Assessment of Platinum Catalyst in Rice Husk Combustion: A Comparative Life Cycle Analysis with Conventional Methods
Abstract
1. Introduction
2. Results and Discussion
2.1. Experimental Results of the Conventional and Catalyst Systems
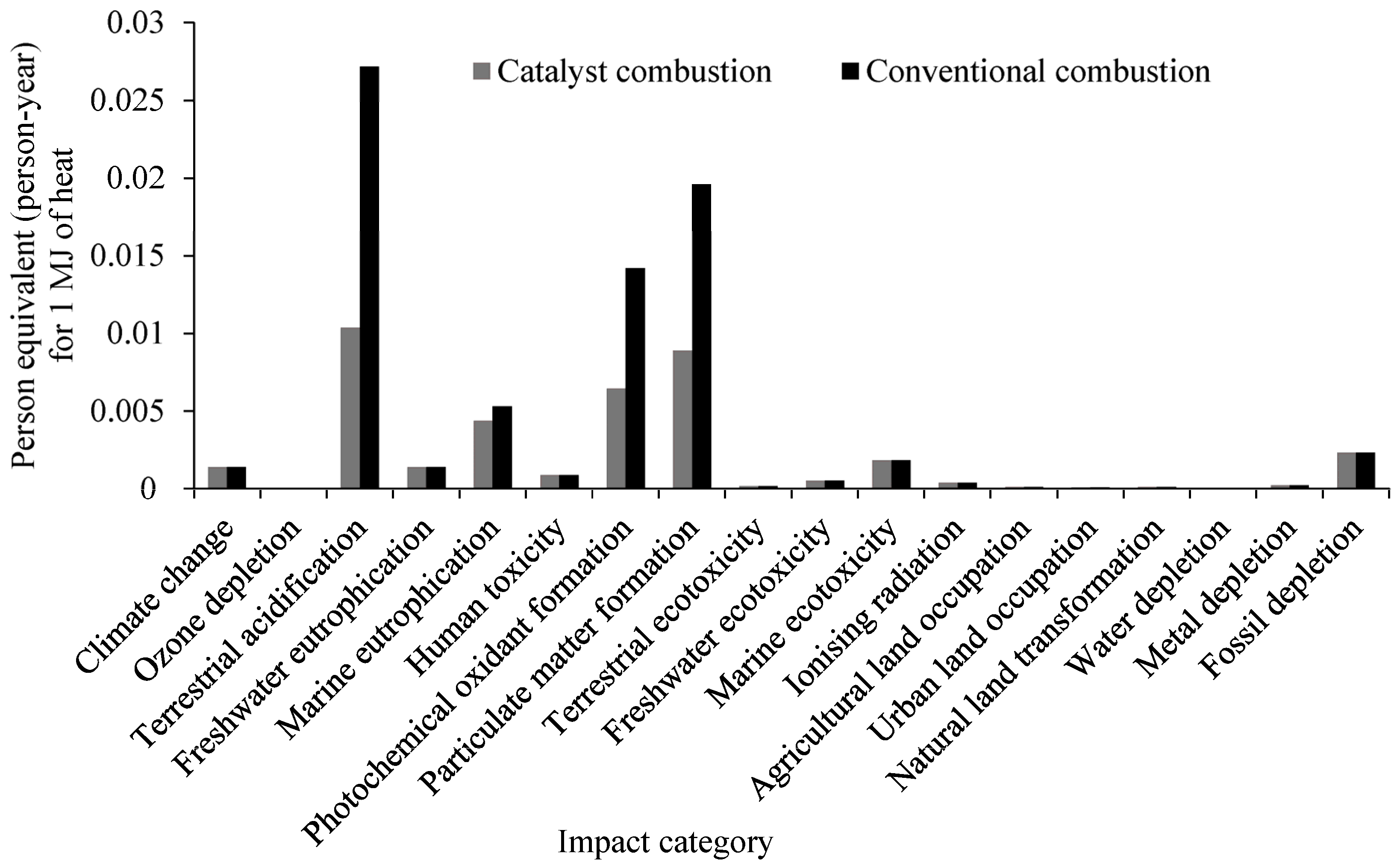
2.2. Scenario 1: LCA Comparison of Characterized Results of Rice Husk Combustion
2.3. Scenario 2: LCA Endpoint Evaluation in Terms of Damage Done to Human Health (DALY)
2.4. Normalized Results
3. Materials and Methods
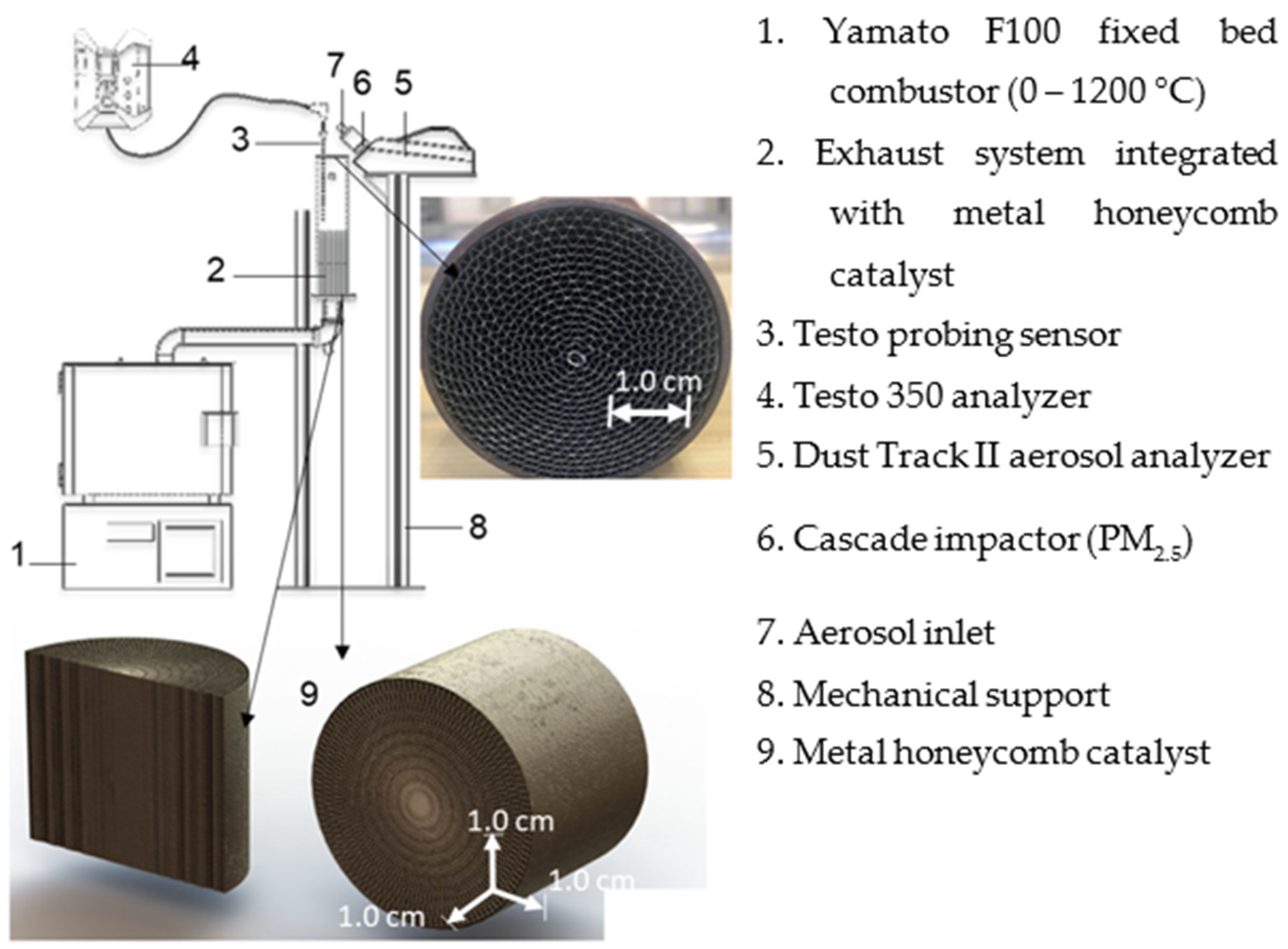
3.1. Experimental Setup and Data Collection
3.2. Goal and Scope Definition
3.3. Inventories for the Conventional Combustion System and the Integrated Catalyst Combustion System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IEA Bioenergy. IEA Bioenergy Annual Report 2017-Task 42; IEA Bioenergy: Valencia, Spain, 2018; p. 144. Available online: http://task42.ieabioenergy.com/publications/iea-bioenergy-annual-report-2017 (accessed on 17 July 2025).
- Rebryk, A.; Kozyatnyk, I.; Njenga, M. Emission of volatile organic compounds during open fire cooking with wood biomass: Traditional three-stone open fire vs. gasifier cooking stove in rural Kenya. Sci. Total Environ. 2024, 934, 173183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, K.; Huang, J.; Feng, Y.; Yellezuome, D.; Zhao, R.; Chen, T.; Wu, J. Synergistic effect and volatile emission characteristics during co-combustion of biomass and low-rank coal. Energy 2024, 289, 130015. [Google Scholar] [CrossRef]
- Jha, G.; Soren, S.; Mehta, K.D. Life cycle assessment of sintering process for carbon footprint and cost reduction: A comparative study for coke and biomass-derived sintering process. J. Clean. Prod. 2020, 259, 120889. [Google Scholar] [CrossRef]
- Piyathissa, S.D.S.; Kahandage, P.D.; Namgay; Zhang, H.; Noguchi, R.; Ahamed, T. Introducing a Novel Rice Husk Combustion Technology for Maximizing Energy and Amorphous Silica Production Using a Prototype Hybrid Rice Husk Burner to Minimize Environmental Impacts and Health Risk. Energies 2023, 16, 1120. [Google Scholar] [CrossRef]
- Emmanuel, O.; Ezeji, T.C. Utilization of biomass-based resources for biofuel production: A mitigating approach towards zero emission. Sustain. Chem. One World 2024, 2, 100007. [Google Scholar] [CrossRef]
- Mörtenkötter, H.; Kerscher, F.; Schönsteiner, M.; DeYoung, S.; Fendt, S.; Spliethoff, H. Potassium release and mitigation by additives in different biomass combustion systems. Fuel 2024, 369, 131800. [Google Scholar] [CrossRef]
