Highly Efficient and Stable Ni-Cs/TS-1 Catalyst for Gas-Phase Propylene Epoxidation with H2 and O2
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Catalysts
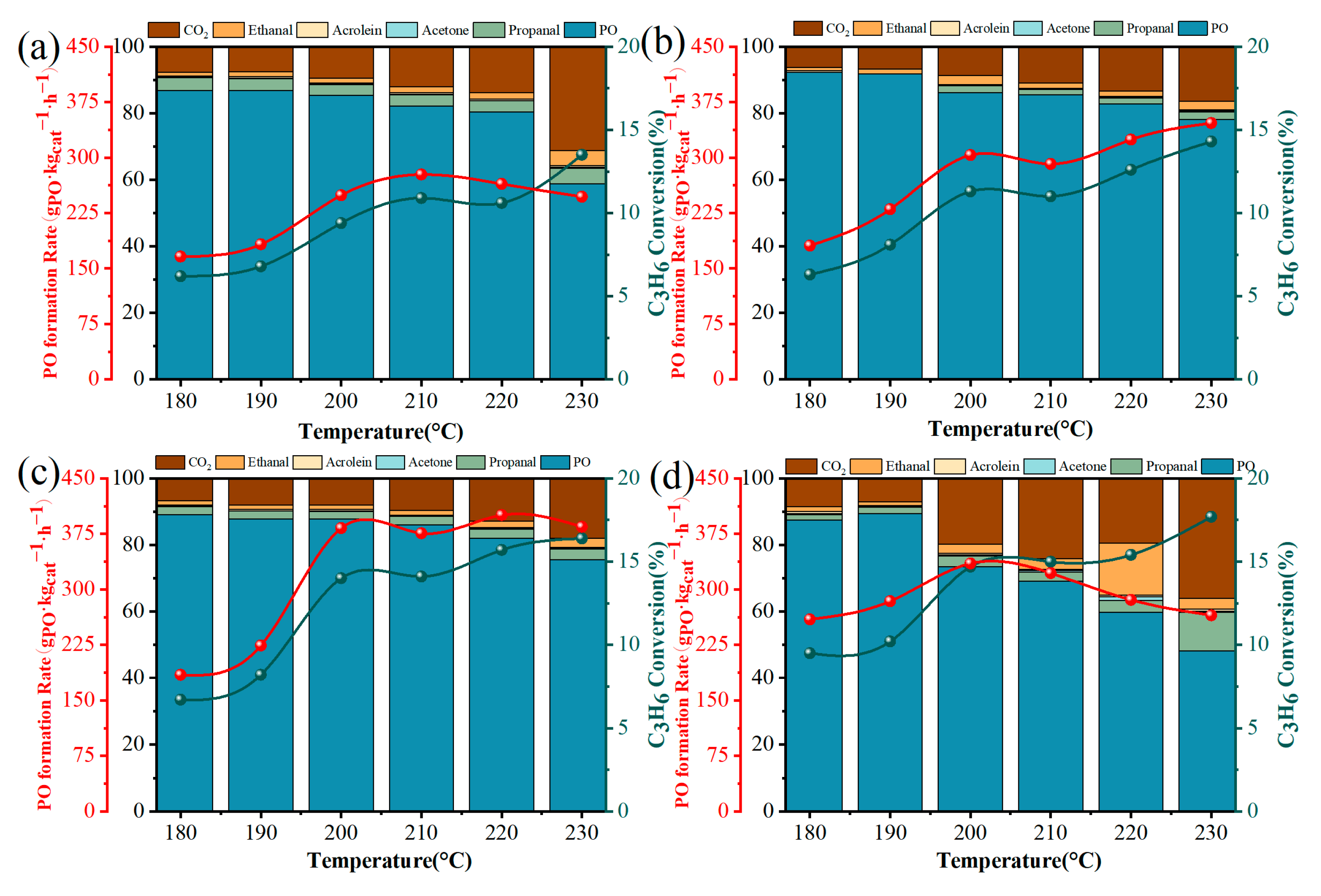
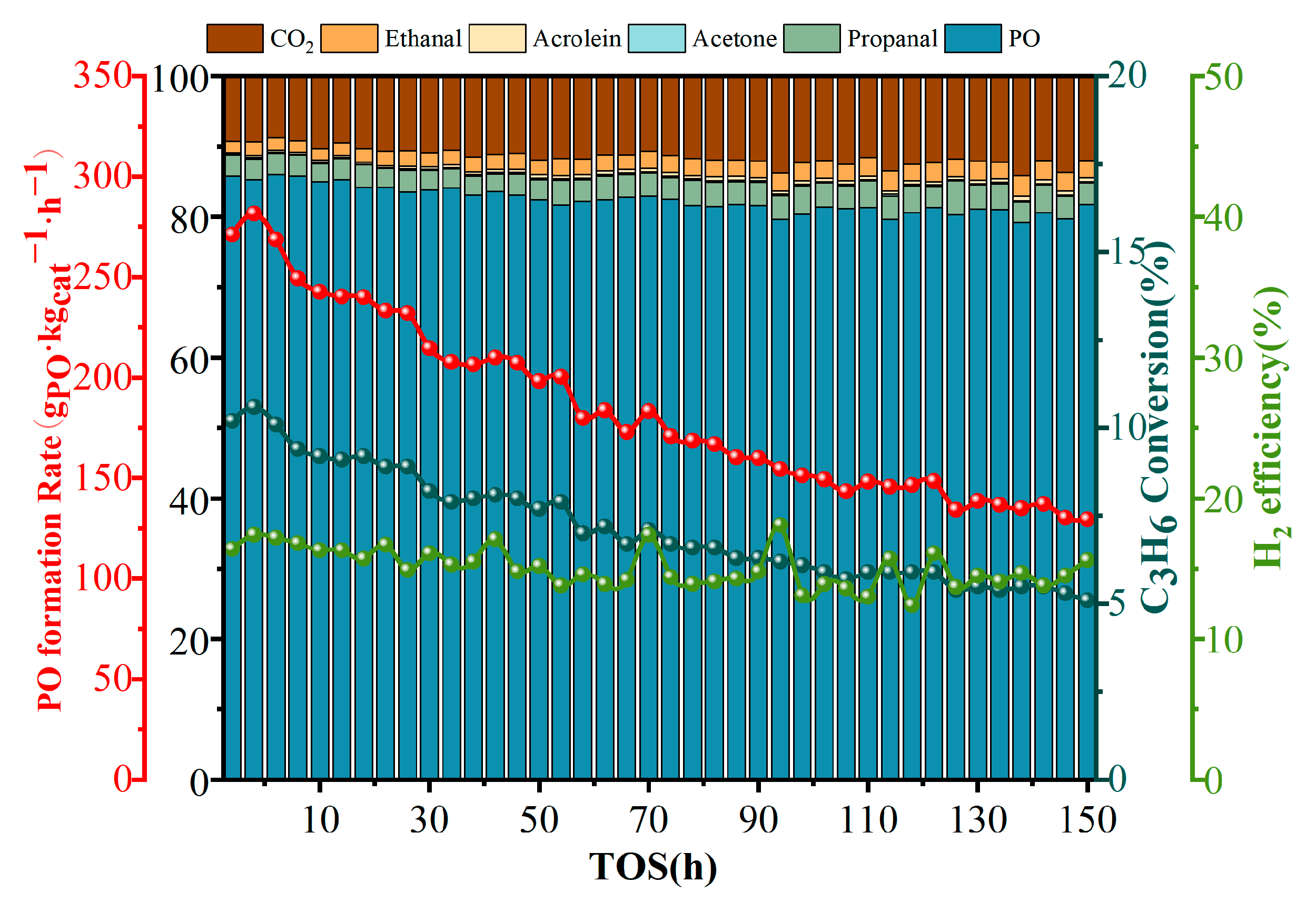
2.2. Catalytic Performance
3. Materials and Methods
3.1. Materials
3.2. Synthesis of TS-1
3.3. Catalyst Preparation
3.4. Catalyst Characterizations
3.5. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.S.; Sharma, V.S.; Kaur, H.; Varma, R.S. Supported Heterogeneous Nanocatalysts in Sustainable, Selective and Eco-Friendly Epoxidation of Olefins. Green Chem. 2020, 22, 5902–5936. [Google Scholar] [CrossRef]
- Feng, X.; Yang, J.; Duan, X.; Cao, Y.; Chen, B.; Chen, W.; Lin, D.; Qan, G.; Chen, D.; Yang, C.; et al. Enhanced Catalytic Performance for Propene Epoxidation with H2 and O2 over Bimetallic Au-Ag/Uncalcined Titanium Silicate-1 Catalysts. ACS Catal. 2018, 8, 7799–7808. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, B.; Zhang, Q.; Deng, W.; Wang, Y.; Yang, Y. Recent Advances in Heterogeneous Selective Oxidation Catalysis for Sustainable Chemistry. Chem. Soc. Rev. 2014, 43, 3480–3524. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, S.; Liu, Y.; Zhou, L.; Wen, H.; Wei, H.; Shen, R.; Wu, X.; Jiang, J.; Li, B. Review and Perspectives on TS-1 Catalyzed Propylene Epoxidation. iScience 2024, 27, 109064. [Google Scholar] [CrossRef] [PubMed]
- Hui, T.; Wang, X. Reconstruction of High-Salt Propylene Epoxide Production Wastewater Treatment. China Water Wastewater 2017, 33, 95–97. [Google Scholar]
- Khatib, S.J.; Oyama, S.T. Direct Oxidation of Propylene to Propylene Oxide with Molecular Oxygen: A Review. Catal. Rev.-Sci. Eng. 2015, 57, 306–344. [Google Scholar] [CrossRef]
- Kuz’micheva, G.M.; Domoroshchina, E.N.; Kravchenko, G.V.; Chernyshev, V.V.; Khramov, E.V.; Chumakov, R.G.; Pavlov, I.S.; Vasil’ev, A.L.; Pirutko, L.V.; Kustov, A.L.; et al. Preparation, Composition, Catalytic Properties of Mfi-Type Zeolite Solid Solutions Hx [Al3+X Ti4+Y Si4+12−X−Y O24] with Al (X < 0.06) and Ti (Y < 0.13) Content. J. Porous Mater. 2025, 32, 1149–1164. [Google Scholar] [CrossRef]
