Cocatalyst-Tipped One-Dimensional Nanorods for Enhanced Photocatalytic Hydrogen Production
Abstract
1. Introduction
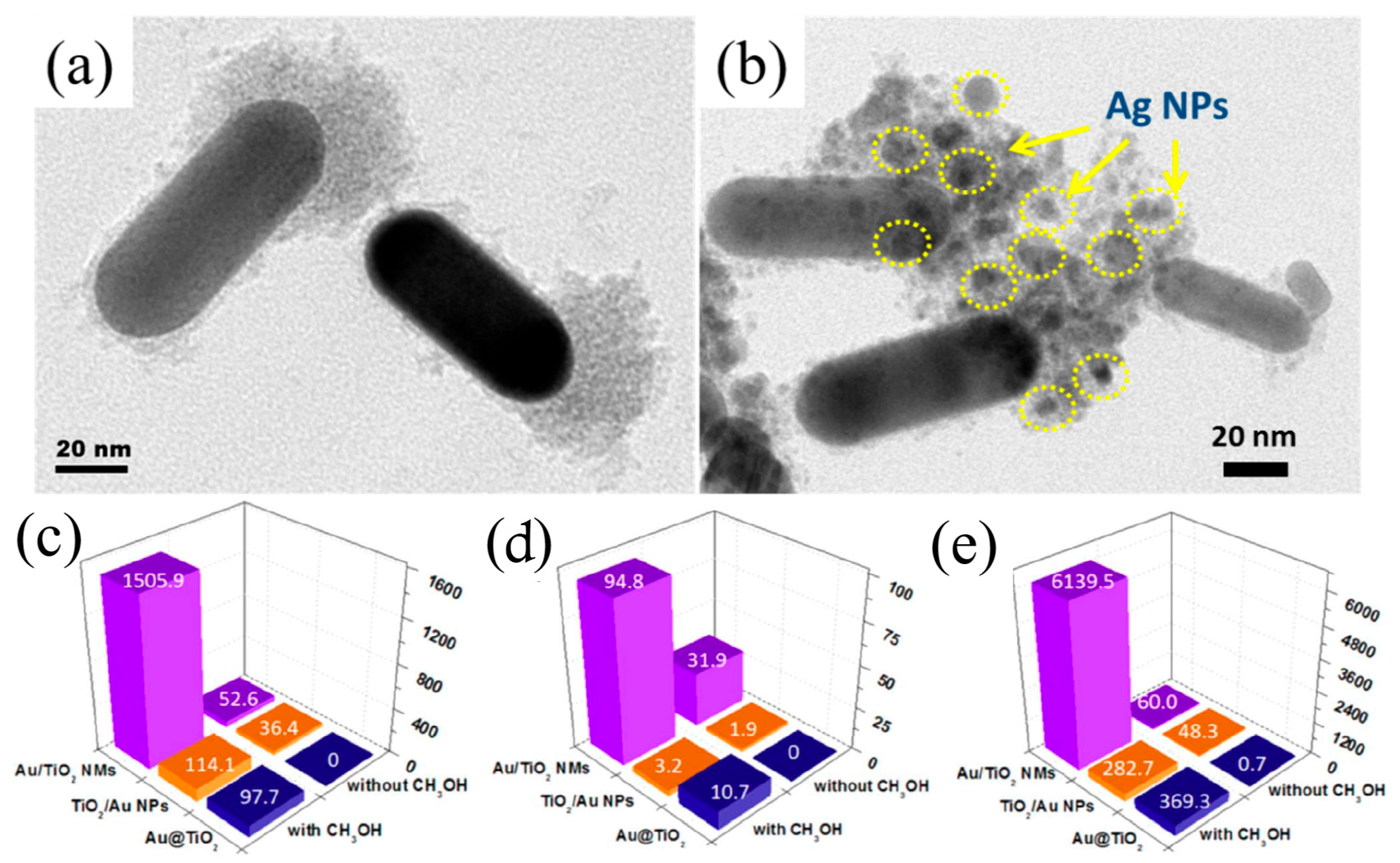
2. Non-Centrosymmetric 1D Metal–Semiconductor Nanostructures
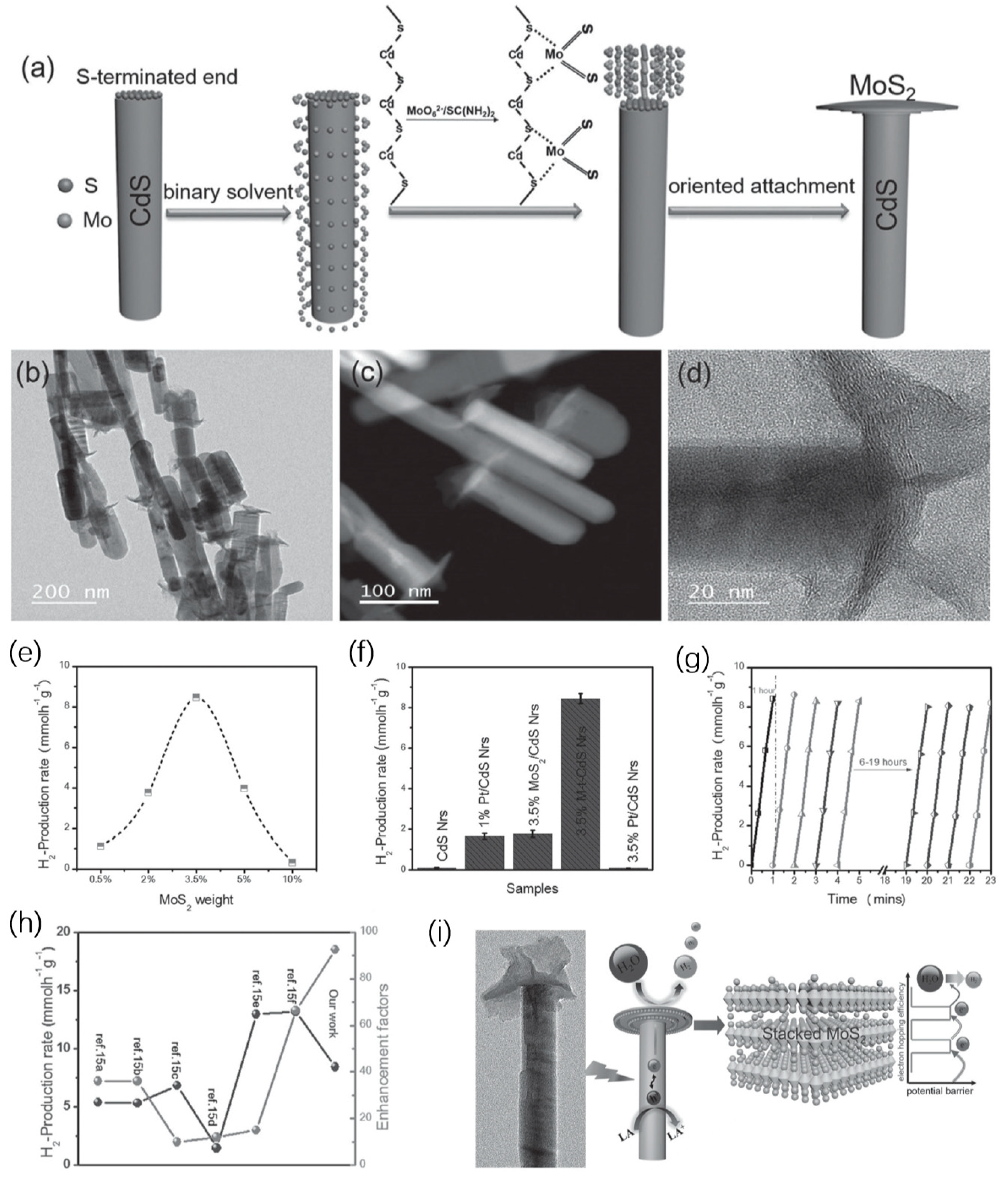
3. Non-Centrosymmetric 1D Semiconductor-Semiconductor Nanostructures
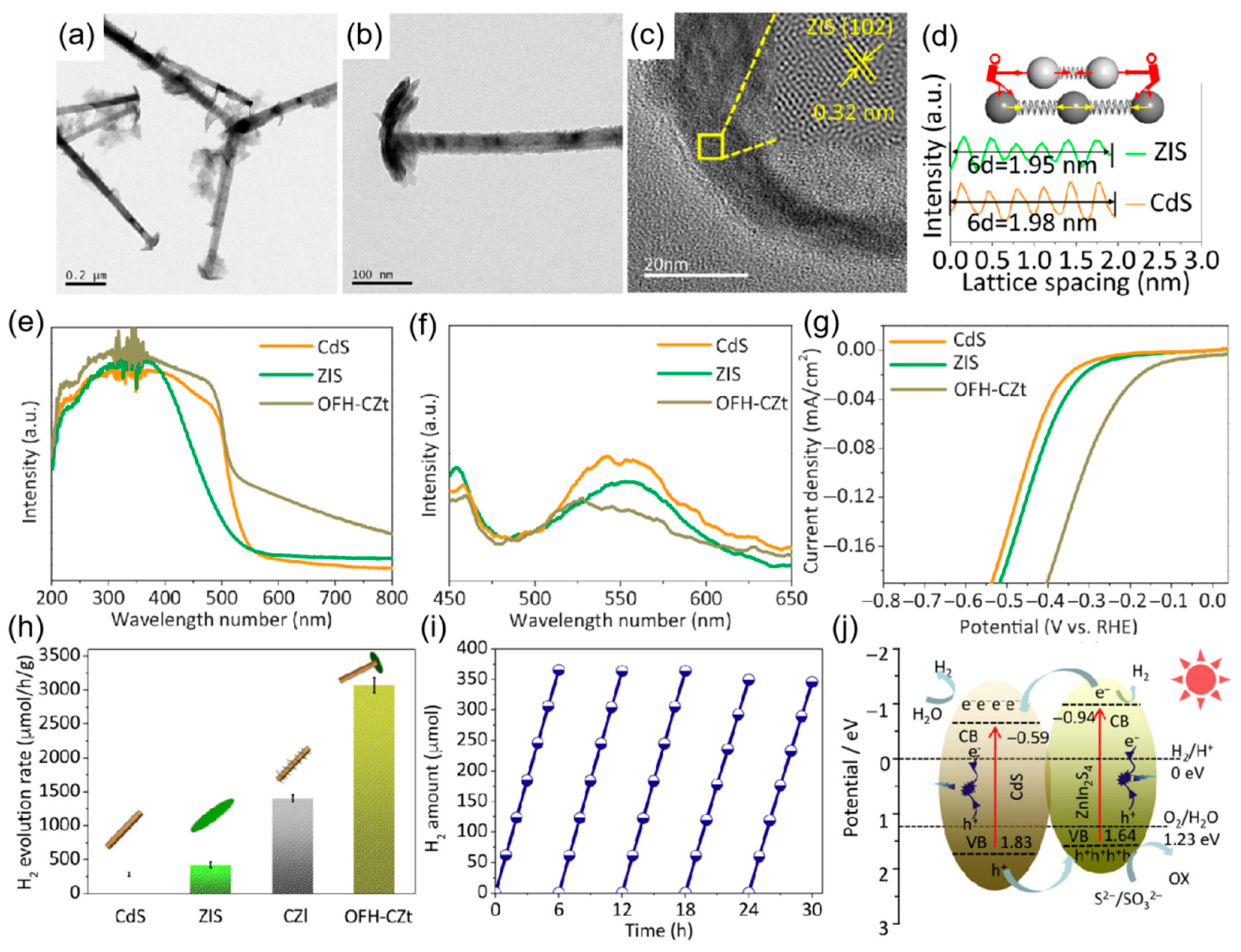
4. Symmetric One-Dimensional Semiconductor–Semiconductor Nanostructures
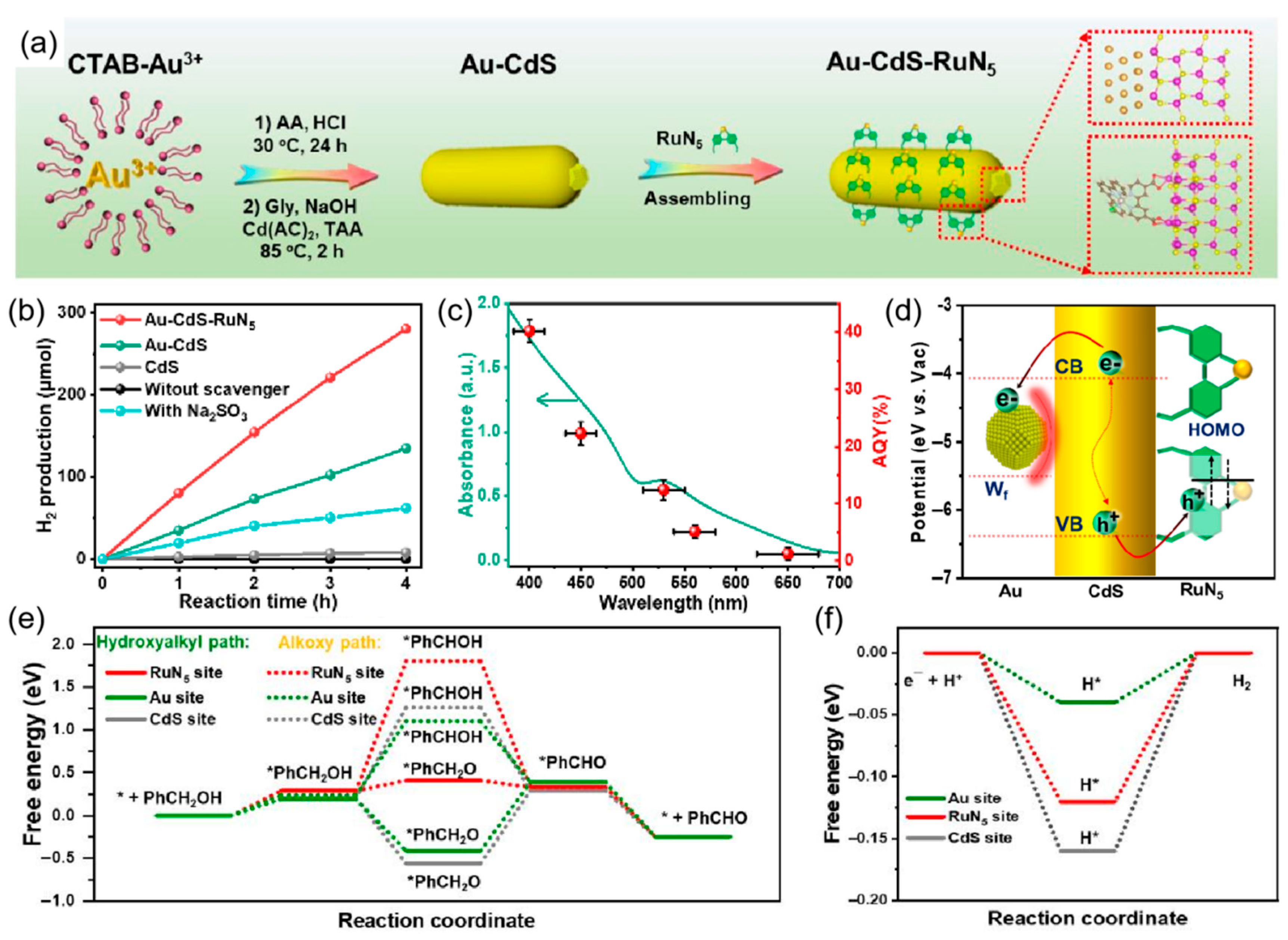
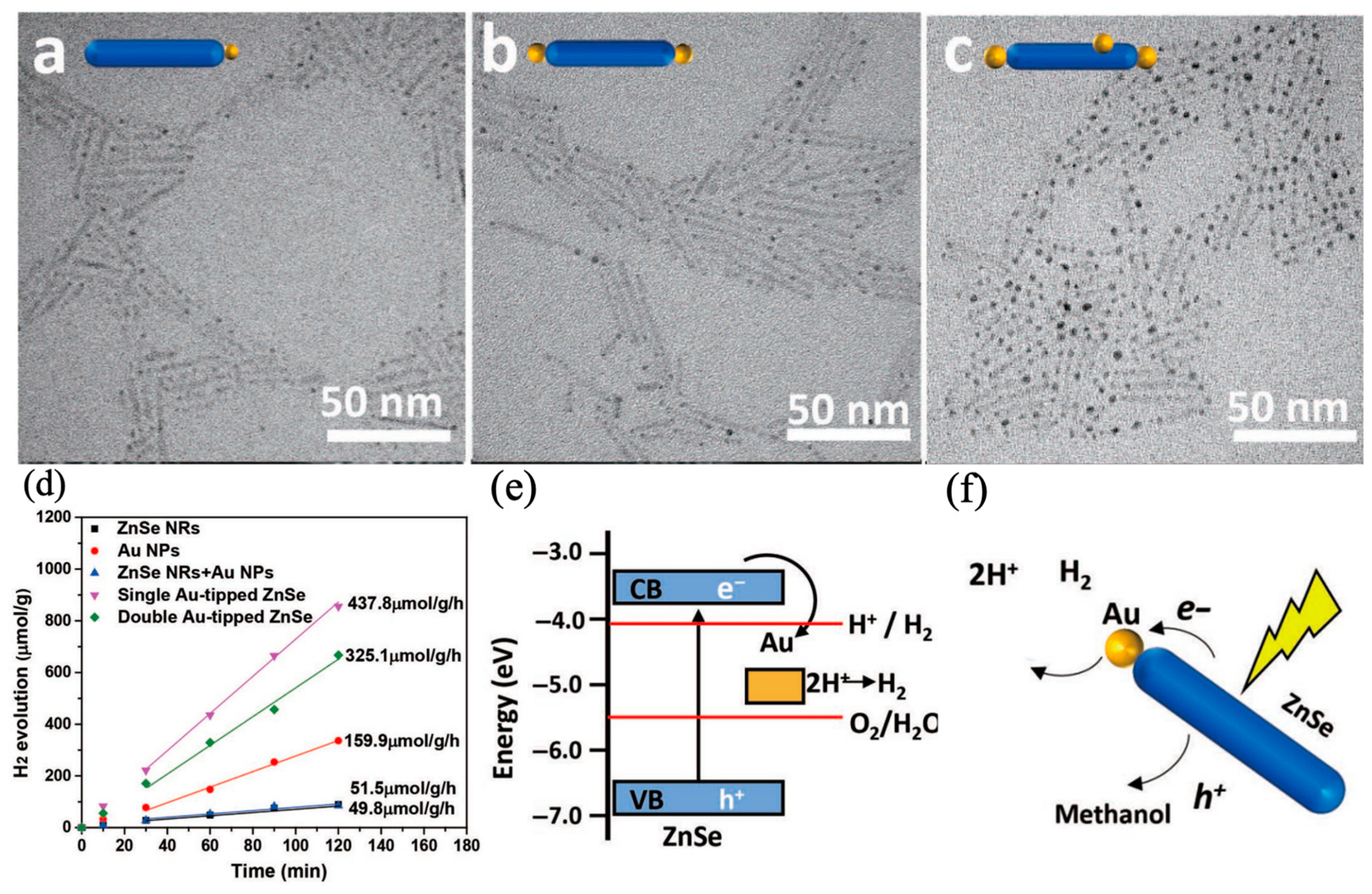
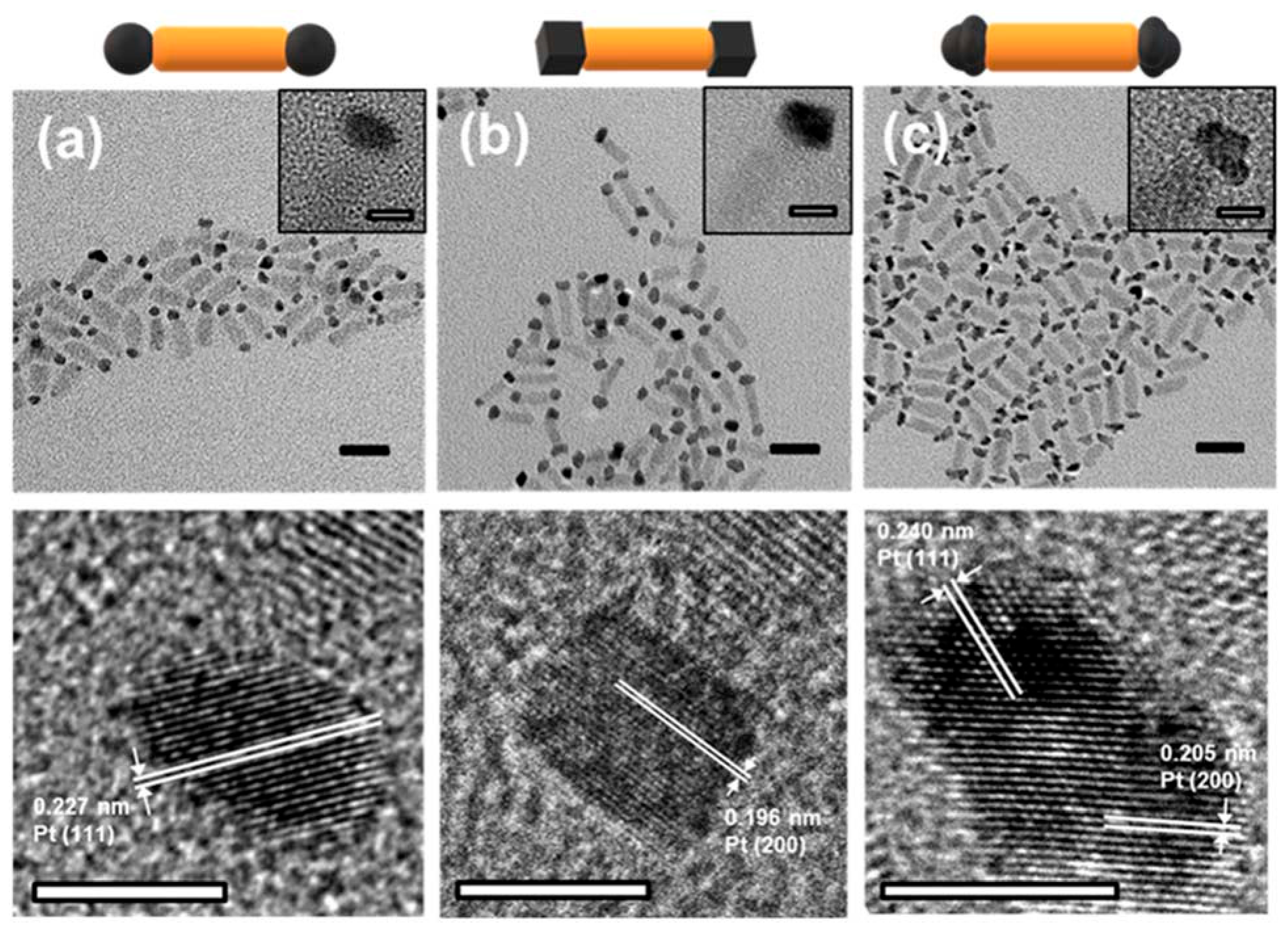
5. Cocatalyst-Tipped One-Dimensional Nanorods
6. Conclusions
Funding
Conflicts of Interest
References
- Chen, J.; Wu, X.J.; Yin, L.; Li, B.; Hong, X.; Fan, Z.; Chen, B.; Xue, C.; Zhang, H. One-pot synthesis of CdS nanocrystals hybridized with single-layer transition-metal dichalcogenide nanosheets for efficient photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2015, 54, 1210–1214. [Google Scholar] [CrossRef]
- Shi, X.; Fujitsuka, M.; Kim, S.; Majima, T. Faster Electron Injection and More Active Sites for Efficient Photocatalytic H2 Evolution in g-C3N4/MoS2 Hybrid. Small 2018, 14, e1703277. [Google Scholar] [CrossRef]
- Yin, X.L.; He, G.Y.; Sun, B.; Jiang, W.J.; Xue, D.J.; Xia, A.D.; Wan, L.J.; Hu, J.S. Rational design and electron transfer kinetics of MoS2/CdS nanodots-on-nanorods for efficient visible-light-driven hydrogen generation. Nano Energy 2016, 28, 319–329. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Liu, C.; Luo, S.; Wang, L.; Cai, T.; Zeng, Y.; Yuan, J.; Dong, W.; Pei, Y.; et al. MoS2 Quantum Dot Growth Induced by S Vacancies in a ZnIn2S4 Monolayer: Atomic-Level Heterostructure for Photocatalytic Hydrogen Production. ACS Nano 2018, 12, 751–758. [Google Scholar] [CrossRef]
- Liu, Q.; Shang, Q.; Khalil, A.; Fang, Q.; Chen, S.; He, Q.; Xiang, T.; Liu, D.; Zhang, Q.; Luo, Y.; et al. In situ Integration of a Metallic 1T-MoS2/CdS Heterostructure as a Means to Promote Visible-Light-Driven Photocatalytic Hydrogen Evolution. ChemCatChem 2016, 8, 2614–2619. [Google Scholar] [CrossRef]
- Xu, H.; Yi, J.; She, X.; Liu, Q.; Song, L.; Chen, S.; Yang, Y.; Song, Y.; Vajtai, R.; Lou, J.; et al. 2D heterostructure comprised of metallic 1T-MoS2/Monolayer O-g-C3N4 towards efficient photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 220, 379–385. [Google Scholar] [CrossRef]