- Huo, X.; Jia, H.; Song, C.; Yun, F.; Hao, S.; Ding, Y.; Liu, S.; Lei, M. Investigation of mitigation of nitric oxide emission characteristics and slagging properties from biomass combustion by the additive of coal gangue. J. Environ. Chem. Eng. 2022, 10, 107573. [Google Scholar] [CrossRef]
- Norizam, N.N.A.N.; Szuhánszki, J.; Ahmed, I.; Yang, X.; Ingham, D.; Milkowski, K.; Gheit, A.; Heeley, A.; Ma, L.; Pourkashanian, M. Impact of the blending of kaolin on particulate matter (PM) emissions in a biomass field-scale 250 kW grate boiler. Fuel 2024, 374, 132454. [Google Scholar] [CrossRef]
- Cheng, W.; Zhu, Y.; Shao, Y.; Zhang, W.; Wu, G.; Jiang, H.; Hu, J.; Huang, Z.; Chen, H. Mitigation of ultrafine particulate matter emission from agricultural biomass pellet combustion by the additive of phosphoric acid modified kaolin. Renew Energy 2021, 172, 177–187. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, J.; Chen, T.; Yellezuome, D.; Zhao, R.; Wu, J. Oxy-fuel co-combustion properties and N-containing pollutants release characteristics of biomass/coal blends. J. Energy Inst. 2024, 117, 101800. [Google Scholar] [CrossRef]
- Parisi, A.; Di Natale, F. An analytical model-based assessment of Wet Electrostatic Scrubbing for mitigating fine and ultrafine particles emissions in domestic biomass boilers. J. Electrostat. 2024, 129, 103921. [Google Scholar] [CrossRef]
- Medina, P.; Mora, A.; Beltrán, A. Combustion efficiency and CO/NOX emissions for a biomass plancha-type stove: Effect of the air excess ratio. Therm. Sci. Eng. Prog. 2024, 48, 102411. [Google Scholar] [CrossRef]
- Abah, E.O.; Ahamed, T.; Noguchi, R. Investigative Study for Low Particulate Matter Emission in Rice Husk Combustion Emmanuel. J. Jpn. Inst. Energy 2020, 99, 220–229. [Google Scholar] [CrossRef]
- Abah, E.O.; Iwai, K.; Sakurai, K.; Noguchi, R. Comparative Real-time Assessment of Particulate Matter Emissions in Rice Husk Combustion. J. Jpn. Inst. Energy 2018, 97, 222–225. [Google Scholar] [CrossRef]
- He, K.; Shen, Z.; Yang, Y.; Zhang, B.; Sun, J.; Xu, H.; Ho, S.S.H.; Cao, J. Insight into emission reduction effect of coal and biomass mixed briquette fuel. J. Clean. Prod. 2024, 471, 143419. [Google Scholar] [CrossRef]
