F.J. Insights into the KOH Activation Parameters in the Preparation of Corncob-Based Microporous Carbon for High-Performance Supercapacitors. Diam. Relat. Mater. 2022, 129, 109331. [Google Scholar] [CrossRef]
- Gale, M.; Nguyen, T.; Moreno, M.; Gilliard-Abdulaziz, K.L. Physiochemical Properties of Biochar and Activated Carbon from Biomass Residue: Influence of Process Conditions to Adsorbent Properties. ACS Omega 2021, 6, 10224–10233. [Google Scholar] [CrossRef]
- Schmitz, E.; Karlsson, E.N.; Adlercreutz, P.; Schmitz, C.E.; Leegood, E.R. Warming Weather Changes the Chemical Composition of Oat Hulls. Plant Biol. 2020, 22, 1086–1091. [Google Scholar] [CrossRef]
- Martinez-Toledo, C.; Valdes-Vidal, G.; Calabi-Floody, A.; Gonzalez, M.E.; Reyes-Ortiz, O. Evaluation of Rheological Properties of Asphalt Binder Modified with Biochar from Oat Hulls. Materials 2024, 17, 4312. [Google Scholar] [CrossRef]
- Han, L.P.; Wang, W.H.; Eneji, A.E.; Liu, J. Phytoremediating Coastal Saline Soils with Oats: Accumulation and Distribution of Sodium, Potassium, and Chloride Ions in Plant Organs. J. Clean. Prod. 2015, 90, 73–81. [Google Scholar] [CrossRef]
- González, M.E.; Cea, M.; Reyes, D.; Romero-Hermoso, L.; Hidalgo, P.; Meier, S.; Benito, N.; Navia, R. Functionalization of Biochar Derived from Lignocellulosic Biomass Using Microwave Technology for Catalytic Application in Biodiesel Production. Energy Convers. Manag. 2017, 137, 165–173. [Google Scholar] [CrossRef]
- Vasić, K.; Podrepšek, G.H.; Knez, Ž.; Leitgeb, M. Biodiesel Production Using Solid Acid Catalysts Based on Metal Oxides. Catalysts 2020, 10, 237. [Google Scholar] [CrossRef]
- Olutoye, M.A.; Hameed, B.H. Kinetics and Deactivation of a Dual-Site Heterogeneous Oxide Catalyst during the Transesterification of Crude Jatropha Oil with Methanol. J. Taibah Univ. Sci. 2016, 10, 685–699. [Google Scholar] [CrossRef]
- Chaos-Hernández, D.; Reynel-Avila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Aguayo-Villarreal, I.A. Functionalization and Activation of Carbon-Based Catalysts with KOH and Calcium and Their Application in Transesterification to Produce Biodiesel: Optimization of Catalytic Properties and Kinetic Study. Fuel 2022, 310, 122066. [Google Scholar] [CrossRef]
- Sarve, A.N.; Varma, M.N.; Sonawane, S.S. Ultrasound Assisted Two-Stage Biodiesel Synthesis from Non-Edible Schleichera Triguga Oil Using Heterogeneous Catalyst: Kinetics and Thermodynamic Analysis. Ultrason. Sonochem 2016, 29, 288–298. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, H.; Wang, Y.; Shan, R. Transesterification of Vegetable Oil on Low Cost and Efficient Meat and Bone Meal Biochar Catalysts. Energy Convers. Manag. 2017, 150, 214–221. [Google Scholar] [CrossRef]
- Osman, A.I.; Ayati, A.; Krivoshapkin, P.; Tanhaei, B.; Farghali, M.; Yap, P.S.; Abdelhaleem, A. Coordination-Driven Innovations in Low-Energy Catalytic Processes: Advancing Sustainability in Chemical Production. Coord. Chem. Rev. 2024, 514, 215900. [Google Scholar] [CrossRef]
- Motasemi, F.; Ani, F.N. A Review on Microwave-Assisted Production of Biodiesel. Renew. Sustain. Energy Rev. 2012, 16, 4719–4733. [Google Scholar] [CrossRef]