- Guo, L.; Yang, Z.; Marcus, K.; Li, Z.; Luo, B.; Zhou, L.; Wang, X.; Du, Y.; Yang, Y. MoS2/TiO2 heterostructures as nonmetal plasmonic photocatalysts for highly efficient hydrogen evolution. Energy Environ. Sci. 2018, 11, 106–114. [Google Scholar] [CrossRef]
- Han, B.; Liu, S.; Zhang, N.; Xu, Y.J.; Tang, Z.R. One-dimensional CdS@MoS2 core-shell nanowires for boosted photocatalytic hydrogen evolution under visible light. Appl. Catal. B Environ. 2017, 202, 298–304. [Google Scholar] [CrossRef]
- Li, Y.; Huang, L.; Li, B.; Wang, X.; Zhou, Z.; Li, J.; Wei, Z. Co-nucleus 1D/2D Heterostructures with Bi2S3 Nanowire and MoS2 Monolayer: One-Step Growth and Defect-Induced Formation Mechanism. ACS Nano 2016, 10, 8938–8946. [Google Scholar] [CrossRef]
- Ma, F.; Wu, Y.; Shao, Y.; Zhong, Y.; Lv, J.; Hao, X. 0D/2D nanocomposite visible light photocatalyst for highly stable and efficient hydrogen generation via recrystallization of CdS on MoS2 nanosheets. Nano Energy 2016, 27, 466–474. [Google Scholar] [CrossRef]
- Su, T.; Shao, Q.; Qin, Z.; Guo, Z.; Wu, Z. Role of Interfaces in Two-Dimensional Photocatalyst for Water Splitting. ACS Catal. 2018, 8, 2253–2276. [Google Scholar] [CrossRef]
- Yin, X.L.; Li, L.L.; Jiang, W.J.; Zhang, Y.; Zhang, X.; Wan, L.J.; Hu, J.S. MoS2/CdS Nanosheets-on-Nanorod Heterostructure for Highly Efficient Photocatalytic H2 Generation under Visible Light Irradiation. ACS Appl. Mater. Interfaces. 2016, 8, 15258–15266. [Google Scholar] [CrossRef]
- Zhang, X.H.; Li, N.; Wu, J.; Zheng, Y.Z.; Tao, X. Defect-rich O-incorporated 1T-MoS2 nanosheets for remarkably enhanced visible-light photocatalytic H2 evolution over CdS: The impact of enriched defects. Appl. Catal. B Environ. 2018, 229, 227–236. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Zhang, M.; Yuan, Q.; Dong, B. Ultrathin hexagonal SnS2 nanosheets coupled with g-C3N4 nanosheets as 2D/2D heterojunction photocatalysts toward high photocatalytic activity. Appl. Catal. B Environ. 2015, 163, 298–305. [Google Scholar] [CrossRef]
- Fu, W.; He, H.; Zhang, Z.; Wu, C.; Wang, X.; Wang, H.; Zeng, Q.; Sun, L.; Wang, X.; Zhou, J.; et al. Strong interfacial coupling of MoS2/g-C3N4 van de Waals solids for highly active water reduction. Nano Energy 2016, 27, 44–50. [Google Scholar] [CrossRef]