- Al-qazzaz, A.; Eidgah, E.E.F.; Alfatlawi, A.-W.; Masroori, A.; Abed, A.M.; Kianifar, A. An approach of analyzing gas and biomass combustion: Positioned of flame stability and pollutant reduction. Results Eng. 2024, 23, 102823. [Google Scholar] [CrossRef]
- Avor, E.P.; Supap, T.; Narku-Tetteh, J.; Muchan, P.; Natewong, P.; Appiah, F.A.; Idem, R. Achieving net-zero CO2 emissions from indirect co-combustion of biomass and natural gas with carbon capture using a novel amine blend. Int. J. Greenh. Gas Control. 2023, 130, 104005. [Google Scholar] [CrossRef]
- Vento, E.; Hartikainen, A.; Tikka, A.; Lamberg, H.; Sippula, O.; Kilpeläinen, A. Integrating aerosol emissions of forest biomass into a life cycle assessment of forest-based production. Biomass Bioenergy 2024, 183, 107156. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, L.; Chen, M.; Yu, Z.; Tang, J.; Tu, Y. Advanced orthogonal analysis of multi-factorial interactions influencing co-combustion performance and pollution reduction potential of coal gangue and biomass in oxy-fuel combustion conditions. Process Saf. Environ. Prot. 2024, 190, 1188–1201. [Google Scholar] [CrossRef]
- Kahandage, P.D.; Piyathissa, S.D.S.; Ariesca, R.; Namgay; Ishizaki, R.; Kosgollegedara, E.J.; Weerasooriya, G.V.T.V.; Ahamed, T.; Noguchi, R. Comparative Analysis of Paddy Harvesting Systems toward Low-Carbon Mechanization in the Future: A Case Study in Sri Lanka. Processes 2023, 11, 1851. [Google Scholar] [CrossRef]
- Simonov, A.D.; Mishenko, T.I. Combustion and processing of rice husk in the vibrofluidized bed of catalyst or inert material. Chem. Sustain. Dev. 2003, 11, 277–283. [Google Scholar]
- Abah, E.O.; Ahamed, T.; Noguchi, R. Catalytic Temperature Effects on Conversion Efficiency of PM2.5 and Gaseous Emissions from Rice Husk Combustion. Energies 2021, 14, 6131. [Google Scholar] [CrossRef]
- Kahandage, P.D.; Rupasinghe, C.P.; Ariyawansha, K.T.; Piyathissa, S.D.S. Assessing environmental impacts of chemical fertilizers and organic fertilizers in Sri Lankan paddy fields through life cycle analysis. J. Dry Zone Agric. 2023, 9, 45–65. [Google Scholar] [CrossRef]
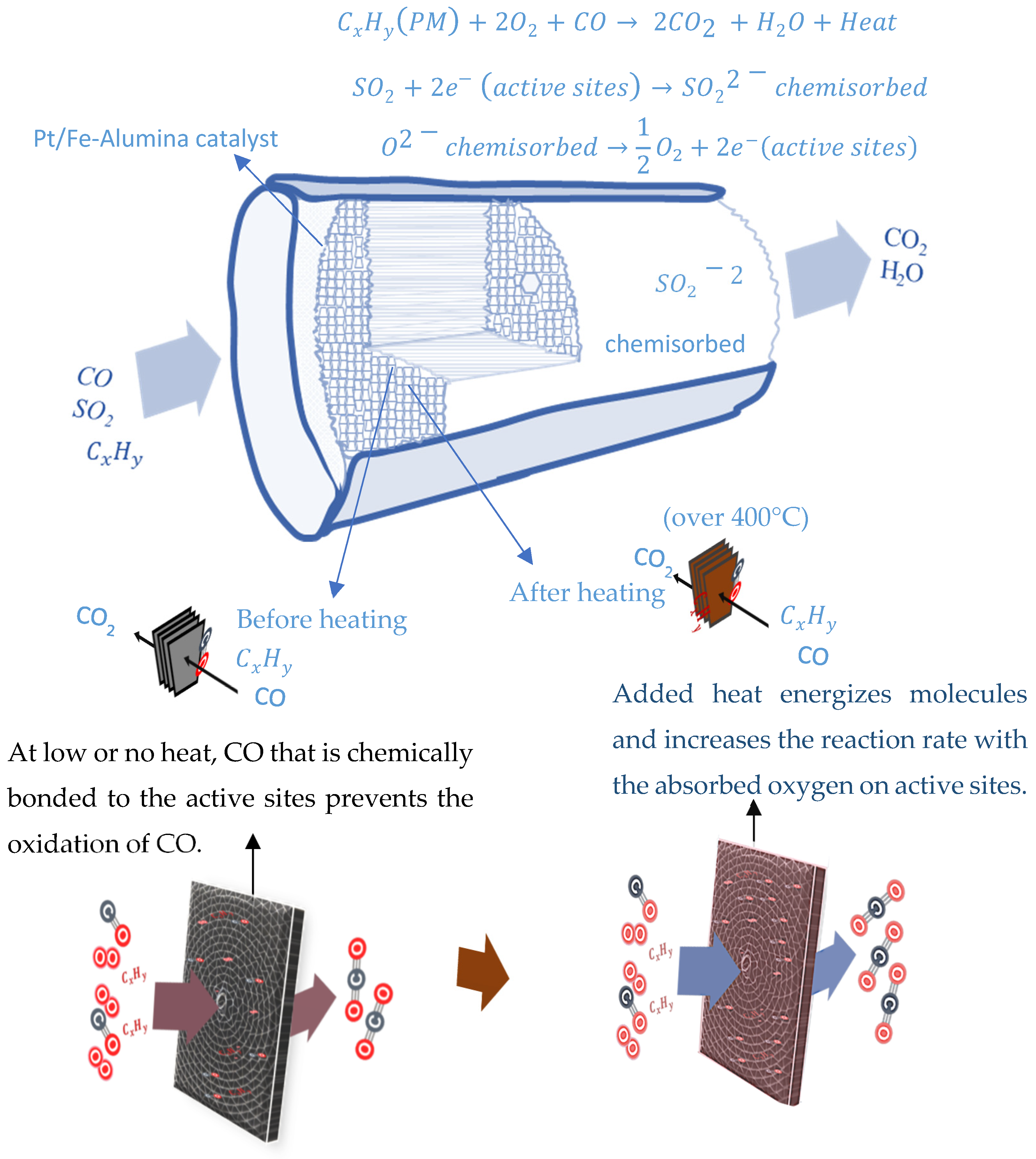
- Tomita, A.; Shimizu, K.; Kato, K.; Akita, T.; Tai, Y. Mechanism of Low-Temperature CO Oxidation on Pt/Fe-Containing Alumina Catalysts Pretreated with Water. J. Phys. Chem. C 2013, 117, 1268–1277. [Google Scholar] [CrossRef]
- Engel, T.; Ertl, G. Surface residence times and reaction mechanism in the catalytic oxidation of CO on Pd(111). Chem. Phys. Lett. 1978, 54, 95–98. [Google Scholar] [CrossRef]