- Kuz’micheva, G.; Chernyshev, V.; Kravchenko, G.; Pirutko, L.; Khramov, E.; Bruk, L.; Pastukhova, Z.; Kustov, A.; Kustov, L.; Markova, E. Impact of Composition and Structural Parameters on the Catalytic Activity of Mfi Type Titanosilicalites. Dalton Trans. 2022, 51, 3439–3451. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, S.; Keshri, K.S.; van Hoof, A.J.F.; d’Angelo, M.F.N.; Schouten, J.C.; Nijhuis, T.A.; Hensen, E.J.M.; Chowdhury, B. Silylation Enhances the Performance of Au/Ti-SiO2 Catalysts in Direct Epoxidation of Propene Using H2 and O2. J. Catal. 2016, 344, 434–444. [Google Scholar] [CrossRef]
- Shi, S.; Du, W.; Zhang, Z.; Duan, X.; Zhou, X. Modulating Ti Coordination Environment in Ti-Containing Materials by Sulfation for Synergizing with Au Sites to Facilitate Propylene Epoxidation. Chem. Eng. J. 2024, 500, 156364. [Google Scholar] [CrossRef]
- Gao, B.; Zhu, Q.; Yi, Y.; Li, Y.; Wu, G.; Guo, Y.; Feng, Z.; Guo, H. Catalytic Performance of Amorphous Ti/SiO2 in the Gas-Phase Epoxidation of Propylene with H2O2. Eur. J. Inorg. Chem. 2023, 26, e202200661. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, X.; Bravo-Suarez, J.J.; Bando, K.K.; Fujitani, T.; Oyama, S.T. Direct Propylene Epoxidation over/Barium-Promoted Au/Ti-Tud Catalysts with H2 and O2: Effect of Au Particle Size. J. Catal. 2007, 250, 350–359. [Google Scholar] [CrossRef]
- Sheng, N.; Song, Z.; Yuan, J.; Liang, W.; Zhao, J.; Zhang, J.; Xu, W.; Sun, B.; Feng, X.; Yang, Z.; et al. Effective Regulation of the Au Spatial Position in a Hierarchically Structured Au/HTS-1 Catalyst: To Boost the Catalytic Performance of Propene Epoxidation with H2 and O2. ACS Sustain. Chem. Eng. 2022, 10, 9515–9524. [Google Scholar] [CrossRef]
- Kalvachev, Y.A.; Hayashi, T.; Tsubota, S.; Haruta, M. Selective Partial Oxidation of Propylene to Propylene Oxide on Au/Ti-Mcm Catalysts in the Presence of Hydrogen and Oxygen. In 3rd World Congress on Oxidation Catalysis; Grasselli, R.K., Oyama, S.T., Gaffney, A.M., Lyons, J.E., Eds.; Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1997; Volume 110, pp. 965–972. [Google Scholar]
- Tang, K.; Hou, W.; Wang, X.; Xu, W.; Lu, X.; Ma, R.; Fu, Y.; Zhu, W. Enhanced Catalytic Performance of Trimethylsilylated Ti-Mww Zeolites for the Liquid-Phase Epoxidation of Propylene with H2O2. Microporous Mesoporous Mater. 2021, 328, 111492. [Google Scholar] [CrossRef]
- Yin, J.; Jin, X.; Xu, H.; Guan, Y.; Peng, R.; Chen, L.; Jiang, J.; Wu, P. Structured Binder-Free Mww-Type Titanosilicate with Si-Rich Shell for Selective and Durable Propylene Epoxidation. Chin. J. Catal. 2021, 42, 1561–1575. [Google Scholar] [CrossRef]