- Hao, X.; Jin, Z.; Yang, H.; Lu, G.; Bi, Y. Peculiar synergetic effect of MoS2 quantum dots and graphene on Metal-Organic Frameworks for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2017, 210, 45–56. [Google Scholar] [CrossRef]
- He, J.; Chen, L.; Wang, F.; Liu, Y.; Chen, P.; Au, C.T.; Yin, S.F. CdS Nanowires Decorated with Ultrathin MoS2 Nanosheets as an Efficient Photocatalyst for Hydrogen Evolution. ChemSusChem 2016, 9, 624–630. [Google Scholar] [CrossRef]
- Hong, S.; Kumar, D.P.; Kim, E.H.; Park, H.; Gopannagari, M.; Reddy, D.A.; Kim, T.K. Earth abundant transition metal-doped few-layered MoS2 nanosheets on CdS nanorods for ultra-efficient photocatalytic hydrogen production. J. Mater. Chem. A 2017, 5, 20851–20859. [Google Scholar] [CrossRef]
- Hu, X.; Lu, S.; Tian, J.; Wei, N.; Song, X.; Wang, X.; Cui, H. The selective deposition of MoS2 nanosheets onto (101) facets of TiO2 nanosheets with exposed (001) facets and their enhanced photocatalytic H2 production. Appl. Catal. B Environ. 2019, 241, 329–337. [Google Scholar] [CrossRef]
- Jia, L.; Wang, D.H.; Huang, Y.X.; Xu, A.W.; Yu, H.Q. Highly Durable N-Doped Graphene/CdS Nanocomposites with Enhanced Photocatalytic Hydrogen Evolution from Water under Visible Light Irradiation. J. Phys. Chem. C 2011, 115, 11466–11473. [Google Scholar] [CrossRef]
- Jia, T.; Kolpin, A.; Ma, C.; Chan, R.C.; Kwok, W.M.; Tsang, S.C. A graphene dispersed CdS-MoS2 nanocrystal ensemble for cooperative photocatalytic hydrogen production from water. Chem. Commun. 2014, 50, 1185–1188. [Google Scholar] [CrossRef]
- Kumar, D.P.; Hong, S.; Reddy, D.A.; Kim, T.K. Ultrathin MoS2 layers anchored exfoliated reduced graphene oxide nanosheet hybrid as a highly efficient cocatalyst for CdS nanorods towards enhanced photocatalytic hydrogen production. Appl. Catal. B Environ. 2017, 212, 7–14. [Google Scholar] [CrossRef]
- Li, J.; Zhan, G.; Yu, Y.; Zhang, L. Superior visible light hydrogen evolution of Janus bilayer junctions via atomic-level charge flow steering. Nat. Commun. 2016, 7, 11480. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Fan, X.; Wu, M.; Du, Y.; Wang, M.; Kong, Q.; Zhang, L.; Shi, J. Dual synergetic effects in MoS2/pyridine-modified g-C3N4 composite for highly active and stable photocatalytic hydrogen evolution under visible light. Appl. Catal. B Environ. 2016, 190, 36–43. [Google Scholar] [CrossRef]