- Ren, J.; Cao, J.-P.; Yang, F.-L.; Liu, Y.-L.; Tang, W.; Zhao, X.-Y. Understandings of Catalyst Deactivation and Regeneration during Biomass Tar Reforming: A Crucial Review. ACS Sustain. Chem. Eng. 2021, 9, 17186–17206. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Catalyst deactivation and regeneration. In Hydrogen Science and Engineering; Wiley-VCH: Weinheim, Germany, 2002; pp. 577–593. [Google Scholar]
- Gomez, A.; Mahinpey, N. Kinetic study of coal steam and CO2 gasification: A new method to reduce interparticle diffusion. Fuel 2015, 148, 160–167. [Google Scholar] [CrossRef]
- Tomita, A.; Miki, T.; Tai, Y. Effect of water treatment and Ce doping of Pt/Al2O3 catalysts on Pt sintering and propane oxidation. Res. Chem. Intermed. 2021, 47, 2935–2950. [Google Scholar] [CrossRef]
- AIST: Catalyst Preparation Technology to Improve the Oxidation Ability of Platinum Catalysts. Available online: https://www.aist.go.jp/aist_j/new_research/2012/nr20120412/nr20120412.html (accessed on 17 July 2025).
- UNECE’s Life Cycle Assessment of Electricity Generation Options-Nucleation Capital. Available online: https://nucleationcapital.com/portfolio/uneces-life-cycle-assessment-of-electricity-generation-options (accessed on 17 July 2025).
- AR5 Climate Change 2014: Mitigation of Climate Change—IPCC. Available online: https://www.ipcc.ch/report/ar5/wg3 (accessed on 17 July 2025).
- Omrani, M.; Goriaux, M.; Liu, Y.; Martinet, S.; Jean-Soro, L.; Ruban, V. Platinum group elements study in automobile catalysts and exhaust gas samples. Environ. Pollut. 2020, 257, 113477. [Google Scholar] [CrossRef]
- Golunski, S.E. Final analysis. Platin Met. Rev. 2007, 51, 162. [Google Scholar] [CrossRef]
- Anand, S.S.; Philip, B.K.; Mehendale, H.M. Volatile Organic Compounds. Encycl. Toxicol. Third Ed. 2014, 3, 967–970. [Google Scholar] [CrossRef]
- Pandey, B.; Choudhary, K.K. Air pollution: Role in climate change and its impact on crop plants. In Climate Change and Agricultural Ecosystems; Woodhead Publishing: Cambridge, UK, 2019; pp. 211–247. [Google Scholar] [CrossRef]
- Kwofie, E.M.; Ngadi, M. A comparative lifecycle assessment of rural parboiling system and an integrated steaming and drying system fired with rice husk. J. Clean. Prod. 2017, 140, 622–630. [Google Scholar] [CrossRef]
- ReCiPe-PRé Sustainability. Available online: https://pre-sustainability.com/articles/recipe (accessed on 17 July 2025).