- Jin, F.; Wu, Y.; Liu, S.; Lin, T.-H.; Lee, J.-F.; Cheng, S. Effect of Ti Incorporated Mww Supports on Au Loading and Catalytic Performance for Direct Propylene Epoxidation. Catal. Today 2016, 264, 98–108. [Google Scholar] [CrossRef]
- Li, X.; Gong, X.; Wang, J.; Jin, S.; Xu, H.; Wu, P. Post-Treatment of Ti-Mww Zeolite with Potassium Fluoride for Propylene Epoxidation. Front. Chem. Sci. Eng. 2024, 18, 88. [Google Scholar] [CrossRef]
- Young, J.W.; Soo, H.S.; Lim, K.T.; Lee, G.D.; Park, S.S. Photocatalytic Decomposition of Methylene Blue over Sm Ion Doped Ti-Sba-15 Catalysts. J. Environ. Sci. 2011, 20, 511–517. [Google Scholar][Green Version]
- Zhang, Z.; Zhao, X.; Wang, G.; Xu, J.; Lu, M.; Tang, Y.; Fu, W.; Duan, X.; Qian, G.; Chen, D.; et al. Uncalcined Ts-2 Immobilized Au Nanoparticles as a Bifunctional Catalyst to Boost Direct Propylene Epoxidation with H2 and O2. AICHE J. 2020, 66, e16815. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, C.; Sun, B.; Zhu, H.; Xu, W. Preparation and Modification of Au/TS-1 Catalyst in the Direct Epoxidation of Propylene with H2 and O2. Appl. Catal. A-Gen. 2021, 624, 118329. [Google Scholar] [CrossRef]
- Hao, D.; Li, X.; He, G.; Bai, R.; Bai, Y.; Zhang, T.; Bai, L.; Li, J.; Zhang, Q.; Mei, D.; et al. Ethanol-Assisted Synthesis of Titanium-Rich TS-1 Zeolite: A New Hexa-Coordinated Ti Site for Efficient Propylene Epoxidation. Chem. Sci. 2025, 16, 4661–4667. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, W.; Yuan, Z.; Xu, Y.; Sun, D.; Li, Q. Tannic Acid Assisted Construction of Au-Ti Integrated Catalyst Regulating the Local Environment of Framework Ti toward Propylene Epoxidation under H2/O2. Mol. Catal. 2023, 545, 113206. [Google Scholar] [CrossRef]
- Sheng, N.; Lin, D.; Liang, W.; Zhao, C.; Liu, Y.; Zhu, Y.; Song, Z.; Jiang, J.; Xu, W.; Yang, Z.; et al. Intracrystalline Diffusion Regulation of Au/Hierarchical TS-1 for Simultaneously Enhanced Stability and Selectivity in Propene Epoxidation with H2 and O2. Chem. Eng. Sci. 2024, 300, 120538. [Google Scholar] [CrossRef]
- Lin, M.; Su, Z.; Ma, W. Effect on the Introduction of Hollow Structure on Au/TS-1 to Improve the Stability for Propylene Epoxidation. Chem. Phys. 2024, 581, 112259. [Google Scholar] [CrossRef]
- Ping, C.; Lin, M.; Guo, Q.; Ma, W. The Competition between Propylene Epoxidation and Hydrogen Oxidation and the Effect of Water on Au/TS-1/Mox Catalysts. Microporous Mesoporous Mater. 2024, 368, 113034. [Google Scholar] [CrossRef]