- Qin, N.; Xiong, J.; Liang, R.; Liu, Y.; Zhang, S.; Li, Y.; Li, Z.; Wu, L. Highly efficient photocatalytic H2 evolution over MoS2/CdS-TiO2 nanofibers prepared by an electrospinning mediated photodeposition method. Appl. Catal. B Environ. 2017, 202, 374–380. [Google Scholar] [CrossRef]
- Tang, C.; Xu, W.; Chen, C.; Li, Y.; Xu, L. Unraveling the Orientation of MoS2 on TiO2 for Photocatalytic Water Splitting. Adv. Mater. Interfaces 2017, 4, 1700361. [Google Scholar] [CrossRef]
- Wu, A.; Tian, C.; Jiao, Y.; Yan, Q.; Yang, G.; Fu, H. Sequential two-step hydrothermal growth of MoS2/CdS core-shell heterojunctions for efficient visible light-driven photocatalytic H2 evolution. Appl. Catal. B Environ. 2017, 203, 955–963. [Google Scholar] [CrossRef]
- Xie, S.; Shen, Z.; Deng, J.; Guo, P.; Zhang, Q.; Zhang, H.; Ma, C.; Jiang, Z.; Cheng, J.; Deng, D.; et al. Visible light-driven C-H activation and C-C coupling of methanol into ethylene glycol. Nat. Commun. 2018, 9, 1181. [Google Scholar] [CrossRef]
- Xu, J.; Cao, X. Characterization and mechanism of MoS2/CdS composite photocatalyst used for hydrogen production from water splitting under visible light. Chem. Eng. J. 2015, 260, 642–648. [Google Scholar] [CrossRef]
- Zong, X.; Yan, H.; Wu, G.; Ma, G.; Wen, F.; Wang, L.; Li, C. Enhancement of Photocatalytic H2 Evolution on CdS by Loading MoS2 as Cocatalyst under Visible Light Irradiation. J. Am. Chem. Soc. 2008, 130, 7176–7177. [Google Scholar] [CrossRef]
- Yao, T.; An, X.; Han, H.; Chen, J.Q.; Li, C. Photoelectrocatalytic Materials for Solar Water Splitting. Adv. Energy Mater. 2018, 8, 1800210. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Chen, D.; Yang, S.; Yang, L.X.; Wang, J.J.; Cao, D.; Tu, W.; Yu, Z.T.; Zou, Z.G. Constructing noble-metal-free Z-scheme photocatalytic overall water splitting systems using MoS2 nanosheet modified CdS as a H2 evolution photocatalyst. J. Mater. Chem. A 2017, 5, 21205–21213. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Wang, W.Y.; Chen, J.J.; Liu, C.; Li, Q.X.; Zhang, X.; Li, W.W.; Si, Y.; Yu, H.Q. Epitaxial facet junctions on TiO2 single crystals for efficient photocatalytic water splitting. Energy Environ. Sci. 2018, 11, 1444–1448. