Abstract
N-heterocyclic carbenes (NHCs) have demonstrated their versatility as catalysts for new activations and synthetic transformations of aldehydes. NHCs were originally applied in benzoin condensation and the Stetter reaction, while the development of new protocols under oxidative conditions has further expanded the potential of this methodology for the formation of carbon−carbon and carbon−heteroatom bonds. Among these reactions, NHCs are recognized as promising organocatalysts for the aerobic oxidation of aldehydes to carboxylic acids. However, to our knowledge, a comparison with other metal-free protocols has never been conducted. This review is intended to provide a perspective on aldehyde oxidation into the corresponding carboxylic acid catalyzed by NHCs, from its first practical description in 2009 until the beginning of 2025, and to compare it with other metal-free methods.
1. Introduction
N-heterocyclic carbenes (NHCs) have emerged as powerful organocatalysts in numerous carbon–carbon and carbon–heteroatom bond formation reactions [1,2]. In particular, NHCs have demonstrated their versatility as catalysts for new activations and synthetic transformations of aldehydes [2,3,4,5]. Among these transformations, NHCs have been identified as promising organocatalysts for the aerobic oxidation of aldehydes to carboxylic acids. Since the pioneering work of Yoshida et al. in 2009 [6], numerous protocols utilizing various oxidants and conditions have been developed for this transformation. However, to our knowledge, no comparison of the efficiency of NHCs for this particular reaction to metal-free protocols has been made, and studies have even questioned some results obtained using NHCs [7,8,9].
To understand how these different protocols have been developed, it is important to review the two main aldehyde transformation mechanisms involving NHCs, focusing on ester and carboxylic acid formation, specifically NHC-mediated carbonyl umpolung (Section 2.1) and the oxidation of the Breslow intermediate (Section 2.2) [10]. Next, the various procedures for the transformation of aromatic aldehydes into the corresponding carboxylic acids will be reviewed (Section 3). The results obtained will be discussed and compared to other metal-free protocols (Section 4).
2. NHCs as Catalysts for Transformation of Aldehydes
2.1. Products from NHC-Mediated Carbonyl Umpolung
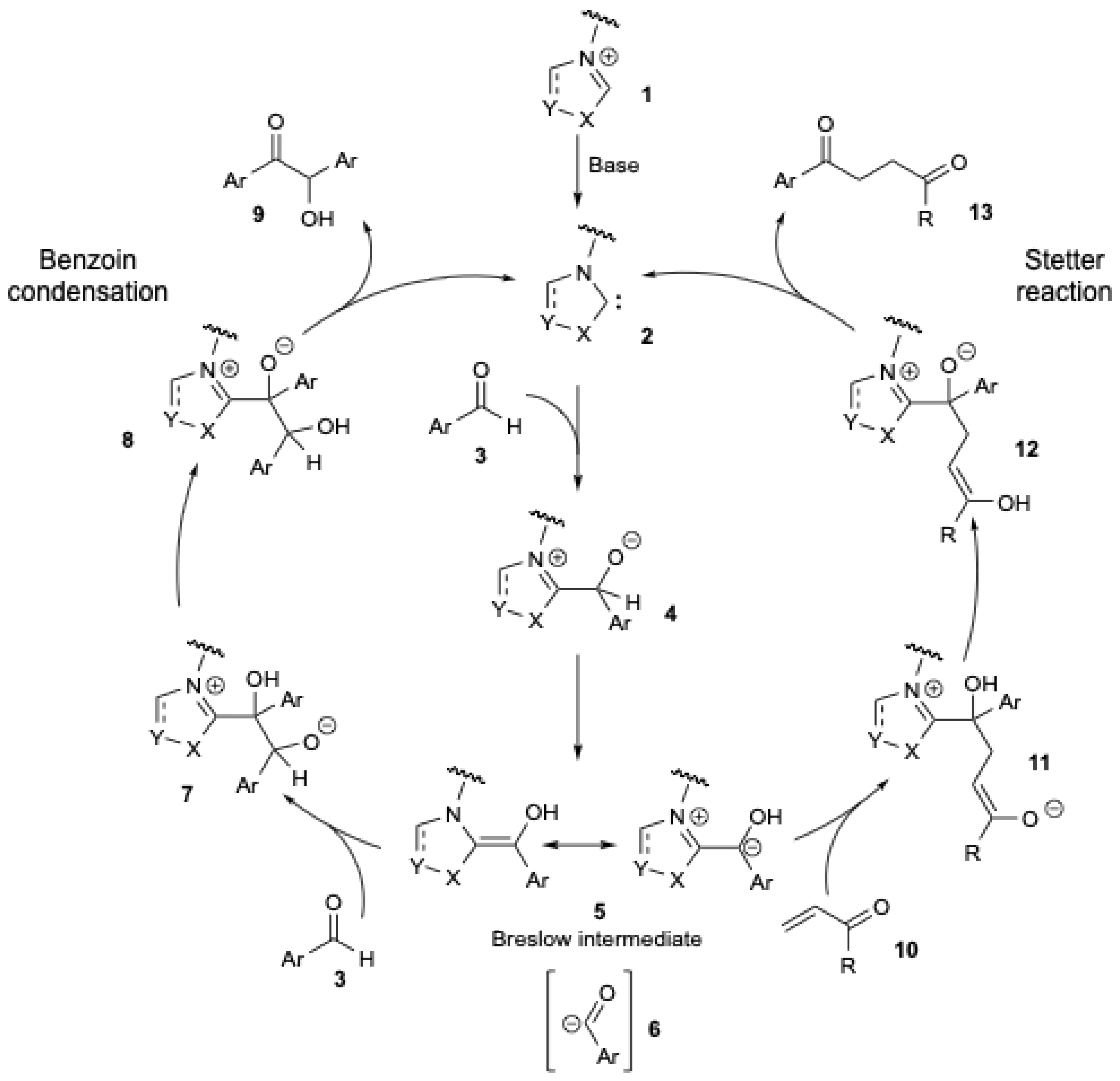
The benzoin condensation (Figure 1, left) constitutes one of the earliest known carbon–carbon bond-forming reactions catalyzed by NHCs [11]. Breslow postulated a mechanistic pathway for the benzoin condensation in 1958 using vitamin B1 as an NHC pre-catalyst [12], and Figure 1 (on the left) describes the general pathway. Deprotonation of a precursor salt 1 generates the NHC 2. Nucleophilic addition of NHC 2 to an aldehyde 3 generates the tetrahedral intermediate 4. The latter then undergoes a proton shift to form the nucleophilic enaminol intermediate, known as the Breslow intermediate 5. Thus, NHC-organocatalyzed umpolung reactions of aldehydes lead to the formation of nucleophilic acyl anion intermediates 6, which can react with various electrophiles, including aldehydes (benzoin condensation), ketones [13], imines [14], and activated, polarized C = C double bonds (Stetter reaction) [15,16]. In the benzoin condensation (Figure 1, left), intermediate 5 reacts with the same aldehyde 3, resulting in the formation of an alkoxide intermediate 7. Following a proton transfer that leads to 8, a subsequent release of NHC 2 affords the final product, an α-hydroxy ketone 9. The Stetter reaction [15,16] (Figure 1, right) involves the 1,4-addition of 5 to an α,β-unsaturated carbonyl compound 10, forming the intermediate 11. After a proton transfer resulting in 12, a subsequent release of NHC 2 yields the final product, a 1,4-diketone 13.
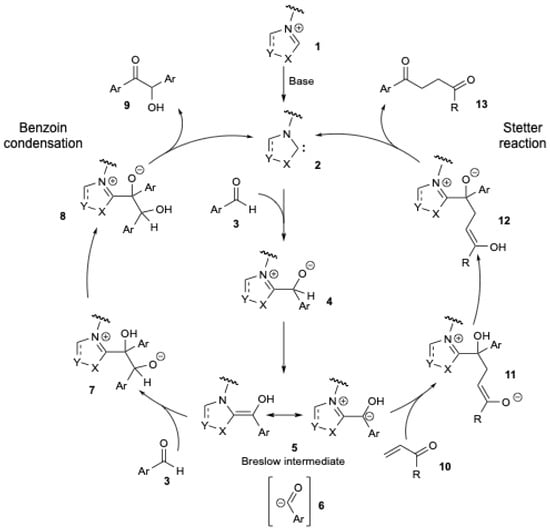
Figure 1.
The NHC-mediated carbonyl umpolung of aldehydes in the benzoin condensation (left) and Stetter reaction (right).
The reaction of the acyl anion intermediates 6 with sp2-carbon-centered electrophiles (Figure 1, right) has been used for the preparation of unique reactive intermediates [17]. However, comparable reactions with sp3-centered electrophiles have been less reported [18,19,20,21].
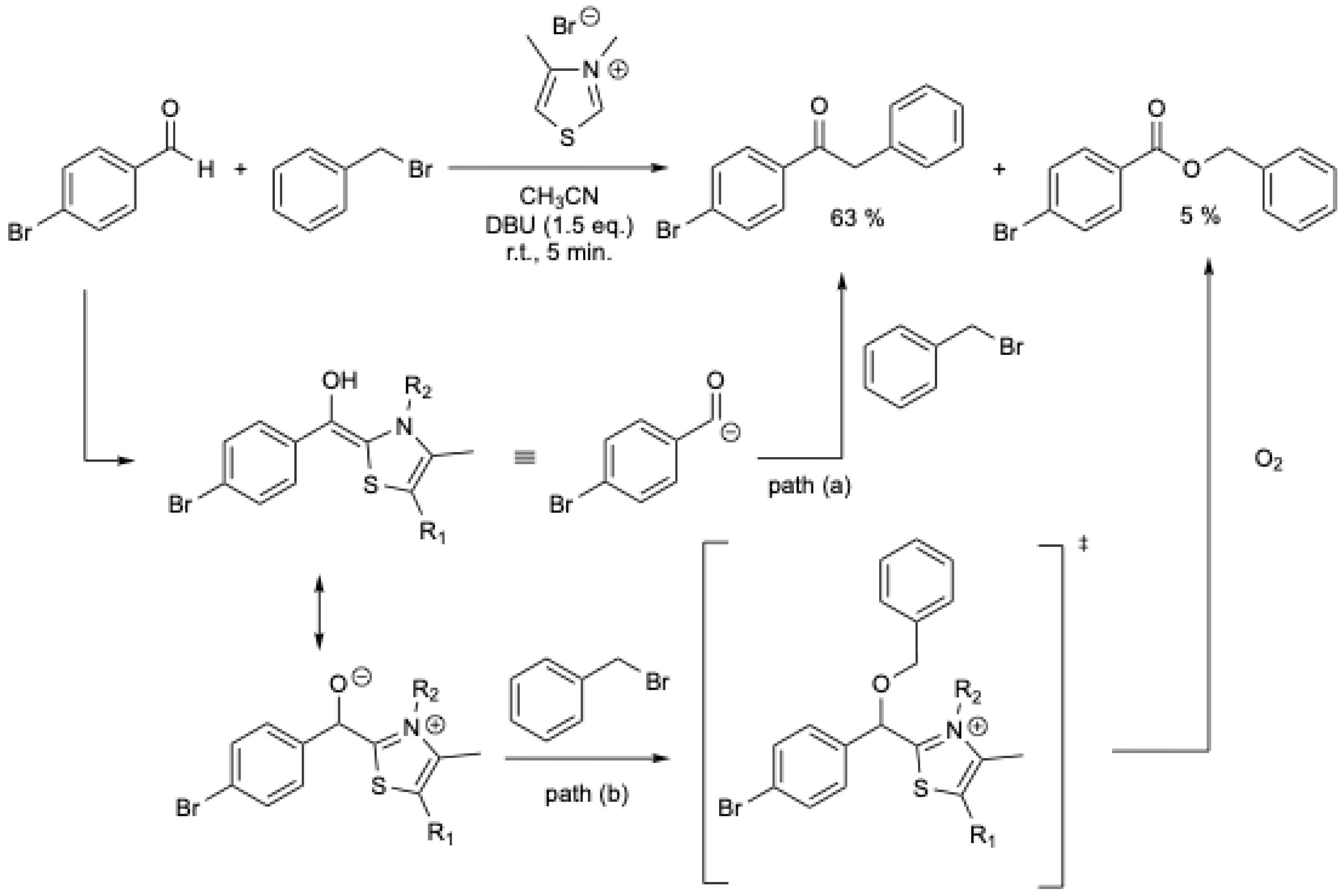
To synthesize ketones, W. Du and W.-P. Deng [18] studied the reaction of the Breslow intermediate with activated halides. It was expected that the equivalent of the Breslow intermediate 6 would react directly with benzyl bromide to yield the desired aryl ketone (Scheme 1, path (a). However, a mixture of ketone and ester was obtained. To explain the formation of the ester, alkylation of the tetrahedral intermediate by the halide, followed by oxidation by O2 from air, was proposed (Scheme 1, path (b). Under N2 and using a stoichiometric amount of NHC, various aryl ketones were obtained in 22–63% yields, but trace amounts of esters (0 to 15%) were still present [18]. For p-bromobenzaldehyde, 63% of ketone was obtained with 5% of ester (Scheme 1).
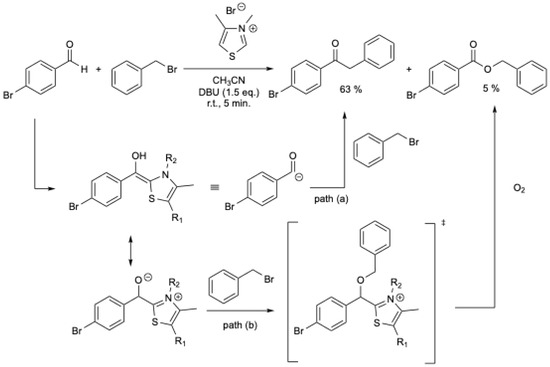
Scheme 1.
NHCs-catalyzed synthesis of ketones from aromatic aldehydes and benzyl bromide [18].
Using catalytic NHC, F. Glorius et al. [19] described an efficient method for the formation of diaryl acetophenone derivatives in good yields. However, reactions carried out using benzyl bromide as the electrophile in the alkylation reaction did not yield any alkylated product [19]. More recently, the synthesis of ketones from the deprotonated Breslow intermediates has been described, starting from alkyl iodines via a single-electron reduction process [21].