- Zheng, J.; Feng, Y.; Yang, T.; Liu, C.; Li, C.; Sunarso, J.; Yang, L.; Wang, X. Uncalcined TS-1 Supported Au Catalyst Nabh4 Reduction Method for Propylene Epoxidation: Insights into the H2 Pretreatment Effect on Catalytic Performance. Appl. Catal. A-Gen. 2024, 670, 119555. [Google Scholar] [CrossRef]
- Wang, Q.; Sang, K.; Liu, C.; Zhang, Z.; Chen, W.; Ji, T.; Li, L.; Lian, C.; Qian, G.; Zhang, J.; et al. Nanoparticles as an Antidote for Poisoned Gold Single-Atom Catalysts in Sustainable Propylene Epoxidation. Nat. Commun. 2024, 15, 3249. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhang, Z.; Wang, J.; Song, N.; Duan, X.; Zhou, X. Kinetic Insights into Reaction Pathways of Acrolein Formation in Propylene Epoxidation by H2 and O2 over Au/TS-1 Catalyst. Chem. Eng. J. 2024, 487, 150512. [Google Scholar] [CrossRef]
- Sun, S.; Liu, S.; Zhou, W.; Wang, Z.; Li, Q.; Sun, D. Defect Engineering of Black TS-1 for Anchoring Electron-Rich Au with Enhanced H2 Efficiency in Propene Epoxidation. Chem. Eng. J. 2025, 504, 158931. [Google Scholar] [CrossRef]
- Hamada, Y.; Yonemori, T.; Ishimaru, Y.; Kawakami, T.; Yamanaka, S.; Okumura, M. Theoretical Investigation of Propylene Epoxidation Using H2 and O2 over Titanosilicate-Supported Au Catalysts. Catal. Lett. 2024, 154, 5948–5954. [Google Scholar] [CrossRef]
- Lin, D.; Xu, Y.; Zheng, X.; Sheng, W.; Liu, Z.; Yan, Y.; Cao, Y.; Liu, Y.; Feng, X.; Chen, D.; et al. Engineering Sodium-Decorated Bifunctional Au-Ti Sites to Boost Molecular Transfer for Propene Epoxidation with H2 and O2. AICHE J. 2023, 69, 17999. [Google Scholar] [CrossRef]
- Lv, Z.; Ding, Y.; Kang, L.; Li, L.; Liu, X.Y.; Wang, A.; Zhang, T. Light-Enhanced Direct Epoxidation of Propylene by Molecular Oxygen over CuOx/TiO2 Catalyst. Acta Phys.-Chim. Sin. 2025, 41, 100038. [Google Scholar] [CrossRef]
- Sangolkar, A.A.; Pawar, R. Enhanced Selectivity of the Propylene Epoxidation Reaction on a Cu Monolayer Surface Via Eley-Rideal Mechanism. ChemPhysChem 2022, 23, e202200334. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Gu, X.-K.; Zhang, Z.; Chai, P.; Zang, Y.; Yu, Z.; Li, D.; Zhang, H.; Liu, Z.; Huang, W. Fine Cubic Cu2O Nanocrystals as Highly Selective Catalyst for Propylene Epoxidation with Molecular Oxygen. Nat. Commun. 2021, 12, 5921, Erratum in Nat. Commun. 2021, 12, 6429. [Google Scholar] [CrossRef] [PubMed]