- Patil, P.D.; Gude, V.G.; Reddy, H.K.; Muppaneni, T.; Deng, S.; Patil, P.D.; Gude, V.G.; Reddy, H.K.; Muppaneni, T.; Deng, S. Biodiesel Production from Waste Cooking Oil Using Sulfuric Acid and Microwave Irradiation Processes. J. Environ. Prot. 2012, 3, 107–113. [Google Scholar] [CrossRef]
- Hincapié, G.M.; Valange, S.; Barrault, J.; Moreno, J.A.; López, D.P. Effect of Microwave-Assisted System on Transesterification of Castor Oil with Ethanol. Univ. Sci. 2014, 19, 193–200. [Google Scholar] [CrossRef]
- Salema, A.A.; Yeow, Y.K.; Ishaque, K.; Ani, F.N.; Afzal, M.T.; Hassan, A. Dielectric Properties and Microwave Heating of Oil Palm Biomass and Biochar. Ind. Crops Prod. 2013, 50, 366–374. [Google Scholar] [CrossRef]
- Devasan, R.; Ruatpuia, J.V.L.; Gouda, S.P.; Kodgire, P.; Basumatary, S.; Halder, G.; Rokhum, S.L. Microwave-Assisted Biodiesel Production Using Bio-Waste Catalyst and Process Optimization Using Response Surface Methodology and Kinetic Study. Sci. Rep. 2023, 13, 2570. [Google Scholar] [CrossRef]
- Kataria, J.; Mohapatra, S.K.; Kundu, K. Biodiesel Production from Frying Oil Using Zinc-Doped Calcium Oxide as Heterogeneous Catalysts. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 861–866. [Google Scholar] [CrossRef]
- Samart, C.; Sreetongkittikul, P.; Sookman, C. Heterogeneous Catalysis of Transesterification of Soybean Oil Using KI/Mesoporous Silica. Fuel Process. Technol. 2009, 90, 922–925. [Google Scholar] [CrossRef]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar Surface Functional Groups as Affected by Biomass Feedstock, Biochar Composition and Pyrolysis Temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Huang, H.; Tang, J.; Gao, K.; He, R.; Zhao, H.; Werner, D. Characterization of KOH Modified Biochars from Different Pyrolysis Temperatures and Enhanced Adsorption of Antibiotics. RSC Adv. 2017, 7, 14640–14648. [Google Scholar] [CrossRef]
- Stoytcheva, M.; Montero, G.; Stoytcheva, M.; Montero, G. Biodiesel—Feedstocks and Processing Technologies; IntechOpen Limited: London, UK, 2011. [Google Scholar] [CrossRef]
- Antonioli, D.; Ghisetti, C.; Mazzanti, M.; Nicolli, F. Sustainable Production: The Economic Returns of Circular Economy Practices. Bus. Strategy Environ. 2022, 31, 2603–2617. [Google Scholar] [CrossRef]
- Demir, V.G.; Yuksel, H.; Koten, H.; Zafer Gul, M.; Soyhan, H.S. Microwave-Assisted Pilot-Scale Biodiesel Production and Engine Tests. Proc. Inst. Civ. Eng.-Energy 2019, 172, 1–11. [Google Scholar] [CrossRef]
- Rhithuparna, D.; Ghosh, N.; Lalthazuala Rokhum, S.; Halder, G. Current Progress and Future Outlooks of Microwave-Irradiated Biodiesel Production: A Holistic Review. Chem. Eng. J. 2024, 482, 149033. [Google Scholar] [CrossRef]
- Dąbrowska, S.; Chudoba, T.; Wojnarowicz, J.; Łojkowski, W. Current Trends in the Development of Microwave Reactors for the Synthesis of Nanomaterials in Laboratories and Industries: A Review. Crystals 2018, 8, 379. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Granados, M.; Sentellas, S.; Saurina, J. Microwave-Assisted Extraction with Natural Deep Eutectic Solvents for Polyphenol Recovery from Agrifood Waste: Mature for Scaling-Up? Sci. Total Environ. 2024, 912, 168716. [Google Scholar] [CrossRef] [PubMed]