2.2. Products from Oxidation of Breslow Intermediate
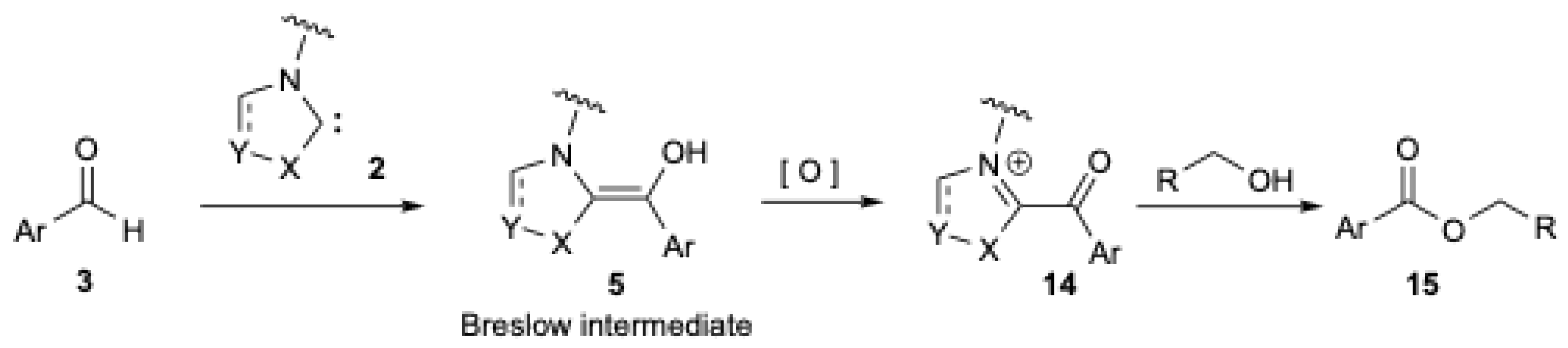
The scope of NHCs chemistry may be further expanded with protocols under oxidative conditions to access a wide range of organic compounds [22,23,24]. The oxidative NHC-catalytic cycle pathway (Figure 1) relies on the oxidation of the initially formed nucleophilic NHC-aldehyde adduct (Breslow intermediate 5) into an acyl azolium intermediate 14 (Figure 2). The latter is electrophilic at the carbonyl carbon and undergoes acyl substitutions with nucleophiles [2,3,4,5], including alcohols, to give the corresponding ester 15 and regenerates NHC 2. The process can be viewed as a double umpolung.
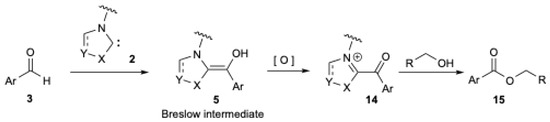
Figure 2.
NHCs chemistry under oxidative conditions to prepare esters from aldehydes.
von Wangelin et al. [10] have classified these reactions as being either “oxidative” (i.e., the oxidation of the Breslow intermediate 5 by an added oxidant) or “oxygenative” (i.e., the oxidation of the Breslow intermediate 5 by oxygen or oxygen from air). Other authors have used the same terms to describe different pathways during the aerobic oxidation of aldehydes [25,26]. To ensure clarity, the nomenclature of von Wangelin et al. will be used in this review [10].
2.2.1. Oxidative NHC Catalysis
The key step in this protocol is the oxidation of the Breslow intermediate 5 (Figure 1). Various nucleophiles can be used, and under these conditions, efficient oxidative esterification of aromatic aldehydes catalyzed by N-heterocyclic carbenes at room temperature has been reported using stoichiometric oxidants, such as nitrobenzene [27], azobenzene [28], DPQ [29,30,31], PIDA [32], or TEMPO and TEMPO derivatives [33] (Scheme 2). However, these oxidants are used in stoichiometric amounts, resulting in large amounts of waste, which limits the scalability of these reactions, even if TEMPO derivatives could be regenerated after the reaction using O2 [33].
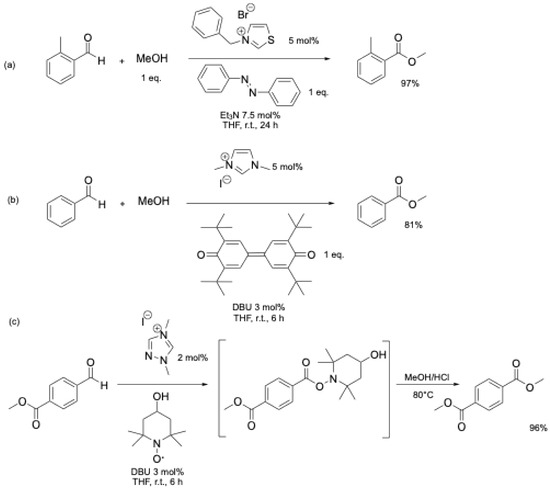
Scheme 2.
Oxidative esterification reaction of aromatic aldehydes using stoichiometric oxidant: (a) azobenzene [28]; (b) DPQ [29]; (c) TEMPO derivative [33].
2.2.2. Oxygenative NHC Catalysis
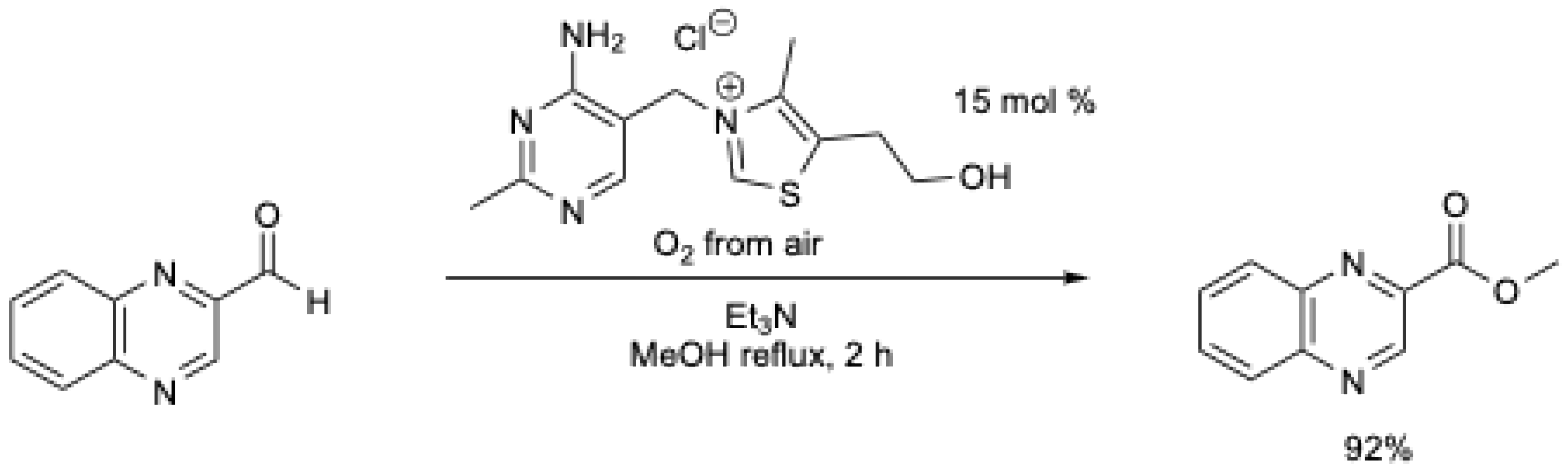
The reactivity of the Breslow intermediate with oxygen or with atmospheric oxygen has been noticed several times in the literature [18,20,34]. In 2009, Goswami et al. [34] described the first use of air as the oxidant for the oxidative esterification reaction of heterocyclic aldehydes using thiamine hydrochloride (Scheme 3). High yields were obtained in under mild conditions (MeOH at reflux, air), but the reaction was limited to heterocyclic aldehydes. Under N2, no ester was obtained [34].
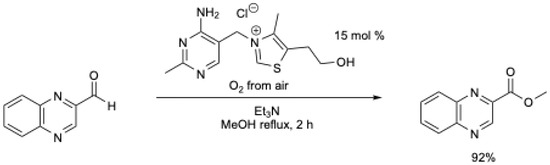
Scheme 3.
Aerobic oxidative esterification of heterocyclic aldehydes using thiamine hydrochloride [34].
The oxidation of the Breslow intermediate by trace amounts of oxygen was also supposed by W.P. Deng et al. [18] and X.-W. Liu et al. [20] to explain the formation of ester during the NHCs-catalyzed synthesis of ketones from aromatic aldehydes. However, the first practical protocol was published by Sudalai et al. in 2013 [35]. They described the use of oxygen as an oxidant for the oxidative aerobic esterification reaction of aromatic aldehydes [35]. The reaction could be performed in air, but the best results were obtained under pure O2 (Scheme 4). The absence of a carboxylic acid intermediate was confirmed by the lack of esterification between carboxylic acid and methanol under the reaction conditions [35]. This protocol could be extended to the synthesis of lactones [36,37].
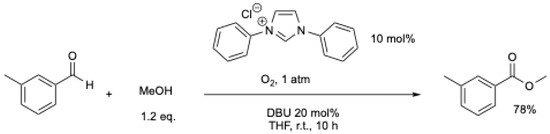
Scheme 4.
Oxidative aerobic esterification reaction of aromatic aldehydes [35].
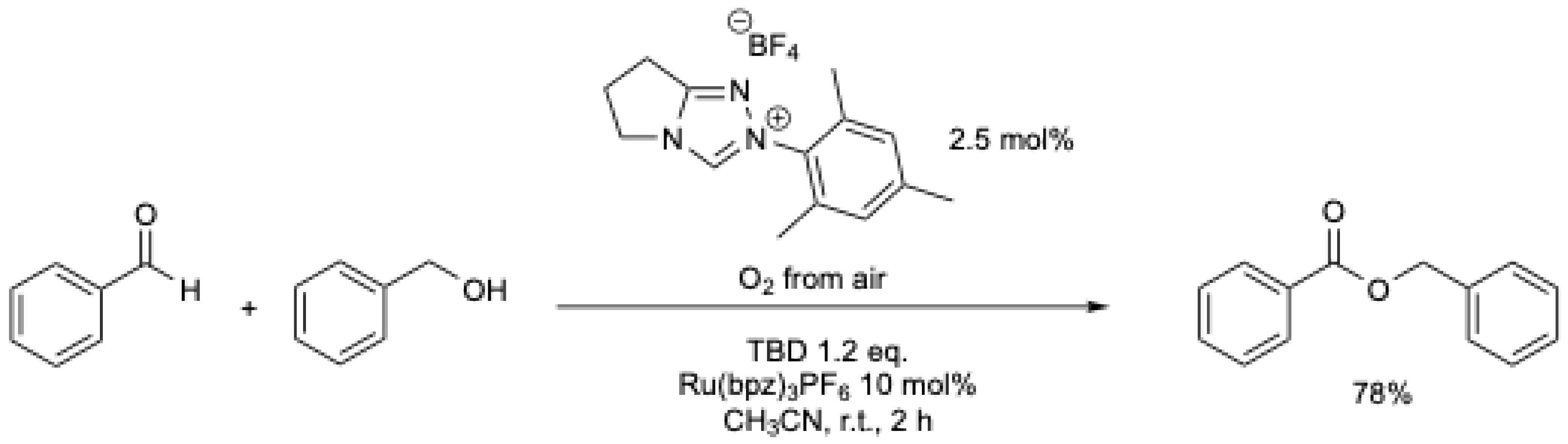
Shortly thereafter, A. Studer et al. [38] described the oxidative esterification of aldehydes using air as the terminal oxidant, employing a ruthenium-based redox catalysis to oxidize the Breslow intermediate (Scheme 5).
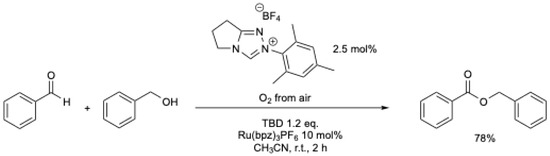
Scheme 5.
Oxidative esterification of aldehydes using air as the terminal oxidant and a ruthenium-based redox catalyst [38].
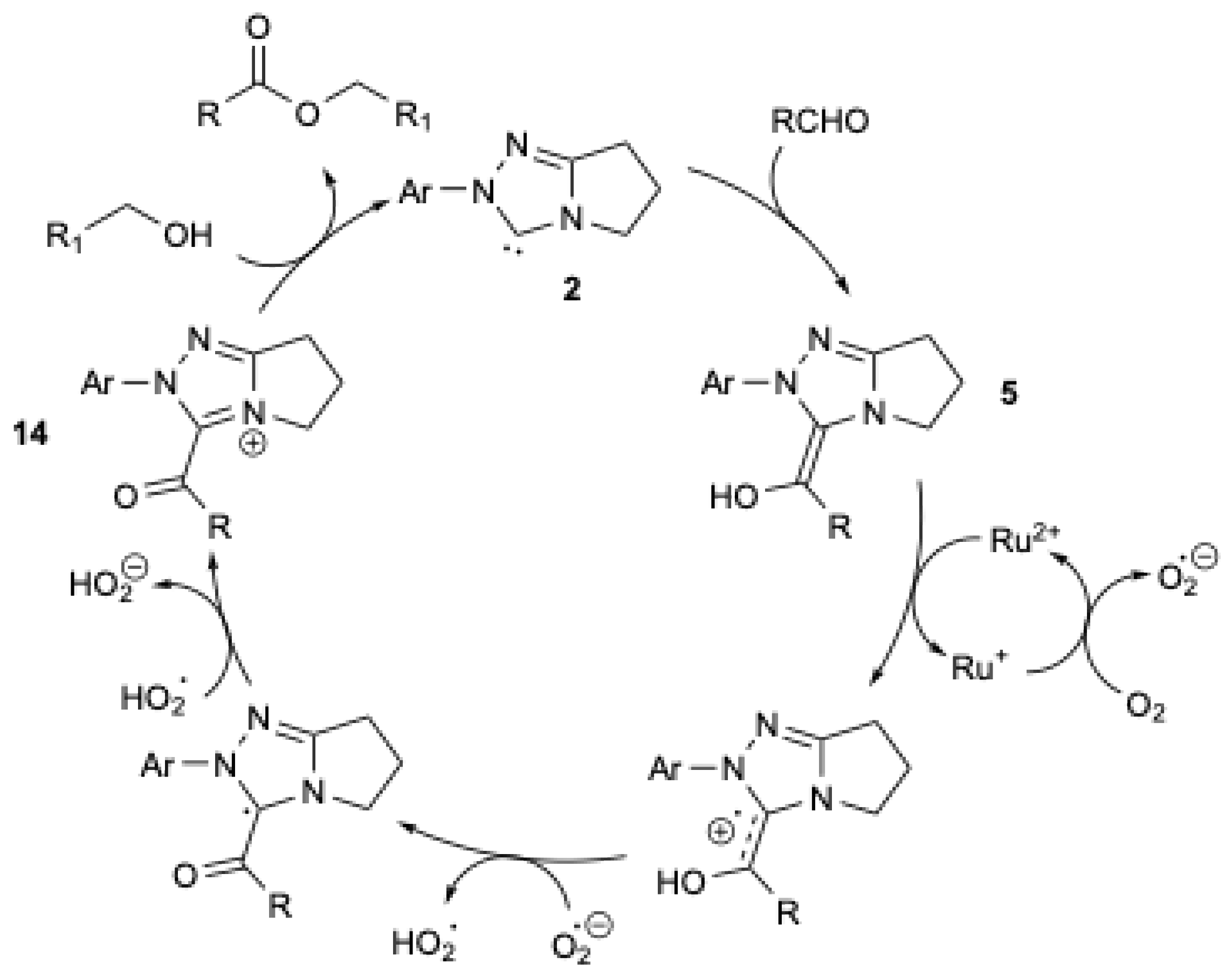
A mechanism (Figure 3) was proposed in which the Breslow intermediate 5 is oxidized by Ru/O2, leading to the formation of the acylazolium ion 14, which reacts with the alcohol to yield the corresponding ester and regenerate the NHC 2. It should be mentioned that, in the absence of the Ru catalyst and alcohol, aromatic and aliphatic aldehydes, under otherwise identical conditions, are oxidized to the corresponding acids (Scheme 12b) [38].