- Prasara-A, J. Comparative Life Cycle Assessment of Rice Husk Utilization in Thailand. Ph.D. Thesis, RMIT University, Melbourne, Australia, 2009. [Google Scholar]
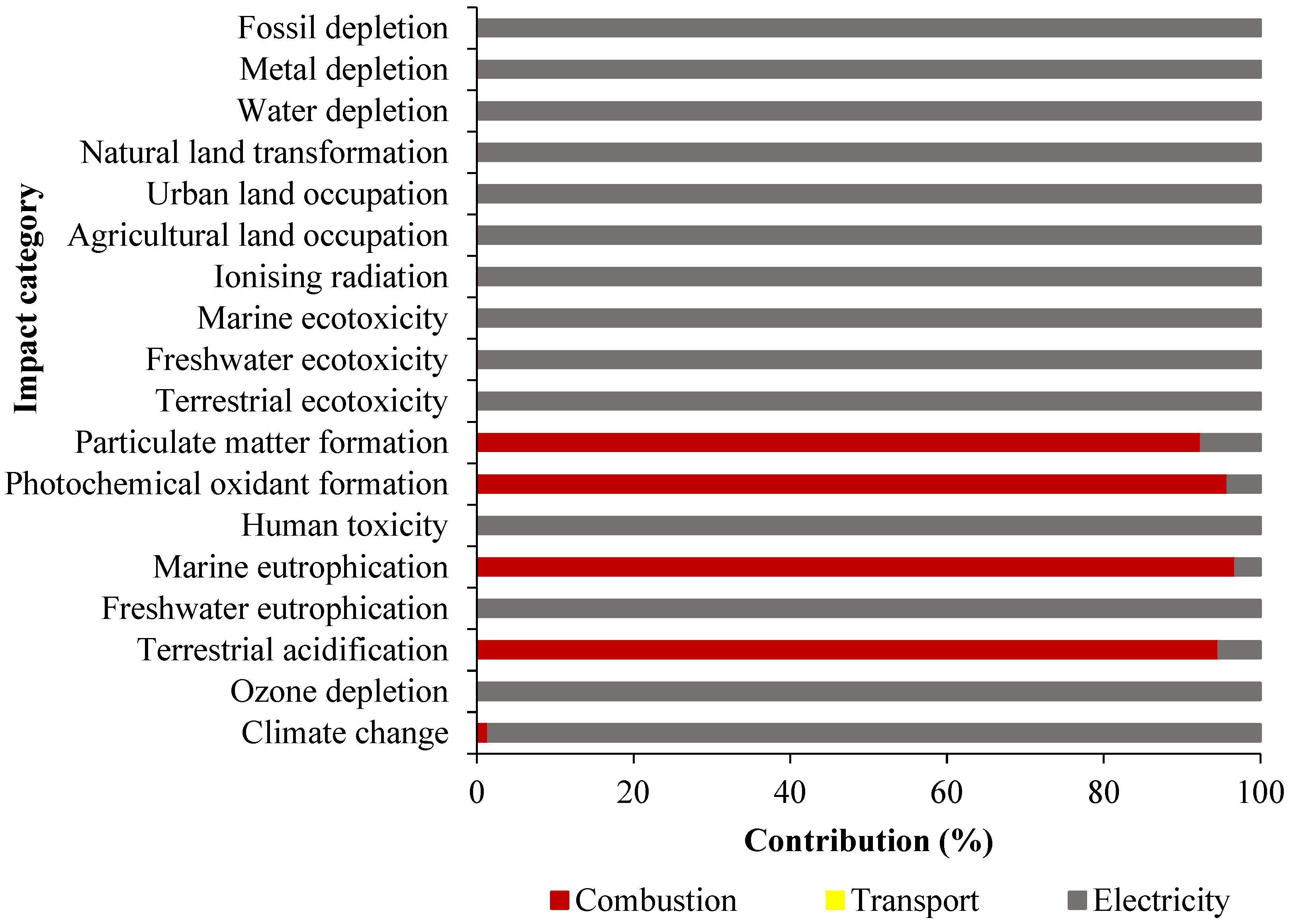
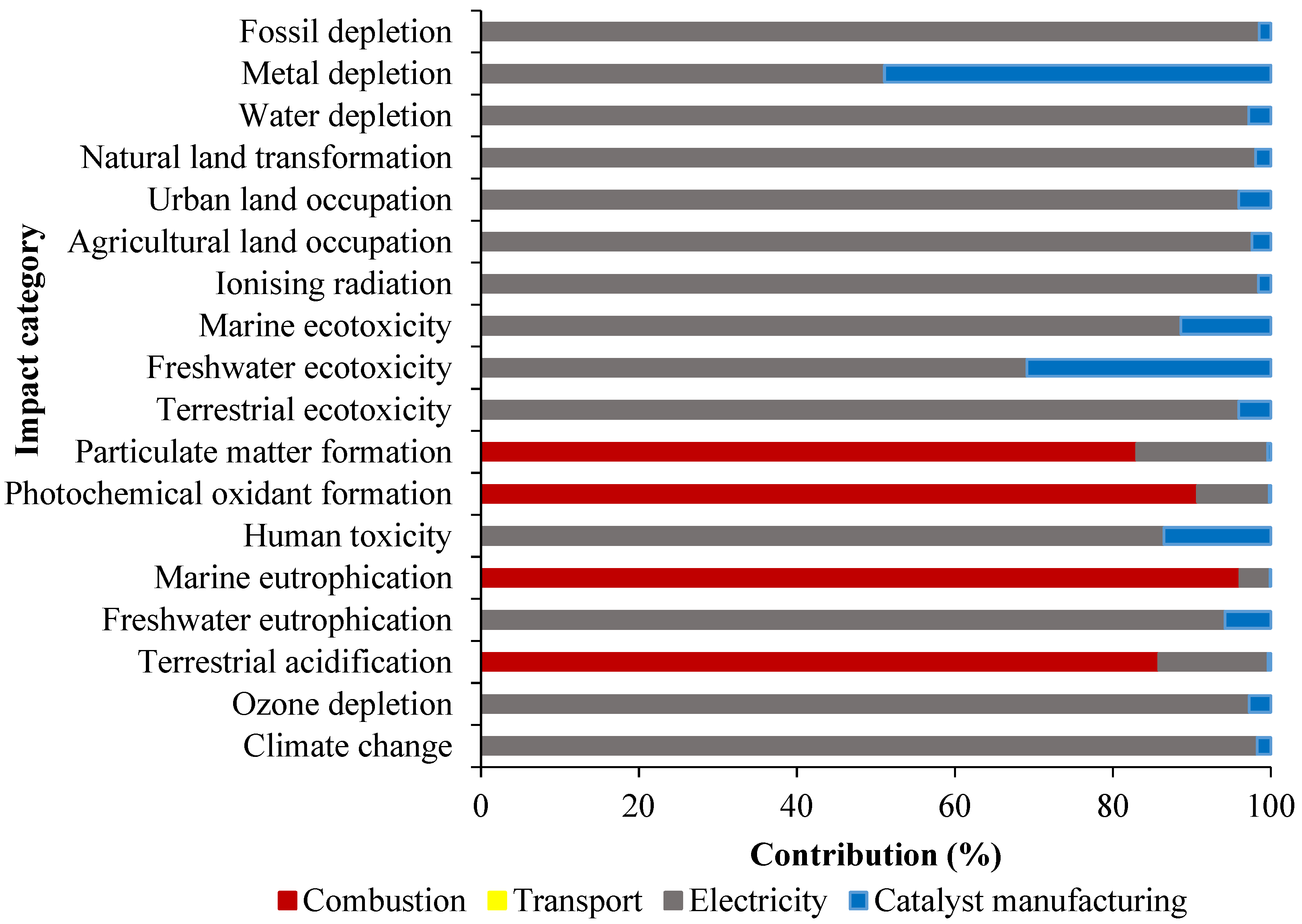
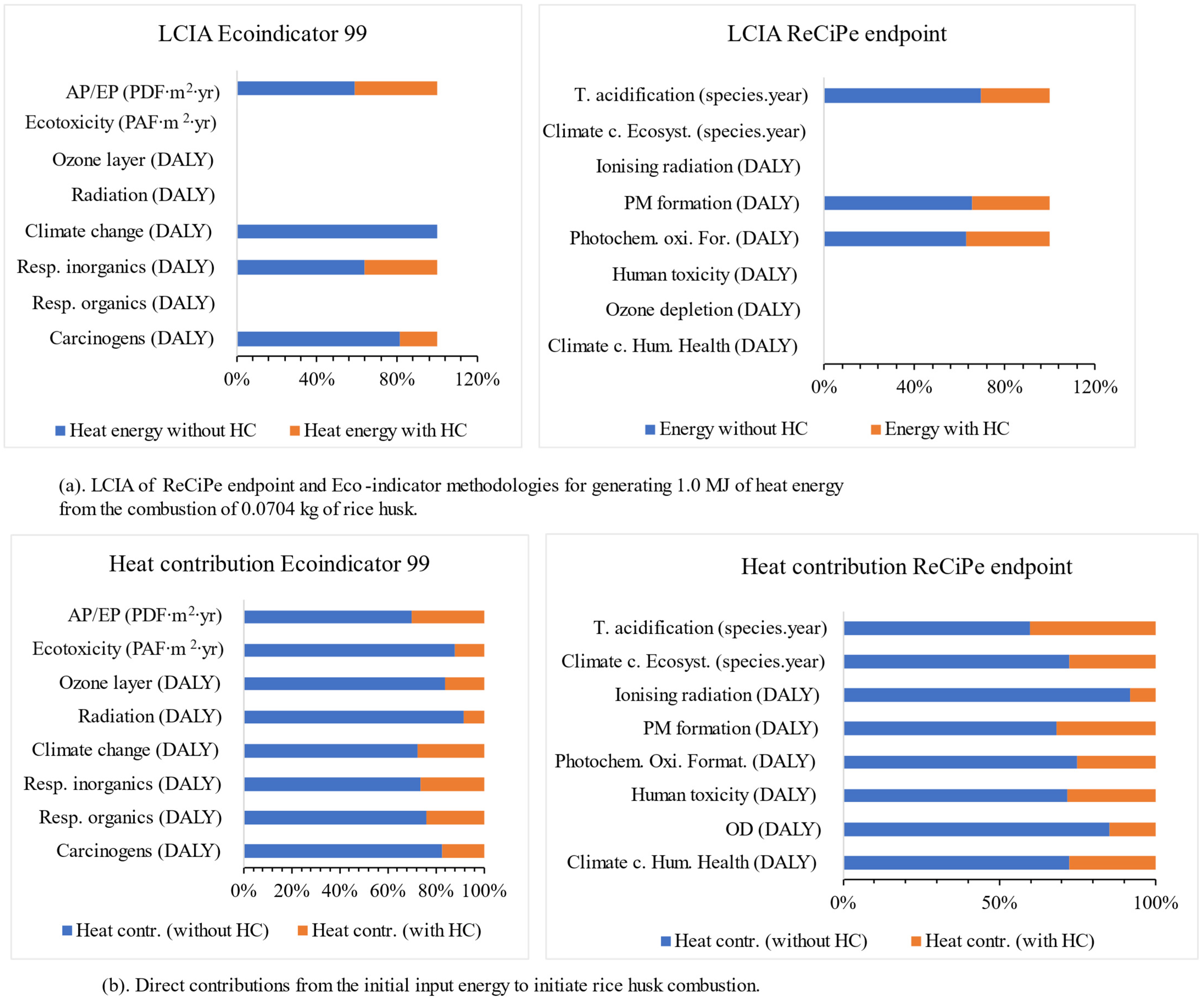
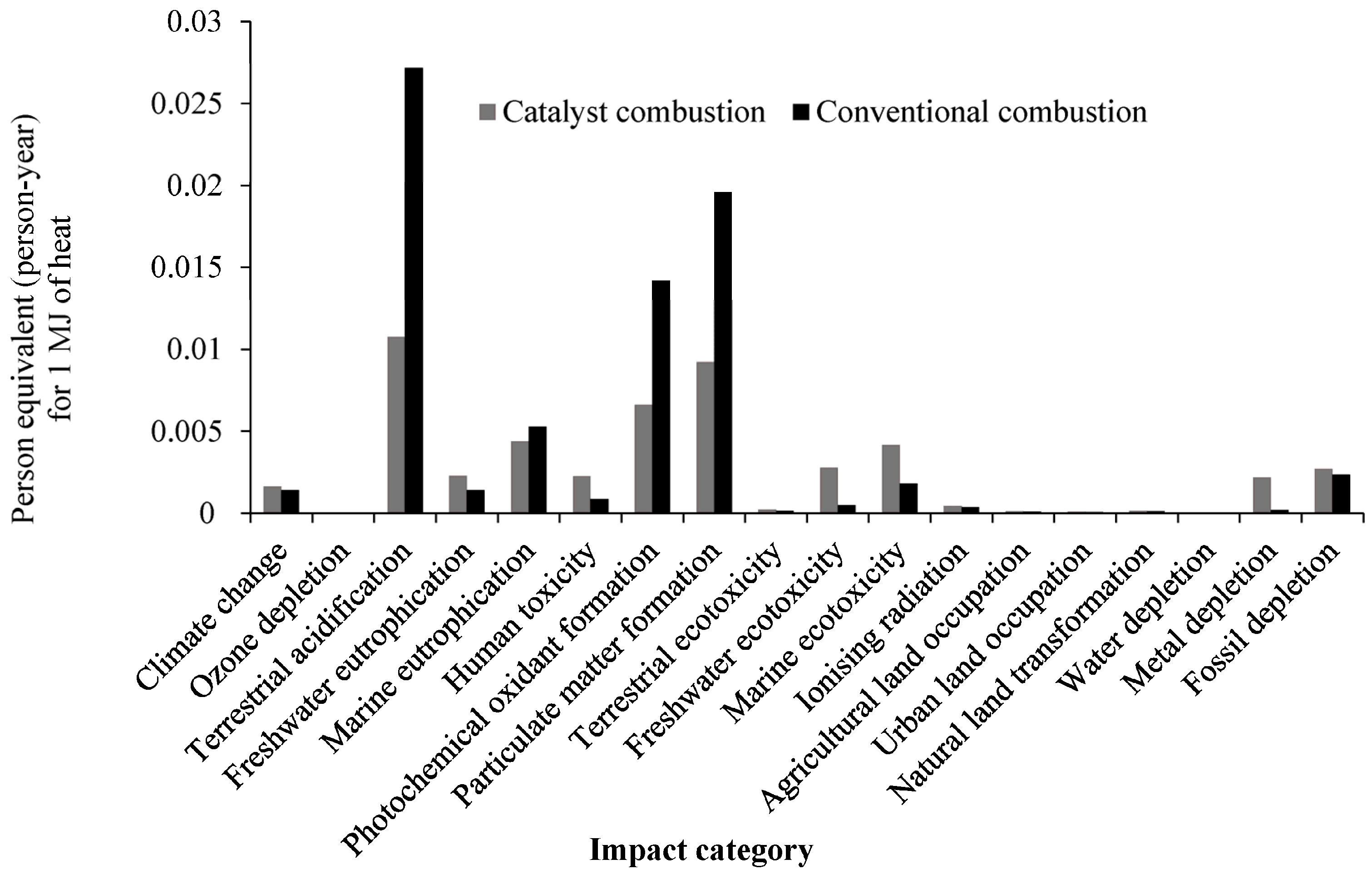

| Temperature °C | HC temperature | 380.1 | 481.3 | 490.3 | 534.1 |
| Combustion temp | 600 | 700 | 800 | 900 | |
| RH_CO (ppm) | HC | 23,941 | 0 | 0 | 56 |
| Conventional | 72,453 | 5043 | 1095 | 4345 | |
| RH_SO2 (ppm) | HC | 40 | 162 | 188 | 170 |
| Conventional | 416 | 711 | 4 | 468 | |
| RB_CO (ppm) | HC | 124,953 | 0 | 0 | 0 |
| Conventional | 433 | 774 | 265 | 141 | |
| RB_SO2 (ppm) | HC | 74 | 0 | 0 | 2 |
| Conventional | 138 | 140 | 98 | 65 | |
| RH_PM2.5 (mg/m3) | HC | 22.50 | 43.39 | 128.16 | 165.04 |
| Conventional | 354.58 | 372.05 | 629.60 | 826.59 | |
| RB_PM2.5 (mg/m3) | HC | 18.95 | 170.07 | 265.75 | 856.05 |
| Conventional | 1169.91 | 1572.22 | 1103.21 | 1209.56 |
| Impact Category | Unit | Catalyst Integrated Combustion | Conventional Combustion |
|---|---|---|---|
| Climate change | kg CO2 eq | 9.7255 | 9.6952 |
| Ozone depletion | kg CFC-11 eq | 1.25 × 10−6 | 1.21 × 10−6 |
| Terrestrial acidification | kg SO2 eq | 0.3978 | 1.0400 |
| Freshwater eutrophication | kg P eq | 0.0004 | 0.0004 |
| Marine eutrophication | kg N eq | 0.0321 | 0.0389 |
| Human toxicity | kg 1,4-DB eq | 0.3277 | 0.2834 |
| Photochemical oxidant formation | kg NMVOC | 0.3679 | 0.8068 |