- Liow, M.Y.; Gourich, W.; Chang, M.Y.; Loh, J.M.; Chan, E.S.; Song, C.P. Towards Rapid and Sustainable Synthesis of Biodiesel: A Review of Effective Parameters and Scale-up Potential of Intensification Technologies for Enzymatic Biodiesel Production. J. Ind. Eng. Chem. 2022, 114, 1–18. [Google Scholar] [CrossRef]



| Run | Identification Sample | YBET (m2/g) | YVp (cm3/g) | YPd (nm) |
|---|---|---|---|---|
| 1 | AB1 | 411.94 | 0.40 | 3.51 |
| 2 | AB2 | 99.91 | 0.17 | 4.17 |
| 3 | AB3 | 87.56 | 0.11 | 3.84 |
| 4 | AB4 | 36.30 | 0.06 | 3.93 |
| 5 | AB5 | 482.71 | 0.70 | 3.33 |
| 6 | AB6 | 131.45 | 0.28 | 3.97 |
| 7 | AB7 | 50.49 | 0.07 | 4.07 |
| 8 | AB8 | 119.02 | 0.18 | 3.97 |
| 9 | AB9 | 19.75 | 0.05 | 4.94 |
| 10 | AB10 | 637.28 | 0.348 | 3.10 |
| 11 | AB11 | 58.65 | 0.01 | 4.09 |
| Factors or Interactions | p-Value of Factors of YBET |
|---|---|
| A | 0.0101 * |
| B | 0.0076 * |
| C | 0.0145 * |
| AB | 0.3842 |
| AC | 0.0016 * |
| BC | 0.7709 |
| ABC | 0.0080 * |
| Model | 0.0068 * |
| R2 | 0.9981 |
| Parameters | Predicted by Model | Experimental Value |
|---|---|---|
| Specific surface area (SSA) BET for AB (m2/g) | 748.63 | 637.28 ± 8.32 |
| Samples | Elemental Analysis | Ash (%) | |||||
|---|---|---|---|---|---|---|---|
| %C | %H | %N | %S | %O | |||
| Activated biochar (AB) | AB1 | 71.2 | 2.0 | 0.8 | n.d. | 15.2 | 10.8 |
| AB2 | 73.2 | 2.1 | 1.0 | n.d. | 16.0 | 7.7 | |
| AB3 | 75.6 | 2.3 | 1.2 | n.d. | 14.2 | 6.7 | |
| AB4 | 74.3 | 2.4 | 1.2 | n.d. | 15.9 | 6.2 | |
| AB5 | 76.2 | 1.9 | 1.0 | n.d. | 14.0 | 6.9 | |
| AB6 | 74.8 | 2.0 | 1.4 | n.d. | 15.8 | 6.0 | |
| AB7 | 71.7 | 2.0 | 0.9 | n.d. | 17.9 | 9.9 | |
| AB8 | 71.0 | 2.7 | 1.3 | n.d. | 18.6 | 6.4 | |
| AB9 | 74.3 | 2.6 | 1.4 | n.d. | 18.6 | 3.1 | |
| AB10 | 78.1 | 2.2 | 1.2 | n.d. | 13.4 | 5.1 | |
| AB11 | 74.8 | 2.3 | 1.4 | n.d. | 15.6 | 5.9 | |
| Biochar pyrolyzed at 600 °C | B600 | 78.3 | 4.7 | 1.9 | n.d. | 5.3 | 9.8 |
| Material | SSA BET (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) | Potassium Content (%) |
|---|---|---|---|---|
| B600 | 108.28 | 0.008 | 2.24 | 1.72 |
| AB | 637.28 | 0.348 | 1.10 | 9.40 |
| Run | Identification Sample | YFAME (%) |
|---|---|---|
| 1 | B1 | 84 |
| 2 | B2 | 59 |
| 3 | B3 | 100 |
| 4 | B4 | 100 |
| 5 | B5 | 55 |
| 6 | B6 | 94 |
| 7 | B7 | 65 |
| 8 | B8 | 61 |
| 9 | B9 | 84 |
| 10 | B10 | 64 |
| 11 | B11 | 100 |
| 12 | B12 | 100 |
| 13 | B13 | 100 |
| 14 | B14 | 83 |
| 15 | B15 | 59 |
| Factor or interactions | p-value of YFAME | |
| A | 0.0185 * | |
| B | <0.0001 * | |
| C | <0.0001 * | |
| AB | <0.0001 * | |
| Model | <0.0001 * | |
| R2 | 0.9895 | |
| Mass (kg) | Volume (L) | Price (USD) | Qs (MJ) | MJ/kg | Time (h) | Energy Required (kW) | |
|---|---|---|---|---|---|---|---|
| Oat hull | 4 | 0.23 | |||||
| KOH | 8 | 134.50 | |||||
| Mixing water | 10 | 10 | 0.02 | ||||
| Biomass drying | 0.34 | 7.78 | |||||