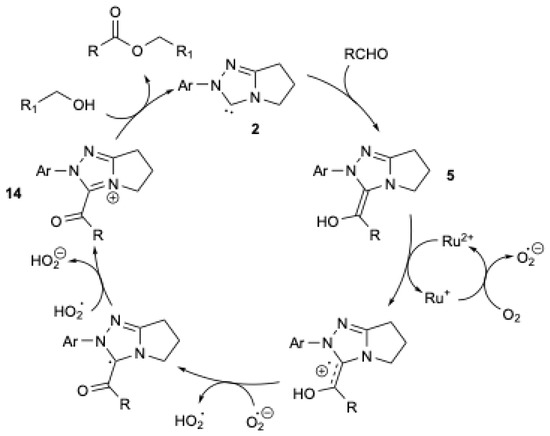
Figure 3.
Mechanism of oxidative esterification catalyzed by ruthenium-based redox catalysis, adapted from [38].
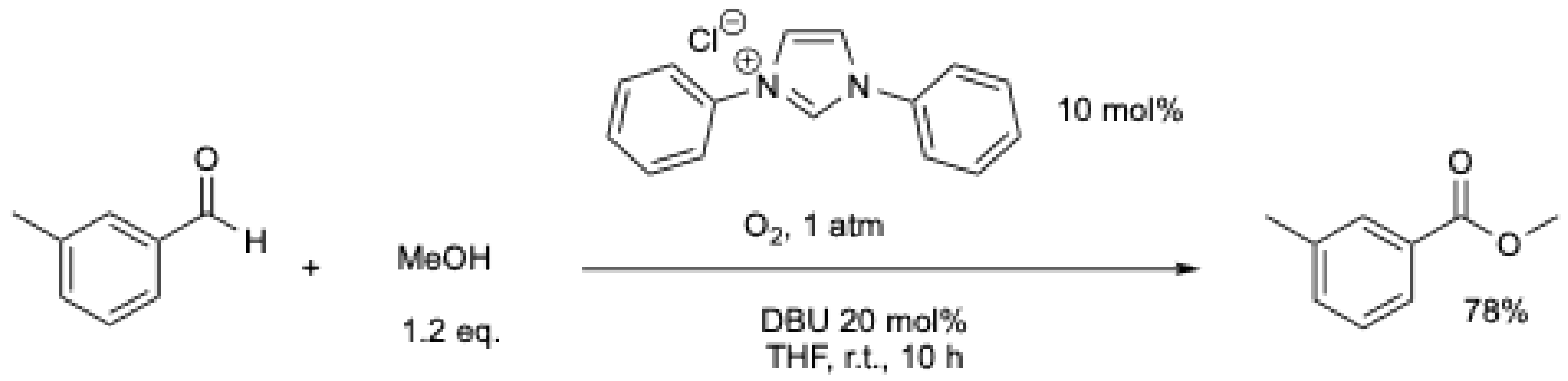
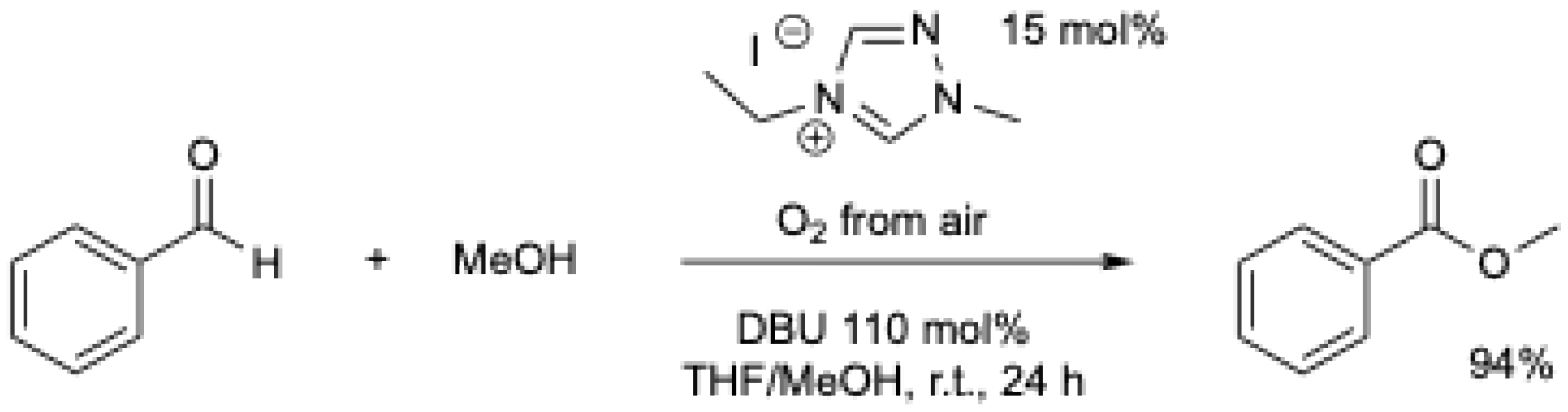
The same year, S. J. Connon et al. showed that by using different NHCs and a higher amount of base, high yields of the corresponding ester could be obtained under air (Scheme 6) [39].
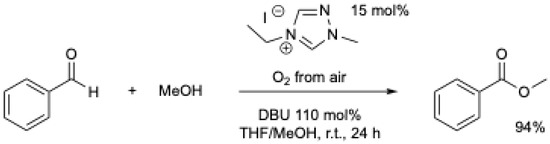
Scheme 6.
NHC-mediated oxidative esterification using atmospheric oxygen at r.t. [39].
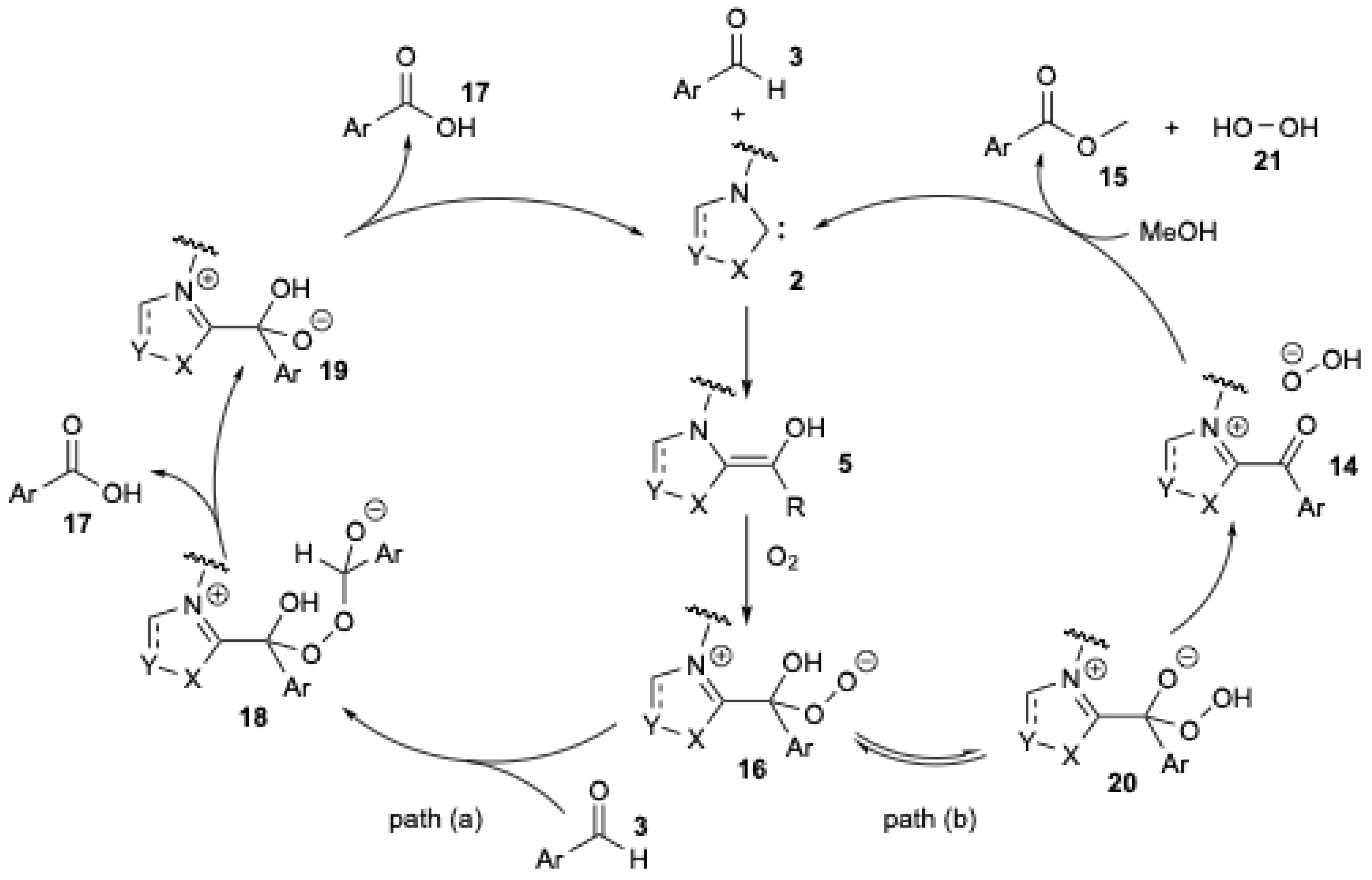
A detailed mechanistic investigation of the NHC-catalyzed aerobic esterification of bromobenzaldehyde was conducted by O. Bortolini et al. [25,26] (Figure 4) in order to explain the formation of carboxylic acid during the aerobic esterification of aromatic aldehydes.
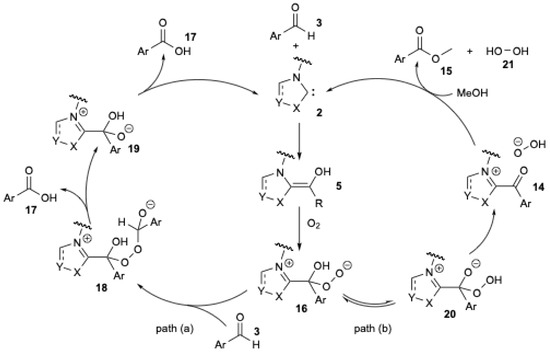
Figure 4.
Mechanistic pathway proposed for the formation of benzoic acid during NHC-catalyzed oxidation−esterification of benzaldehyde (adapted from O. Bortolini et al. [25,26]).
The formation of carboxylic acid can be explained by the competition between two pathways (Figure 4). As previously described, NHC 2 reacts with aromatic aldehyde 3 to form the Breslow intermediate 5. In the presence of O2, a single-electron transfer process is thought to occur, leading to the formation of a peroxide anion 16 (Figure 4). In path (a), 16 could react with a second molecule of aldehyde 3 to yield 18. The rearrangement of 18 releases one molecule of carboxylic acid 17 and 19. The regeneration of carbene 2 occurs with the release of a second molecule of carboxylic acid 17. In path (b), a tautomeric equilibrium gives the hydroperoxy intermediate 20 [25], which is then converted into the acyl cation equivalent 14. Substitution by methanol yields the bromomethyl benzoate 15 and hydrogen peroxide 21.
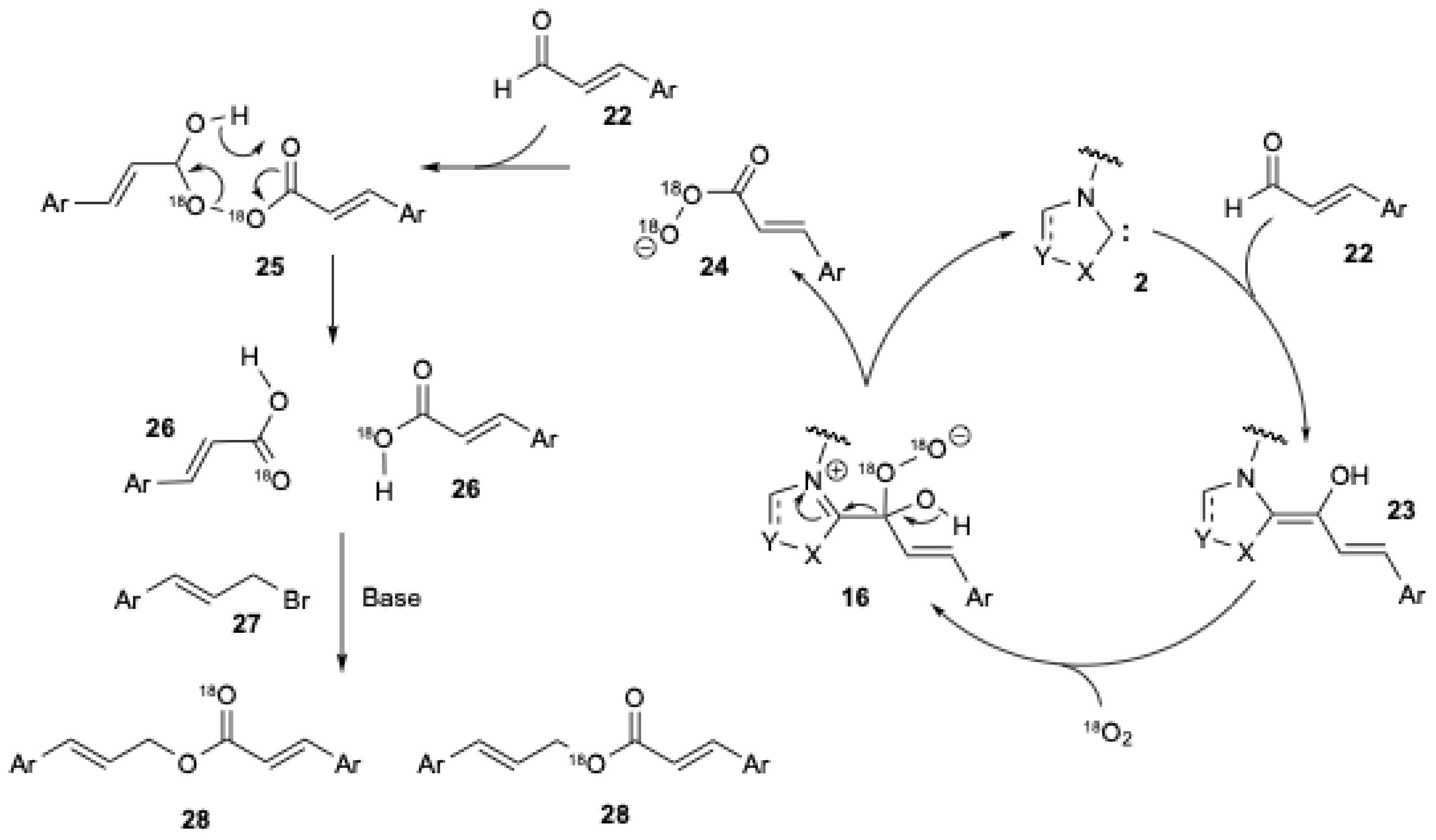
A similar mechanism has already been proposed by several authors [10,18,25]. They also suggested the formation of the peroxide anion 16 (Figure 4) to explain the ester formation during the NHC-catalyzed oxidative esterification of aldehydes using sp3-centered electrophiles, i.e., halides [40]. X.-W. Liu et al. [20] studied the experiment in which 18O2 was used to elucidate the mechanism (Figure 5) [20].