| Particulate matter formation | kg PM10 eq | 0.1255 | 0.2755 |
| Terrestrial ecotoxicity | kg 1,4-DB eq | 0.0009 | 0.0009 |
| Freshwater ecotoxicity | kg 1,4-DB eq | 0.0032 | 0.0022 |
| Marine ecotoxicity | kg 1,4-DB eq | 0.0051 | 0.0045 |
| Ionizing radiation | kBq U235 eq | 0.5064 | 0.4986 |
| Agricultural land occupation | m2a | 0.6011 | 0.5867 |
| Urban land occupation | m2a | 0.0559 | 0.0537 |
| Natural land transformation | m2 | 0.0015 | 0.0014 |
| Water depletion | m3 | 0.0749 | 0.0728 |
| Metal depletion | kg Fe eq | 0.1780 | 0.0909 |
| Fossil depletion | kg oil eq | 3.0553 | 3.0102 |
| Impact Category | HC Catalyst | Electricity (Co Product) | |
|---|---|---|---|
| Eco-indicator 99 Indirect contributions | Carcinogens (DALY) | 8.18969 × 10−6 | 8.50956 × 10−13 |
| Resp. organics (DALY) | 2.29701 × 10−8 | 2.09766 × 10−11 | |
| Resp. inorganics (DALY) | 2.5282 × 10−5 | 2.45066 × 10−10 | |
| Climate change (DALY) | 6.31224 × 10−6 | 1.98898 × 10−10 | |
| Radiation (DALY) | 4.86307 × 10−8 | 5.41186 × 10−14 | |
| Ozone layer (DALY) | 5.77073 × 10−9 | 6.49188 × 10−15 | |
| Ecotoxicity (PAF∙m2∙yr) | 46.38897098 | 3.21522 × 10−6 | |
| AP/EP (PDF∙m2∙yr) | 0.745041133 | 1.53112 × 10−5 | |
| ReCiPe Indirect contributions | Climate c. Hum. Health (DALY) | 4.21338 × 10−5 | 1.32426 × 10−9 |
| OD (DALY) | 1.44176 × 10−8 | 1.60624 × 10−14 | |
| Human toxicity (DALY) | 5.76533 × 10−6 | 2.8175 × 10−11 | |
| Photochem. Oxi. Format. (DALY) | 5.07189 × 10−9 | 2.72641 × 10−13 | |
| PM formation (DALY) | 1.79374 × 10−5 | 1.6064 × 10−10 | |
| Ionising radiation (DALY) | 3.79754 × 10−8 | 4.25363 × 10−14 | |