- Horvath, B.; Sotak, T.; Hronec, M. Gas-Phase Epoxidation of Propylene over Nanostructured Iron-Containing Catalysts. Appl. Catal. A-Gen. 2011, 405, 18–24. [Google Scholar] [CrossRef]
- Garcia-Aguilar, J.; Miguel-Garcia, I.; Juan-Juan, J.; Such-Basanez, I.; San Fabian, E.; Cazorla-Amoros, D.; Berenguer-Murcia, A. One Step-Synthesis of Highly Dispersed Iron Species into Silica for Propylene Epoxidation with Dioxygen. J. Catal. 2016, 338, 154–167. [Google Scholar] [CrossRef]
- Li, W.; Wu, G.; Hu, W.; Dang, J.; Wang, C.; Weng, X.; da Silva, I.; Manuel, P.; Yang, S.; Guan, N.; et al. Direct Propylene Epoxidation with Molecular Oxygen over Cobalt-Containing Zeolites. J. Am. Chem. Soc. 2022, 144, 4260–4268. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Q.; Luo, M.F.; Li, C. Direct Epoxidation of Propylene on Nacl-Modified Cu/SiO2 Catalysts Using Air as Oxidant. Chin. J. Catal. 2004, 25, 5–9. [Google Scholar]
- He, J.; Zhai, Q.; Zhang, Q.; Deng, W.; Wang, Y. Active Site and Reaction Mechanism for the Epoxidation of Propylene by Oxygen over CuOx/SiO2 Catalysts with and without Cs+ Modification. J. Catal. 2013, 299, 53–66. [Google Scholar] [CrossRef]
- Ananieva, E.; Reitzmann, A. Direct Gas-Phase Epoxidation of Propene with Nitrous Oxide over Modified Silica Supported FeOx Catalysts. Chem. Eng. Sci. 2004, 59, 5509–5517. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, W.; Yang, L.; Wang, X.; Zhang, Q. Iron-Containing Heterogeneous Catalysts for Partial Oxidation of Methane and Epoxidation of Propylene. Catal. Today 2006, 117, 156–162. [Google Scholar] [CrossRef]
- Li, W.; Chen, L.; Qiu, M.; Li, W.; Zhang, Y.; Zhu, Y.; Li, J.; Chen, X. Highly Efficient Epoxidation of Propylene with in Situ-Generated H2O2 over a Hierarchical TS-1 Zeolite-Supported Non-Noble Nickel Catalyst. ACS Catal. 2023, 13, 10487–10499. [Google Scholar] [CrossRef]
- Garcia-Aguilar, J.; Fernandez-Catala, J.; Juan-Juan, J.; Such-Basanez, I.; Chinchilla, L.E.; Calvino-Gamez, J.J.; Cazorla-Amoros, D.; Berenguer-Murcia, A. Novelty without Nobility: Outstanding Ni/Ti-SiO2 Catalysts for Propylene Epoxidation. J. Catal. 2020, 386, 94–105. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Cao, X.; Chen, L.; Qin, Y.; Zhu, Y.; Zhang, Y.; Miao, G.; Kong, L.; Li, J.; et al. Efficient Epoxidation of Propylene over Non-Noble Nickel-Based Catalyst Promoted by Alkali Metals. Sci. China-Chem. 2024, 67, 3697–3705. [Google Scholar] [CrossRef]