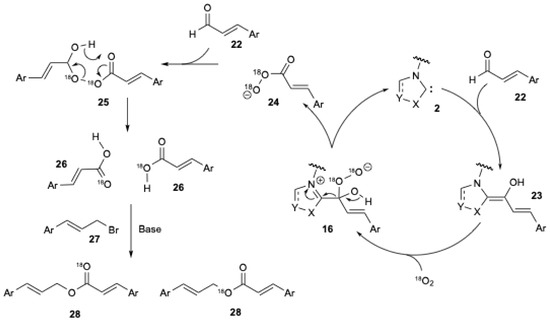
Figure 5.
Mechanism illustrating the insertion of 18O2 into the ester during the NHC-catalyzed oxidative esterification of cinnamaldehyde with cinnamyl bromide (adapted from X.-W. Liu et al. [20]).
In the presence of NHC 2, cinnamaldehyde 22 yields the corresponding Breslow intermediate 23. In the presence of 18O2, the peroxide anion 16 is formed (Figure 5). Rearrangement of the latter produces peroxyacid anion 24, which can react with another cinnamaldehyde 22 to give a Criegee intermediate 25. The rearrangement of 25 results in the formation of two carboxylic acids 26. In the presence of a base and cinnamyl bromide 27, the corresponding ester 28 is formed. The same intermediate was also confirmed by an isotopic labelling experiment using 18O2, which involved the NHC-catalyzed aerobic oxidation of aromatic aldehydes to aryl esters using boronic acids [41].
3. Oxidation of Aromatic Aldehydes into Carboxylic Acids Using an Oxygenative NHC-Catalytic Cycle
As previously noted, the presence of carboxylic acids during oxygenative NHC catalysis has been reported by several authors but was regarded as an intermediate in the oxidative esterification of aldehydes using halides [20] or a by-product [25,26,38].
In 1977, J. Castells et al. [42] studied the oxidative esterification of aldehydes catalyzed by the conjugate bases of thiazolium ions in methanol. Carboxylic acid was obtained in the absence of alcohol, but it was not quantified.
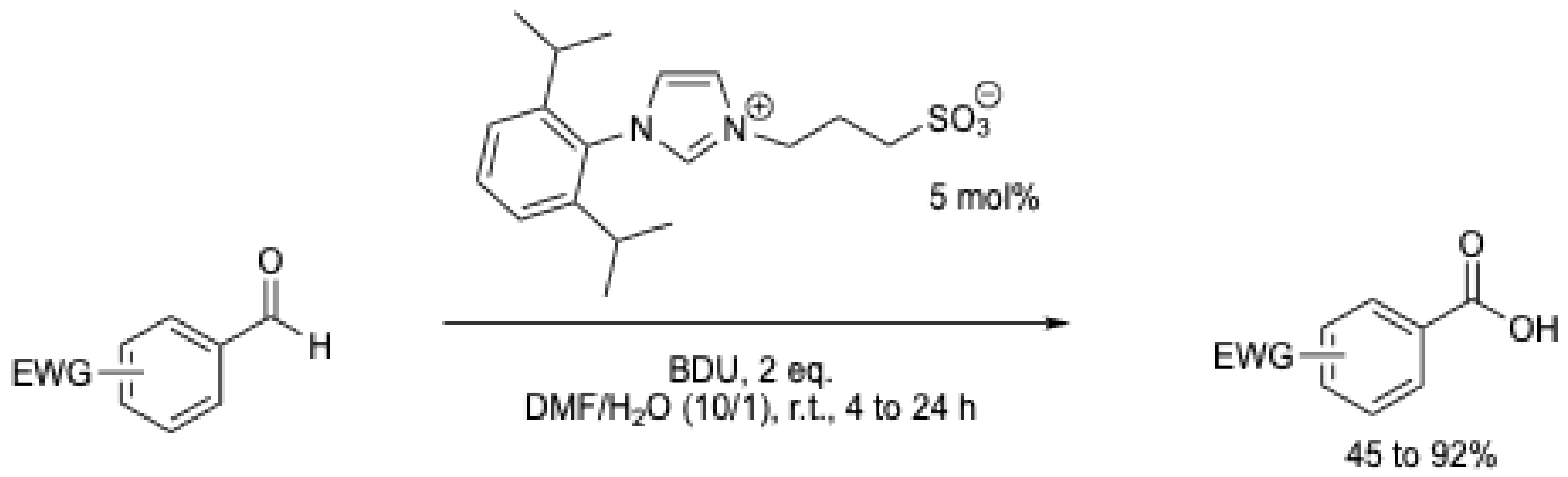
In 2009, M. Yoshida et al. [6] were the first to report the oxidation of aromatic aldehydes with an electron-withdrawing group using an NHC catalyst (Scheme 7). It should be mentioned that the authors suggested a nucleophilic addition of water to the Breslow intermediate. The possibility of oxidation by atmospheric oxygen cannot be excluded, as the reaction was performed at r.t. for 10 to 24 h without mention of the use of an inert atmosphere. Under these conditions, benzoic acid was obtained in less than 10% yield (Table 1, entry 1). Using methanol or dimethylamine, the corresponding ester and amide were obtained in 68 and 60% yield, respectively.
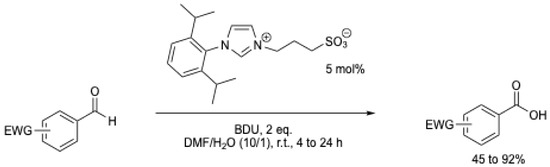
Scheme 7.
NHC-catalyzed oxidative carboxylation of arylaldehydes [6].
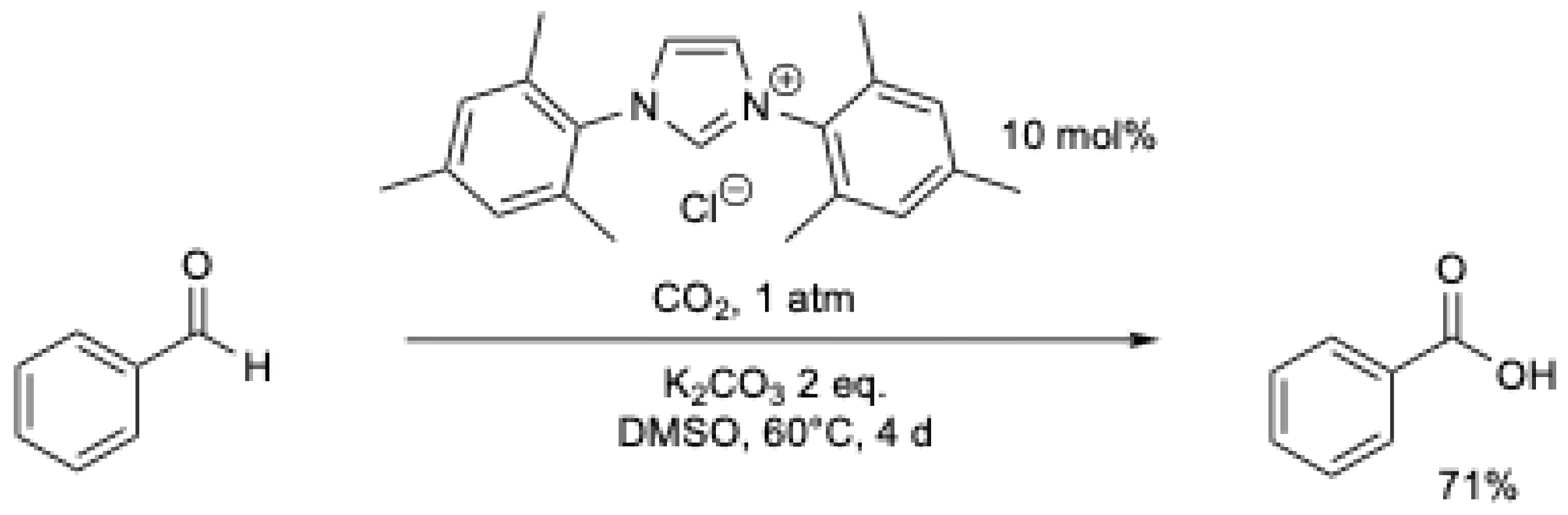
In the same year, Y. Zhang et al. [43] described the oxidation of aromatic aldehydes with NHC using CO2 as the oxidant and 2 as the precursor (Scheme 8). The reaction takes 3 to 5 days at r.t. under an atmospheric pressure of CO2. It was proposed that NHC reacts with CO2, generating an intermediate that can react with the aldehyde, leading to the formation of carboxylic acid and the release of CO.
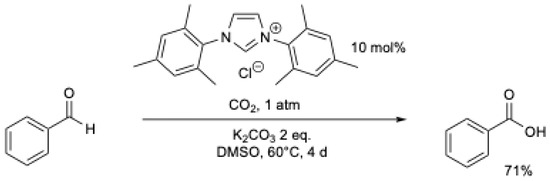
Scheme 8.
NHC-catalyzed oxidation of arylaldehydes using CO2 as an oxidant.
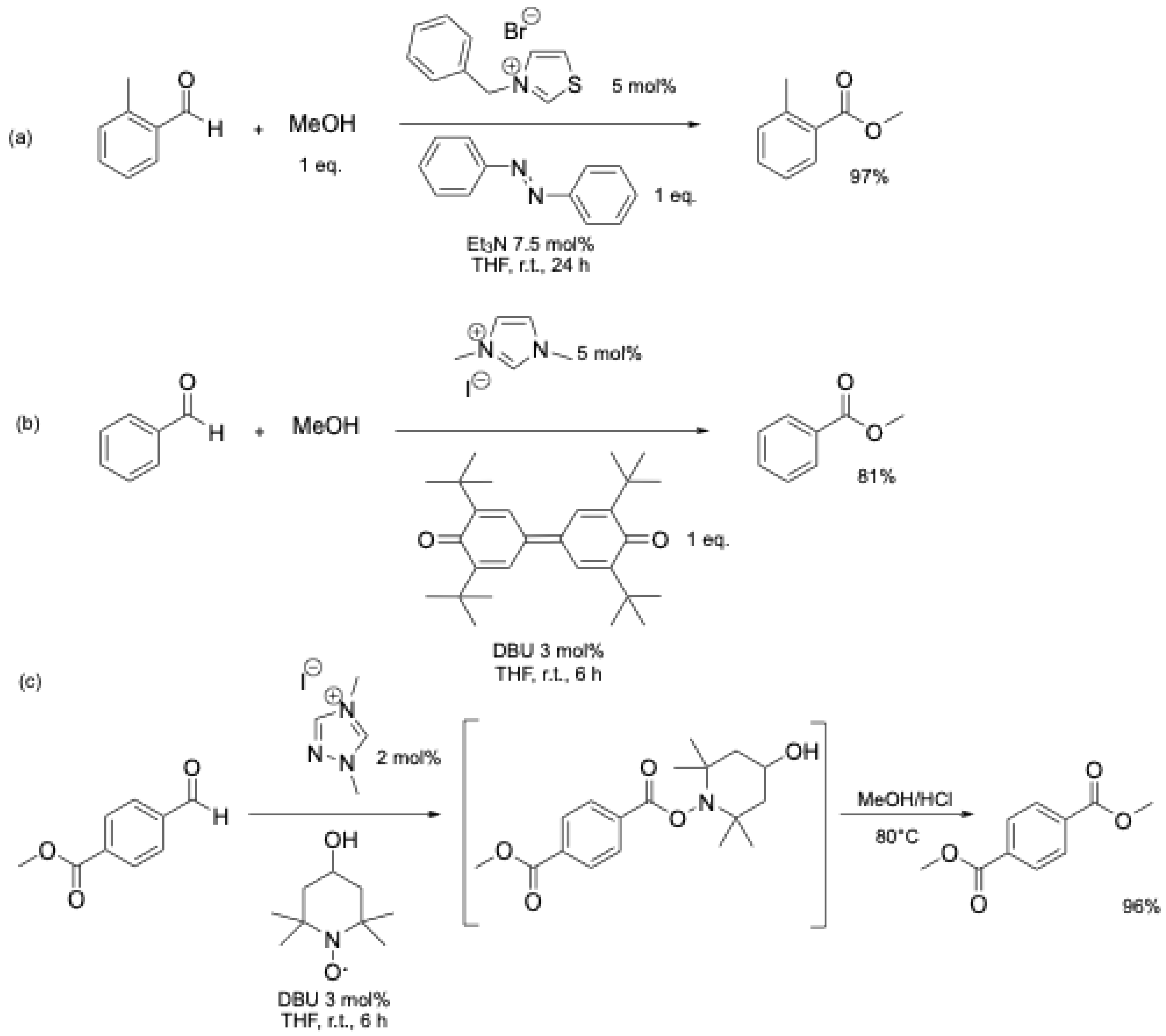
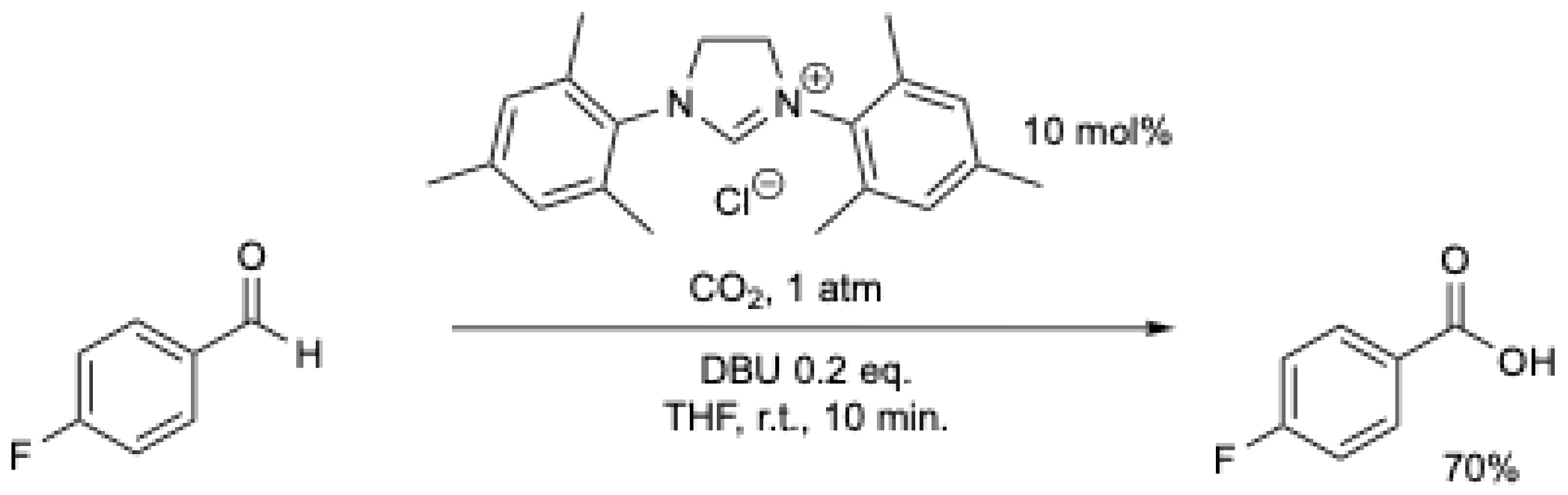
Shortly after, V. Nair et al. [44] described an improved protocol for the NHC-mediated oxidation of aromatic aldehydes using a stream of carbon dioxide (Scheme 9). In less than 10 min. at r.t., a high yield (36–92%) of the corresponding carboxylic acid was obtained (Scheme 9). The best results were obtained in THF at r.t., with the exception being benzaldehyde and 3,4-dimethoxybenzaldehyde which gave better results in acetonitrile under reflux [44].