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Z.; Feng, X. Construction of two-dimensional MoS2/CdS p-n nanohybrids for highly efficient photocatalytic hydrogen evolution. Chemistry 2014, 20, 10632–10635. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, H.; Gao, H.; Cao, R.; Huang, J.; Xu, X. One-pot Synthesis of CdS Irregular Nanospheres Hybridized with Oxygen-Incorporated Defect-Rich MoS2 Ultrathin Nanosheets for Efficient Photocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2017, 9, 23635–23646. [Google Scholar] [CrossRef]
- Zhao, L.; Jia, J.; Yang, Z.; Yu, J.; Wang, A.; Sang, Y.; Zhou, W.; Liu, H. One-step synthesis of CdS nanoparticles/MoS2 nanosheets heterostructure on porous molybdenum sheet for enhanced photocatalytic H2 evolution. Appl. Catal. B Environ. 2017, 210, 290–296. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, J.; Zhang, Q.; Xiong, Y. Steering charge kinetics in photocatalysis: Intersection of materials syntheses, characterization techniques and theoretical simulations. Chem. Soc. Rev. 2015, 44, 2893–2939. [Google Scholar] [CrossRef]
- Bai, S.; Wang, L.; Li, Z.; Xiong, Y. Facet-Engineered Surface and Interface Design of Photocatalytic Materials. Adv. Sci. 2017, 4, 1600216. [Google Scholar] [CrossRef]
- Bai, S.; Xiong, Y. Recent Advances in Two-Dimensional Nanostructures for Catalysis Applications. Sci. Adv. Mater. 2015, 7, 2168–2181. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Liu, B.; Cheng, H.M. Preparation of 2D material dispersions and their applications. Chem. Soc. Rev. 2018, 47, 6224–6266. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, B.; Zou, X.; Cheng, H.M. Chemical Vapor Deposition Growth and Applications of Two-Dimensional Materials and Their Heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [CrossRef]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-Based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Gao, C.; Wang, J.; Xu, H.; Xiong, Y. Coordination chemistry in the design of heterogeneous photocatalysts. Chem. Soc. Rev. 2017, 46, 2799–2823. [Google Scholar] [CrossRef]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef]