- Khan, I.; Chu, X.; Liu, Y.; Khan, S.; Bai, L.; Jing, L. Synthesis of Ni2+ Cation Modified TS-1 Molecular Sieve Nanosheets as Effective Photocatalysts for Alcohol Oxidation and Pollutant Degradation. Chin. J. Catal. 2020, 41, 1589–1602. [Google Scholar] [CrossRef]
- Wu, M.; Chou, L.; Song, H. Epoxidation of Butadiene over Nickel Modified TS-1 Catalyst. Catal. Lett. 2012, 142, 627–636. [Google Scholar] [CrossRef]
- Chowdhury, B.; Bando, K.K.; Bravo-Suarez, J.J.; Tsubota, S.; Haruta, M. Activity of Silylated Titanosilicate Supported Gold Nanoparticles Towards Direct Propylene Epoxidation Reaction in the Presence of Trimethylamine. J. Mol. Catal. A-Chem. 2012, 359, 21–27. [Google Scholar] [CrossRef]
- Ren, Y.-g.; Huang, J.; Lv, Q.; Xie, Y.; Lu, A.-H.; Haruta, M. Dual-Component Gas Pretreatment for Au/TS-1: Enhanced Propylene Epoxidation with Oxygen and Hydrogen. Appl. Catal. A-Gen. 2019, 584, 117172. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Z.; Wang, G.; Duan, X.; Qian, G.; Zhou, X. Zeolite Crystal Size Effects of Au/Uncalcined TS-1 Bifunctional Catalysts on Direct Propylene Epoxidation with H2 and O2. Chem. Eng. Sci. 2020, 227, 115907. [Google Scholar] [CrossRef]
- Song, Z.; Feng, X.; Sheng, N.; Lin, D.; Li, Y.; Liu, Y.; Chen, X.; Chen, D.; Zhou, X.; Yang, C. Cost-Efficient Core-Shell TS-1/Silicalite-1 Supported Au Catalysts: Towards Enhanced Stability for Propene Epoxidation with H2 and O2. Chem. Eng. J. 2019, 377, 119927. [Google Scholar] [CrossRef]
- Feng, X.; Sheng, N.; Liu, Y.; Chen, X.; Chen, D.; Yang, C.; Zhou, X. Simultaneously Enhanced Stability and Selectivity for Propene Epoxidation with H2 and O2 on Au Catalysts Supported on Nano-Crystalline Mesoporous TS-1. ACS Catal. 2017, 7, 2668–2675. [Google Scholar] [CrossRef]
- Song, W.; Xiong, G.; Long, H.; Jin, F.; Liu, L.; Wang, X. Effect of Treatment with Different Bases on the Catalytic Properties of TS-1/SiO2 Extrudates in Propylene Epoxidation. Microporous Mesoporous Mater. 2015, 212, 48–55. [Google Scholar] [CrossRef]
- Sheng, N.; Liu, Z.; Song, Z.; Lin, D.; Feng, X.; Liu, Y.; Chen, X.; Chen, D.; Zhou, X.; Yang, C. Enhanced Stability for Propene Epoxidation with H2 and O2 over Wormhole-Like Hierarchical TS-1 Supported Au Nanocatalyst. Chem. Eng. J. 2019, 377, 119954. [Google Scholar] [CrossRef]