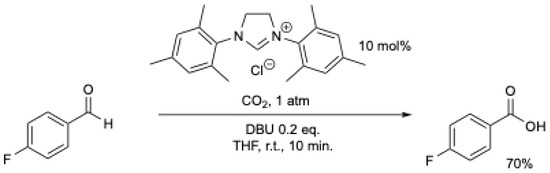
Scheme 9.
NHC-catalyzed conversion of aromatic aldehydes to acids using CO2 as an oxidant [44]. See Scheme 8 for comparison.
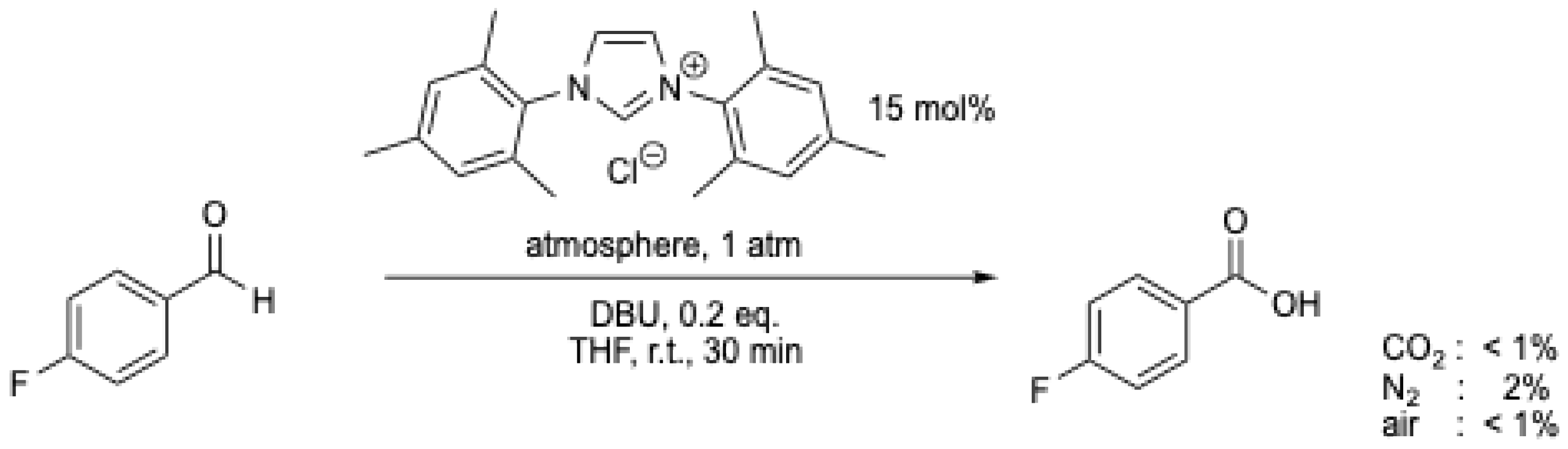
The mechanism suggested for this transformation involves the transfer of an oxygen atom from CO2 to the aldehyde, representing a process that is counterintuitive and thermodynamically questionable [9,45]. Furthermore, the reaction was carefully checked by J.W. Bode et al. [7], and the results of Y. Zhang et al. [43] and V. Nair et al. [44] could not be reproduced (Scheme 10) [7]. It was demonstrated by J.W. Bode et al. that exogenous O2 was the effective oxidant [7]. H. Wang et al. [8] also challenged the role of CO2, concluding that this reaction cannot be performed at room temperature, which further questions the work of Y. Zhang et al. [43] and V. Nair et al. [44]. However, following these works, the use of CO2 as an oxidant for the transformation of aldehydes into the corresponding carboxylic acids continues to be explored, but these results have also faced scrutiny [9].
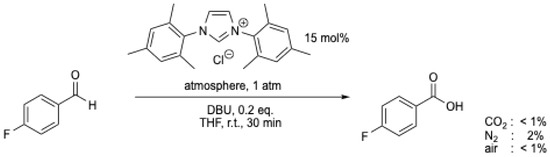
Scheme 10.
NHC-catalyzed oxidation of 4-fluorobenzaldehyde under different atmospheres [7]. See Scheme 9 for comparison.
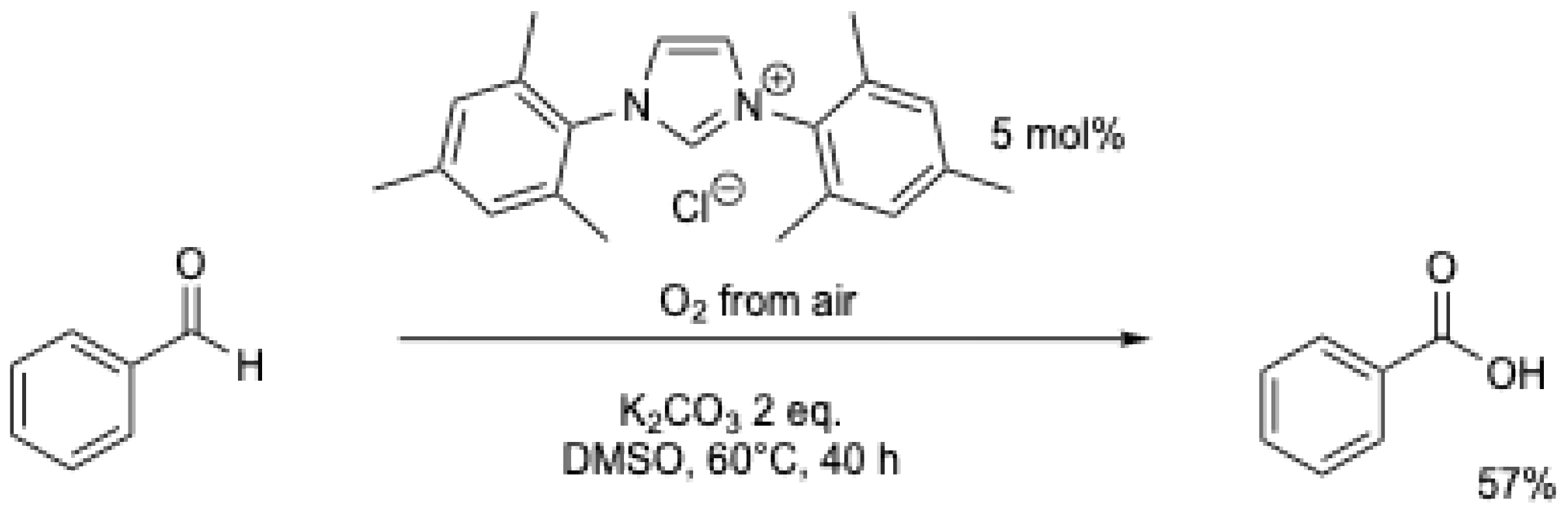
Under air, the results of M. Yoshida et al. [6] were reproduced and improved by J.W. Bode et al. (Scheme 11) [7]. Benzoic acid could be obtained using air with a 57% yield at 60 °C over 40 h (Table 1, entry 2). Using acetonitrile at reflux, a similar yield could be obtained in 14 h, but using 0.4 eq. of DBU (Table 1, entry 3) [36].
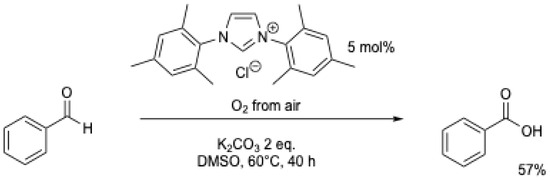
Scheme 11.
NHC-catalyzed oxidation of benzaldehyde under air [7].
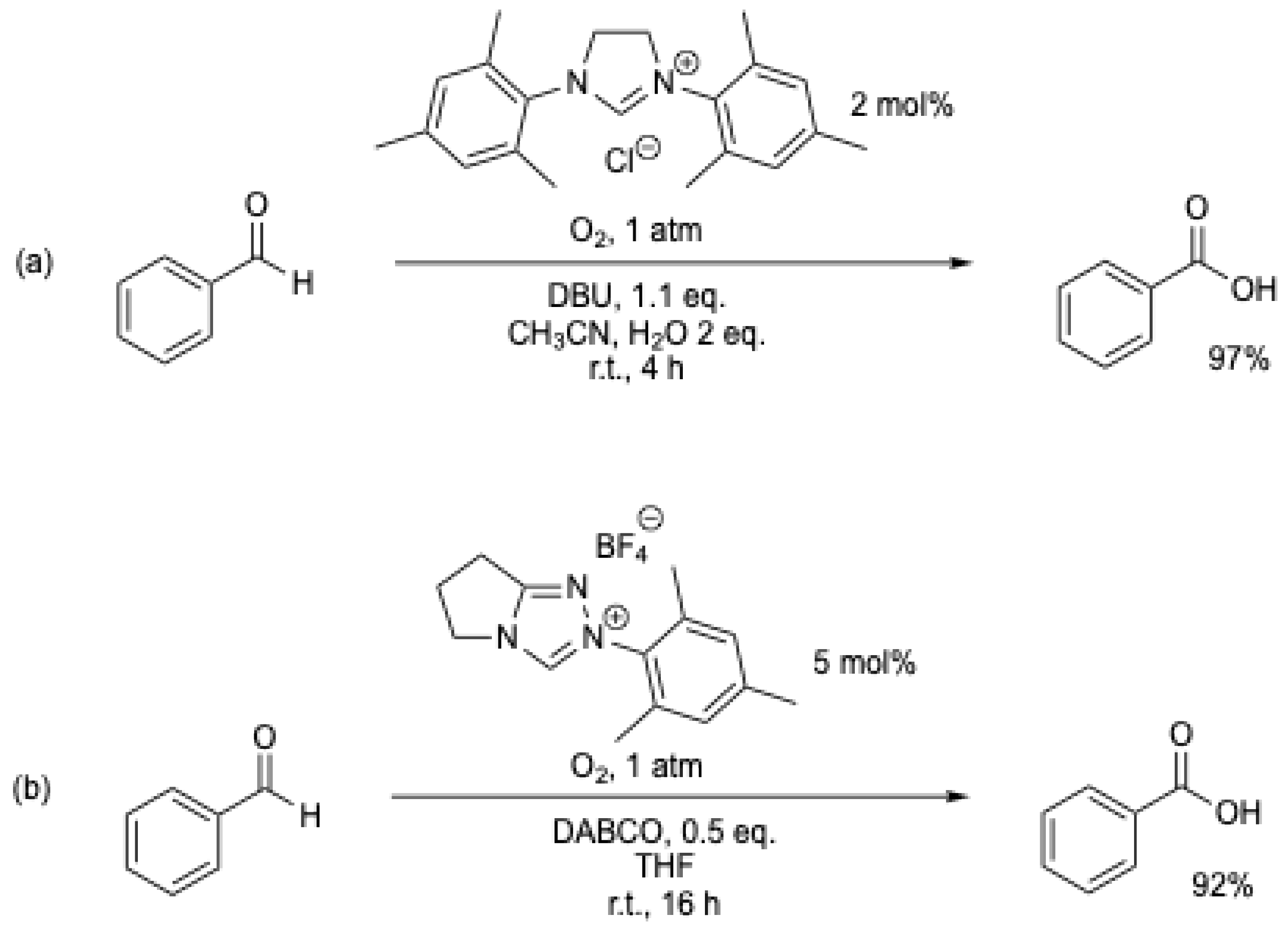
In 2013, W.-F. Fu et al. [46], A. Studer et al. [38], and S. J. Connon et al. [47] (Scheme 12) simultaneously reinvestigated the work of M. Yoshida et al. [6]. Using new NHCs and similar experimental conditions (r.t., solvent/H2O, air [38,46,47], or oxygen [48]), various aromatic aldehydes were oxidized in high yield to their corresponding carboxylic acids (Table 1, entries 4 to 9). Under these conditions, yields of up to 95% of benzoic acid could be obtained using atmospheric oxygen as the oxidant (Scheme 12).