| Pyrolysis | 1.39 | 32.40 | 3 | 3 | |||
| HCl 1N | 8.16 | 8 | 39.30 | 545.62 | 545.62 | ||
| Wash water | 24 | 24 | 0.89 | 7022.40 | 7022.4 | ||
| Catalysts drying | 0.34 | 7.78 | |||||
| Total | 176.97 | 7615.9 |
| Mass (kg) | Volume (L) | Price (USD) | Qs (MJ) | QL (MJ) | Et (MJ) | Energy Required (kW) | |
|---|---|---|---|---|---|---|---|
| Catalyst | 0.025 | 4.43 | |||||
| WCO | 1 | 1.1 | 0.44 | ||||
| Methanol | 0.38 | 0.49 | 4.03 | 126.46 | 0.42 | 126.88 | |
| Microwave | <0.01 | 0.01 | 0.20 | ||||
| Total | 8.90 | 126.90 |
| Level | Factors | |||
|---|---|---|---|---|
| X1 Temperature (°C) | X2 Residence Time (h) | X3 KOH/Biomass Ratio | ||
| Low (−1) | 400 | 1.0 | 0.5 | |
| Middle (0) | 500 | 2.0 | 1.25 | |
| High (+1) | 600 | 3.0 | 2.0 | |
| Run | Identification Sample | X1 (°C) | X2 (h) | X3 |
| 1 | AB1 | 600 | 1 | 2 |
| 2 | AB2 | 400 | 1 | 0.5 |
| 3 | AB3 | 500 | 2 | 1.25 |
| 4 | AB4 | 400 | 3 | 0.5 |
| 5 | AB5 | 400 | 3 | 2 |
| 6 | AB6 | 500 | 2 | 1.25 |
| 7 | AB7 | 600 | 3 | 0.5 |
| 8 | AB8 | 500 | 2 | 1.25 |
| 9 | AB9 | 400 | 1 | 2 |
| 10 | AB10 | 600 | 3 | 2 |
| 11 | AB11 | 600 | 1 | 0.5 |
| Level | Factors | |||
|---|---|---|---|---|
| X1 Temperature (°C) | X2 Catalyst Dosage (%) | X3 WCO/MeOH Molar Ratio | ||
| Low (−1) | 150 | 2.5 | 1/12 | |
| Middle (0) | 200 | 6.25 | 1/30 | |
| High (+1) | 250 | 10 | 1/48 | |
| Run | Identification Sample | X1 (°C) | X2 (%) | X3 |
| 1 | B1 | 200 | 6.25 | 1/30 |
| 2 | B2 | 250 | 10 | 1/12 |
| 3 | B3 | 250 | 2.5 | 1/48 |
| 4 | B4 | 150 | 2.5 | 1/12 |
| 5 | B5 | 250 | 10 | 1/12 |
| 6 | B6 | 250 | 2.5 | 1/48 |
| 7 | B7 | 150 | 10 | 1/12 |
| 8 | B8 | 150 | 10 | 1/48 |
| 9 | B9 | 200 | 6.25 | 1/30 |
| 10 | B10 | 150 | 10 | 1/48 |
| 11 | B11 | 250 | 2.5 | 1/48 |
| 12 | B12 | 150 | 2.5 | 1/12 |
| 13 | B13 | 150 | 2.5 | 1/12 |
| 14 | B14 | 200 | 6.25 | 1/30 |
| 15 | B15 | 250 | 10 | 1/48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ñanculeo, J.; Nahuelcura, B.; Cea, M.; Abreu, N.; Garrido-Miranda, K.; Meier, S.; Romero-García, J.M.; González, M.E. Microwave-Assisted Biodiesel Production Using Activated Oat Hull-Derived Biochar as Catalyst. Catalysts 2025, 15, 729. https://doi.org/10.3390/catal15080729
Ñanculeo J, Nahuelcura B, Cea M, Abreu N, Garrido-Miranda K, Meier S, Romero-García JM, González ME. Microwave-Assisted Biodiesel Production Using Activated Oat Hull-Derived Biochar as Catalyst. Catalysts. 2025; 15(8):729. https://doi.org/10.3390/catal15080729
Chicago/Turabian StyleÑanculeo, Jaime, Benjamín Nahuelcura, Mara Cea, Norberto Abreu, Karla Garrido-Miranda, Sebastián Meier, Juan Miguel Romero-García, and María Eugenia González. 2025. "Microwave-Assisted Biodiesel Production Using Activated Oat Hull-Derived Biochar as Catalyst" Catalysts 15, no. 8: 729. https://doi.org/10.3390/catal15080729
APA StyleÑanculeo, J., Nahuelcura, B., Cea, M., Abreu, N., Garrido-Miranda, K., Meier, S., Romero-García, J. M., & González, M. E. (2025). Microwave-Assisted Biodiesel Production Using Activated Oat Hull-Derived Biochar as Catalyst. Catalysts, 15(8), 729. https://doi.org/10.3390/catal15080729