- Guo, Y.; Park, T.; Yi, J.W.; Henzie, J.; Kim, J.; Wang, Z.; Jiang, B.; Bando, Y.; Sugahara, Y.; Tang, J.; et al. Nanoarchitectonics for Transition-Metal-Sulfide-Based Electrocatalysts for Water Splitting. Adv. Mater. 2019, 31, e1807134. [Google Scholar] [CrossRef]
- Gupta, U.; Gopalakrishnan, K.; Rao, C.N.R. Synthesis and properties of graphene and its 2D inorganic analogues with potential applications. Bull. Mater. Sci. 2018, 41, 129. [Google Scholar] [CrossRef]
- Si, Y.; Cao, S.; Wu, Z.; Ji, Y.; Mi, Y.; Wu, X.; Liu, X.; Piao, L. The effect of directed photogenerated carrier separation on photocatalytic hydrogen production. Nano Energy 2017, 41, 488–493. [Google Scholar] [CrossRef]
- Han, C.; Zeng, Z.; Zhang, X.; Liang, Y.; Kundu, B.K.; Yuan, L.; Tan, C.L.; Zhang, Y.; Xu, Y.J. All-in-One: Plasmonic Janus Heterostructures for Efficient Cooperative Photoredox Catalysis. Angew. Chem. Int. Ed. 2024, 136, e202408527. [Google Scholar] [CrossRef]
- Micheel, M.; Dong, K.; Amirav, L.; Wächtler, M. Lateral Charge Migration in 1D Semiconductor–Metal Hybrid Photocatalytic Systems. J. Chem. Phys. 2023, 158, 154701. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Tu, W.; Ye, J.; Zou, Z. State-of-the-Art Progress in Diverse Heterostructured Photocatalysts toward Promoting Photocatalytic Performance. Adv. Func. Mater. 2015, 25, 998–1013. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, C.; Huang, P.; Cheng, M.; Xie, Y. Regulating the Charge and Spin Ordering of Two-Dimensional Ultrathin Solids for Electrocatalytic Water Splitting. Chem 2018, 4, 1263–1283. [Google Scholar] [CrossRef]
- Mao, J.; Wang, Y.; Zheng, Z.; Deng, D. The rise of two-dimensional MoS2 for catalysis. Front. Phys. 2018, 13, 138118. [Google Scholar] [CrossRef]
- Sun, X.; Chen, Y.; Liu, K.; Ding, Y.; Zeng, M.; Fu, L. Atomic Scale Materials for Emerging Robust Catalysis. Small Methods 2018, 2, 1800181. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]
- Xu, X.; Li, S.; Chen, J.; Cai, S.; Long, Z.; Fang, X. Design Principles and Material Engineering of ZnS for Optoelectronic Devices and Catalysis. Adv. Funct. Mater. 2018, 28, 1802029. [Google Scholar] [CrossRef]
- Yi, H.; Huang, D.; Qin, L.; Zeng, G.; Lai, C.; Cheng, M.; Ye, S.; Song, B.; Ren, X.; Guo, X. Selective prepared carbon nanomaterials for advanced photocatalytic application in environmental pollutant treatment and hydrogen production. Appl. Catal. B Environ. 2018, 239, 408–424. [Google Scholar] [CrossRef]