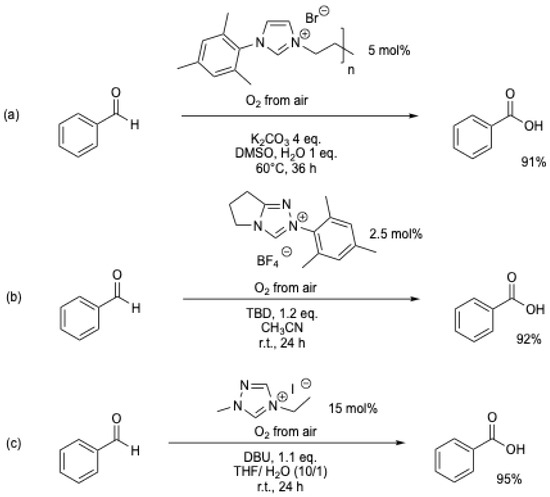
Scheme 12.
NHC-catalyzed transformation of aromatic aldehydes into acids using atmospheric oxygen as an oxidant: (a) [46]; (b) [38]; (c) [47].
Scheme 12.
NHC-catalyzed transformation of aromatic aldehydes into acids using atmospheric oxygen as an oxidant: (a) [46]; (b) [38]; (c) [47].
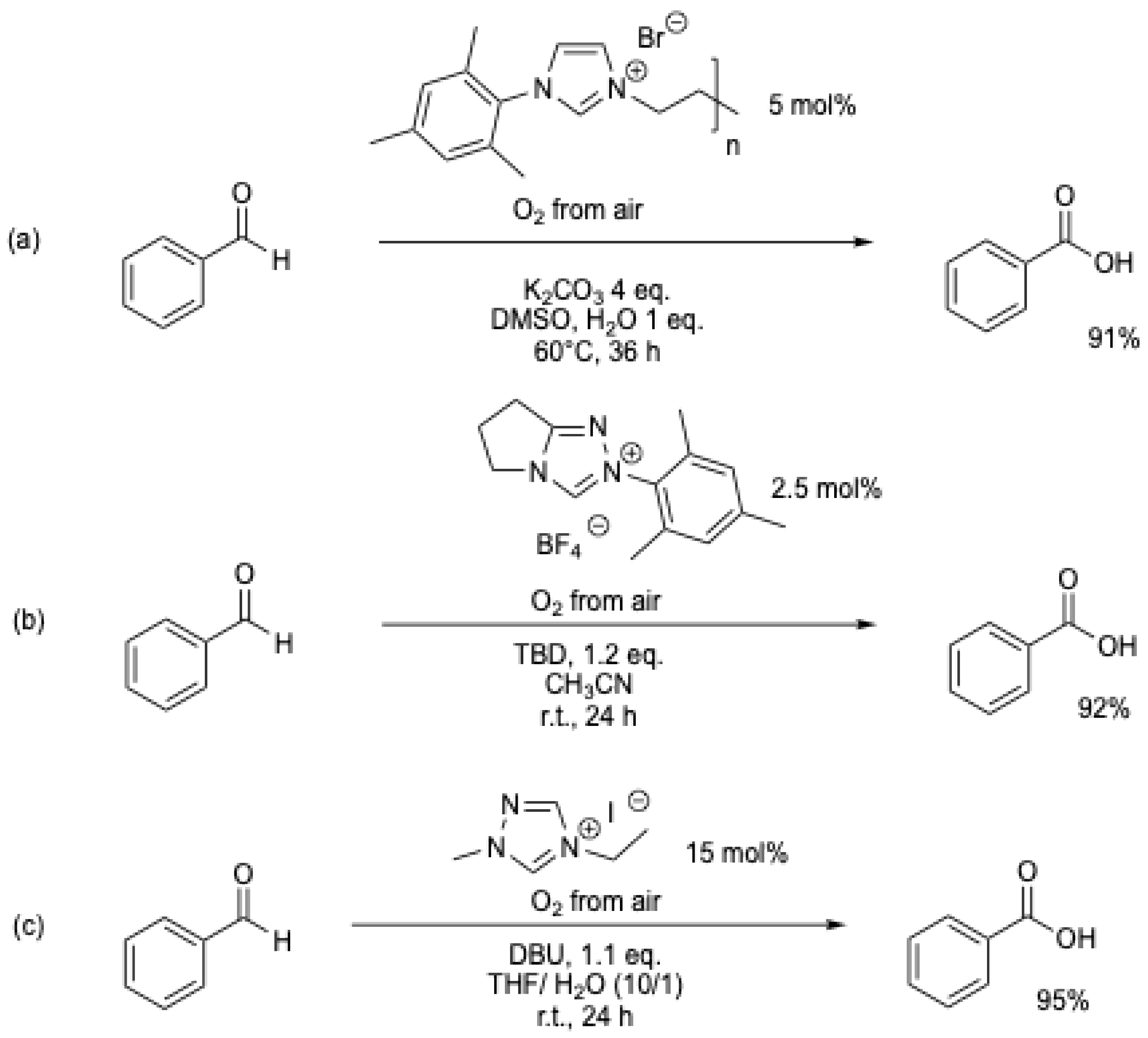
O2 at atmospheric pressure can also be used as an oxidant (Scheme 13) [48,49], but the impact on the chemical reaction rate could only be observed with one example (Table 1, entries 8 and 9) [48].
Scheme 13.
NHC-catalyzed transformation of aromatic aldehydes into acids using oxygen as an oxidant: (a) [48]; (b) [49].
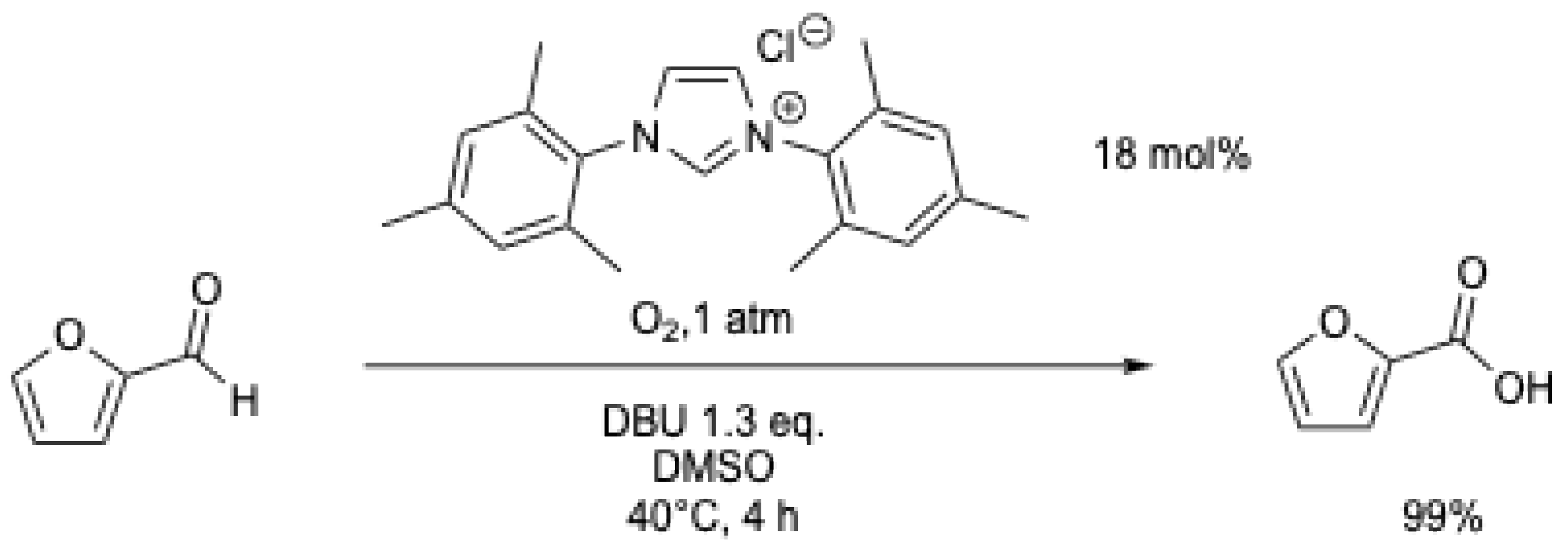
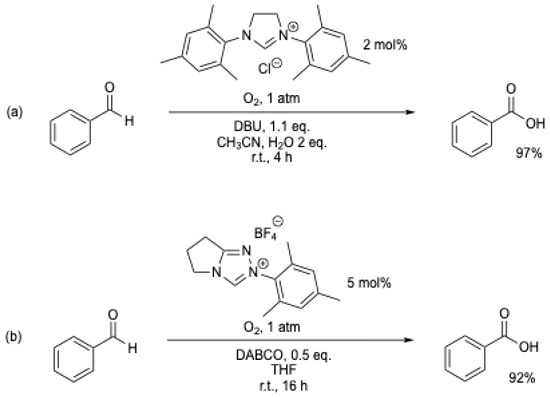
The aerobic oxidation of biomass-derived furfural to furoic acid was studied under oxygen using an NHC catalyst (Scheme 14) [50]. Quantitative yields were obtained under mild conditions (40 °C, 1 atm of O2), and the procedure was extended to other biomass-derived furfurals (5-methylfurfural, HMF, etc.).
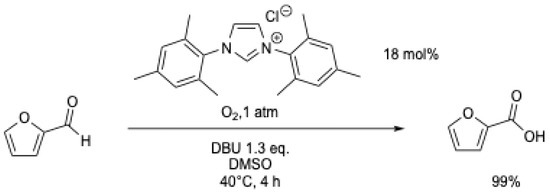
Scheme 14.
Aerobic oxidation of furfural to furoic acid with an NHC catalyst [50].
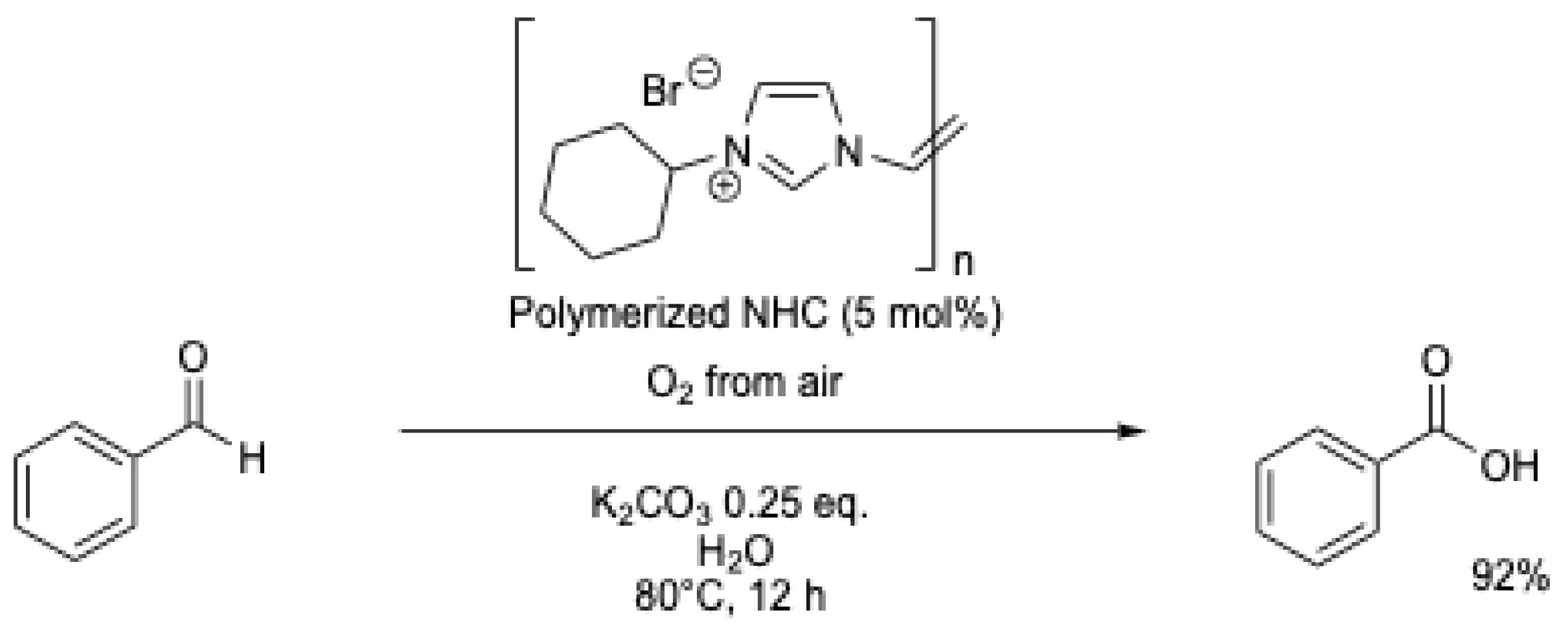
A polymerized NHC catalyst was evaluated in a continuous-flow reactor for the oxidation of benzaldehyde (Scheme 15) [51]. Water containing 0.25 eq. of K2CO3 was used as the solvent, and the mixture was continuously pumped through a reaction tube maintained at 80 °C, which contained the catalyst (∅ = 10 mm) and fed back to a stirred batch open to air. The solution was continuously recycled, and after 12 h, 96% of benzoic acid was obtained (Table 1, entry 10).
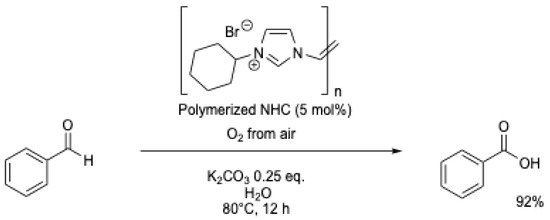
Scheme 15.
Aerobic oxidation of benzaldehyde using a polymerized NHC [51].
To compare the different systems described in the literature, the aerobic oxidation of benzaldehyde to benzoic acid using an NHC catalyst will be considered, and the results are summarized in Table 1. The yields of benzoic acid obtained are good to excellent. However, it is striking to observe that the concentration of NHC precursor salts, the type of base (inorganic/organic), and the amount of base used to form the NHCs are highly variable, ranging from 0.4 to 20 mol% and 0.25 to 80 eq., respectively (Table 1). The same applies to the experimental conditions, which vary from room temperature to 80 °C, using air or O2 as an oxidant. It is worth noting that these oxidations occur in organic solvents, unlike reactions that do not utilize NHCs, which primarily proceed in water (Table 2).
To go beyond yield or reaction time, the productivity of these reactions, determined as the amount of benzaldehyde converted by volume of liquid phase by hour and for 0.21 atm of O2 (i.e., air), could be calculated according to Equations (1) and (2) [52].
Using atmospheric oxygen, Equation (1) was used:
Using oxygen, Equation (2) was used:
Using atmospheric oxygen as the oxidant, productivity correlates with the concentration of benzaldehyde used, with one exception (Table 1, entry 5) [38], which shows the highest productivity, regardless of the type of oxidant used. Based on these observations, it is impossible to propose standard conditions for this type of transformation or to justify the use of pure oxygen. There is only one experiment [49] that compares the use of oxygen with air (Table 1, entries 8 and 9), but surprisingly, the productivity calculated with oxygen is lower than with air, despite a higher yield (for comparison, see Table 2, entries 1 and 2).