- Kapoor, M.P.; Sinha, A.K.; Seelan, S.; Inagaki, S.; Tsubota, S.; Yoshida, H.; Haruta, M. Hydrophobicity Induced Vapor-Phase Oxidation of Propene over Gold Supported on Titanium Incorporated Hybrid Mesoporous Silsesquioxane. Chem. Commun. 2002, 2902–2903. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.W.; Hirsekorn, K.F.; Neidig, M.L.; Yang, X.; Tilley, T.D. Mechanism of the Decomposition of Aqueous Hydrogen Peroxide over Heterogeneous Tisba15 and TS-1 Selective Oxidation Catalysts: Insights from Spectroscopic and Density Functional Theory Studies. ACS Catal. 2011, 1, 1665–1678. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, X.; Huang, J.; Zhang, L.; Zhang, B.; Haruta, M.; Lu, A.-H. Dual-Component Sodium and Cesium Promoters for Au/TS-1: Enhancement of Propene Epoxidation with Hydrogen and Oxygen. Ind. Eng. Chem. Res. 2020, 59, 8155–8163. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, Q.; Qin, Z.; Li, Q.; Feng, X.; Song, Z.; Cai, Z.; Liu, Y.; Chen, X.; Chen, D.; et al. Reversing Titanium Oligomer Formation Towards High-Efficiency and Green Synthesis of Titanium-Containing Molecular Sieves. Angew. Chem. Int. Ed. Engl. 2021, 60, 3443–3448. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Chen, S.-L.; Jia, A.-P.; Lu, J.-Q.; Huang, W.-X. Gas Phase Propylene Epoxidation over Au Supported on Titanosilicates with Different Ti Chemical Environments. Appl. Surf. Sci. 2017, 393, 11–22. [Google Scholar] [CrossRef]
- Khomane, R.B.; Kulkarni, B.D.; Paraskar, A.; Sainkar, S.R. Synthesis, Characterization and Catalytic Performance of Titanium Silicalite-1 Prepared in Micellar Media. Mater. Chem. Phys. 2002, 76, 99–103. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Zhang, J.; Wang, D.; Ma, W. Better Performance for Gas-Phase Epoxidation of Propylene Using H2 and O2 at Lower Temperature over Au/TS-1 Catalyst. Catal. Commun. 2017, 90, 87–90. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, S.; Tang, Y.; Xu, J.; Du, W.; Wang, Q.; Yu, D.; Liao, Y.; Song, N.; Duan, X.; et al. Using Ammonia Solution to Fabricate Highly Active Au/Uncalcined Ts 1 Catalyst for Gas-Phase Epoxidation of Propylene. J. Catal. 2022, 416, 410–422. [Google Scholar] [CrossRef]
- Hou, L.; Yan, S.; Liu, L.; Liu, J.; Xiong, G. Fabrication of Gold Nanoparticles Supported on Hollow Microsphere of Nanosized TS-1 for the Epoxidation of Propylene with H2 and O2. Chem. Eng. J. 2024, 481, 148676. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, Y.; Du, W.; Xu, J.; Wang, Q.; Song, N.; Qian, G.; Duan, X.; Zhou, X. Engineering Gold Impregnated Uncalcined TS-1 to Boost Catalytic Formation of Propylene Oxide. Appl. Catal. B-Environ. Energy 2022, 319, 121837. [Google Scholar] [CrossRef]