- Zhang, K.; Kim, J.K.; Ma, M.; Yim, S.Y.; Lee, C.L.; Shin, H.; Park, J.H. Delocalized Electron Accumulation at Nanorod Tips: Origin of Efficient H2 Generation. Adv. Func. Mater. 2016, 26, 4527–4534. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, J.; Shao, L.; Xia, X.; Liu, Y.; Wang, L. Oriented Facet Heterojunctions on CdS Nanowires with High Photoactivity and Photostability for Water Splitting. Appl. Catal. B Environ. 2020, 268, 118744. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Li, H.; Liu, Z. Freestanding atomically-thin two-dimensional materials beyond graphene meeting photocatalysis: Opportunities and challenges. Nano Energy 2017, 35, 79–91. [Google Scholar] [CrossRef]
- Di, J.; Xiong, J.; Li, H.; Liu, Z. Ultrathin 2D Photocatalysts: Electronic-Structure Tailoring, Hybridization, and Applications. Adv. Mater. 2018, 30, 1704548. [Google Scholar] [CrossRef]
- Di, J.; Yan, C.; Handoko, A.D.; Seh, Z.W.; Li, H.; Liu, Z. Ultrathin two-dimensional materials for photo- and electrocatalytic hydrogen evolution. Mater. Today 2018, 21, 749–770. [Google Scholar] [CrossRef]
- Zhang, K.; Qian, S.F.; Kim, W.J.; Kim, J.K.; Sheng, X.W.; Lee, J.Y.; Park, J.H. Double 2-dimensional H2-evoluting catalyst tipped photocatalyst nanowires: A new avenue for high-efficiency solar to H2 generation. Nano Energy 2017, 34, 481–490. [Google Scholar] [CrossRef]
- Chang, K.; Hai, X.; Pang, H.; Zhang, H.; Shi, L.; Liu, G.; Liu, H.; Zhao, G.; Li, M.; Ye, J. Targeted Synthesis of 2H- and 1T-Phase MoS2 Monolayers for Catalytic Hydrogen Evolution. Adv. Mater. 2016, 28, 10033–10041. [Google Scholar] [CrossRef]
- Chang, K.; Li, M.; Wang, T.; Ouyang, S.; Li, P.; Liu, L.; Ye, J. Drastic Layer-Number-Dependent Activity Enhancement in Photocatalytic H2 Evolution over nMoS2/CdS (n ≥ 1) Under Visible Light. Adv. Energy Mater. 2015, 5, 1402279. [Google Scholar] [CrossRef]
- Chang, K.; Mei, Z.; Wang, T.; Kang, Q.; Ouyang, S.; Ye, J. MoS2/Graphene Cocatalyst for Efficient Photocatalytic H2 Evolution under Visible Light Irradiation. ACS Nano 2014, 8, 7078–7087. [Google Scholar] [CrossRef]