Table 1.
Comparison of experimental conditions for the oxidation of benzaldehyde using NHC catalysts and calculated productivity.
Table 1.
Comparison of experimental conditions for the oxidation of benzaldehyde using NHC catalysts and calculated productivity.
 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Conc. (mol/L) | Precur. Salt (a) | Base | Oxidant | Solvent(s) | Temp. (°C) | Time (h) | Yield (%) | Prod. (b) (mM L−1 h−1) | Ref. |
| 1 | 0.34 | 5 mol% | BDU (2 eq.) | air | DMF/H2O | r. t. | 24 | 10 (b) | 1.43 | [6] |
| 2 | 0.10 | 5 mol% | K2CO3 (2 eq.) | air | DMSO/1 eq. H2O | 60 °C | 40 | 57 | 1.43 | [7] |
| 3 | 0.20 | 20 mol% | DBU (0.4 eq.) | air | CH3CN | 80 °C | 14 | 65 | 9.29 | [36] |
| 4 | 0.20 | 5 mol% | K2CO3 (80 eq.) | air | DMSO/1 eq. H2O | 60 °C | 36 | 91 | 5.06 | [46] |
| 5 | 0.25 | 2.5 mol% | TBD (1.2 eq.) | air | CH3CN | r. t. | 24 | 92 | 115.00 | [38] |
| 6 | 0.40 | 15 mol% | DBU (7.3 eq.) | air | THF/H2O (10/1) | r. t. | 24 | 95 | 15.83 | [47] |
| 7 | 1.00 | 2 mol% | DBU (1.1 eq.) | O2 | CH3CN /2 eq. H2O | r. t. | 4 | 97 | 48.50 | [48] |
| 8 | 0.17 | 5 mol% | DABCO (10 eq.) | O2 | THF | r. t. | 16 | 92 | 1.92 | [49] |
| 9 | 0.17 | 5 mol% | DABCO (10 eq.) | air | THF | r. t. | 16 | 76 | 7.92 | [49] |
| 10 | 1.00 | 0.2 mol% (c) | K2CO3 (0.25 eq.) | air | H2O | 80 °C | 12 (d) | 96 | 80.00 | [51] |
| 11 | 0.33 | 1 mol% | K3PO4 (4 eq.) | air (e) | H2O | 80 °C | 24 | 92 | 12.78 | [53] |
| 12 | 1.00 | 10 mol% | DBU (1.2 eq.) | O2 | THF | 25 °C | 16 | 40 (f) | 5.00 | [54] |
(a) The structure of the precursor salt could be found in Scheme 7, Scheme 8, Scheme 9, Scheme 10, Scheme 11, Scheme 12, Scheme 13, Scheme 14 and Scheme 15. (b) Productivity was calculated as mM aldehyde converted per volume of liquid phase per hour at 0.21 atm O2 (i.e., air), see Equations (1) and (2). (c) A polymerized catalyst (see Scheme 15) was used in a continuous-flow reactor. (d) The aqueous reaction mixture was continuously pumped through a reaction tube containing the catalyst (∅ = 10 mm) and fed back to the stirred reaction mixture open to air. (e) Pd(OAc)2 (1 mol%) was added. (f) Carboxylic acid was transformed in situ into organotin (IV) carboxylates with nBu3SnCl. Quantitative yield was assumed for this reaction.

Table 2.
Comparison of experimental conditions for the aerobic oxidation of benzaldehyde using metal-free, light-induced transformation, and base metal catalysts.
Table 2.
Comparison of experimental conditions for the aerobic oxidation of benzaldehyde using metal-free, light-induced transformation, and base metal catalysts.
| Entry | Conc. (mol/L) | Catalyst | Activation Additive | Oxidant | Solvent | Temp. | Time (h) | Yield (%) | Prod. (a) (mM L−1 h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.33 | ∅ | ∅ | air | H2O | r.t. | 12 h | 83% | 23.05 | [55] |
| 2 | 0.33 | ∅ | ∅ | O2 | H2O | r.t. | 2 h | 81% | 27.67 | [55] |
| 3 | 0.05 | ∅ | ∅ | O2 | H2O | 37 °C | 24 h | 90% | 0.07 | [56] |
| 4 | 0.01 | ∅ | ∅ | O2 | H2O | 37 °C | 24 h | 99% | 0.02 | [56] |
| 5 | 0.025 | ∅ | 365 nm LED | O2 | H2O | r.t. | 30 h | 86% | 0.14 | [57] |
| 6 | 0.2 | CF3SO2Na 25 mol% | 400 nm LED | O2 | CH3CN | r.t. | 12 h | 95% | 3.17 | [58] |
| 7 | 0.3 | ∅ | 370 nm LED | air | Acetone/10%H2O | r.t. | 7 h | 87% | 37.66 | [59] |
| 8 | 0.75 | FeIIIMo6 0.1 mol% | Na2CO3 0.1 eq. | O2 | H2O | 70 °C | 8 h | 96% | 18.00 | [60] |
| 9 | 0.1 | [Cu(acac)2]SIMes 10 mol% | NaOH 1 eq. | O2 | H2O | 50 °C | 12 h | 99% | 1.65 | [61] |
(a) Productivity was calculated as mM aldehyde converted per volume of liquid phase per hour at 0.21 atm O2 (i.e., air), see Equations (1) and (2). The symbol ∅ indicates that no catalyst, activation method, or additive is used.
4. Other Metal-Free Aerobic Oxidation of Aromatic Aldehydes into Carboxylic Acids
More than 195 years ago, it was recognized that benzaldehyde is prone to aerobic oxidation, yielding benzoic acid (autoxidation) [62,63]. Since then, numerous procedures have been described [64], and we have recently discussed the mechanism of this oxidation [65]. Table 2 lists recent publications for the metal-free aerobic oxidation of benzaldehyde. Additional publications were added, i.e., light-induced transformation and base metal catalysts.
Among these catalyst- and activation-free methods, the work of A. Vigalok et al. [55] is a landmark publication in the field of autoxidation of benzaldehyde. They rediscovered that by simply mixing benzaldehyde with water in the presence of air or O2, high yields of benzoic acid could be obtained (Table 2, entries 1 and 2). It should be noted that by using the correction for the partial pressure of O2, similar productivity can be obtained for the same reaction performed with O2 or air (Table 2, entries 1 and 2), i.e., ∼ approximately 20 mM L−1 h−1. However, a total conversion could not be obtained under those conditions, despite using O2. This can be attributed to the poor aqueous solubility of benzoic acid (i.e., 0.03 M) [66]. For high benzaldehyde conversion, crystals of benzoic acid are expected under those conditions. Benzaldehyde could form a host−guest complex in these crystals or form a complex with benzoic acid, protecting it from oxidation [67]. It was later demonstrated that total conversion could be obtained using a diluted solution (Table 2, entries 3 and 4) [56], but under these conditions, productivity drops down to 0.1 mM L−1 h−1. To achieve total conversion in water for higher concentrations, a base is necessary to form benzoate, which has a solubility of up to 4 M in water (Table 2, entry 8).
The photochemical aerobic catalyst-free oxidation of benzaldehyde to benzoic acid has been studied. Using water as a solvent, very low productivities were obtained (Table 2, entries 5 and 6), despite the use of a photosensitizer [58]. Using acetone/water as a solvent, G. Kokotos et al. [59] obtained an impressive productivity of up to 35 mM L−1 h−1 (Table 2, entry 7). A detailed mechanism study suggests a solvent-assisted oxidative transformation.
5. Conclusions
The aerobic oxidation of benzaldehyde into benzoic acid is a spontaneous process (autoxidation). Under these conditions, the formation of benzoic acid in the presence of oxygen (or air) does not indicate a catalytic activity, especially for reaction times up to 12 h. To compare different catalytic systems, we propose using productivity, which is defined by the amount of benzaldehyde converted per volume of liquid phase per hour at 0.21 atm of O2 (i.e., atmospheric oxygen) [52]. The results obtained by A. Vigalok et al. [55], achieved by simply stirring benzaldehyde in water in the presence of air, can serve as a reference. For productivity below 20 mM L−1 h−1 (Table 2, entries 1 and 2), the impact of the catalytic systems on the oxidation of benzaldehyde may be questioned.
According to data from the literature, catalytic systems using NHCs demonstrate particularly interesting activities [38,48,51], which justify their significance (Table 1, entries 5, 7, and 10). However, many other catalytic systems exhibit low productivity, which fails to distinguish them from the natural autoxidation process. These conclusions also extend to other catalytic systems where only two catalytic systems show similar [60] or better productivity [59] than A. Vigalok et al. [55] (Table 2, entries 2, 7, and 8).
In addition to the well-established NHCs catalysis, the aerobic oxidation of aromatic aldehydes into carboxylic acids using NHCs expands the capabilities and applications of organocatalysis. Despite these exciting advances, several challenges still warrant special attention. (1) The structure of the NHC precursor needs justification. In this review, the structure of the NHC precursor has been simplified because, in the literature, structure–performance relationships have never been studied or the structure of the NHC precursor was never justified from previous studies. (2) The amounts of NHCs precursor and base need optimization. In particular, the number of base equivalents should be optimized to minimize the formation of by-products. The nature of the base (whether organic or inorganic) should also be rationalized. (3) The choice of solvent is equally important. Biobased polar solvents, solvent mixtures, and water-miscible solvents should be explored.
I hope that this critical review will attract more attention to NHCs’ catalysis applied to the aerobic oxidation of benzaldehyde and lay the groundwork for the further development of efficient catalysts for this important transformation.
Funding
This research was funded by French National Research Agency (ANR) as part of the IRSIS project “Intensified Reactor Structures for Intrinsically Secure processes” (ANR-16-CE07-0008) and “France Relance Program” (ANR-21-PRRD-0064-01).
Acknowledgments
I would like to thank my colleagues and students from CPE Lyon-Université Claude Bernard Lyon 1 for their encouragement and questions regarding this lengthy study on “simple and fast oxidation” for the characterization of continuous flow reactors, and in particular, L. Vanoye, R. Philippe, F. Bornette, and C. De Bellefon.
Conflicts of Interest
The author declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| acac | Acetylacetonate |
| bpz | 2,2′-bipyrazine |
| DBU | 1,8-Diazabicyclo [5.4.0]undec-7-ene |
| DPQ | 3,3′,5,5′-tetra-tert-butyldiphenoquinone |
| LED | light-emitting diode |
| SIMes | 1,3-Bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidene |
| PIDA | phenyliodine(III) diacetate |
| TBD | Triazabicyclodecene (1,5,7-triazabicyclo [4.4.0]dec-5-ene) |
| TEMPO | (2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl |
References
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An Overview of N-Heterocyclic Carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Flanigan, D.M.; Romanov-Michailidis, F.; White, N.A.; Rovis, T. Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes. Chem. Rev. 2015, 115, 9307–9387. [Google Scholar] [CrossRef] [PubMed]
- Dzieszkowski, K.; Rafiński, Z. N-Heterocyclic Carbene Catalysis Under Oxidizing Conditions. Catalysts 2018, 8, 549. [Google Scholar] [CrossRef]
- Liu, K.; Schwenzer, M.; Studer, A. Radical NHC Catalysis. ACS Catal. 2022, 12, 11984–11999. [Google Scholar] [CrossRef]
- Chakraborty, S.; Barik, S.; Biju, A.T. N-Heterocyclic Carbene (NHC) Organocatalysis: From Fundamentals to Frontiers. Chem. Soc. Rev. 2025, 54, 1102–1124. [Google Scholar] [CrossRef]
- Yoshida, M.; Katagiri, Y.; Zhu, W.B.; Shishido, K. Oxidative Carboxylation of Arylaldehydes with Water by a Sulfoxylalkyl-Substituted N-Heterocyclic Carbene Catalyst. Org. Biomol. Chem. 2009, 7, 4062–4066. [Google Scholar] [CrossRef]
- Chiang, P.-C.; Bode, J.W. On the Role of CO2 in NHC-Catalyzed Oxidation of Aldehydes. Org. Lett. 2011, 13, 2422–2425. [Google Scholar] [CrossRef]
- Ren, X.; Yuan, Y.; Ju, Y.; Wang, H. Oxidation Ability of CO2 for the Transformation of Cinnamic Aldehydes to Acids Catalyzed by N-Heterocyclic Carbene: Combining Computational and Experimental Studies. ChemCatChem 2012, 4, 1943–1951. [Google Scholar] [CrossRef]
- Favre-Réguillon, A. Comment on “CO2 as Oxidant: An Unusual Light-Assisted Catalyst Free Oxidation of Aldehydes to Acids Under Mild Conditions” by S. R. Khan, S. Saini, K. Naresh, A. Kumari, V. Aniya, P.K. Khatri, A. Ray and S. L. Jain, Chem. Commun., 2022, 58, 2208. Chem. Commun. 2023, 59, 14033–14035. [Google Scholar] [CrossRef]
- Knappke, C.E.I.; Imami, A.; Jacobi von Wangelin, A. Oxidative N-Heterocyclic Carbene Catalysis. ChemCatChem 2012, 4, 937–941. [Google Scholar] [CrossRef]
- Menon, R.S.; Biju, A.T.; Nair, V. Recent Advances in N-Heterocyclic Carbene (NHC)-Catalysed Benzoin Reactions. Beilstein J. Org. Chem. 2016, 12, 444–461. [Google Scholar] [CrossRef] [PubMed]
- Breslow, R. On the Mechanism of Thiamine Action. IV. Evidence from Studies on Model Systems. J. Am. Chem. Soc. 1958, 80, 3719–3726. [Google Scholar] [CrossRef]