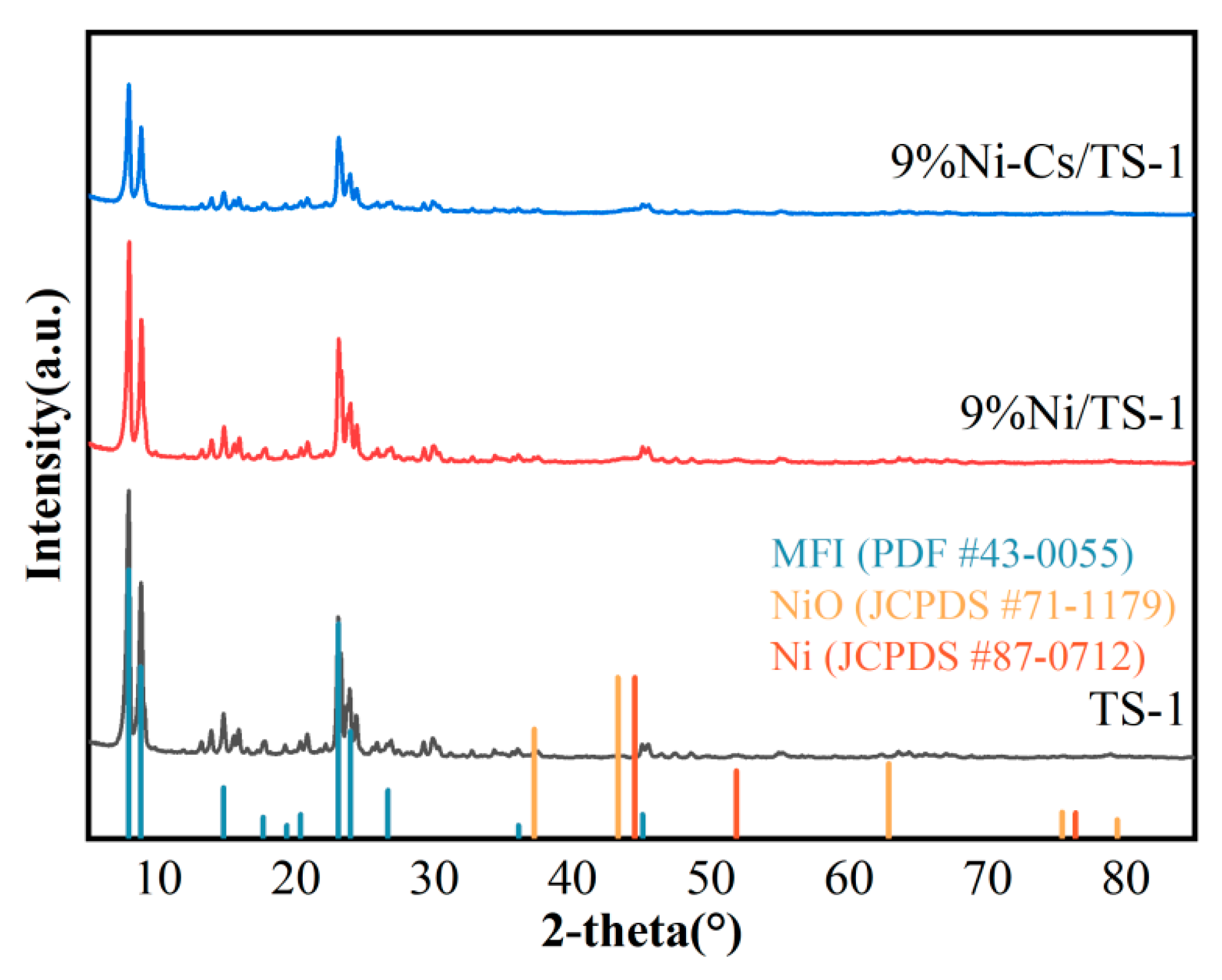
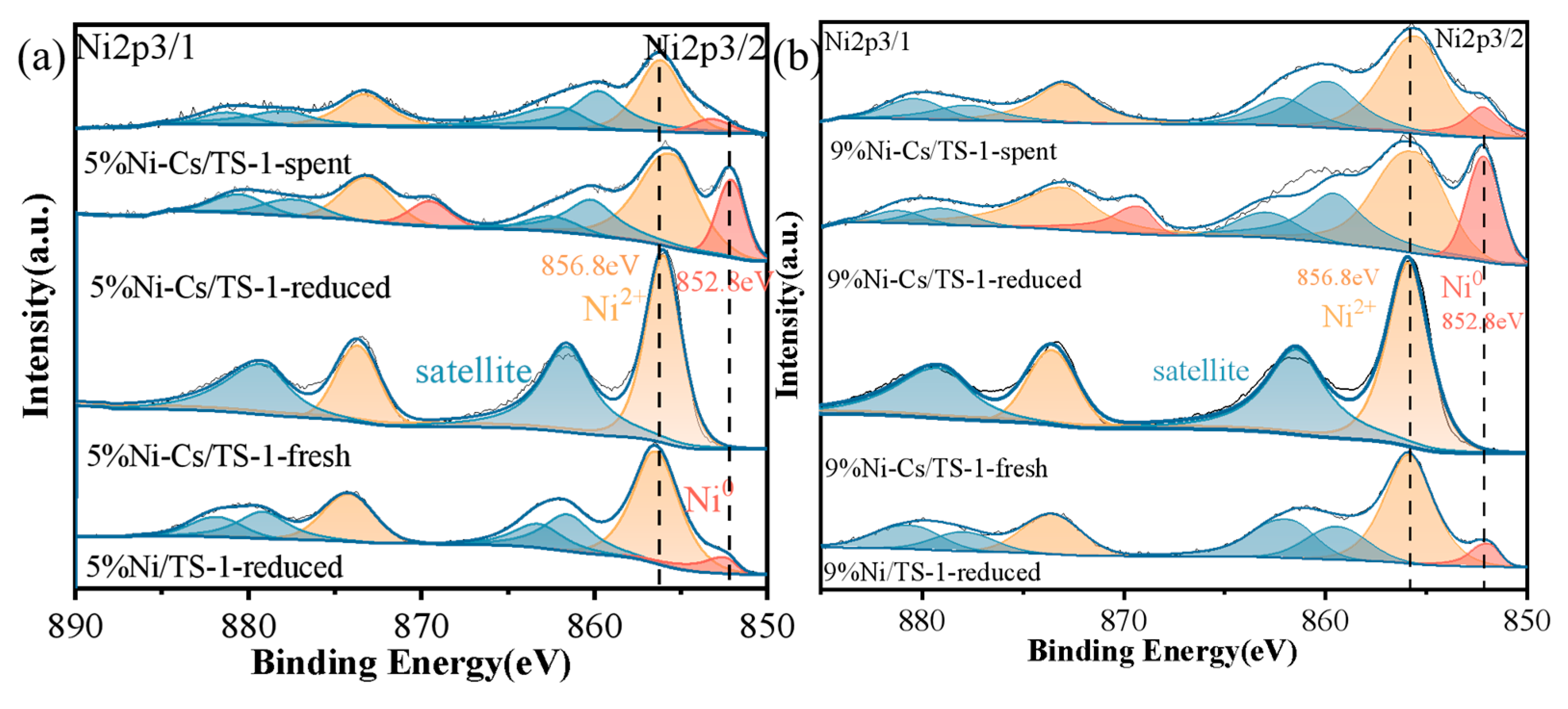
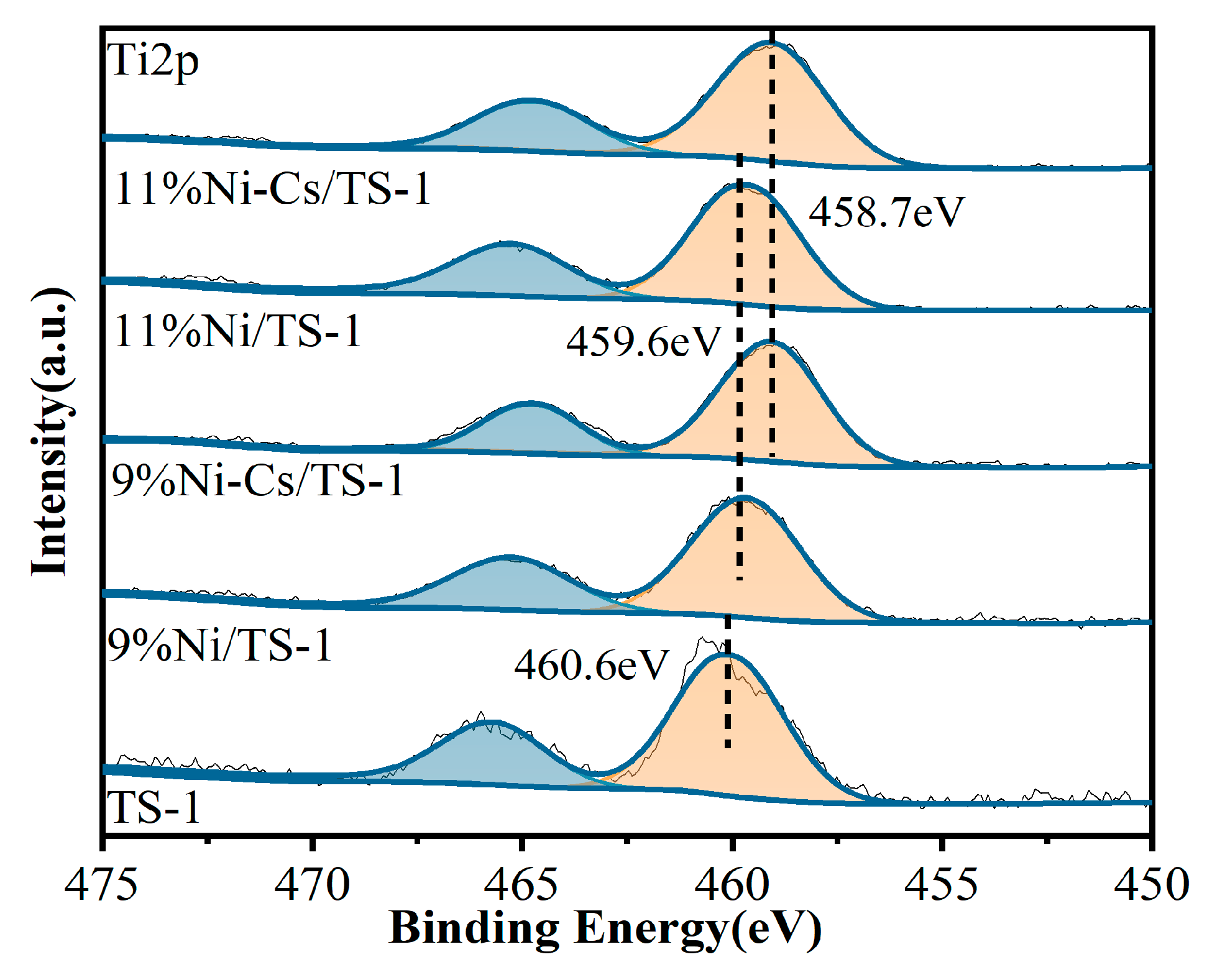



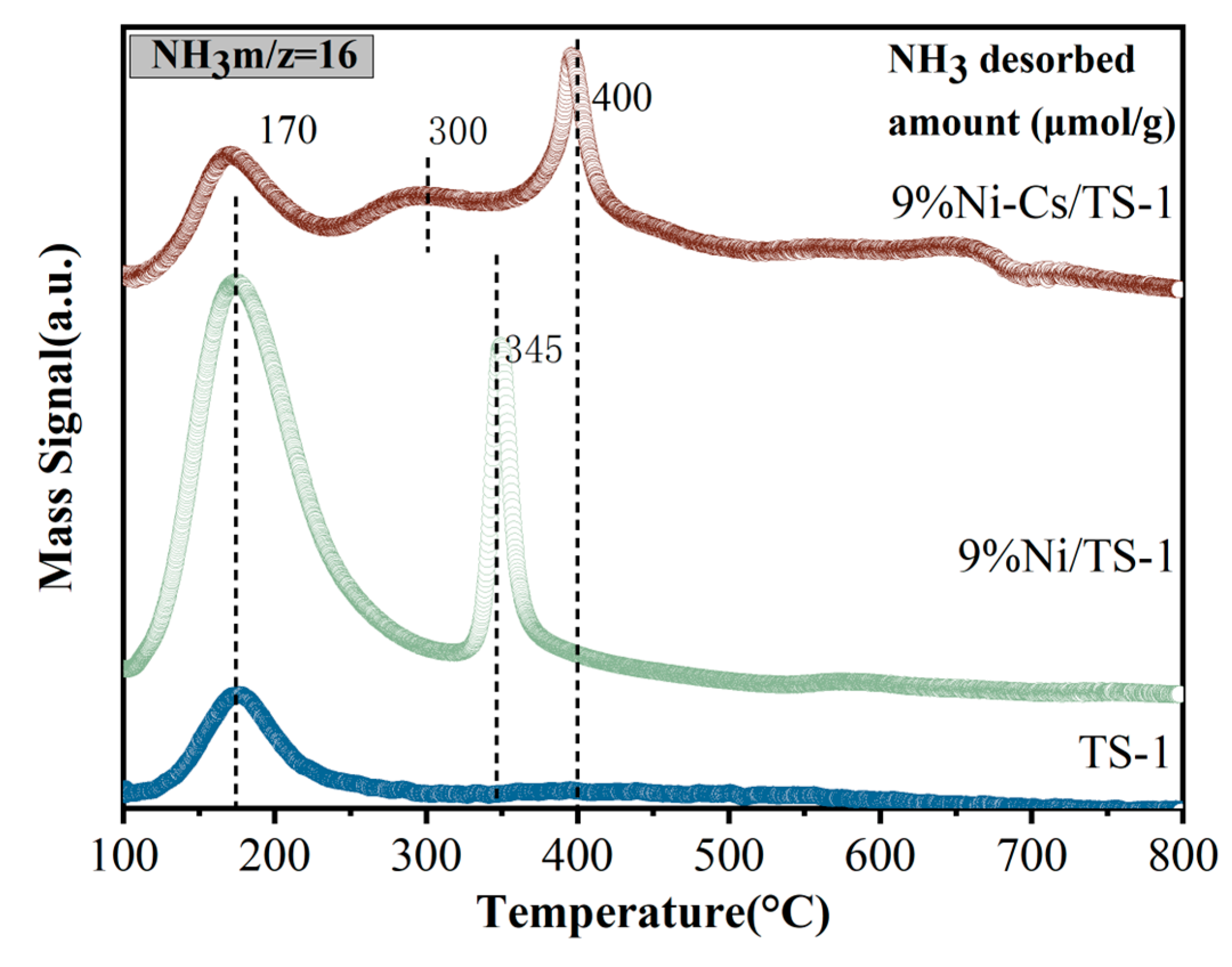
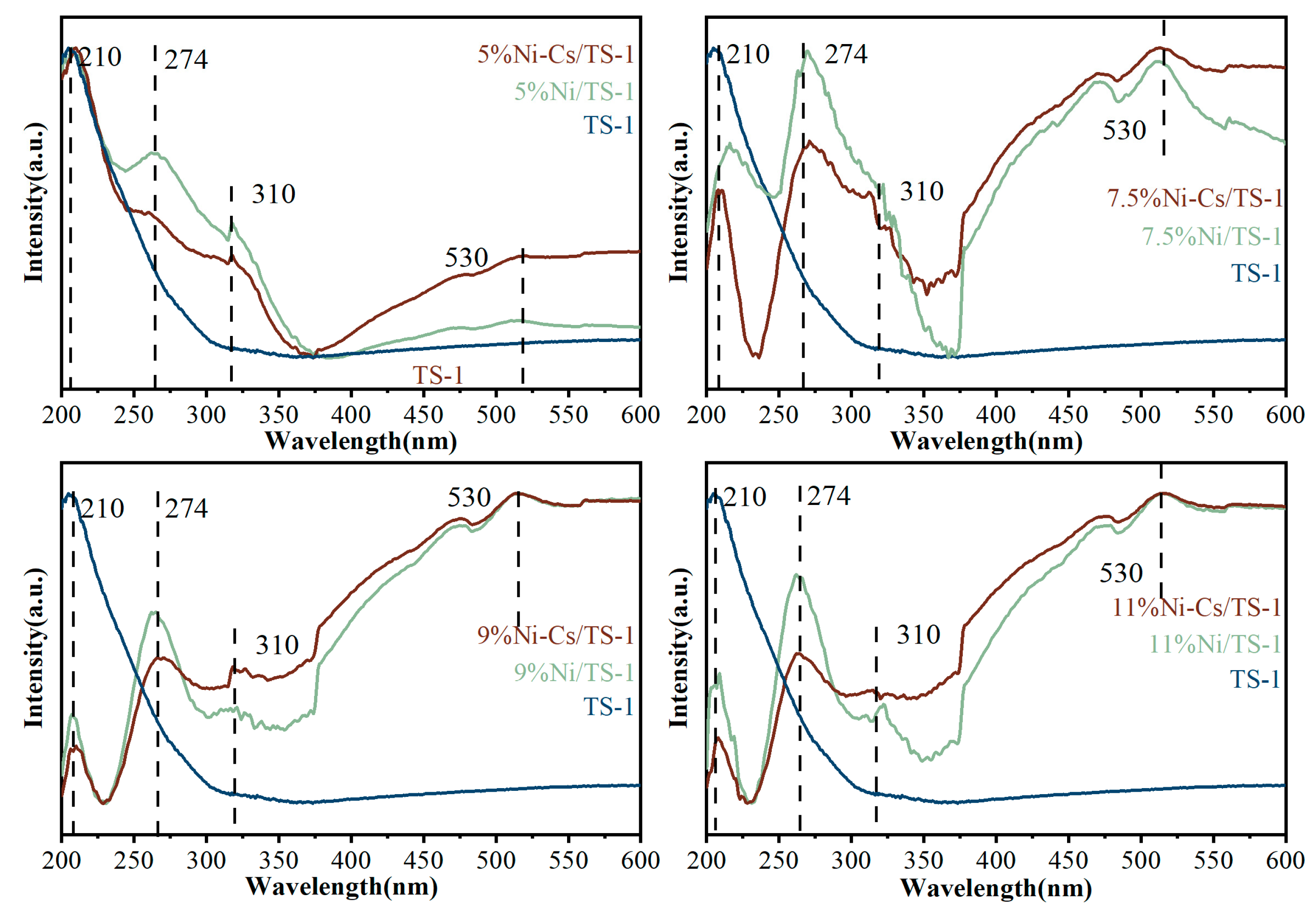
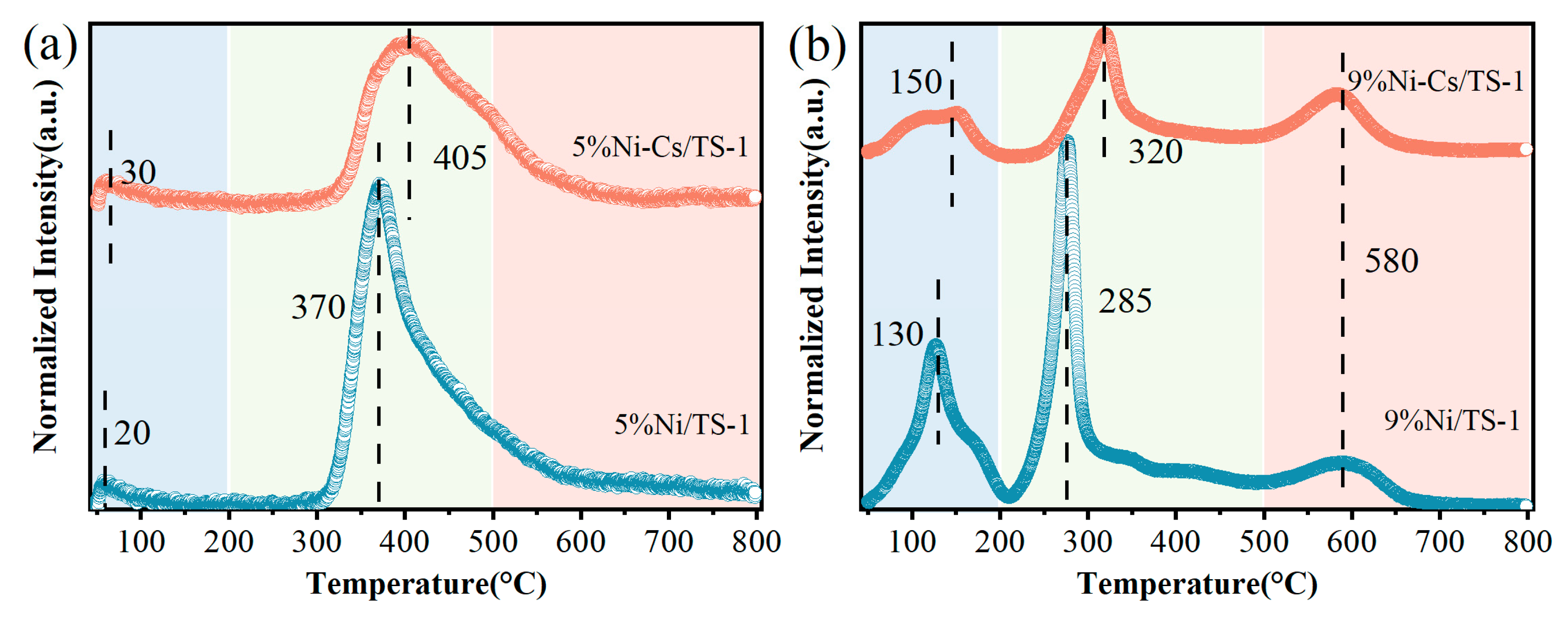

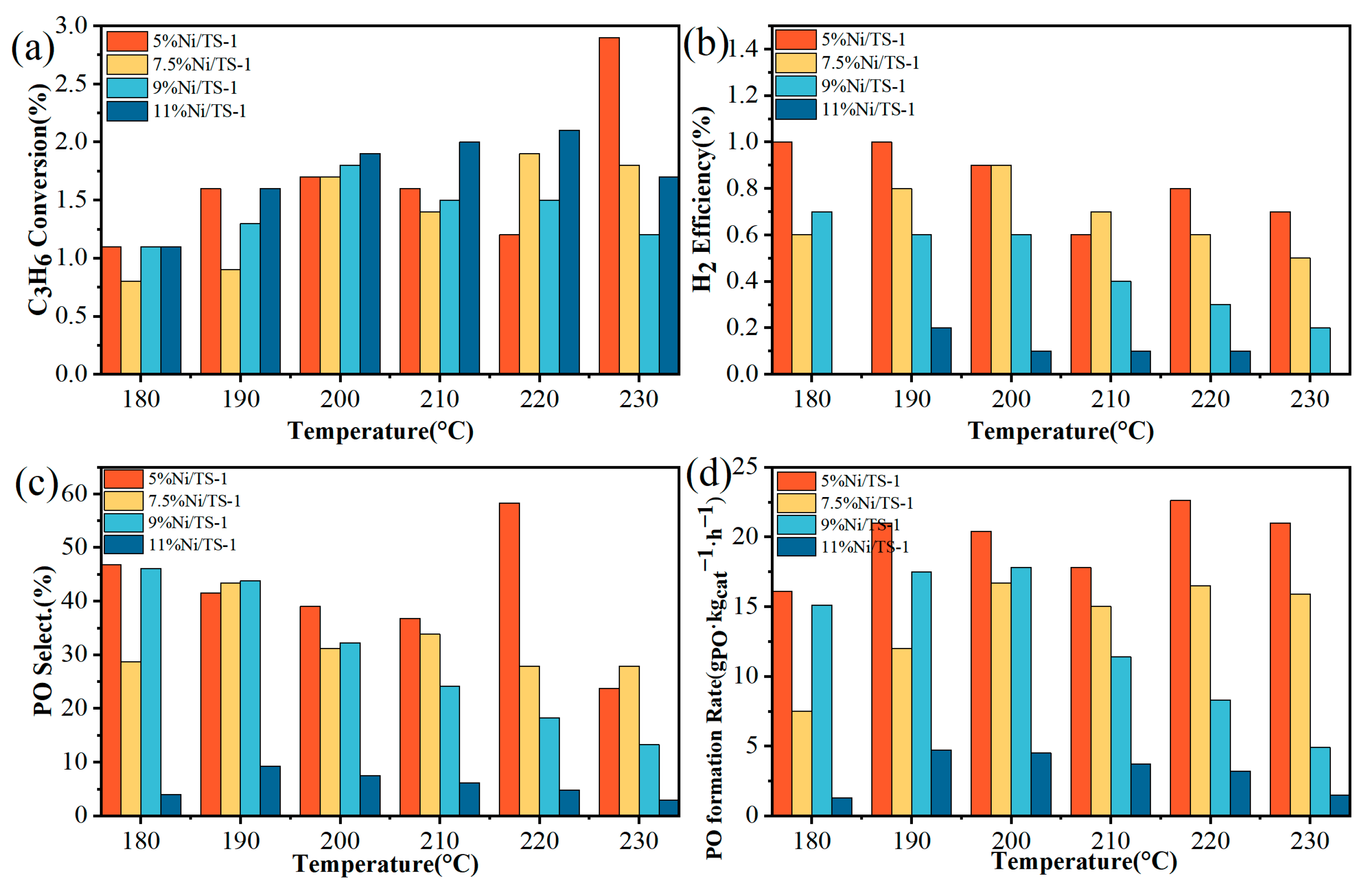
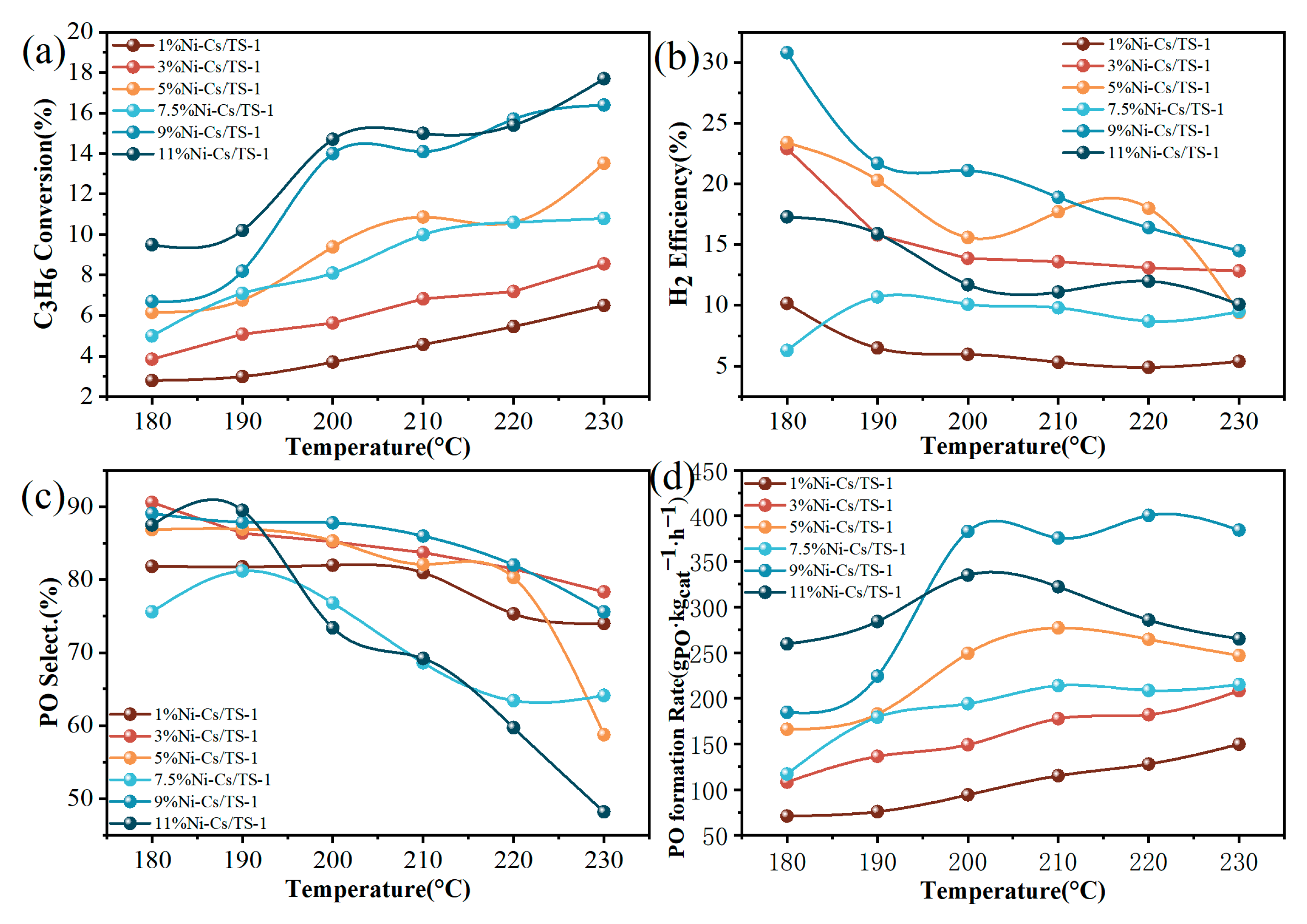
| Samples | SBET a (m2/g) | VTotal b (cm3/g) | Vmicro b (cm3/g) | Pore Size c (nm) | Ni Loading d (wt.%) | Ti Loading d (wt.%) |
|---|---|---|---|---|---|---|
| TS-1 | 463 | 0.48 | 0.07 | 4.0 | - | 1.08 |
| 1%Ni/TS-1 | 424 | 0.64 | 0.12 | 3.0 | 0.56 | 1.09 |
| 1%Ni-Cs/TS-1 | 364 | 0.60 | 0.11 | 3.0 | 0.66 | 1.05 |
| 3%Ni/TS-1 | 419 | 0.64 | 0.13 | 3.3 | 2.04 | 1.20 |
| 3%Ni-Cs/TS-1 | 352 | 0.58 | 0.11 | 3.1 | 1.70 | 1.14 |
| 5%Ni/TS-1 | 386 | 0.50 | 0.13 | 3.1 | 3.12 | 1.17 |
| 5%Ni-Cs/TS-1 | 367 | 0.47 | 0.08 | 4.2 | 3.43 | 1.27 |
| 7.5%Ni/TS-1 | 435 | 0.51 | 0.05 | 4.0 | 5.50 | 1.14 |
| 7.5%Ni-Cs/TS-1 | 311 | 0.40 | 0.04 | 4.2 | 4.16 | 1.09 |
| 9%Ni/TS-1 | 418 | 0.63 | 0.10 | 3.1 | 5.91 | 1.23 |
| 9%Ni-Cs/TS-1 | 305 | 0.51 | 0.08 | 3.9 | 6.68 | 1.26 |
| 11%Ni/TS-1 | 408 | 0.42 | 0.10 | 4.2 | 7.46 | 1.15 |
| 11%Ni-Cs/TS-1 | 363 | 0.51 | 0.03 | 3.0 | 8.81 | 1.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, Z.; Long, H.; Jia, Y.; Ma, Y.; Miao, C.; Xie, Y.; Zhu, X.; Huang, J. Highly Efficient and Stable Ni-Cs/TS-1 Catalyst for Gas-Phase Propylene Epoxidation with H2 and O2. Catalysts 2025, 15, 694. https://doi.org/10.3390/catal15070694
Mi Z, Long H, Jia Y, Ma Y, Miao C, Xie Y, Zhu X, Huang J. Highly Efficient and Stable Ni-Cs/TS-1 Catalyst for Gas-Phase Propylene Epoxidation with H2 and O2. Catalysts. 2025; 15(7):694. https://doi.org/10.3390/catal15070694
Chicago/Turabian StyleMi, Ziyan, Huayun Long, Yuhua Jia, Yue Ma, Cuilan Miao, Yan Xie, Xiaomei Zhu, and Jiahui Huang. 2025. "Highly Efficient and Stable Ni-Cs/TS-1 Catalyst for Gas-Phase Propylene Epoxidation with H2 and O2" Catalysts 15, no. 7: 694. https://doi.org/10.3390/catal15070694
APA StyleMi, Z., Long, H., Jia, Y., Ma, Y., Miao, C., Xie, Y., Zhu, X., & Huang, J. (2025). Highly Efficient and Stable Ni-Cs/TS-1 Catalyst for Gas-Phase Propylene Epoxidation with H2 and O2. Catalysts, 15(7), 694. https://doi.org/10.3390/catal15070694