| Climate c. Ecosyst. (species.year) | 2.38651 × 10−7 | 7.50086 × 10−12 | |
| T. acidification (species.year) | 1.34701 × 10−9 | 9.47752 × 10−15 |
| Element/Parameter | JPN | RB |
|---|---|---|
| C (wt.% db) | 37.53 | 39.37 |
| H (wt.% db) | 5.05 | 5.41 |
| N (wt.% db) | 0.18 | 0.34 |
| S (wt.% db) | 0.23 | 0.11 |
| Catalyst Type | Metal Honeycomb |
|---|---|
| Material composition | Pt/Al2O3 |
| Platinum loading | 1 wt% |
| BET surface area (m2/g) | 180 (support) |
| Pore volume (cm3/g) | 0.45 |
| Thermal stability | Stable up to 800 °C (confirmed by TGA and XRD data) |
| Pt particle size | 2.5 nm (average, from TEM analysis) |
| Heating temperature range of the catalyst (°C) | 100–600 |
| Combusted fuel samples | Rice husk and briquette |
| Fuel sample weight (g) | 3.0 |
| Fuel combustion temperature range (°C) | 600–1000 |
| Combustor air intake (m/s) | 1.5 |
| Sample combustion duration (min) | 3.0 |
| Number of repetitions for each data point | 3.0 |
| Item | Unit | Conventional Rice Husk Combustion | Catalyst Rice Husk Combustion | |||
|---|---|---|---|---|---|---|
| Combustion | Waste Disposal | Production of Catalyst | Combustion | Waste Disposal | ||
| Input (material) | ||||||
| Iron oxide | kg | na | na | 0.155 | na | na |
| Platinum | kg | na | na | 0.00039 | na | na |
| Input (energy) | ||||||
| Rice husk | kg | 0.0704 | na | na | 0.0704 | na |
| Electricity | kWh | 1 | na | na | 1 | na |
| Transportation | t.km | na | 0.000705 | 0.00311 | na | 0.000705 |
| Output (emission) | ||||||