- Hai, X.; Zhou, W.; Wang, S.; Pang, H.; Chang, K.; Ichihara, F.; Ye, J. Rational design of freestanding MoS2 monolayers for hydrogen evolution reaction. Nano Energy 2017, 39, 409–417. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.J.; Gong, Y.; Zhu, Y.; Yang, Z.; Li, B.; Lu, Q.; Yu, Y.; Han, S.; Zhang, Z.; et al. Edge Epitaxy of Two-Dimensional MoSe2 and MoS2 Nanosheets on One-Dimensional Nanowires. J. Am. Chem. Soc. 2017, 139, 8652–8660. [Google Scholar] [CrossRef]
- Tan, C.; Chen, J.; Wu, X.J.; Zhang, H. Epitaxial growth of hybrid nanostructures. Nat. Rev. Mater. 2018, 3, 17089. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, H. Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem. Soc. Rev. 2015, 44, 2713–2731. [Google Scholar] [CrossRef]
- Zhang, K.; Kim, J.K.; Park, B.; Qian, S.; Jin, B.; Sheng, X.; Zeng, H.; Shin, H.; Oh, S.H.; Lee, C.L.; et al. Defect-Induced Epitaxial Growth for Efficient Solar Hydrogen Production. Nano Lett. 2017, 17, 6676–6683. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Wang, F.; Javaid, S.; Pang, Y.; Chen, J.; Yin, Z.; Wang, S.; Li, Y.; Jia, G. Nonepitaxial Gold-Tipped ZnSe Hybrid Nanorods for Efficient Photocatalytic Hydrogen Production. Small 2020, 16, e1902231. [Google Scholar] [CrossRef]
- Park, B.; Park, W.W.; Choi, J.Y.; Choi, W.; Sung, Y.M.; Sul, S.; Kwon, O.H.; Song, H. Pt cocatalyst morphology on semiconductor nanorod photocatalysts enhances charge trapping and water reduction. Chem. Sci. 2023, 14, 7553–7558. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, K.; Sun, J.; Gu, C.; Luo, Y.; Wang, S. Cocatalyst-Tipped One-Dimensional Nanorods for Enhanced Photocatalytic Hydrogen Production. Catalysts 2025, 15, 711. https://doi.org/10.3390/catal15080711
Wang L, Wang K, Sun J, Gu C, Luo Y, Wang S. Cocatalyst-Tipped One-Dimensional Nanorods for Enhanced Photocatalytic Hydrogen Production. Catalysts. 2025; 15(8):711. https://doi.org/10.3390/catal15080711
Chicago/Turabian StyleWang, Longlu, Kun Wang, Junkang Sun, Chen Gu, Yixiang Luo, and Shiyan Wang. 2025. "Cocatalyst-Tipped One-Dimensional Nanorods for Enhanced Photocatalytic Hydrogen Production" Catalysts 15, no. 8: 711. https://doi.org/10.3390/catal15080711
APA StyleWang, L., Wang, K., Sun, J., Gu, C., Luo, Y., & Wang, S. (2025). Cocatalyst-Tipped One-Dimensional Nanorods for Enhanced Photocatalytic Hydrogen Production. Catalysts, 15(8), 711. https://doi.org/10.3390/catal15080711