- Gaggero, N.; Pandini, S. Advances in Chemoselective Intermolecular Cross-Benzoin-Type Condensation Reactions. Org. Biomol. Chem. 2017, 15, 6867–6887. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-Q.; Dai, L.-X.; You, S.-L. Thiazolium-Derived N-Heterocyclic Carbene-Catalyzed Cross-Coupling of Aldehydes with Unactivated Imines. Chem. Commun. 2007, 852–854. [Google Scholar] [CrossRef]
- Stetter, H. Catalyzed Addition of Aldehydes to Activated Double Bonds—A New Synthetic Approach. Angew. Chem. Int. Ed. Engl. 1976, 15, 639–647. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V.; Kafshdarzadeh, K.; Amiri, Z. Recent Advances in Stetter Reaction and Related Chemistry: An Update. Asian J. Org. Chem. 2020, 9, 1999–2034. [Google Scholar] [CrossRef]
- Nair, V.; Vellalath, S.; Babu, B.P. Recent Advances in Carbon–Carbon Bond-Forming Reactions Involving Homoenolates Generated by NHC Catalysis. Chem. Soc. Rev. 2008, 37, 2691–2698. [Google Scholar] [CrossRef]
- Lin, L.; Li, Y.; Du, W.; Deng, W.-P. The NHCs-Mediated Cross-Coupling of Aromatic Aldehydes with Benzyl Halides: Synthesis of α-Aryl Ketones. Tetrahedron Lett. 2010, 51, 3571–3574. [Google Scholar] [CrossRef]
- Padmanaban, M.; Biju, A.T.; Glorius, F. N-Heterocyclic Carbene-Catalyzed Cross-Coupling of Aromatic Aldehydes with Activated Alkyl Halides. Org. Lett. 2011, 13, 98–101. [Google Scholar] [CrossRef]
- Maji, B.; Vedachalan, S.; Ge, X.; Cai, S.; Liu, X.-W. N-Heterocyclic Carbene-Mediated Oxidative Esterification of Aldehydes: Ester Formation and Mechanistic Studies. J. Org. Chem. 2011, 76, 3016–3023. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Z.; Zhao, L.; Bertrand, G.; Yan, X. Mesoionic Carbene-Catalyzed Formyl Alkylation of Aldehydes. Angew. Chem. Int. Ed. 2023, 62, e202303478. [Google Scholar] [CrossRef] [PubMed]
- Stenkvist, S.; Bacaicoa, S.; Goossens, E.; Sunden, H. Aerobic Oxidative N-Heterocyclic Carbene Catalysis. Chem. Rec. 2023, 23, e202300091. [Google Scholar] [CrossRef]
- De Risi, C.; Brandolese, A.; Di Carmine, G.; Ragno, D.; Massi, A.; Bortolini, O. Oxidative N-Heterocyclic Carbene Catalysis. Chem.- Eur. J. 2023, 29, e202202467. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Miyabe, H. NHC-Catalyzed Reaction of Aldehydes for C(Sp2)–O Bond Formation. Catalysts 2024, 14, 219. [Google Scholar] [CrossRef]
- Bortolini, O.; Chiappe, C.; Fogagnolo, M.; Giovannini, P.P.; Massi, A.; Pomelli, C.S.; Ragno, D. An Insight into the Mechanism of the Aerobic Oxidation of Aldehydes Catalyzed by N-Heterocyclic Carbenes. Chem. Commun. 2014, 50, 2008–2011. [Google Scholar] [CrossRef]
- Bortolini, O.; Chiappe, C.; Fogagnolo, M.; Massi, A.; Pomelli, C.S. Formation, Oxidation, and Fate of the Breslow Intermediate in the N-Heterocyclic Carbene-Catalyzed Aerobic Oxidation of Aldehydes. J. Org. Chem. 2017, 82, 302–312. [Google Scholar] [CrossRef]
- Castells, J.; Pujol, F.; Llitjós, H.; Moreno-Mañas, M. Oxidative Benzoin Reactions. Tetrahedron 1982, 38, 337–346. [Google Scholar] [CrossRef]
- Noonan, C.; Baragwanath, L.; Connon, S.J. Nucleophilic Carbene-Catalysed Oxidative Esterification Reactions. Tetrahedron Lett. 2008, 49, 4003–4006. [Google Scholar] [CrossRef]
- De Sarkar, S.; Grimme, S.; Studer, A. NHC Catalyzed Oxidations of Aldehydes to Esters: Chemoselective Acylation of Alcohols in Presence of Amines. J. Am. Chem. Soc. 2010, 132, 1190–1191. [Google Scholar] [CrossRef]
- Samanta, R.C.; Studer, A. N-Heterocyclic Carbene Catalysed Oxidative Esterification of Aliphatic Aldehydes. Org. Chem. Front. 2014, 1, 936–939. [Google Scholar] [CrossRef]
- Harnying, W.; Sudkaow, P.; Biswas, A.; Berkessel, A. N-Heterocyclic Carbene/Carboxylic Acid Co-Catalysis Enables Oxidative Esterification of Demanding Aldehydes/Enals, at Low Catalyst Loading. Angew. Chem. Int. Ed. 2021, 60, 19631–19636. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Zhang, C.-S.; Yi, L.; Gao, Z.-H.; Wang, Z.-X.; Ye, S. Intramolecular α-Oxygenation of Amines via N-Heterocyclic Carbene-Catalyzed Domino Reaction of Aryl Aldehyde: Experiment and DFT Calculation. CCS Chem. 2019, 1, 343–351. [Google Scholar] [CrossRef]
- Guin, J.; De Sarkar, S.; Grimme, S.; Studer, A. Biomimetic Carbene-Catalyzed Oxidations of Aldehydes Using TEMPO. Angew. Chem. Int. Ed. 2008, 47, 8727–8730. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Hazra, A. One-Step Direct Conversion of Heterocyclic Aldehydes to Esters. Chem. Lett. 2009, 38, 484–485. [Google Scholar] [CrossRef]
- Kiran, I.N.C.; Lalwani, K.; Sudalai, A. N-Heterocyclic Carbene Catalyzed Esterification of Aromatic Aldehydes with Alcohols Under Aerobic Conditions. RSC Adv. 2013, 3, 1695–1698. [Google Scholar] [CrossRef]
- Park, J.H.; Bhilare, S.V.; Youn, S.W. NHC-Catalyzed Oxidative Cyclization Reactions of 2-Alkynylbenzaldehydes Under Aerobic Conditions: Synthesis of O-Heterocycles. Org. Lett. 2011, 13, 2228–2231. [Google Scholar] [CrossRef]
- Rose, C.A.; Zeitler, K. Efficient Catalytic, Oxidative Lactonization for the Synthesis of Benzodioxepinones Using Thiazolium-Derived Carbene Catalysts. Org. Lett. 2010, 12, 4552–4555. [Google Scholar] [CrossRef]
- Zhao, J.; Mück-Lichtenfeld, C.; Studer, A. Cooperative N-Heterocyclic Carbene (NHC) and Ruthenium Redox Catalysis: Oxidative Esterification of Aldehydes with Air as the Terminal Oxidant. Adv. Synth. Catal. 2013, 355, 1098–1106. [Google Scholar] [CrossRef]
- Delany, E.G.; Fagan, C.-L.; Gundala, S.; Mari, A.; Broja, T.; Zeitler, K.; Connon, S.J. NHC-Catalysed Aerobic Aldehyde-Esterifications with Alcohols: No Additives or Cocatalysts Required. Chem. Commun. 2013, 49, 6510–6512. [Google Scholar] [CrossRef]
- Xin, Y.-C.; Shi, S.-H.; Xie, D.-D.; Hui, X.-P.; Xu, P.-F. N-Heterocyclic Carbene-Catalyzed Oxidative Esterification Reaction of Aldehydes with Alkyl Halides Under Aerobic Conditions. Eur. J. Org. Chem. 2011, 2011, 6527–6531. [Google Scholar] [CrossRef]
- Arde, P.; Ramanjaneyulu, B.T.; Reddy, V.; Saxena, A.; Anand, R.V. N-Heterocyclic Carbene Catalysed Aerobic Oxidation of Aromatic Aldehydes to Aryl Esters Using Boronic Acids. Org. Biomol. Chem. 2012, 10, 848–851. [Google Scholar] [CrossRef]
- Castells, J.; Llitjos, H.; Moreno-Mañas, M. Nitrobenzene Aldehyde Oxidations Catalyzed by the Conjugate Bases of Thiazolium Ions. Tetrahedron Lett. 1977, 18, 205–206. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, Y. Unexpected CO2 Splitting Reactions To Form CO with N-Heterocyclic Carbenes as Organocatalysts and Aromatic Aldehydes as Oxygen Acceptors. J. Am. Chem. Soc. 2010, 132, 914–915. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Varghese, V.; Paul, R.R.; Jose, A.; Sinu, C.R.; Menon, R.S. NHC Catalyzed Transformation of Aromatic Aldehydes to Acids by Carbon Dioxide: An Unexpected Reaction. Org. Lett. 2010, 12, 2653–2655. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Xiao, T.; Kuznetsov, V.L.; Edwards, P.P. Turning Carbon Dioxide into Fuel. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2010, 368, 3343–3364. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Gou, G.-Z.; Wang, Y.; Fu, W.-F. Abnormal Bis-NHC Mediated Aerial Oxidation of Arylaldehydes: Highly Efficient Transformation of Arylaldehydes to the Corresponding Carboxylic Acids Catalyzed by Organic Catalysts. RSC Adv. 2013, 3, 6334–6338. [Google Scholar] [CrossRef]
- Delany, E.G.; Fagan, C.L.; Gundala, S.; Zeitler, K.; Connon, S.J. Aerobic Oxidation of NHC-Catalysed Aldehyde Esterifications with Alcohols: Benzoin, Not the Breslow Intermediate, Undergoes Oxidation. Chem. Commun. 2013, 49, 6513–6515. [Google Scholar] [CrossRef]
- Möhlmann, L.; Ludwig, S.; Blechert, S. NHC-Catalysed Highly Selective Aerobic Oxidation of Nonactivated Aldehydes. Beilstein J. Org. Chem. 2013, 9, 602–607. [Google Scholar] [CrossRef]
- Khatana, A.K.; Singh, V.; Gupta, M.K.; Tiwari, B. A Highly Efficient NHC-Catalyzed Aerobic Oxidation of Aldehydes to Carboxylic Acids. Synthesis 2018, 50, 4290–4294. [Google Scholar] [CrossRef]
- Gupta, N.K.; Fukuoka, A.; Nakajima, K. Metal-Free and Selective Oxidation of Furfural to Furoic Acid with an N-Heterocyclic Carbene Catalyst. ACS Sustain. Chem. Eng. 2018, 6, 3434–3442. [Google Scholar] [CrossRef]
- Fan, C.; Zhao, X.; Gopireddy, R.; Guo, Y.; Wang, J.; Yuan, J.; Luo, M.; Shi, T.; Yang, L.; He, J.; et al. Unveiling the Hidden Reactivity in the N-Heterocyclic Carbene-Catalyzed Aerobic Oxidation of Aldehydes: Unlocking Its Powerful Catalytic Performance. J. Org. Chem. 2024, 89, 18344–18352. [Google Scholar] [CrossRef]
- Vanoye, L.; Favre-Réguillon, A.; Aloui, A.; Philippe, R.; De Bellefon, C. Insights in the Aerobic Oxidation of Aldehydes. RSC Adv. 2013, 3, 18931. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, T.; Chiu, C.; Lu, T.; Lee, D. Pd(OAc)2 Promoted bis-N-heterocyclic Carbene-catalyzed Oxidative Transformation of Aldehydes. J. Chin. Chem. Soc. 2020, 67, 202–205. [Google Scholar] [CrossRef]
- Reddi, R.N.; Malekar, P.V.; Sudalai, A. N-Heterocyclic Carbene Catalyzed Oxidative Stannylation of Aldehydes: A Facile Entry to Organotin(IV) Carboxylates. Tetrahedron Lett. 2013, 54, 2679–2681. [Google Scholar] [CrossRef]
- Shapiro, N.; Vigalok, A. Highly Efficient Organic Reactions “on Water”, “in Water”, and Both. Angew. Chem. Int. Ed. 2008, 47, 2849–2852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, Y.; Cai, H.; He, S.; Shan, Q.; Zhao, H.; Chen, Y.; Wang, B. Catalyst-Free Aerobic Oxidation of Aldehydes into Acids in Water Under Mild Conditions. Green Chem. 2017, 19, 5708–5713. [Google Scholar] [CrossRef]
- Xu, J.; Yue, X.; He, L.; Shen, J.; Ouyang, Y.; Liang, C.; Li, W. Photoinduced Protocol for Aerobic Oxidation of Aldehydes to Carboxylic Acids Under Mild Conditions. ACS Sustain. Chem. Eng. 2022, 10, 14119–14125. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Liu, C.; Yang, H.; Fu, H. Light and Oxygen-Enabled Sodium Trifluoromethanesulfinate-Mediated Selective Oxidation of C–H Bonds. Green Chem. 2020, 22, 4357–4363. [Google Scholar] [CrossRef]
- Batsika, C.S.; Koutsilieris, C.; Koutoulogenis, G.S.; Kokotou, M.G.; Kokotos, C.G.; Kokotos, G. Light-Promoted Oxidation of Aldehydes to Carboxylic Acids Under Aerobic and Photocatalyst-Free Conditions. Green Chem. 2022, 24, 6224–6231. [Google Scholar] [CrossRef]
- Yu, H.; Ru, S.; Dai, G.; Zhai, Y.; Lin, H.; Han, S.; Wei, Y. An Efficient Iron(III)-Catalyzed Aerobic Oxidation of Aldehydes in Water for the Green Preparation of Carboxylic Acids. Angew. Chem. Int. Ed. 2017, 56, 3867–3871. [Google Scholar] [CrossRef]
- Liu, M.; Li, C.J. Catalytic Fehling’s Reaction: An Efficient Aerobic Oxidation of Aldehyde Catalyzed by Copper in Water. Angew. Chem. Int. Ed. 2016, 55, 10806–10810. [Google Scholar] [CrossRef] [PubMed]
- Robiquet, P.J.; Boutron-Charlard, A. Nouvelles Expériences Sur Les Amandes Amères et Sur l’Huile Volatile Qu’elles Fournissent. Ann. Chim. Phys. 1830, 44, 352–382. [Google Scholar]
- Wisniak, J. Pierre-Jean Robiquet. Educ. Quím. 2013, 24, 139–149. [Google Scholar] [CrossRef]
- Favre-Réguillon, A. Synthesis of Carboxylic Acids from Aldehydes and Ketones. Sci. Synth. Knowl. Updat. 2024, 3, 259–289. [Google Scholar] [CrossRef]
- Vanoye, L.; Favre-Réguillon, A. Selective Aerobic Oxidation of Aliphatic Aldehydes: The Critical Role of Percarboxylate Anion on the Selectivity. React. Chem. Eng. 2023, 8, 1043–1050. [Google Scholar] [CrossRef]
- Muhammad, S.; Sanam, S.; Khan, H.; Muhammad, A.; Sultana, S. Temperature Dependent Solubility of Benzoic Acid in Aqueous Phase and Aqueous Mixtures of Aliphatic Alcohols. Z. Für. Phys. Chem. 2020, 234, 1771–1787. [Google Scholar] [CrossRef]
- Rizzo, P.; Cozzolino, A.; Guerra, G. Chemical Stabilization of Hexanal Molecules by Inclusion as Guests of Nanoporous-Crystalline Syndiotactic Polystyrene Crystals. Macromolecules 2019, 52, 2255–2264. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).