| CO | kg | 0.355 | nm | nm | 0 | nm |
| SO2 | kg | 0.050 | nm | nm | 0.0114 | nm |
| CH4 | kg | 0.0004 | 0.00001 | |||
| NO | kg | 0.022 | nm | 0.0215 | ||
| NO2 | kg | 0.0062 | nm | nm | 0.00057 | nm |
| NOX | kg | 0.028 | nm | nm | 0.022 | nm |
| PM2.5 | kg | 0.00041 | nm | nm | 0.00009225 | nm |
| Output (waste disposal) | ||||||
| Residual ash | kg | 0.0141 | na | nm | 0.0141 | na |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abah, E.O.; Kahandage, P.D.; Noguchi, R.; Ahamed, T.; Adigun, P.; Idogho, C. Assessment of Platinum Catalyst in Rice Husk Combustion: A Comparative Life Cycle Analysis with Conventional Methods. Catalysts 2025, 15, 717. https://doi.org/10.3390/catal15080717
Abah EO, Kahandage PD, Noguchi R, Ahamed T, Adigun P, Idogho C. Assessment of Platinum Catalyst in Rice Husk Combustion: A Comparative Life Cycle Analysis with Conventional Methods. Catalysts. 2025; 15(8):717. https://doi.org/10.3390/catal15080717
Chicago/Turabian StyleAbah, Emmanuel Owoicho, Pubudu D. Kahandage, Ryozo Noguchi, Tofael Ahamed, Paul Adigun, and Christian Idogho. 2025. "Assessment of Platinum Catalyst in Rice Husk Combustion: A Comparative Life Cycle Analysis with Conventional Methods" Catalysts 15, no. 8: 717. https://doi.org/10.3390/catal15080717
APA StyleAbah, E. O., Kahandage, P. D., Noguchi, R., Ahamed, T., Adigun, P., & Idogho, C. (2025). Assessment of Platinum Catalyst in Rice Husk Combustion: A Comparative Life Cycle Analysis with Conventional Methods. Catalysts, 15(8), 717. https://doi.org/10.3390/catal15080717










